Abstract
Pre-mRNA processing protein 40 (Prp40) is a nuclear protein that has a role in pre-mRNA splicing. Prp40 possesses two leucine-rich nuclear export signals, but little is known about the function of Prp40 in the export process. Another protein that has a role in protein export is centrin, a member of the EF-hand superfamily of Ca2+-binding proteins. Prp40 was found to be a centrin target by yeast-two-hybrid screening using both Homo sapiens centrin 2 (Hscen2) and Chlamydomonas reinhardtii centrin (Crcen). We identified a centrin-binding site within H. sapiens Prp40 homolog A (HsPrp40A), which contains a hydrophobic triad W1L4L8 that is known to be important in the interaction with centrin. This centrin-binding site is highly conserved within the first nuclear export signal consensus sequence identified in Saccharomyces cerevisiae Prp40. Here, we examine the interaction of HsPrp40A peptide (HsPrp40Ap) with both Hscen2 and Crcen by isothermal titration calorimetry. We employed the thermodynamic parameterization to estimate the polar and apolar surface area of the interface. In addition, we have defined the molecular mechanism of thermally induced unfolding and dissociation of the Crcen-HsPrp40Ap complex using two-dimensional infrared correlation spectroscopy. These complementary techniques showed for the first time, to our knowledge, that HsPrp40Ap interacts with centrin in vitro, supporting a coupled functional role for these proteins in pre-mRNA splicing.
Introduction
In the nuclei of eukaryotic cells, precursor mRNA (pre-mRNA) undergoes splicing, a process in which the introns are removed from the primary transcripts and the exons are combined to generate mRNA. This process is carried out by the spliceosome, a multi-protein complex composed of U1, U2, U4, U5, and U6 small nuclear ribonucleoproteins and numerous other proteins (1). Recently, the structure of the spliceosome in Schizosaccharomyces pombe was obtained using cryogenic electron microscopy (2). Pre-mRNA processing protein 40 (Prp40), first identified in Saccharomyces cerevisiae, has an essential role in the initiation step of pre-mRNA splicing (3, 4, 5). In higher eukaryotes, such as Homo sapiens and M. musculus, there are two putative homologs of Prp40: homolog A (Prp40A) and homolog B (Prp40B), also known as HYPA/FBP11 and HYPC, respectively (6, 7, 8, 9, 10, 11, 12, 13). Based on phylogenetic analysis, Prp40A is more closely related to S. cerevisiae Prp40 (ScPrp40) than to Prp40B (9). Both homologs contain two WW domains followed by six tandem FF domains (FF1–FF6), which mediate Prp40-target interactions. The WW-domain-mediated interactions of H. sapiens Prp40A (HsPrp40A) and Prp40B (HsPrp40B) are also implicated in genetic disorders such as Huntington’s disease and Rett syndrome (7, 8, 14, 15). In addition to the WW and FF domains, Murphy et al. (16) found two nuclear export signals (NESs) within ScPrp40 whose sequences are 274LKELREYLNGI284 (NES1) and 340LQNKLNELRL349 (NES2). Mutations in NES1 or both NES1 and NES2 result in deficient in vivo splicing, suggesting that these NES sequences are required for efficient pre-mRNA splicing. Moreover, little is known about the function of Prp40 in the export process.
Centrin has been found to play a role in protein and mRNA export. Centrin is a member of the EF-hand superfamily of Ca2+-binding proteins (17, 18, 19, 20, 21), and in H. sapiens, it has four isoforms: centrin 1 (Hscen1), centrin 2 (Hscen2), centrin 3 (Hscen3), and the pseudogene centrin 4 (Hscen4) (22, 23, 24, 25, 26). Hscen1 is localized in male germ cells, neurons, and ciliary cells (23, 27). In contrast, Hscen2 and Hscen3 are ubiquitously expressed in all somatic cells. Hscen1 and Hscen2 have 80% sequence identity, yet exhibit different affinities for Ca2+, whereas Chlamydomonas reinhardtii centrin (Crcen) shares ∼70% sequence identity with Hscen1 and Hscen2 (26, 28).
Centrin has two independent domains, each containing two EF-hands that are composed of helix-loop-helix motifs (26, 28, 29, 30, 31, 32). Ca2+ binding by centrin induces an open conformation, allowing buried hydrophobic residues to be exposed to the solvent. In general, centrins have different relative affinities for Ca2+ (29, 33, 34, 35).
In H. sapiens, Hscen2 has been localized to the centriole and the nucleus (19, 36, 37). In the centrosome, Hscen2 has many targets, such as Sfi1, a 1242-aa protein with 23 tandem centrin-binding sites (CBSs). The centrin-Sfi1 complex was found to be essential for centriole duplication (34, 38). H. sapiens POC5 (HsPOC5) is localized to the distal end of the centriole and contains only three CBSs. HsPOC5 has been associated with the assembly of the distal half of centrioles and is required for centriole elongation (39).
Within the nucleus, Hscen2 participates in DNA nucleotide excision repair via its interaction with Xeroderma pigmentosum group C (XPC) and RAD23 homolog B. This complex functions as a main damage sensor in global genome nucleotide excision repair (40). Hscen2 enhances the affinity of XPC for the damaged DNA segment (40, 41, 42). In addition, Hscen2 is a component of the TREX-2 complex, which is vital for mRNA export (19, 20, 21, 43, 44, 45).
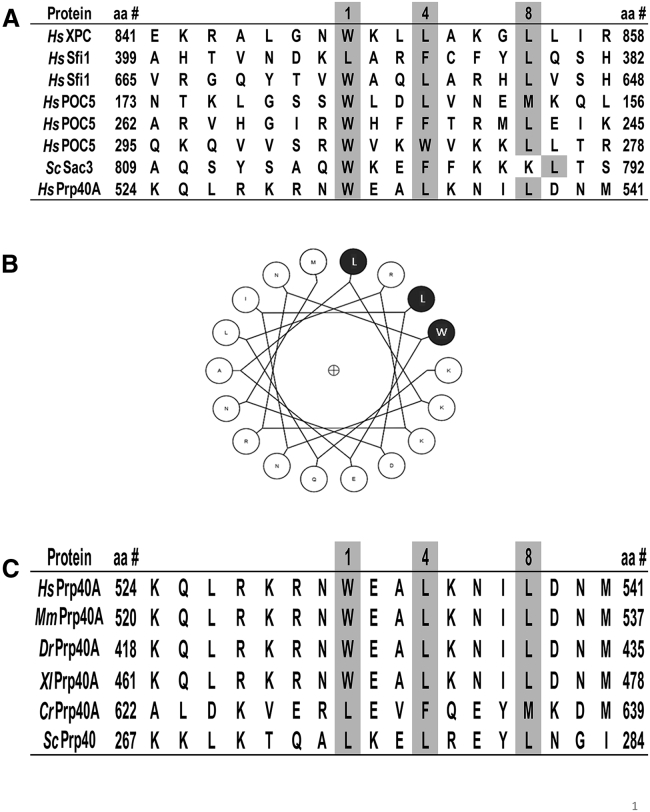
The basis for centrin’s target selectivity is the consensus sequence W1xxL4xxxL8 (where x represents any residue), also known as the hydrophobic triad W1L4L8. This sequence has been found in all available centrin-target complex structures (Fig. 1 A) (29, 33, 34, 35, 43, 46). In this hydrophobic triad, W1 is a well conserved residue among centrin targets, L4 can usually be substituted with another hydrophobic residue such as phenylalanine or tryptophan, and L8 is the least conserved residue. High-resolution structural studies of Hscen2 in complex with XPC or Sfi1, as well as the ternary Sac3-Cdc31-Sus1 complex, have W1 interacting within a hydrophobic pocket within the Hscen2 (or Cdc31, a yeast homolog) C-terminal domain (33, 34, 43, 47).
Figure 1.
