Abstract
Importance
Telomere length has been associated with dementia and psychological stress, but its relationship with human brain size is unknown.
Objective
To determine if peripheral blood telomere length is associated with brain volume.
Design, Setting, and Participants
Peripheral blood leukocyte telomere length and brain volumes were measured for 1960 individuals in the Dallas Heart Study, a population-based, probability sample of Dallas County, Texas, residents, with a median (25th-75th percentile) age of 50 (42-58) years. Global and 48 regional brain volumes were assessed from the automated analysis of magnetic resonance imaging.
Main Outcomes and Measures
Telomere length and global and regional brain volumes.
Results
Leukocyte telomere length was associated with total cerebral volume (β [SE], 0.06 [0.01], P <.001) including white and cortical gray matter volume (β [SE], 0.04 [0.01], P = .002; β [SE], 0.07 [0.02], P <.001, respectively), independent of age, sex, ethnicity, and total intracranial volume. While age was associated with the size of most subsegmental regions of the cerebral cortex, telomere length was associated with certain subsegmental regions. Compared with age, telomere length (TL) explained a sizeable proportion of the variance in volume of the hippocampus, amygdala, and inferior temporal region (hippocampus: βTL [SE], 0.08 [0.02], R2, 0.91% vs βage [SE], −0.16 [0.02], R2, 3.80%; amygdala: βTL [SE], 0.08 [0.02], R2, 0.78% vs βage [SE], −0.19 [0.02], R2,4.63%; inferior temporal: βTL [SE], 0.07 [0.02], R2, 0.92% vs βage [SE], −0.14 [0.02], R2, 3.98%) (P <.001 for all). The association of telomere length and the size of the inferior and superior parietal, hippocampus, and fusiform regions was stronger in individuals older than 50 years than younger individuals (inferior parietal: β>50 [SE], 0.13 [0.03], P <.001 vs β≤50 [SE], 0.02 [0.02], P = .51, P for interaction = .001; superior parietal: β>50 [SE], 0.11 [0.03], P <.001 vs β≤50 [SE], 0.01 [0.02], P = .71, P for interaction = .004; hippocampus: β>50 [SE], 0.10 [0.03], P = .004 vs β≤50 [SE], 0.05 [0.02], P = .07, P for interaction = .04; fusiform: β>50 [SE], 0.09 [0.03], P = .002, β≤50 [SE], 0.03 [0.02], P = .31, P for interaction = .03). The volume of the hippocampus, amygdala, superior and inferior temporal, precuneus, lateral orbitofrontal, posterior cingulate, thalamus and ventral diencephalon were independently associated with telomere length after adjustment for all covariates (age, gender, ethnicity, total intracranial volume, body mass index, blood pressure, diabetes, smoking status, and APOE genotype).
Conclusions and Relevance
To our knowledge, this is the first population-based study to date to evaluate telomere length as an independent predictor of global and regional brain size. Future studies are needed to determine how telomere length and anatomic structural changes are related to cognitive function, dementia, and psychological disease.
Brain atrophy remains an ominous and incompletely understood consequence of aging. Magnetic resonance (MR) imaging of the brain in cross-sectional and longitudinal cohort investigations has demonstrated that brain atrophy is a sensitive indicator of mild cognitive impairment and dementia. Whole-brain atrophy, and hippocampus atrophy in particular, is greater in individuals with Alzheimer disease compared with healthy aged control subjects.1-3 Findings from studies4-6 of individuals at risk for familial Alzheimer disease suggest that atrophy of certain brain regions may precede the generalized cerebral atrophy seen at the time of diagnosis. The mechanisms that underlie accelerated global and regional brain atrophy are incompletely understood.
Telomeres, the specialized structures at the ends of chromosomes, undergo shortening with each cell division. Telomere length has emerged as a promising biomarker of biological age and an indicator of susceptibility to age-related diseases.7 Previous studies have demonstrated an association between shorter leukocyte telomere length and age-related cognitive function8 and Alzheimer disease.9,10 However, little is known about the association between leukocyte telomere length and structural brain changes in humans. We hypothesized that leukocyte telomere length would help explain interindividual differences in age-related brain atrophy. To test this hypothesis, we measured leukocyte telomere lengths and global and subsegmental brain volumes of participants in the Dallas Heart Study (DHS).
