Abstract
Objective
To determine the influence of the sonic hedgehog (shh) pathway and its receptor smoothened (smo), on the survival and functionality of dopaminergic (DA) neurons.
Background
During early development, shh induces the differentiation of DA neurons. However, it is unknown whether shh signaling is required in the maturation or maintenance of DA neurons during later development and adulthood due to the lethality of traditional shh knockout models.
Methods
We utilized the cre-loxP system to achieve late developmental stage and cell type-specific deletion of the shh receptor, smo, in DA neurons by crossing DATcre (dopamine transporter) mice with SmoloxP/loxP mice. We assessed for differences between knockout (ko) and wildtype (wt) mice using combined histochemistry, gene expression analysis, and behavioral evaluation. Number and size of DA neurons in ventral midbrain and the DA neural terminal density in striatum were measured using unbiased stereological quantification. The survival of DA neurons under neurotoxin challenge was examined in the unilateral 6-hydroxydopamine (6-OHDA) Parkinson's disease animal model and the more subtle function under challenge of the dopaminergic system was examined by methamphetamine single- and repeated challenge in wt and ko mice.
Results
Tyrosine hydroxylase (TH) positive neuronal counts and neuronal size in substantia nigra (SN) and ventral tegmental area (VTA) showed no difference between wt and DAT-Smo ko mice in young (5 months) or aged (22 months) mice. There was also no difference in the striatal DA projections between wt and ko mice in both age groups. In unilateral striatal 6-OHDA lesions modeling Parkinson's disease, using stereotaxic injection of 6-OHDA intrastriatally led to loss of dopaminergic neurons in SN and diminished TH positive projections in striatum. However, there was no differences in survival of DA neurons between wt and ko mice.
DAT-Smo ko mice demonstrated hyperactivity compared to wt mice at 5 months, but showed no difference in activity at 22 months. When injected with a one-time bolus of methamphetamine (METH), despite the higher basal locomotion activity, DAT-Smo ko mice showed a diminished response to a single METH challenge. In METH sensitization testing, ko mice showed decreased sensitization compared to wt mice without evidence of a delayed shift in dynamics of sensitization. Gene expression analysis showed decreased gene expression of smo, Gli 1 (known target gene of smo) and BDNF (brain-derived neurotrophic factor) in the SN. Gene expression was not altered in striatum for the genes examined in this study including dopamine receptor genes, neurotropic genes such as Glial cell line-derived neurotrophic factor (GDNF), and bone morphogenetic protein 7 (BMP7).
Conclusion
Our study showed the smo receptor function is not required for the maturation and survival of DA neurons during late development, aging or under stress challenge. However, smo function has an influence on behavior in young adult mice and in responses of mice to a drug that modulates DA neurochemistry through regulation of gene expression in DA neurons. Since young adult DAT-smo ko mice show hyperactivity and altered response to a psychostimulant drug (METH), this may indicate the involvement of the shh pathway in the development of functional changes that manifest as alterations in DA pathway dynamics.
Keywords: dopaminergic neurons, sonic hedgehog, smoothened, Parkinson's disease, 6-OHDA
Introduction
During development, the sonic hedgehog (shh) signaling pathway regulates the early induction and expansion of progenitors in the ventral forebrain, midbrain, and midbrain/hindbrain boundary (MHB) (Blaess et al., 2006; Fuccillo et al., 2004; Hynes et al., 1995; Sousa and Fishell, 2010). Ventral expression of shh along the neural tube in combination with the FGF8, which is produced at the MHB, define the induction of dopaminergic fate in ventral midbrain dopaminergic progenitor cells (Ye et al., 1998). Besides its role during development and early induction of dopaminergic progenitor cells, shh has been implicated in the maturation and survival of dopaminergic neurons in vitro. Shh is commonly used to promote the numbers of DA progenitors in cultures (Kim et al., 2003; Wang et al., 1995; Wu et al., 2012). Treatment with transforming growth factor-beta (TGF-β) and shh significantly increased the number and survival of tyrosine hydroxylase (TH)-immunoreactive neurons in embryonic day 14 (E14) primary midbrain dopaminergic neuronal cultures (Roussa et al., 2004). However, whether shh signaling pathway is also required in the maturation of dopaminergic neurons during later developmental stages is unkown due to the lethality of traditional shh knockout in mice. A neurotrophic effect of shh has been suggested both in vitro and in vivo. To determine unambiguously whether shh signaling mediated by its receptor Patch/smoothened (smo) in DA neurons is critical for the maturation of DA neurons during later developmental stages and the survival of DA neurons in adulthood during aging, we generated a dopaminergic neuronal specific conditional knockout model for the smo gene (DAT-Smo-KO). Smo gene was inactivated in DAT expression neurons starting from E16.5, allowing us to examine the maturation and survival of DA neurons without affecting the initial induction of DA progenitors. In addition, prior studies have shown that the shh signaling pathway might be involved in the protection of DA neurons under neurotoxic challenges. Miao et al showed that shh is neurotrophic in ventral midbrain (VM) dopaminergic cultures (Miao et al., 1997). In the presence of 1-Methyl-4-phenylpyridinium ion (MPP+), a dopaminergic neuron specific neurotoxin, shh prevented dopaminergic neuron death. Injection of shh peptide in the DA neuronal terminals in striatum also increased the survival of DA neurons in response to the neurotoxin 6-hydroxydopamine (6-OHDA) used to model Parkinson's Disease (Tsuboi and Shults, 2002). Whether these trophic and protective effects of shh are mediated by direct protection of DA neurons through the shh receptor smo-mediated molecular pathway is unknown. Therefore, in this study we examined the survival of DA neurons under the challenge of 6-OHDA to determine whether the endogenous shh-smo signaling pathway is critical for the survival of DA neurons under such challenges. Additional functional alterations in DAT-Smo ko mice reported in this study suggest a link of adult shh signaling in regulation of animal behaviors and their response to psychostimulant drugs.
Methods
Animal Models
All animal protocols were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. The mice were housed in the animal facility of Case Western Reserve University on a 12-h light/dark diurnal cycle. Food was provided ad libitum. For the conditional inactivation of smo gene in dopaminergic neurons, a transgenic line, Slc6a3Cre, in which Cre recombinase is driven by the DA transporter promoter starting at about embryonic day 16, was used (Backman et al., 2006). To minimize interference with gene function by preservation of both alleles, Cre recombinase expression was driven from the 3′ untranslated region (3′ UTR) of the endogenous DAT gene by means of an IRES sequence. Dopaminergic neuronal specific Smo knockout (ko) mice (DAT-Smo ko, Fig 1A) were generated by crossing SmoloxP/loxP mice (Long et al., 2001) with Slc6a3Cre knock-in mice to obtain DATcre(wt/+)/Smo loxP/loxP (DAT-Smo ko mice) or DATcre (+/-)/wt/wt (DAT Smo wildtype (wt) mice. The DAT-IRES-Cre gene was genotyped using primers (5′ TGG CTG TTG GTG TAA AGT GG -3′; 5′ GGA CAG GGA CAT GGT TGA CT -3′; 5′ CCA AAA GAC GGC AAT ATG GT -3′). Smo floxed alles were genotyped by the primer set (5′ GGC CTG CGC TGC TCA ACA TGG-3′; 5′ GCA ACT GCA GCA GCC TCC GG-3′). Olfactory bulb, hippocampus, cortex, ventral tegmental area (VTA), cerebellum, striatum and substantia nigra (SN) tissue were punched out using the Paxinos atlas, as we have previously described (Filichia et al., 2015), and DNA was extracted for PCR analysis. Deletion of the smo gene results in amplification of a ΔloxP band, using a set of primers flanking the floxed regeion (5′ GGC CTG CGC TGC TCA ACA TGG-3′ and 5′ GAGCTTGGGTCACGTGTCCGGCAG-3′. This primer set expand a 2000bp sequence in the loxP flanked conditioned allele and generates a 600bp PCR product only when gene deletion occurs (Fig 1B).
Figure 1. Generation of dopaminergic neuron-specific smo ko mice.
