Abstract
Background
Individuals who use illicit drugs are at heightened risk for HIV and/or Hepatitis C Virus (HCV). Despite the medical consequences of drinking for drug-using individuals with these infections, many do drink. In other studies, how individuals perceive their health relates to their engagement in risk behaviors such as drinking. However, among drug-using individuals with HIV and HCV, whether perceived health relates to drinking is unknown.
Objective
We examine the association between perceived health and drinking among drug-using individuals with HIV and/or HCV.
Methods
In a large, cross-sectional study, we utilized samples of individuals with HIV (n=476), HCV (n=1145), and HIV/HCV co-infection (n=180), recruited from drug treatment centers from 2005–2013. In each sample, we investigated the relationship between perceived health and drinking, using ordinal logistic regressions. We present uncontrolled models as well as models controlled for demographic characteristics.
Results
Among samples of drug using individuals with HIV and with HCV, poorer perceived health was associated with risky drinking only when demographic characteristics were taken into account (Adjusted Odds Ratios: 1.32 [1.05, 1.67] and 1.16 [1.00, 1.34], respectively). In the smaller HIV/HCV co-infected sample, the association of similar magnitude was not significant (AOR=1.32 [0.90, 1.93]).
Conclusions
Drug using patients with HIV or HCV with poor perceived health are more likely to drink heavily, which can further damage health. However, when demographics are not accounted for, these effects can be masked. Patients’ reports of poor health should remind providers to assess for health risk behaviors, particularly heavy drinking.
Keywords: HIV, Hepatitis C, alcohol, drug use, perceived health
1. INTRODUCTION
Illicit use of drugs such as opioids, amphetamines, and/or cocaine increases the risk for infection with HIV and/or Hepatitis C Virus (HCV)1 (Degenhardt, Hall, Warner-Smith, & Lynskey, 2004), due to risky injection and/or sexual behaviors (Des Jarlais, Arasteh, & Friedman, 2011). In addition to these infections, heavy drinking is also common among individuals who use illicit drugs (Field et al., 2012). Heavy drinking can pose substantial health risks for drug-using individuals with HIV and/or HCV. Among those with HIV, heavy drinking increases the risk for liver problems (Barve et al., 2010), poor medication adherence (Azar, Springer, Meyer, & Altice, 2010) and shorter survival (Braithwaite et al., 2007). For individuals with HCV, heavy drinking contributes to liver damage (American Association for the Study of Liver Diseases & The Infectious Diseases Society of America, 2014) and may interfere with access to HCV treatment (Ghany, Strader, Thomas, Seeff, & American Association for the Study of Liver, 2009; US Department of Veterans Affairs, 2012). Although heavy drinking is clearly a threat to the health and survival of drug-using individuals infected with HIV and/or HCV, how these individuals perceive their health (i.e., whether they feel well or poorly), and how this relates to their drinking, remains unknown. Determining this association could both (a) provide insight into factors that underlie heavy drinking in these medical populations and (b) help providers identify “red flags” for heavy drinking from information they likely already have about the patient.
Several studies indicate that, in other populations, poor perceived health is associated with substance use, as well as other risky and self-injurious behaviors. For example, men with tuberculosis who perceive their health as poor are more likely to be problem drinkers (Peltzer et al., 2012) and Latina women who perceived their health as poor are more likely to misuse prescription sedatives (Rojas et al., 2013). Taiwanese adolescents who perceive their health as poor are more likely to be sexually active (Chiao & Yi, 2011), and U.S. adolescents who perceive their health as poor are more likely to evidence suicidality (Eaton et al., 2011). These studies suggest that individuals who feel poorly may not see value in avoiding risk behaviors, perhaps due to hopelessness or perceived futility. However, other studies show poor perceived health to be associated with positive health behaviors, including adherence to medication among individuals with tuberculosis (Naidoo et al., 2013), and HIV testing among Canadian aboriginal women (Orchard et al., 2010). This alternate possibility, that poor health may incur health-promoting behaviors to offset current problems, should also be considered. Because the previous literature is not entirely consistent in whether poor perceived health is associated with health promoting or health destructive behaviors, it is unclear whether drug-using individuals with HIV/HCV are more or less likely to drink when they perceive their health to be poor.
