Abstract
Purpose of Review
Multiple myeloma remains an incurable disease, largely due to the tumor-supportive role of the bone marrow microenvironment. Bone marrow adipose tissue (BMAT) is one component of the fertile microenvironment which is believed to contribute to myeloma progression and drug resistance, as well as participate in a vicious cycle of osteolysis and tumor growth.
Recent Findings
MicroRNAs (miRNAs) have recently emerged as instrumental regulators of cellular processes that enable the development and dissemination of cancer. This review highlights the intersection between two emerging research fields and pursues the scientific and clinical implications of miRNA transfer between BMAT and myeloma cells.
Summary
This review provides a concise and provocative summary of the evidence to support exosome-mediated transfer of tumor-supportive miRNAs. The work may prompt researchers to better elucidate the mechanisms by which this novel means of genetic communication between tumor cells and their environment could someday yield targeted therapeutics.
Keywords: Multiple Myeloma, microRNA, Exosome, Bone Marrow Adipose Tissue
1. Introduction
Multiple Myeloma and the Bone Marrow Microenvironment
Multiple myeloma is a clonal proliferation of plasma cells that grows in and spread throughout the bone marrow (BM), where it causes severe osteolytic lesions [1]. Myeloma is the second most common hematological malignancy worldwide, affecting nearly 100,000 people in the United States alone, and remains incurable [2, 3]. The bone marrow microenvironment (BMME), an intricate and dynamic niche composed of extracellular matrix (ECM) proteins and cells of hematopoietic lineage, serves as an instrumental contributor to myeloma disease progression [4]. Understanding the mechanisms of crosstalk between bone marrow stromal cells (BMSCs) and myeloma cells is essential for developing therapeutic agents and protecting the quality of life of those living with myeloma [5, 6]. BMSCs, fibroblasts, macrophages, endothelial cells, bone marrow adipocytes, osteoblasts, and osteoclasts form a complex framework with ECM proteins, growth factors, and hypoxia to enable myeloma cell colonization [5]. Bone marrow adipose tissue (BMAT), a component of the BMME, may be a novel contributor to myeloma oncogenesis and disease progression and affect the metabolism, immune action, inflammation, and angiogenesis of myeloma cells [1].
microRNAs
microRNAs (miRNAs) are a class of endogenous, single-stranded, non-coding RNAs that silence the expression of their target genes either by inhibiting protein translation or triggering mRNA decay [7, 8].
Although these molecules are tiny (only 19-25 nucleotides long), they have huge biological significance, regulating as much as a third of the human genome [9]. Ensconced in RNA binding proteins or in micro vesicles such as exosomes, miRNAs circulate through biological fluids act as signaling molecules within their target cells by changing the expression of specific target genes [6]. Many miRNAs play an important role in bone homeostasis and skeletal development, and even serve as diagnostic and prognostic biomarkers in tumors [9]; both functions make them pertinent to myeloma [10]. miRNAs can function as either tumor suppressors or tumor-causing oncogenes [11, 12]. Subsequently, the deregulation of miRNAs can disrupt cell cycle regulation, proliferation, apoptosis, invasion, and angiogenesis and is associated with cancer formation and progression [13]. The highly specific differential expression of miRNAs in tumors could one day aid in the diagnosis and treatment of cancer [11]. Additionally, miRNAs may mediate crosstalk between the BMME and myeloma cells [6]. In a study conducted by Lee et al., BMSCs were observed to deliver miRNA mimics carried by exosomes to glioma tumor cells, independent of cell to cell contact or gap junctions [13].This study constitutes a novel precedent for the investigation of exosome-mediated miRNA transfer between the BMME and myeloma cells as a means of tumor progression (see Figure 1).
Figure 1.
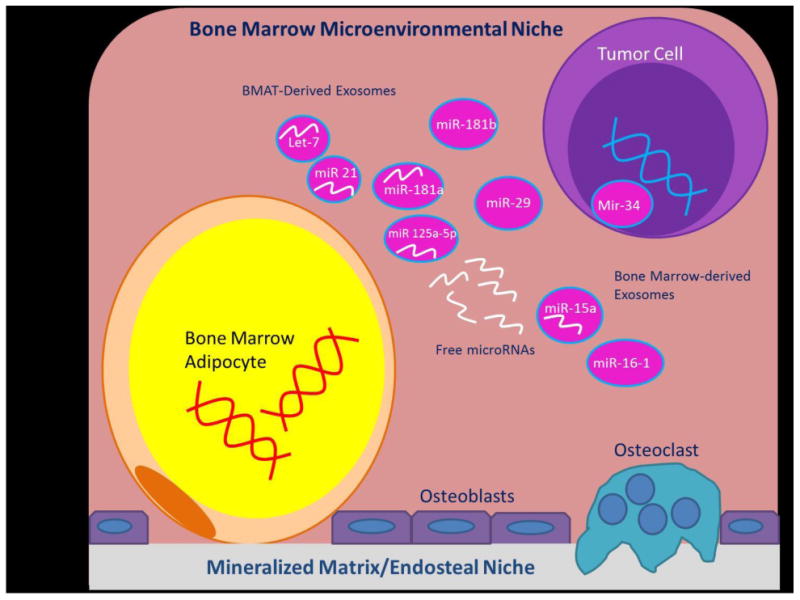
A summary of the interactions within the bone marrow microenvironment between bone marrow adipocytes, miRNAs, and tumor cells that may enable multiple myeloma disease progression. Note that a tumor-supportive cycle may emerge between the microenvironment and adjacent MM cells. miR-15a inhibits tumor suppressors, while miR-16-1 enhances oncogenes. DNA hypermethylation inhibits tumor suppressor miR-34 while miR-29 induces inflammation, supporting pro-inflammatory, tumor supportive pathways such as IL-6 and STAT3 signaling. miR-125a-5p encourages tumor progression and manipulates cell differentiation to favor adipocyte lineages. Exosome encapsulated miRNAs such as miR-let-7, miR-21, and miR-181 a and b may transport myeloma-supportive factors from bone marrow adipocytes to MM cells.