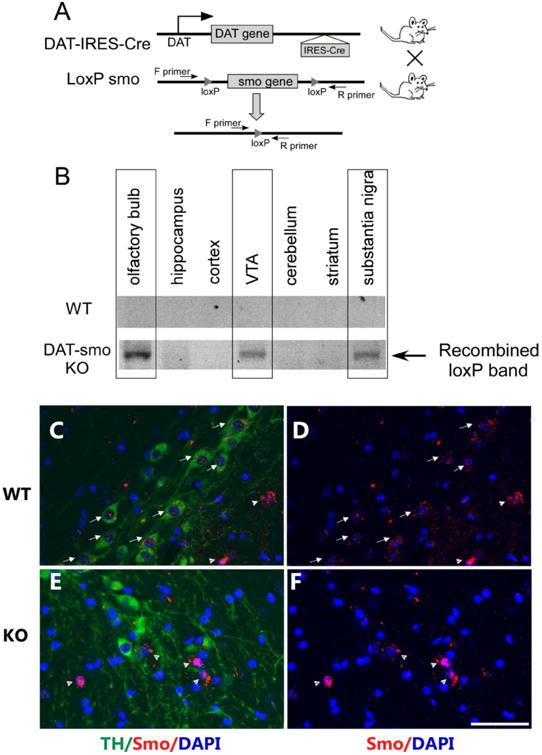
A. Strategy for dopaminergic neuronal specific deletion of the smo gene in DAT-Smo ko mice. B. Detection of recombined alleles (indicating deletion of smo gene) in olfactory bulb, substantia nigra and the ventral tegmental area but not in hippocampus, cortex, cerebellum or striatum of ko mice (lower panel). There is no recombination in different brain areas in wt mice (upper panel). (C) Double immunostaining for TH (green) and Smo (red) shows expression of both Smo protein in TH positive cells (arrow) and TH negative cells (arrow head) in wt mice. (D) shows Smo immunostaining only. However, in ko mice (E) and (F), smo expression is not detected in TH positive cells but is detected in TH negative cells (arrow head). Scale bar= 50um.
Immunostaining
Animals were anesthetized and perfused transcardially with saline followed by 4% PFA in phosphate buffer (PB; 0.1 M; pH 7.2) at different ages or after different treatments. The brains were removed, dissected, postfixed in PFA for 16 hours, and transferred to 20% and 30% sucrose in 0.1 M PB sequentially. Serial sections of the entire brain were sliced at 30 or 40 μm thickness in a cryostat. One series from every 4th section was stained for each antibody used. In order to control for staining variability, specimens from all experimental groups were included in every batch and reacted together in a net well tray under the same conditions. Sections were rinsed in 0.1M PB, and blocked with 4% bovine serum albumin (BSA) and 0.3% Triton x-100 in 0.1M PB. Sections were then incubated in a primary antibody solution of rabbit anti-TH (1:1000, Chemicon, Temecula, CA) diluted in 4% BSA and 0.3% Triton x-100 in 0.1M PB for 24 hours at 4°C. Sections were rinsed in 0.1M PB and incubated in biotinylated goat anti-rabbit IgG for TH primary antibody in the buffer (1:200; Vector Laboratories, Burlingame CA) for 1 hour, followed by incubation for 1 hour with avidin-biotin-horseradish peroxidase complex. Staining was developed with 2,3′ diaminobenzidine tetrahydrochloride (0.5 mg/mL in 50 mM Tris-HCl buffer 7.4). Control sections were incubated without primary antibody. Sections were mounted on slides, and cover slipped. Histological images were acquired using an Infinity3 camera and NIKON 80i microscope. TH immunoreactivity in striatum was visualized with the use of a Nikon super-coolscan 9000 scanner. The optical density of TH immunoreactivity in striatum was analyzed using Scion Image and averaged from 3 sections with a visualized anterior commissure (AP: +0.26 mm, +0.14 mm, +0.02 mm to bregma), as previously described (Luo et al., 2010). Observers, who were blinded to the experimental groups, performed all immunohistochemical measurements. Slight variations in background staining were corrected by subtracting background density of cortical regions from striatal density measurements.
For smoothened (Smo), dopamine transporter (DAT), AADC, VMAT and Nurr1 immunostaining, adult wt or ko mice were perfused transcardially with a solution of 4% paraformaldehyde (PFA, pH 7.2) in 0.1 M phosphate buffer (PB, pH 7.2). Brains were processed for paraffin embedding and sectioned at 5-μm thickness. Paraffin-embedded sections were immunostained with the following antibodies rabbit anti-Smo (1:500, Abcam), rat anti-DAT (1:1000, Millipore), rabbit anti-AADC (1:1000, Millipore), rabbit anti-VMAT (1:500, Millipore) and rabbit anti-Nurr1 (1:100, Santa Cruz Biotechnology). These antibodies have been used previously and specificity has been confirmed in previous publications (Aarnisalo et al., 2002; Petralia et al., 2011; Seo et al., 2011; Zhang et al., 2012). After incubation with primary antibody solution, the sections were washed and incubated for one hour at room temperature in diluted secondary antibody prepared with blocking solution [donkey anti-rabbit, anti-rat 488 or 555 (1:1000/500; Life Technologies). Images were acquired using an Olympus microscope. Omission of the primary or secondary antibodies resulted in no staining and served as negative controls.
Stereologic Analysis
Unbiased stereological counts of TH-positive (TH+) neurons within the substantia nigra pars compacta (SNpc) were performed using stereological principles and analyzed with StereoInvestigator software (Microbrightfield, Williston, VT), as previously described (Luo et al., 2010). Optical fractionator sampling (West and Gundersen, 1990) was carried out on a Leica DM5000B microscope (Leica Microsystems, Bannockburn, IL) equipped with a motorized stage and Lucivid attachment (40× objective). Midbrain dopaminergic groups were outlined on the basis of TH immuno-labeling, with reference to a coronal atlas of the mouse brain (Franklin and Paxinos, (Franklin KBJ, 1997)). For each tissue section analyzed, section thickness was assessed at each sampling site and guard zones of 2.5μm were used at the top and bottom of each section. Pilot studies were used to determine suitable counting frame and sampling grid dimensions prior to counting. The following stereologic parameters were used in the final study: grid size, (X) 220 μm, (Y) 166 μm; counting frame, (X) 68.2 μm, (Y) 75 μm, depth was 20 μm. Gundersen coefficients of error for m=1 were all less than 0.10. Stereologic estimations were performed with the same parameters in the SNpc of wt or DAT-Smo ko mice at different ages. At the same time, the nucleator function was initiated to calculate the size of the nucleus while counting the total number of cells (n=5-6 mice for both wt and ko at each time points were analyzed).
6-Hydroxydopamine (6-OHDA) lesioning and amphetamine-induced rotation
At least 30 min prior to surgery, desipermine 25mg/kg was injected intraperitoneally to prevent unspecific damage of neostriatal and cerebellar noradrenergic neurons. Mice were then anesthetized with chloral hydrate (0.4 gm/kg, i.p.) and placed in a stereotaxic frame. A burr hole is drilled to introduce a syringe for a single injection of the 6-OHDA The 6-OHDA solution (2μL of a 3.3mg/mL 6-OHDA solution in normal saline containing 0.2 mg/ml ascorbic acid) was injected unilaterally over 4 min into the striatum with coordinates (anteroposterior +0.5mm, mediolateral ±2.0 relative to the bregma, and -2.5mm below the dura) on one side with the needle left in place for 5 minutes before withdrawal. This 6-OHDA injection regimen was chosen because it results in partial lesion of the striatum, which allows for the detection of any potential further damage in the DA system due to the smo knockout in DA neurons. 10 wt and 8 ko mice were subjected to the unilaterally 6-OHDA lesion and 8 wt and 6 ko mice survived and were tested for rotational behavior in response to subcutaneous methamphetamine injections (2.5mg/kg) in an automated rotometer (Omnitech electronics) one month after lesioningMouse were perfused 1 day after rotation tests and brains were analyzed by immunohistochemistry.