Amino acid sequence analysis of HsPrp40A. (A) Sequence alignment of CBSs of known centrin targets including HsXPC (UniProt: Q01831), HsSfi1 (UniProt: A8K8P3), HsPOC5 (UniProt: Q8NA72), ScSac3 (UniProt: P46674), and the novel target HsPrp40A (UniProt: O75400). HsSfi1, HsPOC5, and ScSac3 are in a reversed orientation (C- to N-terminal end) to highlight the conserved hydrophobic residues. (B) Helical wheel of the CBS within HsPrp40A comprising the amino acid sequence beginning at Lys524 through Met541. The hydrophobic triad W1L4L8 is identified in gray. The helical wheel representation was generated using an online resource at http://kael.net/helical.htm. (C) Sequence alignment of Prp40A in H. sapiens, M. musculus (UniProt: Q9R1C7), D. renio (UniProt: Q7ZUE4), X. laevis (UniProt: Q08AZ7), and C. reinhardtii (available at the Orthologous Matrix database under accession number CHLRE00747), including ScPrp40 (UniProt: P33203).
Prp40 was first found to be a potential centrin target in yeast-two-hybrid (Y2H) screening assays using both Hscen2 and Crcen. Here, we examine, to our knowledge, a novel centrin target, HsPrp40A peptide (HsPrp40Ap) interaction. In this work, the location of the CBS in HsPrp40A is validated experimentally. We describe fragment-based peptide design to determine the interaction of HsPrp40Ap with different centrins using a combination of isothermal titration calorimetry (ITC) and two-dimensional infrared (2D IR) correlation spectroscopy (35). The first allows the determination of the thermodynamics governing binding, whereas the second determines the molecular requirements of binding and stability.
Materials and Methods
Recombinant protein expression, isolation, and purification
Expression and purification of recombinant centrin were performed as described in Sosa et al. (35) and Pastrana-Ríos et al. (26, 35).
Y2H screen
A Y2H screen for full-length and single-domain constructs of H. sapiens and C. reinhardtii centrin was performed at the Yeast Resource Center (48). One of the major validated hits in the screen was Prp40.
HsPrp40A peptide
HsPrp40Ap consisting of the amino acid sequence Ac-524KQLRKRNWEALKNILDNMANVTYSTTWSEAQQY556-CONH2 was purchased from Bio-Synthesis. (Lewisville, TX) as a custom synthetic product. MS and HPLC results are shown in Fig. S1 and were performed by Bio-Synthesis to validate the molecular mass (4041.40 m/z) and the purity (>94%) of the desired peptide. To remove the trifluoroacetic acid from the peptide, the sample was subjected to repeated lyophilizations in the presence of 0.1 N HCl followed by an exhaustive dialysis against 50 mM HEPES, 150 mM NaCl, 4 mM CaCl2, and 4 mM MgCl2 at pH 7.4. The HsPrp40Ap molar extinction coefficient is ε = 13,980 M−1 cm−1 at 280 nm. The calibration curve of HsPrp40Ap for different concentration ranges is shown in Fig. S2.
CD spectroscopy
HsPrp40Ap (12 μM) in 8 mM HEPES, 50 mM NaCl, 2 mM CaCl2, and 2 mM MgCl2 at pH 7.4 was used to acquire far-UV CD spectra on a Jasco J-810 spectropolarimeter (Jasco, Tokyo, Japan). Five scans within the spectral range of 250–195 nm were collected at a scan rate of 200 nm/min, a response time of 2 s, a bandwidth of 2 nm, and a temperature of 25°C. Baseline correction was performed over the spectral range of 250–240 nm. The CD absorbance was converted to mean residue molar ellipticity to analyze the secondary structure contribution of the peptide. The experiment was carried out in triplicate (n = 3), and the results are shown in Fig. S3.
ITC
Titrations were carried out using a VP-ITC microcalorimeter from MicroCal (Northampton, MA). Protein samples were exhaustively dialyzed separately against the desired buffer: 50 mM HEPES, 150 mM NaCl, 4 mM CaCl2, and 4 mM MgCl2 at pH 7.4. The protein and peptide concentrations were determined using a Jasco model V-560 UV/Vis spectrophotometer. The calculated molar extinction coefficient (ε) was the same for Hscen1, Hscen2, and Crcen: ε = 1490 M−1 cm−1 at 280 nm. In a typical experiment, 6–10 μM HsPrp40Ap within the sample cell was titrated with 36–144 μM centrin by automatic injection with volumes ranging from 5 to 10 μL. The first injection was set at 2 μL and was ignored in the final data analysis. All of the solutions were degassed for at least 10 min before use.
The ITC data were fitted with a one-binding-site model, using MicroCal Origin software (Northampton, MA). The values for the change in enthalpy of binding (ΔHB), the change in entropy of binding (ΔSB), the association constant (Ka), and the stoichiometry of binding (n) were determined. The change in Gibbs free energy (ΔGB) was calculated using Eq. 1:
| (1) |
For a one-binding-site model, the C value was calculated using Eq. 2:
| (2) |
where [P]t is the concentration of the peptide located in the cell and Kd is the dissociation constant. The change in heat capacity (ΔCp) was obtained by the linear best fit for the relative-change-in-enthalpy plot shown in Fig. S4. The estimation of ΔH (60°C) was calculated using ΔH (60°C) = −0.22 kcal mol−1°C−1 (60°C) −5.7 kcal mol−1.
We used the structural parameterization of unfolding energetics to estimate the solvent accessibility for the Crcen-HsPrp40Ap complex. The polar and apolar surface areas (ΔASApolar and ΔASAapolar) were estimated using Eqs. 3 and 4, first introduced by Xie and Freire (49, 50):
| (3) |
| (4) |
where a′ = 5.37 cal mol−1°C−1 Å−2, b′ = −3.10 cal mol−1°C−1 Å−2, a(60°C) = 31.4 cal mol−1 Å−2, and b(60°C) = −8.44 cal mol−1 Å−2. These values were obtained from a statistical analysis of thermodynamic and structural database of proteins (49, 50).
Fourier transform IR spectroscopy
13C-Crcen-HsPrp40Ap complex (1:1 molar ratio) was prepared under the desired buffer conditions (25 mM HEPES, 150 mM NaCl, 2 mM CaCl2, and 2 mM MgCl2 at pD 6.6) using D2O after complete hydrogen-to-deuterium (H→D) exchange via freeze drying as per Sosa et al. (35). 13C-Crcen-HsPrp40Ap complex (8.25 mg/mL) was deposited onto a round 49 × 4 mm custom-milled CaF2 window with a fixed pathlength of 40 μm (Spectral Systems, Hopewell Junction, NY); the reference cell contained buffer only. Both cells were set in a custom dual-chamber cell holder. The temperature within the cell holder was controlled with a Neslab RTE-740 refrigerated circulating bath (Thermo Electron, Newington, NH). The temperature was monitored with a thermocouple positioned in close contact with the cell. Spectral data acquisition was performed at the desired preset temperature. Once the temperature of the cell was reached, 10 min was allowed for thermal equilibrium. The temperature range studied was 5–90°C with 5°C intervals. The instrument used was a Jasco Fourier transform (FT-IR) spectrophotometer model 6200 equipped with an MCT detector, a sample shuttle, and an interface. In this experiment, 640 scans were co-added, apodized with a triangular function, and Fourier transformed to provide a resolution of 4 cm−1 with the data encoded every 2 cm−1.
2D IR correlation spectroscopy
This technique was developed by Dr. Isao Noda (51, 52) and has been extensively used by our research group (26, 30, 35, 53, 54, 55). We used the FT-IR series of acquired sequential spectra as a function of temperature (5–90°C). The spectral data set was subjected to the subtraction of the corresponding 5°C acquired spectrum of the 13C-Crcen-HsPrp40Ap complex. This resulted in the generation of the difference-spectral data set using Eq. 5:
| (5) |
where, Ā is the initial spectrum of the data set to generate the difference spectra. Synchronous two-dimensional correlation intensities that change in-phase are defined by Eq. 6:
| (6) |
The resulting correlation intensity as a function of two independent wavenumber axes, ν1 and ν2, results in the synchronous plot. Thus, effectively spreading the acquired spectral data in two dimensions.
