Abstract
Background and objective
Dietary beetroot juice (BR) supplementation has been shown to reduce the oxygen (O2) consumption of standardized exercise and reduce resting blood pressure (BP) in healthy individuals. However, the physiological response of BR in chronic obstructive pulmonary disease (COPD) remains controversial. The objective was to test exercise performance in COPD, supplementing with higher doses of BR for a longer duration compared to previous trials in this patient group.
Methods
Fifteen COPD patients consumed concentrated BR (2×70 mL twice daily, each containing 300 mg nitrate) or placebo (PL) (2×70 mL twice daily, nitrate-negligible) in a randomized order for 6 consecutive days. On day 7, participants consumed either BR or PL 150 min before testing. BP was measured before completing 6-minute walk test (6MWT) and two trials of submaximal cycling. The protocol was repeated after a minimum washout of 7 days.
Results
Plasma nitrite concentration was higher in the BR condition compared to PL (P<0.01). There was no difference between the BR and PL conditions regarding the covered distance during the 6MWT (mean ± standard error of the mean: 515±35 m (BR) vs 520±38 m (PL), P=0.46), O2 consumption of submaximal exercise (trial 1 P=0.31 vs trial 2 P=0.20), physical activity level (P>0.05), or systolic BP (P=0.80). However, diastolic BP (DBP) was reduced after BR ingestion compared to baseline (mean difference: 4.6, 95% CI: 0.1–9.1, P<0.05).
Conclusion
Seven days of BR ingestion increased plasma nitrite concentrations and lowered DBP in COPD patients. However, BR did not increase functional walking capacity, O2 consumption during submaximal cycling, or physical activity level during the intervention period.
Keywords: nitric oxide, chronic obstructive pulmonary disease, exercise, nitrate, beetroot juice, blood pressure, nitrite
Introduction
Chronic obstructive pulmonary disease (COPD) patients often experience shortness of breath, which might limit their daily physical activity level (PAL) compared with healthy age-matched controls.1–3 These symptoms, along with mitochondrial dysfunction and reduced lung diffusion capacity, might contribute to a sedentary life. PA reductions may contribute to exhaustion, and in avoidance, PAL may further decrease.2,4–6
Beetroot contains high quantities of nitrate (NO3−).7 Dietary NO3− -supplementation has shown to improve efficiency of energy production per unit oxygen (O2), by limiting proton leakage in the respiratory chain, thus improving the mitochondrial respiration.8–10 Hypothetically, this will decrease exercise O2-consumption in COPD patients and improve PAL.
Nitric oxide (NO) mainly facilitates the effects on the mitochondrial respiration9 and is important for smooth muscle cell relaxation in the arterial walls,11 blood pressure (BP),12 and skeletal muscle contraction.13 NO is mainly synthesized endogenously through an ongoing process, which is dependent on nitric oxide synthase (NOS) and O2. However, NO can additionally be synthesized from an O2-and NOS-independent pathway.10
Exogenous dietary NO3− from beetroot juice (BR) is reduced to nitrite (NO2−) by facultative bacteria in the oral cavity, followed by NO formation by the acidic stomach environment.10 Hypoxemia and acidosis facilitate the NO3—NO2− –NO pathway, thereby complementing the classical pathway, during exercise.10 Ingestion of NO3− -rich vegetables will, therefore, contribute to an increased NO3− and NO2− concentration in the bloodstream.14,15
BR supplementation has shown to reduce O2 consumption during standardized exercise in healthy individuals,16–18 and increase exercise performance in COPD patients14,15 and peripheral arterial disease (PAD).19 Therefore, BR supplementation might improve exercise capacity in individuals with reduced O2 consumption.
Studies demonstrate an improved exercise performance following BR supplementation in COPD patients14,15 although lacking an optimal placebo (PL). Later, a study using ideal PL, but suboptimal dose, was not able to observe improvements in O2 consumption during exercise, walking performance, or BP.20
The purpose of this study is to evaluate exercise performance using the 6-minute walk test (6MWT), supplementing with higher doses of dietary NO3− as BR for 7 days; secondarily, to evaluate the O2 consumption of submaximal cycling, amendments in BP, and PAL.