Locomotion
Spontaneous locomotor functions were examined using automated infra-red locomotor activity chambers, as previously described (Jin et al., 2015). Locomotor function was assessed during a 60-min trial in an open field crossed by a grid of photobeams (16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart, VersaMax system, AccuScan Instruments). Counts were taken of the number of photobeams broken during the trial at 5 min intervals, with separate measures for total horizontal and vertical activity, total distance travelled, and total horizontal and vertical movement number over an hour. Locomotion function was measured in wt and DAT-Smo ko mice at different ages (2, 5, 8, 12-15 and 18-21 months) in two separate cohort groups. One group of mice (wt=11, ko=11 with one dead during the course of this study) were tested for locomotion at all of the time points listed above (2months- 21 months). A second group of mice (wt=10, ko=14) were tested for locomotion at 5 months and 8 months and were sacrificed to harvest brain tissues for other analysis. Results from two groups were combined.
Methamphetamine single injection and sensitization
Locomotor activity was measured in activity chambers as described above. 4-5 month old wt or DAT-smo ko mice (n=8 each group) were placed in the locomotor chambers and their activity was measured for 60-min at 10-min intervals. After 60-min of habituation, a single methamphetamine injection (2.5mg/kg, s.c.) was given to each mouse and its locomotion activity was recorded for another 60-min to monitor the response of wt and DAT-Smo ko mice to single methamphetamine challenge. For repeated methamphetamine sensitization (Kubota 2002), the animals were habituated for 60-min in the chamber every day; the animals were first injected with saline on day 1 and day 2 (habituation saline 1 and saline 2). On following 5 days (D1-D5), methamphetamine injection (2.5mg/kg, s.c.) was given to each mouse after the 60min habituation period and the locomotion measurements continued for another 60-min. In summary, the treatment schedule consisted of injections with saline (habituation) daily for 2 days, followed by methamphetamine (2.5 mg/kg, s.c.) daily for 5 days. In the challenge experiment, mice were kept in the home cage after the last treatment (D5) until 7- or 30-days later, when they were challenged with the same dosage of methamphetamine (2.5mk/kg).The sensitization experiment was repeated in another group of wt and ko mice (n=8 for each group) with similar results and this group of mice were sacrificed and their brains harvested after the last methamphetamine injection on D5 for qRT-PCR analysis as described below.
Quantitative reverse transcription-PCR (qRT-PCR)
Brain tissues were harvested for qRT-PCR analysis at 1-hour post last methamphetamine injection (D5) in wt and DAT-Smo ko mice (n=8 for each group). Mice were euthanized and the brains were immediately removed and chilled on ice. The striatum or ventral midbrain was dissected using the Paxinos atlas and total RNA was extracted following the instructions from the manufacturer (RNAqueous, Ambion) as described previously (Luo et al., 2010). Total RNA (1 ug) was treated with RQ-1 Rnase-free Dnase I and reverse transcribed into cDNA using random hexamers by Superscript III reverse transcriptase (Life Sciences). cDNA levels for HPRT1 (hypoxanthine phosphoribosyltransferase 1), Hmbs (hydroxymethylbilane synthase) and various target genes were determined, using specific universal probe Library primer probe sets (Roche), by quantitative RT-PCR using a Roche Light Cycler II 480. A relative expression level was calculated using the delta Ct method compared to Hprt1 as a reference gene, with n=7-8 for each group. Primers and 6-carboxyfluorescein (FAM) labeled probes used in the quantitative RT-PCR for each gene are listed in Table 1.
Table 1. Primer/ probe sets used in qRT-PCR.
| Primer/probe set: | ||
|---|---|---|
| Hmbs: | Forward Primer: | 5′-TCC CTG AAG GAT GTG CCT AC-3′ |
| Reverse Primer: | 5′-ACA AGG GTT TTC CCG TTT G-3′ | |
| Probe: | Universal Probe Library: Probe 79 - Roche | |
| DAT: | Forward Primer: | 5′-CAA CCT GTA CTG GCG GCT AT-3′ |
| Reverse Primer: | 5′-ATG CTG ACC ACG ACC ACA TA-3′ | |
| Probe: | Universal Probe Library: Probe 38 - Roche | |
| smo | Forward Primer: | 5′- GCA AGC TCG TGC TCT GGT-3′ |
| Reverse Primer: | 5′- GGG CAT GTA GAC AGC ACA CA-3′ | |
| Probe: | Universal Probe Library: Probe 3 - Roche | |
| shh: | Forward Primer: | 5′-ACC CCG ACA TCA TAT TTA AGG A-3′ |
| Reverse Primer: | 5′-TTA ACT TGT CTT TGC ACC TCT GA-3′ | |
| Probe: | Universal Probe Library: Probe 32 - Roche | |
| Gli | Forward Primer: | 5′-CTG ACT GTG CCC GAG AGT G-3′ |
| Reverse Primer: | 5′-CGC TGC TGC AAG AGG ACT-3′ | |
| Probe: | Universal Probe Library: Probe 84 - Roche | |
| BDNF: | Forward Primer: | 5′-CAC TTT TGA GCA CGT CAT CG-3′ |
| Reverse Primer: | 5′-TCC TTA TGG TTT TCT TCG TTG G-3′ | |
| Probe: | Universal Probe Library: Probe 42 - Roche | |
| Drd1a: | Forward Primer: | 5′-TCT GGT TTA CCT GAT CCC TCA-3′ |
| Reverse Primer: | 5′-GCC TCC TCC CTC TTC AGG T-3′ | |
| Probe: | Universal Probe Library: Probe 82 - Roche | |
| Drd2: | Forward Primer: | 5′-CTC TTT GGA CTC AAC AAC ACA GA-3′ |
| Reverse Primer: | 5′-AAG GGC ACG TAG AAC GAG AC-3′ | |
| Probe: | Universal Probe Library: Probe 25 - Roche | |
| GDNF: | Forward Primer: | 5′-TCC AAC TGG GGG TCT ACG-3′ |
| Reverse Primer: | 5′-GAC ATC CCA TAA CTT CAT CTT AGA GTC-3′ | |
| Probe: | Universal Probe Library: Probe 70 - Roche | |
| BMP7: | Forward Primer: | 5′-CGA GAC CTT CCA GAT CAC AGT-3′ |
| Reverse Primer: | 5′-CAG CAA GAA GAG GTC CGA CT-3′ | |
| Probe: | Universal Probe Library: Probe 1 - Roche |
Statistical analysis
Comparisons between different age and gentotype groups in DA neuronal number, size and striatal TH fiber density were conducted suing two-way ANOVA to compare the effect of age or genotype on various measurements with Tukey post hoc test. For behavioral analysis, the Bonferroni correction was used for repeated measures. For 6-OHDA lesion experiment, treatment groups (6-OHDA injected or contralateral side) and wt or ko groups were conducted using two-way ANOVA to compare the effect of 6-OHDA injection vs contralateral side or genotype on vafious measurements with Tukey post hoc test. For experiments with only two groups, Student's t-test is used for comparison between two different groups. All statistical analyses and bar graph displays were performed using Sigma Plot and Stat version 2.0 from Jandel Scientific, San Diego, CA. Data are presented as mean ± standard error of the mean (S.E.M.) values, and statistical significance (*p<0.05, ** p<0.01 or *** p<0.001) is noted in the legend of each figure.
Results
Characterization of mice with specific deletion of smo gene in DAT-expressing DA neurons
Our strategy for deletion of smo gene in DAT expressing neurons is shown in Fig 1A. To achieve dopaminergic-specific smo deletion, we used the Slc6a3Cre line, a cre knock-in animal model in which a cre recombinase gene is specifically inserted into the DAT locus of the 3′ UTR of the endogenous DAT gene following an IRES (internal ribosome entry site sequence). This provides dopaminergic neuron-specific cre expression with minimal interference to endogenous DAT gene expression (Backman et al., 2006), which is important when assessing the dopaminergic system. In this mouse line, Cre recombinase is driven by the DA transporter promoter starting at embryonic day 16 (Backman et al., 2006). By crossing this line with a LacZ reporter strain carrying the reporter cassette in the Rosa 26 locus (Soriano, 1999), it has been shown that Cre recombinase activity is sufficiently robust to mediate genomic recombination restricted to dopaminergic neurons, as β-galactosidase expression was detectable in the ventral mesencephalon soon after DAT expression at E16 (Backman et al., 2006), and virtually all DAT-expressing cells co-localized with β-galacosidase-stained neurons in the SN and VTA. In this study, to confirm the specific deletion of the smo gene in DAT-expressing dopaminergic neurons, primers specific to the recombined DNA sequence (ΔloxP) were used to amplify the recombined smo allele. As expected, only brain tissues obtained from the olfactory bulb, ventral tegmental area (VTA) and substantia nigra (SN) contained the recombined ΔloxP, thereby confirming the deletion of the smo gene in these brain areas (Fig 1B). In contrast, the cortex, hippocampus, striatum and cerebellum were found to be negative for ΔloxP, indicating lack of smo gene deletion in these areas in our DAT-Smo ko mice. No recombination was seen in wt mice. Furthermore, double immunostaining of TH and Smo using a smo antibody whose specificity has been demonstrated in previous studies (Petralia et al., 2011; Seo et al., 2011; Zhang et al., 2012), we showed that many TH positive and TH negative cells in ventral midbrain expresses smo protein in wt mice (Fig 1 C and D), however, in DAT-Smo ko mice, smo protein is mostly detected in TH negative cells (Fig 1E and F), further confirming the specific deletion of smo gene in midbrain dopaminergic neurons.