Little is known about the relationship of perceived health with risk behaviors among individuals with HIV and/or HCV in particular. Although asymptomatic individuals with HIV may perceive their health to be comparable to that of non-infected persons (Podraza et al., 1994), others who are symptomatic may have poorer perceived health. HIV-infected persons with poor perceived health are at increased risk for depression (Slot et al., 2015), a known risk factor for drinking among those with HIV (Cook et al., 2009; Cook et al., 2013). HIV-infected persons with poor perceived health also take longer to stop smoking (Hessol et al., 2014), perhaps indicating an overall risk for substance use. These studies suggest that individuals with HIV who are in poorer health may drink more. Yet, no studies known to us have examined the relationship of perceived health to drinking in drug-using individuals with HIV. Further, perceived health is understudied among individuals with HCV, and we know of no studies of the relationship of poor perceived health with drinking or other similar risk behaviors among individuals with HCV. Further elucidating these associations could help identify factors underlying heavy drinking, a risky behavior in these important medical populations, and could help identify those in need of intervention.
The purpose of the current study is to better understand the association between perceived health and heavy drinking among drug-using individuals with HIV, HCV, and HIV/HCV co-infection. Therefore, in three sets of analyses, we examined whether poor perceived health was associated with more or less drinking among drug-using individuals who are infected (a) with HIV, (b) with HCV, and (c) with both HIV and HCV.
2. MATERIALS AND METHODS
2.1 Participants
From 2005–2013, respondents were recruited from Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City to participate in an ongoing, large, serial cross-sectional study of HIV risk factors among individuals who use drugs (Des Jarlais et al., 2011). Patients who reported injection or non-injection use of heroin, cocaine, and/or amphetamines within the past six months, and who provided informed consent, were included in the study (Des Jarlais et al., 2011; Des Jarlais, McKnight, Arasteh, Feelemyer, Perlman, Hagan, Dauria, et al., 2014). Participants completed interviews and provided blood samples that were used to test for HIV and HCV infection status, as described previously (Des Jarlais, McKnight, Arasteh, Feelemyer, Perlman, Hagan, & Cooper, 2014). The study was approved by the Institutional Review Board at Beth Israel Medical Center.
Based on the blood tests, we selected for analysis two primary samples of drug-using individuals with data on perceived health and drinking: the 476 patients who tested positive for HIV and the 1145 patients who tested positive for HCV. We also conducted a sub-analysis of the 180 patients who tested positive for both HIV and HCV infections in a separate model. Our participants were mostly male, minority, in mid-adulthood, and with less than a high school education (for all descriptive characteristics, see Table 1). Participants generally reported good-to-fair health. The majority of HIV-infected patients were risky drinkers; the HCV and HIV/HCV samples were largely split between abstainers and risky drinkers. All groups reported relatively small percentages of non-risky drinkers.
Table 1.
Sample characteristics of HIV- and HCV-infected individuals who use drugs.
| HIV-infected (n=476) | HCV-infected (n=1145) | HIV/HCV co-infected (n=180) | |
|---|---|---|---|
|
| |||
| M (s. d.) | M (s. d.) | M (s. d.) | |
| Age | 43.95 (7.38) | 43.13 (9.31) | 45.82 (7.73) |
| Years of education | 11.09 (2.12) | 11.10 (2.20) | 10.86 (1.99) |
| Perceived healtha | 2.53 (0.84) | 2.53 (0.80) | 2.57 (0.82) |
|
| |||
| n (%) | n (%) | n (%) | |
|
| |||
| Gender | |||
| Male | 332 (69.75%) | 928 (81.05%) | 134 (74.44%) |
| Female | 140 (29.41%) | 213 (18.60%) | 44 (24.44%) |
| Transgender | 4 (0.84%) | 4 (0.35%) | 2 (1.11%) |
| Ethnicity | |||
| White | 30 (6.30%) | 255 (22.27%) | 16 (8.89%) |
| Black | 283 (59.45%) | 319 (27.86%) | 81 (45.00%) |
| Latino/a | 155 (32.56%) | 540 (47.16%) | 78 (43.33%) |
| Other | 8 (1.68%) | 31 (2.71%) | 5 (2.78%) |
| Drinking status | |||
| Abstainer | 138 (28.99%) | 550 (48.03%) | 76 (42.22%) |
| Drinker | 57 (11.97%) | 99 (8.65%) | 17 (9.44%) |
| Risky Drinker | 281 (59.03%) | 496 (43.32%) | 87 (48.33%) |
Note.