2. miRNAs in Multiple Myeloma
Please refer to Table 1 for a list of miRNAs differentially expressed in myeloma cells that may be derived from BMAT or the BM microenvironment and have been shown to be carried by exosomes. For a comprehensive review of dysregulated miRNAs in myeloma, see these excellent reviews: [14–17].
Table 1. MiRNAs Differentially Expressed in Myeloma and Carried by Exosomes with a Role in Adipose Tissue.
| miRNAs of interest and reference | Upregulated or downregulated in MM | Role in adipose tissue (BMAT or WAT) | Reference demonstrating miR carried by exosomes |
|---|---|---|---|
| miR-15b [15] | Upregulated | Inhibits osteoblast differentiation [9] | [30] |
| miR-181b [6, 15–17, 25, 27] | Upregulated | Associated with inflammation in AT [36, 43] | [30] |
| miR-21 [6, 16, 17, 25, 26] | Upregulated | Adhesion to the BMME upregulates in myeloma cells, upregulated in obese WAT [6, 36] | [29] |
| miR-222 [17, 27] | Upregulated | Associated with inflammation in AT [36] | [29] |
| Let-15a [6] | Downregulated | Biomarker for type 2 diabetes and morbid obesity, inhibits adipogenic differentiation [36, 43] | [30] |
| Let-7 family (let-7a, let-7b-5p, let-7f, let-7g, let-7i) [6, 46, 56] | Downregulated | Downregulated in obese WAT, associated with inflammation in AT, all AT expresses let-7a-1-5p and let-7f-5p [36, 43] | [29, 30] |
miRNA Deregulation and Epigenetic Targets
Put simply, cancer is an accumulation of genetic mutations that disables certain cell cycle processes that regulate proliferation, cell cycle checkpoints, and apoptosis. Preceding tumorigenesis, epigenetic changes occur that cause cells to exhibit the characteristics of cancer, namely unchecked proliferation and the evasion of apoptosis [18]. Accordingly, myeloma is not unique among cancers in that it is characterized by genomic instability and chromosomal abnormalities [15]. One mechanism of genetic damage is DNA hypermethylation, which in myeloma can inactivate tumor-suppressive miRNAs and tumor suppressor genes [18, 19]. Apart from constituting one form of genetic injury that impels a cell to become cancerous, miRNAs may facilitate cancer progression by transmitting the cancer-supportive tendencies of neighboring cells.
miRNAs can act either as tumor suppressors or oncogenes in myeloma cells by preventing the expression of specific target genes [10]. Downregulated miRNAs act as tumor suppressors by inhibiting cell checkpoint functionality or cell division processes, while upregulated miRNAs act as oncogenes by coding for pro-proliferative or anti-apoptotic mechanisms in their target cells [16]. In addition, miRNAs can directly target tumor suppressors and oncogenes in myeloma cells and exert their myeloma-supportive qualities by either decreasing the expression of tumor suppressor genes or enhancing the expression of oncogenes [16]. The primary tumor suppressor gene influenced by miRNAs in myeloma is TP53, a gene that codes for the P53 tumor suppressor protein which is often inhibited or absent in myeloma and other cancers [16]. Adhesion of myeloma cells to BMSCs deregulates the expression of P53, highlighting an essential role of the BM microenvironment in myeloma progression through rendering ineffective safeguards against uncontrolled proliferation [20].
3. Bone Marrow Adipose Tissue
BMAT is currently thought to originate from an osterix-positive BMSC lineage that is common to osteoblasts, chondrocytes, and other BMSCs [1]. A key regulator of skeletal biology, hematopoiesis, and metabolic activity, BMAT is also involved in endocrine functions and the secretion of fatty acids, chemokines, and adipokines [21]. BMAT volume increases with age and occupies a significant portion of the BM, as well as composing over 10% of the average person's total adipose tissue mass [21]. Of significance to this review is the finding that elevated regulated BMAT volume, which is located in close proximity to the trabecular bone and hematopoietic elements and increases with age, is directly correlated with bone loss and resultant osteoporosis [22]. A mutually incompatible relationship arises between adipose components of the BM and bone-forming components, as osteoblastogenesis and the factors that support it often inhibit adipogenesis, and vice versa. This is consistent with the inhibition of bone formation often performed by BMAT [21]. This may be due to direct effects of these cells on each other or could be due to lineage competition that results from the fact that both osteoblasts and adipocytes derive from the same precursor cell. Of note, in certain cases, high bone mass occurs in parallel with high BMAT, but this is not typically the case [23].
