Abstract
Many compound collections used in high-throughput screening are composed of members whose structural complexity are considerably lower than natural products. We previously reported a strategy for the synthesis of complex and diverse small molecules from natural products using ring distortion reactions, called complexity-to-diversity (CtD), and herein CtD is applied in the synthesis of 16 diverse scaffolds and 65 total compounds from the alkaloid natural product sinomenine. Chemoinformatic analysis shows that these compounds possess complex ring systems and marked 3-dimensionality.
Graphical abstract
High-throughput screening (HTS) is a common method for identification of starting points for drug discovery projects, and 90% of first-in-class drugs from 1999 to 2008 originated from a screen.1 As such, the compound collections utilized in HTS campaigns have been highly scrutinized, and it is now well appreciated that the typical large (>100,000 members) compound collection available from commercial sources is principally populated by small molecules considered to be structurally simple, with a high percentage of sp2-hybridized carbons and few stereogenic centers.2 For example, Tan and co-workers calculated an average fraction sp3 (Fsp3) of 0.37 for a collection representative of commercial screening collections, whereas natural product drugs have an average Fsp3 of 0.68.3 While structurally simple compounds are valuable and have led to many drugs, especially for targets whose active sites favor the binding of flat molecules (e.g. kinases inhibitors4 and certain tubulin binders5,6) there is a continual need for complementary sets of small molecules that have greater structural complexity; such compounds would be expected to hit different types of targets (e.g. protein-protein interactions).7–10 Consequently, a priority for drug discovery is assembling compound collections populated by complex molecules.
Natural products are a major source of compounds with structural complexity, and many natural products or their derivatives are FDA approved drugs.11 Inspired by the success of natural products in drug discovery, synthetic methods have been developed to rapidly generate complex compounds including diversity–oriented synthesis (DOS),12–17 biology–oriented synthesis (BIOS),18 and construction of natural product–inspired scaffolds.19–23 We have previously reported the Complexity-to-Diversity (CtD) strategy in which the core ring systems of complex, readily available natural products are significantly altered using ring fusion, expansion, cleavage, and rearrangement reactions in the preparation of novel and structurally complex small molecules.24–27 Herein we disclose the manipulation of the alkaloid sinomenine (1, Scheme 1) using the CtD approach to generate a collection of 65 compounds.
Scheme 1.
Key transformations known for sinomenine
Sinomenine (1) is isolated from the roots and stems of the plant Sinomenium acutum, native to Japan and China.28 It is used in Asia for the treatment of rheumatoid arthritis and possesses immunosuppressive and anti-inflammatory activities.29,30 Structurally, sinomenine is composed of four fused rings and contains three contiguous stereogenic centers. The presence of an anisole, tertiary amine, enol ether, and ketone provides ready handles for alteration of three of the four rings and establishes sinomenine as an outstanding candidate for the CtD strategy. Furthermore, the hydrochloride salt of 1 is inexpensive and readily available in gram quantities.
