The overlap between oncology and nephrology is an area of growing importance. A major reason for this is that less than half of cancer patients were long-term survivors years ago, whereas now over two-thirds will live five years or longer. Late effects of cancer treatment include nephrotoxicity and are part of current clinical practice. In addition, cancer is now a known feature of chronic kidney disease (CKD), with increased risk in patients receiving dialysis or with a functioning kidney transplant, as well as in those with earlier stages of the disease. Therefore, oncologists will refer patients to nephrologists, and nephrologists will need to consult oncologists. This Core Curriculum addresses the key issues at this challenging clinical interface.
ASSESSMENT OF KIDNEY FUNCTION
Clinical Practice
Kidney function determines the choice of cancer treatment, and its decline is a serious adverse event for cancer patients. The number of nephrons corresponds closely to the total glomerular filtration rate (GFR) of a given patient. Thus the practical concept of kidney function is usually the same as that of the GFR. The “gold standard” measurement of the GFR is by inulin or iothalamate clearances, but those are rarely done in clinical practice. A 24-hour urine collection enables measurement of urea and creatinine clearances, which can be averaged to estimate the GFR. Formulas can correlate the serum creatinine to iothalamate clearances. The best known estimation formulas are the MDRD (Modification of Diet in Renal Disease) Study equation and the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. The formula-derived value for the GFR is commonly reported by clinical chemistry laboratories. This value is not 100% accurate or precise because of measurement and biological variability. The formula-derived estimated GFR (eGFR) cannot be used for patients whose kidney function is rapidly changing. It also is not reliable in patients who have lost muscle mass, as they have a relatively lower generation of creatinine. This results in lower serum levels of creatinine, causing overestimation of the GFR as compared to the true value. Twenty or more percent of cancer patients may have sarcopenia, ie, significant loss of muscle mass, and will thus have lower-than-expected serum creatinine levels. This can lead to medication dosing that results in side effects and toxicities because the patient’s actual GFR is significantly lower that the eGFR reported by the laboratory. In these patients, determining the true GFR by using 24-hour urine collections (or even the more expensive iothalamate clearance) may be needed.
Laboratory Measurement
Neither the serum creatinine concentration nor the eGFR derived from it has pinpoint accuracy or precision. The critical value difference of the serum creatinine is 0.2 mg/dl when its absolute value is close to normal. That means that a day-to-day change of less than 0.2 mg/dl may just be noise and not significant. The critical value difference for serum creatinine is higher when its absolute value is higher. While most clinical chemistry labs use an enzymatic method, some may use the Jaffe method, which may give an artifactually low serum creatinine value in patients with immunoglobulin (Ig) G paraproteinemias.
Relevant Clinical Investigation
Other ways of assessing kidney function have been tested in patients with cancer. Using the cystatin C assay in the general population may slightly increase the accuracy of eGFR, but not to the extent of justifying its routine use. Seventy-five percent of active clinical cancer research studies registered on ClinicalTrials.gov exclude patients with reduced kidney function, limiting our knowledge of cancer treatments in this population. Patients with CKD and cancer have a higher mortality rate than patients who have cancer but not CKD. While it may be possible to improve the precision and accuracy of clinical assessment of kidney function, a higher priority is to include patients with reduced kidney function in cancer trials.
Tubular Function
Toxicity from chemotherapy may be primarily tubular. Magnesium wasting from cisplatinum or epidermal growth factor inhibitors, and Fanconi syndromes from ifosfamide are well-known. Changes in the renal excretion of ions can be detected by calculation of their fractional excretion from ion and serum creatinine concentrations, ie {[(urine ion)]×(serum creatinine)]/[(urine creatinine)×(serum ion)]}×100, taking care to use the filterable serum ion concentation, and comparing the obtained value to the value expected for the simultaneous level of GFR.
Subtle tubular toxicity from chemo- or radiotherapy may not change the serum urea nitrogen (SUN) or serum creatinine very much, but it may cause tubular injury that affects medication excretion. In such cases there may be evidence of tubular injury, such as increased β2-microglobulin or urinary ion excretion. However, current tests do not quantify abnormalities of tubular handling of medications. Prudent clinical observation will enable medication dose adjustments.