The gene expression pattern of BMAT, as well as its function, overlaps with that of white adipose tissue (WAT) [1]. Similar to WAT, BMAT stores energy in the form of unilocular lipid droplets. As BMAT and WAT volume increases because of caloric excess, which increases the size and quantity of adipocytes, both adipose tissues may be reservoirs for excess energy [1]. However, BMAT and WAT also differ, most significantly in that BMAT has a higher expression of some proteins than WAT (UCP1, PGC-1a, PRDM16, FOXC2, DIO2, PPAR and leptin). Also, BMAT volume increases during starvation while WAT volume decreases [1]. BMAT accumulation, which is most often due to aging, obesity, and radiation, may also be a contributor to cancer, osteoporosis, and bone disease [1]. Seventy to 80% of advanced cases of breast and prostate cancer, tumor cells migrate to and occupy the BM niche, and BMAT may contribute to the fertile soil for tumor growth they encounter there [21]. Specifically, it is very likely that BM adipocytes influence the microenvironment by secreting extracellular vesicles that contain adipogenic mRNA transcripts which could then be taken up by osteoblasts or cancer cells [21].
Contributions to Myeloma Progression
The role of BMAT in myeloma progression is still under investigation, in our lab and others, but may relate to the initial stages of myeloma development before remodeling of the BMME occurs, or during relapse. BMAT contains myeloma growth factors and chemokines, which could contribute to myeloma progression by affecting, migration, proliferation, or apoptosis [5]. A few instances of strong correlation between BMAT and myeloma also exist. First, both BMAT volume and myeloma risk increase with age [5]. Second, leptin, a prominent adipokine expressed by BMAT, affects the proliferation of myeloma cells [5]. Third, in WAT, the most similar adipose tissue to BMAT, enlarged adipocytes as seen in obesity produce more adipokines and inflammatory cytokines and less adiponectin (an anti-myeloma adipokine) [24]. These obese adipocytes also exhibit a decreased ability to store fatty acids which results in dysfunctional WAT, insulin resistance, and the increased release of fatty acids [21]. Finally, lipids are necessary to the production of tumor membrane and provide energy for tumors, often through direct transfer of lipids to tumor cells, suggesting that BMAT may provide energy for adjacent myeloma cells. BMAT can also potentially contribute to tumor progression is though inducing survival signaling pathways in myeloma cells; notable pathways include interleukin-6 (IL-6) [24], Signal Transducer and Activator of Transcription (STAT3) [26], NFκB [12], and MAPK [25]. miRNAs have emerged as potentially instrumental regulators of the dissemination of the pro-myeloma effects of these pathways from the BMAT to myeloma. Just as BMSCs from normal donors have been shown to dramatically differ from BMSCs from myeloma patients [28], careful and rigorous investigation into the differences between BMAT derived from healthy donors versus myeloma patients should also be performed.
4. Exosome Mediated Transfer of miRNAs from BMAT to Myeloma Cells
Exosomes
Exosomes are extracellular vesicles on the nano scale which are formed through exocytosis and released into the extracellular milieu, somewhat like an envelope in a biological postal system between cells [29]. Exosomes can contain protein, lipid, and DNA signatures in addition to mRNA and miRNAs, but only 2-5% of the RNAome is found in exosomes [29]. Exosomes mediate communication between cells by transferring their contents through fusion or endocytosis with their target cell [6]. Reticulocytes, dendritic cells, B cells, T cells, mast cells, epithelial cells, and tumor cells all release exosomes [30]. Cell-to-cell communication can be carried out by exosomes, which are released under certain physiological and pathological conditions such as antigen presentation and the presence of tumors [31]. Exosomes can bind to cells by receptor-ligand interactions and fuse with the membrane of the target cell in order to deliver surface proteins and cytoplasm [30]. Interaction between exosomes and myeloma cells is enabled by the binding of fibronectin to heparin sulfate, as they are expressed on the surfaces of both myeloma cells and exosomes [6].
Exosomal miRNA Transfer
Exosomes contain mRNAs and miRNAs, called exosomal shuttle RNAs (esRNAs), which can be delivered to and function in specific target cells [30]. Exosomes carry about 121 miRNAs, of which the miR-let-7 family, miR-1, the miR-15/miR-16 family, miR-181, and miR-375 have been definitively identified [30]. The miRNAs carried by exosomes have been shown to repress their target genes both in vivo and in vitro [29]. In addition, new proteins found in cells that take up exosomes indicate that mRNAs transferred by exosomes can be successfully translated in the target cell as well [30]. In a study conducted by Valdi et al., exosomes from mouse and human mast cell lines, as well as mouse BM mast cells, were found to contain RNA, including miRNA, from approximately 1,300 genes, many of which were not found in the donor cell [30]. This provides compelling evidence that in addition to obtaining miRNA from the cell of origin, the exosomes can also be influenced by other factors such as other nearby cells of the microenvironment.