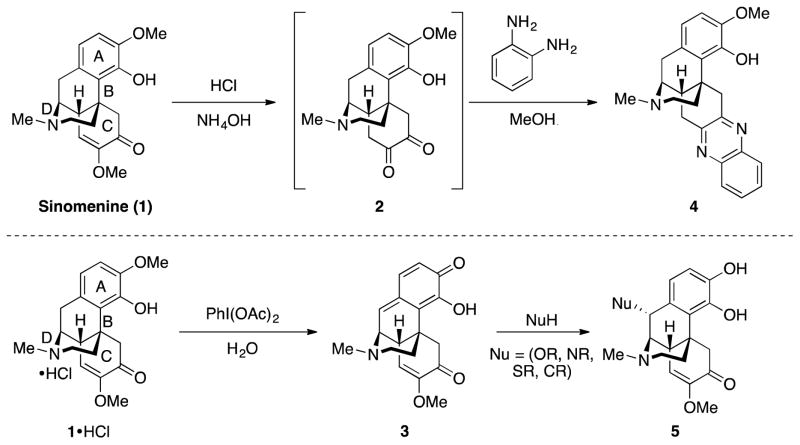
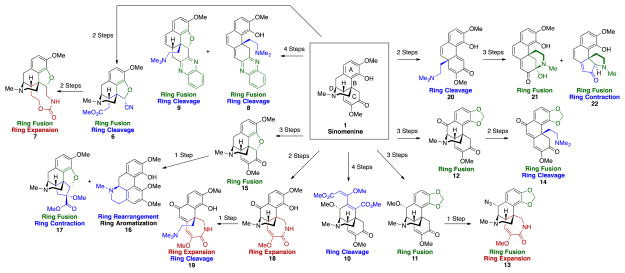
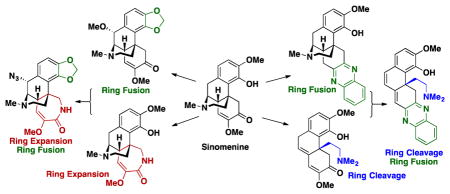
In designing the CtD library, several known transformations of 1 were instructive for priming 1 for ring distortion reactions (Scheme 1). These reactions include hydrolysis of the enol ether on the C-ring of 1 to generate diketone 2,31 and oxidative dearomatization of the HCl salt of 1 to form quinone methide 3.32 Treatment of 2 with o-phenylenediamine produces quinoxaline 4,31 and reaction of 3 with nucleophiles yields various catechols (5) substituted at the benzylic position.32 These known reactions, coupled with anticipated reactivity of the anisole, ketone, enol ether, and tertiary amine on the A, C, and D rings, provided strategic entry points into the core scaffold of 1. An overview of routes to 6–22 from 1 is shown in Scheme 2 and is described in detail below.
Scheme 2.
Overview of scaffolds generated from the alkaloid sinomenine
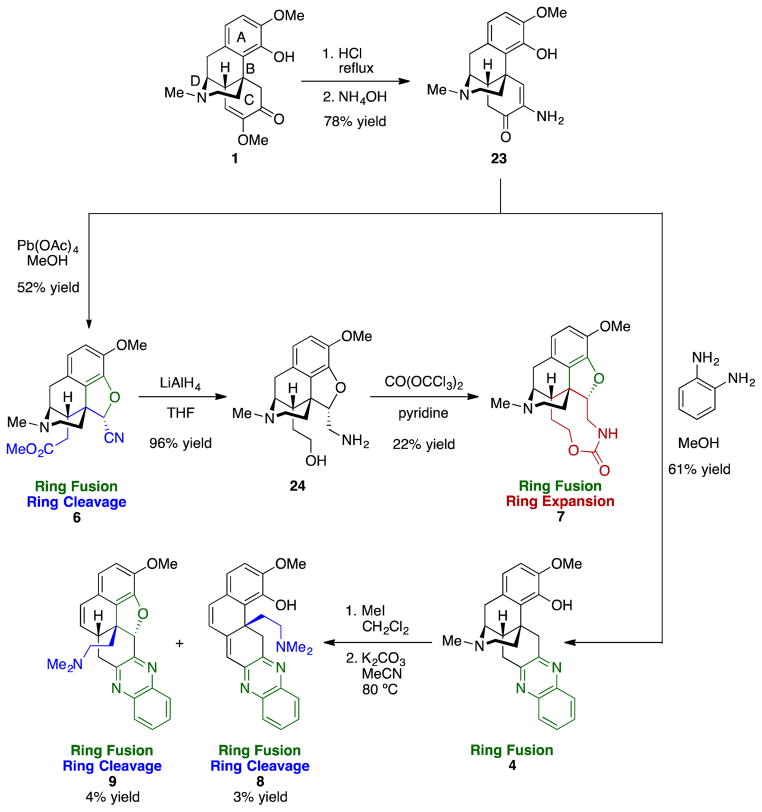
In investigating the hydrolysis of 1 it was discovered that treatment of 1 with hydrochloric acid and ammonium hydroxide resulted in isolation of keto–enamine 23. Compound 23 (Scheme 3) was envisioned as a key intermediate and a lynchpin for the synthesis of new ring systems, through oxidative cleavage of the C ring, and through the condensation to quinoxaline 4. Treatment of 23 with lead tetracetate resulted in a successful C-ring oxidative cleavage and a ring fusion to arrive at nitrile ester 6; isolation of a single diastereomer of 6 suggests that ring fusion occurred before cleavage. Reduction of 6 afforded amino alcohol 24, and exposure to triphosgene led to 9-membered carbamate 7. Condensation of keto-enamine 23 with o-phenylenediamine afforded 4. Hofmann–type elimination was envisioned from 4 by sequential exposure to iodomethane and potassium carbonate. The elimination was carried out successfully to generate conjugated compound 8, albeit in low yield, and the concomitant fusion of the anisole oxygen onto the C-ring to produce 9 was also observed.
