Main Text
One of the most important duties of a kinesin molecular motor is to not fall off its microtubule track. However, despite considerable advances in our understanding of the structure and mechanism of kinesin in recent years, we have remained mostly in the dark about what enables this motor protein to take many steps in a row before detaching—a feature known as processivity. In this issue of the Biophysical Journal, Mickolajczyk and Hancock (1) give us valuable new insight into a kinetic race in the kinesin ATPase cycle that is central to processivity in these motors.
Similar to a hand-over-hand rope climber, kinesin must detach one of its two motor domains (or catalytic heads) to take a step forward (Fig. 1). At this point in the process, kinesin risks falling off completely if the attached motor domain passes through a particularly vulnerable (i.e., weakly attached) biochemical state before the second head reattaches. It has therefore been argued that processivity in kinesin could be controlled by a kinetic race between dissociation of such a vulnerable state and a successful forward step (2). This model has proved challenging to test, due in part to the difficulty of capturing the relevant intermediates during the ATPase cycle—some of which have been too transient to be observed even by the most powerful biophysical methods.
Figure 1.
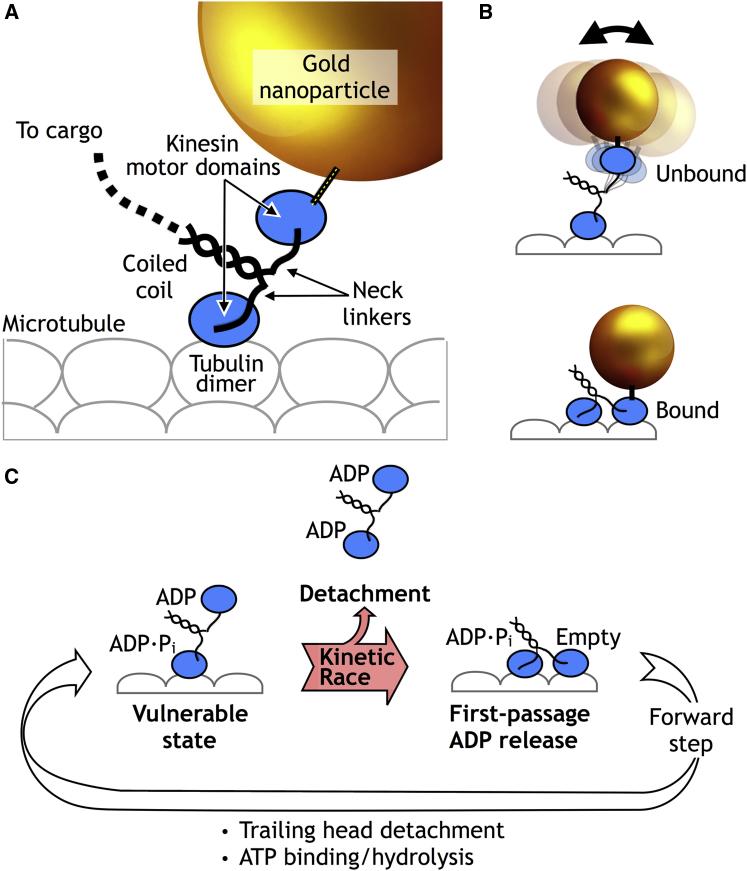
Kinetic race scheme from Mickolajczyk and Hancock (1), providing a model for how long kinesin can walk before falling off the microtubule. (A) Shown here is a schematic of a microtubule-bound kinesin dimer, highlighting key architectural elements including the neck linkers as well as the gold nanoparticle label used for iSCAT tracking (see text). (B) By localizing the gold nanoparticle with high spatial and temporal resolution, iSCAT can discriminate between bound and unbound states of the labeled kinesin motor domain. (C) Shown here is a schematic of the stepping cycle of kinesin, highlighting the key findings of Mickolajczyk and Hancock (1). Kinetic race: the one-head-bound ADP•Pi state is identified as a vulnerable state, from which either 1) a successful forward step is made or 2) the motor detaches to end the processive run. First passage ADP release: when the ADP-bound, tethered head of kinesin initially steps forward, it hangs onto the microtubule long enough for ADP release to lock it into place—thus minimizing reversions to the vulnerable state. To see this figure in color, go online
Mickolajczyk and Hancock (1) now make an important advance in this area by utilizing a new imaging method called “iSCAT”, which is related to interference reflection microscopy and uses laser illumination to achieve high spatial and temporal resolution (3). The potency of the iSCAT method for studying kinesin was recently established by a pair of articles (one of which was led by the current authors (4)) that demonstrated nanometer-scale positional tracking of individual, nanogold-labeled kinesin heads, with time steps as short as 80 μs (5). Consequently, iSCAT measurements are able to precisely quantify the durations that kinesin spends in one-head- versus two-head-bound states during its stepping cycle (Fig. 1, A and B), even when stepping at maximum speed under saturating ATP conditions. This information, which currently cannot be obtained in any other way, is clearly relevant to the kinetic race hypothesis.
In their new study, Mickolajczyk and Hancock (1) use iSCAT to examine a group of engineered kinesin constructs in which a 3-aa insertion is present or absent from the neck linker (Fig. 1 A), a structural element that connects the motor domains to each other. Previous studies on these constructs established that this neck-linker insertion substantially alters processivity, but were unable to definitively link the processivity changes to distinct structural transitions in the kinesin cycle (6). The new iSCAT experiments address this question by showing that the neck-linker insertion specifically affects the duration of the one-head-bound state, but not the two-head-bound state. These results are consistent with the kinetic race model; here, the interpretation would be that the neck linker influences processivity primarily by modulating the time spent in a vulnerable one-head-bound state. However, the iSCAT observations by themselves do not prove the kinetic race hypothesis, although they provide important constraints on how the race could operate.