Contributions to Myeloma Progression
In addition to carrying myeloma-supportive miRNAs, exosomes contribute directly to myeloma progression. In a study conducted by Roccaro et al., myeloma-derived BMSCs increased myeloma cell proliferation significantly more than healthy BMSCs did. In the same study, while myeloma BMSC-derived exosomes increased myeloma cell proliferation by about 30%, healthy BMSC-derived exosomes reduced myeloma cell proliferation by a similar margin [31]. The notable difference between BMSCs in an environment with and without the presence of myeloma demonstrates the ability of myeloma cells to hijack and alter their local environment to induce a positive feedback loop that supports their growth. Additionally, these findings suggest that exosomes inherit the pro- or anti-myeloma inclination of their cell of origin. Thus, BMAT-derived exosomes from healthy or myeloma-corrupted microenvironments may differ greatly in terms of their effects on myeloma cells and therefore should be explored more carefully. Examples of exosomes derived from myeloma-influenced BMSCs being conducive to tumor progression include the downregulation of the tumor suppressor miR-15and elevated levels of oncogenic proteins, cytokines, and adhesion molecules in myeloma-influenced exosomes [31]. Differences in oncogenic protein content between myeloma patient BMSC-derived exosomes and healthy ones may constitute another means of exosome contribution to tumor progression, highlighting the various cargos of exosomes [31]. Exosome transfer between BMSCs and myeloma cells is one way in which BMSCs may support tumor progression and in which tumor cell-derived exosomes can shape the host environment to be more hospitable to cancerous invasion.
A study by Baglio et al. found that BMSCs and adipose tissue mesenchymal stem cells (AT-MSCs) release extracellular vesicles similar to exosomes that contain small RNAs [29]. Interestingly, they found that MSC exosomes are selective about which miRNAs they contain and that RNA composition varies between MSCs and their exosomes [29]. Multiple miRNAs that are highly expressed in MSC exosomes, such as miR-191, miR-222, miR-21, and miR-let-7a, were found to regulate the two processes essential to the development of cancer: cell cycle progression and proliferation [29]. These results seem to suggest that a few recurring miRNAs could be responsible for widespread effects, as the 5 most abundant miRNAs in BMSC exosomes accounted for 50% of the total miRNAs [29].
Cancer patients often have elevated levels of circulating exosomes, suggesting that exosomes may play a role in cancer progression by aiding in tumor dissemination through the transport of oncogenic RNA or proteins [6]. One study illustrated how myeloma tumors grew much better in tissue-engineered bone (TEB) transplanted with myeloma BMSC exosomes than in TEB transplanted with healthy BMSC exosomes [6]. Myeloma BMSC exosomes also spread to distant BM niches more easily than normal BMSC exosomes do [6]. Exosomes produced by myeloma cells in hypoxia are more numerous and quantitatively different than those produced by cells in normoxia. This suggests that the tumor cells' environment may trigger exosomal delivery of RNAs and/or proteins [6]. Finally, exosomes have proven to be biomarkers for cancer, and tumor-derived exosomes have been shown to transfer miRNAs [32, 33].
5. Significant miRNAs in Myeloma with Relevance to BMAT and Exosomal Transfer
miR-21
miR-21, an oncogenic miRNA, is upregulated in many solid and hematological malignancies, including myeloma [26]. miR-21 is known as a pro-survival miR in myeloma and contributes to rapamycin drug resistance [34]. miR-21 targets STAT3 and has been shown to play an important role in the initiation and recurrence of myeloma [26, 35]. Expression of miR-21 is elevated in myeloma cells, as well as BMSCs attached to myeloma cells, and suppression of miR-21can cause apoptosis and inhibit cell proliferation and invasion in myeloma cells [26, 35]. Inhibiting miR-21 in the myeloma microenvironment would also be beneficial through its effects in BMSCs via its effects on osteoprotegerin (OPG), a RANKL decoy protein whose expression is significantly decreased in BMSCs that are adherent to myeloma cells [35]. The inhibition of miR-21 in myeloma-adherent BMSCs restored OPG expression and reduced RANKL production, resulting in decreased osteoclast resorption [35]. In addition, miR-21 is carried by exosomes [29]. Studies show that the adhesion of myeloma cells to BMSCs also causes miR-21 upregulation in myeloma cells through the IL-6/STAT3 pathway and that miR-21 over-expression supports myeloma progression by inhibiting apoptosis [6, 15]. In addition, the over-expression of miR-21 in myeloma cells changed JAK, STAT3, MAPK, NF B, and AKT pathways in BMSCs to favor myeloma progression and drug resistance [26]. These findings indicate that suppression of miR-21, or exosomes that carry it, could be a novel way to protect against myeloma-induced bone loss and implicate the BMME in the upregulation of pro-myeloma miRNAs. Data have demonstrated that miR-21 is also a pro-adipogenic miRNA, is increased in subcutaneous WAT in obesity[36], and is the most represented miRNA in adipose-derived stem cells (ASC) and the eighth most common miRNA in ASC-derived exosomes[29]. Interestingly, miR-21 is also found to be changed during osteogenic differentiation in BMSC, and may act as either an “initiator of osteogenic differentiation”, or an “inhibitor or maintainer of stemness”, demonstrating the complicated and unclear roles of miR-21 in the BMME [37]. Importantly, miR-21 is one of the five most abundant miRs in BMSC (BM adipocyte progenitor) exosomes [29], and hence is potentially also secreted by BM adipocytes into exosomes, but this remains to be determined. These data pose some important questions: How is elevated miRNA expression (e.g., miR-21) signaled from the BMME to adjacent myeloma cells and back? How is miR-21 regulated? What other cells, such as BM adipocytes, induce increased miR-21 in myeloma cells, and is there any direct transfer (free or within exosomes) of this miRNA between cells? These questions remain to be answered.