Scheme 3.
New scaffolds through intermediate 23
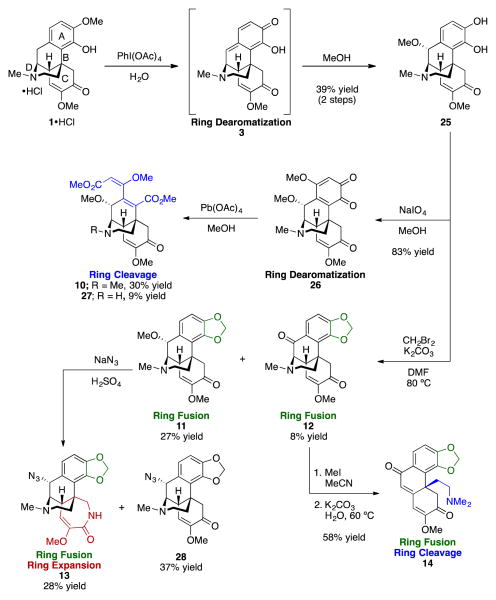
Demethylated catechol 2532 (Scheme 4) was envisioned as an entry point for extensive modification of the A ring of sinomenine. Oxidation of the HCl salt of 1 with diacetoxyiodobenzene in water produced dearomatized compound 3, and after dissolution in methanol catechol 25 was generated as a single diastereomer (Scheme 4) using the known protocol (albeit without purification of 3).32 Oxidative dearomatization of 25 with sodium periodate in methanol led to formation of o-quinone 26, whose cleavage with lead tetracetate formed diester 10 and demethylated analog 27. Catechols are known to undergo methylenation using methylene dihalides to form benzodioxoles.33 Following this precedent, treatment of 25 with dibromomethane and potassium carbonate led to generation of benzodioxole 11 and an oxidized variant, ketone 12. Exposure of 11 to Schmidt reaction conditions led to generation of ring–expanded 13. These reaction conditions also induced stereoretentive displacement of the benzylic methoxy group in 11 with azide en route to 13 and to yield 28. The azide approaches the substrate from the more accessible bottom face of the molecule. Methylation of benzodioxole 12 and subsequent treatment with potassium carbonate led to D-ring–opened 14.
Scheme 4.
