ABSTRACT
Ticks transmit a large number of pathogens capable of causing human disease. In this study, the PCR-reverse line blot (RLB) method was used to screen for pathogens in a total of 554 Ixodes ricinus ticks collected from all provinces of Austria. These pathogens belong to the genera Borrelia, Rickettsiae, Anaplasma/Ehrlichia (including “Candidatus Neoehrlichia”), Babesia, and Coxiella. The pathogens with the highest detected prevalence were spirochetes of the Borrelia burgdorferi sensu lato complex, in 142 ticks (25.6%). Borrelia afzelii (80/142) was the most frequently detected species, followed by Borrelia burgdorferi sensu stricto (38/142) and Borrelia valaisiana (36/142). Borrelia garinii/Borrelia bavariensis, Borrelia lusitaniae, and Borrelia spielmanii were found in 28 ticks, 5 ticks, and 1 tick, respectively. Rickettsia spp. were detected in 93 ticks (16.8%): R. helvetica (39/93), R. raoultii (38/93), R. monacensis (2/93), and R. slovaca (1/93). Thirteen Rickettsia samples remain uncharacterized. “Candidatus Neoehrlichia mikurensis,” Babesia spp. (B. venatorum, B. divergens, B. microti), and Anaplasma phagocytophilum were found in 4.5%, 2.7%, and 0.7%, respectively. Coxiella burnetii was not detected. Multiple microorganisms were detected in 40 ticks (7.2%), and the cooccurrence of Babesia spp. and “Candidatus Neoehrlichia mikurensis” showed a significant positive correlation. We also compared different PCR-RLBs for detection of Borrelia burgdorferi sensu lato and Rickettsia spp. and showed that different detection approaches provide highly diverse results, indicating that analysis of environmental samples remains challenging.
IMPORTANCE This study determined the wide spectrum of tick-borne bacterial and protozoal pathogens that can be encountered in Austria. Surveillance of (putative) pathogenic microorganisms occurring in the environment is of medical importance, especially when those agents can be transmitted by ticks and cause disease. The observation of significant coinfections of certain microorganisms in field-collected ticks is an initial step to an improved understanding of microbial interactions in ticks. In addition, we show that variations in molecular detection methods, such as in primer pairs and target genes, can considerably influence the final results. For instance, detection of certain genospecies of borreliae may be better or worse by one method or the other, a fact of great importance for future screening studies.
KEYWORDS: Austria, Babesia, Borrelia burgdorferi, Ixodes ricinus, method comparison, reverse line blot, Rickettsia, tick screening
INTRODUCTION
Ticks carry and transmit a variety of pathogens (bacteria, protozoa, viruses) that can lead to serious infections in humans (1). In Europe, the majority of vector-borne diseases are caused by tick-borne pathogens; thus, knowledge about such pathogens is of medical relevance.
The hard tick Ixodes ricinus is the most important vector in Europe (2) and the most frequent tick species in Austria (3). The main part of this study deals with determining the presence of the following microbial genera in I. ricinus ticks: Borrelia, Rickettsia, Anaplasma, “Candidatus Neoehrlichia mikurensis,” Babesia, and Coxiella.
Tick-borne diseases, most notably Lyme borreliosis, are highly endemic in Austria (4). In some areas, the seroprevalence of antibodies to Borrelia burgdorferi sensu lato in persons frequently exposed to ticks (e.g., hunters) has reached about 54% (5). Thus far, five borrelial genospecies have been identified in I. ricinus populations in different regions of Austria: B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. bavariensis, and B. valaisiana (6–9). The mean overall infection rate in I. ricinus ticks collected in different regions of Austria ranges from 10.9% to 33.3% (6, 7, 10–13).
Among other pathogens, the prevalence of Rickettsia spp. ranges between 12.4% in the west of Austria (Tyrol) (14) and 17% in the east of the country (12); in a further study, the overall prevalence for Rickettsia helvetica was 35.6% (15). Anaplasma phagocytophilum has been detected in 1.0% to 8.7% of I. ricinus ticks (6, 12, 16, 17), and Babesia spp. have been detected in a surprisingly high 51.7% (18). The emerging pathogen “Candidatus Neoehrlichia mikurensis” has been detected twice in Austrian ticks (6, 19). Coxiella burnetii was detected once in I. ricinus ticks from Tyrol in 1994, by means of the hemocyte test (20); however, there has not been any molecular evidence of its presence (21, 22).
Molecular methods are routinely used for direct detection of microorganisms for clinical and research purposes. The PCR-reverse line blot (RLB) is useful in broad-range studies because it enables simultaneous screening for multiple agents in a single sample (23).
We used this method in our study to investigate the presence of various pathogens in I. ricinus ticks collected in different regions of Austria in 2005 and from Vienna in 2013. The application of the RLB method has provided a more comprehensive insight into the presence of coinfections with different pathogenic agents and has allowed us to extend our earlier results (7, 15, 18). We also compared different RLB-based detection methods for B. burgdorferi sensu lato and Rickettsia spp. The first comparison concerned two previously described RLBs for B. burgdorferi sensu lato (24, 25), both targeting the 5S-23S intergenic spacer (IGS) region but using different primers. The second comparison, for detection of Rickettsia spp., involved two RLBs based on different target genes, in particular, the 16S rRNA gene (26, 27) and the 23S-5S IGS (28). In this study, we compared these methods for their sensitivity and performance and applied them to the same environmental tick samples. During the evaluation, we found high variations in how particular molecular detection methods affect the results. The findings of the current work are therefore of importance for further epidemiological studies.
RESULTS
Detection of B. burgdorferi sensu lato.
DNA of B. burgdorferi sensu lato was detected in 142 (25.6%) of the 554 ticks tested (Table 1). Of the 494 ticks from the 2005 collection, 126 ticks (25.5%) were positive; of the 2013 Vienna collection, 26.7% (16/60) were positive. Furthermore, we identified coinfections with multiple Borrelia species in 39 (27.5%) of the positive ticks; most were dual infections (33/142), five ticks harbored three species, and a single tick was infected with four species (Table 2). Multiple genospecies occurred more often in nymphal ticks (31.8%) than in adults (21.1%); however, the difference was not significant (P = 0.183).
TABLE 1.
Microorganisms detected in ticks collected in the provinces of Austriaa
| Province (no. of ticks tested) | No. of positive ticks |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Borrelia spp. |
Rickettsia spp. |
Babesia spp. |
Anaplasma/Ehrlichia spp. |
||||||||||||||||
| Total | Bbss | Ba | Bg/Bbav | Bv | Blus | Bsp | Total | Rh | Rr | Rm | Rs | Unid. | Total | Bve | Bd | Bm | Ap | CNM | |
| Vienna (60) | 16 | 5 | 4 | 5 | 5 | 30 | 3 | 24 | 2 | 1 | 2 | 2 | 1 | 5 | |||||
| Lower Austria (64) | 12 | 3 | 4 | 2 | 3 | 3 | 12 | 2 | 8 | 2 | 1 | 1 | 2 | ||||||
| Burgenland (60) | 14 | 1 | 11 | 3 | 2 | 4 | 1 | 3 | 3 | ||||||||||
| Upper Austria (60) | 17 | 6 | 8 | 5 | 6 | 6 | 4 | 1 | 1b | 2 | 1 | 1 | 1 | 4 | |||||
| Salzburg (62) | 16 | 1 | 12 | 2 | 3 | 1 | 7 | 7 | 2 | 2 | 1 | ||||||||
| Styria (62) | 17 | 4 | 11 | 2 | 4 | 7 | 5 | 1 | 1 | 1 | 1 | 2 | |||||||
| Carinthia (63) | 12 | 4 | 7 | 6 | 15 | 7 | 4 | 4 | 2 | 1 | 1 | 2 | |||||||
| Tyrol (61) | 17 | 6 | 11 | 5 | 4 | 1 | 3 | 3 | 2 | 2 | 5 | ||||||||
| Vorarlberg (62) | 21 | 8 | 12 | 4 | 3 | 1 | 9 | 7 | 1 | 1 | 3 | 1 | 1 | 1 | 2 | ||||
| Total (554) | 142 | 38 | 80 | 28 | 36 | 5 | 1 | 93 | 39 | 38 | 2 | 1 | 13 | 15 | 8 | 4 | 3 | 4 | 24 |
Abbreviations: Bbss, B. burgdorferi sensu stricto; Ba, B. afzelii; Bg/Bbav, B. garinii/B. bavariensis; Bv, B. valaisiana; Blus, B. lusitaniae; Bsp, B. spielmanii; Rh, R. helvetica; Rr, R. raoultii; Rm, R. monacensis; Rs, R. slovaca; Unid, unidentified; Bve, B. venatorum; Bd, B. divergens; Bm, B. microti; Ap, A. phagocytophilum; CNM, “Candidatus Neoehrlichia mikurensis.”
Unidentified Rickettsia sp. that does not belong to the SFG rickettsiae.
TABLE 2.
Borrelia genospecies detected in Borrelia-positive ticks (n = 142)
| Genospecies | No. (%) of ticks |
|---|---|
| Single infections | 103 (72.5) |
| B. afzelii | 55 (38.7) |
| B. burgdorferi sensu stricto | 18 (12.7) |
| B. garinii/B. bavariensisa | 14 (9.9) |
| B. valaisiana | 13 (9.2) |
| B. lusitaniae | 3 (2.1) |
| Dual infections | 33 (23.2) |
| B. burgdorferi sensu stricto + B. afzelii | 8 (5.6) |
| B. afzelii + B. valaisiana | 8 (5.6) |
| B. garinii/B. bavariensis + B. valaisiana | 7 (4.9) |
| B. burgdorferi sensu stricto + B. valaisiana | 4 (2.8) |
| B. afzelii + B. garinii/B. bavariensis | 3 (2.1) |
| B. burgdorferi sensu stricto + B. spielmanii | 1 (0.7) |
| B. burgdorferi sensu stricto + B. garinii/B. bavariensis | 1 (0.7) |
| B. valaisiana + B. lusitaniae | 1 (0.7) |
| Triple infections | 5 (3.5) |
| B. burgdorferi sensu stricto + B. afzelii + B. garinii/B. bavariensis | 3 (2.1) |
| B. burgdorferi sensu stricto + B. afzelii + B. valaisiana | 2 (1.4) |
| Quadruple infections | 1 (0.7) |
| B. burgdorferi sensu stricto + B. afzelii + B. valaisiana + B. lusitaniae | 1 (0.7) |
B. garinii and B. bavariensis cannot be discriminated with the amplified target gene (5S-23S IGS).
Overall, the most common genospecies among the 142 positive ticks was B. afzelii (56.3%), followed by B. burgdorferi sensu stricto (26.8%) and B. valaisiana (25.4%). B. garinii/B. bavariensis, Borrelia lusitaniae, and Borrelia spielmanii were found in 19.7%, 3.5%, and 0.7%, respectively. In the 2005 tick cohort, the distribution of species within the 126 positive ticks was the same, but with slightly different rates: B. afzelii (60.3%), B. burgdorferi sensu stricto (26.2%), B. valaisiana (24.6%), B. garinii/B. bavariensis (18.3%), B. lusitaniae (4.0%), and B. spielmanii (0.8%). In the 16 positive ticks from Vienna, B. burdorferi sensu stricto, B. garinii/B. bavariensis, and B. valaisiana occurred equally often (31.3%), followed by B. afzelii (25.0%). B. lusitaniae and B. spielmanii were not detected.
