Abstract
The negative symptoms have been described in association with schizophrenia since the early days of it being recognized as an entity. However, their elusive nature kept them unacknowledged until there was a revival of interest in them following the development of specific quantifying measures. Over the past three decades, there has been a tremendous surge in research on their types, measurements, status in the present classificatory system, and their implications. The developments in modern investigatory methods have provided the researchers with fresh insights into the underlying mechanisms, and a distributed functioning of the neuronal networks has emerged as the major abnormality. Accordingly, a variety of pharmacological and other treatment modalities have been developed which go beyond the traditional. Nevertheless, a lot remain unanswered. The present paper summarizes important concepts with regard to negative symptoms in schizophrenia.
Keywords: Negative symptoms, overview, schizophrenia
Initial descriptions of dementia praecox discussed at length about the lack of emotional responsivity, a loss of interest, and inability to “feel” for others in the patients,[1] recognizing this cluster of unique “absent” manifestations as an important part of the disorder. The concept of negative symptoms was subsequently introduced to psychiatry to describe this phenomenon.
However, the identification and quantification of these symptoms in practice proved to be a daunting task because of an inherent subjectivity and the obvious discomfort of the psychiatrist at identifying the “lack” of something. Instead, some easily identifiable and objective “first-rank” symptoms characterizing this disorder grouped together by Schneider[2] caught the fancy of the medical fraternity. They appeared to be the most obvious and dramatic of all the features of schizophrenia and became the focus of the available ameliorating strategies of the day. The demonstrated success in treating these symptoms with antipsychotic medications took away the limelight from an equally basic and perhaps more important and enduring negative symptoms dimension of the illness.
Nevertheless, the negative symptoms persisted. They subsequently staged resurgence through the works of Snezhnevsky, Carpenter, Strauss, Crow, and Andreasen during 1970s and 1980s. While the negative symptoms were finally recognized as a separate symptoms dimension with its own temporal stability, cognitive aspects, and heritability,[3] the development of tools as Positive and Negative Syndrome Scale (PANSS)[4] and the Scale for the Assessment of Negative Symptoms (SANS)[5] allowed for an accurate measurement of these. Such concerted effort led to the inclusion of the negative symptoms in the standard diagnostic criteria for schizophrenia.
THE NEGATIVE SYMPTOMS
Since the negative symptoms signify a lost function, there can be theoretically a large number of potential negative symptoms. Given the variety of cognitive, emotional, and socio-occupational deficits seen in schizophrenia, excepting defects in the domain pertaining to emotion, the rest are not defined as “negative symptoms.”[6] Diagnostic and Statistical Manual of Mental Disorders Fourth Edition - Text Revision (DSM)[5] and DSM-5[7] have enlisted and defined the negative symptoms under Criterion A5. Affective flattening, alogia (poverty of speech), and avolition (an inability to initiate and persist in goal-directed activities) have been included in the definition of schizophrenia while other symptoms such as anhedonia (loss of the ability to find or derive pleasure from activities or relationships) have been described as associated symptoms. The other international classificatory system, International Classification of Diseases (ICD-10),[8] enlists “marked apathy, paucity of speech, and blunting or incongruity of emotional responses” as negative symptoms. The National Institute of Mental Health Measurement and Treatment Research to Improve Cognition in Schizophrenia consensus panel has recently defined five negative symptoms:[9] blunted affect (diminished facial and emotional expression), alogia (decrease in verbal output or verbal expressiveness), asociality (lack of involvement in social relationships of various kinds), avolition (a subjective reduction in interests, desires, and goals and a behavioral reduction of self-initiated and purposeful acts), and anhedonia (inability to experience pleasure from positive stimuli). The current DSM-5 describes negative symptoms as “restricted emotional expression and avolition.” The first term includes reduction in expressions of emotion “in the face, eye contact, intonation of speech (prosody), and movements of the hand, head, and face that normally give an emotional emphasis to speech.”[7] Avolition has been defined as “a decrease in motivated self-initiated purposeful activities.” Certain other negative symptoms have been mentioned and defined in the current system and include alogia, anhedonia, and asociality.
While working with the negative symptoms in schizophrenia, it was recognized early that they might originate secondary to a lot of conditions, some related directly and others indirectly to the disease under study. These included long-term institutionalization, lack of environmental stimuli, poor social support, secondary to positive symptoms, other psychiatric illnesses as depression, and exacerbations of the disease as such and even antipsychotic medications.[10] As primary negative symptoms tend to be chronic, frustrating, and extremely debilitating, this “secondary origin” is important to recognize for the simple reason of potential treatability of a number of these. Both ICD-10 and DSM-5 require the clinician to rule out the secondary causes before concluding on this. DSM-5 recommends a persistence of the symptoms for a “considerable period” of time in spite of directed efforts at resolving the underlying secondary causes to be a reliable indicator for the primacy of the negative symptoms.