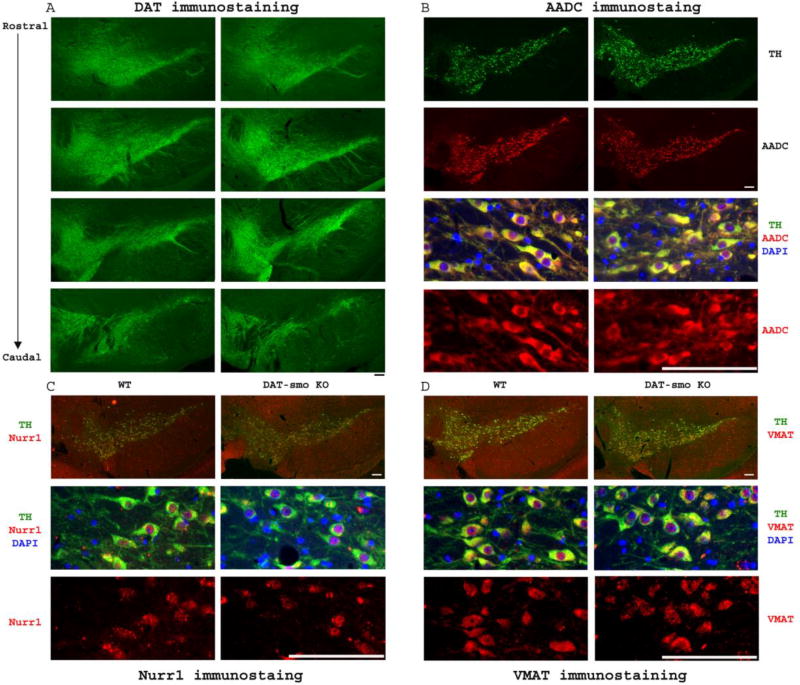
The DAT-Smo ko animals were born at expected Mendelian frequencies and showed normal viability. We first determined if smo gene deletion on E16 in DA neurons affected DA neuronal number and projections. Our results show that deletion of smo gene in post-mitotic DA neurons at E16 does not affect the patterning of the ventral mesencephalon and survival of DA neurons. Loss of the smo gene in DAT positive- dopaminergic neurons did not lead to an abnormal development of ventral midbrain, as demonstrated by the normal structure and number of midbrain dopaminergic neurons within the SN and VTA and by TH positive projections in striatum in DAT-Smo ko mice at 2 month, 5 month and 22 month of age (Fig 2 A, 2 month data not shown). As shown in Fig 2A, TH immunocytochemistry, evaluated at three different levels within the SN, showed no overt morphological differences at either 5 month or 22 month of age. Moreover, unbiased stereological counts of SN and VTA DA neuron numbers (Fig 2B) showed no difference in total TH positive neurons within SN and VTA in WT and DAT-Smo KO mice at 5 or 22 months of age (SN DA neuron number: Two-way ANOVA, genotype: F1, 24 = 0.0005, p=0.981; age: F1, 24 = 2.846, p=0.106, n=5-6 per group; VTA DA neuron number: Two-way ANOVA, genotype: F1, 24 = 0.939, p=0.344; age: F1, 24 = 0.659, p=0.426, n=5-6 per group, no interaction between age and genotype). The size of DA neurons in both SN and VTA showed no difference between wt and DAT-Smo ko mice Fig 2C (SN DA neuron size: Two-way ANOVA, genotype: F1, 24 = 0.883, p=0.363; age: F1, 24 = 2.222, p=0.158, n=5-6 per group; VTA DA neuron size: Two-way ANOVA, genotype: F1, 24 = 2.159, p=0.166; age: F1, 24 = 0.002, p=0.962, n=5-6 per group, no interaction between age and genotype). In addition, striatal DA fiber densities were similar in wt and DAT-Smo ko mice (Fig 2D/E) at both ages (Two-way ANOVA, genotype: F1, 24 = 0.0541, p=0.818 wt vs ko, ANOVA) with 22 months showing decreased density (Two – way ANOVA, age: F1, 24 = 4.8, p=0.04 5 months vs 22 months) but no difference between wt and ko mice (p=0.992 and p=0.759 for wt vs ko at 5months and 22 months, ANOVA, Tukey post hoc analysis, n=5-6 per group, no interaction between age and genotype). To further examine whether other factors involved in the maintenance and function of dopaminergic neurons are affected by smo gene deletion in dopaminergic neurons, we examined the expression of other proteins involved in the maturation (Nurr1), synthesis (AADC), storage/release (VMAT) and reuptake (DAT) of dopamine in young adult (5 month old) WT and DAT-smo KO mice (n=3 for each genotype). As shown in Fig 3A, DAT immunostaining evaluated at different levels within the SN, from rostral to caudal position, showed no overt morphological differences between WT and DAT-Smo KO mice. Similarly, there is no apparent difference in the immunostaining patterns between WT and DAT-Smo KO mice in AADC (Fig 3B), Nurr1 (Fig 3C) and VMAT (Fig 3D) immunostaining at ventral midbrain.
Figure 2. Conditional deletion of smo gene in dopaminergic neurons dose not affect development and aging of dopaminergic neurons.
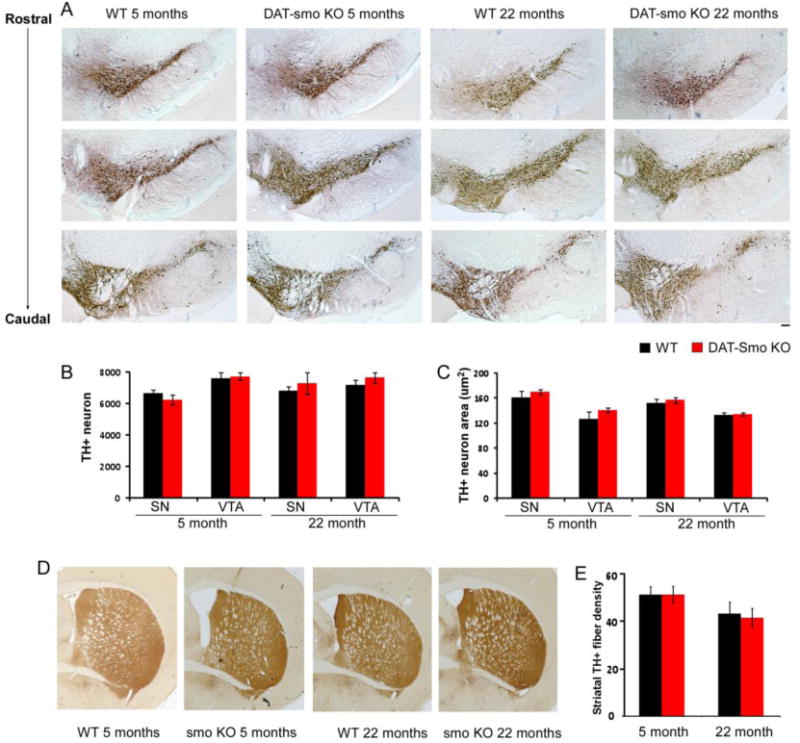
A. Tyrosine hydroxylase staining showed that the general patterning and structure of substantia nigra (SN) and ventral tegmental area (VTA) is similar in wt and DAT-Smo ko mice both at 5 months and 22 months of age. (B) Unbiased stereological counts of DA neurons in SN and VTA in wt or DAT-Smo ko mice at different ages showing no difference between the genotypes or two age groups (p>0.05 among all groups, ANOVA, n=5-6). (C) DA neuron size measured by the nucleator probe showing no difference between the wt and DAT-Smo ko mice in the size of SN and VTA neurons at both age groups. (p>0.05 among all groups, ANOVA, n=5-6) (D). Striatal TH immunostaining showing DA neuronal terminals in wt and DAT-Smo ko mice at different ages. (E) optical density of TH-positive fibers in striatum was quantified and show no difference between wt and DAT-Smo ko at both age groups (p>0.05, ANOVA. n= 5-6 per group). Scale bar= 100um.