New scaffolds through intermediate 25
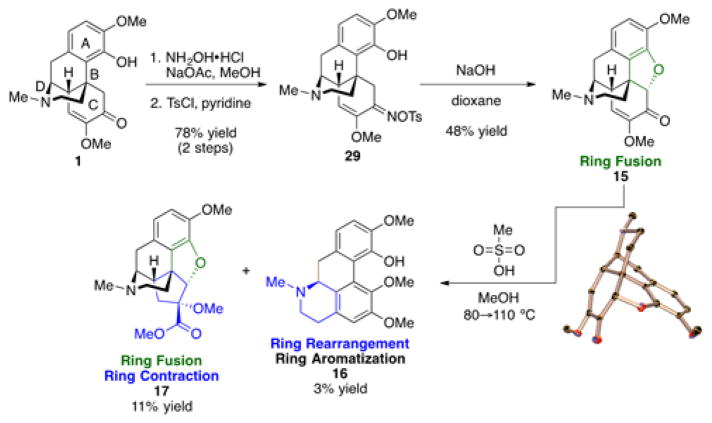
Further manipulation of the C and D-rings of 1 are shown in Scheme 5. Tosyl oxime 29 was synthesized from 1 in two steps. An attempted Beckmann rearrangement via subjection of 29 to 10% sodium hydroxide in dioxane led to unexpected ring fused compound 15, which was characterized by X-ray crystallography. Formation of 15 likely occurs through an azirine intermediate as is observed in the Neber rearrangement.34 Interestingly, 15 resembles the structure of (+)-morphine, and it is known that treatment of naturally occurring (−)-morphine with methanesulfonic acid yields apomorphine.35 Therefore, similar reaction conditions were applied to 15, resulting in a ring rearrangement involving aromatization of the C-ring, rearrangement of the D-ring, and addition and migration of a methoxy group to generate expected product 16 (the enantiomer of the apomorphine scaffold) as well as ring-contracted product 17, which is likely formed through a benzilic acid-type rearrangement.
Scheme 5.
Distortion of C and D ring systems
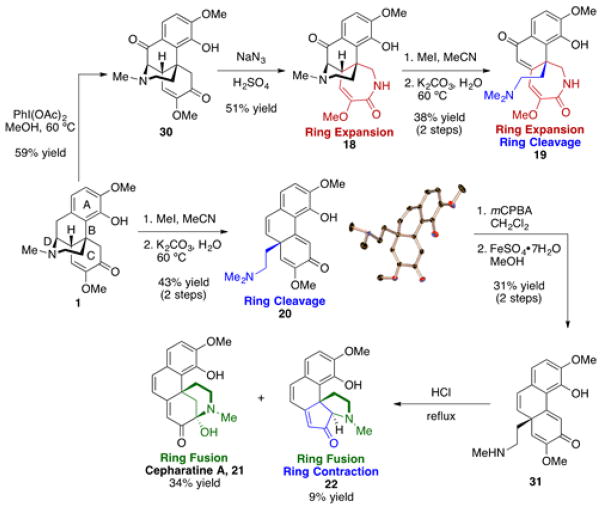
Finally, two of the transformations reported herein—Schmidt ring expansion of the C ring and Hofmann-type ring opening of the D ring—were applied to other complex scaffolds derived from 1. In the presence of diacetoxyiodobenzene, 1 could be oxidized at the benzylic position to afford ketone 30 (Scheme 6).32,36 The Schmidt reaction of 30 resulted in ring expansion (18), which could be followed by a Hofmann–type elimination to provide 19 (Scheme 6). Given the previous success of Hofmann–type eliminations, a direct ring cleavage of 1 was envisioned. Interestingly, treatment of sinomenine with iodomethane and subsequent elimination with potassium carbonate produced unexpected ring–cleaved, alkyl shifted 20, characterized by X-ray crystallography (Scheme 6). N-oxidation followed by iron-mediated demethylation produced secondary amine 31. Exposure of 31 to HCl induced another alkyl shift to arrive at the known natural product cepharatine A (21)37 as well as 22. Previously, 21 had been enantioselectively synthesized in 10 steps,38 making the work reported herein the shortest enantioselective synthesis to date (5 steps).
Scheme 6.