For the first time, we detected B. lusitaniae (five ticks) and B. spielmanii (one tick) in I. ricinus ticks collected in Austria. Three of the B. lusitaniae-positive ticks came from a single collection site in Lower Austria (Ebreichsdorf), one from Salzburg (Goldegg), and one from Tyrol (Imst). The only tick that was positive for B. spielmanii was collected in Vorarlberg (Thüringen) and was coinfected with B. burgdorferi sensu stricto.
More adult ticks (33.1%) than nymphs (22.3%) were infected with B. burgdorferi sensu lato (see Table S4 in the supplemental material), and the difference was significant (P = 0.008).
Detection of Rickettsia spp.
Rickettsia spp. were detected in 93 (16.8%) of the 554 ticks. The percentages of positive ticks were 12.8% (63/494) in the 2005 collection and 50.0% (30/60) in the 2013 Vienna collection.
Overall, the most frequently detected species was R. helvetica in 39 (41.9%) ticks, followed by Rickettsia raoultii in 38 (40.9%), Rickettsia monacensis in two (2.2%), and Rickettsia slovaca in one (1.1%). Thirteen ticks (14.0%) harbored Rickettsia spp. that gave a signal only with the genus-specific probes (Table 1). All of them, with the exception of one tick collected in Niederottensheim in Upper Austria, reacted with the spotted fever group (SFG) Rickettsia-specific probe on the 23S-5S RLB. In the 63 positive ticks from 2005, the most abundant species was R. helvetica (57.1%), followed by R. raoultii (22.2%), R. slovaca (1.6%), and other Rickettsia spp. (19.1%). Among the 30 positive ticks from Vienna, R. raoultii was the most frequently detected species (80.0%), followed by R. helvetica (10.0%), R. monacensis (6.7%), and other not further identified Rickettsia spp. (3.3%). Coexistence of multiple Rickettsia spp. did not occur.
For the first time, we detected Rickettsia spp. other than R. helvetica in I. ricinus ticks in Austria. Of the R. raoultii-positive ticks, the majority (24 ticks) was collected in Vienna in 2013, where the location Lainzer Tiergarten represented a hot spot with 20 positive ticks (66.7% R. raoultii-positive). This species was also detected in eight ticks from Lower Austria (seven from Wolfenreith, one from Hüttendorf), four ticks from Carinthia (two each from Drobollach and Molzbichl), one from Styria (Mürzzuschlag), and one from Upper Austria (Stallhofen). R. monacensis was detected only in Vienna (Prater/Lusthaus) after bidirectional sequencing. R. slovaca was found in one tick from Vorarlberg (Thüringen) and was repeatedly positive in the 23S-5S IGS RLB but not in the 16S RLB.
We observed a significant difference in the infection rates of adult (23.3%) and nymphal (13.9%) ticks (P = 0.010) (Table S4).
Detection of Babesia spp.
We detected Babesia spp. in 2.7% (15/554) of all ticks: 13 ticks (2.6%) from the 2005 collection and two (3.3%) from the 2013 Vienna collection. Babesia venatorum was the only species found in the latter collection.
B. venatorum was also the most frequently detected species, in eight ticks (53.3%; six from 2005, two from 2013), followed by Babesia divergens in four ticks (26.7%), and Theileria (Babesia) microti was detected for the first time in three ticks (20.0%)—one nymph in Upper Austria (Stallhofen), one female in Vorarlberg (Klaus), and one male in Carinthia (Molzbichl).
Adult ticks had an infection rate of 4.1%, nymphs had an infection rate of 2.1% (Table S4); however, the difference was not significant (P > 0.05).
Detection of Anaplasma/Ehrlichia spp.
Only four nymphal ticks (0.7% mean overall prevalence) were positive for A. phagocytophilum with the RLB (Table 1). Among the four different probes used for this species (Table 3), which cover different genotypes of A. phagocytophilum, only the A. phagocytophilum 3 probe (29) yielded a signal. The positive ticks were collected in Carinthia (two ticks), Upper Austria (one), and Vienna (one).
TABLE 3.
Primers and probes used in reverse line blots
| Target species for primer or probea | Target gene | Nucleotide sequence (5′→3′)b | Referencec |
|---|---|---|---|
| Primers | |||
| Borrelia burgdorferi sensu lato | 5S-23S IGS | ACC ATA GAC TCT TAT TAC TTT GAC CA | 24 |
| Biotin-GAG AGT AGG TTA TTG CCA GGG | 24 | ||
| TCA GGG TAC TTA GAT GGT TCA CTT | 25 | ||
| Biotin-GAG TTC GCG GGA GAG TAG GTT ATT | 25 | ||
| Rickettsia spp. | 16S rRNA | GAA CGC TAT CGG TAT GCT TAA CAC A | 27, modified from reference 26 |
| Biotin-CAT CAC TCA CTC GGT ATT GCT GGA | 27, modified from reference 26 | ||
| 23S-5S IGS | GAT AGG TCR GRT GTG GAA GCA C | 28 | |
| Biotin-TCG GGA YGG GAT CGT GTG TTT C | 28 | ||
| Anaplasma/Ehrlichia spp. | 16S rRNA | GGA ATT CAG AGT TGG ATC MTG GYT CAG | 29 |
| Biotin-CGG GAT CCC GAG TTT GCC GGG ACT TYT TCT | 84 | ||
| Babesia/Theileria spp. | 18S rRNA | GAC ACA GGG AGG TAG TGA CAA G | 85 |
| Biotin-CTA AGA ATT TCA CCT CTG ACA GT | 85 | ||
| Coxiella burnetii | htpAB | TAT GTA TCC ACC GTA GCC AGT C | 78 |
| Biotin-CCC AAC AAC ACC TCC TTA TTC | 78 | ||
| Probes, membrane 1 | |||
| B. burgdorferi sensu lato | 5S-23S IGS | CTT TGA CCA TAT TTT TAT CTT CCA | 24 |
| B. burgdorferi sensu lato 2 | C TTC CAT CTC TAY TTT GCC AAT | This paper | |
| B. burgdorferi sensu stricto | AAC ACC AAT ATT TAA AAA ACA TAA | 24 | |
| B. afzelii | AAC ATT TAA AAA ATA AAT TCA AGG | 24 | |
| B. garinii/B. bavariensis | AAC ATR AAC ATC TAA AAA CAT AAA | 24, modified | |
| B. spielmanii | GTC AAT ATC TAT TTT CTT TTT TAT G | This paper | |
| B. valaisiana 2 | CAT GTC AAT ATC TAT TTT ATT TTT TAC ATT A | This paper | |
| B. valaisiana (VS116) | CAT TAA AAA AAT ATA AAA AAT AAA TTT AAG G | 24 | |
| B. valaisiana (VSNE) | TAT ATC TTT TGT TCA ATC CAT GT | 86 | |
| B. lusitaniae | TTT TTA AAT CAA ACA TTC AAA AAA AT | This paper | |
| B. lusitaniae (LusiNE) | TCA AGA TTT GAA GTA TAA AAT AAA A | 86 | |
| B. lusitaniae (LusiNE1) | CAT TCA AAA AAA TAA ACA TTT AAA AAC AT | 31 | |
| B. lusitaniae (LusiNE2) | AAA TCA AAC ATT CAA AAA AAT AAA C | 31 | |
| B. bissetti/B. carolinensis | CAC TAA CAT TTA AAA AAT ATA AAA TAA AAT | This paper | |
| Rickettsia spp. | 23S-5S IGS | TAG CTC GAT TGR TTT ACT TTG | 28 |
| SFG Rickettsiae | ACT CAC AAR GTT ATC AGG T | 28 | |
| Typhus group rickettsiae | GTT ATT CTA TCG TTT TAT GTY ACG | 28 | |
| R. bellii | GTG TTT ATT CTA TAA TAT GTC AG | 28 | |
| R. conorii | GTT ATA TAC TGT AGC CCT G | 28 | |
| R. aeschlimannii | ATA TTA TAC TGT ATG TAG CCC C | 28 | |
| R. rickettsii-R. sibirica | GTT ATA CTG TAG TCC TGC AA | 28 | |
| R. slovaca | GTA GCC CCT GCC ACG ATA | 28 | |
| R. helvetica | CAT GGC TTG ATC CAC GGT A | 28 | |
| R. raoultii | TCA ACT AAT AAA TTT GCT GAG TA | 81 | |
| R. monacensis | CAA TGT CAT ACC GTG GTC AAG | M. Wijnveld, unpublished | |
| C. burnetii | htpAB | GCA AGA ATA CGG ACT CAC GA | 77 |
| Probes, membrane 2 | |||
| Ehrlichia/Anaplasma spp. | 16S rRNA | GGG GGA AAG ATT TAT CGC TA | 84 |
| A. phagocytophilum 1 | TTG CTA TAA AGA ATA ATT AGT GG | 29 | |
| A. phagocytophilum 3 | TTG CTA TGA AGA ATA ATT AGT GG | 29 | |
| A. phagocytophilum 5 | TTG CTA TAA AGA ATA GTT AGT GG | Protocol book UCTD | |
| A. phagocytophilum 7 | TTG CTA TAG AGA ATA GTT AGT GG | Protocol book UCTD | |
| “Ca. Neoehrlichia mikurensis” | GCT GTA GTT TAC TAT GGG TA | 29 | |
| Theileria/Babesia catch-all | 18S rRNA | TAA TGG TTA ATA GGA RCR GTT G | 87 |
| Babesia catch-all 1 | ATT AGA GTG TTT CAA GCA GAC | 87 | |
| Babesia catch-all 2 | ACT AGA GTG TTT CAA ACA GGC | 87 | |
| B. divergens | ACT RAT GTC GAG ATT GCA C | 76 | |
| Theileria (Babesia) microti | GRC TTG GCA TCW TCT GGA | 87 | |
| B. canis canis | TGC GTT GAC GGT TTG AC | 88 | |
| B. canis canis 2 | TGG TTG GTT ATT TCG TTT TCG | 87 | |
| Babesia venatorum | CGA TTT CGC TTT TGG GAT T | 27 | |
| Theileria catch-all | ATT AGA GTG CTC AAA GCA GGC | 87 | |
| Rickettsia spp. | 16S rRNA | TTT AGA AAT AAA AGC TAA TAC CG | 26 |
| R. conorii | CTT GCT CCA GTT AGT TAG T | 26 | |
| R. helvetica | GCT AAT ACC ATA TAT TCT CTA TG | 26 | |
| R. massiliae | TGG GGC TTG CTC TAA TTA GT | 89 | |
| R. raoultii | CTA ATA CCG CAT ATT CTC TAC G | 27 |
All probes were labeled with a C6 amino linker for covalent binding to the membranes.
Bold letter indicates the difference from the original probe: nucleotide ambiguity code uses R to represent A or G to cover more species.
Protocol book UCTD, oligonucleotide sequences obtained from the Utrecht Center for Ticks and Tick-borne Diseases Summer School Protocol Book (2012), no original reference available.
