Abstract
Objective: We had previously demonstrated that the carbohydrate antigen 19-9 (CA19-9), lactate dehydrogenase (LDH), neutrophil lymphocyte ratio (NLR) are prognostic factors for patients with metastatic colorectal cancer (mCRC). In this study, we try to analysis the association of these blood-based biomarkers with bevacizumab efficacy in the first line setting.
Methods: A total of 284 eligible consecutive mCRC patients who received first-line chemotherapy with or without bevacizumab were studied from 2007 to 2014 at Sun Yat-Sen University Cancer Center. The endpoints were overall survival (OS), progression free survival (PFS).
Results: Among all the patients, the initial elevated CA19-9, high LDH, and NLR > 2.47 were confirmed as independent unfavorable prognostic factors. The CA19-9 and LDH levels were significantly associated with PFS. In the high CA19-9 subgroup, patients had favorable OS from bevacizumab administration in the first line therapy (32.1 vs. 20.1 months, P = 0.03), but without PFS benefit. In terms of different levels of LDH, and NLR, there were no survival benefit from bevacizumab treatment.
Conclusions: Our results suggest that the initial CA19-9, LDH, and NLR levels could be independent prognostic biomarkers in mCRC patients. And among all these factors, the initial high CA19-9 level could be a predictor for bevacizumab effect.
Keywords: carbohydrate antigen 19-9, bevacizumab, predictive marker, overall survival, metastatic colorectal cancer.
Introduction
The colorectal cancer is one of the most frequently diagnosed and deadly cancer globally 1. Nearly 40-50% colorectal cancer patients were initially diagnosed with metastatic lesion(s) 2. Due to distinct outcomes among metastatic colorectal cancer (mCRC) patients, various efforts had been attempted for systematic management. In the clinical practice, the measurement of tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) are commonly used during the management of colorectal cancer patients 3. Besides, other readily available indexes associated with the cancer-related metabolism and inflammation processes could be surrogate markers including lactate dehydrogenase (LDH) 4, 5, neutrophil lymphocyte ratio (NLR) 6, 7 for evaluating the prognosis. In our previous study, we have also found that increased CEA 8, high CA19-9 9, and upraised NLR 10 are adverse prognostic indicators.
As an encouraging targeting tumor microvascular monoclonal antibody bevacizumab had been widely administrated in mCRC 11-13. However, as the angiogenesis process in mCRC is not completely discerned, the optimal predictive factor for bevacizumab has not been discovered yet 14, 15. Our earlier study suggested that the LDH in peripheral blood would serve as a potential predictor of bevacizumab therapy 16. Nonetheless, the several isoforms could hardly make LDH a satisfactory biomarker candidate.
The objective of our present study was to assess the association of baseline serum CEA, CA 19-9, LDH, and NLR with outcomes of mCRC patients and bevacizumab effect.
Materials and Methods
Patients
We retrospectively analyzed the intact data of 284 patients who were pathological diagnosed with mCRC and with clinical and/or pathological confirmations of metastasis at Sun Yat-Sen University Cancer Center from January 2005 to March 2014 and finished the integrated course of first-line treatment in this center. Patients in chemotherapy plus bevacizumab group received more than 4 cycles bevacizumab (at a dose of 5 mg/kg on day 1) 17 was administered as a combination with the standard oxaliplatin-based or irinotecan-based first-line chemotherapy. None of the patients had previously or subsequently received other target therapies. Patients who lack of a pathological diagnosis or complete medical history, loss to follow-up, suffering from two or more kinds of tumors asynchronously or synchronously were excluded.
Laboratory measurement, calculation and grouping
Neutrophil and lymphocyte counts were contained in complete blood counts test by the Sysmex XE-5000™ Automated Hematology System (Shanghai, China). NLR was calculated as neutrophil count (number of neutrophils ×109/L) divided by lymphocyte count (number of lymphocytes ×109/L), using the median value 2.47 for cutoff. While LDH was contained in biochemical test by the Hitachi Automatic Analyzer 7600-020 (Tokyo, Japan). Roche Elecsys 2010 Chemistry Analyzer (Basel, Switzerland) was used to test CEA and CA19-9. The upper normal values were used as cutoff: CEA 5 ng/ml; CA 19-9 35 ng/ml; LDH 245 U/ml.