Suggested Readings
-
»
Delanaye P, Cohen EP. Formula-based estimates of the GFR: equations variable and uncertain. Nephron Clin Pract 2008;110(1):c48–53.
-
»
Dalal BI, Brigden ML. Factitious biochemical measurements resulting from hematologic conditions. Am J Clin Pathol 2009 Feb;131(2):195–204.
-
»
Nakai K, Kikuchi M, Fujimoto K, Kaneko Y, Omori S, Nakai K, et al. Serum levels of cystatin C in patients with malignancy. Clin Exp Nephrol 2008 Apr;12(2):132–139.
-
»
Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014 Jan;63(1):23–30.
-
»
Popovtzer MM, Schainuck LI, Massry SG, Kleeman CR. Divalent ion excretion in chronic kidney disease: relation to degree of renal insufficiency. Clin Sci 1970 Mar;38(3):297–307.
WATER AND ELECTROLYTE DISTURBANCES IN CANCER
Electrolyte Imbalances
Electrolyte imbalances can be caused by cancer or its treatment. The following imbalances could be encountered, as summarized in Table 1.
Table 1.
Electrolyte disturbances in cancer
| Abnormality | Risk | Cause |
|---|---|---|
| Hypercalcemia | AKI | Increased GI absorbtion, reduced renal excretion, or increased bone lysis |
| Hypocalcemia | Tetany | Decreased GI absorbtion, increased renal excretion, TLS, bisphosphonates, or osteoblastic metastasis |
| Hyperphosphatemia | Precipitation | Increased GI absorbtion, reduced renal excretion, or redistribution |
| Hypophosphatemia | Weakness | Increased renal excretion |
| Hypernatremia | Thirst, lethargy | Water loss |
| Hyponatremia | Confusion | Water retention |
| Hyperkalemia | Arrhythmia | AKI, TLS, hypoaldosteronism |
| Hypokalemia | Weakness | GI loss, hyperadrenalism |
| Hypermagnesemia | Arrhythmia | AKI |
| Hypomagnesemia | Cramps, hypocalcemia | GI loss, tubulointerstitial disease |
Abbreviations: AKI, acute kidney injury, GI, gastrointestinal; TLS tumor lysis syndrome, SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Hypercalcemia
Seen in up to in 20%–30% of patients with advanced cancer and carrying a poor prognosis, hypercalcemia could result from bone metastasis (osteolytic hypercalcemia), or, in lymphoma, from overproduction of 1,25 vitamin D3. Both of these mechanisms will cause hypercalciuria with elevated fractional excretion of calcium. For reference, the expected fractional excretion of calcium is 1% to 2% in patients with eGFR above 50 mL/min/1.73 m2. However, when hypercalcemia is caused by parathyroid hormone–related peptide (PTHrP), there is a low urinary calcium excretion. Hypercalcemia causes polyuria (24-hour urine volume > 3 liters) due to collecting duct insensitivity to vasopressin, and acute kidney injury (AKI) due to volume depletion that itself aggravates the hypercalcemia. Polyuria may increase urinary potassium, magnesium, and phosphorus.
Hypercalcemia is treated with parenteral saline, which restores sufficient intravascular volume. Treatment with loop diuretics is no longer recommended, unless there is fluid overload. Intravenous bisphosphonates should be given as soon as hypercalcemia is diagnosed, at a dose adjusted for reduced kidney function. More recently, subcutaneous denosumab has been used, which reduces calcium release from bone. Cinacalcet, a calcium receptor sensitizer, could be used in patients with parathyroid cancer. In severe and intractable hypercalcemia in the setting of oliguric kidney failure, hemodialysis therapy with a dialysate calcium concentration < 2.5 mEq/L could be started.
Hypocalcemia
Hypocalcemia is common in patients with cancer. Total serum calcium may be low due to hypoalbuminemia; testing the serum ionized calcium will confirm if true hypocalcemia (serum ionized calcium < 1 mmol/L) is present. If so, one must consider osteoblastic metastatic bone disease, use of bisphosphonates, magnesium depletion following cisplatin administration, or even tumor lysis syndrome with hyperphosphatemia that has caused precipitation of calcium phosphate in tissues. Managing hypocalcemia depends on its severity. For tetany or seizures, urgent intravenous calcium chloride is required (eg, 10 mL of 10 % calcium gluconate in 50mL of 5% dextrose in water given over 10 minutes). In pauci-symptomatic true hypocalcemia due to osteoblastic bone metastases, oral calcium and 1,25-dihydroxyvitamin D could be a therapeutic option. At the same time, one addresses the cause by stopping bisphosphonates, correcting hypomagnesemia, and/or treating hyperphosphatemia.