Twenty-four (4.3%) of the 554 ticks were positive for “Candidatus Neoehrlichia mikurensis,” of which 19 were derived from the 2005 collection (3.8%) and 5 (8.3%) were collected in Vienna 2013. Adult and nymphal ticks had similar infection rates of 4.5% and 4.1%, respectively (P > 0.05) (Table S4). We detected no other genospecies belonging to the Anaplasma/Ehrlichia genera.
Detection of Coxiella burnetii.
None of the 554 ticks tested in this study were positive for C. burnetii.
Coinfections.
Of the 554 I. ricinus ticks tested, 235 (42.4%) harbored at least one pathogen that can cause disease in humans. Coinfections with multiple microorganisms occurred in 40 ticks (7.2%), corresponding to 17.0% of all positive ticks. An overview of the coinfections is shown in Table 4 and a more detailed overview of the dual infections at the species level is shown in Table S5.
TABLE 4.
Putative pathogenic microorganisms and coinfections detected in ticks
| Microorganism(s) | No. of ticks |
|---|---|
| Single infections | 195 |
| Borrelia spp. | 109 |
| Rickettsia spp. | 65 |
| Babesia spp. | 4 |
| “Ca. Neoehrlichia mikurensis” | 14 |
| A. phagocytophilum | 3 |
| Dual infections | 37 |
| Borrelia spp. + Rickettsia spp. | 22 |
| Borrelia spp. + Babesia spp. | 4 |
| Borrelia spp. + A. phagocytophilum | 1 |
| Borrelia spp. + “Ca. Neoehrlichia mikurensis” | 5 |
| Rickettsia spp. + Babesia spp. | 3 |
| Babesia spp. + “Ca. Neoehrlichia mikurensis” | 2 |
| Triple infections | 3 |
| Borrelia spp. + Rickettsia spp. + “Ca. Neoehrlichia mikurensis” | 1 |
| Rickettsia spp. + Babesia spp. + “Ca. Neoehrlichia mikurensis” | 2 |
The most common coinfection was the combination of B. burgdorferi sensu lato and Rickettsia spp. (23 ticks). Triple infections were detected in three ticks: all contained “Candidatus Neoehrlichia mikurensis” DNA, the other species being R. helvetica with B. divergens, R. raoultii with B. venatorum, and Rickettsia spp. with B. afzelii.
Cooccurrences of pairs of tick-borne pathogens were investigated with Pearson's chi-square test. The only significant observation was for cooccurrence of Babesia spp. with “Candidatus Neoehrlichia mikurensis” (P < 0.001); Pearson's correlation analysis showed a positive correlation (r = 0.183, P < 0.001). Unfortunately, the number of Babesia-positive ticks in this survey was low; therefore, it was not possible to determine whether certain Babesia species are more commonly associated with the presence of “Candidatus Neoehrlichia mikurensis” than others or whether the developmental stages of the ticks played a role.
Comparison of Borrelia PCR-RLBs.
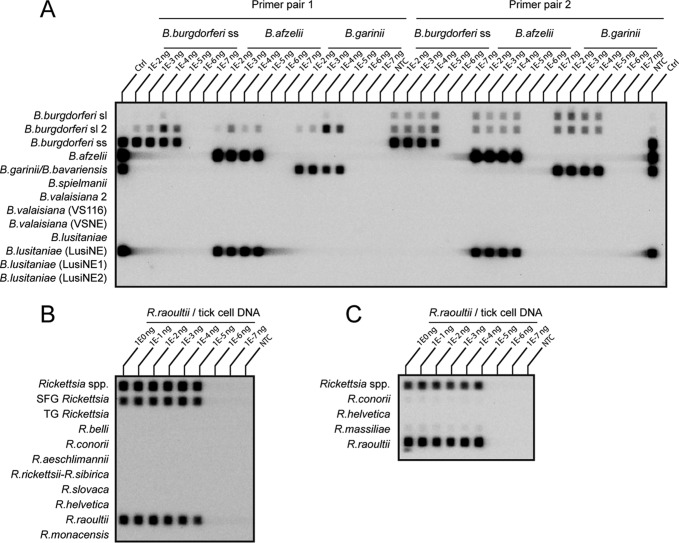
Sensitivity of both PCR-RLBs was evaluated in triplicate for five B. burgdorferi sensu lato strains. All five strains were detected with both methods to a detection limit of 10−5 ng total borrelial DNA (Fig. 1).
FIG 1.
Sensitivity determinations for Borrelia and Rickettsia RLBs. (A) The detection limit for both primer pairs in the Borrelia RLB is 10−5 ng borrelial DNA, as demonstrated here for three of the tested B. burgdorferi sensu lato (sl) strains in a dilution series from 10−2 ng to 10−7 ng DNA. Cross-reaction of our B. afzelii strain with the B. lusitaniae probe LusiNE can be seen on this RLB, also described by Gern et al. (31). ss, sensu stricto. (B and C) The detection limit for R. raoultii was 10−5 ng DNA (containing host cell DNA) on the 23S-5S IGS RLB (B) and on the 16S rRNA gene RLB (C). This corresponds to 18 rickettsial cells, as calculated in qPCR.
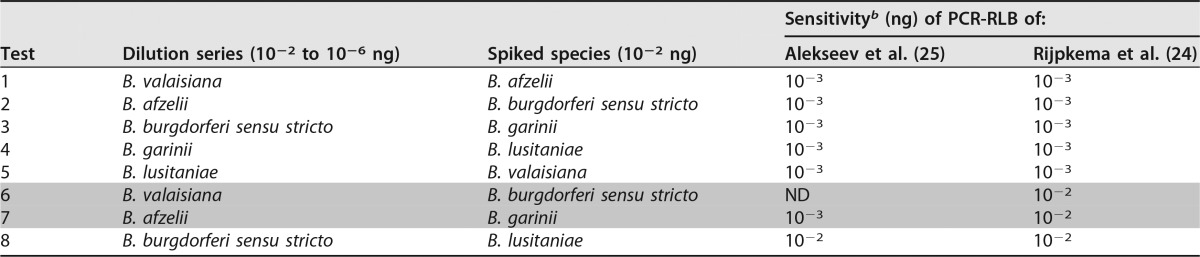
When we spiked a dilution series of one strain with 10−2 ng DNA of another strain, the detection limit of the diluted strain decreased from 10−5 ng to 10−3 ng or in some cases to 10−2 ng (Table 5). When the dilution series of B. valaisiana was spiked with 10−2 ng of B. burgdorferi sensu stricto DNA in the PCR-RLB of Alekseev et al. (25), B. valaisiana was not detected at all, whereas in the PCR-RLB of Rijpkema et al. (24), the detection limit for B. valaisiana simply decreased to 10−2 ng DNA. When the dilution series of B. afzelii was spiked with 10−2 ng DNA of B. garinii, the PCR-RLB of Alekseev et al. performed better (detection limit for B. valaisiana, 10−3 ng) than that of Rijpkema et al. (detection limit for B. valaisiana, 10−2 ng).
TABLE 5.
Sensitivity for the detection of multiple B. burgdorferi sensu lato speciesa
Rows shaded in gray indicate the combination for which a difference in results between the methods was observed.
b Sensitivity of detection for the diluted strain when spiked with 10−2 ng of another strain. ND, not detected.
Next, we compared the primer pairs of Alekseev et al. (25) and Rijpkema et al. (24) (Table 3) on the basis of the tick screening results. As described earlier, B. afzelii was the most common genospecies detected (56.3%) when the detection methods were considered together. This finding remained the same when the PCR-RLBs were analyzed independently (56.9% and 56.0% with the primers of Rijpkema et al. and Alekseev et al., respectively). However, slight changes in the prevalences of other B. burgdoferi sensu lato genospecies occurred when the methods were analyzed separately. With the primer pair of Rijpkema et al., the second most commonly found species was B. valaisiana at 26.3% (36/137), followed by B. burgdorferi sensu stricto at 25.5% (35/137), B. garinii/B. bavariensis at 20.4% (28/137), B. lusitaniae at 3.6% (5/137), and B. spielmanii at 0.7% (1/137). Using the primer pair designed by Alekseev et al., the second most frequently detected species was B. burgdorferi sensu stricto at 24.0% (18/75), followed by B. garinii/B. bavariensis at 20.0% (15/75), B. valaisiana at 16.0% (12/75), and B. lusitaniae at 2.7% (2/75). B. spielmanii was not detected with these primers.
The tick screening results were compared using McNemar's test. The calculation considering all samples showed a significant difference (P < 0.001). A contingency table (Fig. 2) showed that 67 samples were positive only with the primers of Rijpkema et al., whereas five samples (three B. burgdorferi sensu stricto, two B. afzelii) were positive only with the primer pair of Alekseev et al.
FIG 2.
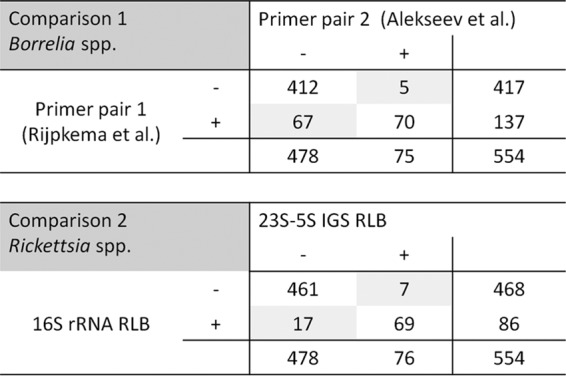
Contingency tables for comparison of the different methods using McNemar's test.
The mean overall B. burgdorferi sensu lato infection rate of ticks, with both methods taken into account, was 25.6%. When the screening tests were analyzed separately, the mean overall prevalences were 13.5% using the primer pair of Alekseev et al. and 24.7% with the primers of Rijpkema et al.
To further investigate whether this difference might relate to particular genospecies being preferentially amplified, we split the data set by the species detected. To avoid distortion of the calculation by coinfections with multiple B. burgdorferi sensu lato genospecies, we filtered the data for single infections only. McNemar's test was used for B. burgdorferi sensu stricto and B. afzelii, as these were the only species detected in sufficient numbers in single infections. The difference between the primer pairs was significant for detection of B. afzelii (P < 0.001) but not for B. burgdorferi sensu stricto (P = 0.344).
Comparison of Rickettsia RLBs.
The sensitivity of the two RLBs was evaluated by testing a 10-fold serial dilution of DNA of R. raoultii in tick cell DNA in triplicate. Using a quantitative PCR (qPCR) targeting the single-copy citrate synthase gene (gltA), we determined the number of genome equivalents by generating a standard curve (slope, −3.004; y intercept, 42.726; R2, 0.988; efficiency, 115.201%) in the Rickettsia-host cell DNA mix. Our tests showed that detection limits for the 16S rRNA gene RLB and the 23S-5S IGS RLB were the same, with a determined number of 18 genome equivalents in 10−5 ng of R. raoultii-host cell DNA. Hence, at least for the tested R. raoultii strain, the RLBs performed equally well.