Follow up and statistical analysis
The latest follow-up was performed on June 30ed, 2016 through telephone interview or medical records review. Overall survival (OS) was measured from date of diagnosis with mCRC to the date of death. Progression free survival (PFS) was measured from the initiation of first line therapy to the progression. Both OS and PFS were estimated by the Kaplan-Meier method and survival differences were analyzed by the log-rank test. The distributions of the baseline characteristics of the patients were assessed by the χ2 test. The P value less than 0.05 was considered with statistically significant.
Human Rights
The protocol of our study was approved by the independent ethics committee of the Sun Yat-Sen University Cancer Center and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. Written informed consent was gathered from each patient.
Results
Patient characteristics
A total of 284 patients were enrolled in this retrospective study, including 59 patients received chemotherapy containing bevacizumab (B+C group) and 225 patients received chemotherapy alone (C group). The patient characteristics between the two groups are shown in Table 1. All of the factors were balanced in statistics. As a main clinic parameter, the bevacizumab treatment was separately statistically compared, making it difficult to judge whether these two groups were comparable according to initial levels of serum indexes (Table 2). The bevacizumab administrations were balanced within different subgroup of several serum indexes.
Table 1.
Patient characteristics
| Characteristics | n (%, total n=284) |
|---|---|
| Males | 187 (65.8) |
| Age (years) | |
| median, range | 55 (19-87) |
| ≤60 | 191 (67.3) |
| >60 | 93 (32.7) |
| Primary Tumor | |
| Right colon | 87 (30.6) |
| Left colon | 95 (33.5) |
| Rectum | 102 (35.9) |
| Pathological type | |
| Well differentiation | 3 (1.1) |
| Moderately differentiation | 177 (63.3) |
| Poorly differentiation | 64 (22.5) |
| Mucinous adenocarcinoma | 35 (12.3) |
| Unknown a | 5 (1.8) |
| Pathologic tumor classification | |
| T1 | 4 (1.4) |
| T2 | 9 (3.2) |
| T3 | 50 (17.6) |
| T4 | 219 (77.1) |
| Unknown b | 2 (0.7) |
| Lymphatic invasion | 201 (70.8) |
| Vascular invasion | 26 (9.2) |
| Perineural invasion | 8 (2.8) |
| First line bevacizumab therapy | |
| With Bevacizumab | 59 (20.8) |
| Without Bevacizumab | 225 (79.2) |
| First line chemotherapy regimen | |
| Oxaliplatin-based | 213 (75.0) |
| Irinotecan-based | 53 (18.7) |
| Fluorouracil alone | 5 (1.8) |
| Oxaliplatin plus irinotecan | 13 (4.5) |
a This part of patients was pathological diagnosed with colorectal adenocarcinoma without the differentiation degree by the biopsy specimen or pathology consultation of specimen from other hospitals; b There were no surgical primary tumor specimen of these patients.
Table 2.