miR-15a, miR-16-1
miR-15a and miR-16-1 are tumor suppressor miRNAs downregulated in myeloma that target oncogenes and regulate genes associated with myeloma proliferation, drug resistance, and apoptosis [15, 38]. They also regulate the growth and proliferation of myeloma cells by inhibiting the AKT, MAPK, ribosomal-protein-S6, and NFκB pathways [27]. miR-15a and miR-16-1 are located on chromosome 13, which is commonly deleted in myeloma, and as a result have little or no expression in those myeloma cells [25]. Our lab also found that miR-15a is decreased in MM patient MSCs vs ND-MSCs [39]. miR-15a and miR-16-1 exert an anti-proliferative, pro-apoptotic influence on myeloma cells even in the presence of BMSCs [16]. BMSCs in proximity to myeloma cells mimic their low expression of miR-15a and miR-16-1 [6]; this suggests that these miRNAs may enable crosstalk between the BM microenvironment and myeloma cells [6]. The anti-myeloma drug bortezomib may in part exert its anti-myeloma effects by restoring expression of miR-15a and miR-16-1, thus inducing apoptosis in myeloma cells and limiting their homing and migration capacities [15]. The disruption of tumor suppressors and anti- myeloma pathways due to downregulation of miR-15a and miR-16-1 seen in myeloma suggests that these miRNAs are important to pro-myeloma effects of the microenvironment. Interestingly, circulating miR-15a has been proposed to be a biomarker for type 2 diabetes mellitus and miR-15a expression levels have been proposed as biomarkers for risk estimation and classification for patients with morbid obesity [36]. The connection between mR-15a, obesity/diabetes and expression of this miR in myeloma cells or bone marrow adipocytes remains to be determined. It is possible that in obesity, a state that correlates with increased BMAT [40] and elevated risk for myeloma, an increase in circulating miRs is related to decreased miR-15a expression in plasma cells that somehow promotes myelomagenesis. Overall, although the inhibition of myeloma cell adhesion to BMSCs through the upregulation of miR-15a and 16-1 may prove to be an effective mechanism of impeding cell-adhesion mediated drug resistance in myeloma, rampant overexpression of miRs 15a/16-1 systemically could have deleterious side-effects.
miR-181a/b
miR-181a and miR-181b are upregulated in myeloma cells versus plasma cells, as well as in drug-resistant myeloma cell lines versus non-drug resistant lines [17, 26], and regulate the P53 tumor suppressor [16, 25]. miR-181a and b inhibit apoptosis and increase cell growth in myeloma cells, an effect which can be prevented with bortezomib treatment [41].There is evidence of a correlation between the dysregulation of these miRNAs in BMSCs and their dysregulation in myeloma cells [42]. Moreover, these miRNAs are associated with inflammation in WAT, suggesting that their pro-myeloma effects may stem from incriminating involvement with tumor- supportive pathways in BMAT [36, 43]. For example, miR-181a expression negatively correlates with levels of adiponectin[44], the adipokine that is decreased in obesity and has anti-myeloma effects [24]. miR-181a and b have also been shown to be carried by exosomes [29]. As miR-181a and b play a pivotal role in myeloma disease progression and are also tied to WAT and BMSCs, these miRNAs are a promising example of potential exosome-mediated exchange between BMAT and myeloma cells that would support myeloma progression.
miR-125a-5p and miR-125b
Multiple studies have asserted that miR-125a-5p is upregulated in myeloma cells as a result of adhesion to BMSCs and supports myeloma progression [45]. Expression of miR-125a-5p downregulated genes involved in the P53 pathway and caused increased tumor growth, proliferation, and migration in myeloma cells [6, 20, 46]. Studies have shown that miR-125a-5p mimics delivered by lentiviral vectors inhibit myeloma growth and survival, and overcome the protective role of BMSCs in vivo [19]. miR-125a-5p and the related miR-125b also seem to function as manipulators of cell differentiation towards adipocyte lineages. miR-125a-5p and miR-125b expression is increased in osteoblasts that are grown in adipocyte-conditioned media and downregulated in adipose-derived stem cells in osteogenic media, demonstrating that these miRNAs can be powerful regulators of the delicate balance between BM adipocytes and osteoblasts [19]. It is possible that their imbalance can upset the health of the microenvironment and make it susceptible to invasion or bone disease [47]. In addition, miR-125a-5p is associated with inflammation in adipose tissue [36]. Moreover, miR-125a was downregulated in males and females in subcutaneous and visceral adipose tissue [45] while miR-125b was found to be upregulated in female subcutaneous adipose in obese versus lean conditions [36]; the functions of these miRs in adipocytes are not established. Thus, it is possible that miR-125a-5p comes from BMAT and creates an inflammatory niche favoring myeloma and bone-resorbing osteoclasts.