Asynchronous two-dimensional correlation peak intensities that change out-of-phase from one another are defined by Eq. 7:
| (7) |
The term is the element of the so-called Hilbert-Noda transformation matrix, given by Eq. 8:
| (8) |
This technique allows for determination of the changes that occur in the spectral region of interest by the synchronous and asynchronous plots, and consequently, it enhances the spectral resolution.
The use of both plots and the application of Noda’s rules (56) provides information regarding the sequence of molecular events during the perturbation of the protein-peptide complex (26, 35, 51, 52, 53, 54). These plots are symmetrical, and for this reason, we will always refer to the top triangle for analysis. We begin with the contour plot that evaluates the out-of-phase peak changes (the asynchronous plot). 1) If the asynchronous cross peak, ν2, is positive, then ν2 is perturbed before ν1 (ν2 → ν1). 2) If the asynchronous cross peak, ν2, is negative, then ν2 is perturbed after ν1 (ν2 ← ν1). 3) If the corresponding synchronous cross peak is positive, then the order of the event is established using the asynchronous plot (rules I and II). 4) However, if the corresponding synchronous cross peak is negative and the asynchronous cross peak is positive, then the order is reversed.
The order of events can be established for each peak observed in the ν2 axis.
The spectral data were not manipulated, and only baseline correction was performed. The baseline correction and 2D IR plots were generated using the Kinetics program of MATLAB (MathWorks, Natick, MA), which was generously provided by Dr. Erik Goormaghtigh from the Free University of Brussels, Belgium.
Results
Identification of the centrin-binding site in HsPrp40A
We analyzed the Prp40A and Prp40B amino acid sequences to identify a W1L4L8 pattern that could act as a potential CBS within Prp40. For this, we analyzed the CBSs of known centrin targets. We found the consensus sequence exclusively in Prp40A. Fig. 1 A shows the CBSs for different protein targets in which the hydrophobic triad is conserved. These CBSs have been validated by different molecular biophysical techniques (such as x-ray crystallography and ITC) or cellular immunofluorescence studies, where co-localization of the desired proteins was observed (33, 34, 38, 39, 43). In HsPrp40A, the hydrophobic triad W1L4L8 comprises W531, L534, and L538 and is located within the third FF (FF3) domain.
Available NMR structures of the FF domains of HsPrp40A and ScPrp40 reveal a bundle of three α-helices, with a 310-helix between the second and third α-helices (57, 58, 59, 60). However, the structure of the FF3 domain has not been determined to date. Fig. 1 B shows the helical wheel representation of HsPrp40A, which suggests that the spatial distribution of the hydrophobic triad is limited to one face of the helix. This result is consistent with previous work showing that the hydrophobic triad of other centrin targets is also arranged on a single side of the helix (29, 33, 34, 35).
To establish the evolutionary conservation of this hydrophobic triad within eukaryotes, an amino acid sequence alignment is shown in Fig. 1 C. In higher eukaryotes (H. sapiens, M. musculus, Danio renio, and Xenopus laevis), the CBSs were observed to be identical. In C. reinhardtii and S. cerevisiae, the tryptophan in position 1 was substituted with leucine, similar to the ninth CBS within HsSfi1, whose interaction with centrin was validated by ITC (47). Interestingly, the leucines in positions 1, 4, and 8 of ScPrp40 correspond to the NES1 as reported by Murphy (16).
Thermodynamic comparative analysis of the interaction between centrins and Prp40Ap
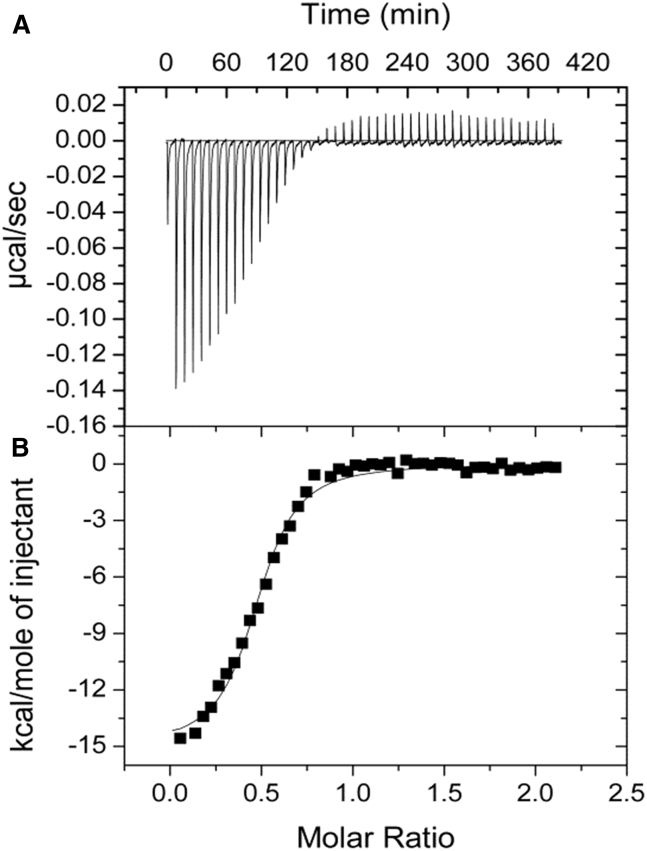
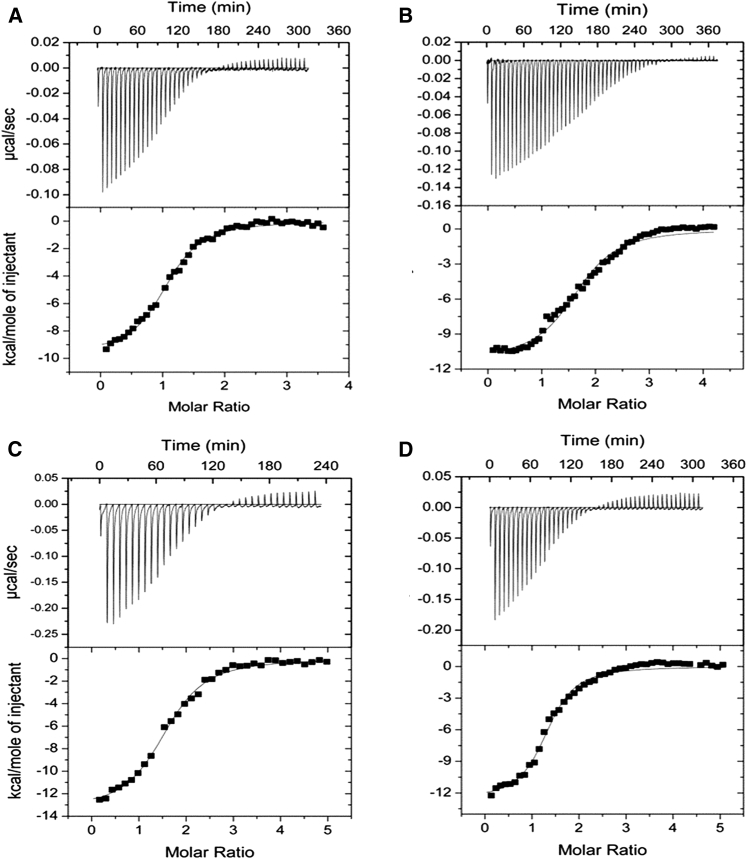
The power data and the integrated enthalpy for the interaction of full-length Hscen2 and HsPrp40Ap at 25°C is shown in Fig. 2, A and B. The binding stoichiometry (n) was close to 0.5. The thermodynamic parameters governing binding for the H. sapiens centrin isoform interaction with HsPrp40Ap are summarized in Table 1. However, we did not observe interaction between Hscen1 and HsPrp40Ap at 25°C and at different molarity ratios (data not shown). Taken together, we can affirm that HsPrp40Ap interacts selectively with Hscen2. We also performed titrations of HsPrp40Ap with full-length Crcen, which has high affinity for Ca2+, to determine whether the interaction between HsPrp40Ap and centrin is dependent on centrin’s affinity for Ca2+ within the C-terminal domain. ITC experiments were carried out at 20, 25, 30, and 35°C to establish the relative affinity for the target (Fig. 3, A–D), and the results are summarized in Table 1. The mean stoichiometry for these experiments was ∼1.44. In general, the C values obtained were ≥10, which suggests that the binding isotherm is sigmoidal and therefore suitable for the determination of the thermodynamic parameters. The exception is Crcen at 30°C, whose C value was determined to be 7. In summary, the binding isotherms were exothermic for all the interactions evaluated