Statistically comparison of bevacizumab treatment according to initial levels of serum indexes
| With bevacizumab n (%) |
Without bevacizumab n (%) |
P | |
|---|---|---|---|
| CEA (ng/ml) | 0.87 | ||
| ≤5 | 15 (19.5) | 62 (80.5) | |
| >5 | 44 (21.3) | 163 (78.7) | |
| CA19-9 (ng/ml) | 0.77 | ||
| ≤35 | 30 (21.7) | 108 (78.3) | |
| >35 | 29 (19.9) | 117 (80.1) | |
| LDH (U/ml) | 0.94 | ||
| ≤245 | 43 (20.7) | 165 (79.3) | |
| >245 | 16 (21.1) | 60 (78.9) | |
| NLR | 0.55 | ||
| ≤2.47 | 29 (20.7) | 111 (79.3) | |
| >2.47 | 30 (20.8) | 114 (79.2) |
CEA carcinoembryonic antigen, CA 19-9 carbohydrate antigen 19-9, LDH lactate dehydrogenase, NLR neutrophil lymphocyte ratio
Relation between initial levels of serum indexes and survival
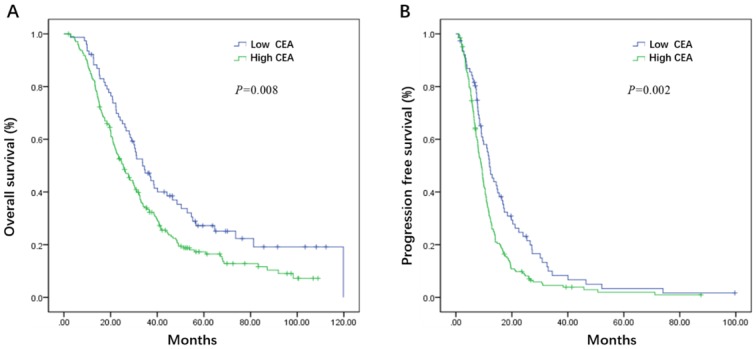
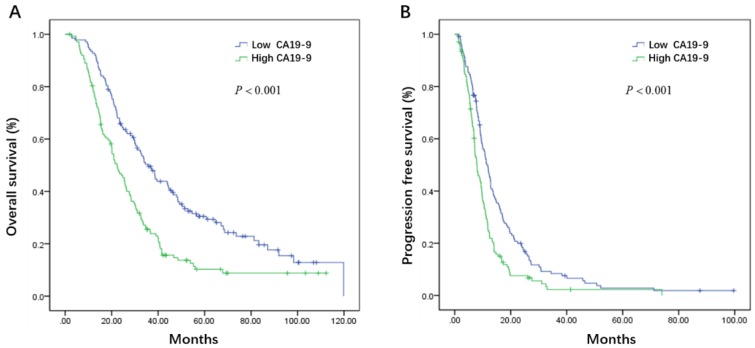
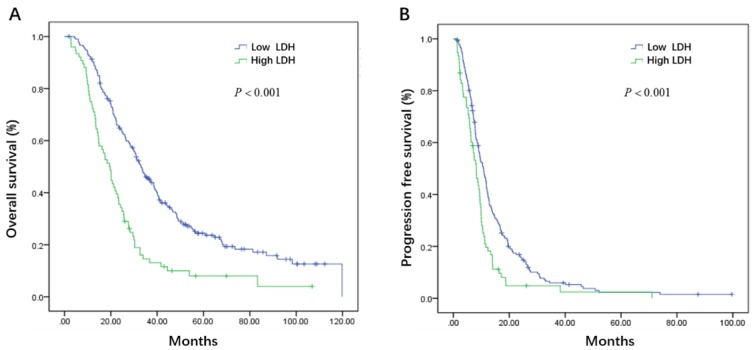
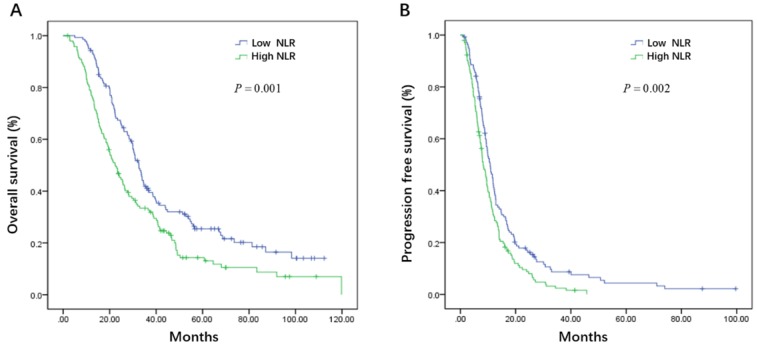
Initial serum levels of CEA, CA 19-9, LDH, and NLR are shown in Table 3. The median PFS and OS for patients with low CEA was 12.0 and 33.7 months compared to 9.1 and 25.5 months for patients with high CEA (P = 0.002 and P = 0.008) (Figure 1A, B). The median PFS and OS for patients with low CA 19-9 was 11.4 and 35.7 compared to 7.9 and 22.3 months for patients with high CA 19-9 (P < 0.001 and P < 0.001) (Figure 2A, B). The median PFS and OS for patients with low LDH was 10.8 and 33.0 compared to 8.2 and 19.5 months for patients with high LDH (P < 0.001 and P < 0.001) (Figure 3A, B). The median PFS and OS for patients with low NLR was 10.8 and 32.6 compared to 8.2 and 22.4 months for patients with high NLR (P = 0.002 and P = 0.001) (Figure 4A, B).