Methods
Participants
Fifteen COPD patients completed the trial. Inclusion criterion was moderate-severe COPD (forced expiratory volume in 1 s [FEV1] <80% of predicted), in accordance with criteria of the American Thoracic Society.21 Exclusion criteria included smoking, failure to complete physical testing, ongoing participation in rehabilitation programs, pacemaker, or use of nicotine products, oxygen mask, beta blockers, antibacterial mouthwash, chewing gum, or stomach-neutralizing medicine during interventions. Participants were recruited from the local hospital, by advertising (http://sundhed.dk), and through telephone calls. All data were collected at the Section of Sport Science, Department of Public Health, Aarhus University. The study was approved by The Central Denmark Region Committees on Health Research Ethics (1–10-72-13-13) and complied with the Declaration of Helsinki. All participants received oral and written information about the study and gave written informed consent prior to inclusion. The study was registered at ClinicalTrials.gov on January 13th, 2017, with the identification number NCT03020862.
Previous trials in healthy individuals have observed significant effects on exercise capacity and physical responses during exercise in randomized controlled crossover trials including only nine individuals17 or eight individuals16 and using a dose three times lower per day than in the present trial. Nevertheless, we were aiming to achieve 15 completers because we expected that the variation in response might be greater in the COPD patients.
Design
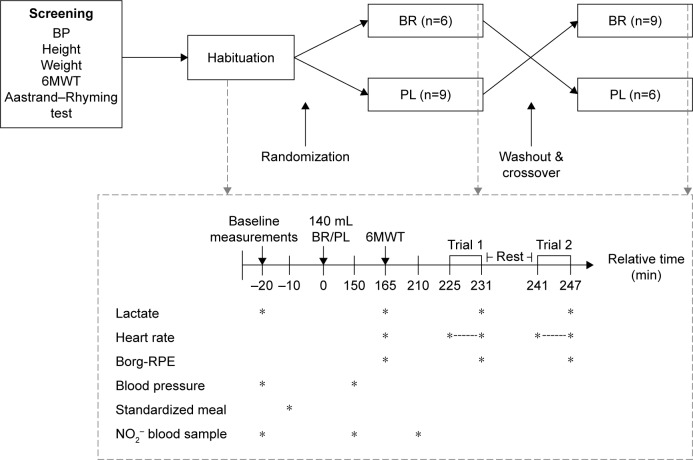
This randomized, double-blinded, PL-controlled, crossover study investigated the acute and accumulated effect of BR supplementation in COPD patients. The participants completed four visits: screening, habituation, and experimental days at the end of each intervention. They consumed 2×70 mL concentrated BR (Beet-it; James White Drinks, Ipswich, UK, 300 mg NO3−) or PL (Beet-it; James White Drinks; NO3− -negligible) each morning and night for six days. During this period, PAL was estimated by accelerometry. The experimental day, outlined in the following sections, was carried out on day 7 (Figure 1).
Figure 1.
Schematic overview of the study protocol (top) and the experimental protocol (bottom) completed during the habituation visit and at day 7 of each intervention period (indicated by dashed arrows).
Note: *indicates the time when the measurements were performed.
Abbreviations: BP, Blood Pressure; 6MWT, 6-minute walk test; BR, Beetroot juice; PL, Placebo; Borg-RPE, Borg scale for ratings of perceived exertion; NO2−, Nitrite.
Study procedure
Participants were randomly allocated to BR or PL using an unrestricted computer-generated sequence, only available to laboratory technicians not involved in the project, except for packaging of the products. PL was an organic NO3− -depleted BR, similar in taste and appearance.
To standardize the NO3− intake, participants were instructed to avoid variation in habitual diet, limit intake of NO3− -rich vegetables, and avoid alcohol and strenuous activities for 24 hours and caffeine for 12 hours before testing. Participants arrived in the morning after overnight fasting and consumed a standardized meal, according to 20% of their individual estimated daily energy requirement.22
At screening, height, weight, and BP were measured, and the participants completed a 6MWT and Aastrand–Rhyming submaximal cycle test (Figure 1).23
Participants were familiarized with the equipment at the habituation visit. Upon arrival, BP was measured after 10 min supine rest (Monitor: BP A100 Plus; Microlife, Taipei, Taiwan) and a blood sample was collected. Afterwards, 6MWT was performed followed by submaximal cycling. Immediately following the physical tests blood lactate was analyzed (1500 SPORT Lactate Analyzer; YSI Life Sciences, Yellow Springs, OH, USA).
At day 7 of the interventions, the participants arrived in the morning after overnight fasting. Details regarding specific tests and analysis are outlined below, but the experimental day consisted of BP measurements (baseline values were measured on the morning of the PL period), blood sampling, and baseline blood lactate measurements (fingerprick test). The participants consumed a standardized meal and 2×70 mL of BR/PL. After 2½ hours, another blood sample was collected and BP was measured. Participants performed a 6MWT followed by blood lactate collections and reported a value on the Borg scale for ratings of perceived exertion (Borg-RPE). After rest (30 min), another blood sample was collected followed by completion of submaximal cycling, blood lactate, and Borg-RPE collection (Figure 1).
