Abstract
Aim: IgG4 is associated with a Th1-to-Th2 switch, which plays a vital role in metastasis, in patients with malignances; thus, we aimed to investigate its clinical significance in predicting hepatocellular carcinoma (HCC) recurrence in the present study.
Methods: The correlation between serum IgG4:IgG ratio and recurrence was analyzed in a cohort of 195 patients undergoing curative resection in 2012. Another 100 patients were analyzed in a prospective independent cohort during 2012-2013 to validate the value of serum IgG4. Serum IgG4 and total IgG concentrations were measured with an automatic immune analyzer and the optimal cutoff value for serum IgG4 levels was determined by X-tile software.
Results: Our data revealed that serum IgG4:IgG were significantly elevated in patients with tumor recurrence (P<0.05). A cutoff IgG:IgG4 ratio of 0.08 was set to stratify HCC patients into high (>0.08) and low (≤0.08) groups. High serum IgG4:IgG ratio correlated with significantly shorter time-to-recurrence (median 11.85 months vs. 39.20, P=0.005). Univariate and multivariate analyses demonstrated that serum IgG4:IgG ratio is an independent indicator of tumor recurrence and this retained its clinical significance even in conventional low-recurrence-risk subgroups, including patients with low α-fetoprotein and early-stage diseases.
Conclusion: Our results demonstrated that elevated serum IgG4:IgG ratio is associated with poor clinical outcomes in HCC patients and therefore, and can serve as a novel prognostic predictor for HCC patients undergoing resection. Analyzing serum IgG4 would be useful to tailor individualized therapies for patients.
Keywords: Hepatocellular carcinoma, serum biomarkers, IgG4, recurrence, prognosis, curative resection, Th1-to-Th2 switch.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignant disease and the third leading cause of cancer-related death worldwide1. Although developments in surgery have improved the survival of patients with HCC, post-surgery survival remains unsatisfactory because of high recurrence and metastasis rates2, 3. Therefore, there is a pressing need for a reliable biomarker that can effectively detect patients with a high risk of relapse after surgery so that additional treatments can be administered.
Recent evidence demonstrates that the immune microenvironment plays an important role in regulating tumor progression and metastasis. A Th2 microenvironment indicates an intratumoral Th2-cell-dominant immune reaction, which characterized by the activation of regulatory cells that produce interleukin-10, and microenvironment with this phenotype exhibited impressing tumor prompting capacity. Moreover in HCC, it has been reported to promote HCC recurrence and metastasis4-8. In addition, circulating Th2 cytokines have been identified as potential predictors of prognosis9-11. A serum biomarker would be ideal for monitoring tumor recurrence in HCC patients.
Human B cells are known to secrete four subclasses of IgG, each of which has different functions12, 13. Although the IgG subclasses have been determined to activate different components of the immune system, their individual effector functions in cancer inflammation remain largely unknown14. The Th2-dependent Immunoglobulin, IgG4, is a minor immunoglobulin subtype that composes 3-6% of circulating IgG in adults. However, the subclass proportions may be altered in the context of certain diseases15. IgG4 has been reported in a range of chronic inflammatory and autoimmune conditions, such as autoimmune pancreatitis, where IgG4-expressing cells infiltrate target organs16, 17. A previous study reported that IgG4 produced in a tumor-induced Th2-based immune response might suppress effector cells. Thereby, IgG4 may induce clinical tolerance in some malignancies16. Moreover, elevated serum IgG4 has been associated with poor prognosis in melanoma18. We assume that this molecule could be an ideal marker for predicting HCC recurrence. However, by far, the clinical utility of serum IgG4 in HCC remains unclear.
We therefore designed this prospective, single-center study with an independent validation cohort to investigate whether serum IgG4 can identify a Th1-to-Th2 switch, which will aid in the prediction of HCC recurrence.
Patients and Methods
Study population
We prospectively recruited a test cohort of patients with HCC who underwent curative resection from 46 ward of Hepatobiliary depart of Zhonghshan Hospital (Fudan University, Shanghai, China) between January and December 2012. Another 100 patients were recruited independently from 47 ward of Hepatobiliary depart of Zhonghshan Hospital (Fudan University, Shanghai, China) from March 2012 to May 2013 to validate the findings in the training cohort (Figure 1). We ensure these two cohorts are entirely independent without any overlapping patients. HCC was defined on the basis of imaging scans and biochemistry tests and was confirmed by histopathology according to the American Association for the Study of Liver Diseases guidelines19. The HCC tumor stage was defined according to the Barcelona Clinic Liver Cancer (BCLC) staging system and BCLC 0+A stage tumors were classified as early stage. Tumor differentiation was determined according to the Edmondson grading system. Liver function was assessed by the Child-Pugh scoring system. Approval for the use of human subjects was obtained from the research ethics committee of Zhongshan hospital and informed consent was obtained from each individual enrolled in the study.