Under the present classificatory systems, schizophrenia remains the only disorder described along with negative symptoms. Perhaps, this approach is rooted in history because, while describing disordered volition in patients with dementia praecox, Kraepelin[1] had simultaneously noted, and emphasized upon, an absence of this in manic-depressive psychosis. As the current evidence gradually blur the once-distinct boundary between the two entities, the exclusivity of negative symptoms in schizophrenia is questioned.
This question was answered in various comprehensive studies, showing the presence of negative symptoms in different psychotic illness, including schizophrenia spectrum disorder as well as substance-induced psychosis. A comparable level of impairment with the presence of negative symptoms was noted even in affective disorder.[11]
All these now lend credibility to the proposition of a specific “deficit syndrome” by Kirkpatrick et al., existing along a trans-categorical plane,[12] and the existence of a separate subtype of schizophrenia with deficit syndrome as a prominent manifestation.[13]
FUNCTIONAL OUTCOME OF NEGATIVE SYMPTOMS
The advent of antipsychotic medications changed the way schizophrenic patients were treated. Amidst the ensuing euphoria of success in reducing the prevalence of positive symptoms, the psychiatrists noted that the patients' socio-occupational functioning remained muted. Thus, the spotlight turned back to the negative symptoms and the cognitive dysfunction dimensions. The “deficit syndrome” is presently estimated to have a prevalence of 15% in first-episode patients and 25%–30% in cases of chronic.[13] In another report, it has been found in 20%–25% clinical samples and 15%–20% of community-based samples of schizophrenia.[9]
Although few of the negative symptoms may be found even in “normal” population, typically, the negative symptoms have been described in long-term institutionalized patients, with limited environmental stimuli. However, the current conceptualizations indicate that primary negative symptoms may predate the positive symptoms by years in an undiagnosed state. These deficits might have a deteriorating, or a fluctuating course with intermittent improvement, and are considered to be a better predictor for functional outcome than the positive or disorganized symptoms.[6] Numerous researchers have tried to look into the issue[14] of a prognostic implication of these symptoms, and a consensus has emerged in recent years regarding a significant correlation between a higher burden of the negative symptoms on the one hand and a poor performance in social interaction, interpersonal relationships, economic functioning, and recreational activities on the other hand. On a longitudinal scale, deficit syndrome and occupational functioning appear to be inversely related, with deterioration in the later as the former increased in severity.[13] A logical question that cropped up from these is whether the domain of negative symptoms behaves as a single entity, or do the subdomains have independent predictive values? Further subanalysis of the individual symptoms revealed conflicting evidence on the interrelationship between anhedonia and functional outcomes, both being favored and refuted[15,16] in research findings. One source of this controversy appears to stem from the questionable validity of the scales used to measure the anhedonia symptoms,[17] and hence, caution is warranted in generalizing these findings.[16] Regarding amotivation and apathy, the studies revealed a positive association between these and the social dysfunction. Similarly, in patients with affective flattening, an increased severity of negative symptoms has been noted. However, no such relationship with either social skills or impaired social functioning could be found.[18]
THE MEASUREMENT OF NEGATIVE SYMPTOMS
During the 1970s and 1980s, psychiatry saw the introduction of a number of scales specially prepared to measure negative symptoms. The major scales included the SANS,[19] PANSS,[4] Brief Psychiatric Rating Scale,[20] and the Schedule for the Deficit Syndrome (SDS).[21]
A detailed discussion of the individual scales will not be considered here further, and the readers are referred to the respective references. However, a few salient points need to be mentioned in this discussion. First, while a number of dimensions were measured using these scales in an overlapping manner, they differed in defining these dimensions and thus exclusivity of definitions to symptoms remained questionable.[17] Furthermore, not all of these were meant primarily for measuring negative symptoms and rightly deviated from the same. While SDS measured the presence or absence of deficit syndrome, its utility for negative symptoms remained contentious. Similarly, later studies showed an overlap in domains measured on the subscales of PANSS, thus defining passive social withdrawal (N4), emotional withdrawal (N2), poor rapport (N3), active social avoidance (G16), lack of spontaneity (N6), blunted affect (N1), and disturbance of volition (G13) as actually measuring negative symptoms, rather than the negative symptom subscale itself.[22] Out of all of these, the SANS had been proposed to be the most comprehensive of all,[9] but a few of its subitems such as attentional impairment and inappropriate affect have been questioned for their inclusion in the definition of negative symptoms[23] subsequently.
Recent researchers have developed a number of scales to measure the negative symptoms. These include the brief negative symptoms scale - a 13-item instrument that measures under six subscales of anhedonia, distress, asociality, avolition, affective blunting, and alogia[24] and the Clinical Assessment Interview for Negative Symptoms that measures motivation, pleasure, and emotional expression.[25] The major limitation of these instruments is that they lack the extensive and time-tested validation of their older counterparts.