Methods
Study Population
This study was approved by the institutional review board at the University of Texas Southwestern Medical Center, and all participants provided written informed consent. The DHS is a population-based, probability sample of Dallas County, Texas, residents, designed to include approximately 50% African American and 50% non–African American participants. The study was initiated in 2000 and was transformed from across-sectional study to a longitudinal study in 2007, when all participants of the first study were invited for a repeat evaluation. The follow-upDHS2cohortwas augmented by voluntary participation of unrelated spouses and friends of the original cohort. It includes 2485 participants from the original study and 916 unrelated individuals. Race/ethnicity was self-reported according to a list of categories used in the US census. Measurement of peripheral blood leukocyte telomere length was performed on the subset of the DHS2 participants, 18 to 85 years of age, who submitted blood for genetic analysis (n = 3302). A subset of the total cohort (n = 2082) underwent MR imaging. Individuals were excluded from MR imaging if they were pregnant, had any history of exposure to metal fragments, had undergone a previous surgical procedure for a brain aneurysm, or had an implanted cardiac pacemaker, defibrillator, cochlear device, spinal cord stimulator, or other internal electrical device. The mean (SD) age of the cohort was 50.0 (10.6) years. Five participants were younger than 20 years, and 2 participants were older than 80 years. Most participants (approximately 95%) wereagedbetween30and 70years, and the majority (approximately 60%) were aged between 40 and 60 years.
Determination of Telomere Length
Genomic DNA was isolated from circulating leukocytes using an automated purification system (Autopure LS; Qiagen). Telomere lengths of genomic DNA isolated from the DHS2 cohort were measured using a quantitative polymerase chain reaction assay as previously described11 and are reported as logarithm-transformed relative ratios of the copy number of telomere DNA to a single-copy gene (T:S ratios). Logarithm-transformed relative T:S ratios were normally distributed. The Spearman rank correlation coefficient between telomere length and age was −0.17 (P < .001) for this study.
MR Imaging Protocol
Brain MR images were obtained on a 3-T MR imaging system (Achieva; Philips Medical Systems) using 3-dimensional magnetization-prepared rapid acquisition gradient. Images were obtained from the vertex of the skull to the foramen magnum in true axial orientation. Axial sections were reconstructed at 1.0-mm section thickness with a repetition time of 9.6 milliseconds, an echo time of 5.8 milliseconds, a flip angle of 12°, and a field of view of 260 × 260 mm.
Image Analysis
Cross-sectional determination of brain atrophy was based on estimating brain size at maturity from total cranial volume.12 Total cranial volume was estimated with SIENAX,13 part of the freely available Functional MRI of the Brain Software Library.14 Segmentation and quantification of global brain volumes, as well as cortical and subcortical structures, were obtained from the fully automated FreeSurfer image analysis suite, version 4.4 (documented and freely available for download online at http://surfer.nmr.mgh.harvard.edu/). The FreeSurfer analysis was performed at the Texas Advanced Computing Center at The University of Texas at Austin. Magnetic resonance images from 107 participants were excluded because of self-reported stroke (n = 37) or because of major structural defects (eg, corpus callosum agenesis, imaging evidence of stroke, or hydrocephalus) or image acquisition errors (metal or motion artifacts) precluding automated analysis (n = 70). Each MR image that generated an error was reviewed by a neuroradiologist (K.S.K.).
Statistical Analysis
All statistical analyses were performed with the R software package, version 3.0.1 (www.r-project.org). Linear regression was used to estimate the association between telomere length and brain volume, with age, sex, race/ethnicity, and total cranial volume included as covariates. Interactions with sex and age were tested by adding telomere length × sex and telomere length × age terms to the model. To investigate whether the association between telomere length and brain size differed by age, we first stratified participants by median age (≤50 vs >50 years). To assess whether differences in the association patterns were statistically significant, we included an interaction term between the indicator variable for age (>50 years) and telomere length in the model. To investigate whether other established risk factors affected the association between telomere length and brain volume, we repeated the analysis with the inclusion of body mass index, systolic blood pressure, diabetes mellitus (DM), and smoking status (1 indicates past or current smoker, and 0 indicates never smoker) as additional covariates. The presence of DM was defined as a self-reported diagnosis of DM, the use of prescription medication for DM, a glycated hemoglobin level of 6.5% or higher, or a fasting glucose level of 126 mg/dL or higher or a nonfasting glucose level of 200 mg/dL or higher (to convert glycated hemoglobin level to the proportion of total hemoglobin, multiply by 0.01; to convert glucose level to millimoles per liter, multiply by 0.0555). Finally, we tested whether APOE genotype had an effect on the association by adding genotype to the model in a subset of participants for whom genotype data were available. To account for the multiple brain regions tested, we adjusted the significance levels using the false discovery rate method.15 All tests were 2-sided; P < .05 was considered statistically significant.