Synthesis of 19 and cepharatine A
In summary, the natural product sinomenine was used as a starting point for the creation of a diverse collection of structurally complex compounds. The combination of ring distortion reactions, such as fusion, contraction, expansion, rearrangement, dearomatization, and aromatization were strategically combined to generate 16 scaffolds and 65 total compounds (all compounds are shown in Figure S1 of the Supporting Information). Of these compounds, 14 are known (1, 3, 4, 21, 25, 30, 49–52, 55–57, 60), while the other 52 compounds are novel (full characterization data for all new compounds is in the Supporting information). In addition to the 28 compounds shown in the manuscript, another 37 compounds were synthesized from sinomenine. For these compounds (32–68 in Figure S1) the synthetic routes are in Figure S2. Most of the 65 compounds were produced on a scale of 25 mg or greater. Chemoinformatic diversity analysis was used to compare this collection to a commercially available screening collection. The set of 66 compounds (including sinomenine) contains the following complexity parameters (average): Fsp3 = 0.48, number of stereogenic centers = 3.1, ring fusion density39 = 0.37, ring complexity index39 = 1.40, and Glob40 = 0.36, which are markedly higher in value compared to Chembridge compounds (combined Diverset™-CL and Diverset™-EXP) Fsp3 = 0.38, number of stereogenic centers = 0.55, ring fusion density = 0.06, ring complexity index = 1.06, and Glob = 0.08. Please see Figure S3 for graphs comparing diversity parameters.
Complex molecules are different from and complementary to compounds in standard screening collections. As we have demonstrated previously with gibberellic acid24, adrenosterone24, quinine24, abietic acid25, pleuromutilin26, and now here with sinomenine, natural products are an outstanding starting point for the construction of complex and diverse small molecules.
Supplementary Material
Acknowledgments
We are grateful to the University of Illinois for support of this work. A.G. is a member of the NIH Chemistry–Biology Interface Training Grant (NRSA 1-T32-GM070421).
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, NMR spectra, and graphs comparing complexity parameters (PDF)
X-ray data for 15 (CIF)
X-ray data for 20 (CIF)
References
- 1.Swinney DC, Anthony J. Nat Rev Drug Discov. 2011;10:507. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 2.Lovering F, Bikker J, Humblet C. J Med Chem. 2009;52:6752. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 3.Stratton CF, Newman DJ, Tan DS. Bioorg Med Chem Lett. 2015;25:4802. doi: 10.1016/j.bmcl.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker A, Kettle JG, Nowak T, Pease JE. Drug Discov Today. 2013;18:298. doi: 10.1016/j.drudis.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Chen J, Xiao M, Li W, Miller DD. Pharm Res. 2012;29:2943. doi: 10.1007/s11095-012-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanpure RP, Nguyen BL, Strecker TE, Aguirre S, Sharma S, Chaplin DJ, Siim BG, Hamel E, Lippert JW, Pettit GR, Trawick ML, Pinney KG. J Nat Prod. 2011;74:1568. doi: 10.1021/np200104t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver LL. Clin Microbiol Rev. 2011;24:71. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arkin MR, Tang Y, Wells JA. Chem Biol. 2014;21:1102. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkin MR, Wells JA. Nat Rev Drug Discov. 2004;3:301. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 10.Milroy LG, Grossmann TN, Hennig S, Brunsveld L, Ottmann C. Chem Rev. 2014;114:4695. doi: 10.1021/cr400698c. [DOI] [PubMed] [Google Scholar]