For detection of Rickettsia spp. in our tick DNA samples, we compared the results of the 16S rRNA gene (27) and the 23S-5S IGS region (28) using McNemar's test; there was no significant difference between the RLBs (P = 0.064). The results were consistent for 461 negative and 69 positive ticks. The differences were due to seven ticks that were positive only with the 23S-5S IGS RLB and 17 ticks that gave a signal only in the 16S rRNA RLB (Fig. 2).
Next, we compared the performances of both methods in relation to the Rickettsia spp. detected. When the data were split according to species, the relevant cell counts were too low for a valid McNemar's test. However, the contingency table showed that the significant difference was caused only by the ticks that reacted with the 16S R. raoultii probe, representing 40.9% of the Rickettsia-positive ticks. Of these, 14 of 38 were positive only with the 16S rRNA gene RLB and 24 were positive with both tests. None of them were positive with the 23S-5S IGS RLB alone. Furthermore, we detected the two R. monacensis-positive ticks with either method but R. slovaca only in the 23S-5S IGS RLB. In detection of R. helvetica, the two tests performed equally well (38 of 39 ticks positive in both tests), and only a single sample was false negative in the 23S-5S IGS RLB.
Analysis of the results of the tick screening separately resulted in an overall Rickettsia prevalence of 13.7% (76/554) with the 23S-5S IGS RLB and 15.7% (86/554) with the 16S RLB, compared with 16.8% when the methods were combined. Single analysis of the RLBs did not have a major impact on the order of frequencies of identified Rickettsia spp. The most common rickettsial species detected using the 16S rRNA-based RLB was R. helvetica, in 45.3% (39/86) of ticks, followed by R. raoultii in 44.2% (38/86) and R. monacensis in 2.3% (2/86). In analysis of the 23S-5S IGS RLB, the order stayed the same, but the frequencies differed: R. helvetica was detected in 50.0% (38/76), R. raoultii in 35.5% (27/76), R. monacensis in 2.6% (2/76), and R. slovaca in 1.3% (1/76). Unidentified Rickettsia spp. were detected in 8.1% (7/86) and 10.5% (8/76) of ticks with the 16S rRNA gene RLB and the 23S-5S IGS RLB, respectively.
DISCUSSION
In this study, the PCR-RLB technique was used to assess the prevalence of various tick-borne pathogens in I. ricinus ticks collected in Austria in 2005 and 2013. Compared to more modern techniques, such as deep sequencing, it is a very feasible tool for large epidemiological studies due to its relatively low costs, widely available laboratory equipment, and ease of use. High numbers (42.4%) of the investigated ticks were positive for microorganisms of medical and veterinary importance.
The most frequently detected pathogen was B. burgdorferi sensu lato, at a prevalence of 25.6% (25.5% in 2005 and 26.7% in Vienna in 2013); this value is within the infection rates reported from the surrounding countries (30–36). Our results are also in accordance with other Austrian studies in the province of Styria (6) and eastern Austria (12). In a previous study (7) on ticks collected from all regions of Austria in 2005, the overall prevalence was only 14.5%; however, nearly a third of those ticks were larvae. In the present study, larval ticks were excluded because transovarial transmission of B. burgdorferi sensu lato does not efficiently contribute to maintain these spirochetes in the natural cycle, and even if vertical transmission occurs, it is inefficient (37, 38). By subtracting the number of larval ticks tested in the previous study by Blaschitz et al. (7), the prevalence increases to 19.6% in their study and is closer to our result.
The comparison of methods in the present study also showed that results differed significantly according to the method used (13.5% versus 24.7% with either of the primer pairs). Moreover, we have clearly shown that some species are preferentially amplified by certain primer pairs, as indicated by our experiment for detection of multiple B. burgdorferi sensu lato genospecies with both primer pairs and by our finding during the tick screening study that B. afzelii was significantly more often detected using the primers of Rijpkema et al. than with the primers of Alekseev et al.
In our study, B. afzelii was the dominating Borrelia species (56.3%). This is in good agreement with many other studies that describe B. afzelii as the most prevalent B. burgdorferi sensu lato species in ticks in Europe (6, 31, 33, 34). In humans, the most common manifestation of Lyme borreliosis is erythema migrans (39). This skin-associated lesion is often linked to the presence of B. afzelii (8, 40, 41), the most common species identified in patient samples in our institute (A. Schötta, M. Reiter, I. Korschinek, A. Müller, G. Khanakah, H. Stockinger, and G. Stanek, presented at the 33rd Annual Meeting of the Austrian Society for Hygiene, Microbiology and Preventive Medicine, Salzburg, Austria, 21 to 24 May 2012).
In the tick screening study mentioned above (7), the dominating species was B. garinii, in 66.9% of the positive ticks; B. afzelii was detected in only 11.3%. This difference might be explained by the use of different detection methods, as demonstrated in the present study where the proportions of the various B. burgdorferi sensu lato genospecies differed according to the method used. Furthermore, different cohorts of ticks from the 2005 collection were used in the two studies, which may also contribute to this difference.
In Austria, five species of the B. burgdorferi sensu lato complex have been reported so far: B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. bavariensis, and B. valaisiana (6, 7, 9). In the present study, we also detected B. lusitaniae and B. spielmanii, and to the best of our knowledge, this is the first report of these genospecies in I. ricinus ticks in Austria.
We detected high numbers of coinfections (27.5%) with multiple genospecies among the B. burgdorferi sensu lato-positive ticks, similarly to other studies (42, 43). In Denmark, coinfections with multiple strains were detected even more frequently than single infections (42). These findings imply that people living in areas where ticks are endemic and that have a high prevalence of B. burgdorferi sensu lato can become infected with different strains at the same time, and indeed cases of human infections with multiple Borrelia strains have been reported (44, 45). In a meta-analysis of the Borrelia genospecies occurring in ticks in Europe (46), about 13% of ticks contained mixed infections, with cooccurrence of B. garinii and B. valaisiana being the most common (46). This combination occurred frequently (7/33 dual infections) in our study, but the most common coinfections detected were B. afzelii with either B. burgdorferi sensu stricto or B. valaisiana (8/33 dual infections). In an extensive investigation of the occurrence and ecology of B. burgdorferi sensu lato coinfections in ticks (47), it was concluded that certain genospecies facilitate or inhibit the spirochete load of other genospecies, depending on their host specificity. B. afzelii and B. burgdorferi sensu stricto, which are both rodent-associated genospecies, appeared to facilitate growth when occurring together, whereas the combination of B. afzelii and B. valaisiana, the latter being an avian-associated genospecies, was described as growth inhibiting (47). In our screening, coinfections of B. afzelii with either B. valaisiana or B. burgdorferi sensu stricto occurred equally often.
We did not investigate the spirochete load within ticks harboring multiple strains to determine which genospecies was dominant. Nevertheless, our results show that in Austria, the prevalence of Borrelia in ticks is high and that high numbers of ticks harbor more than one B. burgdorferi sensu lato species. Human infections with multiple Borrelia strains therefore can result from transmission during a single tick bite or following sequential tick bites in tick-infested areas. So far, there have been no reports of cultivation of more than one Borrelia genospecies from a single infection site, such as from erythema migrans; however, different genospecies have been cultivated from different body sites in individual patients. No evidence exists for an ambiguous course that might impede the clinical diagnosis (48).
We detected Rickettsia spp. as the second most frequently occurring pathogen in Austrian ticks, with a mean overall infection rate of 16.8%; however, the proportion of Rickettsia-positive ticks was 12.8% in the 2005 collection and 50% in the 2013 Vienna collection. The prevalence of Rickettsia spp. in questing I. ricinus ticks in the countries surrounding Austria typically does not exceed 20% (30, 32, 49–54). A previous Austrian study found a higher rate of 35.6% in ticks from the 2005 collection (15). Possible reasons for this difference might be that rickettsiae are transovarially transmitted (55) and that large numbers of larvae were tested in the earlier study. However, subtracting the larvae from the previous study would lead to an increase in positivity to 41.5%. It is possible that in the study of Blaschitz et al. (15), ticks from certain collection sites may have represented hot spots for rickettsiae, a result of the rickettsial life cycle. This was also the case in our screening of ticks collected in Vienna, where one collection site was identified as a hot spot in which 66.7% of ticks tested positive. Another explanation could be the use of different detection methods in the two screenings. In the present study, we compared two RLB approaches in parallel, one targeting the 16S rRNA gene (27) and the other targeting the 23S-5S IGS (28), and detected a significant difference between the two methods in detection of Rickettsia spp. R. raoultii was more often detected with the 16S rRNA gene-based RLB, whereas R. slovaca was detected only when using the 23S-5S IGS RLB. This finding indicates that detection of certain genospecies is better or worse depending on the method used. The use of different assays might also explain why only R. helvetica had been found in Austrian ticks in earlier studies (15, 56).
With the methods used in the present study, we also detected R. monacensis, R. raoultii, and R. slovaca in questing I. ricinus ticks collected from vegetation. The detection of R. monacensis in I. ricinus ticks adds to findings in surrounding countries, where the presence of this species has been confirmed (30, 32, 49, 54). However, finding this high proportion of R. raoultii (40.9% of Rickettsia species-positive ticks) and one R. slovaca-positive I. ricinus tick in our investigation was surprising, as these species are usually associated with Dermacentor ticks (55, 57). The single R. slovaca-positive tick could have fed on a host that was infected with this Rickettsia species. A single I. ricinus tick harboring R. slovaca DNA was found in another epidemiological study (58); nevertheless, the preferential vectors for R. slovaca remain Dermacentor ticks. Growth studies have shown that R. slovaca grown in D. marginatus ticks reaches much higher numbers than it does in I. ricinus ticks. Further, when I. ricinus ticks are artificially infected with R. slovaca, a third of the ticks die (59). It seems unlikely, therefore, that I. ricinus plays a major role in maintaining the life cycle of this Rickettsia species.
This might not be the case for the high number of R. raoultii-positive I. ricinus ticks that we detected in different parts of Austria. Cross-reactions of other Rickettsia spp. with the probe used for species identification on the RLB was ruled out by sequencing the partial 16S rRNA gene of multiple positive ticks; this yielded 100% identity with R. raoultii. Moreover, this is not the first report of detection of R. raoultii in I. ricinus ticks. In a study in Poland (58), 18.2% of I. ricinus ticks contained R. raoultii. The question arises, therefore, whether I. ricinus has vector potential for transmitting R. raoultii to humans after a tick bite. Transmission studies would be of great interest to evaluate whether I. ricinus ticks harboring this agent can constitute a risk to human health. Moreover, seroprevalence studies investigating whether people living in tick-infested regions are in contact with certain tick-borne microorganisms can be of clinical value. As Rickettsia spp. were found in 16.8% of the ticks tested, which is approximately 10 percentage points below the prevalence of B. burgdorferi sensu lato, one would expect that a few clinical cases occur in Austria. However, to the best of our knowledge, no infections with SFG rickettsiae have been reported. Nevertheless, in seroprevalence studies (in Tyrol, for example), 7.7% of 1,200 blood donors had antibodies against R. helvetica (14). Human rickettsiosis might therefore be underdiagnosed in Austria because of etiologically unexplained nonspecific symptoms or a short, self-limiting course of disease (55).