Table 3.
Progression free survival and overall survival according to initial levels of serum indexes
| n (%) | median PFS (months, 95%CI) | P | median OS (months, 95%CI) | P | |
|---|---|---|---|---|---|
| CEA (ng/ml) | 0.002 | 0.008 | |||
| ≤5 | 77 (27.1) | 12.0 (9.6-14.4) | 33.7 (26.9-40.5) | ||
| >5 | 207 (72.9) | 9.1 (8.0-10.2) | 25.5 (21.6-29.4) | ||
| CA19-9 (ng/ml) | <0.001 | <0.001 | |||
| ≤35 | 102 (35.9) | 11.4 (9.6-13.3) | 35.7 (30.5-40.9) | ||
| >35 | 118 (41.1) | 7.9 (6.6-9.3) | 22.3 (18.0-26.5) | ||
| LDH (U/ml) | <0.001 | <0.001 | |||
| ≤245 | 208 (73.3) | 33.0 (28.9-37.2) | 10.8 (9.3-12.3) | ||
| >245 | 76 (26.7) | 19.5 (16.0-23.0) | 8.2 (6.7-9.8) | ||
| NLR | 0.002 | 0.001 | |||
| ≤2.47 | 140 (49.3) | 10.8 (9.3-12.3) | 32.6 (29.7-35.5) | ||
| >2.47 | 144 (50.7) | 8.2 (6.9-9.6) | 22.4 (18.6-26.3) |
CEA carcinoembryonic antigen, CA 19-9 carbohydrate antigen 19-9, LDH lactate dehydrogenase, NLR neutrophil lymphocyte ratio, PFS progression free, OS overall survival, CI confidence interval
Figure 1.
Kaplan-Meier estimates of overall survival (OS) (A) and progression-free survival (PFS) (B) according to the initial levels of carcinoembryonic antigen (CEA)
Figure 2.
Kaplan-Meier estimates of overall survival (OS) (A) and progression-free survival (PFS) (B) according to the initial levels of carbohydrate antigen 19-9 (CA 19-9)
Figure 3.
Kaplan-Meier estimates of overall survival (OS) (A) and progression-free survival (PFS) (B) according to the initial levels of lactate dehydrogenase (LDH)
Figure 4.
Kaplan-Meier estimates of overall survival (OS) (A) and progression-free survival (PFS) (B) according to the initial levels of neutrophil lymphocyte ratio (NLR)
All the prognostic serum factors were assessed together in multivariate Cox proportional hazards model. The multivariate Cox proportional hazards model revealed that the CA19-9 (HR = 1.45, P = 0.004), LDH (HR = 1.37, P = 0.04) were significantly associated with PFS, and the CA19-9 (HR = 1.60, P = 0.01), LDH (HR = 1.84, P < 0.001), NLR (HR = 1.38, P = 0.04) were significantly associated with OS (Table 4).
Table 4.