Perceived health was measured using the question “How would you describe your current health?” with response options of: 1=Excellent, 2=Good, 3=Fair, 4=Poor. HIV=Human Immunodeficiency Virus. HCV=Hepatitis C Virus. Percentages may not sum exactly to 100% due to rounding error. Participant breakdown may not sum exactly to total sample size due to missing demographic data. HIV and HCV columns include both mono-infected and co-infected individuals.
2.2 Measures
2.2.1 Perceived health
Participants were asked: “How would you describe your current health?” Response options included: Excellent (1), Good (2), Fair (3), or Poor (4). This variable was treated as a continuous indicator of perceived health in the current study.
2.2.2 Drinking status
Participants were asked to report their typical weekly consumption of alcohol in the past six months. As participants were recruited soon after admission, this six-month period referenced pre-treatment drinking. Drinks per week of beer, wine, and hard liquor were reported separately, then values were summed to yield total drinks in a typical week. Consistent with prior research in this sample (Arasteh & Des Jarlais, 2009; Arasteh, Des Jarlais, & Perlis, 2008), these data were then used to categorize participants as abstainers (no drinks in a typical week), drinkers (one or more drinks per week but below risky drinking thresholds), or risky drinkers (more than fourteen drinks per week for men; more than seven drinks per week for women), consistent with National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines (National Institute on Alcohol Abuse and Alcoholism, 2016). In the current study, drinking status for abstainers, drinkers, and risky drinkers was coded as 0, 1, and 2, respectively, so that higher values indicated heavier drinking.
2.2.3 Demographic covariates
Patients reported age and education, in years. They also reported their gender (male, female, transgender) and race (White, Black, Latino/a, Asian/Pacific Islander, Native American, Mixed, Other). The latter four racial categories were collapsed into one category (“other”) in the current analyses, due to low frequencies.
2.3 Analyses
Identical procedures were followed for HIV, HCV, and HIV/HCV subsamples. We first summarized perceived health for all drinking groups, and used a Kruskal-Wallis test to assess whether perceived health differed between drinking groups. A figure was used to illustrate the unadjusted distribution of drinking patterns at each perceived health level. An ordinal logistic regression was also used to characterize the unadjusted effect of perceived health on drinking category, yielding an Odds Ratio (OR) and 95% Confidence Interval (95% CI). An adjusted ordinal logistic regression then assessed the effect of perceived health on drinking category, controlling for demographic (age, sex, race, education) characteristics, yielding an Adjusted Odds Ratio (AOR) and 95% CI. In the event of discrepancies between uncontrolled and controlled models, separate models controlling for each covariate in turn were conducted, to determine the role of each individual demographic characteristic. Finally, exploratory models tested potential interactions between health and demographics, in separate models for each demographic characteristic. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, 2012), with proportional odds assumptions tested in all ordinal logistic regressions.
3. RESULTS
3.1 HIV sample
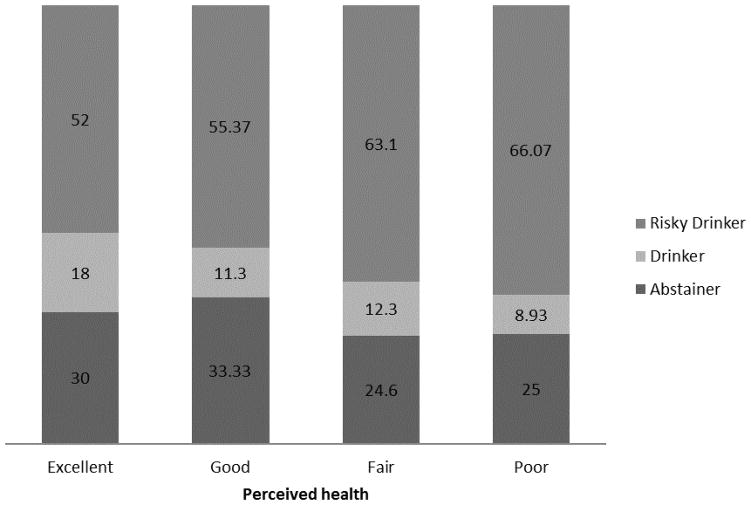
Abstainers and drinkers appeared to have better perceived health than risky drinkers, although this difference was not significant, p=0.11 (Table 2). Among those reporting excellent health, 52% reported risky drinking; among those reporting poor health, 66% reported risky drinking (Figure 1). Although perceived health narrowly missed significance in its association with drinking category in the uncontrolled ordinal logistic regression (OR = 1.24; 95% CI: 0.997, 1.53]), poorer perceived health was associated with greater alcohol use in the ordinal logistic regression controlling for all demographic covariates (AOR = 1.32; 95% CI = 1.05, 1.67) (Table 3). Models controlling for individual covariates indicated that this significant effect emerged when age, race, or education were held constant (Table 3). Perceived health did not interact with any of the demographic variables in interaction models predicting drinking category, ps>0.10.