Results
Baseline characteristics of the probability-based, multiethnic population cohort, the DHS2, are listed in Table 1. The participants ranged in age from 18 to 85 years, with a median age of 50 years. Forty-eight different subsegmental brain volumes were measured using FreeSurfer analyses of brain MR imaging. In a multivariable model that included age, sex, race/ethnicity, total cranial volume, and telomere length, all brain regions except the entorhinal cortex and cerebellar white matter were associated with age (P < .001 for all) (Table 2). In all cases, the volumetric size of brain regions decreased with advancing age. The changes in brain size with age were inversely correlated with changes in ventricle size. Age accounted for a large proportion of the variation in size of the superior frontal region, superior temporal region, thalamus, putamen, cerebral cortex, and lateral ventricle (partial R2 for age >17% for all).
Table 1. Demographic and Clinical Characteristics of the Dallas Heart Study 2 Cohort.
| Variable | Value (n = 1960) |
|---|---|
| Age, median (25th-75th percentile), y | 50 (42-58) |
| Male sex, No. (%) | 807 (41.2) |
| Race/ethnicity, No. (%) | |
| Non-Hispanic white | 728 (37.1) |
| Non-Hispanic black | 911 (46.5) |
| Hispanic | 276 (14.1) |
| Other | 45 (2.3) |
| Blood pressure, mean (SD), mm Hg | |
| Systolic | 130.6 (18.2) |
| Diastolic | 80.4 (8.9) |
| Diabetes mellitus prevalence, No. (%) | 245 (12.5) |
| Body mass index, mean (SD)a | 29.6 (5.6) |
| Smoking status, No. (%)b | (n = 1917) |
| Present | 395 (20.6) |
| Past | 445 (23.2) |
| Never | 1077 (56.2) |
| APOE genotype, No. (%)c | (n = 1409) |
| ε2 Heterozygous | 189 (13.4) |
| ε2 Homozygous | 14 (1.0) |
| ε3 Heterozygous | 550 (39.0) |
| ε3 Homozygous | 63 (4.5) |
| ε4 Heterozygous | 404 (28.7) |
| ε4 Homozygous | 49 (3.5) |
| Telomere length, mean (SD), kbd | 6.3 (0.6) |
| Total cranial volume, mean (SD), mm3 | 904 000 (104 813) |
Calculated as weight in kilograms divided by height in meters squared.
Information regarding smoking status was available for only 1917 participants.
APOE genotypes were available for only 1409 participants.
Conversion of telomere length from logarithm-transformed relative ratios of the copy number of telomere DNA to a single-copy gene to kilobase (kb) was performed as previously described.11
Table 2. Association of Total and Subsegmental Brain Region Size With Telomere Length and Age Among 1960 Patientsa.
| Subsegmental Brain Region | Telomere Length | Age | ||||
|---|---|---|---|---|---|---|
| β Level (SE) | P Value | Partial R2, % | β Level (SE) | P Value | Partial R2, % | |
| Frontal lobe | ||||||
| Pars opercularis | 0.07 (0.02) | <.001 | 0.70 | −0.29 (0.02) | <.001 | 12.28 |
| Medial orbitofrontal | 0.07 (0.02) | <.001 | 0.69 | −0.22 (0.02) | <.001 | 7.23 |
| Lateral orbitofrontal | 0.07 (0.02) | <.001 | 0.96 | −0.21 (0.01) | <.001 | 9.06 |
| Paracentral lobule | 0.06 (0.02) | .005 | 0.48 | −0.23 (0.02) | <.001 | 7.39 |
| Pars orbitalis | 0.05 (0.02) | .01 | 0.37 | −0.23 (0.02) | <.001 | 7.30 |
| Precentral | 0.05 (0.02) | .01 | 0.41 | −0.24 (0.02) | <.001 | 10.34 |
| Superior frontal | 0.04 (0.02) | .03 | 0.29 | −0.31 (0.01) | <.001 | 18.03 |
| Rostral middle frontal | 0.03 (0.02) | .05 | NA | −0.29 (0.02) | <.001 | 14.94 |