Measurements
The 6MWT was conducted in accordance with established guidelines.24 Considering a possible learning effect, the test was performed at screening and habituation visit.24,25
The 2×6 min standardized submaximal cycling test with a constant load (60 rpm) and a 10 min break between trials was performed at each experimental day. Respiratory measurements (AMIS 2001; Innovision, Odense, Denmark) and heart rate (HR) (POLAR RS800CX) were continuously measured. Before testing, the analyzer was calibrated using known O2 concentrations and CO2 concentrations. The same cycle ergometer (Monark 894E) was used for all tests and the submaximal load was equivalent to 50% of the estimated Wattmax. Ending each trial, HR, lactate concentration, respiratory measures, and Borg-RPE were registered. Mean VO2 values in the last 2 min were considered to reflect steady state and were used for analysis of the effect between interventions in addition to analysis for differences over the entire cycling period of each trial.
The Aastrand–Rhyming test was conducted to ensure participants’ ability to cycle continuously for 10 min and to estimate Wattmax,23 which was used for submaximal cycling at the intervention. The workload on the Aastrand–Rhyming test was adjusted so that the participants could cycle for 6 min at an HR between 105 and 115 bpm followed by 4 min with an HR between 125 and 140 bpm. HR and workload were registered every minute until steady-state HR (varied <3–5 bpm). Following the test, HRmax (220 bpm–age) and Wattmax were calculated.23
Daily PAL was monitored using accelerometry (ActiGraph wGT3X-BT) during waking hours (except water activities), on the right hip all 6 intervention days, except on experimental days. The accelerometer was programmed (ActiLife Analysis Software, Pensacola, FL, USA) to store data at 80 Hz with 10 s epoch. Before analysis, data between retiring to bed and wakeup time were removed, as this was expected to be non-awake time. When participants went to bed past midnight, the activity was included in the calculations for the previous day. To estimate intensity and duration of PA, established cutoffs for accelerometers were used.26 Data were considered valid when wear time was >10 h/day for ≥4 days for each intervention.
To determine plasma NO2− concentration, ~4 mL venous blood was collected from the bilateral antecubital vein in 8 mL lithium-heparin vacutainers (ref 367378; Becton, Dickinson and Co, Franklin Lakes, NJ, USA). Samples were centrifuged at 4,000 rpm at 4°C for 10 min and plasma was frozen at −80°C until further analysis.
NO2− concentrations were determined using chemiluminescence. Blood plasma was injected into the reaction vessel and NO3− was reduced by I3−-reagent to gaseous NO and detected by Sievers NO analyzer (NOA, 280i; GE Analytical Instruments, Boulder, CO, USA) using helium as a carrier gas. Calibration was performed using known NO3− amounts according to the manufacturer’s manual.
Statistical analyses
Statistical analyses were performed in STATA 12.1. Data for 6MWT (meters, lactate, and HR), PAL in each activity zone (% of total time), and COPD Assessment Test, and data from cycling trials (VO2 steady state, lactate, HR, and Borg-RPE) were analyzed using two-tailed paired t-tests. Data for NO2− and VO2 of the submaximal cycling trials (all data points) and BP were analyzed using a two-way repeated-measurements analysis of variance. GraphPad Prism version 6.02 was used for figures. Statistical significance was accepted at P<0.05. Results are presented as mean ± standard error of the mean unless stated otherwise.
Results
Nineteen patients were included and randomized. Of these, 15 COPD patients completed the interventions between December 2013 and April 2014. The four patients who did not complete dropped out for reasons not related to the intervention beverages. The flow of participants is illustrated in Figure 2. Participants reported to comply with supplementation, although two reported nausea after ingestion of both PL (n=2) and BR (n=2), but still completed the intervention and tests. Baseline characteristics of the participants are presented in Table 1.
Figure 2.