Hazard ratios from multivariate Cox proportional hazard model for progression free survival and overall survival
| Index | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| CEA | 1.23 (0.91-1.67) | 0.12 | 1.05 (0.75-1.45) | 0.8 |
| CA19-9 | 1.45 (1.12-1.88) | 0.004 | 1.60 (1.21-2.13) | 0.001 |
| LDH | 1.37 (1.02-1.84) | 0.04 | 1.84 (1.35-2.50) | <0.001 |
| NLR | 1.27 (0.96-1.68) | 0.1 | 1.38 (1.01-1.87) | 0.04 |
CEA carcinoembryonic antigen; CA 19-9 carbohydrate antigen 19-9, LDH lactate dehydrogenase, NLR neutrophil lymphocyte ratio, PFS progression free, OS overall survival, HR hazard ratio, CI confidence interval
Relation between initial levels of serum indexes and bevacizumab efficacy
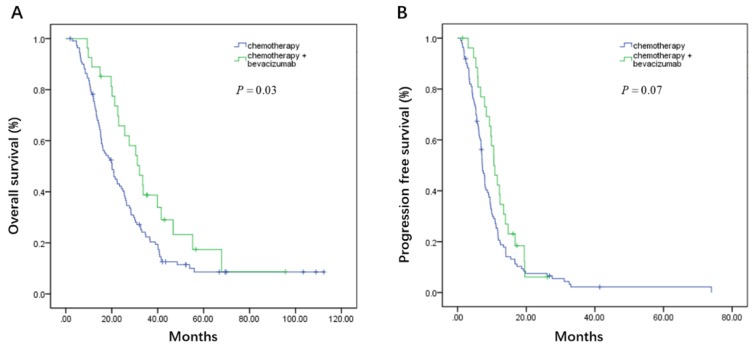
On the basis of whether the first line therapy containing bevacizumab or not, the patients could be further divided into bevacizumab plus chemotherapy (B+C group) and chemotherapy group (C group). In the high CA19-9 subgroup, patients had favorable OS from bevacizumab treatment in the first line (32.1 vs. 20.1 months, P = 0.03, Figure 5A), though without progression free survival benefit (10.6 vs. 7.2 months, P = 0.07, Figure 5B). When it comes to low CA19-9 subgroup, CEA, LDH, and NLR subgroups, there were no statistically survival difference between B+C group and C group (Table 5).
Figure 5.
Kaplan-Meier estimates of overall survival (OS) (A) and progression-free survival (PFS) (B) according to whether adding bevacizumab in the first line setting in the high carbohydrate antigen 19-9 (CA19-9) subgroup
Table 5.
Association of bevacizumab efficacy and initial levels of serum indexes
| median PFS (months, 95%CI) | P | median OS (months, 95%CI) | P | |||
|---|---|---|---|---|---|---|
| B+C group n=59 |
C group n=225 |
B+C group n=59 |
C group n=225 |
|||
| CEA (ng/ml) | ||||||
| ≤5 | 16.4(8.8-24.0) | 12.0(9.0-15.0) | 0.4 | 46.5(25.2-67.9) | 33.7(26.4-41.0) | 0.9 |
| >5 | 10.6(10.0-11.1) | 8.2(7.0-9.4) | 0.3 | 29.6(22.6-36.6) | 24.6(20.7-28.5) | 0.2 |
| CA19-9 (ng/ml) | ||||||
| ≤35 | 12.8(7.8-17.8) | 11.2(8.9-13.5) | 0.80 | 36.9(31.7-42.2) | 30.3(8.6-52.0) | 0.70 |
| >35 | 10.6(8.4-12.7) | 7.2(6.4-8.0) | 0.07 | 32.1(24.8-39.4) | 20.1(15.8-24.4) | 0.04 |
| LDH (U/ml) | ||||||
| ≤245 | 12.2(10.1-14.3) | 9.4(7.3-11.5) | 0.4 | 39.9(24.0-55.8) | 32.3(27.8-36.9) | 0.4 |
| >245 | 8.3(6.0-10.7) | 8.0(6.0-9.9) | 0.3 | 20.1(17.1-23.1) | 17.5(12.6-22.4) | 0.7 |
| NLR | ||||||
| ≤2.47 | 12.8(9.0-16.6) | 9.8(8.3-11.3) | 0.4 | 34.0(22.3-45.6) | 32.3(29.2-35.4) | 0.8 |
| >2.47 | 10.4(8.8-12.0) | 7.8(6.8-9.0) | 0.4 | 23.4(14.8-32.0) | 21.5(16.5-26.6) | 0.2 |
CEA carcinoembryonic antigen, CA 19-9 carbohydrate antigen 19-9, LDH lactate dehydrogenase, NLR neutrophil lymphocyte ratio, PFS progression free, OS overall survival, CI confidence interval, C chemotherapy, B+C bevacizumab plus chemotherapy
Discussion