Table 2.
Comparison of perceived health between drinking groups: Unadjusted associations.
| Abstainers | Drinkers | Risky Drinkers | Kruskal-Wallis test | |
|---|---|---|---|---|
| HIV-infected sample (n=476) | n=134 | n=57 | n=279 | |
| Perceived health | M=2.44 (SD = 0.83) | M=2.42 (SD = 0.86) | M=2.59 (SD = 0.83) | X2(2) = 4.35, p=0.11 |
| HCV-infected sample (n=1145) | n=544 | n=98 | n=493 | |
| Perceived health | M=2.49 (SD = 0.80) | M=2.58 (SD = 0.73) | M=2.56 (SD = 0.82) | X2(2) = 2.84, p=0.24 |
| HIV/HCV co-infected subsample (n=180) | n=75 | n=17 | n=86 | |
| Perceived health | M=2.45 (SD = 0.79) | M=2.82 (SD 0.73) | M=2.63 (SD = 0.85) | X2(2) = 3.20, p=0.20 |
Note. HIV=Human Immunodeficiency Virus. HCV=Hepatitis C Virus. Perceived health was coded such that 1=Excellent, 2=Good, 3=Fair, and 4=Poor.
Figure 1.
Drinking status by perceived health status for drug-using individuals with HIV.
Table 3.
Associations between perceived health and drinking status among HIV- and HCV-infected individuals who use drugs: Odds ratios (ORs) and Adjusted Odds Ratios (AORs).
| Primary models | Exploratory models | |||||
|---|---|---|---|---|---|---|
| Uncontrolled model | Fully controlled model | Age-controlled | Sex-controlled | Race-controlled | Education-controlled | |
| OR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Primary samples | ||||||
| HIV patients | 1.24 (0.997, 1.53) | 1.32 (1.05, 1.67)* | 1.25 (1.00, 1.55)* | 1.22 (0.98, 1.51) | 1.33 (1.07, 1.67)* | 1.25 (1.00, 1.55)* |
| HCV patients | 1.12 (0.98, 1.29) | 1.16 (1.00, 1.34)*a | 1.12 (0.97, 1.29) | 1.13 (0.98, 1.30) b | 1.14 (0.99, 1.32) | 1.13 (0.98, 1.30) |
| Sub-analysis | ||||||
| HIV/HCV co-infected patients | 1.26 (0.89, 1.79) | 1.32 (0.90, 1.93) | 1.26 (0.89, 1.79) | 1.28 (0.90, 1.82) b | 1.26 (0.88, 1.82) | 1.31 (0.92, 1.87) |
Note.
Significant at 95% confidence. Due to convergence errors, gender covariate treats transgender as missing.
Perceived health was coded such that 1=Excellent, 2=Good, 3=Fair, and 4=Poor, and drinking status was coded such that 0=Abstainer, 1=Drinker, 2=Risky drinker; therefore, positive adjusted odds ratios indicate that poorer health is associated with more risky drinking.
HIV=Human Immunodeficiency Virus. HCV=Hepatitis C Virus. 95% CI=95% Confidence Interval.
Indicates that the Proportional Odds Assumption was not met when all covariates were included, requiring omission of the gender covariate; however, models with and without gender were consistent in magnitude, direction, and significance.
Presented models do not meet Proportional Odds Assumption.