miR-34 family
The miR-34 family contains the tumor suppressor miRNAs miR-34a, miR-34b, and miR-34c, which are downregulated in myeloma through epigenetic silencing, primarily by DNA hypermethylation [48, 49]. Multivariate logistical regression analysis of circulating serum microRNAs showed that a combination of miR-34a and let-7e can distinguish multiple myeloma from healthy donors with a sensitivity of 80.6% and a specificity of 86.7%, and monoclonal gammopathy of undetermined significance from healthy donors with a sensitivity of 91.1% and a specificity of 96.7% [50]. Moreover, miR-34a also inhibits osteoclast differentiation [51], suggesting that increasing this miR family in myeloma could be beneficial for patients. However, its expression in other cancers such as colorectal cancer cells, increases drug sensitivity, and miR-34 also inhibits osteoblast differentiation [52], so it is unclear if the myeloma microenvironment would benefit from higher or lower miR-34 levels, or if depends on the cell type [48].
In a study by Lavery et al., miR-34 knockout mice fed a high fat diet were heavier and had a larger increase in WAT than wild type mice did, illustrating that miR-34 also regulates inflammatory pathways and potentially decreases adipogenesis or lipid accumulation and storage [53]. Others have found that miR-34a inhibits differentiation of human adipose tissue-derived stem cells [54] and inhibits beige and brown fat formation in obesity [55]. Thus, miR-34 may mediate communication between BMAT, other cells in the BMME and myeloma, but it is currently not well understood how this may occur. Although the miR-34 family has not yet been found in exosomes, further investigation of the means of miR-34 transfer between BMAT and myeloma cells, and downstream ramifications, could yield promising therapeutic targets.
Let-7 family
Members of the let-7 family appear frequently in discussions of miRNAs in both myeloma and adipose tissues. Both myeloma cells and the BMME display low expression levels of let-7a, let-15a, and let-106b [6, 46]. Let-7d and let-7e are upregulated and let-7i, 7f, and 7g are downregulated in myeloma cells [15]. Additionally, the combination of miR-34a and let-7e in blood serum often distinguishes myeloma and MGUS patients from healthy donors [50]. Let-7b-5p is under-expressed in myeloma and inhibits tumor development and growth. Accordingly, the over-expression of let-7b-5p induces apoptosis and cell cycle arrest and suppresses proliferation in myeloma cells [56]. Let-7b-5p also negatively regulates insulin-like growth factor receptor 1 (IGFR1) by downregulating both protein and mRNA levels [56]. Overall, the let-7 family negatively regulates the Ras family of oncogenes and therefore could serve as a potential therapeutic target for gene therapy to inhibit tumor progression by inhibiting the expression of these oncogenes [11]. The let-7 family is also an important player in adipose tissue development. Universally, adipose tissues contain let-7a-1-5p and let-7f-5p, which are assumed to play a role in adipogenesis [43]. Surprisingly, although let-7a and let-7d are associated with adipose tissue inflammation, let-7a, let-7d, and let-7i are downregulated in the WAT of obese patients [36]. The let-7 family members have also been identified in exosomes [30]. This suggests that the let-7 family is a likely candidate for genetic transfer as a mediator of tumor progression between BMAT and myeloma cells. To elaborate on one particularly compelling piece of evidence for this theory, let-7a is highly expressed in MSC exosomes and regulates cell cycle progression and proliferation processes, which is significant because it enables the malignant transformation of healthy cells into cancerous cells [29]. Additional research in this area is necessary to better elucidate the mechanisms by which exosomal transfer of miRNAs such as the let-7 family members may, perhaps by silencing the expression of anti-inflammatory or cell cycle moderator genes in myeloma cells, promote disease progression.
6. Conclusion
Because of the genetic heterogeneity of myeloma, efforts to target the BM microenvironment in order to halt the progression of tumor growth and bone disease have become the new avenue of treatment research [1]. The ability of exosomes to deliver select miRNA to highly specific target cells indicates that they could be extremely promising potential new targets for therapy, or therapeutics as DNA or RNA-loaded vectors for gene therapy [30]. Exosomes are not targeted by the immune system, which is another advantage for gene therapy [30].
This review demonstrates that miRNAs could serve as a novel means of targeted therapy to inhibit interactions between bone marrow adipocytes and myeloma cells that further tumor progression. The genetic targets of these miRNAs merit further clarification, as do the mechanisms that determine which miRNAs are incorporated into exosomes. The implications of miRNA transfer as a means of microenvironmental contribution to tumor progression are not limited to myeloma. Cancers such as bone metastatic breast cancer, as well as related bone diseases such as osteoporosis, could benefit from research regarding miRNA-based crosstalk between BM stromal cells and their descendants, such as BM adipocytes, and diseased cells. miRNAs may even come to constitute a new line of cancer therapy which could combat the genetic mutations that cause and drive cancer. Due to the potential of novel miRNA targets to inhibit communication between tumor-supportive aspects of the microenvironment and adjacent tumor cells, the frontier of targeted cancer therapy using miRNAs is optimistic.
Acknowledgments
The authors' work is supported by Start-up funds, a pilot project Grant from NIH/NIGMS (P30GM106391), and the NIH/NIDDK (R24DK092759-01) at Maine Medical Center Research Institute. We thank Dr. Michael Erard for help editing the manuscript.