Figure 3. DAT-Smo ko mice shows similar expression pattern of several key genes involved in dopaminergic neuron development and functions in young adult mice.
(A) DAT immunostaining showed that the general patterning and structure of substantia nigra (SN) and ventral tegmental area (VTA) is similar in wt and DAT-Smo ko mice both at 5 months of age on different levels ranging from rostral to caudal positions. Representative images showed coexpression of TH and and similar immunostaining pattern in WT and DAT-Smo ko mice in ventral midbrain for (B) AADC, (C) Nurr1 and (D) VMAT protein. Scale bar= 100um.
DA specific deletion of Smo gene does not affect the survival of DA neurons in a 6-OHDA PD animal model
To assess whether the smo gene is required for the survival of DA neurons under neurotoxin challenge, we compared the survival of DA neurons and function of the nigrostriatal DA system of wt and DAT-Smo ko mice in a partial DA lesion animal model. The 6-OHDA partial lesion, instead of a complete lesion through medial forebrain bundle (MFB), was chosen because it allows for detection of either potential protective effects or vulnerability caused by gene modulation. Unilateral injection of 6-OHDA into the striatum of mice led to similar extents of DA neuronal loss evaluated by TH immunohistochemistry, in SN in both wt and DAT-Smo ko mice (Fig 4A and 4D, 60% DA neuronal loss in wt and 54% DA neuronal loss in DAT-Smo ko mice, Two-way ANOVE, treatment: contralateral side vs lesioned side, F(1, 31)= 43.978, p<0.001, and Two-way ANOVA, genotype: wt vs DAT-Smo ko, F(1, 31)= 0.0237. p=0.879, n=6-8 per group, no interaction between treatment and genotype). Normalized to each animal on its contralateral side, TH+ dopaminergic neuronal terminal loss in the striatum was also similar in wt and DAT-Smo ko mice (Fig 4B and 4E, 72.68+5.71% loss of striatal TH fibers in wt and 69.13+9.56% loss in DAT-Smo ko mice, p= 0.75, Student's t-test, n=6-8 per group). Unilateral lesion of the nigostriatal DA projection results in an imbalance of the two hemispheres and amphetamine induced rotation is widely used as an index of lesion deficits. Methamphetamine injection in 6-OHDA lesioned mice elicited similar ipisilateral rotations to the lesioned side in wt and DAT-Smo ko miced (Fig 4C, 283.71±59.36 turns/hour for wt mice and 280.33+123.68 turns/hour for DAT-Smo ko mice, p=0.97, Student's t-test, n=6-8 per group) consistent with the immunohistochemistry results. Our data therefore suggest that smo gene expression in DA neurons does not affect DA neuronal survival and function in the 6-OHDA partial lesion PD model.
Figure 4. DAT-Smo ko mice have a similar vulnerability to 6-OHDA lesions.
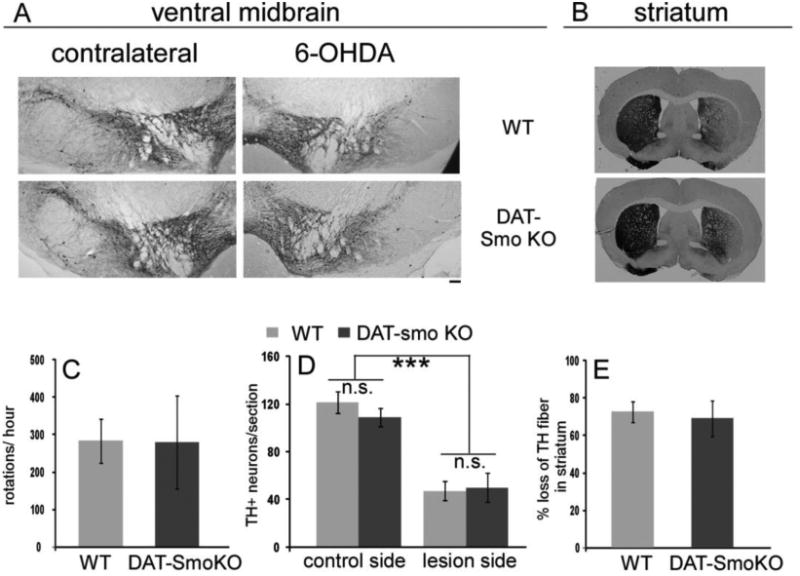
(A) TH immunostaining reveals loss of DA neurons in the SN on the 6-OHDA injected side in both wt and DAT-Smo ko mice, representative images. (B) Unilateral loss of TH neuronal terminal in 6-OHDA injected wt and DAT-Smo ko mice, representative images. (C) Methamphetamine induced ipsilateral rotation in wt and DAT-Smo ko mice showed similar behavioral effects (p=0.979, Student's t-test). (D) and (E) quantification of DA neuronal loss in SN and diminished DA terminals in striatum in wt and DAT-Smo ko mice. No difference is observed between wt an dDAT-Smo ko in all parameters measured (p>0.05, ANOVA, n=6-8.) Scale bar=100um.
DAT-Smo ko mice show hyperactivity as young adults
DA specific smo gene deletion does not affect the DA neuronal survival during normal aging and under stress. However, basal locomotion tests performed in automated behavioral chambers demonstrated hyperactivity in DAT-Smo ko mice at age 5- and 8-months, as measured by total horizontal activity (Fig 5A, Two-way ANOVA, genotype: F1, 156=9.211, p=0.003; age: F4, 156=26.545, p<0.001; Bonferroni post hoc test p<0.001 for 5 month age wt vs. ko and p<0.05 for 8 months, wt vs ko, n=21-25 per group) and at 5-months for total distance traveled (Fig 5B, Two-way ANOVA, genotype: F1, 156=3.066, p=0.082; age: F4, 156=15.049, p<0.001: p<0.01 for wt vs ko at 5 month age, Bonferroni post hoc test, ANOVA), total horizontal (Fig 5C, Two-way ANOVA, genotype: F1, 156=2.190, p=0.141; age: F4, 156=26.744, p<0.001; p<0.05, wt vs ko at 5 month age, Bonferroni post hoc test, ANOVA) and vertical movement numbers (Fig 5D, Two-way ANOVA, genotype: F1, 156=21.635, p=0.203; age: F4, 156=20.12, p<0.001; p<0.05, wt vs ko at 5 month age, Bonferroni post hoc test, ANOVA, n=21-25 per group, no interaction between age and genotype for all parameters measured). There is a general decline of locomotor activity with aging with both genotypes; the hyperactivity observed in younger DAT-Smoko adult (5 months) diminished and activity levels became similar to that of wt animals in 12 or 18-21 month old mice (p>0.05 at 12 or 18-21 month age, Bonferroni post hoc test, ANOVA, for all the parameters measured, wt vs ko, n=10-11 per group). This suggests that although there is no difference in the DA neuronal numbers in wt and DAT-Smo komice at 5 month of age, the DAT-Smo ko display some behavioral differences compared to wt littermates.
Figure 5. Hyperactivity in young DAT-Smo ko mice.