3.2 HCV sample
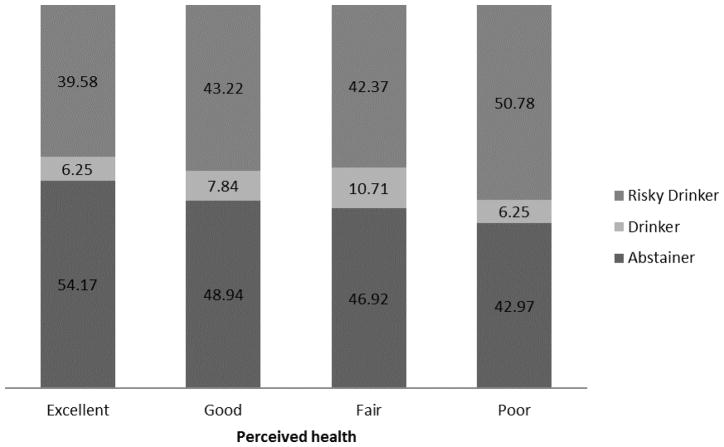
Abstainers appeared to have better perceived health than drinkers or risky drinkers, although this difference was not significant, p=0.24 (Table 2). Among those reporting excellent health, 40% reported risky drinking; among those reporting poor health, 51% reported risky drinking (Figure 2). Although perceived health was not significantly associated with drinking category in the uncontrolled ordinal logistic regression (OR = 1.12; 95% CI: 0.98, 1.29), poorer perceived health was associated with greater alcohol use in the ordinal logistic regression controlling for all demographic covariates (AOR = 1.16; 95% CI = 1.00, 1.34) (Table 3; Proportional Odds Assumption was violated with gender included in model, but results were significant regardless of gender’s inclusion). This effect did not emerge when any individual covariate was held constant; it was only significant when age, race, and education (with or without gender) were controlled. Health interacted with both age (p=0.02) and with race (p=0.02), but not with sex or education (ps>0.70), in interaction models predicting drinking (proportional odds assumptions not met for sex and race models). In regard to age, perceived health was significantly predictive of drinking category for younger (p<0.05) but not older (p>0.70) individuals. In regard to race, perceived health was only predictive of drinking category for Latino and “other” race categories (ps<0.05).
Figure 2.
Drinking status by perceived health status for drug-using individuals with Hepatitis C Virus (HCV) infection.
3.3 Sub-analyses: HIV/HCV subsample
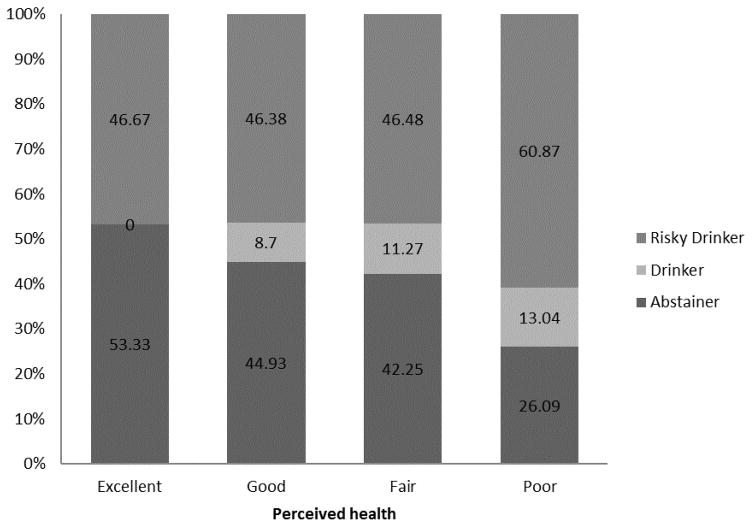
Abstainers appeared to have better perceived health than drinkers or risky drinkers, although this difference was not significant, p=0.20 (Table 2). Among those reporting excellent health, 47% reported risky drinking; among those reporting poor health, 61% reported risky drinking (Figure 3). Perceived health was not significantly associated with drinking category in the uncontrolled (OR=1.26; 95% CI: 0.89, 1.79) or the fully controlled (AOR=1.32, 95% CI: 0.90, 1.93) ordinal logistic regressions (Table 3). Models with single covariates were similarly nonsignificant. However, the direction and magnitude of these associations (1.26–1.32) were similar to that of the larger HIV- and HCV-infected samples, with the direction suggesting that poorer perceived health was associated with greater alcohol use. Perceived health did not interact with any of the demographic variables, ps>0.05 (proportional odds assumption not met for sex interaction model).
Figure 3.
Drinking status by perceived health status for drug-using individuals with HIV and Hepatitis C Virus (HCV) co-infection.
4. DISCUSSION AND CONCLUSIONS
In this study, among HIV- and HCV- infected drug-using individuals, those who perceived their health more negatively generally had greater alcohol use, when all demographic characteristics were held constant. Results for co-infected patients evidenced trends of similar magnitude and direction that did not reach significance in the smaller subsample. This study is important in that it demonstrates that those who report feeling poorly are those also most likely to be drinking at levels that impair their future health, representing a potentially destructive cycle that could threaten the health and well-being of these individuals.