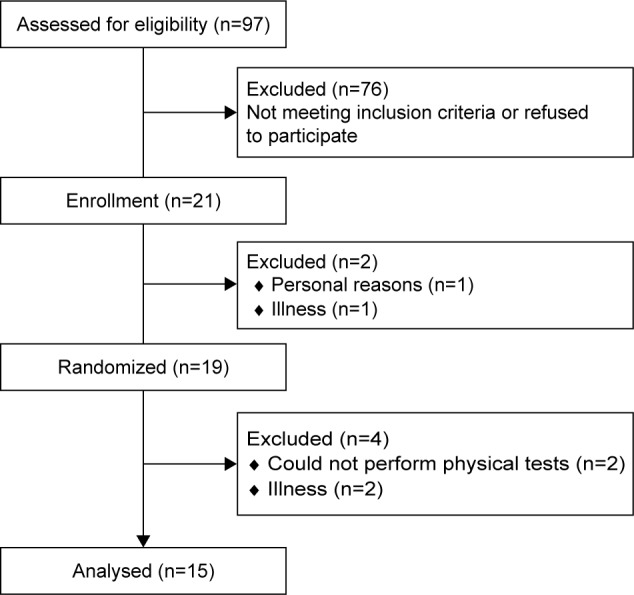
Flow of participants including enrollment, randomization, and completion.
Table 1.
Baseline characteristics of participants
| Characteristics | Mean ± SD | Range |
|---|---|---|
| Male gender, n (m/f) | 9/6 | |
| Age (years) | 63±13 | (27–77) |
| Height (cm) | 171.8±6.4 | (163–182.5) |
| Weight (kg) | 75.8±17.3 | (51–112.6) |
| BMI (kg/m2) | 25.5±4.8 | (19.2–34.8) |
| FEV1 (liters) | 1.4±0.7 | (0.44–2.81) |
| FEV1 (% predicted) | 44.7±15.1 | (22–76) |
| FVC (liters) | 2.7±0.7 | (1.37–4.25) |
| FVC (% predicted) | 74.2±14.7 | (49–97) |
| FEV1/FVC (%) | 0.61±0.19 | (0.31–1.05) |
| MRC score | 2.47±0.74 | (1–4) |
| CAT score (n=14) | 16.4±5.11 | (7–22) |
Note: Data are presented as mean ± SD and with data range (n=15).
Abbreviations: BMI, body mass index; CAT, COPD Assessment Test; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MRC, Medical Research Council Scale; m/f, male/female.
Plasma nitrite concentration
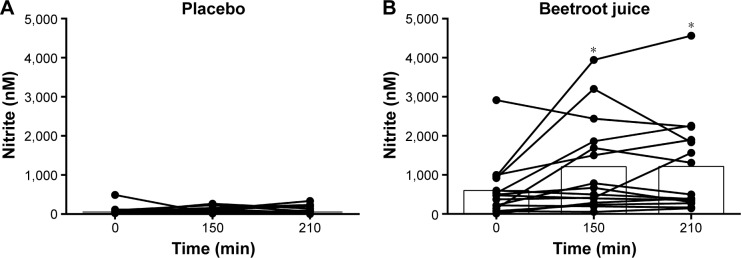
NO2− was significantly higher in the BR condition compared to PL (BR: 538.5±186.6 nM; PL: 140.0±68.9 nM, P<0.01) at the end of the intervention. After PL ingestion, no change in NO2− -concentration was detected (150 min: 193.4±95.4 nM; 210 min: 192.0±124.6 nM, time and interaction P>0.05). However, NO2− concentration increased after BR ingestion during the experimental day, and was significantly higher, 150 min (1,118.4±317.0 nM, P<0.01) and 210 min (1,099.2±318.3 nM, P<0.01), compared to before BR ingestion at day 7 (Figure 3).
Figure 3.
Individual and mean plasma nitrite (nM) at the experimental day at arrival (0 min), 150 and 210 min after ingestion of either (A) placebo (PL) or (B) beetroot juice (BR). Nitrite was significantly higher in the BR period compared to PL (P<0.01), and nitrite increased after ingestion of BR, but not after PL (time P<0.01, interaction P<0.05).
Note: *P<0.01, nitrite higher at 150 and 210 min after ingestion of BR compared to arrival (0 min).
6MWT
All participants (n=15) completed the 6MWT during both interventions. Three participants expressed discomfort during the test. There was no difference between conditions in the covered distance (BR: 515±35 m vs PL: 520±38 m, P=0.46; Figure 4). There was no difference in HR (BR: 119±6 bpm vs PL: 119±5 bpm, P=0.86), lactate concentration (BR: 2.20±0.31 mmol L−1 vs PL: 2.54±0.44 mmol L−1, P=0.16), or Borg-RPE (BR: 14±1 score vs PL: 15±1 score, P=2.20) between conditions.
Figure 4.