“Candidatus Neoehrlichia mikurensis” was detected in 4.3% of the ticks tested, an infection rate in agreement with reports from surrounding countries, where the prevalence in questing I. ricinus ticks ranged from around 1% to nearly 12% (32, 50, 60–62). Interestingly, in a study of ticks collected in Austria (19), the highest prevalence ever recorded in Europe, 23.5%, was reported. Unfortunately, the year in which the ticks were collected was not stated. In our study, we show that “Candidatus Neoehrlichia mikurensis” was present in I. ricinus ticks throughout Austria in 2005, only 6 years after its discovery as an Ehrlichia-like organism in the Netherlands (29). So far, this species has played a pathological role only in immunocompromised patients (63); however, in a Chinese study, “Candidatus Neoehrlichia mikurensis” was identified in the blood of febrile patients with a history of tick bite who were otherwise healthy (64). This organism has not yet been successfully cultivated, and no serological tests are available for evaluation of its seroprevalence in regions where ticks are endemic or for detection of latent infections.
Strikingly, we found a positive correlation for the presence of “Candidatus Neoehrlichia mikurensis” and Babesia spp., but the low numbers of ticks positive for these two microorganisms did not permit further evaluation of whether certain Babesia species are more commonly associated with “Candidatus Neoehrlichia mikurensis” or whether the life stage of the ticks plays a role.
The related pathogen A. phagocytophilum occurred in only 0.7% of ticks tested, well within the range of prevalence detected in neighboring countries, where it rarely exceeded 5% (30, 32, 50, 60, 61). A few patients with human granulocytic anaplasmosis have been described in Austria (65, 66), of which nearly all required hospitalization. One of four A. phagocytophilum-positive ticks in our study also harbored B. burgdorferi sensu lato. According to North American observation, human coinfections with A. phagocytophilum and B. burgdorferi sensu lato can result in a more severe outcome, for example, enhanced migration of the spirochetes through the brain-blood barrier (67).
For Babesia spp., we detected a mean overall prevalence of 2.7% in the questing I. ricinus ticks and, similar to prevalences of other tick-borne pathogens detected in our study, this rate is comparable to those reported in neighboring countries. The prevalence in I. ricinus ticks is usually low and ranges from 0% to around 10% (30, 32, 50, 68, 69). In contrast, in a previous study in Austrian ticks, the mean overall prevalence for Babesia spp. was 51.0% (18). This extraordinary finding was explained by high tick densities at certain sampling sites and by the life cycle of Babesia spp., which possess the potential of transstadial as well as transovarial transmission (70). Once the pathogen is introduced to an area, the probability is high that these areas will become hot spots, meaning that the whole tick population contains Babesia spp. In areas of high tick density, it is more likely that ticks belonging to the same population are collected, and therefore observations of high prevalences, as in the study by Blaschitz et al., are possible. The difference in the present study might be explained by multiple factors: first, we investigated different numbers of ticks per collection site; second, we tested completely different collection sites; and last, we used a different detection method that can have a major influence on the results. This might also be a reason why we were able to detect Theileria (Babesia) microti in questing I. ricinus ticks for the first time in Austria.
We did not detect the pathogen C. burnetii in the I. ricinus ticks tested. Few other studies have investigated I. ricinus ticks for the presence of C. burnetii and either could not detect this microorganism (71, 72) or detected it in very low numbers (73). Nevertheless, in Austria, C. burnetii was detected in Tyrol in 1994 by use of the hemocyte test (20), a commonly used method for detection of rickettsiae and Rickettsia-like organisms in the hemolymph of ticks. Furthermore, 2% of the Austrian adult population carry antibodies against C. burnetii (74), indicating circulation of this organism in the Austrian environment.
In summary, we have identified the borrelial species B. lusitaniae and B. spielmanii for the first time in Austrian I. ricinus ticks. In the same tick collection, we also found for the first time R. raoultii, R. monacensis, and R. slovaca. We also detected A. phagocytophilum and “Candidatus Neoehrlichia mikurensis” in new regions in Austria. Furthermore, in addition to B. divergens and B. venatorum, we detected Theileria (Babesia) microti for the first time in Austrian I. ricinus ticks. An interesting observation in the present study was the significant cooccurrence of Babesia spp. with “Candidatus Neoehrlichia mikurensis.” We also showed that the choice of the detection method influences the outcome of the results, even when the initial evaluation of the methods results in equal performances. The difference in the detection methods is reflected in the infection rates of ticks with different microorganisms and even in the pathogen species detected.
Moreover, we found a high number of tick-borne pathogens circulating in Austrian I. ricinus populations and coinfections with different organisms in 17% of pathogen-positive ticks, which may be of medical relevance because several of the identified pathogens might promote the virulence of others (67).
MATERIALS AND METHODS
Ticks.
Questing I. ricinus ticks in all provinces of Austria were collected from vegetation using the flagging method. The ticks tested in this study were derived from two sampling occasions. The first batch contained 494 ticks (149 adults, 345 nymphs) deriving from a collection made in summer 2005 over a time period of 3 months (beginning of May to beginning of August) from deciduous and mixed woodlands in eight Austrian provinces (Lower Austria, Upper Austria, Styria, Burgenland, Salzburg, Carinthia, Tyrol, Vorarlberg) of which a minimum number of 60 ticks per province (Fig. 3; Table 6) was tested during this study. The second batch comprised 60 ticks (23 adults, 37 nymphs) collected in two deciduous forests in October 2013 from the ninth province, Vienna.
FIG 3.
Tick collection sites in Austria. Names of provinces are underlined. Abbreviations of surrounding countries: D, Germany; CH, Switzerland; I, Italy; SLO, Slovenia; H, Hungary; SK, Slovakia; CZ, Czech Republic; FL, Liechtenstein. Abbreviations of the 27 collection sites: K, Klaus; W, Weiler; T, Thüringen; M, Mils; Im, Imst; F, Fügen; G, Goldegg; A, Adnet; N, Neufahrn; S, Stallhofe; No, Niederottensheim; Di, Dietachdorf; Wo, Wolfenreith; Ir, Irnfritz; SP, St. Pölten; Hd, Hüttendorf; E, Ebreichsdorf; L, Lainzer Tiergarten; P, Prater; B, Breitenbrunn; St, Stoob; O, Oberwart; Mz, Mürzzuschlag; Ad, Admont; SPL, St. Paul im Lavantal; Dr, Drobollach; Mo, Molzbichl.
TABLE 6.
Collection sites and numbers of ticks tested
| Yr of collection | Province | Collection site (abbreviation) | Total no. of ticks | No. of nymphs | No. of adults |
|---|---|---|---|---|---|
| 2005 | Styria | Mürzzuschlag (Mz) | 32 | 11 | 21 |
| Admont (Ad) | 30 | 30 | |||
| Salzburg | Golgegg (G) | 20 | 20 | ||
| Adnet (A) | 20 | 10 | 10 | ||
| Neufahrn (N) | 22 | 16 | 6 | ||
| Lower Austria | Hüttendorf (Hd) | 12 | 12 | ||
| St. Pölten (SP) | 13 | 7 | 6 | ||
| Irnfritz (Ir) | 14 | 8 | 6 | ||
| Ebreichsdorf (E) | 13 | 7 | 6 | ||
| Wolfenreith (W) | 12 | 7 | 5 | ||
| Burgenland | Stoob (St) | 20 | 20 | ||
| Oberwart (O) | 20 | 20 | |||
| Breitenbrunn (B) | 20 | 18 | 2 | ||
| Vorarlberg | Weiler (W) | 21 | 14 | 7 | |
| Klaus (K) | 21 | 3 | 18 | ||
| Thüringen (T) | 20 | 20 | |||
| Tyrol | Imst (Im) | 20 | 9 | 11 | |
| Mils (M) | 21 | 21 | |||
| Fügen (F) | 20 | 18 | 2 | ||
| Upper Austia | Dietachdorf (Di) | 20 | 12 | 8 | |
| Stallhofen (S) | 20 | 18 | 2 | ||
| Niederottenheim (No) | 20 | 10 | 10 | ||
| Carinthia | Molzbichl (Mo) | 20 | 5 | 15 | |
| Drobollach am Faakersee (Dr) | 22 | 19 | 3 | ||
| St. Paul im Lavantal (SPL) | 21 | 10 | 11 | ||
| 2013 | Vienna | Prater/Lusthaus (P) | 30 | 20 | 10 |
| Lainzer Tiergarten (L) | 30 | 17 | 13 |
The ticks from 2005, some of which have been used for previous studies in our institute (7, 15, 18), were stored at −80°C. For the present study, DNA was freshly extracted from ticks from this collection, and thus these ticks represent a cohort independent from the ticks used in the earlier studies. As no more ticks from Vienna in 2005 were available, a new collection was made in 2013. Those ticks were stored at 4°C until DNA extraction on the following day. The ticks were identified using a stereomicroscope and standard taxonomic keys before the DNA was extracted.
DNA extraction.
Ticks were washed in 70% ethanol, dried on microscopic slides, and cut in half using sterile blades. The DNeasy blood and tissue kit (Qiagen, Hilden, Germany) was used for DNA extraction. Briefly, ticks were lysed at 56°C on a thermal shaker for at least 3 h in 180 μl ATL buffer and 20 μl proteinase K solution. AL buffer (200 μl) was added, tubes were incubated for another 10 min at 70°C, and then 200 μl 96% ethanol was added and the samples were centrifuged at 14,000 × g for 5 min. The manufacturer's protocol was followed for the remaining steps. DNA was eluted in 100 μl prewarmed (68°C) AE buffer, and extracts were stored at −20°C until used for PCR. A negative extraction control (reagents without tick DNA) was included in every process of DNA extraction.
Molecular detection of pathogens using reverse line blot.
Using the PCR-RLB hybridization method, we screened the ticks for the presence of the following pathogens: B. burgdorferi sensu lato, Anaplasma/Ehrlichia spp. including “Candidatus Neoehrlichia mikurensis,” Babesia spp., Rickettsia spp., and C. burnetii. To avoid problems of cross-reactivity caused by unspecific amplification during PCR, both genus- and species-specific oligonucleotide probes need to give a signal on RLB. Samples that give a species-specific signal but no signal for the genus-specific probe ("catch-all" probe) are considered negative. In contrast, samples that give only a genus-specific signal are sequenced for determination of the species.
In the RLBs, genus- and species-specific oligonucleotide probes (Table 3) labeled with a 5′ C6 amino linker (Eurofins Genomics GmbH, Ebersberg, Germany) were covalently linked to a nylon membrane (Biodyne C; Pall Laboratories, Crailsheim, Germany) using a miniblotter 45 (Immunetics, Boston, MA, USA) and a previously described protocol (75). In the next step, genus-specific PCRs on target genes that also contained species-specific variable regions were performed for each of the five pathogen groups. RLB hybridization was used for detection of the obtained PCR amplicons. One primer in each PCR was labeled with 5′-biotin (Table 3), which resulted in amplification of biotinylated PCR products if DNA of the investigated pathogen was present. The PCR products were combined and hybridized to the respective membranes. All RLBs were performed as described elsewhere (75). Results were visualized using the ChemiDoc touch imaging system (Bio-Rad, Hercules, USA) in combination with the Pierce ECL Western blotting substrate (Thermo Fisher Scientific, Vienna, Austria).