To our knowledge, this is the first study demonstrating the link between poor perceived health and heavy drinking among HIV- and HCV- infected individuals who use drugs. Considering a previous study showing that poor perceived health was associated with smoking in HIV patients (Hessol et al., 2014), poor perceived health among HIV-infected individuals appears to indicate increased risk for multiple substance use behaviors that further endanger health (Bryant, Nelson, Braithwaite, & Roach, 2010; Pacek, Harrell, & Martins, 2014). Also, given that a previous study showed an association between poor perceived health and problem drinking in men with tuberculosis (Peltzer et al., 2012), it seems that poor perceived health may relate to risky drinking across populations with varied infectious diseases, including HIV, HCV, and tuberculosis. These studies lend support to the general hypothesis that poor perceived health may result in perceived futility in health behaviors, as opposed to inspiring health-promoting lifestyles. However, it is important to note that, in our study, the effects only emerged when demographics were controlled. This suggests that the intersection of age, sex, race, and education—factors which relate to perceived health and/or drinking to varying degrees in the different groups (bivariate correlations available upon request)--may function as suppressor variables that require statistical control.
The current study is subject to certain limitations. First, regarding generalizability, data were collected only in Mount Sinai Beth Israel drug treatment centers in New York City. The degree to which these results apply to those who use drugs but are not in treatment, or to those in less urban or other geographic areas, requires further study. However, patients’ reported drinking reflects pre-admission drinking patterns. Potential differences by drug of choice could have also been examined in larger samples, to ensure that these findings adequately represent all subgroups included in our groups. Second, perceived health was assessed with one question. Future research using more comprehensive, validated measures, and/or measures designed specifically for individuals with HIV and/or HCV, would be useful. Third, we used NIAAA risky drinking guidelines (National Institute on Alcohol Abuse and Alcoholism, 2016) to define risky drinking in our samples. These drinking guidelines may not apply to individuals with HIV or HCV, for whom drinking is likely harmful at lower levels. However, the levels of risky drinking used in this study would almost certainly indicate problematic behavior in these medically ill individuals. Fourth, the current study is cross-sectional. Whether those who drink at risky levels feel ill due to drinking, or whether they drink at risky levels due to hopelessness or to cope with negative feelings (Elliott, Aharonovich, O’Leary, Wainberg, & Hasin, 2014a, 2014b) about their disease should be further clarified in prospective work. Relatedly, research on whether improving drinking leads to improvements in perceived health, or vice versa, requires study. However, the cross-sectional findings are highly relevant to screening, which occurs at one point in time, at which perceived health is often known to providers, and drinking patterns are of high importance to health. Finally, effects are modest and only emerge when demographic variables are controlled. Therefore, although this study demonstrates a link between perceived health and drinking, individuals’ demographic characteristics relate to this association in complex ways, with perceived health being more influential in HCV-infected patients who are young, Latino, or of “other” ethnicity. These study limitations are offset by study strengths, including the importance of identifying factors underlying drinking in these important medical populations, the availability of large samples of HIV- and HCV- infected individuals who use drugs, and attention to important demographic covariates.
In summary, the current study demonstrates an association between poor perceived health and greater alcohol use among HIV- and HCV- infected individuals who use drugs, when the effects of demographics are accounted for. Although all health providers working with such individuals should screen for heavy alcohol use and provide brief intervention and referral to treatment when indicated, this does not always occur. Research shows that HIV providers inadequately screen for heavy drinking (Metsch et al., 2008), and that HIV patients who drink heavily experience poorer quality communication from their providers (Korthuis et al., 2011). However, perceived health is often discussed in such sessions, providing an indicator of who may be at particularly high risk for heavy drinking. Reports of poor perceived health should thus remind providers to inquire about drinking, to explore the reasons for this connection, and to be aware that identity characteristics may impact this association. This could allow HIV or liver clinics, in addition to substance use settings, to serve as a point-of-intervention, providing “teachable moments” (Lawson & Flocke, 2009) regarding alcohol use. Brief intervention messages from providers in these contexts may motivate patients to reduce risky drinking, which could potentially improve their perceived and/or objective (e.g., CD4, ALT) health. The current study thus provides an impetus to intervene with these most severely ill HIV- and HCV- infected individuals who use drugs, who continue to further damage their health (including liver function and medication efficacy) through unhealthy alcohol use.
Highlights.
We assessed perceived health in drug users with HIV or Hepatitis C Virus (HCV).
In drug users with HIV or HCV, those reporting poorer health drank at riskier levels.
These associations were only significant when demographics were controlled.