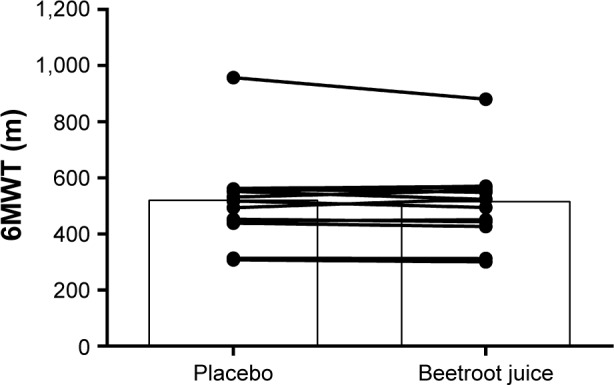
Individual and mean distance covered in meters during 6MWT.
Note: Data are presented as mean ± standard error of the mean (n=15).
Abbreviation: 6MWT, 6-minute walk test.
Oxygen consumption during submaximal cycling
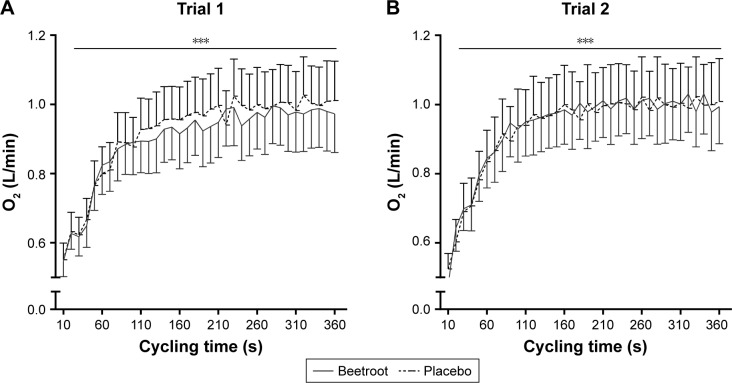
O2 consumption during submaximal cycling is illustrated in Figure 5. Two participants were unable to complete both trials because of discomfort and were excluded from further analysis, resulting in a total of 13 completing the test. O2 consumption increased during each trial (time P<0.001; Figure 5), but was not influenced by treatment (interaction trial 1 P=0.31 vs trial 2 P=0.20).
Figure 5.
Oxygen consumption during submaximal cycling (entire period).
Notes: Data are presented as mean ± standard error of the mean (n=13). (A) Trial 1 (interaction P=0.31; time P<0.001) and (B) trial 2 after 10 min break (interaction P=0.20; time P<0.001). ***indicates a significant increase in oxygen consumption during the trial (P<0.001).
Mean oxygen consumption (steady-state period)
When comparing conditions for both trials, there was no difference between trials (trial 1: BR: 0.96±0.12 L/min, PL: 1.01±0.12 L/min, P=0.23 vs trial 2: BR: 0.99±0.12 L/min, PL: 1.02±0.12, P=0.37). There was no difference between trials (P=0.25), no difference as a consequence of treatment (P=0.27), and no significant interaction between trial and treatment (P=0.33).
Secondary outcomes during submaximal cycling
Lactate concentration during cycling did not differ between conditions for trials (trial 1: BR: 2.37±0.29 mmol/L, PL: 2.07±0.31 mmol/L, P=0.10, n=9 paired data vs trial 2: BR: 1.98±0.38 mmol/L, PL: 1.66±0.28 mmol/L, P=0.15, n=6 paired data). HR during cycling did not differ between trials for conditions (trial 1: BR: 112±4 bpm, PL: 114±5 bpm, P=0.54, n=13 paired data vs trial 2: BR: 115±5 bpm, PL: 116±4 bpm, P=0.63, n=13 paired data). No difference in Borg-RPE was observed during trial 1 (BR: 14±1 vs PL: 13±1, P=0.40, n=15 paired data). Trial 2 Borg-RPE was significantly higher during BR compared to PL (BR: 14±1 vs PL: 13±1, P<0.05, n=13 paired data).
Physical activity level
Data from four participants were excluded because of missing data. Thus, a total of 11 participants were included in the analysis. No difference between time spent in the three PALs was observed for conditions (sedentary: BR: 85%±2% vs PL: 84%±2%, P=0.32, light: BR: 13%±2% vs PL: 14%±2%, P=0.18, moderate-high intensity: BR: 2.0%±0.0% vs PL: 2.0%±0.0%, P=0.85). Mean wear time did not differ between conditions (BR: 15.1±0.4 h vs PL: 14.8±0.3 h, P=0.19), and there was no difference between the number of days data were collected (BR: 5.6±0.2 days vs PL: 5.8±0.2 days, P=0.51).