Bevacizumab, the classical anti-angiogenesis drug, had been widely applied in various cancers 18. Meanwhile, hunting for suitable predictor of bevacizumab efficacy continues. Several surrogates including circulating vascular endothelial growth factor A (VEGF-A) isoforms, vascular endothelial growth factor receptor-1,2 (VEGFR-1, and VEGFR-2) had been explored but not sufficiently validated for routine clinical use15, 19. CEA, CA19-9, LDH, NLR are readily available serum biomarkers in diagnosing and monitoring the mCRC patients. Therefore, we paid attention to the correlation between these indexes and bevacizumab efficacy.
As an intercellular adhesion molecular of immunoglobulin superfamily, CEA promotes the gathering of tumor cells 20. It is reported CEA had a specificity of 90% and a sensitivity of 40% to 75% in colorectal cancer 3. Albeit CEA is a widely accepted prognostic biomarker in colorectal cancer 21, 22, it is still dubious in several other studies 23-25 in which CEA showed no statistically significant prognostic value. In our study, we found a significantly shorter PFS and OS in patients with high initial CEA levels. However, it did not prove to be an independent biomarker in the multivariate Cox proportional hazards model. Moreover, there was no association between baseline CEA levels and the efficacy of bevacizumab based therapy.
Though CA19-9 frequent elevation caused by pancreatitis, obstructive jaundice and pancreatic cancer 26, it's still a critical additional marker to monitor colorectal cancer 27. Almost 35% to 40% of colorectal cancer patients had increased CA19-9 level 28. CA19-9 had been found promoting adhesion of cancer cells to endothelial 29. Similarly, we observed high initial CA19-9 level associated with a significantly poor PFS and OS, and the multivariate Cox proportional hazards model confirmed that CA 19-9 was independent prognostic marker. On the other hand, several studies 30-32 have reported that patients received bevacizumab plus chemotherapy have promising OS compared with chemotherapy alone in the high pre-treatment CA19-9 level group. While there was no similar difference in normal CA19-9 group. Fiala O et al. 33 had observed the analogous results among patients with bevacizumab treatment. Likewise, our data indicated the potential predictive role of CA19-9 in bevacizumab therapy.
LDH, the main catalyst of aerobic glycolysis, reflects the metabolic status of tumor cells. High serum LDH level had been demonstrated to be a unfavorable prognostic factor in several tumors 34-36. Our previous study has also explored the associated of the serum LDH, the outcome and the bevacizumab efficacy 16. In our present study, the lack of correlation between serum LDH and bevacizumab efficacy, may enlighten the further exploration of local LDH in tumor microenvironment.
Increased NLR was associated with poor tumor outcome had been observed before 10, 37, implying the interaction of inflammatory and immune response play a role in tumor progression. Our data here is consistent with this conclusion.
The main limitations of this study are its retrospective design and relatively small number of patients with bevacizumab therapy.