for the Crcen-HsPrp40A complex. As the temperature increased, the ΔHB also increased. We also estimated the theoretical (calculated) change in enthalphy (ΔHcalc) based on structure-based calculations (49, 50) and the difference with our experimental ΔHB was between 0 and 9%. Also, the greatest deviation from linearity was observed for the ΔHB at 35°C which may be due to concentration error due to dilution. The Kd was observed to be in the nanomolar range, so a high-affinity complex was obtained. The highest affinity and stability were determined to be at 35°C, which is close to physiological temperature. Comparing both complexes at near room temperature (25°C), the Hscen2-HsPrp40Ap complex exhibited a twofold higher affinity (Kd = 278 ± 31 versus 588 ± 69 nM) and stability (ΔGB = −8.95 ± 0.07 versus −8.51 ± 0.07 kcal/mol). Also, the –TΔSB for Hscen2 is roughly two times higher than that for Crcen. The relative-change-in-enthalpy plot (Fig. S4; Table S2) resulted in a negative ΔCp (−0.22 ± 0.09 kcal/mol°C), suggesting an increase in the ΔASApolar between Crcen and Prp40Ap during the interaction with the estimated binding interface of the Crcen-HsPrp40Ap complex to be more polar (ΔASApolar = −1815 Å2) than apolar (ΔASAapolar = −1090 Å2). This result is consistent with the result obtained in the hydrophobicity plot for HsPrp40Ap using the Kyte and Doolittle index (61), which shows that HsPrp40Ap possess a higher degree of polar-residue composition (Fig. S5). In addition, the burial of the catalytic triad that was originally exposed in centrin by HsPrp40Ap upon complex formation may account for the negative ΔCp observed.
Figure 2.
Binding isotherm of the titration of HsPrp40Ap by Hscen2 at 25°C. (A) Power data and (B) integrated enthalpy are fitted to a one-binding-site model.
Table 1.
Summary of the Thermodynamic Data for the Interaction between Wild-Type Centrin Homologs and HsPrp40Ap
| Protein | Peptide | Temperature (°C) | Ka (× 106 M−1) | Kd (nM) | ΔGB (kcal/mol) | ΔHB (kcal/mol) | −TΔSB (kcal/mol) | na |
|---|---|---|---|---|---|---|---|---|
| Hscen1 | HsPrp40Ap | 25 | NBb | – | – | – | – | – - |
| Hscen2 | HsPrp40Ap | 25 | 3.6 (0.4) | 278 (31) | −8.95 (0.07) | −15.0 (0.3) | 6.1 (0.3) | 0.481 (0.007) |
| Crcen | HsPrp40Ap | 20 | 2.0 (0.2) | 500 (50) | −8.45 (0.05) | −9.7 (0.1) | 1.2 (0.1) | 1.10 (0.01) |
| Crcen | HsPrp40Ap | 25 | 1.7 (0.2) | 588 (69) | −8.51 (0.07) | −11.2 (0.2) | 2.7 (0.2) | 1.70 (0.02) |
| Crcen | HsPrp40Ap | 30 | 1.3 (0.1) | 769 (59) | −8.48 (0.05) | −13.5 (0.2) | 5.0 (0.2) | 1.6 (0.2) |
| Crcen | HsPrp40Ap | 35 | 2.2 (0.3) | 455 (62) | −8.95 (0.07) | −12.6 (0.2) | 3.6 (0.2) | 1.34 (0.02) |
Numbers in parentheses indicate the mean ± SE.
Number of binding sites for HsPrp40Ap.
NB, no binding observed.
Figure 3.
Relative affinities of the interaction between Crcen with HsPrp40Ap at (A) 20°C, (B) 25°C, (C) 30°C, and (D) 35°C, showing the power data (upper) and the integrated enthalpy (lower) fitted to a one-binding site model.
Infrared analysis of the Crcen-HsPrp40Ap complex
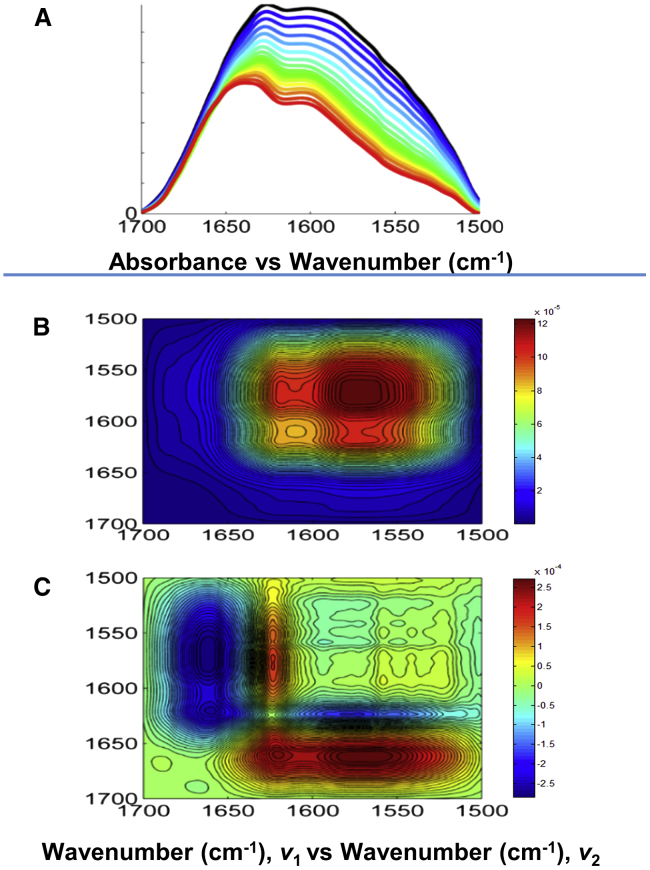
FT-IR was selected as a technique of choice, due to its high selectivity and sensitivity, to study the conformational dynamics that occur within the Crcen-HsPrp40Ap complex. The spectral region of interest was 1700–1500 cm−1. Both the protein and the peptide samples were fully H→D exchanged to simplify the amide I′ band located typically within 1690–1600 cm−1 (53, 54, 62). This band is comprised of primarily carbonyl stretching vibrations (ν(C=O)) located within the peptide bond (63). Also, the H→D exchange results in a shift of the amide II band from 1550 to ∼1450 cm−1, known as the amide II′ band, due to N-D deformation modes within the peptide bond. A summary of the H→D exchange effects on HsPrp40Ap vibrational modes within the spectral region of interest is summarized in Table S2. Also, the buffered solution had a pD of 6.6, which ensures the presence of the deprotonated species of the acidic amino acids (Glu and Asp), evidence of which is shown in Fig. S6. Thus, the band observed in the 1600–1500 cm−1 range is attributable to side chains, primarily Arg, Glu−, and Asp− vibrational modes (35, 53, 54, 55, 62, 63, 64, 65, 66). In the case of Crcen, we homogeneously labeled the protein with 13C. This difference in atomic mass causes the 13C-labeled carbonyl stretching vibrations (ν(13C=O)), also referred to as amide I′∗, to shift to lower wavenumbers (67, 68). Consequently, a simultaneous study of both the target peptide (HsPrp40Ap) and the full-length centrin can be achieved (35). We also observed the shifts of carboxylate modes (ν(13COO−)) within side chains such as Glu− and Asp−residues. As a result, we were able to distinguish between vibrational modes pertaining to the protein (ν(13C=O) and ν(13COO−)) and those pertaining to the peptide (ν(C=O) and ν(COO−)), providing detailed molecular behavior for each component within the complex. This experimental strategy has proven useful in past work by our group, and the band assignments were made in agreement with previous work (35, 53).