Investigators in the past two decades have also tried to categorize negative symptoms into relevant and valid subdomains. Foussias and Remington,[17] based on a review of the past studies, have proposed two basic subdomains, one with diminished expression, affective flattening, and poverty of speech (expressive component) and the other with amotivation, avolition/apathy, and anhedonia/asociality (involvement with the surrounding environment). When analyzed further, these two subdomains demonstrated a moderately strong interrelationship (interfactor correlation coefficients between 0.47 and 0.57) leading to speculations that these varied phenomenological manifestations might have a common underlying abnormality.
A recent study by Liemburg et al.[26] with 664 patients with schizophrenia did an exploratory factor analysis of the PANSS items after a diagnostic interview. The search yielded two-factor structure of the negative symptoms, with one consisting of affective flattening, avolition, poor rapport, mannerism, posturing, motor retardation, and lack of spontaneity and the other of emotional withdrawal, passive social withdrawal, and active social avoidance. The study findings corroborate with the recent DSM-5[7] proposal of expressive deficits and avolition/asociality domains for negative symptoms.
BIOLOGICAL ABNORMALITIES AND NEGATIVE SYMPTOMS
Structural and functional studies
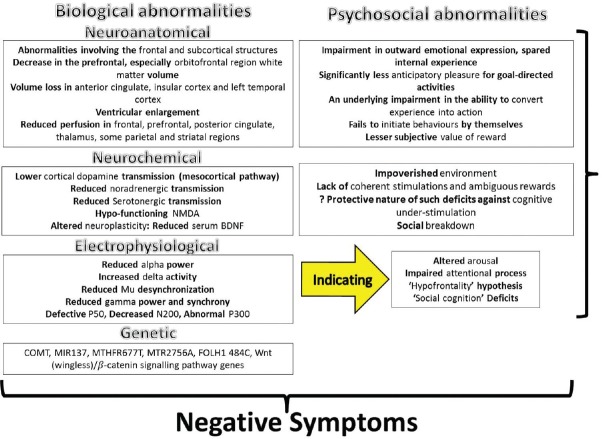
The neuropathologic origin of the negative symptoms has been postulated in various neuroimaging studies. Although different structures such as basal ganglia (particularly caudate nucleus), subcortical and right hemisphere lesions were implicated, the correlations were often found to be inconsistent. As a result, involvement of brain networks, rather than any specific anatomical structure, is proposed for genesis of these symptoms.[27] Neuroimaging studies have pointed out a myriad of both structural and functional alterations associated with negative symptoms since Crow's[3] description of Type 1 and 2 schizophrenia. A decrease in the prefrontal, especially orbitofrontal region, white matter volume along with volume loss in anterior cingulate, insular cortex, left temporal cortex, and ventricular enlargement[28] is associated with severity of negative symptoms, and this loss is found to start even before the appearance of the negative symptoms and continues with the course of illness. Similarly, reduced cerebral blood perfusions in frontal, prefrontal, posterior cingulate, thalamus, some parietal, and striatal regions have been shown to correlate with an increased negative symptom scores in the patients.[29]
With concurrent developments in the neuroelectrophysiological method, negative symptoms in schizophrenia were found to correlate with reduced alpha power, as well as an increased delta activity in the frontal, temporal, and central regions, increased frontal theta and an increased beta (18.5–30 Hz) activity at frontal, temporal, and central electrodes in those with prominent negative symptoms as compared to normal controls. The increased low-frequency activity was thought of as a vindication for the “hypofrontality” hypothesis.[30] The increased frontal delta activity is also found in high-risk population for the development of psychotic and negative symptoms, which might be a manifestation of a reduced activity at the “nonmotor cortico-basal ganglia-thalamocortical circuits.”[31] A study on specific delta wave activity using low-resolution brain electromagnetic tomography suggested that electrophysiological changes in areas such as the prefrontal cortex might be responsible for the manifestation of negative symptoms.[32]
A reduced event-related desynchronization of the mu wave, apparently tapping the “social cognition” circuits, in response to biological motion has been recently demonstrated and has been found to strongly correlate with negative symptoms. Similarly, reduced gamma-band power and synchrony has been shown to be related to negative symptom in recent research work. Studies on the event-related potentials in relation to negative symptoms of schizophrenia demonstrated defective p50 gating showing the abnormal gating mechanisms operating in this condition.[33] A recent study with magnetoencephalography correlated a poor right hemispheric sensory gating in patients with prominent negative symptoms,[34] implicating the right superior temporal gyrus. Decreased N200 amplitude and abnormal P300 wave have been noted in association with the negative symptoms in a number of past studies[35] and replicated numerous times since then. In summary, the neuroelectrophysiological findings support the hypothesis of abnormal network functioning rather than particular anatomical aberrations in the genesis of the negative symptoms.