Figure 1.
Distribution of patients enrolled in this study.
Follow up
Patients were monitored prospectively by serum α-fetoprotein (AFP) testing, abdomen ultrasonography and chest X-ray, every 1-6 months, as previously described19, 20. Follow up was ended in August 2016. Time to recurrence (TTR) was defined as the interval between surgery and the diagnosis of any type of recurrence, including intrahepatic or extrahepatic recurrence as identified by magnetic resonance imaging or computed tomography.
Determination of serum IgG4 and total IgG concentrations
Peripheral blood samples from HCC patients were collected three days before resection. Serum was immediately separated by centrifugation and stored at -80°C until testing. Commercially available IgG4 and IgG kits (BN* System, Siemens Healthcare Diagnostics, Germany), which based on the scattering immunoturbidimetric assay, were used to determine serum IgG4 and total IgG concentrations for each patient using the BNII instrument according to the manufacturer's instructions. Test quality control was conducted with manufacturer's quality control materials, and clinical samples would not be tested until the detection system was under control.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM, Chicago, USA). Experimental values are presented as the mean ± SEM for continuous variables. χ2 tests, Fisher's exact probability tests and Student's t-tests A were used for comparison between groups, as appropriate. If variances within groups were not homogeneous, the nonparametric Mann-Whitney U test or Wilcoxon signed-rank test was used. The optimal cutoff value for the IgG4:IgG ratio was estimated by X-tile software as we previous did21, 22. The IgG4:IgG ratio and TTR were analyzed using Kaplan-Meier survival curves and log-rank tests, respectively. Univariate and multivariate proportional analyses were performed with the Cox proportional hazard regression model.
Results
Clinical characteristics of patients
The characteristics of the study participants are summarized in Table 1. At the time of analysis, the median follow-up time was 25.80 months (range 0.60-55.00 months) for the training cohort and 33.90 months (range 2.00-53.00months) for the validation cohort. In training cohort, 140 patients were stratified into BCLC 0+A stage (0: 16; A: 124), while 55 patients were stratified into BCLC B+C stage (B: 41; C: 14). On the other hand, in validation cohort, 82 patients were considered as BCLC 0+A stage (0: 6; A: 72), and 18 patients were considered as BCLC B+C stage (B: 9; C: 9). All clinical characteristics were well balanced between the training and validation cohort, excluding the hepatitis B surface antigen (HBsAg) status (P<0.01, Table 1).
Table 1.
Patient characteristics in training and validation cohorts
| Characteristic | No. of patients | Training cohort | Validation cohort | P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Total No. of patients | 295 | 195 | 100 | ||||
| Age (mean±SD) | 54.90±9.72 | 53.55±12.35 | 0.304 | ||||
| SEX | Female | 42 | 23 | 11.79 | 19 | 19 | 0.094 |
| Male | 253 | 172 | 88.21 | 81 | 81 | ||
| AFP (ng/mL) |
≤400 | 207 | 139 | 71.28 | 68 | 68 | 0.056 |
| >400 | 88 | 56 | 28.72 | 32 | 32 | ||
| ALT (U/L) |
≤75 | 272 | 176 | 90.26 | 96 | 96 | 0.082 |
| >75 | 23 | 19 | 9.74 | 4 | 4 | ||
| HBsAg | Negative | 30 | 12 | 6.15 | 18 | 18 | 0.001 |
| Positive | 265 | 183 | 93.85 | 82 | 82 | ||
| Liver cirrhosis | No | 67 | 38 | 19.49 | 29 | 29 | 0.065 |
| Yes | 228 | 157 | 80.51 | 71 | 71 | ||
| No.of tumor | Single | 235 | 150 | 76.92 | 85 | 85 | 0.103 |
| Multiple | 60 | 45 | 23.08 | 15 | 15 | ||
| Tumor size, cm | ≤5 | 181 | 114 | 58.46 | 67 | 67 | 0.154 |
| >5 | 114 | 81 | 41.54 | 33 | 33 | ||
| Tumor encapsulation | Complete | 183 | 124 | 63.59 | 59 | 59 | 0.442 |
| None | 112 | 71 | 36.41 | 41 | 41 | ||
| Satellite lesion | No | 269 | 179 | 91.79 | 90 | 90 | 0.607 |
| Yes | 26 | 16 | 8.21 | 10 | 10 | ||
| Vascular invasion | No | 180 | 123 | 63.08 | 57 | 57 | 0.311 |
| Yes | 115 | 72 | 36.92 | 43 | 43 | ||
| Edmondson stage | I-II | 182 | 119 | 61.03 | 63 | 63 | 0.741 |
| III-IV | 113 | 76 | 38.97 | 37 | 37 | ||
| Child-Pugh score | A | 286 | 189 | 96.92 | 97 | 97 | 1.000 |
| B | 9 | 6 | 3.08 | 3 | 3 | ||
| BCLC stage | 0+A | 222 | 140 | 71.79 | 82 | 82 | 0.055 |
| B+C | 73 | 55 | 28.21 | 18 | 18 | ||
| Recurrence | No | 134 | 88 | 45.13 | 46 | 46 | 0.902 |
| Yes | 161 | 107 | 54.87 | 54 | 54 | ||
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; BCLC, Barcelona Clinic Liver Cancer
Vascular invasion contains both portal invasion and microvascular invasion.