Fig. 4 A shows the overlaid FT-IR spectra of the 13C-Crcen-HsPrp40Ap complex within the spectral region 1700–1500 cm−1 for the temperature range 5–90°C. The FT-IR spectral band assignments (67, 69, 70, 71) for the amide I′ band (1690–1600 cm−1) are as follows: for HsPrp40Ap, β-turn (1680 cm−1), random coil (1660 cm−1), and α-helix (1649 cm−1); and for the amide I′∗ band in the spectral region of 1620–1550 cm−1, 13C-Crcen’s loop (1638 cm−1), π-helix (1623 cm−1), α-helix (1610 cm−1), and β-sheet (1592 cm−1). Three side-chain bands of HsPrp40Ap were observed: two glutamates ν(COO−) (1541 and 1549 cm−1) and one aspartate ν(COO−) (1567 cm−1) (65, 66). These carboxylates within HsPrp40Ap have distinct vibrational modes. We have assigned the 1549 cm−1 band to Glu532, which would potentially be participating in an intra-molecular salt-bridge interaction with the positively charged residues located at the N-terminal end of the peptide after a helical conformation was adopted by the peptide upon complex formation (53). We assigned the 1567 cm−1 band to Asp539, which is located at the center of the peptide, and the 1541 cm−1 band to Glu552, which is located near the C-terminal end of the peptide. These residues can serve as probes for the inter-molecular interaction between HsPrp40Ap and 13C-Crcen. 13C-centrin’s side chains were assigned as follows: glutamates, 1523 cm−1; arginines’ symmetric νs(13C-N) and anti-symmetric νa(13C-N) stretches, 1557 and 1577 cm−1, respectively; and aspartates, 1536 cm−1. The 13C-Crcen glutamates and aspartates are located mainly within the Ca2+-binding site (CaBS) (35).
Figure 4.
2D IR correlation spectroscopy of the 13C-Crcen-HsPrp40Ap complex (1:1 molar ratio). (A) Overlaid FT-IR spectra and (B) synchronous and (C) asynchronous plots within the spectral region 1700–1500 cm−1 and over the 5–90°C temperature range with 5°C temperature intervals. In (A), the black and red lines correspond to 5°C and 90°C, respectively.
2D IR correlation spectroscopy provides the advantage of enhancing the resolution of the spectral region of interest via the asynchronous plot. In Fig. 4, B and C, the synchronous and asynchronous plots are shown. Both plots confirm molecular evidence of the interaction between Crcen and HsPrp40Ap. In the synchronous plot (Fig. 4 B), there is a strong correlation of 13C-Crcen’s α-helix (1610 cm−1) with Asp539 of HsPrp40Ap (1567 cm−1). In the asynchronous plot (Fig. 4 C), we observed that HsPrp40Ap’s random coil (1660 cm−1) interacts with 13C-Crcen’s β-sheet (1592 cm−1). This random coil of HsPrp40Ap also correlates with the π-helix (1623 cm−1) located at the C-terminal end of 13C-Crcen, suggesting that this interaction occurs at the C-terminal domain of centrin. Also, HsPrp40Ap’s Asp539 (1567 cm−1) correlates with 13C-Crcen’s arginine’s νa(13C-N) (1577 cm−1), which suggests the existence of an inter-molecular salt bridge.
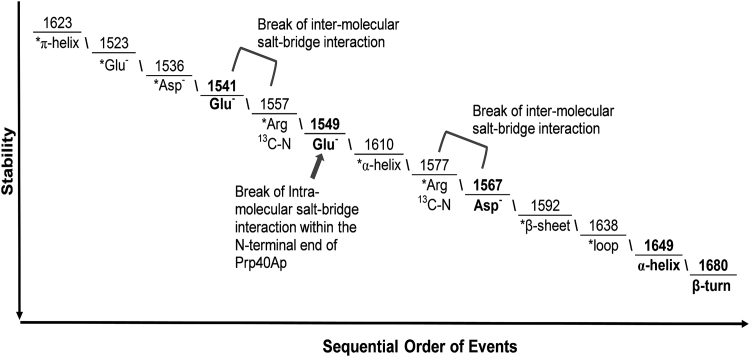
The synchronous plot contains only positive cross peaks; therefore, the sequential molecular order of events for 13C-Crcen-HsPrp40Ap complex dissociation and unfolding with increasing temperature is defined solely by the asynchronous plot, applying Noda’s rules (51, 52). The order of events is summarized in Fig. 5 and Table S3. The perturbation begins with 13C-Crcen’s C-terminal π-helix (1623 cm−1), followed by centrin’s glutamates (1523 cm−1) and aspartates (1536 cm−1), most of which are located within the CaBS of the protein (35). Increasing temperature then affects the inter-molecular salt-bridge interaction between HsPrp40Ap’s Glu552 (1541 cm−1) and 13C-Crcen’s arginine νs(13C-N) (1557 cm−1), followed by HsPrp40Ap’s Glu532 (1549 cm−1), which is involved in an intra-molecular salt-bridge interaction with the N-terminal end of the peptide, and lastly 13C-Crcen’s α-helix (1610 cm−1). This perturbation within the α-helix of centrin affects the inter-molecular salt-bridge interaction between the 13C-Crcen’s arginine νa(13C-N) (1577 cm−1) and HsPrp40Ap’s Asp539 (1567 cm−1), which is consistent with the cross peaks observed in Fig. 4 C. Once both inter-molecular salt bridges break, then centrin’s EF-hand β-sheet segments (1592 cm−1) and loops (1638 cm−1), located within the CaBS, are perturbed, indicating the unfolding of centrin. Finally, the HsPrp40Ap unfolds, as indicated by the perturbation of its α-helix (1649 cm−1) and then its β-turn (1680 cm−1). These results suggest that the HsPrp40Ap is more stable than centrin within the complex. The most stable β-turn may be the one located within the loop between the first and second α-helices of the FF domain of Prp40A (57, 58).
Figure 5.
Sequential order of molecular events during the thermal perturbation of the 13C-Crcen-HsPrp40Ap complex (1:1 molar ratio) over the temperature range 5–90°C. The key to complex formation is the inter-molecular salt bridge interactions between HsPrp40Ap’s negatively charged residues and centrin’s Arg residues, as well as the requirement of the hydrophobic interaction within the binding interface. The text in bold corresponds to HsPrp40Ap’s vibrational modes.