In conclusion, the initial CA19-9, LDH level were independently associated with PFS, while the baseline CA19-9, LDH, and NLR level independently associated with OS in first line treatment. The pre-treatment CA19-9 level could potentially serve as a predictor of bevacizumab. Prospective studies on the predictive role of serum markers should be conducted to certify the results.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Guangdong, China (2015A030313010), Science and Technology Program of Guangzhou, China (1563000305) and National Natural Science Foundation of China (81272641and 81572409).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M. et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England) 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 3.Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clinical colorectal cancer. 2014;13:239–44. doi: 10.1016/j.clcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:3402–7. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 5.Kostakis ID, Vaiopoulos AG, Philippou A, Papavassiliou AG, Koutsilieris M, Kouraklis G. Preoperative serum lactate dehydrogenase levels in colorectal and gastric cancer: a hospital-based case-control study. Biomarkers in medicine. 2013;7:131–7. doi: 10.2217/bmm.12.86. [DOI] [PubMed] [Google Scholar]
- 6.Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT. et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? International journal of colorectal disease. 2012;27:1347–57. doi: 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- 7.Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K. et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. European journal of cancer (Oxford, England: 1990) 2009;45:1950–8. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 8.He WZ, Jiang C, Yin CX, Guo GF, Rong RM, Qiu HJ. et al. Prognostic model built on blood-based biomarkers in patients with metastatic colorectal cancer. Asian Pacific journal of cancer prevention: APJCP. 2014;15:7327–31. doi: 10.7314/apjcp.2014.15.17.7327. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Liao F, Huang Y, Jiang C, Liu S, He W. et al. Longterm effects of palliative local treatment of incurable metastatic lesions in colorectal cancer patients. Oncotarget. 2016;7:21034–45. doi: 10.18632/oncotarget.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Yin C, Guo G, Jiang C, Wang F, Qiu H. et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Medical oncology (Northwood, London, England) 2013;30:439. doi: 10.1007/s12032-012-0439-x. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Yin C, Liao F, Huang Y, He W, Jiang C. et al. Bevacizumab plus chemotherapy as third- or later-line therapy in patients with heavily treated metastatic colorectal cancer. OncoTargets and therapy. 2015;8:2407–13. doi: 10.2147/OTT.S88679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E. et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. The Lancet Oncology. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 14.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. The Lancet Oncology. 2010;11:1172–83. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 15.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1219–30. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 16.Yin C, Jiang C, Liao F, Rong Y, Cai X, Guo G. et al. Initial LDH level can predict the survival benefit from bevacizumab in the first-line setting in Chinese patients with metastatic colorectal cancer. OncoTargets and therapy. 2014;7:1415–22. doi: 10.2147/OTT.S64559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WHB. A Potential Administration-time Dependent Effect of Bevacizumab in Improving Overall Survival and Increasing Metastasis in Metastatic Colorectal Cancer. Chemotherapy Open Access; 2013. p. 02. [Google Scholar]
- 18.Yin C, Ma G, Rong Y, Kong P, Yang Q, Jiang C. et al. The Efficacy of Bevacizumab in Different Line Chemotherapy for Chinese Patients with Metastatic Colorectal Cancer. Journal of Cancer. 2016;7:1901–6. doi: 10.7150/jca.15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Azuma M, Lurje G, Gordon MA, Yang D, Pohl A. et al. Molecular predictors of combination targeted therapies (cetuximab, bevacizumab) in irinotecan-refractory colorectal cancer (BOND-2 study) Anticancer research. 2010;30:4209–17. [PubMed] [Google Scholar]