Biochemical and genetic studies
Biochemically, the origin of the negative symptoms has been inferred from the demonstrated alterations in various neurotransmitters in the affected brains. A lower plasma homovanillic acid level predicted a higher negative symptom score.[36] Based on this finding, a dopaminergic hypothesis of the negative symptoms has been proposed, where a lower cortical dopamine transmission (mesocortical pathway) gives rise to negative symptoms while an excess dopamine in the subcortical structures elicits positive symptoms.[37] Deficient noradrenergic transmission in patients with negative symptoms was based on the demonstration of beneficial effects of drugs such as imipramine.[38] Besides this, decreased cerebrospinal fluid noradrenaline level and urinary 3-methoxy-4-hydrophenylglycol (noradrenaline metabolite) level[39] commensurate with symptom severity. Reduced serotonergic transmission is also implicated for the emergence of negative symptoms, which is further bolstered by the evidence of improvement of the same by the antipsychotics with action on serotonin receptors.[12] A combined approach has been taken by Goff and Evins[40] in proposing an interaction between the cortical dopaminergic and the serotonergic system in determining the manifestation of the negative symptoms. Taking into account a similar clinical profile of reduced motor initiative and impaired performance in cognitive tasks requiring a higher level of attention, along with similar profile of abnormalities in major depressive disorder and Parkinson's disease,[29] researchers have now proposed an impaired functioning of fronto-striato-limbic circuits in the genesis of these negative symptoms.
Recent works on the neurogenesis and neuroplasticity pathways have generated special interest in their role in negative symptomatology. That neuroinflammation in adulthood might lead to the development of negative symptoms subsequently has been proposed on the basis of the finding of elevated C-reactive protein in these patients[41] and evidence of herpes simplex virus infection.[42] A study by Yoshimura et al.[39] found a reduced serum brain-derived neurotrophic factor (BDNF)-like immunoreactivity in chronic schizophrenics, probably as a result of long-standing neurodegeneration and simultaneous reparative process in the brain. As BDNF is involved in various stages of nervous system development and functioning, its involvement in various manifestations of the disorder is imperative. However, convincing results are lacking and further exploration is needed. Similarly, a hypofunctioning N-methyl-D-aspartate (NMDA) system which plays a role in the long-term potentiation and promoting neuroplasticity has been implicated in the genesis of the negative symptoms. It is hypothesized that reduced NMDA transmission, possibly in face of neuroinflammation, might be detrimental at the functional level, and supporting evidence in form of a demonstrated improvement in symptoms with NMDA agonists such as D-cycloserine are encouraging.[43]
The proposal that the genetic factors are involved in the “genesis” of the negative symptoms has been supported by the results of a meta-analysis by Esterberg et al.[44] where the authors found a small but significant increase in the severity of negative symptoms in those with a positive family history. These findings also indicate a multifactorial etiology or additive influence of small genetic changes in multiple loci rather than an involvement of single gene or genetic pathway behind emergence of negative symptoms.[45] The genes related to dopaminergic transmissions (DA receptor subtypes, DA transporter, and catechol-O-methyl transferase [COMT]), related to glutaminergic transmission (NMDA receptors, NR1 subunits, and metabotropic receptors), DISC1, dysbindin, FGFR1, GSK3 β, and neuregulin1, etc., have been proposed. The rs4633-rs4680 haplotype of COMT gene was shown to be significantly associated with the overall score of negative symptoms. The folate deficiency in patients with negative symptoms intrigued researchers to look for genetic variations in folate metabolism, which led to identification of MTHFR 677T, MTR 2756 A, FOLH1 484C, and COMT 675A, to be independent and significant predictors of negative symptom severity.[46] Single-nucleotide polymorphism (rs1625579 SNP) at MIR137 involved in adult neurogenesis and neuronal maturation has been reported recently[47] to be significantly associated with the negative symptomatology, emphasizing the “abnormal neurodevelopment” hypothesis. Another finding is a significant correlation between the Wnt (wingless)/β-catenin signaling pathway genes (glycogen synthase kinase 3 beta and disheveled 2) and negative symptoms.[48] Nevertheless, these reports are sparse and sporadic, and confirmation awaits further refinement.
NEUROCOGNITIVE AND PSYCHO-SOCIAL ABNORMALITIES
Various neurocognitive abnormalities have been definitely demonstrated in patients with schizophrenia with negative symptoms. Whether these represent overlapping dimensions is an interesting proposition. In various studies, negative symptoms and cognitive deficits have been found to correlate weakly, and no clear link between these two has been demonstrated in terms of longitudinal evolution of the symptoms. Harvey et al.[49] in their analysis of the domains based on four theoretical models concluded that negative and cognitive symptoms of schizophrenia were apparently related but potentially separable domains, proposing that either each of these symptom dimensions has a separate but related etiology or these symptom dimensions are distinct from each other, with completely separate etiologies.