Blood pressure
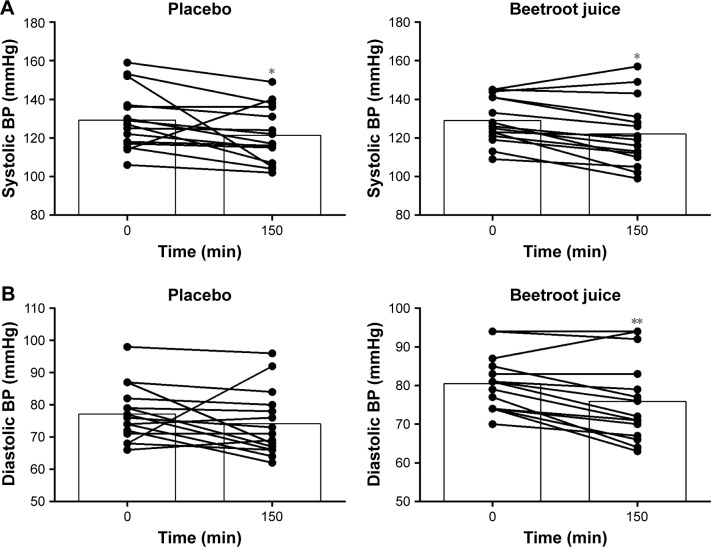
Measures of systolic BP (SBP) and diastolic BP (DBP) for all participants (n=15) are included in the BP analysis. SBP was significantly lower 150 min after PL ingestion compared to arrival at the lab (0 min: 129±4 mmHg and 150 min: 121±4 mmHg, P<0.05). SBP was reduced 150 min after BR ingestion (0 min: 129±3 mmHg and 150 min: 122±4 mmHg, P<0.05). The reduction in SBP was not influenced by treatment (interaction P=0.80; Figure 6).
Figure 6.
Individual and mean response in (A) systolic blood pressure (mmHg) and (B) diastolic blood pressure (mmHg) (n=15).
Notes: *P<0.01 denotes difference between baseline and 150 min after ingestion of both placebo and BR. **P<0.05 denotes difference between baseline and 150 min after ingestion of BR.
Abbreviations: BP, blood pressure; BR, beetroot juice.
DBP was significantly lower 150 min after ingestion of beverages compared to arrival at the lab (time P=0.03). The reduction was significant after BR ingestion compared to baseline (mean difference: −4.6, 95% CI: −9.1–(−0.1), P<0.05), but not after PL ingestion (mean difference: −3.1, 95% CI: −7.6–1.4, P=0.24; Figure 6). No significant interaction was observed (P=0.56; Figure 6).
Discussion
BR intake (2×140 mL/day) for 7 days, and just before testing, did not increase functional capacity and PAL or reduce O2 consumption of submaximal cycling. However, DBP was reduced 150 min following BR ingestion compared to baseline.
BR contains NO3−, which after conversion to NO2− should introduce physiological beneficial effects in COPD patients. We did not directly measure NO3− concentration in the batch, but based on prior studies,15,27 the content of 2×70 mL aliquots is ~0.6 mg (7.6–9.6 mmol). As expected, plasma NO2− concentration significantly increased following BR administration, although with large variations between participants. Thus, the concern regarding the age-related changes in gastrointestinal function and facultative bacteria utility, potentially impairing the formation of NO2−, remains unresolved. Plasma NO2− concentration following BR supplementation was similar to the findings in PAD patients19 and COPD patients14,15 using similar doses. Using smaller dosing regimens, the plasma NO2− concentration was additionally comparable to that in active young men, indicating that COPD patients might have an impaired capacity for the conversion of dietary ingested NO3− to NO2− compared to healthy individuals.13,28–31
To our knowledge, participants in the present study ingested the highest dose used in studies including COPD patients. This protocol was chosen because an accumulated effect of BR in reducing the O2 consumption of submaximal exercise of healthy individuals has been observed.16,18,28 Heterogeneous plasma NO2− responses to BR supplementation were observed in the present study, which may reflect the heterogeneous nature of COPD patients. Our results, showing no significant effect of BR on PAL, walking performance, and O2 consumption may, therefore, partly be explained by non-responders (six of 15 reached NO2− levels >1,000 nM). Our study was not statistically powered to perform subanalysis, merely including patients having a marked increase in plasma NO2−. Hence, future investigations should try identifying specific COPD phenotypes obtaining high plasma NO2− levels after BR ingestion and who, with higher probability, could benefit physiologically. In addition, the trial was not designed for performing stratified analyses depending on degree of COPD. Nevertheless, we repeated the statistical analysis for the primary outcome parameter 6MWT separately, based on data from the group of patients with an FEV1 <50% (n=5) and in the group of patients with an FEV1 >50%. The walking distance was not different between the two stratified groups of COPD patients. Furthermore, no significant effect of BR was observed in either of the groups.