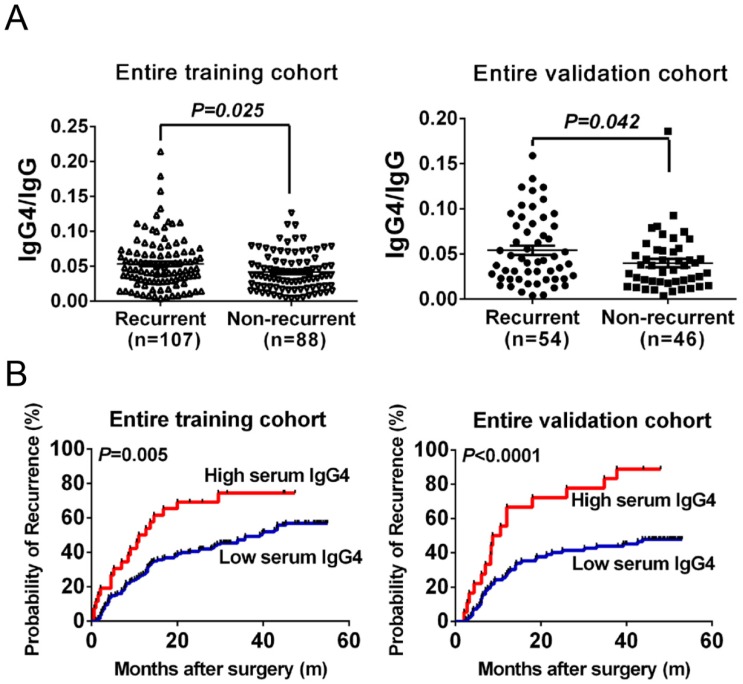
Serum IgG4:IgG ratio are significantly elevated in recurrent HCC patients
Because the IgG subclass IgG4, which associated with tumor inflammatory microenvironments, might be affected by the total IgG concentration in HCC patients. Hence, we first investigated the correlation between IgG4 and IgG in the training cohort. Serum IgG4 concentrations significantly correlated with the serum IgG concentrations in HCC patients (r2=0.44, P<0.01). This indicates that comparing IgG4 to IgG might reduce individual variation and, therefore, we applied IgG4:IgG ratio to reflect serum IgG4 levels. In the training cohort, the IgG4:IgG ratio was significantly higher in recurrent patients than in non-recurrent patients (P<0.05, Figure 2A). This indicates that IgG4:IgG ratio was significantly elevated in recurrent HCC patients, which suggests it could be used for predicting HCC recurrence.
Figure 2.
Prediction of recurrence with serum IgG4 levels in HCC patients undergoing curative resection. (A) Distribution of serum IgG4 levels in recurrent and non-recurrent patients from the training cohort (left) and from the validation cohort (right). (B) Kaplan-Meier analysis of HCC patients according to serum IgG4 levels in the training cohort (left) and in the validation cohort (right). Cutoff value of IgG4 levels (IgG4 concentration:total IgG concentration) was set as 0.08 according to X-tile software.
Determination of Optimal Cutoff Value for predicting Recurrence
As serum IgG4 showed potential as a predictor of HCC recurrence, we next evaluated the optimized cutoff value for serum IgG4:IgG ratio for recurrence prediction by using X-tile 3.6.1 software (Yale University, New Haven, CT, USA) as our group did previously21, 22. The cutoff value was set according to the most significant P value based on log-rank test and results demonstrated that a cutoff value of 0.08 showed the most significant capability to predict recurrence.