| Frontal pole | 0.03 (0.02) | .27 | NA | −0.22 (0.02) | <.001 | 5.29 |
| Caudal middle frontal | 0.02 (0.02) | .31 | NA | −0.20 (0.02) | <.001 | 6.03 |
| Pars triangularis | 0.01 (0.02) | .67 | NA | −0.36 (0.02) | <.001 | 16.15 |
| Parietal lobe | ||||||
| Precuneus | 0.09 (0.02) | <.001 | 1.62 | −0.24 (0.02) | <.001 | 11.31 |
| Inferior parietal | 0.08 (0.02) | <.001 | 1.01 | −0.26 (0.02) | <.001 | 11.54 |
| Posterior central | 0.07 (0.02) | <.001 | 0.92 | −0.21 (0.02) | <.001 | 7.73 |
| Superior parietal | 0.06 (0.02) | .002 | 0.60 | −0.22 (0.02) | <.001 | 7.48 |
| Supramarginal | 0.03 (0.02) | .07 | NA | −0.29 (0.02) | <.001 | 13.45 |
| Temporal lobe | ||||||
| Hippocampus | 0.08 (0.02) | <.001 | 0.91 | −0.16 (0.02) | <.001 | 3.80 |
| Amygdala | 0.08 (0.02) | <.001 | 0.78 | −0.19 (0.02) | <.001 | 4.63 |
| Inferior temporal | 0.07 (0.02) | <.001 | 0.92 | −0.14 (0.02) | <.001 | 3.98 |
| Fusiform | 0.06 (0.02) | <.001 | 0.69 | −0.21 (0.02) | <.001 | 7.85 |
| Superior temporal | 0.05 (0.02) | .005 | 0.49 | −0.34 (0.02) | <.001 | 19.36 |
| Transverse temporal | 0.05 (0.02) | .01 | 0.38 | −0.31 (0.02) | <.001 | 13.25 |
| Middle temporal | 0.04 (0.02) | .01 | 0.40 | −0.24 (0.01) | <.001 | 12.58 |
| Bank superior temporal sulcusb | 0.04 (0.02) | .07 | NA | −0.26 (0.02) | <.001 | 9.79 |
| Entorhinal cortex | 0.03 (0.02) | .11 | NA | 0.03 (0.02) | .07 | NA |
| Parahippocampal gyrus | 0.02 (0.02) | .32 | NA | −0.14 (0.02) | <.001 | 2.47 |
| Temporal pole | 0.02 (0.02) | .36 | NA | −0.08 (0.02) | <.001 | 0.78 |
| Occipital lobe | ||||||
| Lateral occipital | 0.05 (0.02) | .01 | 0.36 | −0.13 (0.02) | <.001 | 3.27 |
| Cuneus cortex | 0.01 (0.02) | .62 | NA | −0.18 (0.02) | <.001 | 4.98 |
| Pericalcarine | 0.01 (0.02) | .62 | NA | −0.17 (0.02) | <.001 | 3.95 |
| Lingual | 0.01 (0.02) | .62 | NA | −0.13 (0.02) | <.001 | 2.79 |
| Insula | 0.05 (0.02) | .003 | 0.56 | −0.25 (0.02) | <.001 | 12.09 |
| Cingulate gyrus | ||||||
| Posterior cingulate | 0.07 (0.02) | <.001 | 0.92 | −0.27 (0.02) | <.001 | 12.15 |
| Rostral anterior | 0.06 (0.02) | .004 | 0.51 | −0.19 (0.02) | <.001 | 5.94 |
| Caudal anterior | 0.05 (0.02) | .02 | 0.31 | −0.12 (0.02) | <.001 | 1.98 |
| Isthmus of cingulate | 0.04 (0.02) | .02 | 0.30 | −0.21 (0.02) | <.001 | 7.16 |
| Deep nuclei | ||||||
| Thalamus | 0.08 (0.02) | <.001 | 1.26 | −0.31 (0.01) | <.001 | 18.54 |
| Ventral diencephalon | 0.06 (0.02) | <.001 | 0.68 | −0.21 (0.02) | <.001 | 8.83 |
| Caudate | 0.04 (0.02) | .05 | 0.24 | −0.12 (0.02) | <.001 | 2.12 |
| Nucleus accumbens | 0.04 (0.02) | .09 | NA | −0.27 (0.02) | <.001 | 8.87 |
| Putamen | 0.03 (0.02) | .15 | NA | −0.34 (0.02) | <.001 | 17.01 |
| Pallidum | 0.02 (0.02) | .22 | NA | −0.32 (0.02) | <.001 | 14.94 |
| Cerebellum | ||||||
| White matter | 0.06 (0.02) | .01 | 0.36 | −0.03 (0.02) | .14 | NA |
| Cortex | 0.03 (0.02) | .17 | NA | −0.21 (0.02) | <.001 | 6.86 |
| Cerebral | ||||||
| White matter | 0.04 (0.01) | .002 | 0.59 | −0.15 (0.01) | <.001 | 8.09 |
| Cortex | 0.07 (0.02) | <.001 | 1.16 | −0.30 (0.01) | <.001 | 17.26 |
| Inferior lateral ventricle | –0.06 (0.02) | .01 | 0.38 | 0.24 (0.02) | <.001 | 6.99 |
| Lateral ventricle | –0.03 (0.02) | .17 | NA | 0.37 (0.02) | <.001 | 17.07 |
| Total cerebral volumec | 0.06 (0.01) | <.001 | 1.38 | –0.24 (0.01) | <.001 | 20.20 |
Abbreviation: NA, not applicable.