For the smaller HIV/HCV sample, the similar association did not reach significance.
HIV and HCV-infected drug users in poor health should be screened for risky drinking.
Acknowledgments
We thank Courtney McKnight, DrPH, and Kamyar Arasteh, PhD for their assistance in coordinating collaboration. We also thank Melanie Wall, PhD, and Malka Stohl, MS, for their statistical guidance.
This work was supported by National Institutes of Health (NIH) grants R01DA003574 (Des Jarlais), K23AA023753 (Elliott), R01AA023163 (Hasin), and the New York State Psychiatric Institute. The funding sources had no further involvement in this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Contributors:
DDJ conducted the primary study from which data were taken. JCE conducted analyses and drafted the initial version of the current manuscript. JCE, DSH, and DDJ participated in interpretation of data and critical revisions. All authors approve submission of this manuscript.
Conflict of Interest:
No conflict declared.
Role of Funding Sources:
Financial support was provided by NIH grants R01DA003574 (Des Jarlais), K23AA023753 (Elliott), R01AA023163 (Hasin), and the New York State Psychiatric Institute. The funding sources had no further involvement in this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Association for the Study of Liver Diseases, & The Infectious Diseases Society of America. HCV testing and linkage to care. 2014 Retrieved July 22, 2016, from http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care.
- Arasteh K, Des Jarlais DC. HIV testing and treatment among at-risk drinking injection drug users. Journal of the International Association of Physicians in AIDS Care. 2009;8(3):196–201. doi: 10.1177/1545109709336222. [DOI] [PubMed] [Google Scholar]
- Arasteh K, Des Jarlais DC, Perlis TE. Alcohol and HIV sexual risk behaviors among injection drug users. Drug and Alcohol Dependence. 2008;95(1–2):54–61. doi: 10.1016/j.drugalcdep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence. 2010;112(3):178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, … McClain CJ. Focus on the Liver: Alcohol Use, Highly Active Antiretroviral Therapy, and Liver Disease in Hiv-Infected Patients. Alcohol research & health. 2010;33(3):229–236. [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, … Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KJ, Nelson S, Braithwaite RS, Roach D. Integrating HIV/AIDS and alcohol research. Alcohol research & health. 2010;33(3):167–178. [PMC free article] [PubMed] [Google Scholar]
- Chiao C, Yi CC. Adolescent premarital sex and health outcomes among Taiwanese youth: perception of best friends’ sexual behavior and the contextual effect. AIDS Care. 2011;23(9):1083–1092. doi: 10.1080/09540121.2011.555737. [DOI] [PubMed] [Google Scholar]
- Cook RL, Zhu F, Belnap BH, Weber K, Cook JA, Vlahov D, … Cohen MH. Longitudinal trends in hazardous alcohol consumption among women with human immunodeficiency virus infection, 1995–2006. American Journal of Epidemiology. 2009;169(8):1025–1032. doi: 10.1093/Aje/Kwp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Zhu F, Belnap BH, Weber KM, Cole SR, Vlahov D, … Cohen MH. Alcohol consumption trajectory patterns in adult women with HIV infection. AIDS and Behavior. 2013;17(5):1705–1712. doi: 10.1007/s10461-012-0270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Warner-Smith M, Lynskey M. Illicit drug use. In: Ezzati M, Lopez A, Rodgers A, Murray C, editors. Comparative quantification of health risks: global and regional burden of diseases attributable to selected major risk factors. Geneva: World Health Organization; 2004. [Google Scholar]
- Des Jarlais DC, Arasteh K, Friedman SR. HIV among drug users at Beth Israel Medical Center, New York City, the first 25 years. Substance Use and Misuse. 2011;46(2–3):131–139. doi: 10.3109/10826084.2011.521456. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Arasteh K, Feelemyer J, Perlman DC, Hagan H, Cooper HL. Transitions from injecting to non-injecting drug use: potential protection against HCV infection. Journal of Substance Abuse Treatment. 2014;46(3):325–331. doi: 10.1016/j.jsat.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Arasteh K, Feelemyer J, Perlman DC, Hagan H, … Cooper HL. A perfect storm: crack cocaine, HSV-2, and HIV among non-injecting drug users in New York City. Substance Use and Misuse. 