IgG4:IgG ratio as a prognostic marker in the training cohort
The prognostic significance of IgG4:IgG ratio in patients receiving curative resection was investigated with a cohort of 195 patients. We followed these patients over a median of 25.80 months. During this time, 54.87% (107/195) of these patients suffered recurrence. When stratified into two groups based on IgG4:IgG ratio, patients with higher serum IgG4:IgG ratio had significantly shorter TTR (median 11.85 months vs. 39.20, P=0.005, Figure 2B) and higher recurrence rates than those with low ratio (73.08% vs. 52.07%). A univariate analysis indicated that the IgG4:IgG ratio, AFP levels, tumor size, vascular invasion including portal invasion as well as microvascular invasion, and BCLC stage correlated with HCC recurrence (P<0.050, Table 2). Considering the BCLC stage was associated with several clinical characteristics including tumor burden and liver function, it was excluded from the multivariate analyses to avoid potential bias. The multivariate analysis also revealed that IgG4:IgG ratio is a significant indicator for TTR (hazard ratio of 1.89; 95% confidential interval: 1.08-3.32; P=0.03; Table 2). In addition, AFP levels and a tumor size greater than 5 cm were also independent indicators of TTR (Table 2).
Table 2.
Univariate and Multivariate Cox proportional hazard regression analysis of factors associated with recurrence in training and validation cohort
| Variables | Training cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| IgG4/IgG | >0.08 VS ≤0.08 | 2.385 (1.433-3.969) | 0.001 | 1.950 (1.261-3.014) | 0.004 | 2.478 (1.300-4.725) | 0.006 | 3.416 (1.754-6.655) | 0.000 |
| Sex | Male VS female | 0.369 (0.150-0.911) | 0.031 | 0.396 (0.160-0.979) | 0.045 | 0.808 (0.401-1.629) | 0.552 | NA | |
| AFP | >400ng/ml VS ≤400ng/ml | 2.026 (1.321-3.107) | 0.001 | 1.682 (1.081-2.617) | 0.021 | 1.572 (0.873-2.828) | 0.132 | NA | |
| No. of tumor | Multiple VS Single | 1.533 (0.970-2.424) | 0.068 | NA | 1.073 (0.479-2.401) | 0.864 | NA | ||
| Tumor size | >5cm VS ≤5cm | 2.257 (1.482-3.437) | 0.000 | 1.726 (1.161-2.814) | 0.003 | 2.621 (1.462-4.697) | 0.001 | 2.643 (1.47-4.830) | 0.002 |
| Satellite lesion | Present VS absent | 1.849 (0.957-3.574) | 0.067 | NA | 2.510 (1.160-5.430) | 0.019 | 2.456 (1.110-5.434) | 0.027 | |
| Vascular invasion | Present VS absent | 1.483 (1.972-2.261) | 0.067 | NA | 1.481 (0.831-2.640) | 0.183 | NA | ||
| Edmondson stage | III-IV VS I-II | 1.119 (0.731-1.711) | 0.605 | NA | 2.614 (1.459-4.683) | 0.001 | 2.396 (1.312-4.374) | 0.004 | |
| Child-Pugh score | B VS A | 1.931 (0.707-5.275) | 0.199 | NA | 3.150 (0.970-10.225) | 0.056 | NA | ||
| BCLC stage | B+C VS 0+A | 1.927 (1.254-2.961) | 0.003 | NA | 2.033 (1.050-3.936) | 0.035 | NA | ||
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; BCLC, Barcelona Clinic Liver Cancer