Covariates in the multivariable model include age, sex, race/ethnicity, telomere length, and total cranial volume. The effect size of telomere length (β level) is reported as the mean difference in each regional brain size associated with a 1-SD unit difference in telomere length. The β coefficients for age correspond to the mean differences in each regional brain size per 10 years of age. P values for telomere length and age have been corrected for multiple comparisons.
FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) term for the banks of the superior temporal sulcus.
Total cerebral volume includes all subregional brain volumes, excluding the ventricles and cerebellum. The P values for the association of telomere length and age with total cerebral volume were not adjusted for multiple comparisons.
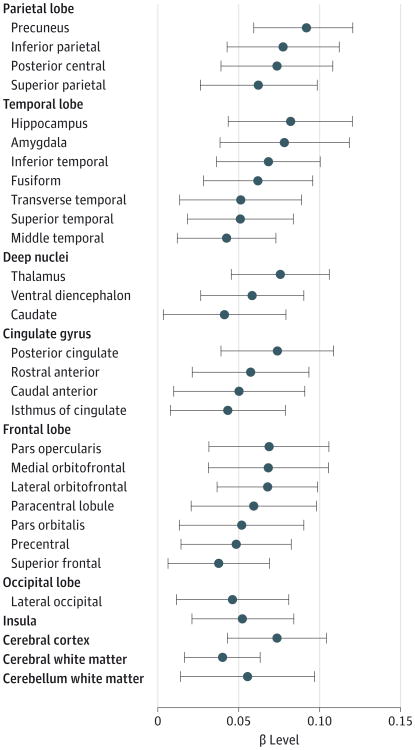
In contrast, the sizes of only certain subsegmental regions were associated with telomere length independent of age or the other covariates (Figure and Table 2). Regions in which telomere length had the largest effect on size included the precuneus and inferior and posterior central regions of the parietal lobe, as well as the hippocampus, amygdala, fusiform, and inferior segment of the temporal lobe (P < .001 for all). Telomere length was also independently associated with the size of the thalamus, ventral diencephalon, posterior cingulate, and 3 subsegmental regions of the frontal lobe (P < .001 for all). Telomere length was a significant predictor of total cerebral volume, cortical white matter volume, and cortical gray matter volume (P < .001, P = .002, and P < .001, respectively). Telomere length was associated with cerebellar white matter volume (P = .01) but not with cerebellar gray matter (P = .17). In contrast, age was not significantly correlated with atrophy of cerebellar white matter (P = .14) but was significantly associated with cerebellar gray matter volume (P < .001). Areas of the brain in which telomere length compared with age explained a large proportion of variation in size included the hippocampus, amygdala, and inferior temporal region (R2 = 0.91%, R2 = 0.78%, and R2 = 0.92%, respectively, for telomere length; and R2 = 3.80%, R2 = 4.63%, and R2 = 3.98%, respectively, for age).
Figure. Brain Size Associated With Telomere Length in the Dallas Heart Study 2 Cohort.
The effect size of telomere length (β level with 95% CI) is shown for each global or subsegmental brain region. Only regions that are significantly associated with telomere length independent of age, sex, race/ethnicity, and total cranial volume after correction for multiple testing are shown.
The effect of telomere length on regional brain size varied by sex (eTable 1 in the Supplement). For some of the subsegmental regions (eg, the lateral orbitofrontal region and the inferior temporal region), the association between telomere length and size was seen only in men (P < .001 for both); no association was seen in women after correction for multiple testing. No significant difference for associations of telomere length were observed between right and left hemisphere segmental brain volumes (data not shown).
The effect of telomere length on brain size varied by age (Table 3 and eTable 2 in the Supplement). For the inferior and superior regions of the parietal lobe, the association between telomere length and size was significant only for those older than 50 years (P < .001 for both). Similarly, associations between telomere length and the hippocampus and fusiform regions of the temporal lobe were seen only for those older than 50 years (P = .004 and P = .002, respectively).
Table 3. Interaction of Telomere Length and Certain Subsegmental Brain Regions by Agea.
| Subsegmental Brain Region | Age≤50y (n = 1013) | Age >50 y (n = 947) | P Value for Interaction | ||||
|---|---|---|---|---|---|---|---|
| β Level (SE) | P Value | Partial R2, % | β Level (SE) | P Value | Partial R2, % | ||
| Parietal | |||||||
| Inferior parietal | 0.02 (0.02) | .51 | NA | 0.13 (0.03) | <.001 | 2.78 | .001 |
| Superior parietal | 0.01 (0.02) | .71 | NA | 0.11 (0.03) | <.001 | 1.81 | .004 |
| Temporal | |||||||
| Hippocampus | 0.05 (0.02) | .07 | NA | 0.10 (0.03) | .004 | 1.28 | .04 |
| Fusiform | 0.03 (0.02) | .31 | NA | 0.09 (0.03) | .002 | 1.45 | .03 |
Abbreviation: NA, not applicable.