Before moving on with the discussion, the interrelationship between anticipatory, consummatory pleasure, and anhedonia needs to be reviewed. Working on anticipatory (that an activity will be enjoyable) and consummatory pleasure (engaging in enjoyable activities), Gard et al.[16] found that those with schizophrenia reported significantly less anticipatory pleasure for goal-directed activities (making dinner, working/studying, etc.) compared to nongoal-directed activities such as eating and sleeping. They also found that anticipatory pleasure scale scores were significantly correlated with clinical ratings of anhedonia and impaired community functioning. Similarly, Heerey and Gold[50] proposed that motivational deficits in schizophrenia were a manifestation of an underlying impairment in the ability to convert experience into action, based on their demonstration of a deficit in the ability of the patients to behave commensurate to the motivational property of the stimulus, while in effect, they maintained an equivalent arousal ratings as of control participants. The findings suggest these individuals with schizophrenia might actually be experiencing amotivation instead of anhedonia.
On these lines, the neurocognitive model proposes a deficit in the ability to “initiate” actions by themselves while the ability to respond to and perform “stimulus-driven” actions is more or less preserved. At the heart of this deficit has been described a dysfunctional Shallice's Supervisory Attentional System[51] which fails to initiate behaviors by itself in the absence of an environmental stimulus. Studies demonstrated that patients with more negative symptoms show reduced subjective value for rewards which might be responsible for their inability to learn from positive outcome in spite of preserved ability to learn from negative outcome of an action.[52] Interestingly, these specific deficits were found to be independent of the general impairment in neuropsychological performance of the respondents, and results suggest “a complex relationship between negative and cognitive symptoms in determining goal-directed behavior in schizophrenia as well as a detrimental impact of motivational deficits in cognitive functioning.”[17]
From a psychosocial perspective, the negative symptoms have been conceptualized as a manifestation of an impoverished environment, lack of coherent stimulations, and ambiguous rewards.[53] It was proposed that the negative symptoms might actually be a result of persistent cognitive understimulation and elimination of pleasurable and reinforcing stimuli due to institutional care or subsistent level of existence.[6] The main drawback of the proposition is it failed to explain the occurrence of negative symptoms in persons living in apparently enriched environments as well as the lack of response to an environmental enrichment in all studied.
NEGATIVE SYMPTOMS: MANAGEMENT
Somatic strategies
The primary negative symptoms remain a frustrating and obstinate adversary for a worldwide effort at achieving a breakthrough in the management of schizophrenia. A variety of treatment proposals have been considered in the past, based on the etiological theories of the negative symptoms.
When the role of serotonin was demonstrated, for example, the agents having an action on this system attracted great fanfare. The antipsychotics with an action of this system theoretically appeared to be the wonder drugs. Amisulpride, olanzapine, quetiapine, along with newer molecules such as asenapine, paliperidone, and lurasidone[54] were tested in patient population and all demonstrated effectiveness in the studies. However, they suffered from limited sample sizes. Large-scale studies such as CUtLASS[55] failed to show any significant difference between the second and the first generation antipsychotics, in spite of the latter having only D2-receptor action. Hence, it can be concluded that even if the atypical antipsychotics are beneficial, the benefit is only modest. The 2009 PORT recommendations reflect these observations and acknowledge that “the level of evidence is currently insufficient to support a treatment recommendation for any pharmacological treatment of negative symptoms in schizophrenia.”[56]
A search for an alternative quickly suggested antidepressants. This again was in line with the proposed spectrum of neurotransmitter abnormalities noted in negative symptoms and their clinical similarities to depression. Singh et al.[57] did a meta-analysis of the available literature on 23 randomized controlled trials in this regard, with an overall sample size of 819 participants. The authors found a “justification for the use of antidepressants in negative symptoms in chronic schizophrenia.” They further analyzed the efficacy of individual antidepressants and found a statistically significant role of fluoxetine, trazodone, and ritanserin while an equivocal role of mirtazapine, sertraline, paroxetine, reboxetine, fluvoxamine, mianserin, and citalopram. The number needed to treat was found to be 10 for the antidepressants as a group, 11 for fluoxetine, 6 for trazodone, and 5 for ritanserin, which appear promising and clinically useful. While one possibility remains that the benefit was actually due to an alleviation of the negative symptoms which were secondary to a primary depression in these patients, it is difficult to disentangle. There is also a possibility of increased side effects and cost of treatment when this strategy is applied in combination with the primary antipsychotic and recommends caution in such endeavors. The PORT guideline,[56] however, is less convinced, and it considers the evidence available to be insufficient for a strongly founded recommendation on this strategy.
The third approach has been the use of psychostimulants. The rationale was a proposed dopaminergic dysfunction in the mesocortical pathway and at the prefrontal cortex through D1-dopaminergic receptors.[37] A review of the related studies conducted between 1984 and 2011 by Lindenmayer et al. found that the results favored the adjunctive use of dopamine agonists such as amphetamine and methylphenidate for improvement of the negative symptoms under the cover of an adequate dose of antipsychotics, with an overall improvement between 37% and 67% in the symptom scores. However, a large number of studies had a small sample size, and a few did report negative to neutral improvement in overall symptomatology,[58] thus appearing to be major deterrents in recommendations for an utility or a dose range for these agents. Of course, the chances of exacerbating the positive symptoms by increasing transmission at the D2 receptors were also very real, hence the authors emphasized that only “clinically stable patients in the nonacute phase of illness who are reliably receiving antipsychotic therapy and presenting with minimal positive symptomatology are at a relatively reduced risk for the psychotogenic effects of psychostimulants.”