- 20.Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R. et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. European journal of cancer (Oxford, England: 1990) 2003;39:718–27. doi: 10.1016/s0959-8049(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 21.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS. et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Annals of oncology: official journal of the European Society for Medical Oncology. 2010;21(Suppl 5):v93–7. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LN, OuYang PY, Xiao WW, Yu X, You KY, Zeng ZF. et al. Elevated CA19-9 as the Most Significant Prognostic Factor in Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Medicine. 2015;94:e1793. doi: 10.1097/MD.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filella X, Molina R, Grau JJ, Pique JM, Garcia-Valdecasas JC, Astudillo E. et al. Prognostic value of CA 19.9 levels in colorectal cancer. Annals of surgery. 1992;216:55–9. doi: 10.1097/00000658-199207000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouri M, Pyrhonen S, Kuusela P. Elevated CA19-9 as the most significant prognostic factor in advanced colorectal carcinoma. Journal of surgical oncology. 1992;49:78–85. doi: 10.1002/jso.2930490204. [DOI] [PubMed] [Google Scholar]
- 26.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ. et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PloS one. 2014;9:e94928. doi: 10.1371/journal.pone.0094928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tampellini M, Ottone A, Alabiso I, Baratelli C, Forti L, Berruti A. et al. The prognostic role of baseline CEA and CA 19-9 values and their time-dependent variations in advanced colorectal cancer patients submitted to first-line therapy. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:1519–27. doi: 10.1007/s13277-014-2693-3. [DOI] [PubMed] [Google Scholar]
- 28.Hanke B, Riedel C, Lampert S, Happich K, Martus P, Parsch H. et al. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA) Annals of oncology: official journal of the European Society for Medical Oncology. 2001;12:221–6. doi: 10.1023/a:1008378412533. [DOI] [PubMed] [Google Scholar]
- 29.Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL. et al. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World journal of gastroenterology. 2001;7:431–4. doi: 10.3748/wjg.v7.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formica V, Massara MC, Portarena I, Fiaschetti V, Grenga I, Del Vecchio Blanco G. et al. Role of CA19.9 in predicting bevacizumab efficacy for metastatic colorectal cancer patients. Cancer biomarkers: section A of Disease markers. 2009;5:167–75. doi: 10.3233/CBM-2009-0101. [DOI] [PubMed] [Google Scholar]
- 31.Narita Y, Taniguchi H, Komori A, Nitta S, Yamaguchi K, Kondo C. et al. CA19-9 level as a prognostic and predictive factor of bevacizumab efficacy in metastatic colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Cancer chemotherapy and pharmacology. 2014;73:409–16. doi: 10.1007/s00280-013-2367-7. [DOI] [PubMed] [Google Scholar]
- 32.Petrioli R, Licchetta A, Roviello G, Pascucci A, Francini E, Bargagli G. et al. CEA and CA19.9 as early predictors of progression in advanced/metastatic colorectal cancer patients receiving oxaliplatin-based chemotherapy and bevacizumab. Cancer investigation. 2012;30:65–71. doi: 10.3109/07357907.2011.629380. [DOI] [PubMed] [Google Scholar]
- 33.Fiala O, Finek J, Buchler T, Matejka VM, Holubec L, Kulhankova J. et al. The Association of Serum Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, Thymidine Kinase, and Tissue Polypeptide Specific Antigen with Outcomes of Patients with Metastatic Colorectal Cancer Treated with Bevacizumab: a Retrospective Study. Targeted oncology. 2015;10:549–55. doi: 10.1007/s11523-015-0365-x. [DOI] [PubMed] [Google Scholar]
- 34.Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K. et al. Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagnostic pathology. 2010;5:22. doi: 10.1186/1746-1596-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danner BC, Didilis VN, Wiemeyer S, Stojanovic T, Kitz J, Emmert A. et al. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer research. 2010;30:1347–51. [PubMed] [Google Scholar]
- 36.Hong J, Yoon HH, Ahn HK, Sym SJ, Park J, Park PW. et al. Prognostic role of serum lactate dehydrogenase beyond initial diagnosis: a retrospective analysis of patients with diffuse large B cell lymphoma. Acta haematologica. 2013;130:305–11. doi: 10.1159/000353127. [DOI] [PubMed] [Google Scholar]
- 37.Jiang C, Hu WM, Liao FX, Yang Q, Chen P, Rong YM. et al. Elevated preoperative neutrophil-to-lymphocyte ratio is associated with poor prognosis in gastrointestinal stromal tumor patients. OncoTargets and therapy. 2016;9:877–83. doi: 10.2147/OTT.S90569. [DOI] [PMC free article] [PubMed] [Google Scholar]