Genus-specific PCRs used for RLB.
The Phire hot start II polymerase kit (Thermo Fisher Scientific, Vienna, Austria) and a C1000 touch thermal cycler (Bio-Rad, Hercules, USA) were used for all PCRs for the RLB. The reaction mixtures (total volume of 25 μl) contained 5 μl 5× Phire reaction buffer, including 1.5 mM MgCl2 (Thermo Fisher Scientific, Vienna, Austria), 200 nM deoxynucleoside triphosphates (dNTPs) (Solis Biodyne, Tartu, Estonia), 400 nM each primer, 0.125 U Phire II polymerase (Thermo Fisher Scientific, Vienna, Austria), PCR-grade H2O (Sigma-Aldrich, Darmstadt, Germany), and 2.5 μl template DNA.
For detection of B. burgdorferi sensu lato, two previously described PCRs (24, 25) using different primer pairs, both amplifying the 5S-23S IGS region, were compared (see below). The PCRs were carried out as described previously (29) with minor modifications: an initial denaturation step at 98°C for 30 s, followed by 10 touchdown cycles of 98°C for 5 s, 60°C for 5 s, and 72°C for 10 s in which the annealing temperature was lowered from 60°C to 50°C by 1°C per cycle. The touchdown cycles were followed by 45 additional cycles (each 5 s at 98°C, 5 s at 50°C, and 10 s at 72°C) and a final extension step of 1 min at 72°C. Results for the general tick screening were considered positive when both PCRs gave a signal on the RLB. If only one PCR gave a signal, both tests were repeated; the sample was considered positive if the previously positive test gave the same result, even if the second test remained negative.
The PCRs for Anaplasma/Ehrlichia spp. (V1 hypervariable region of the 16S rRNA gene), Babesia/Theileria spp. (V4 hypervariable region of the 18S rRNA gene), and Rickettsia spp. (hypervariable region of the 16S rRNA gene) were performed as described previously (27, 29, 76) with minor differences: no uracil DNA glycosylase (UDG) was used, and during the touchdown cycles the annealing temperature was lowered by 1°C per cycle from 67°C to 57°C instead of 2°C every two cycles. In addition, results of a PCR targeting the 23S-5S IGS of Rickettsia spp. (28) were compared with those of the Rickettsia PCR described above; results were interpreted in the same way as for B. burgdorferi sensu lato.
C. burnetii was detected using primers and a specific probe described previously (77, 78). PCR amplification reactions were as follows: 98°C for 30 s and then 45 cycles of 5 s at 98°C, 5 s at 60°C, and 10 s at 72°C, followed by a final step at 72°C for 30 s. An overview of the primers and RLB probes used in this study is provided in Table 3.
Comparison of methods for B. burgdorferi sensu lato and Rickettsia spp. RLBs.
We compared the different methods with regard to sensitivity for certain strains and the results of the tick screening.
In the case of B. burgdorferi sensu lato, two RLBs based on the 5S-23S IGS region have been published, the main difference being the primer pair used for amplification. One set of primers was derived from a report by Rijpkema et al. (24), the other was designed by Alekseev et al. a few years later (25). As both primer pairs span similar regions on the 5S-23S IGS, the same RLB probes could be used for the identification of positive samples. For determination of test sensitivity, the following strains were used: B. burgdorferi sensu stricto (in-house isolate Lenz from human heart [79]), B. afzelii (in-house isolate H2 from human skin), B. garinii (in-house isolate H4 from human skin), B. valaisiana (VS116), and B. lusitaniae (PotiB2). Strains VS116 and PotiB2 were obtained from the National Reference Centre for Borrelia in Oberschleißheim, Bavaria, Germany (courtesy of Volker Fingerle). Strains were grown in modified BSK-2 medium (80), and DNA was extracted from 3 ml of a late-logarithmic-phase culture using the Qiagen DNeasy blood and tissue kit (Qiagen, Hilden, Germany). Sensitivity was determined by testing a 10-fold dilution series (10−2 ng to 10−7 ng total borrelial DNA) of the above-mentioned strains in triplicate. Initial dilutions were made in PCR-grade H2O and spiked with 100 ng of Borrelia-free tick DNA to resemble real tick samples that might contain PCR inhibitors. In addition, we evaluated whether coinfections with multiple B. burgdorferi sensu lato strains influenced the tests and whether one of the primer pairs gave an advantage in detection of those. For this, we used the same dilution series as described above and spiked them with 10−2 ng DNA of a randomly selected other strain (Table 5).
For Rickettsia spp., two completely independent RLBs have been published, one based on the highly conserved 16S rRNA gene (27) and the other based on the more variable 23S-5S IGS region of Rickettsia spp. (28). The sensitivities of the two available RLBs were tested using R. raoultii strain Jongejan cultured as described previously (81) in BME/CTVM2 tick cells (82) provided by Lesley Bell-Sakyi (The Tick Cell Biobank, The Pirbright Institute, United Kingdom). DNA was extracted from 200 μl infected tick cell culture (Qiagen blood and tissue kit; Qiagen, Hilden, Germany), and the concentration was measured in a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Vienna, Austria). A dilution series in PCR-grade H2O from 100 ng to 10−6 ng spiked with 100 ng Rickettsia-free tick DNA was used for comparing the two RLBs. The extracted DNA represented a mixture of R. raoultii and host cell DNA, reflecting the intracellular life style of Rickettsia spp. A qPCR based on the single-copy chromosomal citrate synthase gene (gltA) (83) was therefore used for determination of the genome equivalents of Rickettsia spp. present in a defined quantity of DNA extract. First, we used a conventional PCR with the qPCR primers CS-5 and CS-6 (83) and cloned the amplified 147-bp fragment, after PCR purification (QIAquick PCR purification kit; Qiagen, Hiden, Germany), into a pJET1.2/blunt cloning vector (CloneJET PCR cloning kit; Thermo Scientific). Cloning was performed according to the manufacturer's instruction with DH5α cells, and plasmids were extracted using the GeneJET plasmid miniprep kit (Thermo Fisher Scientific, Vienna, Austria). The plasmids were checked by sequencing (Microsynth, Vienna, Austria) for the presence of the correct insert. In a second step, the qPCR was performed using defined numbers of plasmids (105 to 10−1 plasmids per PCR) in triplicate to generate a standard curve. Some modifications to the original qPCR of Labruna et al. (83) were made. We used 10 μl GoTaq probe qPCR master mix (Promega, Mannheim, Germany) in a total reaction volume of 20 μl, primers CS-5 and CS-6 at final concentrations of 500 nM each, a fluorogenic probe (5′ 6-FAM-CAT TGT GCC ATC CAG CCT ACG GT-TAMRA-3′) at a final concentration of 250 nM, 0.25 μl CXR reference dye (Promega) per reaction, 6.5 μl molecular-grade H2O (Promega), and finally 2 μl template. The qPCR was run in a QuantStudio 5 real-time PCR system (Thermo Fisher Scientific, Vienna, Austria) with the following program: 95°C for 2 min, followed by 50 cycles of 95°C for 15 s and 50°C for 1 min. A diluted DNA extract of R. raoultii in a total of 1 ng DNA (including host cell DNA) per qPCR (in triplicate) was used to determine the copy number of the rickettsial gltA gene according to the standard curve generated.
PCR-RLB controls.
For quality control during tick screening, DNA extracts of bacteria (in-house culture of B. burgdorferi sensu stricto from tick pool; R. helvetica DNA from Ingenetix, Vienna, Austria) or pathogen-positive ticks/samples (A. phagocytophilum, “Candidatus Neoehrlichia mikurensis,” B. canis, C. burnetii) were used in every PCR and RLB. Nontemplate controls were included in the PCRs and RLBs to ensure contamination-free processing of the samples.
Sequencing.
Samples that produced a genus-specific signal on the RLB but no species-specific signal were sequenced. Samples that were positive for genospecies detected for the first time in Austria were also sequenced to confirm the results. PCRs were therefore repeated with nonbiotinylated primers. The reaction volume of 25 μl contained 5 μl 5× Phire reaction buffer, including 1.5 mM MgCl2 (Thermo Fisher Scientific, Vienna, Austria), 400 nM dNTPs (Solis Biodyne, Tartu, Estonia), 400 nM each primer, 0.5 U Phire II Polymerase (Thermo Fisher Scientific, Vienna, Austria), PCR-grade H2O (Sigma-Aldrich, Darmstadt, Germany), and 2.5 μl template. Amplicons were purified using the QIAquick PCR purification kit or the Qiagen gel extraction kit (Qiagen, Hilden, Germany) and sent for bidirectional sequencing to Eurofins Genomics GmbH (Ebersberg, Germany). The obtained sequences were aligned and analyzed using CLC Main Workbench (CLC Bio, version 7.6) and BLAST provided by the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistics.
The collected data set was analyzed using IBM SPSS 19.0 software. Differences within nominal categories (e.g., life stage of tick and infection rate, coinfections with pathogens) were calculated using Fisher's exact test or Pearson's chi-square test. Two-tiered P values of <0.05 for a 95% confidence interval were considered significant. McNemar's test for paired nominal data was used for comparison of two different methods applied to the same sample size (e.g., tests for B. burgdorferi sensu lato and Rickettsia spp.). Results were considered significant if P was <0.05.
Accession number(s).
We submitted the sequences obtained in this study to GenBank under the following accession numbers: KX161763 (R. raoultii 16S rRNA gene), KX161764 (Babesia venatorum 18S rRNA gene), KX161765 [Theileria (Babesia) microti 18S rRNA gene], KX161766 (R. slovaca 23S-5S IGS), KX161767 (R. monacensis 16S rRNA gene), KX161768 (R. monacensis 23S-5S IGS), and KX161769 (R. raoultii 23S-5S IGS).
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00489-17.