When the most trusted groups of psychopharmacological armamentarium failed, the researchers looked beyond. Addressing novel systems in the brain, glycine transporter 1 (GlyT1) inhibitor sarcosine (N-methylglycine) was found to be effective in reducing negative symptoms in both acute as well as chronic cases.[59] In a Phase IIb trial, a noncompetitive GlyT1 inhibitor bitopertin was found to be significantly associated with an improvement in negative symptoms and a functional improvement during the preprotocol analysis among 323 patients with schizophrenia.[60] Both these have been hypothesized to work through an interaction with the NMDA-mediated glutaminergic transmission in the brain. Similar encouraging findings involving the cholinergic system have also emerged in recent times. A Phase II double-blind, randomized, crossover trial with selective α7 nicotinic acetylcholine receptors agonist dimethoxybenzylidene anabaseine showed improvements in negative symptoms at statistically significant levels.[61] Interestingly, minocycline, a second-generation tetracycline antibiotic, appeared to attenuate negative symptoms in a 16-week double-blind, randomized placebo control trial, possibly through its primary action on the glutaminergic system as well as to antioxidant, antiapoptotic, and neuroprotective effects.[62]
A number of other agents such as memantine, donepezil, dehydroepiandrosterone, ondansetron, and nutrients such as Ginkgo biloba have been used in the past to address the issue of negative symptoms. While they have mainly been validated in small trials, guidelines lack for their use based on available evidence.[59]
The use of electroconvulsive therapy is controversial and not recommended for negative symptoms.[56] The role of repetitive transcranial magnetic stimulation (rTMS) in this regard has been proposed on the theoretical model of its ability to improve the excitability of the dorsolateral prefrontal cortex (dlPFC) which has been found to be hypoactive in these patients. Conclusive results lack on this strategy though Shi et al.[63] undertook a meta-analysis of the available data on this and found a strong effect size for high-frequency rTMS (10 Hz) over left dlPFC and 110% MT, the relatively small sample size remains constraint for generalization of finding. However, given the lack of other proven strategies, this appears to be a method worth exploring.
Preventive approaches
The preventive approaches to the development of negative symptoms are limited. The identification of the secondary causes giving rise to such deficits needs to be searched for thoroughly and addressed. An early intervention for the psychotic illness has also been useful in reducing the chances of developing negative symptoms longitudinally.[64]
Psychotherapeutic approaches
The NICE guidelines[65] recommend the use of cognitive behavior therapy along with family intervention for all patients with schizophrenia. Especially for the negative symptoms, arts therapies have been recommended. The arts therapies are an umbrella term encompassing strategies that have central nonverbal components, such as body psychotherapy. A number of studies in the past[66] have found favorable outcomes with these techniques on the negative symptom domain. The guidelines recommend it in a group setting, and the strategies “combine psychotherapeutic techniques with activity aimed at promoting creative expression, which is often unstructured and led by the service user.” The aims of this include improving the self-image and ways, in which the patients related to others and to improve the expressions and organization of the experiences into a coherent whole. However, the guidelines currently require a further exploration of these strategies before recommending them with an evidence base. Routine use of supportive psychotherapy, social skill training, and counseling is discouraged, as is adherence therapy; a clinician's judgment is warranted in these cases. Another useful approach is the use of environmental-stimulating strategies, such as supported employment, for an understimulation might be giving rise to the pathology.[53]
CONCLUSION
The interest in the negative symptoms has seen a resurgence over the past three decades, and with improvements in the understanding of the mechanisms involved in their genesis, the approach to them is bound to change in the near future. The negative symptoms are presently being considered as a separate dimension of psychopathology and seem to be pervading across the categorical diagnostic classes. Rather than a single structural or neurochemical abnormality, present evidence point toward a distributed dysfunctioning of neuronal networks in the manifestations, originating at various stages of the developmental process [Figure 1]. Likewise, the therapeutic approaches have moved beyond the conventional antipsychotic and antidepressant models, and the field has seen trials with novel neuropeptides, anti-inflammatory agents,[67] NMDA agonists, and rTMS. With that, newer psychosocial interventions have addressed the specific etiological models and proved effective in tackling a number of associated deficits.
Figure 1.