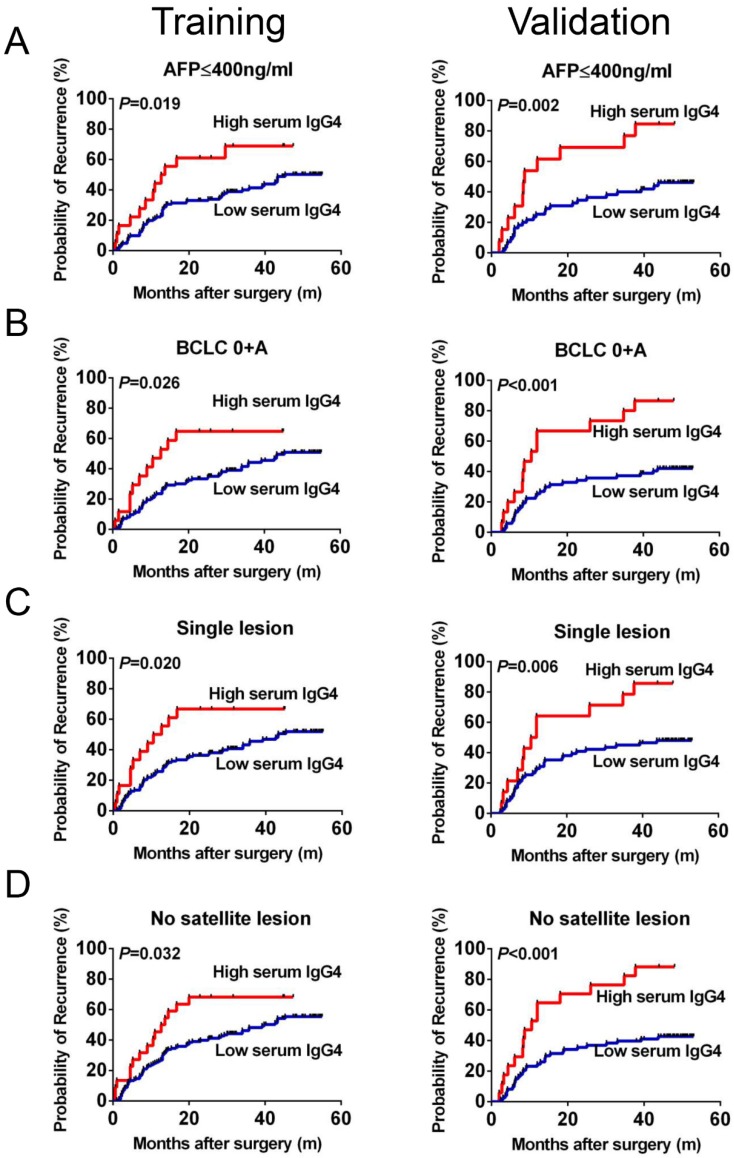
Predicting HCC recurrence with serum IgG4:IgG ratio in subgroups of training cohort
The prognostic significance of serum IgG4:IgG ratio within low recurrence risk subgroups was further investigated. Patients with AFP ≤400 ng/ml (low-AFP) and with a high preoperative IgG4:IgG ratio had a higher probability of recurrence or metastasis (median 13.2 months vs. 45.30, P=0.019, Figure 3A). Similar results were observed in other conventional low risk subgroups, including patients with BCLC stage 0+A (median 12.7 months vs. 45.30, P=0.026, Figure 3B), single tumor (median 11.6 months vs. 43.25, P=0.020, Figure 3C) and no satellite lesions (median 13.2 months vs. 39.75, P=0.032, Figure 3D).
Figure 3.
Recurrence prediction values using serum IgG4 levels in patients with low-recurrence risk. Kaplan-Meier analysis of HCC patients with AFP <400 ng/ml (A), BCLC 0+A (B), single tumor (C), and no satellite lesion (D) in the training and validation cohorts.
Correlation between serum IgG4:IgG ratio and clinical characteristics of HCC recurrence in the training cohort
Patients with high IgG4:IgG ratio were more likely to have a larger tumor than those with low ratio. However, it did not reach statistical significance (P=0.07, Table 3). There was no significant difference in other clinicopathological characteristics including tumor size and pathological differentiation between high and low IgG4 level groups in the training cohort.
Table 3.
Correlation between serum IgG4 levels and clinical characteristics
| Clinical characteristics | Training cohort | Validation cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients (N=195) | IgG4/IgG≤0.08 (N=169) |
IgG4/IgG>0.08 (N=26) |
P | No. of patients (N=100) | IgG4/IgG≤0.08 (N=82) |
IgG4/IgG>0.08 (N=18) |
P | |
| Age | ||||||||
| ≤50 | 60 | 48 | 12 | 0.068 | 46 | 39 | 7 | 0.504 |
| >50 | 135 | 121 | 14 | 54 | 43 | 11 | ||
| Sex | ||||||||
| Male | 172 | 147 | 25 | 0.177 | 81 | 66 | 15 | 1.000 |
| Female | 23 | 22 | 1 | 19 | 16 | 3 | ||
| AFP, ng/mL | ||||||||
| ≤400 | 139 | 121 | 18 | 0.804 | 68 | 55 | 13 | 0.672 |
| >400 | 56 | 48 | 8 | 32 | 27 | 5 | ||
| ALT, U/L | ||||||||
| ≤75 | 176 | 154 | 22 | 0.492 | 96 | 78 | 18 | 0.770 |
| >75 | 19 | 15 | 4 | 4 | 4 | 0 | ||
| HBsAg | ||||||||
| Negative | 12 | 11 | 1 | 0.930 | 18 | 15 | 3 | 1.000 |
| Positive | 183 | 158 | 25 | 82 | 67 | 15 | ||
| Liver cirrhosis | ||||||||
| No | 38 | 35 | 3 | 0.272 | 30 | 27 | 3 | 0.173 |
| Yes | 157 | 134 | 23 | 70 | 55 | 15 | ||
| No. of tumor | ||||||||
| Single | 150 | 132 | 18 | 0.453 | 85 | 71 | 14 | 0.560 |
| Multiple | 45 | 37 | 8 | 15 | 11 | 4 | ||
| Tumor size, cm | ||||||||
| ≤5 | 114 | 103 | 11 | 0.073 | 67 | 54 | 13 | 0.603 |
| >5 | 81 | 66 | 15 | 33 | 28 | 5 | ||
| Tumor encapsulation | ||||||||
| Complete | 124 | 105 | 19 | 0.280 | 59 | 51 | 8 | 0.166 |