REFERENCES
- 1.Ginsberg HS, Faulde MK. 2008. Ticks, p 303–305. In Bonnefoy X, Kampen H, Sweeney K (ed), Public health significance of urban pests. World Health Organization, Copenhagen, Denmark. [Google Scholar]
- 2.Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, Losson B, Saegerman C, Donoso-Mantke O, Niedrig M, Papa A. 2010. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther 8:33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 3.Radda A, Burger I, Stanek G, Wewalka G. 1986. Austrian hard ticks as vectors of Borrelia burgdorferi, overview. Zentralbl Bakteriol Mikrobiol Hyg A 263:79–82. [DOI] [PubMed] [Google Scholar]
- 4.Stanek G, Flamm H, Groh V, Hirschl A, Kristoferitsch W, Neumann R, Schmutzhard E, Wewalka G. 1987. Epidemiology of borrelia infections in Austria. Zentralbl Bakteriol Mikrobiol Hyg A 263:442–449. [DOI] [PubMed] [Google Scholar]
- 5.Cetin E, Sotoudeh M, Auer H, Stanek G. 2006. Paradigm Burgenland: risk of Borrelia burgdorferi sensu lato infection indicated by variable seroprevalence rates in hunters. Wien Klin Wochenschr 118:677–681. doi: 10.1007/s00508-006-0694-y. [DOI] [PubMed] [Google Scholar]
- 6.Glatz M, Müllegger RR, Maurer F, Fingerle V, Achermann Y, Wilske B, Bloemberg GV. 2014. Detection of Candidatus Neoehrlichia mikurensis, Borrelia burgdorferi sensu lato genospecies and Anaplasma phagocytophilum in a tick population from Austria. Ticks Tick Borne Dis 5:139–144. doi: 10.1016/j.ttbdis.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Walochnik J, Stanek G. 2008. Borrelia burgdorferi sensu lato genospecies in questing Ixodes ricinus ticks in Austria. Int J Med Microbiol 298:168–176. doi: 10.1016/j.ijmm.2007.10.001. [DOI] [Google Scholar]
- 8.Stünzner D, Pierer K, Hubalek Z, Halouzka J, Aberer E, Millner MM, Marth E. 1999. Species identification of Borrelia burgdorferi sensu lato from tick and human isolates in Styria (Austria) by PCR-RFLP analysis. Wien Klin Wochenschr 111:994–996. [PubMed] [Google Scholar]
- 9.Markowicz M, Ladstätter S, Schötta AM, Reiter M, Pomberger G, Stanek G. 2015. Oligoarthritis caused by Borrelia bavariensis, Austria, 2014. Emerg Infect Dis 21:1052–1054. doi: 10.3201/eid2106.141516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stünzner D, Hubálek Z, Halouzka J, Wendelin I, Sixl W, Marth E. 2006. Prevalence of Borrelia burgdorferi sensu lato in the tick Ixodes ricinus in the Styrian mountains of Austria. Wien Klin Wochenschr 118:682–685. doi: 10.1007/s00508-006-0695-x. [DOI] [PubMed] [Google Scholar]
- 11.Stünzner D, Hubalek Z, Halouzka J, Postic D, Pierer K, Marth E. 1998. Prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus ticks from Styria (Austria) and species identification by PCR-RFLP Analysis. Zentralbl Bakteriol 288:471–478. doi: 10.1016/S0934-8840(98)80063-7. [DOI] [PubMed] [Google Scholar]
- 12.Leschnik MW, Khanakah G, Duscher G, Wille-Piazzai W, Hörweg C, Joachim A, Stanek G. 2012. Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Med Vet Entomol 26:440–446. doi: 10.1111/j.1365-2915.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 13.Hubálek Z, Stünzner D, Halouzka J, Sixl W, Wendelin I, Juricová Z, Sanogo YO. 2003. Prevalence of borreliae in ixodid ticks from a floodplain forest ecosystem. Wien Klin Wochenschr 115:121–124. doi: 10.1007/BF03040291. [DOI] [PubMed] [Google Scholar]
- 14.Sonnleitner ST, Simeoni J, Lang S, Dobler G, Speck S, Zelger R, Schennach H, Lass-Flörl C, Walder G. 2013. Spotted fever group—rickettsiae in the Tyrols: evidence by seroepidemiology and PCR. Zoonoses Public Health 60:284–290. doi: 10.1111/j.1863-2378.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 15.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Walochnik J, Stanek G. 2008. First detection of Rickettsia helvetica in Ixodes ricinus ticks in Austria. Vector Borne Zoonotic Dis 8:561–563. doi: 10.1089/vbz.2007.0250. [DOI] [PubMed] [Google Scholar]
- 16.Polin H, Hufnagl P, Haunschmid R, Gruber F, Ladurner G. 2004. Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J Clin Microbiol 42:2285–2286. doi: 10.1128/JCM.42.5.2285-2286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sixl W, Petrovec M, Marth E, Wüst G, Stünzner D, Schweiger R, Avsic-Zupanc T. 2003. Investigation of Anaplasma phagocytophila infections in Ixodes ricinus and Dermacentor reticulatus ticks in Austria. Ann N Y Acad Sci 990:94–97. doi: 10.1111/j.1749-6632.2003.tb07343.x. [DOI] [PubMed] [Google Scholar]
- 18.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Stanek G, Walochnik J. 2008. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol 74:4841–4846. doi: 10.1128/AEM.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derdáková M, Václav R, Pangrácova-Blaňárová L, Selyemová D, Koči J, Walder G, Špitalská E. 2014. Candidatus Neoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of Central Europe. Parasit Vectors 7:160. doi: 10.1186/1756-3305-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehácek J, Kaaserer B, Urvölgyi J, Lukácová M, Kovácová E, Kocianová E. 1994. Isolation of Coxiella burnetii and of an unknown rickettsial organism from Ixodes ricinus ticks collected in Austria. Eur J Epidemiol 10:719–723. doi: 10.1007/BF01719288. [DOI] [PubMed] [Google Scholar]
- 21.Schabereiter-Gurtner C, Lubitz W, Rölleke S. 2003. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J Microbiol Methods 52:251–260. doi: 10.1016/S0167-7012(02)00186-0. [DOI] [PubMed] [Google Scholar]
- 22.Rehácek J, Kocianová E, Lukácová M, Stanek G, Khanakah G, Vyrosteková V, Valková D. 1997. Detection of spotted fever group (SFG) rickettsia in Ixodes ricinus ticks in Austria. Acta Virol 41:355–356. [PubMed] [Google Scholar]
- 23.Kong F, Gilbert GL. 2006. Multiplex PCR-based reverse line blot hybridization assay (mPCR/RLB)—a practical epidemiological and diagnostic tool. Nat Protoc 1:2668–2680. doi: 10.1038/nprot.2006.404. [DOI] [PubMed] [Google Scholar]
- 24.Rijpkema SG, Molkenboer MJ, Schouls LM, Jongejan F, Schellekens JF. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol 33:3091–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alekseev AN, Dubinina HV, Van De Pol I, Schouls LM. 2001. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J Clin Microbiol 39:2237–2242. doi: 10.1128/JCM.39.6.2237-2242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christova I, Van De Pol J, Yazar S, Velo E, Schouls L. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group Rickettsiae in ticks from southeastern Europe. Eur J Clin Microbiol Infect Dis 22:535–542. doi: 10.1007/s10096-003-0988-1. [DOI] [PubMed] [Google Scholar]
- 27.Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, Jebbink F, Jongejan F. 2007. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis 7:585–595. doi: 10.1089/vbz.2007.0130. [DOI] [PubMed] [Google Scholar]
- 28.Jado I, Escudero R, Gil H, Jiménez-Alonso MI, Sousa R, García-Pérez AL, Rodríguez-Vargas M, Lobo B, Anda P. 2006. Molecular method for identification of Rickettsia species in clinical and environmental samples. J Clin Microbiol 44:4572–4576. doi: 10.1128/JCM.01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol 37:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshoo MW, Crowder CD, Carolan HE, Rounds M a, Ecker DJ, Haag H, Mothes B, Nolte O. 2014. Broad-range survey of tick-borne pathogens in southern Germany reveals a high prevalence of Babesia microti and a diversity of other tick-borne pathogens. Vector Borne Zoonotic Dis 14:584–591. doi: 10.1089/vbz.2013.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gern L, Douet V, López Z, Rais O, Cadenas FM. 2010. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis 1:23–29. doi: 10.1016/j.ttbdis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Capelli G, Ravagnan S, Montarsi F, Ciocchetta S, Cazzin S, Porcellato E, Babiker AM, Cassini R, Salviato A, Cattoli G, Otranto D. 2012. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: a cost-effectiveness analysis in north-eastern Italy. Parasit Vectors 5:61. doi: 10.1186/1756-3305-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strle F, Cheng Y, Nelson JA, Picken MM, Bouseman JK, Picken RN. 1995. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto in Slovenia. Eur J Clin Microbiol Infect Dis 14:994–1001. doi: 10.1007/BF01691382. [DOI] [PubMed] [Google Scholar]
- 34.Szekeres S, Coipan EC, Rigó K, Majoros G, Jahfari S, Sprong H, Földvári G. 2015. Eco-epidemiology of Borrelia miyamotoi and Lyme borreliosis spirochetes in a popular hunting and recreational forest area in Hungary. Parasit Vectors 8:309. doi: 10.1186/s13071-015-0922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smetanová K, Burri C, Pérez D, Gern L, Kocianová E. 2007. Detection and identification of Borrelia burgdorferi sensu lato genospecies in ticks from three different regions in Slovakia. Wien Klin Wochenschr 119:534–537. doi: 10.1007/s00508-007-0851-y. [DOI] [PubMed] [Google Scholar]
- 36.Danielová V, Daniel M, Rudenko N, Golovchenko M. 2004. Prevalence of Borrelia burgdorferi sensu lato genospecies in host-seeking Ixodes ricinus ticks in selected South Bohemian locations (Czech Republic). Cent Eur J Public Health 12:151–156. doi: 10.1007/s10389-003-0013-2. [DOI] [PubMed] [Google Scholar]
- 37.Nefedova VV, Korenberg EI, Gorelova NB, Kovalevskii YV. 2004. Studies on the transovarial transmission of Borrelia burgdorferi sensu lato in the taiga tick Ixodes persulcatus. Folia Parasitol (Praha) 51:67–71. doi: 10.14411/fp.2004.010. [DOI] [PubMed] [Google Scholar]
- 38.Richter D, Debski A, Hubalek Z, Matuschka F-R. 2012. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector Borne Zoonotic Dis 12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 39.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 40.Stupica D, Lusa L, Maraspin V, Bogovič P, Vidmar D, O'Rourke M, Traweger A, Livey I, Strle F. 2015. Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One 10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt FC, Ertas B, Falk TM, Metze D, Böer-Auer A. 2014. Genotyping of Borrelia from formalin-fixed paraffin-embedded skin biopsies of cutaneous borreliosis and tick bite reactions by assays targeting the intergenic spacer region, ospA and ospC genes. Br J Dermatol 171:528–543. doi: 10.1111/bjd.12855. [DOI] [PubMed] [Google Scholar]
- 42.Vennestrøm J, Egholm H, Jensen PM. 2008. Occurrence of multiple infections with different Borrelia burgdorferi genospecies in Danish Ixodes ricinus nymphs. Parasitol Int 57:32–37. doi: 10.1016/j.parint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 43.May K, Jordan D, Fingerle V, Strube C. 2015. Borrelia burgdorferi sensu lato and co-infections with Anaplasma phagocytophilum and Rickettsia spp. in Ixodes ricinus in Hamburg, Germany. Med Vet Entomol 29:425–429. doi: 10.1111/mve.12125. [DOI] [PubMed] [Google Scholar]
- 44.Floris R, Menardi G, Bressan R, Trevisan G, Ortenzio S, Rorai E, Cinco M. 2007. Evaluation of a genotyping method based on the ospA gene to detect Borrelia burgdorferi sensu lato in multiple samples of Lyme borreliosis patients. New Microbiol 30:399–410. [PubMed] [Google Scholar]