Footnotes
Compliance with Ethical Guidelines: Conflict of Interest: Carolyne Falank, Luna Soley, and Michaela Reagan declare no conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Falank C, Fairfield H, Reagan MR. Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Front Endocrinol (Lausanne) 2016;7:67. doi: 10.3389/fendo.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.122. Provides an exhaustive summary of dysregulated miRNAs in myeloma. [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Cao DD, Li Q, et al. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;5 doi: 10.18632/oncotarget.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheideler M, Elabd C, Zaragosi LE, et al. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 2008;9:340. doi: 10.1186/1471-2164-9-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caers J, Deleu S, Belaid Z, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–4. doi: 10.1038/sj.leu.2404658. [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo L, Desantis V, Solimando AG, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7:60698–60711. doi: 10.18632/oncotarget.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Deng S, Zhang S, et al. The role of miRNAs in the differentiation of adipose-derived stem cells. Curr Stem Cell Res Ther. 2014;9:268–79. doi: 10.2174/1574888x09666140213203309. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Li F, Saha MN, Abdi J, Qiu L, Chang H. miR-137 and miR-197 Induce Apoptosis and Suppress Tumorigenicity by Targeting MCL-1 in Multiple Myeloma. Clin Cancer Res. 2015;21:2399–2411. doi: 10.1158/1078-0432.CCR-14-1437. [DOI] [PubMed] [Google Scholar]
- 9.Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136–48. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Slack FJ. Oncomirs — microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Guo Y, Yu J, et al. miRNA-202 in bone marrow stromal cells affects the growth and adhesion of multiple myeloma cells by regulating B cell-activating factor. Clin Exp Med. 2016;16:307–316. doi: 10.1007/s10238-015-0355-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–61. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glavey S, Manier S, Sacco A, Rossi G, Ghobrial IM, Roccaro AM. The Role of miRNAs in Plasma Cell Dyscrasias. 2013;2:165–173. doi: 10.2174/2211536602666131126002144. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos K, Gimsing P, Grønbaek K. Aberrant microRNA expression in multiple myeloma. Eur J Haematol. 2013;91:95–105. doi: 10.1111/ejh.12124. [DOI] [PubMed] [Google Scholar]
- 16.Lionetti M, Agnelli L, Lombardi L, Tassone P, Neri A. MicroRNAs in the Pathobiology of Multiple Myeloma. Curr Cancer Drug Targets. 2012;12:823–837. doi: 10.2174/156800912802429274. [DOI] [PubMed] [Google Scholar]
- 17.Rossi M, Tagliaferri P, Tassone P. MicroRNAs in multiple myeloma and related bone disease. Ann Transl Med. 2015;3:334. doi: 10.3978/j.issn.2305-5839.2015.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Wang YE, Zhang Y, et al. Global epigenetic regulation of microRNAs in multiple myeloma. PLoS One. 2014;9:e110973. doi: 10.1371/journal.pone.0110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Martino MT, Guzzi PH, Caracciolo D, et al. Integrated analysis of microRNAs, transcription factors and target genes expression discloses a specific molecular architecture of hyperdiploid multiple myeloma. Oncotarget. 2015;6:19132–47. doi: 10.18632/oncotarget.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leotta M, Biamonte L, Raimondi L, et al. A p53-Dependent Tumor Suppressor Network Is Induced by Selective miR-125a-5p Inhibition in Multiple Myeloma Cells. J Cell Physiol. 2014;229:2106–2116. doi: 10.1002/jcp.24669. [DOI] [PubMed] [Google Scholar]
- 21•.Morris EV, Edwards CM. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front Endocrinol (Lausanne) 2016;7:90. doi: 10.3389/fendo.2016.00090. Discusses bone marrow adipose tissue in the context of the bone marrow microenvironment with particular attention to potential contributions to myeloma progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.McDonald MM, Fairfield H, Falank C, Reagan MR. Adipose, Bone, and Myeloma: Contributions from the Microenvironment. Calcif Tissue Int. 2016 doi: 10.1007/s00223-016-0162-2. Discuss bone marrow adipose tissue in the context of the bone marrow microenvironment with particular attention to potential contributions to myeloma progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. lancet Diabetes Endocrinol. 2015;3:141–147. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler JA, Lwin ST, Drake MT, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagliaferri P, Rossi M, Di Martino MT, et al. Promises and Challenges of MicroRNA-based Treatment of Multiple Myeloma. Curr Cancer Drug Targets. 2012;12:838–846. doi: 10.2174/156800912802429355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Liu S, Wang Y. MicroRNA-21 and multiple myeloma: small molecule and big function. Med Oncol. 2014;31:94. doi: 10.1007/s12032-014-0094-5. [DOI] [PubMed] [Google Scholar]