Hypophosphatemia
Hypophosphatemia could result from malnutrition in advanced cancer, from paraneoplastic PTHrP secretion, or chemotherapy inducing renal phosphate wasting (ie, a fractional excretion of phosphate > 15% when the eGFR is in the normal range). Ifosfamide is a known culprit; a similar Fanconi syndrome could also occur after cisplatin or pamidronate use. The multi-target tyrosine kinase inhibitors imatinib, sunitinib, and sorafenib can generate hypophosphatemia as well, through inhibition of bone remodelling and phosphaturia. Oncogenic osteomalacia related to tumoral fibroblast growth factor 23 (FGF-23) secretion causes phosphaturia leading to hypophosphatemia, and can be debilitating.
Hypophosphatemia should be treated by oral phosphate supplementation (eg, potassium phosphate packets of 8 mmol each up to 4 times a day), or, for marked hypophosphatemia (< 1 mg/dl) by intravenous administration of phosphate (eg, 0.25 mmol/kg given over 6 hours and repeated as necessary).
Hyperphosphatemia
Hyperphosphatemia could be the result of cellular injury from rhabdomyolysis or TLS. Kidney failure is also a common cause. Extreme increases in serum phosphate concentration may be associated with hypocalcemia due to calcium-phosphate tissue precipitation, particularly within the kidney, with a risk of acute obstructive nephropathy. Oral phosphate binders may be used for treatment, along with parenteral crystalloid. Dialysis will be needed for patients experiencing kidney failure.
Hyponatremia
Hyponatremia is very common in malignancy and increases morbidity and mortality. The initial step in evaluating hyponatremia is assessment of serum osmolality to distinguish pseudohyponatremia due to hyperglycemia, hyperlipidemia, or hyperproteinemia from hypoosmolar hyponatremia. The clinical symptoms depend on the speed and magnitude of hyponatremia and its cause. The condition can be observed in paraneoplastic syndromes due to inappropriate secretion of anti-diuretic hormone (SIADH). Those patients will be euvolemic on physical exam, and have a urine sodium concentration and osmolality higher than 30 mmol/l and100 mOsm/kg, respectively. The unregulated ADH production could be the direct result of cancers, especially those of the lung or brain. It can also result from drugs such as cyclophosphamide or vincristine. Volume depletion from tubular toxicity induced by chemotherapy or caused by nausea, vomiting, or diarrhea could lead to non-osmotic secretion of ADH. This will be associated with a urine sodium less than 30 mmol/L and a high urine osmolarity. Finally, hyponatremia may occur in cancer patients for the same reasons it does in the general population, for instance from the use of thiazide diuretics or carbamazepine. The management of hyponatremia depends on the pathophysiologic mechanism and volume status. Hypovolemia requires use of parenteral saline. Correction of hyponatremia that has lasted more than 48 hours must be at a rate of 8 mmol/L per day or less to avoid brain demyelination syndromes. Three percent (hypertonic) saline should only be used if there are seizures or change in mental status from hyponatremia.
Hypernatremia
Hypernatremia could be the result of thirst impairment, inability to drink water, or the presence of central or nephrogenic diabetes insipidus. Central diabetes insipidus could be caused by leukemic infiltration of the pituitary gland, or primary or metastatic tumors at that site. Nephrogenic diabetes insipidus could result from hypercalcemia, hypokalemia, or urinary tract obstruction. Treatment must restore extracellular volume by use of hypotonic fluids, the amounts of which are calculated to decrease the serum sodium by <10 mmol/l over the initial 24 hours.