Covariates in the multivariable model include age, sex, race/ethnicity, telomere length, and total cranial volume. The effect size of telomere length (β level) is reported as the mean difference in each regional brain size associated with a 1-SD unit difference in telomere length. P values for the main effect of telomere length have been corrected for multiple comparisons.
We also sought to determine whether telomere-related atrophy was independent of other risk factors linked to brain atrophy.16 Adjusting for hypertension, obesity, DM, and smoking status had only a marginal effect on the association between telomere length and brain volume (eTable 3 in the Supplement).
APOE genotypes were available for approximately 70% of the participants. These genotypes were not independently associated with any of the subsegmental brain volumes after adjustment for all covariates (data not shown). No association was observed between telomere length and APOE genotype (data not shown). The addition of each of the different APOE genotypes (ε2, ε3, or ε4) to a multivariable model that included all other covariates did not substantially affect the association between telomere length and brain volume (eTable 4 in the Supplement). Cortical white and gray matter volumes were still independently associated with telomere length (P = .004 and P < .001, respectively), while cerebellar white and gray matter volumes were not. The volumes of the hippocampus, amygdala, superior and inferior temporal, precuneus, posterior central parietal, lateral orbitofrontal, posterior cingulate, thalamus, and ventral diencephalon regions were independently associated with telomere length after adjustment for all covariates, including APOE genotype (P < .001 for all).
Discussion
We present herein the first population-based analysis, to our knowledge, of brain MR imaging and its cross-sectional analysis with age and telomere length. While almost all subsegmental regions of the brain decreased in size with advancing age, telomere length was associated with the volumes of only certain subsegmental regions. The association with telomere length was independent of total cranial volume and age and other demographic factors, although a significant interaction with age was seen for the hippocampus, fusiform, and inferior and superior parietal regions. The structural changes associated with shortened telomeres may lead to an increased vulnerability to dementia and psychological disease.
More than half of the different regional brain volumes were independently associated with telomere length (Table 2). The associations between telomere length and some regions were more robust than others because they were observed in all subgroup analyses of the total cohort stratified by sex (eTable 1 in the Supplement) or age (eTable 2 in the Supplement) or after adjustment for additional covariates (eTable 3 and eTable 4 in the Supplement). The biological significance of these associations is unclear. However, the results of prior studies8-10,17 have suggested a link among leukocyte telomere length, cognitive function, and Alzheimer disease. In the analysis of the full cohort, we observed a significant association between shorter telomere length and the size of the hippocampus, a risk factor for Alzheimer disease18 and other forms of dementia.19 The volumes of other regions of the brain implicated in Alzheimer disease, including the amygdala, temporal lobe, parietal lobe, precuneus, and posterior cingulate,20-22 were also independently associated with telomere length. All these regions are susceptible to amyloid plaque deposition and belong to the default mode network, a functional connectivity of cortical regions sensitive to changes in brain cell activity.23,24 Several regions of the brain that show atrophy in Alzheimer disease but are not classically thought to have an abundance of amyloid plaque deposition (thalamus, ventral diencephalon, and fusiform cortex)25-27 were also associated with telomere length in this study. Their atrophy and neuronal loss may be due to a loss of reciprocal connections from and within the cortex. Other regions such as the entorhinal cortex and parahippocampal gyrus, which are closely related to the hippocampus and are known to atrophy in patients with Alzheimer disease,28,29 were not significantly associated with telomere length. So, brain volumes directly associated with telomere length imperfectly overlap those regions that atrophy in patients with Alzheimer disease.
Leukocyte telomere length has also been associated with life stressors.30,31 Adversity and stress have been associated with smaller volumes of corticostriatal-limbic regions, including the hippocampus, orbitofrontal cortex, insula, and anterior cingulate regions.32-34 The sizes of all these regions were found herein to be independently associated with telomere length. Therefore, the pattern of telomere-related regional brain volumes overlaps areas of the brain associated with dementia and stress-related psychopathology.
Few studies have explored the association between telomere length and human brain size. Shorter leukocyte telomere length was associated with mild cognitive impairment and decreased hippocampus volume in 29 participants of the Nurses' Health Study35 who underwent MR imaging. In contrast, a Swedish study36 of 57 individuals found longer leukocyte telomere length to be associated with smaller hippocampal volumes among APOE ε3/ε3 carriers but not among ε4 carriers. The same group later demonstrated evidence of a link between shorter leukocyte telomere length and subcortical brain size in 102 older individuals (age range, 64-75 years).37 The study was limited by the use of a simple 4-point Visual Atrophy Rating Scale, which may explain the lack of a significant association between telomere length and cortical volumes. The Cardiovascular Health Study38 obtained brain MR imaging white matter grade scores to indicate small-vessel vascular disease in 419 participants; these scores were not associated with telomere length after adjustment for age, sex, and race/ethnicity.