| None | 71 | 64 | 7 | 41 | 31 | 10 | ||
| Satellite lesion | ||||||||
| No | 179 | 157 | 22 | 0.152 | 87 | 72 | 15 | 0.901 |
| Yes | 16 | 12 | 4 | 13 | 10 | 3 | ||
| Vascular invasion | ||||||||
| No | 123 | 107 | 16 | 0.861 | 57 | 46 | 11 | 0.697 |
| Yes | 72 | 62 | 10 | 43 | 36 | 7 | ||
| Edmondson stage | ||||||||
| I-II | 119 | 102 | 17 | 0.624 | 63 | 50 | 13 | 0.371 |
| III-IV | 76 | 67 | 9 | 37 | 32 | 5 | ||
| Child-Pugh score | ||||||||
| A | 189 | 164 | 25 | 1.000 | 97 | 80 | 17 | 1.000 |
| B | 6 | 5 | 1 | 3 | 2 | 1 | ||
| BCLC stage | ||||||||
| 0+A | 140 | 123 | 17 | 0.435 | 82 | 67 | 15 | 1.000 |
| B+C | 55 | 46 | 9 | 18 | 15 | 3 | ||
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; BCLC, Barcelona Clinic Liver Cancer. Vascular invasion contains both portal invasion and microvascular invasion.
Validation of serum IgG4:IgG ratio as a prognostic marker for HCC recurrence
After exploring the preliminary significance of serum IgG4, we next independently recruited another 100 HCC patients to construct a cohort to validate the training cohort findings. The TTR for patients with high IgG4:IgG ratio was significantly shorter compared with low-risk patients (median 9.6 months vs. not reached, P<0.01, Figure 2B). This was found for all HCC patients as well as within the low-recurrence-risk subgroups (low-AFP, median 8.7 months vs. not reached, P=0.04; BCLC 0+A, median 10.5 months vs. not reached, P<0.01; single tumor, median 11.25 months vs. not reached, P=0.03; no satellite lesion, median 10.5 months vs. not reached, P<0.01; Figure 3A-D). Therefore, these data support the hypothesis that serum IgG4:IgG ratio is an independent indictor of HCC recurrence (HR=3.29; 95%CI: 1.65-6.55; P=0.01; Table 2). In addition, tumor size is also a significant indicator of HCC recurrence.
Discussion
IgG4 is a significant prognostic indicator in extrahepatic cholangiocarcinomas, melanoma and pancreatic cancers16,18,23. In the present study, we described the clinical significance of serum IgG4 in HCC. Our data showed that serum IgG4 were significantly elevated in patients with recurrent HCC, which was also demonstrated to be an independent predictor of recurrence for HCC patients after curative resection in two cohorts, including an independent validation cohort. IgG4 retained its prediction value in several conventional low-recurrence-risk subgroups, thereby strengthening the clinical utility of this circulating biomarker for predicting HCC recurrence. Moreover, serum IgG4 and IgG could be analyzed using standard commercial kits, which are approved by the FDA for in vitro clinical diagnostic use and are routinely used in clinical laboratories. Thus, the detection of serum IgG4 levels could easily be standardized to provide accurate, universal and important information for early decision-making to tailor the most effective therapy for each HCC patient.