Hyperkalemia
While it_is an important abnormality in cancer patients, hyperkalemia may also be an artefact in such patients, for instance from leukemic cell lysis. After ruling out this possibility, the next step is to identify excess potassium intake (oral or intravenous) or deficient excretion (chronic kidney failure, urinary tract obstruction, volume depletion, use of drugs causing hypoaldosteronism), or transcellular potassium shifts (eg, from TLS). For reference, the expected fractional excretion of potassium is 10% in subjects with normal kidney function. Lower-than-expected values indicate impaired renal potassium excretion. Treatment for hyperkalemia in cancer patients is the same in any other patient.
Hypokalemia
Hypokalemia can frequently occur as well. Excessive potassium losses could be gastrointestinal or renal. For instance, hypokalemia may be secondary to Fanconi syndrome due to multiple myeloma or drugs, and thus associated with other electrolyte abnormalities such as hypophosphatemia. Hypokalemia with urinary potassium > 20 mmol/24 h or a higher–than-expected fractional excretion is caused by kidney disorders. Abiraterone, used in metastatic castration-refractory prostate cancer, can cause hypokalemia related to excess concentration of mineralocorticoid through adrenal CYP17 inhibition and reactive corticotropin (ACTH) secretion. Fluid retention and hypertension may occur. Paraneoplastic ACTH secretion or adrenal cortical cancers are rare but challenging causes of hypokalemia.
The treatment of hypokalemia is urgent in the presence of weakness or arrythmias. The intravenous route for potassium administration should then be used, but at a concentration of no more than 40 mEq/L and a rate no higher than 10 mEq/h if using a peripheral vein. In a less severe situation, it is better to replace potassium deficit orally. Magnesium supplementation is needed when hypokalemia is caused by hypomagnesemia. Spironolactone or amiloride could be used for patients with hypokalemia caused by persistent cancer-related corticosteroid secretion. Hypokalemia due to abiraterone can be corrected by parallel use of prednisone.
Hypomagnesemia
Hypomagnesemia is due to kidney or gastrointestinal losses. Kidney losses could be the result of chemotherapeutic agents including cisplatin, carboplatin, oxaliplatin, ifosfamide, and epidermal-growth factor receptor antibodies. For reference, the fractional excretion of magnesium is ~4% for a GFR in the normal range. If hypomagnesemia is not corrected, hypocalcemia and hypokalemia may ensue. Hypomagnesemia also could be induced by kidney wasting in the presence of hypercalcemia due to a competition in the loop of Henle for paracellular reabsorption. The treatment is oral magnesium supplementation, but parenteral magnesium is required if there is arrhythmia or tetany.
Hypermagnesemia
Hypermagnesemia could rarely occur. It is sometimes noted in advanced kidney failure and high magnesium intake, and is treated by stopping magnesium supplementation.
Tumor lysis syndrome
TLS combines hyperkalemia, hyperphosphatemia, severe hyperuricemia, and secondary hypocalcemia. It has been reported in every cancer type but is primarily seen in tumors with a large burden or high proliferative rate such as in hematologic malignancies. Its incidence varies from sporadic case reports in certain solid tumors to more than 25% in high-grade B-cell acute lymphoblastic leukemia. TLS is due to rapid release into the extracellular space of substances from lysing malignant cells, with the rapid serum increase of phosphate, potassium, and uric acid, the latter derived from the breakdown of nucleic acids. This can lead to severe oliguric AKI due to tubular obstruction with uric acid crystals possibly associated with intrarenal deposition of calcium phosphate. Kidney failure then limits the clearance of potassium, phosphate, and uric acid, aggravating these abnormalities and leading to secondary hypocalcemia due to calcium-phosphate deposits in tissues. Prevention of TLS may be more effective than treatment. One must identify those at high risk in whom preventative measures must be applied.
TLS should be considered in any patient with AKI and a significant burden of malignant disease, particularly in the setting of hyperuricemia, hyperkalemia, and hyperphosphatemia. Accurate risk assessment is vital to prevent TLS. Patients at risk of TLS should receive at least 3 L/d of oral or intravenous fluid before initiation of chemotherapy, provided they have no contraindications to volume expansion, to induce a high urine output. Among patients at medium or high risk of developing TLS, a prophylactic xanthine oxidase inhibitor like allopurinol (or febuxostat in cases of allopurinol hypersensitivity or reduced kidney function) should be started. In patients with high-risk tumor types, consensus guidelines suggest the prophylactic use of recombinant urate oxidase (rasburicase) before chemotherapy. Using intravenous bicarbonate perfusion to avoid uric acid precipitation by inducing extracellular alkalinisation is no longer advised because of the high risk for calcium phosphate deposition in tissues and secondary severe symptomatic hypocalcemia.