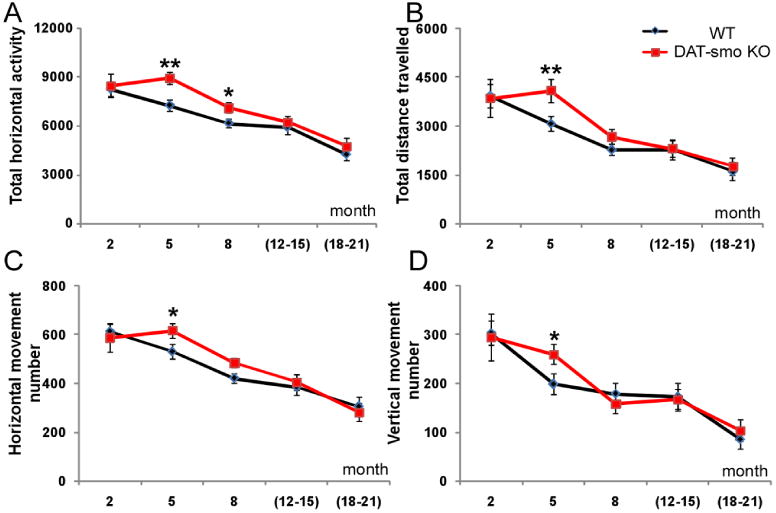
Spontaneous locomotion was measured in open field chambers for 60 min. DAT-Smo ko mice show hyperactivity at 5 months for (A) Total horizontal activity (B) total distance travelled (C) Total horizontal and (D) total vertical movement numbers. Hyperactivity was diminished during aging and there was no difference in all four parameters measured in wt and DAT-Smo ko mice at 12-21 months. * indicates p<0.05, ANOVA and ** indicates p<0.01, ANOVA. n>11 per group.
DA specific Smo gene deletion lead to attenuated responses to single and repeated psychostimulant (methamphetamine) challenges possibly through modulating BDNF gene expression
Since psychostimulants act as antihyperkinetic agents in humans with hyperkinetic disorders such as attention-deficit hyperactivity disorder (ADHD) and can attenuate hyperactivity in both human and genetic mouse models (Sontag et al., 2010; Tanaka et al., 2006), we evaluated wt and DAT-Smo ko mice response to a single methamphetamine challenge (2.5mg/kg). Interestingly, despite the higher basal locomotion activity, DAT-Smo ko mice showed diminished responses to single METH challenge (Fig 6A, Repeated measure two way ANOVA, genotype: F1, 191= 1.456, p=0.248 and time point F11, 191= 26.926, p<0.001 and an interaction between genotype and time point, F11, 191= 3.219, p<0.001. For individual time point, Bonferroni post-hoc analysis: p<0.01 for 20-30 minutes and 30-40 min after METH injection and p<0.05 for 40-50min and 50-60 min after METH injection, n=8). To determine whether this diminished response to METH is sustained or delayed with multiple METH administration, we carried out a classic METH sensitization analysis. Animals received the same dose of METH (2.5mg/kg) in the activity chamber for 5 consecutive days. This repeated exposure of METH has been previously reported to induce behavioral sensitization in rodents. With METH sensitization testing, ko mice showed a decreased locomotion response compared to wt mice on all 5 days without evidence of a delayed shift in the dynamics of the induction of the behavioral sensitization (Fig 6B, Repeated measure two way ANOVA, genotype: F1, 95= 10.788, p=0.005 and time point F5, 95= 11.505, p<0.001 and no interaction between genotype and time point, F5, 95= 1.820, p=0.12. For individual time point, Bonferroni post-hoc anylysis: p=0.016 for D1, p=0.043 for D 2 and p=0.008 for D5 wt vs ko, n=8 per group). Next, the expression of behavioral sensitization was measured on days 7 and 30 after repeated injection of the drug, respectively. On days 7 and 30, both wt and DAT-Smo ko mice demonstrated elevated locomotion responses to METH compared to D1 levels (Fig 6B, Bonferroni post-hoc analysis: p<0.001 and p=0.003 for C7 vs D1 and C30 vs D1 for wt and p=0.003 and p=0.011 for C7 vs D1 and C30 vs D1 for DAT-Smo ko mice, n=8 per group), indicating that behavioral sensitization can last at least 30 days in both wt and ko mice. Importantly, DAT-Smo ko mice had consistently lower levels of locomotion response to METH on both challenge day 7 and challenge day 30 (Fig 6B, Bonferroni post-hoc analysis: p<0.001 and p=0.008 for wt vs ko, ANOVA, n=8 per group) indicating a sustained lower response to METH exposure. We also examined gene expression differences in the wt and DAT-Smo ko mice after 5 days of repeated METH exposure. Gene expression analysis showed decreased gene expression for smo (p=0.001, Student's t-test) and Gli 1 (known target gene of smo, p=0.009, Student's t-test) in DAT-Smo ko mice, confirming the DA specific deletion of smo gene and alteration in the shh-smo-Gli1 signaling pathway in the ventral midbrain (Fig 7A). Brain-derived neurotrophic factor (BDNF) has been implicated in DA system function, including addiction and responses to METH (Bousman et al., 2009; Iamjan et al., 2015; Itoh et al., 2005; Manning et al., 2015; Manning and van den Buuse, 2013; Sim et al., 2010), and has recently been identified as a potential target gene for the shh pathway in smooth muscle cells (Radzikinas et al., 2011). To examine whether it is also regulated by shh signaling in dopaminergic neurons, we examined the mRNA levels of BDNF in ventral midbrain tissues from wt and DAT-Smo ko mice. Interestingly, BDNF expression levels in the ventral midbrain was attenuated in DAT-Smo ko mice compared to wt mice (Fig 7A, p=0.013, Student's t-test, n=8 per group). Striatal gene expression analysis showed that the expression of dopamine receptors (Drd1, Drd2) were not altered in DAT-Smo ko mice (Fig 7B, p>0.05, Student's t-test).
Figure 6. DA specific deletion of the Smo gene affects behavioral responses to methamphetamine in mice.
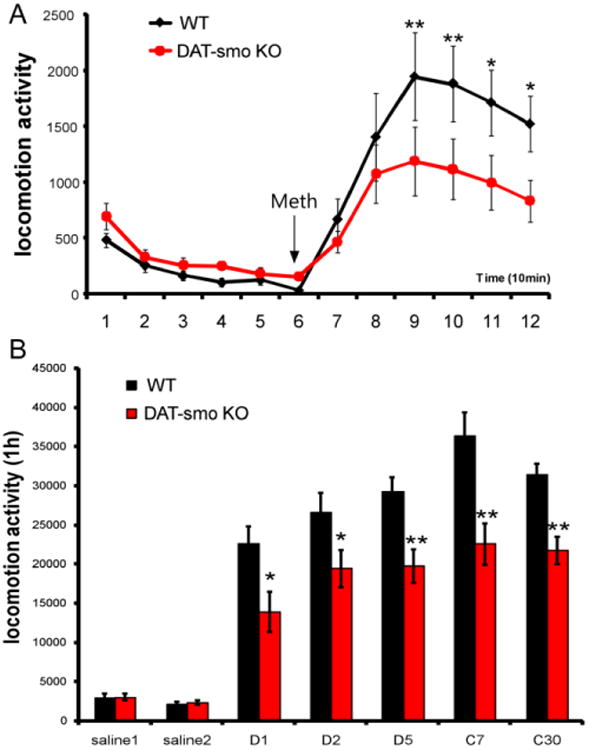
(A) DAT-Smo ko mice showed an attenuated response to single methamphetamine challenge (2.5mg/kg). (**, p<0.01 and * p<0.05, Repeated measurement two way ANOVA, n= 8). (B) DAT-Smo ko mice showed attenuated locomotion activity in response to repeated methamphetamine injection in days during the development (D1,2,5) and the expression (challenge day 7 and d30) of sensitization (**, p<0.01 and * p<0.05 for Repeated measurement two way ANOVA, n= 8).
Figure 7. Gene expression alterations in DAT-Smo ko mice after multiple METH exposures.