2014;49(7):783–792. doi: 10.3109/10826084.2014.880176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Foti K, Brener ND, Crosby AE, Flores G, Kann L. Associations between risk behaviors and suicidal ideation and suicide attempts: do racial/ethnic variations in associations account for increased risk of suicidal behaviors among Hispanic/Latina 9th- to 12th-grade female students? Archives of suicide research. 2011;15(2):113–126. doi: 10.1080/13811118.2011.565268. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives among HIV primary care patients. AIDS and Behavior. 2014a;18(7):1315–1323. doi: 10.1007/s10461-013-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives as prospective predictors of outcome in an intervention trial with heavily drinking HIV patients. Drug and Alcohol Dependence. 2014b;134:290–295. doi: 10.1016/j.drugalcdep.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CA, Klimas J, Barry J, Bury G, Keenan E, Lyons S, … Cullen W. Alcohol screening and brief intervention among drug users in primary care: a discussion paper. Irish Journal of Medical Science. 2012;181(2):165–170. doi: 10.1007/s11845-011-0748-7. [DOI] [PubMed] [Google Scholar]
- Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessol NA, Weber KM, D’Souza G, Burton D, Young M, Milam J, … Cohen MH. Smoking cessation and recidivism in the Women’s Interagency Human Immunodeficiency Virus Study. American Journal of Preventive Medicine. 2014;47(1):53–69. doi: 10.1016/j.amepre.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Saha S, Chander G, McCarty D, Moore RD, Cohn JA, … Beach MC. Substance use and the quality of patient-provider communication in HIV clinics. AIDS and Behavior. 2011;15(4):832–841. doi: 10.1007/s10461-010-9779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Education and Counseling. 2009;76(1):25–30. doi: 10.1016/j.pec.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Pereyra M, Colfax G, Dawson-Rose C, Cardenas G, McKirnan D, Eroglu D. HIV-positive patients’ discussion of alcohol use with their HIV primary care providers. Drug and Alcohol Dependence. 2008;95(1–2):37–44. doi: 10.1016/j.drugalcdep.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Naidoo P, Peltzer K, Louw J, Matseke G, McHunu G, Tutshana B. Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health. 2013;13:396. doi: 10.1186/1471-2458-13-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. What’s “at-risk” or “heavy” drinking? 2016 Retrieved July 22, 2016, from http://rethinkingdrinking.niaaa.nih.gov/isyourdrinkingpatternrisky/whatsatriskorheavydrinking.asp.
- Orchard TR, Druyts E, McInnes CW, Clement K, Ding E, Fernandes KA, … Hogg RS. Factors behind HIV testing practices among Canadian Aboriginal peoples living off-reserve. AIDS Care. 2010;22(3):324–331. doi: 10.1080/09540120903111510. [DOI] [PubMed] [Google Scholar]
- Pacek LR, Harrell PT, Martins SS. Cigarette smoking and drug use among a nationally representative sample of HIV-positive individuals. The American journal on addictions. 2014;23(6):582–590. doi: 10.1111/j.1521-0391.2014.12145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Louw J, McHunu G, Naidoo P, Matseke G, Tutshana B. Hazardous and harmful alcohol use and associated factors in tuberculosis public primary care patients in South Africa. International Journal of Environmental Research and Public Health. 2012;9(9):3245–3257. doi: 10.3390/ijerph9093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podraza AM, Bornstein RA, Whitacre CC, Para MF, Fass RJ, Rice RR, Jr, Nasrallah HA. Neuropsychological performance and CD4 levels in HIV-1 asymptomatic infection. Journal of Clinical and Experimental Neuropsychology. 1994;16(5):777–783. doi: 10.1080/01688639408402691. [DOI] [PubMed] [Google Scholar]
- Rojas P, Dillon FR, Ravelo GJ, Malow R, Duan R, De La Rosa MR. Non-medical prescription sedative use among adult Latina mothers and daughters. Journal of Psychoactive Drugs. 2013;45(4):329–339. doi: 10.1080/02791072.2013.825513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT, Version 9.4. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- Slot M, Sodemann M, Gabel C, Holmskov J, Laursen T, Rodkjaer L. Factors associated with risk of depression and relevant predictors of screening for depression in clinical practice: a cross-sectional study among HIV-infected individuals in Denmark. HIV Medicine. 2015;16(7):393–402. doi: 10.1111/hiv.12223. [DOI] [PubMed] [Google Scholar]
- US Department of Veterans Affairs. Viral hepatitis: Pretreatment assessments: Update on the Management and Treatment of Hepatitis C Virus Infection. 2012 Retrieved July 22, 2016, from http://www.hepatitis.va.gov/provider/guidelines/2012HCV-pretreatment-assessments.asp.