Dietary NO3− has shown to optimize mitochondria efficiency and blood flow in young healthy participants.9,32 BR reduced O2 consumption in healthy participants by 10% and 19% during submaximal exercise.16,18 To the contrary, we did not observe reduced O2 consumption during submaximal exercise even though we used equal or higher doses and longer supplementation periods.16,18 The lacking physiological effects were underlined by significantly higher Borg-RPE ratings during BR supplementation. Our findings are supported in a recent study20 investigating the effect of BR in COPD, which failed to detect a significant O2 reduction during submaximal cycling. Thus, we cannot exclude that age/disease-related causes might reduce the responsiveness to BR supplementation.
COPD patients are susceptible to weather factors, which are conveyed as impaired respiration, potentially impacting cycling and 6MWT considerably. Two participants failed to complete cycling because of respiration problems, during the PL condition. The susceptibilities might attenuate measurability of the effect of BR supplementation in COPD, and our study might be statistically underpowered because of greater day-to-day variation in this group compared to healthy individuals.
Distance covered in the 6MWT and the secondary outcomes were not changed by BR ingestion, although an improved maximal walking performance in PAD patients following acute NO3− supplementation has been observed.19 Nevertheless, similar to our observation, no change in the 6MWT compared to PL has been reported in COPD20 and in older healthy adults;33 which was attributed to a compromised ability of older individuals to perform maximally.33 Although lactate and HR following 6MWT were enhanced compared to rest in the present study, the increases were minor and not different than after cycling. These observations are questionable as to whether the 6MWT reflects maximal performance. The reduced ability to perform maximally may partly be caused by other health-related challenges, as three of 15 participants reported having knee pains unrelated to COPD. In support, a negative correlation between pain severity and 6MWT distance has been reported.4
When testing COPD patients on a shuttle walk test (SWT) following an acute consumption of BR, an 11% increase in walking distance was observed (P=0.005).14 However, these results remain questionable, as a true PL was lacking and the energy content of the drinks was unequal.14 Conversely, following a 3-day BR supplementation and using a lower BR dose, Leong et al34 failed to observe a significant increase in walking distance or time-to-fatigue, thereby questioning the superiority of the SWT compared to the 6MWT in this patient group.
PAL measured in the present study corresponded with the previous measurements in COPD patients.35 Previous studies show that the effect of NO3− supplementation, if continuously supplemented, is maintained for 6–15 days in healthy individuals.16,18,28 Therefore, we hypothesized that COPD patients would increase PAL in the BR period, and unfortunately this was not observed. This is not surprising, as O2 consumption during cycling was not reduced, and we cannot exclude that PAL is primarily based on scheduled habitual activity plans instead of small variations in physical capacity. The accelerometer might not be sensitive in detecting small changes in this patient/age group, partly explaining the lack of effect on PAL.
BR supplementation reduced DBP compared to baseline, which was not observed in the PL condition. This observation agrees with the observations in healthy older adults,33 PAD patients,19 and COPD patients.14,15,35 In contrast to these findings,14,15,33 we did not observe a reduction in SBP following NO3− supplementation. Another study in COPD failed to show a reduction in both SBP and DBP,20 which may have been due to a lower BR dose compared with the present and previous studies in COPD patients.14,15
Consistent with the previous findings, we observed large variations in response to BR supplementation in COPD patients14,15,27 compared to healthy controls.31 We included a small heterogeneous COPD group, which potentially limits the statistical power for detecting small, but relevant, differences. The included group largely reflects the nature of COPD patients, adding to the external validity of the results. However, future research in this area should try to identify specific COPD phenotypes and include a larger and more homogeneous group, with respect to age, body mass index, and severity of their COPD, thus increasing the internal validity.
Submaximal cycling was an uncommon activity for the majority of the COPD patients. Although the habituation was completed to eliminate a learning effect, the participants did not become familiar with cycling, thus potentially affecting respiration during testing. Taking the learning effect into consideration, performing two 6MWT, and selecting the best distance, thus omitting submaximal cycling, might be a more suitable approach in future research on physical performance in this patient group.
Conclusion
BR supplementation increased plasma NO2− concentration and lowered DBP in moderate-severe COPD patients. However, BR supplementation two times/day for 7 days did not increase functional walking capacity or PAL during the intervention. Submaximal cycling O2 consumption was not significantly influenced by BR supplementation. Further studies are warranted to clarify the therapeutic role of BR supplementation in COPD patients.