- 45.Rijpkema SGT, Tazelaar DJ, Molkenboer MJCH, Noordhoek GT, Plantinga G, Schouls LM, Schellekens JFP. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect 3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 46.Rauter C, Hartung T. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann C, Gern L, Voordouw MJ. 2013. Species co-occurrence patterns among Lyme borreliosis pathogens in the tick vector Ixodes ricinus. Appl Environ Microbiol 79:7273–7280. doi: 10.1128/AEM.02158-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruzic-Sabljic E, Arnez M, Logar M, Maraspin V, Lotric-Furlan S, Cimperman J, Strle F. 2005. Comparison of Borrelia burgdorferi sensu lato strains isolated from specimens obtained simultaneously from two different sites of infection in individual patients. J Clin Microbiol 43:2194–2200. doi: 10.1128/JCM.43.5.2194-2200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Špitalská E, Boldiš V, Derdáková M, Selyemová D, Rusňáková Taragel'ová V. 2014. Rickettsial infection in Ixodes ricinus ticks in urban and natural habitats of Slovakia. Ticks Tick Borne Dis 5:161–165. doi: 10.1016/j.ttbdis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Venclíková K, Mendel J, Betášová L, Blažejová H, Jedličková P, Straková P, Hubálek Z, Rudolf I. 2016. Neglected tick-borne pathogens in the Czech Republic, 2011-2014. Ticks Tick Borne Dis 7:107–112. doi: 10.1016/j.ttbdis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Beninati T, Lo N, Noda H, Esposito F, Rizzoli A, Favia G, Genchi C. 2002. First detection of spotted fever group rickettsiae in Ixodes ricinus from Italy. Emerg Infect Dis 8:983–986. doi: 10.3201/eid0809.020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichon B, Kahl O, Hammer B, Gray JS. 2006. Pathogens and host DNA in Ixodes ricinus nymphal ticks from a German forest. Vector Borne Zoonotic Dis 6:382–387. doi: 10.1089/vbz.2006.6.382. [DOI] [PubMed] [Google Scholar]
- 53.Klubal R, Kopecky J, Nesvorna M, Sparagano OAE, Thomayerova J, Hubert J. 2016. Prevalence of pathogenic bacteria in Ixodes ricinus ticks in Central Bohemia. Exp Appl Acarol 68:127–137. doi: 10.1007/s10493-015-9988-y. [DOI] [PubMed] [Google Scholar]
- 54.Floris R, Yurtman AN, Margoni EF, Mignozzi K, Boemo B, Altobelli A, Cinco M. 2008. Detection and identification of Rickettsia species in the northeast of Italy. Vector Borne Zoonotic Dis 8:777–782. doi: 10.1089/vbz.2008.0006. [DOI] [PubMed] [Google Scholar]
- 55.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier P-E, Raoult D. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobler G, Essbauer S, Terzioglu R, Thomas A, Wölfel R. 2008. Häufigkeit des Frühsommer-Meningoenzephalitis-Virus und von Rickettsien in Zecken aus dem Burgenland (Österreich). Wien Klin Wochenschr 120:45–48. doi: 10.1007/s00508-008-1074-6. [DOI] [PubMed] [Google Scholar]
- 57.Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D. 2009. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis 15:1105–1108. doi: 10.3201/eid1507.081449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chmielewski T, Podsiadly E, Karbowiak G, Tylewska-Wierzbanowska S. 2009. Rickettsia spp. in ticks, Poland. Emerg Infect Dis 15:486–488. doi: 10.3201/eid1503.080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boldiš V, Špitalská E. 2010. Dermacentor marginatus and Ixodes ricinus ticks versus L929 and Vero cell lines in Rickettsia slovaca life cycle evaluated by quantitative real time PCR. Exp Appl Acarol 50:353–359. doi: 10.1007/s10493-009-9322-7. [DOI] [PubMed] [Google Scholar]
- 60.Szekeres S, Claudia Coipan E, Rigó K, Majoros G, Jahfari S, Sprong H, Földvári G. 2015. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in natural rodent and tick communities in Southern Hungary. Ticks Tick Borne Dis 6:111–116. doi: 10.1016/j.ttbdis.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Obiegala A, Pfeffer M, Pfister K, Tiedemann T, Thiel C, Balling A, Karnath C, Woll D, Silaghi C. 2014. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum: prevalences and investigations on a new transmission path in small mammals and ixodid ticks. Parasit Vectors 7:563. doi: 10.1186/PREACCEPT-2021441884132711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maurer FP, Keller PM, Beuret C, Joha C, Achermann Y, Gubler J, Bircher D, Karrer U, Fehr J, Zimmerli L, Bloemberg GV. 2013. Close geographic association of human neoehrlichiosis and tick populations carrying “Candidatus Neoehrlichia mikurensis” in eastern Switzerland. J Clin Microbiol 51:169–176. doi: 10.1128/JCM.01955-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, Plantard O, Vayssier-Taussat M, Bonnet S, Spitalská E, Kazimírová M. 2014. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health 2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Jiang J, Liu W, Zheng Y, Huo Q, Tang K, Zuo S, Liu K, Jiang B, Yang H, Cao W-C. 2012. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg Infect Dis 18:1636–1639. doi: 10.3201/eid1810.120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walder G, Fuchs D, Sarcletti M, Berek K, Falkensammer B, Huber K, Petrovec M, Dierich MP, Würzner R. 2006. Human granulocytic anaplasmosis in Austria: epidemiological, clinical, and laboratory findings in five consecutive patients from Tyrol, Austria. Int J Med Microbiol 296(Suppl):297–301. doi: 10.1016/j.ijmm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Vogl UM, Presterl E, Stanek G, Ramharter M, Gattringer K-B, Graninger W. 2010. First described case of human granulocytic anaplasmosis in a patient in Eastern Austria. Wien Med Wochenschr 160:91–93. doi: 10.1007/s10354-009-0733-1. [DOI] [PubMed] [Google Scholar]
- 67.Grab DJ, Nyarko E, Barat NC, Nikolskaia OV, Dumler JS. 2007. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin Vaccine Immunol 14:1420–1424. doi: 10.1128/CVI.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svehlová A, Berthová L, Sallay B, Boldiš V, Sparagano OAE, Spitalská E. 2014. Sympatric occurrence of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks and Rickettsia and Babesia species in Slovakia. Ticks Tick Borne Dis 5:600–605. doi: 10.1016/j.ttbdis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Duh D, Petrovec M, Avsic-Zupanc T. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J Clin Microbiol 39:3395–3397. doi: 10.1128/JCM.39.9.3395-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonnet S, Jouglin M, Malandrin L, Becker CAM, Agoulon A, L'Hostis M, Chauvin A. 2007. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology 134:197–207. doi: 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- 71.Quarsten H, Skarpaas T, Fajs L, Noraas S, Kjelland V. 2015. Tick-borne bacteria in Ixodes ricinus collected in southern Norway evaluated by a commercial kit and established real-time PCR protocols. Ticks Tick Borne Dis 6:538–544. doi: 10.1016/j.ttbdis.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bødker R, Fach P, Moutailler S. 2014. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol 4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berthová L, Slobodník V, Slobodník R, Olekšák M, Sekeyová Z, Svitálková Z, Kazimírová M, Špitalská E. 2016. The natural infection of birds and ticks feeding on birds with Rickettsia spp. and Coxiella burnetii in Slovakia. Exp Appl Acarol 68:299–314. doi: 10.1007/s10493-015-9975-3. [DOI] [PubMed] [Google Scholar]
- 74.Tobudic S, Nedomansky K, Poeppl W, Müller M, Faas A, Mooseder G, Allerberger F, Stanek G, Burgmann H. 2014. Seroprevalence for Coxiella burnetii, Francisella tularensis, Brucella abortus and Brucella melitensis in Austrian adults: a cross-sectional survey among military personnel and civilians. Ticks Tick Borne Dis 5:315–317. doi: 10.1016/j.ttbdis.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Nijhof AM, Pillay V, Steyl J, Prozesky L, Stoltsz WH, Lawrence JA, Penzhorn BL, Jongejan F. 2005. Molecular characterization of Theileria species associated with mortality in four species of African antelopes. J Clin Microbiol 43:5907–5911. doi: 10.1128/JCM.43.12.5907-5911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nijhof AM, Penzhorn BL, Lynen G, Mollel JO, Morkel P, Bekker CPJ, Jongejan F. 2003. Babesia bicornis sp. nov. and Theileria bicornis sp. nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). J Clin Microbiol 41:2249–2254. doi: 10.1128/JCM.41.5.2249-2254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barandika JF, Hurtado A, García-Esteban C, Gil H, Escudero R, Barral M, Jado I, Juste RA, Anda P, García-Pérez AL. 2007. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl Environ Microbiol 73:6166–6171. doi: 10.1128/AEM.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willems H, Thiele D, Frölich-Ritter R, Krauss H. 1994. Detection of Coxiella burnetii in cow's milk using the polymerase chain reaction (PCR). Zentralbl Veterinarmed B 41:580–587. [DOI] [PubMed] [Google Scholar]
- 79.Stanek G, Klein J, Bittner R, Glogar D. 1990. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med 322:249–252. doi: 10.1056/NEJM199001253220407. [DOI] [PubMed] [Google Scholar]
- 80.Reiter M, Schötta A-M, Müller A, Stockinger H, Stanek G. 2015. A newly established real-time PCR for detection of Borrelia miyamotoi in Ixodes ricinus ticks. Ticks Tick Borne Dis 6:303–308. doi: 10.1016/j.ttbdis.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Wijnveld M, Schötta A-M, Pintér A, Stockinger H, Stanek G. 2016. Novel Rickettsia raoultii strain isolated and propagated from Austrian Dermacentor reticulatus ticks. Parasit Vectors 9:567. doi: 10.1186/s13071-016-1858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell-Sakyi L. 2004. Ehrlichia ruminantium grows in cell lines from four ixodid tick genera. J Comp Pathol 130:285–293. doi: 10.1016/j.jcpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Labruna MB, Whitworth T, Horta MC, Bouyer DH, Mcbride JW, Pinter A, Popov V, Gennari SM, Walker DH. 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42:90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bekker CPJ, de Vos S, Taou A, Sparagano OAE, Jongejan F. 2002. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol 89:223–238. doi: 10.1016/S0378-1135(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 85.Georges K, Loria GR, Riili S, Greco A, Caracappa S, Jongejan F, Sparagano O. 2001. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet Parasitol 99:273–286. doi: 10.1016/S0304-4017(01)00488-5. [DOI] [PubMed] [Google Scholar]
- 86.Poupon M-A, Lommano E, Humair P-F, Douet V, Rais O, Schaad M, Jenni L, Gern L. 2006. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl Environ Microbiol 72:976–979. doi: 10.1128/AEM.72.1.976-979.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matjila PT, Leisewitz AL, Jongejan F, Bertschinger HJ, Penzhorn BL. 2008. Molecular detection of Babesia rossi and Hepatozoon sp. in African wild dogs (Lycaon pictus) in South Africa. Vet Parasitol 157:123–127. doi: 10.1016/j.vetpar.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 88.Matjila PT, Penzhorn BL, Bekker CPJ, Nijhof AM, Jongejan F. 2004. Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Vet Parasitol 122:119–125. doi: 10.1016/j.vetpar.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 89.Hornok S, Fuente J, Horváth G, Fernández de Mera IG, Wijnveld M, Tánczos B, Farkas R, Jongejan F. 2013. Molecular evidence of Ehrlichia canis and Rickettsia massiliae in ixodid ticks of carnivores from South Hungary. Acta Vet Hung 61:42–50. doi: 10.1556/AVet.2012.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.