Hemodialysis, continuous or intermittent, may be needed for AKI caused by TLS, as in any case of AKI.
Suggested Readings
-
»
Rosner MH and Dalkin AC. Electrolyte disorders associated with cancer. Advances in chronic Kidney Dis. 2014;21 :7–17.
-
»
Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;352 :373–379.
-
»
Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi’s syndrome. Am J Kidney Dis. 2003;41 :292–309.
-
»
Doshi S, Shah P, Lei X, et al. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012;59:222–228.
-
»
Sterns R. Disorders of Plasma Sodium - Causes, Consequences, and Correction. N Engl J Med 2015; 372: 55–65.
-
»
Garofeanu C, Weir M, Rosas-Arellano P, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45 :626–637.
-
»
Saif MW. Management of hypomagnesemia in cancer patients receiving chemotherapy. J Support Oncol. 2008;6:243–248.
-
»
Wilson P and Berns J. Tumor Lysis Syndrome: New Challenges and Recent Advances. Adv Chronic Kidney Dis 2014; 21:18–26
ACUTE KIDNEY INJURY
Epidemiology
AKI is a common condition that is associated with increases in costs, length of hospital stay, morbidity, and mortality. A Danish population-based study reported the incidence of AKI (defined as a doubling of serum creatinine) to be 18% in the first year after cancer diagnosis. This is very much higher than the incidence of AKI in the general population, which is about one per thousand per year. Patients at higher risk for developing AKI include those with kidney cancer, multiple myeloma, liver cancer, and acute leukemia and lymphoma undergoing induction chemotherapy. Patients undergoing hematopoietic stem cell (HSC) transplantation or nephrectomy for renal cell cancer and those admitted to the intensive care unit (ICU) are also at higher risk. In addition, diabetes, chemotherapy, intravenous contrast, hyponatremia, and antibiotics are associated with an increased risk of AKI. A recent study from the MD Anderson Cancer Center reported that 12% of hospitalized patients developed AKI, a quarter of whom required dialysis. In this series, more than half of patients developed AKI more than 2 days after hospitalization. This points to a window of opportunity for preventive or mitigating interventions to optimize the renal status of the patient before chemotherapy, perhaps by hydration and removal of nephrotoxic medications.
Causes and Treatment of AKI
The causes of AKI can be divided into cancer-specific and cancer non-specific causes. Cancer-specific causes include nephrotoxic chemotherapy, cast nephropathy, obstructive nephropathy, hypercalcemia, lymphomatous infiltration of the kidney, hepatic sinusoidal obstruction syndrome, thrombotic microangiopathy, and TLS. Cancer non-specific causes are volume depletion, medication (diuretics, angiotensin-converting enzyme [ACE] inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs]), and contrast nephropathy. The work-up and treatment is similar to that of AKI in the general population and focuses on establishing the prerenal, renal, or post-renal nature of AKI (Table 2), optimizing volume status, treating the underlying cause, and—if necessary—renal replacement therapy. As in the general population, the dominant causes of AKI in critically ill patients with cancer are sepsis and hypotension (Figure 1). Thus, improving the diagnosis and treatment of AKI in the general population will also benefit patients with cancer and AKI.
Table 2.
Types of Acute Kidney Injury
| Site of injury | Cause |
|---|---|
| Prerenal | Hypovolemia, cardiac failure, hepatorenal syndrome |
| Renal | |
| Glomeruli | Small vessel disease: TMA, vasculitis, atheroembolism, light-chain associated glomerular disease |
| Vasculature | |
| Vein | Renal vein thrombosis |
| Artery | Arterial occlusion, large/medium vessel vasculitis |
| Interstitium | Drugs, infections, systemic diseases |
| Tubules | |
| Acute tubular necrosis | Ischemia, nephrotoxins, rhabdomyolysis, radiocontrast |
| Intratubular obstruction | Cast, crystalluria, tumor lysis syndrome, drugs |
| Postrenal | Tumoral invasion of ureter, retroperitoneal fibrosis, bladder outlet obstruction, renal calculi, papillary necrosis |
Abbreviations: TMA, thrombotic microangiopathy.