- 27.Roccaro AM, Sacco A, Thompson B, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009;113:6669–80. doi: 10.1182/blood-2009-01-198408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Reagan MR, Ghobrial IM. Multiple Myeloma-Mesenchymal Stem Cells: Characterization, Origin, and Tumor-Promoting Effects. Clin Cancer Res. 2012;18:342–9. doi: 10.1158/1078-0432.CCR-11-2212. Characterizes the ability of exosomes to mediate the exchange of genetic material, specifically miRNAs, between cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. Provides compelling evidence for this review's interest in exosomal transfer of miRNAs as a potential means of microenvironment contribution to myeloma progression by elucidating the mechanisms by which exosomes originating from bone marrow mesenchymal stromal cells can facilitate tumor-stroma cross-talk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 31.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Øverbye A, Skotland T, Koehler CJ, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–76. doi: 10.18632/oncotarget.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trindade AJ, Medvetz DA, Neuman NA, et al. MicroRNA-21 is induced by rapamycin in a model of tuberous sclerosis (TSC) and lymphangioleiomyomatosis (LAM) PLoS One. 2013;8:e60014. doi: 10.1371/journal.pone.0060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitari MR, Rossi M, Amodio N, et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget. 2015;6:27343–58. doi: 10.18632/oncotarget.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11:276–288. doi: 10.1038/nrendo.2015.25. [DOI] [PubMed] [Google Scholar]
- 37.Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: A Novel Panel of MicroRNA Biomarkers in Osteoblastic and Osteocytic Differentiation from Mesencymal Stem Cells. PLoS One. 2013;8:e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Xu Y, Deng S, et al. MicroRNA-15a/16-1 cluster located at chromosome 13q14 is down-regulated but displays different expression pattern and prognostic significance in multiple myeloma. Oncotarget. 2015;6:38270–82. doi: 10.18632/oncotarget.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reagan MR, Mishima Y, Glavey SV, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124:3250–9. doi: 10.1182/blood-2014-02-558007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardouin P, Rharass T, Lucas S. Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front Endocrinol (Lausanne) 2016;7:85. doi: 10.3389/fendo.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Thakur A, Zhang S, et al. Expressions of miR-181a and miR-20a in RPMI8226 cell line and their potential as biomarkers for multiple myeloma. Tumor Biol. 2015;36:8545–8552. doi: 10.1007/s13277-015-3600-2. [DOI] [PubMed] [Google Scholar]
- 42.Jones CI, Zabolotskaya MV, King AJ, et al. Identification of circulating microRNAs as diagnostic biomarkers for use in multiple myeloma. Br J Cancer. 2012;107:1987–96. doi: 10.1038/bjc.2012.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J, Jiang Z, He S, et al. Intrinsic features in microRNA transcriptomes link porcine visceral rather than subcutaneous adipose tissues to metabolic risk. PLoS One. 2013;8:e80041. doi: 10.1371/journal.pone.0080041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klöting N, Berthold S, Kovacs P, et al. MicroRNA Expression in Human Omental and Subcutaneous Adipose Tissue. In: Polidori C, editor. PLoS One. Vol. 4. 2009. p. e4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raimondi L, De Luca A, Morelli E, et al. MicroRNAs: Novel Crossroads between Myeloma Cells and the Bone Marrow Microenvironment. Biomed Res Int. 2016;2016:6504593. doi: 10.1155/2016/6504593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seckinger A, Meiβner T, Moreaux J, et al. miRNAs in multiple myeloma--a survival relevant complex regulator of gene expression. Oncotarget. 2015;6:39165–83. doi: 10.18632/oncotarget.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin PJ, Haren N, Ghali O, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs) BMC Cell Biol. 2015;16:10. doi: 10.1186/s12860-015-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siemens H, Jackstadt R, Kaller M, Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4:1399–415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bi C, Chung TH, Huang G, et al. Genome-wide pharmacologic unmasking identifies tumor suppressive microRNAs in multiple myeloma. Oncotarget. 2015;6:26508–18. doi: 10.18632/oncotarget.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubiczkova L, Kryukov F, Slaby O, et al. Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica. 2014;99:511–8. doi: 10.3324/haematol.2013.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krzeszinski JY, Wan Y. New therapeutic targets for cancer bone metastasis. Trends Pharmacol Sci. 2015 doi: 10.1016/j.tips.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, HolmstrØm K, Qiu W, et al. MicroRNA-34a Inhibits Osteoblast Differentiation and In Vivo Bone Formation of Human Stromal Stem Cells. Stem Cells. 2014;32:902–912. doi: 10.1002/stem.1615. [DOI] [PubMed] [Google Scholar]
- 53.Lavery CA, Kurowska-Stolarska M, Holmes WM, et al. miR-34a(-/-) mice are susceptible to diet-induced obesity. Obesity (Silver Spring) 2016;24:1741–51. doi: 10.1002/oby.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park H, Park H, Pak H-J, et al. miR-34a inhibits differentiation of human adipose tissue-derived stem cells by regulating cell cycle and senescence induction. Differentiation. 2015;90:91–100. doi: 10.1016/j.diff.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Fu T, Seok S, Choi S, et al. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol. 2014;34:4130–42. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Liu C, Zhang Y, et al. Let-7b-5p regulates proliferation and apoptosis in multiple myeloma by targeting IGF1R. Acta Biochim Biophys Sin (Shanghai) 2014;46:965–72. doi: 10.1093/abbs/gmu089. [DOI] [PubMed] [Google Scholar]
- 57.Kosaka N, Takeshita F, Yoshioka Y, et al. Exosomal tumor-suppressive microRNAs as novel cancer therapy. Adv Drug Deliv Rev. 2013;65:376–382. doi: 10.1016/j.addr.2012.07.011. [DOI] [PubMed] [Google Scholar]