The actual mechanisms connecting leukocyte telomere length and regional brain size remain unclear. Leukocyte telomere shortening is associated with oxidative stress and inflammation.39,40 Insulin resistance, obesity, decreased physical activity, and smoking status have been linked to oxidative stress, telomere shortening,41,42 and structural changes of the brain.43-45 Although we found that the association between subsegmental brain volumes and telomere length remained significant even after adjusting for DM, body mass index, and smoking status, leukocyte telomere length may be a bio-marker for undetermined mediators of inflammation. Also, telomeres may be interacting with other genetic determinants to influence brain size. We did not find herein that the associations between brain volumes and telomere length were substantially altered after adjusting for APOE genotype, which has been previously linked to telomere length36,46 and Alzheimer disease risk.47 Because telomere length mirrors endogenous telomerase activity, leukocyte telomere length may also be a surrogate marker of brain cell proliferation potential. Neural stem cells of the adult brain are located in 2 major niches, the subventricular zone48,49 and the dentate gyrus of the hippocampus,50,51 and are capable of cell division throughout adulthood. Telomerase reactivation has also been shown to reverse neurodegeneration and restore proliferation of neuronal progenitors in animal models with deficient telomerase activity.52 Critical support cells in the brain capable of undergoing cell division in the adult (eg, microglia, astrocytes, oligodendrocytes, and pericytes) may be even more susceptible to deleterious effects of short telomeres. In mice, damage to pericytes led to neuronal damage and neurofibrillary tangle deposition in the hippocampus and cerebral cortex.53 Therefore, telomere shortening is a plausible biological mechanism underlying changes in regional brain volumes.
The strengths of this study include a middle-aged, population-based, multiethnic cohort and the availability of detailed medical phenotypes, biomarkers, leukocyte telomere lengths, and volumetric brain MR imaging measurements. Brain volumes were calculated using automated software tools that have been previously found to provide robust and accurate results,13,14 and all regional sizes were scaled to total cranial volume to adjust for total brain size at maturity. Telomere length was measured using a quantitative polymerase chain reaction assay to accommodate the limited amounts of available DNA and were estimated relative to a single-copy gene. While the age of the participants ranged from 18 to 85 years, with a mean age of 50 years, only 40 participants (2.0%) were older than 70 years, and only 5 participants (0.3%) were older than 75 years. The young age of our cohort is an asset in identifying early changes in brain structure associated with telomere length. The use of a multiethnic study population allows generalizability of our findings.
Because this is a cross-sectional study, we were unable to determine how the brain size changes over time. Another limitation is the lack of APOE genotypes for the entire cohort, although they were analyzed for 1372 participants, representing 70.0% of the total cohort. The addition of the APOE genotypes as a covariate did not change the seminal findings of this work. Finally, clinically validated measures of cognitive function or validated measures of stress-related phenotypes are not yet available for this cohort, so the associations with telomere length cannot be extrapolated to disease. In the absence of these phenotypes, the association between regional brain sizes and telomere lengths may reflect individual variability rather than functional atrophy associated with disease.
Conclusions
Alterations of several different biological pathways may underlie the pathogenesis of brain atrophy. We identified leukocyte telomere length as a biomarker associated with regional brain size independent of age. Additional phenotyping will be needed to assess whether the patterns of regional brain volumes in populations are associated with vulnerability to cognitive decline, dementia, or mood disorders. Longitudinal evaluations of this cohort will be essential in determining the predictive effect of these associations.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants UL1TR000451 and KL2TR000453 from the National Center for Advancing Translational Sciences and by grant R01HL093096 from the National Institutes of Health (Dr Garcia). Additional funding for this research was provided by grant P3012300-19 from the National Institutes of Health/National Institute on Aging Alzheimer's Disease Centers (Dr Rosenberg).
Role of the Sponsor: None of the funding organizations or sponsors had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr King had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: King, Rosenberg, Garcia.
Acquisition, analysis, or interpretation of data: King, Kozlitina, Peshock, McColl, Garcia.
Drafting of the manuscript: King, Kozlitina, Rosenberg, Garcia.
Critical revision of the manuscript for important intellectual content: King, Kozlitina, Peshock, McColl, Garcia.
Statistical analysis: King, Kozlitina, Garcia.
Obtained funding: Peshock, Garcia.
Administrative, technical, or material support: Peshock, McColl, Garcia.
Study supervision: Garcia.
Conflict of Interest Disclosures: Dr Rosenberg is the editor of JAMA Neurology and reported having a patent entitled “Amyloid and Gene Vaccines.” No other disclosures were reported.