Figure 1.
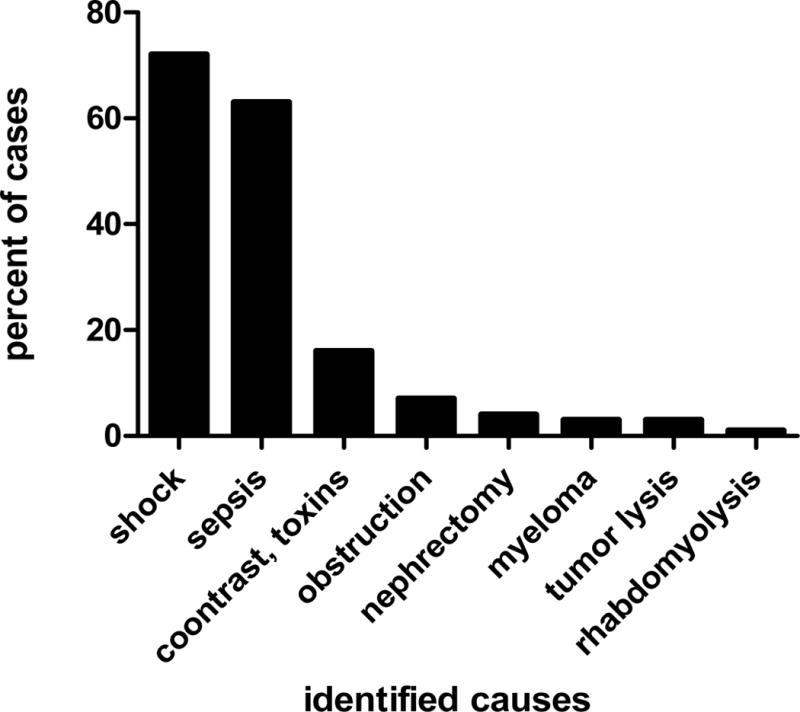
Causes of acute kidney injury in critically ill cancer patients. Data from Soares et al (Prognosis of critically ill patients with cancer and acute renal dysfunction. J Clin Oncol. 2006;24:4003–4010).
Role of Kidney Biopsy
In a recent report, only 0.66% of the kidney biopsies performed at Brigham and Women’s Hospital in Boston were done in patients with cancer. Nephrologists appear reluctant to perform a kidney biopsy in patients with cancer, but tubulointerstitial nephritis is an underrecognized yet treatable entity in cancer patients that may only be apparent on biopsy. Ifosfamide, BCG, tyrosine kinase inhibitors, premetrexed, and anti-CTLA4 antibodies have been associated with tubulointerstitial nephritis in patients receiving chemotherapy. Glucocorticosteroids may be effective in treating the condition.
Treatment by Dialysis
The available data suggest that hemodialysis should be offered to patients in the ICU with both cancer and AKI. Kidney recovery is possible and survival of selected patients with cancer is similar to that of non-cancer ICU patients with AKI requiring hemodialysis. In patients with cancer and AKI, sustained low efficiency dialysis may be the treatment of choice in the ICU.
Consequences of AKI
AKI can result both in under-treatment and over-treatment; the former due to delay or cancellation of chemotherapy and the latter due to unadjusted dosing of chemotherapy. A recent study showed that the occurrence of AKI was associated with a decreased rate of cancer remission. AKI after HSC transplantation is associated with later development of CKD, but whether this is true for cancer patients in general is not known. Because survival is improving in cancer patients, the development of CKD after AKI could have a major impact on morbidity and mortality. Automated detection of the occurrence of AKI, early involvement of a nephrologist, and better preventive, mitigating, and treatment strategies may improve the outcome of AKI in patients with cancer.
Suggested Readings
-
»
Christiansen CF, Johansen MB, Langeberg WJ, Fryzek JP, Sorensen HT. Incidence of acute kidney injury in cancer patients: a Danish population-based cohort study. Eur J Intern Med 2011 ;22:399–406.
-
»
Salahudeen AK, Doshi SM, Pawar T, Nowshad G, Lahoti A, Shah P. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol 2013;8:347–354.