Discussion
We combined bioinformatics and molecular biophysical approaches (ITC and 2D IR correlation spectroscopy) to determine both the thermodynamics governing binding and the molecular requirements for the interaction between centrin and Prp40Ap. Bioinformatics guided the design of the HsPrp40A-fragment-based peptide on the putative CBS containing the hydrophobic triad (W1L4L8) and its convergence with both the NES1 and the FF3 domain. Furthermore, we performed a comparative thermodynamic analysis using several centrin homologs (Hscen1, Hscen2, and Crcen), which enabled us to ascertain the requirement of HsPrp40Ap for high Ca2+ affinity at the C-terminal end of centrin to allow for formation of the complex. No binding was observed for Hscen1, which, unlike Hscen2 and Crcen, lacks high Ca2+ affinity in its third EF-hand. More importantly, the ITC results showed that both Hscen2 and Crcen bound the peptide with Kd values in the nanomolar range and exothermic binding. We then proceeded with establishing the relative affinity for Crcen. Also, the −TΔS in all cases studied suggested a conformational rearrangement upon formation of the complex. The results for –TΔSB may be due to the transition within HsPrp40Ap from an unordered to a more helical structure as it binds. Also, the thermodynamic parameterization approach confirms that the ΔASApolar within the binding interface is greater than that of ΔASAapolar which confirms the molecular description defined by 2D IR correlation analysis. This result is also consistent with the combined CD and 2D IR correlation spectroscopy results obtained for HsPrp40Ap, which show that this peptide has a predominantly random-coil structure in the absence of centrin, whereas in the presence of centrin, HsPrp40Ap adopts a primarily helical structure, as defined exclusively by 2D IR correlation spectroscopy. Regarding the stoichiometry (n) being consistently >1 and the negative double-digit ΔHB values obtained for the Crcen-HsPrp40Ap interactions at different temperatures, one can reconcile these values with the complex dissociation mechanism determined by 2D IR correlation analysis. The complex involves three different types of weak interaction, 1) target selection involving the hydrophobic triad, which requires high calcium affinity to allow for the open conformation of the EF-hand at the C-terminal end of centrin, and 2) two inter-molecular salt-bridge interactions; all three of these interactions would contribute to the negative double-digit ΔHB values determined for the Crcen-HsPrp40Ap complex. The stoichiometry values defined may also be due to the unusually large binding interface that involves all of the interactions stated herein. Furthermore, the high-resolution structures for several centrin-Sfi1p complexes have been determined, and when one considers the binding interface for these complexes, it involves the twisting of centrin about its target (34). If a similar structure were observed for the centrin-Prp40A complex, it would also account for n values that are higher than an integer. The change in enthalpy of binding may also require a hydrogen-bonding interaction, and this may account for similar values for the partial binding observed for the Hscen2 and HsPrp40Ap interaction at 25°C. Its stoichiometry value was <1, and target selection was achieved due to access to the hydrophobic triad. This rationale has also been used by others studying protein-peptide complexes, and we believe it represents a potential model that will need to be further investigated (72, 73, 74, 75). One final point is that in every case, the affinity constants determined were all in the nanomolar range. We will continue to pursue further explanations for these differences by determining the high-resolution structures for these different complexes with known dynamic behavior and their stability as defined by 2D IR correlation analysis.
2D IR correlation spectroscopy is especially suited for characterizing conformational changes, since it enhances the resolution of the spectral region of interest (1700–1500 cm−1) and provides the sequence of molecular events that occur during thermally induced perturbation. The correlation analysis identified the existence of three salt-bridge interactions as requirements for formation of the complex, in addition to high Ca2+ affinity in the centrin C-terminal domain and the hydrophobic triad within the target (26, 28, 29, 30, 54). The salt bridges are both inter- and intra-molecular in nature and involve the three negatively charged residues in HsPrp40Ap. The molecular description of dissociation of the complex provides clues as to the relative orientation of the HsPrp40Ap with respect to centrin. Briefly, centrin’s π-helix, located at the C-terminal end, is perturbed initially, followed by its glutamates and aspartates; then, the first inter-molecular salt bridge interaction, involving Glu552 of the peptide and an arginine in centrin, is perturbed. This Glu552 is located at the C-terminal end of the peptide, suggesting that HsPrp40Ap is oriented tail to tail with respect to centrin. Glu532, located at the N-terminal end of HsPrp40Ap and adjacent to a stretch of positively charged residues, is perturbed next; presumably, Glu532 is involved in an intra-molecular salt-bridge interaction. This event is followed by centrin’s α-helical motifs being perturbed, then by the breaking of the second inter-molecular salt bridge, which involves an arginine from centrin and the single Asp539 residue at the center of HsPrp40Ap. Once the salt-bridge interactions between centrin and HsPrp40Ap no longer exist, centrin’s short antiparallel β-sheets and associated loops within the Ca2+-binding sites are perturbed, presumably within the C-terminal EF-hand. Then, and only then, does the peptide target begin to unfold.
In summary, our multidisciplinary approach allowed for fragment-based peptide design using, as a basis for the rationale, the hydrophobic triad consistently observed in known centrin targets. As in other centrin-target interactions studied previously, the hydrophobic triad W1L4L8 in the CBS of HsPrp40A plays a role in the selectivity of the target, as does the high-affinity CaBS within the C-terminal domain (29, 33, 35). We used full-length centrin and the HsPrp40Ap as representative of the “in vivo complex,” since the available high-resolution structure for other centrin targets requires both centrin domains for complex formation. However, the high-affinity CaBSs within the C-terminal domain promote the open conformation of the EF-hand domain, exposing the Phe residues that drive the hydrophobic interaction along with the hydrophobic triad found on one face of the peptide’s helix, which may be essential for target selectivity. Also crucial were the inter-molecular salt-bridge interaction between centrin and Prp40Ap toward the formation of the complex. These results provide direct evidence that Prp40 is, to our knowledge, a novel centrin target. The next step will be to determine the high-resolution structure of the Hscen2-HsPrp40Ap complex, which will be used to generate representative models of the complex and, along with the empirically defined molecular dynamics from the 2D IR correlation spectroscopy, normal-mode simulations of the complex.
Further evidence, Hscen2 was found to have a regulatory role in nuclear protein export by its association with the NES of the HIV protein Rev, a protein that also has been known to have a role in the regulation of viral RNA splicing (19, 21, 76). Moreover, Hscen2 interacts with Galectin-3, a β-galactoside-binding protein that also has a NES and is involved in pre-mRNA splicing (77, 78). Taken together, these observations also may implicate Hscen2 in the pre-mRNA splicing mechanism. Additionally, the evolutionary conservation of this CBS in Prp40A among many organisms confirms the potential biological importance of this segment. In conclusion, these studies raise new questions regarding the putative regulatory role of Hscen2 in HsPrp40A export from the nucleus and pre-mRNA splicing. Our group will pursue the biophysical characterization of Galectin-3 as a centrin target.
Author Contributions
A.D.C. conducted the experiments presented in this article, analyzed the results, and wrote most of the article. W.J.C. conceived of and arranged for the yeast-two-hybrid screen that showed that Prp40 was a potential centrin target. B.P.-R. conceived the idea for the project and wrote the article with input from A.D.C. and W.J.C.
Acknowledgments
The authors thank Susan M. Meyn for preparing the vectors for the Y2H screen, and Dr. Lea Starita and Professor Stanley Field for the Y2H screens performed at the Yeast Resource Center at the University of Washington. They also thank Melissa Stauffer for her editorial review of the manuscript. We also thank the University of Puerto Rico at Mayagüez.
This work was supported by the National Institutes of Health grant SO6GM08103 (B.P.-R.), a Henry Dreyfus Teacher Scholar Award (B.P.-R.), Hector Collazo, Esq., funds provided through the International Health Games (B.P.-R.), the Alfred P. Sloan Foundation Scholar Award (A.D.C.), and a National Institutes of Health-Research Initiative for Scientific Enhancement Scholarship (A.D.C.).
Editor: Enrique De La Cruz.
Footnotes
Six figures and three tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30555-6.