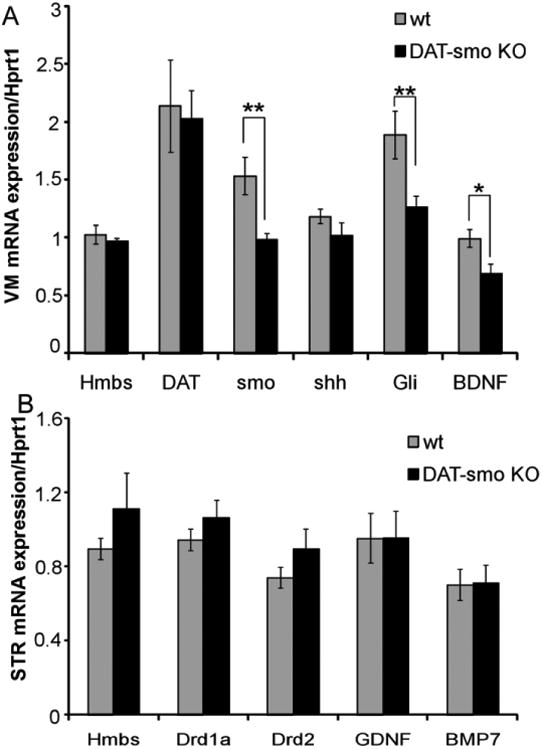
Gene expression was analyzed using qRT-PCR after 5 days of repeated METH injection in wt and DAT-Smo ko mice. (A) gene expression analysis in ventral midbrain (VM) shows that Smo gene deletion in DA neurons leads to decreased mRNA levels for Smo and target gene Gli in SN and VTA. BDNF mRNA levels are also significantly downregulated in DAT-Smo ko mice (**, P<0.01, * P<0.05, Student's t-test. n= 8). (B) gene expression analysis in striatum (STR) shows no difference in dopamine receptor (Drd1 and Drd2) and neurotrophic factors glial cell line-derived neurotrophic factor (GDNF) and bone morphogenic protein 7 (BMP7) genes, p>0.05 Student's t-test, n=8)
Similarly genes that have been shown to have neurotrophic effects in the nigrostriatal system such as Glial cell line-derived neurotrophic factor (GDNF) and bone morphogenetic protein 7(BMP7) had similar expression levels in wt and DAT-Smo ko mice (Fig 7B, p>0.05, Student's t-test, n=8 per group).
Discussion/Conclusions
The role of shh signaling pathway in the early induction of DA progenitors has been well established (Blaess et al., 2006; Fuccillo et al., 2004; Hynes et al., 1995; Perez-Balaguer et al., 2009; Sousa and Fishell, 2010; Ye et al., 1998). Conditional deletion of the shh gene (Blaess et al., 2006) or the receptor Smo gene (Perez-Balaguer et al., 2009), using engrailed-cre (En-cre) at early developmental stages (E9.0), leads to substantial reduction of the midbrain DA cell population. After the initial induction of ventral midbrain DA progenitors, at E10.5 to E12.5, shh expressing cells are detected in the dorsal and lateral part of the midbrain neural tube, adjacent to and partially overlapping with the developing Nurr1+/ TH+dopaminergic neurons (Ferri et al., 2007; Tang et al., 2013). This suggests this signaling pathway might continue to play a role in the maturation and survival of DA neurons. Evidence supporting this speculation include the continuous expression of shh pathway genes in the nigrostriatal systems in adulthood (Traiffort et al., 1999; Traiffort et al., 1998) and the neuroprotective effects of exogenously delivered shh peptide in 6-OHDA Parkinson's Disease (PD) animal models (Tsuboi and Shults, 2002). However, whether shh signaling mediated through smo receptor in DA neurons is required for the survival and function of these cells in adulthood has not been thoroughly examined. To answer these questions, a better-controlled disruption of shh signaling pathway genes in late embryonic developmental stages is needed because shh can signal in both a paracrine and autocrine fashion, affecting adjacent cells as well as the cells in which it is produced (Cai et al., 2008), DA specific deletion of the smo gene enabled us to examine the role of this pathway specifically in DA neurons in contrast to DAT-shh ko mice, which might affect other target cells. Utilizing the DA specific smo conditional knockout mice, our data demonstrate that during late development and adulthood, shh-smo pathway activity does not affect the survival of dopaminergic neurons. DAT-Smo ko mice have normal expression pattern for several key genes that are important for the development and function of ventral midbrain dopaminergic neurons including TH, DAT, Nurr1, AADC and VMAT. Previous studies have suggested that shh-smo-Gli pathway might not be required in the late development of ventral midbrain since Shh-Cre:Smo conditional knockout mice have normal ventral midbrain at P0 (Tang et al., 2013). Our results are in agreement with this hypothesis and further demonstrate that shh signaling mediated by smo is not critical in the survival of SN and VTA dopaminergic neurons in adulthood or during aging. In addition to its role under physiological conditions, it is also speculated that shh signaling might be involved in the cellular and molecular responses to DA neurotoxin and challenges. Shh gene expression is transiently up-regulated after 6-OHDA lesions (Gonzalez-Reyes et al., 2012) and exogenously delivered shh peptide is neuroprotective in 6-OHDA animal models. Our data using the DAT-Smo ko mice in a 6-OHDA partial lesion model showed that smo gene deletion in dopaminergic neurons does not result in increased vulnerability in ko mice. Because a partial lesion model was used in this study, the absence of an increased vulnerability in ko mice was not due to saturation ceiling effect of the lesion in mice. Therefore, our data supports the conclusion that smo mediated shh signaling might not be critical or directly involved in the survival of DA neurons during late development, aging or under neurotoxic stress.
In contrast to a direct role in the survival of midbrain dopaminergic neurons, our data suggest a role of the shh-smo-Gli1 pathway in the function of dopaminergic system in adult mice. DAT-Smo ko mice are hyperactive at 5 months of age, as measured by multiple parameters in open field testing. Although having higher basal locomotor activity, ko mice showed an attenuated response to the psychostimulant, methamphetamine both in a single bolus injection and in the repeated regimen that induces behavioral sensitization. DAT-Smo ko mice showed significantly less sensitization to METH than wt mice both during the initial induction (first 5 days) as well as during the expression of sensitization (challenged after 7 days or 30 days withdrawal) (Fig5). The mechanisms maintaining cellular and neurochemical homeostasis in the mature mesostriatal and mesocortical DA system in the healthy brain have not been fully elucidated. A recent study reported that mesencephalic DA mecencephalic neurons express shh throughout life and DA neuron-produced shh is necessary for the long-term structural and functional maintenance of mesencephalic DA neurons (Gonzalez-Reyes et al., 2012). The effect of smo gene deletion in DA neurons on the function of DA system has not been characterized. Behavioral differences in DAT-Smo ko mice in basal locomotive activity as well as different responses to single or repeated exposure to methamphetamine suggest that ablation of shh signaling pathway in DA neurons may alter DA neurotransmission through regulation of primary or secondary target genes. Our data on midbrain tissue gene expression analysis following 5 days of repeated methamphetamine demonstrated that 1). smo gene and its target gene (Gli) expression is substantially and significantly decreased in midbrain in DAT-Smo ko mice compared to their wt littermates while shh gene expression is not affected in DAT-Smo ko mice. This suggests DA specific deletion of the smo gene is specific and leads to a decreased functional shh pathway response in the midbrain. 2). DAT gene expression is not different between wt and DAT-Smo ko, demonstrating that this particular DATcre knock-in transgene does not interfere with DAT expression in DA neurons. 3), more importantly, our data showed that BDNF expression levels in midbrain tissue is significantly less abundant in DAT-Smo ko mice compared to wt mice after repeated methamphetamine sensitization treatment. Since BDNF is thought to play critical roles in regulation of addictive behavior and behavioral responses to psychostimulants such as methamphetamine (Bousman et al., 2009; Iamjan et al., 2015; Itoh et al., 2005; Manning et al., 2015; Manning and van den Buuse, 2013; Sim et al., 2010), its alteration by smo deletion in DA neurons might account for the behavioral differences we observed in DAT-Smo ko mice. A recent study showed that DAT-Shh ko mice have altered GDNF expression in striatum, which suggested reciprocal, trophic factor signaling between mesencephalic DA and striatal acetylcholine (Ach) and fast-spiking (FS) GABAergic neurons (Gonzalez-Reyes et al., 2012). We also examined striatal tissue gene expression and did not find any differences in Drd1 receptor, Drd2 receptor or GDNF expression levels. This is consistent with our speculation that smo gene deletion might cause an intrinsic alteration in molecular changes in DA neurons themselves and shh gene deletion might exert its effects through regulating its target cells. To further explore these possibilities, further studies utilizing DA neuron specific shh gene ko mice and examination of DA storage and release in vivo will be necessary. In this study our data demonstrate that loss of shh signaling in DA neurons results in decreased levels of BDNF transcripts, supporting a previous report that the shh pathway regulates BDNF transcription in smooth muscle cells (Radzikinas et al., 2011). Interestingly, BDNF heterozygous mice showed attenuated response in behavioral sensitization to psychomotor stimulants (Horger et al., 1999) similar to what we observed in our DAT-Smo ko mice. These data suggest that shh signaling might not affect the survival of DA neurons, but may modulate function and homeostasis of the DA system, possibly through regulation of important genes or pathways such as BDNF.