Because IgG4 is secreted by plasma cells, the predictive value of serum IgG4 for tumor recurrence could be demonstrated by the function of this IgG subclass. IgG4 exhibits a limited capacity for activating the immune system and is considered as a non-activating IgG subclass24-26. In addition, IgG4 antibodies are able to interact with other IgG antibodies, impeding their immune activating function16. Furthermore, IgG4 is a marker of the immune system shifting from a Th1 to a Th2 response27, in which regulatory T cells are highly activated and several negative immune regulatory factors, including IL-10, are secreted8,28. Thus, intratumoral IgG4 can greatly hinder the antitumor function of immune cells that have infiltrated a tumor, thereby creating a suitable microenvironment for tumor growth and resulting in a high incidence of recurrence caused by tumor cell spreading. Given that IgG4 is a secreted protein, detection of serum IgG4 levels might be an ideal tool to reflect the intratumoral immune status and to predict patient prognosis. In support of this, serum IgG4 was associated with poor prognosis in melanoma patients. This indicates that serum IgG4 could serve as a prognostic prediction marker with the advantages of cost efficient and non-invasive, compared with detecting IgG4 by immunohistochemistry16. In HCC, the impact of the immune status of the tumor microenvironment on patient prognosis has raised extensive attention in recent years29-33. A pilot study showed that the immune response within the tumor microenvironment underwent a unique Th1-to-Th2 switch in metastatic HCC. In these cases, the secretion of Th2-like cytokines was significantly elevated, which strongly inhibited the inflammatory response within the tumor microenvironment and facilitated HCC metastasis8. Additionally, individual serum levels of several Th2 cytokines have been reported to be significantly associated with the recurrence of HCC10,11.
In light of the above considerations, we proposed that serum IgG4 could serve as an indicator of a Th1-to-Th2 switch within HCC patients and act as a powerful prognostic biomarker. We found a significant correlation between IgG4 and IgG, as the level of IgG4 would affect the total IgG level. Hence, we analyzed the IgG4:IgG ratio instead of IgG4 alone to reduce individual bias16. Our data showed that 0.08 according to X-tile software was the optimal cutoff value for predicting HCC recurrence. High preoperative IgG4 was an independent recurrence indicator and this was validated with an independent cohort of patients in our study. Our results strongly suggest that analyzing preoperative serum IgG4:IgG ratio could effectively identify patients who have a high risk of recurrence. These patients could then receive additional adjuvant therapies to reduce the recurrence risk and improve their prognosis. Taken together, the detection of serum IgG4 could indicate the intratumoral immune status and thereby identify patients who require further treatment following resection.
In clinical practice, it is difficult to predict which individuals would have tumor recurrence after curative resection for early-stage HCC, such as BCLC 0+A-stage patients22. From our data, we observed that serum IgG4 levels retained significant recurrence prediction in BCLC 0+A-stage patients with HCC. Currently, AFP is the most widely used serum biomarker for HCC. However, about 30 to 40% of patients have normal serum AFP levels at diagnosis, which is a significant limitation for the clinical use of this biomarker34, 35. Thus, we investigated the prediction value of IgG4 in the low-AFP subgroup. We found that low-AFP patients could be stratified into two groups according to serum IgG4 with substantially different recurrence rates. Our results indicate that IgG4 is a powerful prognostic marker for HCC, especially for patients with early-stage disease and normal AFP levels. We identified a novel serum biomarker for patients whose prognosis is difficult to predict by conventional indexes. Analyzing IgG4 can significantly improve the ability of clinicians to identify patients at high risk of recurrence that require targeted adjuvant therapy.
We enrolled an independent cohort of patients to validate the clinical utility of serum IgG4, and the clinical characteristics between the training and validation cohort were similar, which indicates the reliability and universality of our findings. However, there are still some limitations of present study. It should be noted that most patients with HCC in China have a hepatitis B virus-positive background, which differs greatly from the patient population in previous studies in the United States, Europe and Japan. Therefore, the prognostic significance of serum IgG4 needs to be validated in patients with HCC from those geographic areas. Moreover, several conventional prognostic factors such as vascular invasion and satellite lesions, this might resulted from the relatively small cohort of patients enrolled in this study and short time of follow-up. What should also be mentioned was that all patients recruited in present study received curative resection which indicated they shared almost similar severity of tumor. Thus, a multi-center, large-scale, systematic clinical trial should be conducted in the future. In addition, we did not comprehensively investigate the mechanism of IgG4 in promoting tumor cell spreading, but this work is underway in our laboratory.
To our knowledge, this is the first report to demonstrate the recurrence prediction value of serum IgG4 in HCC patients. Further investigation into the function of IgG4 in regulating the immune microenvironment might provide a new insight into the mechanism of HCC recurrence and metastasis. This information may identify novel therapeutic strategies that promote a Th1 microenvironment to improve the prognosis of HCC patients.
Acknowledgments
We thank the participating patients for the source of clinical samples.
Funding/Support
This study was supported by grants from the National High Technology Research and Development Program (863 Program) of China (2015AA020401), the State Key Program of National Natural Science of China (81530077), the National Natural Science Foundation of China (81472676, 81572823, 81372317 and 81572064), the Projects from the Shanghai Science and Technology Commission (13140901900, 134119a1201, 14DZ1940300, 14411970200 and 14140902301), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12010202), Specialized Research Fund for the Doctoral Program of Higher Education and Research Grants Council Earmarked Research Grants Joint Research Scheme (20130071140008), Key Developing Disciplines of Shanghai Municipal Commission of Health and Family Planning (2015ZB0201), Research Project of Shanghai Municipal Commission of Health and Family Planning (201540052), The funding plan for outstanding youth doctors training of Shanghai (2016-01), the National Natural Science Foundation of China (81572064), and the Research funding of Shanghai Municipal Commission of Health and Family Planning (201440389).