Supporting Material
References
- 1.Wahl M.C., Will C.L., Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Yan C., Hang J., Shi Y. Structure of a yeast spliceosome at 3.6-Å resolution. Science. 2015;349:1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 3.Kao H.-Y., Siliciano P.G. Identification of Prp40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol. Cell. Biol. 1996;16:960–967. doi: 10.1128/mcb.16.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abovich N., Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. doi: 10.1016/s0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 5.Berglund J.A., Chua K., Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 6.Chan D.C., Bedford M.T., Leder P. Formin binding proteins bear WWP/WW domains that bind proline-rich peptides and functionally resemble SH3 domains. EMBO J. 1996;15:1045–1054. [PMC free article] [PubMed] [Google Scholar]
- 7.Faber P.W., Barnes G.T., MacDonald M.E. Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 8.Passani L.A., Bedford M.T., MacDonald M.E. Huntingtin’s WW domain partners in Huntington’s disease post-mortem brain fulfill genetic criteria for direct involvement in Huntington’s disease pathogenesis. Hum. Mol. Genet. 2000;9:2175–2182. doi: 10.1093/hmg/9.14.2175. [DOI] [PubMed] [Google Scholar]
- 9.Becerra S., Andrés-León E., Suñé C. Prp40 and early events in splice site definition. Wiley Interdiscip. Rev. RNA. 2016;7:17–32. doi: 10.1002/wrna.1312. [DOI] [PubMed] [Google Scholar]
- 10.Bedford M.T., Reed R., Leder P. WW domain-mediated interactions reveal a spliceosome-associated protein that binds a third class of proline-rich motif: the proline glycine and methionine-rich motif. Proc. Natl. Acad. Sci. USA. 1998;95:10602–10607. doi: 10.1073/pnas.95.18.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstrohm A.C., Albrecht T.R., Garcia-Blanco M.A. The transcription elongation factor CA150 interacts with RNA polymerase II and the pre-mRNA splicing factor SF1. Mol. Cell. Biol. 2001;21:7617–7628. doi: 10.1128/MCB.21.22.7617-7628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin K.T., Lu R.M., Tarn W.Y. The WW domain-containing proteins interact with the early spliceosome and participate in pre-mRNA splicing in vivo. Mol. Cell. Biol. 2004;24:9176–9185. doi: 10.1128/MCB.24.20.9176-9185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerra S., Montes M., Suñé C. Prp40 pre-mRNA processing factor 40 homolog B (PRPF40B) associates with SF1 and U2AF65 and modulates alternative pre-mRNA splicing in vivo. RNA. 2015;21:438–457. doi: 10.1261/rna.047258.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y.J., Che M.X., Hu H.Y. Interaction with polyglutamine-expanded huntingtin alters cellular distribution and RNA processing of huntingtin yeast two-hybrid protein A (HYPA) J. Biol. Chem. 2011;286:25236–25245. doi: 10.1074/jbc.M110.216333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buschdorf J.P., Strätling W.H. A WW domain binding region in methyl-CpG-binding protein MeCP2: impact on Rett syndrome. J. Mol. Med. (Berl.) 2004;82:135–143. doi: 10.1007/s00109-003-0497-9. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M.W., Olson B.L., Siliciano P.G. The yeast splicing factor Prp40p contains functional leucine-rich nuclear export signals that are essential for splicing. Genetics. 2004;166:53–65. doi: 10.1534/genetics.166.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kretsinger R.H. Calcium-binding proteins. Annu. Rev. Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama S., Moncrief N.D., Kretsinger R.H. Evolution of EF-hand calcium-modulated proteins. II. Domains of several subfamilies have diverse evolutionary histories. J. Mol. Evol. 1992;34:416–448. doi: 10.1007/BF00162998. [DOI] [PubMed] [Google Scholar]
- 19.Resendes K.K., Rasala B.A., Forbes D.J. Centrin 2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. Mol. Cell. Biol. 2008;28:1755–1769. doi: 10.1128/MCB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jani D., Lutz S., Wickramasinghe V.O. Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. Nucleic Acids Res. 2012;40:4562–4573. doi: 10.1093/nar/gks059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham C.N., Schmidt C.A., Resendes K.K. Human TREX2 components PCID2 and centrin 2, but not ENY2, have distinct functions in protein export and co-localize to the centrosome. Exp. Cell Res. 2014;320:209–218. doi: 10.1016/j.yexcr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Lee V.D., Huang B. Molecular cloning and centrosomal localization of human caltractin. Proc. Natl. Acad. Sci. USA. 1993;90:11039–11043. doi: 10.1073/pnas.90.23.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart P.E., Glantz J.N., Salisbury J.L. Testis-specific murine centrin, Cetn1: genomic characterization and evidence for retroposition of a gene encoding a centrosome protein. Genomics. 1999;60:111–120. doi: 10.1006/geno.1999.5880. [DOI] [PubMed] [Google Scholar]
- 24.Middendorp S., Küntziger T., Bornens M. A role for centrin 3 in centrosome reproduction. J. Cell Biol. 2000;148:405–416. doi: 10.1083/jcb.148.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavet O., Alvarez C., Bornens M. Centrin4p, a novel mammalian centrin specifically expressed in ciliated cells. Mol. Biol. Cell. 2003;14:1818–1834. doi: 10.1091/mbc.E02-11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastrana-Ríos B., Reyes M., Colón M. Relative stability of human centrins and its relationship to calcium binding. Biochemistry. 2013;52:1236–1248. doi: 10.1021/bi301417z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salisbury J.L. A mechanistic view on the evolutionary origin for centrin-based control of centriole duplication. J. Cell. Physiol. 2007;213:420–428. doi: 10.1002/jcp.21226. [DOI] [PubMed] [Google Scholar]
- 28.Veeraraghavan S., Fagan P.A., Chazin W.J. Structural independence of the two EF-hand domains of caltractin. J. Biol. Chem. 2002;277:28564–28571. doi: 10.1074/jbc.M112232200. [DOI] [PubMed] [Google Scholar]
- 29.Hu H., Chazin W.J. Unique features in the C-terminal domain provide caltractin with target specificity. J. Mol. Biol. 2003;330:473–484. doi: 10.1016/s0022-2836(03)00619-3. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz M., Sanoguet Z., Pastrana-Rios B. Dynamics of hydrogen-deuterium exchange in Chlamydomonas centrin. Biochemistry. 2005;44:2409–2418. doi: 10.1021/bi0484419. [DOI] [PubMed] [Google Scholar]
- 31.Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 32.Lewit-Bentley A., Réty S. EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 33.Thompson J.R., Ryan Z.C., Kumar R. The structure of the human centrin 2-xeroderma pigmentosum group C protein complex. J. Biol. Chem. 2006;281:18746–18752. doi: 10.1074/jbc.M513667200. [DOI] [PubMed] [Google Scholar]
- 34.Li S., Sandercock A.M., Kilmartin J.V. Structural role of Sfi1p-centrin filaments in budding yeast spindle pole body duplication. J. Cell Biol. 2006;173:867–877. doi: 10.1083/jcb.200603153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sosa L. del V., Alfaro E., Pastrana-Ríos B. The structure, molecular dynamics, and energetics of centrin-melittin complex. Proteins. 2011;79:3132–3143. doi: 10.1002/prot.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paoletti A., Moudjou M., Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- 37.Salisbury J.L., Suino K.M., Springett M. Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 2002;12:1287–1292. doi: 10.1016/s0960-9822(02)01019-9. [DOI] [PubMed] [Google Scholar]