Disclaimer: Dr Rosenberg is the Editor of JAMA Neurology and serves on the editorial board of JAMA. He was not involved in the editorial evaluation or decision to accept this article for publication.
Additional Contributions: Hannah Bereuter, BS, provided technical excellence in measuring telomere lengths. We thank Helen Hobbs, MD, and the other members of the Dallas Heart Study steering committee.
Correction: This article was corrected on October 30, 2014, to fix an error in the Funding/Support section.
References
- 1.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J Magn Reson Imaging. 1997;7(6):1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51(4):993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72(11):999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358(9277):201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 5.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer's disease. Lancet. 1999;353(9170):2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Alzheimer's Disease Neuroimaging Initiative. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132(pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe K, Lindquist K, Kluse M, et al. Health ABC Study. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panossian LA, Porter VR, Valenzuela HF, et al. Telomere shortening in T cells correlates withAlzheimer's disease status. Neurobiol Aging. 2003;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas P, O'Callaghan NJ, Fenech M. Telomerelength in white blood cells, buccal cells and braintissue and its variation with ageing and Alzheimer'sdisease. Mech Ageing Dev. 2008;129(4):183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Kozlitina J, Garcia CK. Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS One. 2012;7(12):e51046. doi: 10.1371/journal.pone.0051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sgouros S, Natarajan K, Hockley AD, Goldin JH, Wake M. Skull base growth in childhood. Pediatr Neurosurg. 1999;31(5):259–268. doi: 10.1159/000028873. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 16.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69(10):1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouiha A, Duchesne S. Alzheimer's Disease Neuroimaging Initiative. Hippocampal atrophy rates in Alzheimer's disease: automated segmentation variability analysis. Neurosci Lett. 2011;495(1):6–10. doi: 10.1016/j.neulet.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22(6):474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporalgyrus in mild cognitive impairment and Alzheimer'sdisease. J Alzheimers Dis. 2011;24(3):547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer's disease. Neurobiol Aging. 2010;31(1):25–33. doi: 10.1016/j.neurobiolaging.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49(12):967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 23.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarei M, Patenaude B, Damoiseaux J, et al. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer's disease. Neuroimage. 2010;49(1):1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Murphy EA, Holland D, Donohue M, et al. Alzheimer's Disease Neuroimaging Initiative. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. Neuroimage. 2010;53(4):1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westman E, Simmons A, Muehlboeck JS, et al. AddNeuroMed Consortium; Alzheimer's Disease Neuroimaging Initiative. AddNeuroMed and ADNI: similar patterns of Alzheimer's atrophy and automated MRI classification accuracy in Europe and North America. Neuroimage. 2011;58(3):818–828. doi: 10.1016/j.neuroimage.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 28.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41(1):51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Kerchner GA, Bernstein JD, Fenesy MC, et al. Shared vulnerability of two synaptically-connected medial temporal lobe areas to age and cognitive decline: a seven tesla magnetic resonance imaging study. J Neurosci. 2013;33(42):16666–16672. doi: 10.1523/JNEUROSCI.1915-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladwig KH, Brockhaus AC, Baumert J, et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8(7):e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage. 2008;40(2):788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grodstein F, van Oijen M, Irizarry MC, et al. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the Nurses' Health Study. PLoS One. 2008;3(2):e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wikgren M, Karlsson T, Lind J, et al. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE ε3/ε3 subjects. PLoS One. 2012;7(4):e34292. doi: 10.1371/journal.pone.0034292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wikgren M, Karlsson T, Soderlund H, et al. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age Ageing. 2014;43(2):212–217. doi: 10.1093/ageing/aft172. [DOI] [PubMed] [Google Scholar]
- 38.Sanders JL, Fitzpatrick AL, Boudreau RM, et al. Leukocyte telomere length is associated with noninvasively measured age-related disease: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2012;67(4):409–416. doi: 10.1093/gerona/glr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donovan A, Pantell MS, Puterman E, et al. Health Aging and Body Composition Study. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masi S, Nightingale CM, Day IN, et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler Thromb Vasc Biol. 2012;32(8):2029–2034. doi: 10.1161/ATVBAHA.112.250589. [DOI] [PubMed] [Google Scholar]
- 41.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 42.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 43.Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi K, Kurioka S, Yamaguchi T, et al. Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(2):180–185. doi: 10.1016/j.diabres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Wikgren M, Karlsson T, Nilbrink T, et al. APOE ε4 is associated with longer telomeres, and longer telomeres among ε4 carriers predicts worse episodic memory. Neurobiol Aging. 2012;33(2):335–344. doi: 10.1016/j.neurobiolaging.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 49.Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gage FH, Coates PW, Palmer TD, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92(25):11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 52.Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.