The origins of negative symptoms
The authors envision further improvements in the genetic and functional understanding, along with a refinement in the understanding of the environmental influences, which would herald a better management approach toward these symptoms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh: Livingstone; 1919. [Google Scholar]
- 2.Schneider K. Clinical Psychopathology. New York: Grune and Stratton; 1959. [Google Scholar]
- 3.Crow TJ. The two-syndrome concept: Origins and current status. Schizophr Bull. 1985;11:471–86. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- 4.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition Text Revision. Washington D.C: American Psychiatric Association; 2000. [Google Scholar]
- 6.Lewis S, Escalona PR, Keith SJ. Phenomenology of Schizophrenia. In: Sadock BJ, Sadock V, Ruiz P, editors. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C: American Psychiatric Association; 2013. [Google Scholar]
- 8.World Health Organization. The Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) Geneva: World Health Organization; 1993. [Google Scholar]
- 9.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirschner M, Aleman A, Kaiser S. Secondary negative symptoms - A review of mechanisms, assessment and treatment. Schizophr Res. 2016 doi: 10.1016/j.schres.2016.05.003. pii: S0920-996430224-9. [DOI] [PubMed] [Google Scholar]
- 11.Lyne J, O'Donoghue B, Owens E, Renwick L, Madigan K, Kinsella A, et al. Prevalence of item level negative symptoms in first episode psychosis diagnoses. Schizophr Res. 2012;135:128–33. doi: 10.1016/j.schres.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Möller HJ. Management of the negative symptoms of schizophrenia: New treatment options. CNS Drugs. 2003;17:793–823. doi: 10.2165/00023210-200317110-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–71. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 14.Rosenheck R, Leslie D, Keefe R, McEvoy J, Swartz M, Perkins D, et al. Barriers to employment for people with schizophrenia. Am J Psychiatry. 2006;163:411–7. doi: 10.1176/appi.ajp.163.3.411. [DOI] [PubMed] [Google Scholar]
- 15.Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophr Res. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–60. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foussias G, Remington G. Negative symptoms in schizophrenia: Avolition and Occam's razor. Schizophr Bull. 2010;36:359–69. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca-Pedrero E, Gooding DC, Paino M, Lemos-Giráldez S, Muñiz J. Anhedonia: A Comprehensive Handbook Volume II. Netherlands: Springer; 2014. Measuring anhedonia in schizophrenia-spectrum disorders: A selective update; pp. 19–54. [Google Scholar]
- 19.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 20.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 21.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The schedule for the deficit syndrome: An instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–23. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 22.Emsley R, Rabinowitz J, Torreman M RIS-INT- Early Psychosis Global Working Group. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61:47–57. doi: 10.1016/s0920-9964(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 23.Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff D, et al. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90:316–24. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: Psychometric properties. Schizophr Bull. 2011;37:300–5. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Am J Psychiatry. 2013;170:165–72. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Two subdomains of negative symptoms in psychotic disorders: Established and confirmed in two large cohorts. J Psychiatr Res. 2013;47:718–25. doi: 10.1016/j.jpsychires.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Levitt JJ, Rosow LK, Nestor PG, Pelavin PE, Swisher TM, McCarley RW, et al. A volumetric MRI study of limbic, associative and sensorimotor striatal subregions in schizophrenia. Schizophr Res. 2013;145:11–9. doi: 10.1016/j.schres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12:53–69. doi: 10.1586/ern.11.173. [DOI] [PubMed] [Google Scholar]
- 29.Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, Bradshaw JL, White OB. Negative symptoms: A review of schizophrenia, melancholic depression and Parkinson's disease. Brain Res Bull. 2006;70:312–21. doi: 10.1016/j.brainresbull.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.John JP, Rangaswamy M, Thennarasu K, Khanna S, Nagaraj RB, Mukundan CR, et al. EEG power spectra differentiate positive and negative subgroups in neuroleptic-naive schizophrenia patients. J Neuropsychiatry Clin Neurosci. 2009;21:160–72. doi: 10.1176/jnp.2009.21.2.160. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie S, Schäfer MR, Whitford TJ, Benninger F, Feucht M, Klier CM, et al. Frontal delta power associated with negative symptoms in ultra-high risk individuals who transitioned to psychosis. Schizophr Res. 2012;138:206–11. doi: 10.1016/j.schres.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Itoh T, Sumiyoshi T, Higuchi Y, Suzuki M, Kawasaki Y. LORETA analysis of three-dimensional distribution of delta band activity in schizophrenia: Relation to negative symptoms. Neurosci Res. 2011;70:442–8. doi: 10.1016/j.neures.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Featherstone RE, McMullen MF, Ward KR, Bang J, Xiao J, Siegel SJ. EEG biomarkers of target engagement, therapeutic effect, and disease process. Ann N Y Acad Sci. 2015;1344:12–26. doi: 10.1111/nyas.12745. [DOI] [PubMed] [Google Scholar]