- 11.Newman DJ, Cragg GM. J Nat Prod. 2016;79:629. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 12.Tan DS, Foley MA, Shair MD, Schreiber SL. J Am Chem Soc. 1998;120:8565. [Google Scholar]
- 13.Ibbeson BM, Laraia L, Alza E, O’Connor CJ, Tan YS, Davies HML, McKenzie G, Venkitaraman AR, Spring DR. Nat Commun. 2014:5. doi: 10.1038/ncomms4155. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor CJ, Beckmann HSG, Spring DR. Chem Soc Rev. 2012;41:4444. doi: 10.1039/c2cs35023h. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Kim H, Park SB. J Am Chem Soc. 2014;136:14629. doi: 10.1021/ja508343a. [DOI] [PubMed] [Google Scholar]
- 16.Burke MD, Schreiber SL. Angew Chem, Int Ed. 2004;43:46. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer GI, Perez JR, Duvall JR, Stanton BZ, Shamji AF, Schreiber SL. J Am Chem Soc. 2013;135:9675. doi: 10.1021/ja400034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hattum H, Waldmann H. J Am Chem Soc. 2014;136:11853. doi: 10.1021/ja505861d. [DOI] [PubMed] [Google Scholar]
- 19.McLeod MC, Singh G, Plampin JN, Iii, Rane D, Wang JL, Day VW, Aubé J. Nat Chem. 2014;6:133. doi: 10.1038/nchem.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenning AJ, Snyder JK, Porco JA. Org Lett. 2014;16:792. doi: 10.1021/ol4035269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber M, Owens K, Masarwa A, Sarpong R. Org Lett. 2015;17:5432. doi: 10.1021/acs.orglett.5b02797. [DOI] [PubMed] [Google Scholar]
- 22.McLeod MC, Aubé J. Tetrahedron. 2016;72:3766. doi: 10.1016/j.tet.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth JD, Craven PGE, Lilburn M, Pahl A, Marsden SP, Nelson A. Chem Commun. 2016;52:9837. doi: 10.1039/c6cc04662b. [DOI] [PubMed] [Google Scholar]
- 24.Huigens RW, III, Morrison KC, Hicklin RW, Flood TA, Jr, Richter MF, Hergenrother PJ. Nat Chem. 2013;5:195. doi: 10.1038/nchem.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafferty RJ, Hicklin RW, Maloof KA, Hergenrother PJ. Angew Chem, Int Ed. 2014;53:220. doi: 10.1002/anie.201308743. [DOI] [PubMed] [Google Scholar]
- 26.Hicklin RW, López Silva TL, Hergenrother PJ. Angew Chem, Int Ed. 2014;53:9880. doi: 10.1002/anie.201404765. [DOI] [PubMed] [Google Scholar]
- 27.Morrison KC, Hergenrother PJ. Nat Prod Rep. 2014;31:6. doi: 10.1039/c3np70063a. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki H. Acta Med Okayama. 1976;30:1. [PubMed] [Google Scholar]
- 29.Xu M, Liu L, Qi C, Deng B, Cai X. Planta Med. 2008;74:1423. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Li XK. Int Immunopharmacol. 2011;11:373. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Teng P, Liu HL, Deng ZS, Shi ZB, He YM, Feng LL, Xu Q, Li JX. Biorg Med Chem. 2011;19:3096. doi: 10.1016/j.bmc.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Lou YT, Ma LY, Wang M, Yin D, Zhou TT, Chen AZ, Ma Z, Bian C, Wang S, Yang ZY, Sun B, Yao ZJ. Tetrahedron. 2012;68:2172. [Google Scholar]
- 33.Yang T, Zhao K-m, Zhang L, Fang P-h, Jiang W-w. Agrochem. 2012:647. [Google Scholar]
- 34.O’Brien C. Chem Rev. 1964;64:81. [Google Scholar]
- 35.Csutoráas C, BeréAnyi S, Makleit S. Synth Commun. 1996;26:2251. [Google Scholar]
- 36.Compound 30 is known and can be accessed in two steps from 1 in an overall 6% yield.32
- 37.He L, Zhang YH, Guan HY, Zhang JX, Sun QY, Hao XJ. J Nat Prod. 2011;74:181. doi: 10.1021/np1005696. [DOI] [PubMed] [Google Scholar]
- 38.Chuang KV, Navarro R, Reisman SE. Angew Chem, Int Ed. 2011;50:9447. doi: 10.1002/anie.201104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todeschini R, Consonni V. Vol. 41 John Wiley & Sons; Hoboken: 2009. [Google Scholar]
- 40.Kuenemann MA, Bourbon LML, Labbé CM, Villoutreix BO, Sperandio O. J Chem Inf Model. 2014;54:3067. doi: 10.1021/ci500487q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.