- 38.Kilmartin J.V. Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J. Cell Biol. 2003;162:1211–1221. doi: 10.1083/jcb.200307064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azimzadeh J., Hergert P., Bornens M. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009;185:101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marteijn J.A., Lans H., Hoeijmakers J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014;15:465–481. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 41.Araki M., Masutani C., Hanaoka F. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 2001;276:18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- 42.Nishi R., Okuda Y., Hanaoka F. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol. Cell. Biol. 2005;25:5664–5674. doi: 10.1128/MCB.25.13.5664-5674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jani D., Lutz S., Stewart M. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol. Cell. 2009;33:727–737. doi: 10.1016/j.molcel.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer T., Rodríguez-Navarro S., Hurt E. Yeast centrin Cdc31 is linked to the nuclear mRNA export machinery. Nat. Cell Biol. 2004;6:840–848. doi: 10.1038/ncb1163. [DOI] [PubMed] [Google Scholar]
- 45.González-Aguilera C., Tous C., Aguilera A. The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability. Mol. Biol. Cell. 2008;19:4310–4318. doi: 10.1091/mbc.E08-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popescu A., Miron S., Craescu C.T. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J. Biol. Chem. 2003;278:40252–40261. doi: 10.1074/jbc.M302546200. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Sanz J., Kateb F., Craescu C.T. Structure, dynamics and thermodynamics of the human centrin 2/hSfi1 complex. J. Mol. Biol. 2010;395:191–204. doi: 10.1016/j.jmb.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 48.Riffle M., Malmström L., Davis T.N. The yeast resource center public data repository. Nucleic Acids Res. 2005;33:D378–D382. doi: 10.1093/nar/gki073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie D., Freire E. Structure based prediction of protein folding intermediates. J. Mol. Biol. 1994;242:62–80. doi: 10.1006/jmbi.1994.1557. [DOI] [PubMed] [Google Scholar]
- 50.Murphy K.P., Freire E. Thermodynamics of structural stability and cooperative folding behavior in proteins. Adv. Protein Chem. 1992;43:313–361. doi: 10.1016/s0065-3233(08)60556-2. [DOI] [PubMed] [Google Scholar]
- 51.Noda I. Advances in two-dimensional correlation spectroscopy. Vib. Spectrosc. 2004;36:143–165. [Google Scholar]
- 52.Noda I. Recent advancement in the field of two-dimensional correlation spectroscopy. J. Mol. Struct. 2008;883–884:2–26. [Google Scholar]
- 53.Pastrana-Rios B. Understanding the mechanism of peptide unfolding. Biochemistry. 2001;40:9074–9081. doi: 10.1021/bi0155145. [DOI] [PubMed] [Google Scholar]
- 54.Pastrana-Rios B., Ocaña W., Salisbury J.L. Centrin: its secondary structure in the presence and absence of cations. Biochemistry. 2002;41:6911–6919. doi: 10.1021/bi0157971. [DOI] [PubMed] [Google Scholar]
- 55.Pastrana-Rios B. Simulation of FT-IR and 2D-COS analysis for the thermal perturbation of apo-centrin. J. Mol. Struct. 2006;799:163–167. [Google Scholar]
- 56.Noda I. Techniques to two-dimensional (2D) correlation spectroscopy useful in life science research. Biomed. Spectrosc. Imaging. 2015;4:109–127. [Google Scholar]
- 57.Allen M., Friedler A., Bycroft M. The structure of an FF domain from human HYPA/FBP11. J. Mol. Biol. 2002;323:411–416. doi: 10.1016/s0022-2836(02)00968-3. [DOI] [PubMed] [Google Scholar]
- 58.Gasch A., Wiesner S., Macias M.J. The structure of Prp40 FF1 domain and its interaction with the crn-TPR1 motif of Clf1 gives a new insight into the binding mode of FF domains. J. Biol. Chem. 2006;281:356–364. doi: 10.1074/jbc.M508047200. [DOI] [PubMed] [Google Scholar]
- 59.Bonet R., Ramirez-Espain X., Macias M.J. Solution structure of the yeast URN1 splicing factor FF domain: comparative analysis of charge distributions in FF domain structures-FFs and SURPs, two domains with a similar fold. Proteins. 2008;73:1001–1009. doi: 10.1002/prot.22127. [DOI] [PubMed] [Google Scholar]
- 60.Bonet R., Ruiz L., Macias M.J. Solution structure of the fourth FF domain of yeast Prp40 splicing factor. Proteins. 2009;77:1000–1003. doi: 10.1002/prot.22547. [DOI] [PubMed] [Google Scholar]
- 61.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 62.Venyaminov SYu, Kalnin N.N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers. 1990;30:1243–1257. doi: 10.1002/bip.360301309. [DOI] [PubMed] [Google Scholar]
- 63.Bandekar J. Amide modes and protein conformation. Biochim. Biophys. Acta. 1992;1120:123–143. doi: 10.1016/0167-4838(92)90261-b. [DOI] [PubMed] [Google Scholar]
- 64.Chirgadze Y.N., Fedorov O.V., Trushina N.P. Estimation of amino acid residue side-chain absorption in the infrared spectra of protein solutions in heavy water. Biopolymers. 1975;14:679–694. doi: 10.1002/bip.1975.360140402. [DOI] [PubMed] [Google Scholar]
- 65.Mizuguchi M., Nara M., Nitta K. FT-IR study of the Ca2+-binding to bovine α-lactalbumin. Relationships between the type of coordination and characteristics of the bands due to the Asp COO- groups in the Ca2+-binding site. FEBS Lett. 1997;417:153–156. doi: 10.1016/s0014-5793(97)01274-x. [DOI] [PubMed] [Google Scholar]
- 66.Nara M., Morii H., Tanokura M. Coordination to divalent cations by calcium-binding proteins studied by FTIR spectroscopy. Biochim. Biophys. Acta. 2013;1828:2319–2327. doi: 10.1016/j.bbamem.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 67.Barth A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Haris P.I., Robillard G.T., Chapman D. Potential of 13C and 15N labeling for studying protein-protein interactions using Fourier transform infrared spectroscopy. Biochemistry. 1992;31:6279–6284. doi: 10.1021/bi00142a016. [DOI] [PubMed] [Google Scholar]
- 69.Arrondo L.R., Muga A., Goñi F.M. Quantitative studies of the structure of proteins in solution by Fourier-transform infrared spectroscopy. Prog. Biophys. Mol. Biol. 1993;59:23–56. doi: 10.1016/0079-6107(93)90006-6. [DOI] [PubMed] [Google Scholar]
- 70.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. (Shanghai) 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 71.Haris P.I., Chapman D. The conformational analysis of peptides using Fourier transform IR spectroscopy. Biopolymers. 1995;37:251–263. doi: 10.1002/bip.360370404. [DOI] [PubMed] [Google Scholar]
- 72.Velazquez-Campoy A., Leavitt S.A., Freire E. Characterization of protein-protein interactions by isothermal titration calorimetry. Methods Mol. Biol. 2004;261:35–54. doi: 10.1385/1-59259-762-9:035. [DOI] [PubMed] [Google Scholar]
- 73.Turnbull W.B., Daranas A.H. On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J. Am. Chem. Soc. 2003;125:14859–14866. doi: 10.1021/ja036166s. [DOI] [PubMed] [Google Scholar]
- 74.Freyer M.W., Lewis E.A. Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods Cell Biol. 2008;84:79–113. doi: 10.1016/S0091-679X(07)84004-0. [DOI] [PubMed] [Google Scholar]
- 75.Myszka D.G., Abdiche Y.N., Doyle M.L. The ABRF-MIRG’02 study: assembly state, thermodynamic, and kinetic analysis of an enzyme/inhibitor interaction. J. Biomol. Tech. 2003;14:247–269. [PMC free article] [PubMed] [Google Scholar]
- 76.Powell D.M., Amaral M.C., Greene W.C. HIV Rev-dependent binding of SF2/ASF to the Rev response element: possible role in Rev-mediated inhibition of HIV RNA splicing. Proc. Natl. Acad. Sci. USA. 1997;94:973–978. doi: 10.1073/pnas.94.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koch A., Poirier F., Delacour D. Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis. Mol. Biol. Cell. 2010;21:219–231. doi: 10.1091/mbc.E09-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S.Y., Davidson P.J., Arnoys E.J. Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology. 2006;16:612–622. doi: 10.1093/glycob/cwj089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.