References
- 1.Siegel R, Ma J, Zou Z. et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya T, Shimada M, Kudo M. et al. A Comparison of the Surgical Outcomes Among Patients With HBV-Positive, HCV-Positive, and Non-B Non-C Hepatocellular Carcinoma: A Nationwide Study of 11,950 Patients. Ann Surg. 2015;261:513–520. doi: 10.1097/SLA.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 4.Marabelle A, Kohrt H, Caux C. et al. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–56. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makkouk A, Weiner GJ. Cancer Immunotherapy and Breaking Immune Tolerance: New Approaches to an Old Challenge. Cancer Res. 2015;75:5–10. doi: 10.1158/0008-5472.CAN-14-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero I, Garrido F, Garcia-Lora AM. Metastases in Immune-Mediated Dormancy: A New Opportunity for Targeting Cancer. Cancer Res. 2014;74:6750–57. doi: 10.1158/0008-5472.CAN-14-2406. [DOI] [PubMed] [Google Scholar]
- 7.Ruffell B, Chang-Strachan D, Chan V. et al. Macrophage IL-10 Blocks CD8(+) T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–37. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budhu A, Forgues M, Ye QH. et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Sanmamed MF, Carranza-Rua O, Alfaro C. et al. Serum Interleukin-8 Reflects Tumor Burden and Treatment Response across Malignancies of Multiple Tissue Origins. Clin Cancer Res. 2014;20:5697–707. doi: 10.1158/1078-0432.CCR-13-3203. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZY, Wei W, Guo ZX. et al. Using multiple cytokines to predict hepatocellular carcinoma recurrence in two patient cohorts. Br J Cancer. 2014;110:733–40. doi: 10.1038/bjc.2013.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SL, Mo FK, Wong CS. et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012;118:3984–92. doi: 10.1002/cncr.26726. [DOI] [PubMed] [Google Scholar]
- 12.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–66. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Papadea C, Check IJ. Human immunoglobulin G and immunoglobulin G subclasses: biochemical, genetic, and clinical aspects. Crit Rev Clin Lab Sci. 1989;27:27–58. doi: 10.3109/10408368909106589. [DOI] [PubMed] [Google Scholar]
- 14.Steplewski Z, Sun LK, Shearman CW. et al. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc Natl Acad Sci U S A. 1988;85:4852–56. doi: 10.1073/pnas.85.13.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French M. Serum IgG subclasses in normal adults. Monogr Allergy. 1986;19:100–107. [PubMed] [Google Scholar]
- 16.Karagiannis P, Gilbert AE, Josephs DH. et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–74. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Takahashi H, Shinomura Y. Mechanisms and assessment of IgG4-related disease: lessons for the rheumatologist. Nat Rev Rheumatol. 2014;10:148–59. doi: 10.1038/nrrheum.2013.183. [DOI] [PubMed] [Google Scholar]
- 18.Cipponi A, Mercier M, Seremet T. et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Yang XR, Sun YF. et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform. Clin Cancer Res. 2014;20:4794–805. doi: 10.1158/1078-0432.CCR-14-0251. [DOI] [PubMed] [Google Scholar]
- 21.Sun YF, Xu Y, Yang XR. et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–68. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Yang XR, Xu Y. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 23.Harada K, Shimoda S, Kimura Y. et al. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56:157–64. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]
- 24.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aalberse RC, Stapel SO, Schuurman J. et al. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 26.Labrijn AF, Aalberse RC, Schuurman J. When binding is enough: nonactivating antibody formats. Curr Opin Immunol. 2008;20:479–85. doi: 10.1016/j.coi.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 28.O'Garra A, Barrat FJ, Castro AG. et al. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 29.Yeung OW, Lo C, Ling C. et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62:607–16. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Sprinzl MF, Reisinger F, Puschnik A. et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358–68. doi: 10.1002/hep.26328. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Kuang DM, Pan WD. et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57:1107–16. doi: 10.1002/hep.26192. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C, Teufel A, Yevsa T. et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut. 2012;61:1733–43. doi: 10.1136/gutjnl-2011-301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu XD, Zhang JB, Zhuang PY. et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 34.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PubMed] [Google Scholar]
- 35.Shen Q, Fan J, Yang XR. et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–26. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]