Highlights.
- DA Smoothened gene ko does not affect maturation of dopaminergic neurons.
- Smo gene deletion in DA neurons does not affect their survival during aging.
- DA smo ko mice do not show increased vulnerability to a 6-OHDA lesion.
- DA smo ko mice are hyperactive in young adulthood.
- DA smo ko mice show altered responses to psychostimulants such as methamphetamine.
Acknowledgments
This study is supported by NINDS R01NS094152, the Spitz Brain Health Innovation Award, AANS medical student fellowship (AANS MSSRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnisalo P, Kim CH, Lee JW, Perlmann T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. The Journal of biological chemistry. 2002;277:35118–35123. doi: 10.1074/jbc.M201707200. [DOI] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Glatt SJ, Everall IP, Tsuang MT. Genetic association studies of methamphetamine use disorders: A systematic review and synthesis. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2009;150B:1025–1049. doi: 10.1002/ajmg.b.30936. [DOI] [PubMed] [Google Scholar]
- Cai C, Thorne J, Grabel L. Hedgehog serves as a mitogen and survival factor during embryonic stem cell neurogenesis. Stem cells. 2008;26:1097–1108. doi: 10.1634/stemcells.2007-0684. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA, Ang SL. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134:2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- Filichia E, Shen H, Zhou X, Qi X, Jin K, Greig N, Hoffer B, Luo Y. Forebrain neuronal specific ablation of p53 gene provides protection in a cortical ischemic stroke model. Neuroscience. 2015;295:1–10. doi: 10.1016/j.neuroscience.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, P G. The mouse brain in stereotaxic coordinates. Academic Press; 1997. [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes LE, Verbitsky M, Blesa J, Jackson-Lewis V, Paredes D, Tillack K, Phani S, Kramer ER, Przedborski S, Kottmann AH. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron. 2012;75:306–319. doi: 10.1016/j.neuron.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Iamjan SA, Thanoi S, Watiktinkorn P, Nudmamud-Thanoi S, Reynolds GP. BDNF (Val66Met) genetic polymorphism is associated with vulnerability for methamphetamine dependence. Pharmacogenomics. 2015;16:1541–1545. doi: 10.2217/pgs.15.96. [DOI] [PubMed] [Google Scholar]
- Itoh K, Hashimoto K, Shimizu E, Sekine Y, Ozaki N, Inada T, Harano M, Iwata N, Komiyama T, Yamada M, Sora I, Nakata K, Ujike H, Iyo M. Association study between brain-derived neurotrophic factor gene polymorphisms and methamphetamine abusers in Japan. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2005;132B:70–73. doi: 10.1002/ajmg.b.30097. [DOI] [PubMed] [Google Scholar]
- Jin Y, Raviv N, Barnett A, Bambakidis NC, Filichia E, Luo Y. The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model. PloS one. 2015;10:e0124657. doi: 10.1371/journal.pone.0124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TE, Lee HS, Lee YB, Hong SH, Lee YS, Ichinose H, Kim SU, Lee MA. Sonic hedgehog and FGF8 collaborate to induce dopaminergic phenotypes in the Nurr1-overexpressing neural stem cell. Biochemical and biophysical research communications. 2003;305:1040–1048. doi: 10.1016/s0006-291x(03)00879-9. [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Luo Y, Wang Y, Kuang SY, Chiang YH, Hoffer B. Decreased level of Nurr1 in heterozygous young adult mice leads to exacerbated acute and long-term toxicity after repeated methamphetamine exposure. PloS one. 2010;5:e15193. doi: 10.1371/journal.pone.0015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EE, Halberstadt AL, van den Buuse M. BDNF-Deficient Mice Show Reduced Psychosis-Related Behaviors Following Chronic Methamphetamine. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2015 doi: 10.1093/ijnp/pyv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EE, van den Buuse M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Frontiers in cellular neuroscience. 2013;7:92. doi: 10.3389/fncel.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao N, Wang M, Ott JA, D'Alessandro JS, Woolf TM, Bumcrot DA, Mahanthappa NK, Pang K. Sonic hedgehog promotes the survival of specific CNS neuron populations and protects these cells from toxic insult In vitro. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:5891–5899. doi: 10.1523/JNEUROSCI.17-15-05891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Balaguer A, Puelles E, Wurst W, Martinez S. Shh dependent and independent maintenance of basal midbrain. Mechanisms of development. 2009;126:301–313. doi: 10.1016/j.mod.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Schwartz CM, Wang YX, Mattson MP, Yao PJ. Subcellular localization of Patched and Smoothened, the receptors for Sonic hedgehog signaling, in the hippocampal neuron. The Journal of comparative neurology. 2011;519:3684–3699. doi: 10.1002/cne.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussa E, Farkas LM, Krieglstein K. TGF-beta promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiology of disease. 2004;16:300–310. doi: 10.1016/j.nbd.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS genetics. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim MS, Mohamed Z, Hatim A, Rajagopal VL, Habil MH. Association of brain-derived neurotrophic factor (Val66Met) genetic polymorphism with methamphetamine dependence in a Malaysian population. Brain research. 2010;1357:91–96. doi: 10.1016/j.brainres.2010.08.053. [DOI] [PubMed] [Google Scholar]
- Sontag TA, Tucha O, Walitza S, Lange KW. Animal models of attention deficit/hyperactivity disorder (ADHD): a critical review. Attention deficit and hyperactivity disorders. 2010;2:1–20. doi: 10.1007/s12402-010-0019-x. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Fishell G. Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Current opinion in genetics & development. 2010;20:391–399. doi: 10.1016/j.gde.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Shintani N, Hashimoto H, Kawagishi N, Ago Y, Matsuda T, Hashimoto R, Kunugi H, Yamamoto A, Kawaguchi C, Shimada T, Baba A. Psychostimulant-induced attenuation of hyperactivity and prepulse inhibition deficits in Adcyap1-deficient mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5091–5097. doi: 10.1523/JNEUROSCI.4376-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Luo SX, Tang V, Huang EJ. Temporal and spatial requirements of Smoothened in ventral midbrain neuronal development. Neural development. 2013;8:8. doi: 10.1186/1749-8104-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. The European journal of neuroscience. 1999;11:3199–3214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. Journal of neurochemistry. 1998;70:1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Shults CW. Intrastriatal injection of sonic hedgehog reduces behavioral impairment in a rat model of Parkinson's disease. Experimental neurology. 2002;173:95–104. doi: 10.1006/exnr.2001.7825. [DOI] [PubMed] [Google Scholar]
- Wang MZ, Jin P, Bumcrot DA, Marigo V, McMahon AP, Wang EA, Woolf T, Pang K. Induction of dopaminergic neuron phenotype in the midbrain by Sonic hedgehog protein. Nature medicine. 1995;1:1184–1188. doi: 10.1038/nm1195-1184. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. The Journal of comparative neurology. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Wu SM, Tan KS, Chen H, Beh TT, Yeo HC, Ng SK, Wei S, Lee DY, Choo AB, Chan KK. Enhanced production of neuroprogenitors, dopaminergic neurons, and identification of target genes by overexpression of sonic hedgehog in human embryonic stem cells. Stem cells and development. 2012;21:729–741. doi: 10.1089/scd.2011.0134. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Human molecular genetics. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]