Acknowledgments
The authors would like to thank all of the study participants for their time, dedication, and enthusiasm. In addition, we thank the employees at the Department of Respiratory Diseases and Allergy, Aarhus University Hospital for their help with recruitment of participants and Professor, MD, Thorsten Ingemann Hansen from the Section of Sport Science, Department of Public Health, Aarhus University, for his medical expertise. We would also like to acknowledge the laboratory technicians Janni Mosgaard Jensen and Gitte Kaiser Hartvigsen for their ineffable expertise and help during laboratory work and data collection and Ulf Simonsen who analyzed the serum samples for NO2−. Additionally, we would like to thank Thomas Maribo for his guidance in choosing relevant physiological tests for the trial. The trial is funded by Krista and Viggos Foundation (DKK 35,000).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arne M, Janson C, Janson S, et al. Physical activity and quality of life in subjects with chronic disease: chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scand J Prim Health Care. 2009;27(3):141–147. doi: 10.1080/02813430902808643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 3.Schönhofer B, Ardes P, Geibel M, Köhler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10(12):2814–2819. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- 4.Hajghanbari B, Jayne Garland S, Road JD, Darlene Reid W. Pain and physical performance in people with COPD. Respir Med. 2013;107(11):1692–1699. doi: 10.1016/j.rmed.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Hajghanbari B. Limitations in Physical Performance in People with Chronic Obstructive Pulmonary Disease [Thesis] Iran: Tarbiat Modares University; 2013. [Google Scholar]
- 6.Hartman JE, Boezen HM, de Greef MH, Bossenbroek L, ten Hacken NH. Consequences of physical inactivity in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2010;4(6):735–745. doi: 10.1586/ers.10.76. [DOI] [PubMed] [Google Scholar]
- 7.Santamaria P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J Sci Food Agric. 2006;86(1):10–17. [Google Scholar]
- 8.Clerc P, Rigoulet M, Leverve X, Fontaine E. Nitric oxide increases oxidative phosphorylation efficiency. J Bioenerg Biomembr. 2007;39(2):158–166. doi: 10.1007/s10863-007-9074-1. [DOI] [PubMed] [Google Scholar]
- 9.Larsen FJ, Schiffer TA, Borniquel S, et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 11.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jajja A, Sutyarjoko A, Lara J, et al. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr Res. 2014;34(10):868–875. doi: 10.1016/j.nutres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Bailey SJ, Fulford J, Vanhatalo A, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109(1):135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 14.Kerley CP, Cahill K, Bolger K, et al. Dietary nitrate supplementation in COPD: An acute, double-blind, randomized, placebo-controlled, crossover trial. Nitric Oxide. 2015;44:105–111. doi: 10.1016/j.niox.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Berry MJ, Justus NW, Hauser JI, et al. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 17.Lansley KE, Winyard PG, Fulford J, et al. Dietary nitrate supplementation reduces the O 2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110(3):591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 18.Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 19.Kenjale AA, Ham KL, Stabler T, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd AI, Wilkerson DP, Dobson L, et al. The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: A double blind placebo controlled, randomised control trial. Nitric Oxide. 2015;48:31–37. doi: 10.1016/j.niox.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 22.Nordic Council of Ministers . Nordic Nutrition Recommendations 2012. 2014. [Google Scholar]
- 23.Åstrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during submaximal work. J Appl Physiol. 1954;7(2):218–221. doi: 10.1152/jappl.1954.7.2.218. [DOI] [PubMed] [Google Scholar]
- 24.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 26.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Leong P, Basham JE, Yong T, et al. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:52. doi: 10.1186/s12890-015-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansley KE, Winyard PG, Fulford J, et al. Dietary nitrate supplementation reduces the O 2 cost of walking and running: a placebo-controlled study. 2011;110(3):591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 29.Lansley KE, Winyard PG, Bailey SJ, et al. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011;43(6):1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 30.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc. 2014;46(1):143–150. doi: 10.1249/MSS.0b013e3182a1dc51. [DOI] [PubMed] [Google Scholar]
- 31.Wylie LJ, Kelly J, Bailey SJ, et al. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol. 2013;115(3):325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 32.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81(1):209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 33.Kelly J, Fulford J, Vanhatalo A, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 34.Leong P, Basham JE, Yong T, et al. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:52. doi: 10.1186/s12890-015-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis KJ, O’Brien KA, Tanner RJ, et al. Acute dietary nitrate supplementation and exercise performance in COPD: a double-blind, placebo-controlled, randomised controlled pilot study. PLoS One. 2015;10(12):e0144504. doi: 10.1371/journal.pone.0144504. [DOI] [PMC free article] [PubMed] [Google Scholar]