- 34.Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar C, et al. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res. 2005;73:311–8. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–84. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Ribeyre JM, Lesieur P, Varoquaux O, Dollfus S, Pays M, Petit M. A comparison of plasma homovanillic acid in the deficit and nondeficit subtypes of schizophrenia. Biol Psychiatry. 1994;36:230–6. doi: 10.1016/0006-3223(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 37.Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: Making sense of it all. Curr Psychiatry Rep. 2007;9:329–36. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- 38.Siris SG, Bermanzohn PC, Gonzalez A, Mason SE, White CV, Shuwall MA. The use of antidepressants for negative symptoms in a subset of schizophrenic patients. Psychopharmacol Bull. 1991;27:331–5. [PubMed] [Google Scholar]
- 39.Yoshimura R, Hori H, Katsuki A, Atake K, Nakamura J. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1. doi: 10.1186/s12991-015-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goff DC, Evins AE. Negative symptoms in schizophrenia: Neurobiological models and treatment response. Harv Rev Psychiatry. 1998;6:59–77. doi: 10.3109/10673229809000313. [DOI] [PubMed] [Google Scholar]
- 41.Fawzi MH, Fawzi MM, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res. 2011;190:91–7. doi: 10.1016/j.psychres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, et al. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: A longitudinal study. Am J Psychiatry. 2011;168:822–30. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goff DC. D-cycloserine in Schizophrenia: New strategies for improving clinical outcomes by enhancing plasticity. Curr Neuropharmacol. 2017;15:21–34. doi: 10.2174/1570159X14666160225154812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esterberg ML, Trotman HD, Holtzman C, Compton MT, Walker EF. The impact of a family history of psychosis on age-at-onset and positive and negative symptoms of schizophrenia: A meta-analysis. Schizophr Res. 2010;120:121–30. doi: 10.1016/j.schres.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Potkin SG, Macciardi F, Guffanti G, Fallon JH, Wang Q, Turner JA, et al. Identifying gene regulatory networks in schizophrenia. Neuroimage. 2010;53:839–47. doi: 10.1016/j.neuroimage.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zai G, Robbins TW, Sahakian BJ, Kennedy JL. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci Biobehav Rev. 2017;72:50–67. doi: 10.1016/j.neubiorev.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Green MJ, Cairns MJ, Wu J, Dragovic M, Jablensky A, Tooney PA, et al. Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol Psychiatry. 2013;18:774–80. doi: 10.1038/mp.2012.84. [DOI] [PubMed] [Google Scholar]
- 48.Bousman CA, Glatt SJ, Chandler SD, Lohr J, Kremen WS, Tsuang MT, et al. Negative symptoms of psychosis correlate with gene expression of the Wnt/ß-catenin signaling pathway in peripheral blood. Psychiatry J. 2013;2013:852930. doi: 10.1155/2013/852930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophr Bull. 2006;32:250–8. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116:268–78. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 51.Shallice T. From Neuropsychology to Mental Structure. New York: Cambridge University Press; 1988. [Google Scholar]
- 52.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–21. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpenter WT, Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophr Bull. 1985;11:440–52. doi: 10.1093/schbul/11.3.440. [DOI] [PubMed] [Google Scholar]
- 54.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 55.Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life of second- vs. first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–87. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 56.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh SP, Singh V, Kar N, Chan K. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: Meta-analysis. Br J Psychiatry. 2010;197:174–9. doi: 10.1192/bjp.bp.109.067710. [DOI] [PubMed] [Google Scholar]
- 58.Lindenmayer JP, Nasrallah H, Pucci M, James S, Citrome L. A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: Challenges and therapeutic opportunities. Schizophr Res. 2013;147:241–52. doi: 10.1016/j.schres.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Arango C, Garibaldi G, Marder SR. Pharmacological approaches to treating negative symptoms: A review of clinical trials. Schizophr Res. 2013;150:346–52. doi: 10.1016/j.schres.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Umbricht D, Martin-Facklam M, Pizzagalli F, Youssef E, Yoo K, Doerflinger E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: Results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Schizophr Bull. 2011;37(Suppl 1):324. [Google Scholar]
- 61.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang B, et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: A double blind, randomized, controlled trial. Schizophr Res. 2014;153:169–76. doi: 10.1016/j.schres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Shi C, Yu X, Cheung EF, Shum DH, Chan RC. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: A meta-analysis. Psychiatry Res. 2014;215:505–13. doi: 10.1016/j.psychres.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melle I, Larsen TK, Haahr U, Friis S, Johannesen JO, Opjordsmoen S, et al. Prevention of negative symptom psychopathologies in first-episode schizophrenia: Two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry. 2008;65:634–40. doi: 10.1001/archpsyc.65.6.634. [DOI] [PubMed] [Google Scholar]
- 65.NICE National Clinical Guideline Number 82: Schizophrenia. National Institute for Health and Clinical Excellence. 2009. [Last accessed on 2013 Oct 09]. Available from: http://www.nice.org.uk/CG82fullguideline .
- 66.Seruya BB. The effects of training on body-size estimation of schizophrenics. Dissertation Abstr Int. 1977;38:1421B. [Google Scholar]
- 67.Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–24. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]