Mickolajczyk and Hancock (1) then use a clever combination of bulk biochemical rate measurements and Markov-state models to dissect how ADP release couples with a forward step from kinesin’s one-head-bound state. These experiments reveal that in the one-head-bound state, the tethered head rarely detaches after its initial encounter with the forward stepping site; instead, the tethered head strongly prefers to transition to a tightly bound state by releasing ADP, a behavior that is denoted “first passage ADP release”. This novel observation supplies the authors with an additional, key piece of information—namely, that the one-head-bound vulnerable state preceding the hypothesized kinetic race would only be visited once per step (Fig. 1 C).
By combining the iSCAT and first passage observations with other measured rate parameters, Mickolajczyk and Hancock (1) are finally able to assemble a strong case in favor of the kinetic race hypothesis, resulting in the overall scheme shown in Fig. 1 C. It is worth noting that their final argument turns out to depend on the assumption that the neck-linker insertion used in their experiments does not strongly affect the rate at which a kinesin motor domain catalyzes ATP hydrolysis. Although unproven, this assumption is nevertheless reasonable, given that the insertion is most likely to affect structural transitions involving a two-heads-bound state of the motor, whereas current evidence indicates that hydrolysis occurs in a one-head-bound state (6).
A key strength of the analysis by Mickolajczyk and Hancock (1) is that they are able to estimate values of most of the microscopic rate parameters that underlie the kinetic race, allowing the authors to quantitatively test their predictions through the use of a Markov-state model. This task, however, is complicated by the fact that detachment from the vulnerable state can occur in two different ways: either before or after phosphate release (for simplicity, these alternate pathways are not depicted in Fig. 1 C). Moreover, the model developed by Mickolajczyk and Hancock (1) explicitly considers the possibility that first passage ADP release does not occur. Modeling these additional features of the kinetic race turns out to require three distinct detachment rates (each involving different motor geometries and/or nucleotide states) that have not been measured separately. The authors are therefore forced to assume that these rates are all the same. With these caveats in mind, it is still impressive that in the end the Markov-state model quantitatively reproduces the processivity behavior of their constructs, under all tested experimental conditions. It is especially noteworthy that the rate constants utilized in this final model are derived more or less directly from biophysical rate measurements rather than, for example, global fits of the data. This detail significantly strengthens the overall conclusions of Mickolajczyk and Hancock (1).
The kinetic race model sheds light on key aspects of kinesin motility (notably processivity), but also highlights some puzzling unanswered questions. A quiet revolution in the kinesin field has recently contradicted the longstanding idea that the forward step by one-head-bound kinesin is triggered by ATP binding (7), establishing that the forward step instead occurs after hydrolysis of ATP (6). The kinetic race model builds on this recent development by elaborating on the details of the vulnerable ADP•Pi state of the motor that precedes a forward step. However, it is difficult to reconcile this newly established behavior of kinesin’s ADP•Pi state with high-resolution structures of kinesin obtained using x-ray crystallography and cryo-electron microscopy (8, 9). In particular, structure studies have generally been interpreted to show that ATP binding initiates a large structural change in the motor domain that drives docking of the neck linker, which in turn would immediately lead to a forward step. This prediction has seemingly been contradicted by recent observations, notably including the finding that ATPγS, a very slowly hydrolyzing nucleotide analog, fails to promote a forward step until after hydrolysis (4). Additional data, including the structure of kinesin in its ADP•Pi state (which has proven elusive), will be needed to address this key aspect of the kinesin mechanism. Another puzzle, not related to processivity per se, is why the recent iSCAT studies of kinesin-1 seem to contradict each other on whether kinesin stays in a one-head-bound state while it waits for ATP to bind (4, 5). For now, it seems safe to say that kinesin still remains one step ahead of our best efforts to understand it—even when anchored to a golden ball.
Editor: Steven Rosenfeld.
References
- 1.Mickolajczyk, K. J., and W. O. Hancock. Kinesin processivity is determined by a kinetic race from a vulnerable one-head-bound state. Biophys. J. 112:2615–2623. [DOI] [PMC free article] [PubMed]
- 2.Hancock W.O., Howard J. Kinesin’s processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA. 1999;96:13147–13152. doi: 10.1073/pnas.96.23.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortega-Arroyo J., Kukura P. Interferometric scattering microscopy (iSCAT): new frontiers in ultrafast and ultrasensitive optical microscopy. Phys. Chem. Chem. Phys. 2012;14:15625–15636. doi: 10.1039/c2cp41013c. [DOI] [PubMed] [Google Scholar]
- 4.Mickolajczyk K.J., Deffenbaugh N.C., Hancock W.O. Kinetics of nucleotide-dependent structural transitions in the kinesin-1 hydrolysis cycle. Proc. Natl. Acad. Sci. USA. 2015;112:E7186–E7193. doi: 10.1073/pnas.1517638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isojima H., Iino R., Tomishige M. Direct observation of intermediate states during the stepping motion of kinesin-1. Nat. Chem. Biol. 2016;12:290–297. doi: 10.1038/nchembio.2028. [DOI] [PubMed] [Google Scholar]
- 6.Hancock W.O. The kinesin-1 chemomechanical cycle: stepping toward a consensus. Biophys. J. 2016;110:1216–1225. doi: 10.1016/j.bpj.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice S., Lin A.W., Vale R.D. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 8.Shang Z., Zhou K., Sindelar C.V. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. eLife. 2014;3:e04686. doi: 10.7554/eLife.04686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W., Cao L., Knossow M. Kinesin, 30 years later: recent insights from structural studies. Protein Sci. 2015;24:1047–1056. doi: 10.1002/pro.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]