-
»
Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol 2012;7:1692–1700.
-
»
Airy M, Raghavan R, Truong LD, Eknoyan G. Tubulointerstitial nephritis and cancer chemotherapy: update on a neglected clinical entity. Nephrol Dial Transplant 2013;28:2502–2509.
-
»
Raghavan R, Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol 2014;82:149–162.
-
»
Darmon M, Thiery G, Ciroldi M, Porcher R, Schlemmer B, Azoulay E. Should dialysis be offered to cancer patients with acute kidney injury? Intensive Care Med 2007;33:765– 772.
-
»
Salahudeen AK, Kumar V, Madan N et al. Sustained low efficiency dialysis in the continuous mode (C-SLED): dialysis efficacy, clinical outcomes, and survival predictors in critically ill cancer patients. Clin J Am Soc Nephrol 2009;4:1338–1346.
-
»
Canet E, Zafrani L, Lambert J et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: impact on remission and survival. PLoS One 2013;8:e55870.
CANCER CHEMOTHERAPY NEPHROTOXICITY
Obvious renal toxicity may result from hemodynamic changes, parenchymal injury, and/or urinary obstruction. More subtle kidney damage (eg, acid–base abnormalities, disorders of water balance disorders, electrolyte imbalances, mild urinary sediment abnormalities, tubulopathy) are frequently unrecognized, and therefore the true incidence of nephrotoxicity is difficult to determine. Most episodes of medication-induced GFR loss are reversible, with kidney function returning to baseline when the drug is withdrawn. CKD can occur via glomerular scarring or tubulointerstitial inflammation. This review is limited to frequently used agents, such as platinum, methotrexate, and gemcitabine, and to more recent molecules such as tyrosine kinase inhibitors. Other anticancer drug-induced nephrotoxicities are summarized in Table 3.
Table 3.
Nephrotoxicity related to some anticancer drugs
| Drug | Nephrotoxicity profile | Prevention or Management |
|---|---|---|
| Azacitidine | Fanconi syndrome, nephrogenic diabetes insipidus | Discontinuation of the drug |
| Carmustine | Decreased kidney perfusion induced by hypotension (resolves a few hours after completion of carmustine administration) CKD 1 to 24 mo following completion of therapy |
Administration of supplemental crystalloid fluid; reduction of the carmustine infusion rate by 50% or drug discontinuation Vasopressor administration; antihypertensive medication should be discontinued 24 h preceding and withheld on the day of carmustine administration |
| Cyclophosphamide | SIADH, nephrogenic diabetes insipidus Hemorrhagic cystitis | Discontinuation of the drug Aggressive hydration and mesna use |
| Ifosfamide | Fanconi syndrome, CKD, SIADH, nephrogenic diabetes insipidus; risk factors include cumulative ifosfamide dose > 50 g/m2, preexisting GFR loss and/or nephrectomy, age ≤ 12 y, Wilms tumor, previous cisplatin treatment | If possible, ifosfamide should be discontinued in patients developing signs of moderate to severe AKI during therapy; oral and/or IV fluid and electrolyte supportive therapy should be provided |
| Interferon | Acute tubular necrosis and variable glomerular lesions, proteinuria in up 5%–20% of patients; normalisation of sCr level generally occurs within weeks to months of drug discontinuation; SIADH TMA |
If possible, treatment with interferon alfa should be discontinued with the onset of AKI Prompt diagnosis, early discontinuation of the drug and supportive treatment may improve the outcome |
| IL-2 | Decreased kidney perfusion induced by capillary leak syndrome; time and dose dependent; occurs 24–48 h after initiating therapy; risk factors include baseline CKD, previous hypertension, male sex, sepsis, cardiac dysfunction | Supplemental crystalloid fluid administration; diuresis; discontinuation of antihypertensive therapy prior and during IL-2 infusion |
| Mercaptopurine | Fanconi syndrome | Discontinuation of the drug |
| Mitomycin C | TMA; risk at a cumulative dose > 30 mg/m2; generally arises 4–8 wk after the end of therapy but the onset may occur immediately after treatment or up to 9 mo later; recovery is possible but CKD is usually progressive and dialysis is required in almost 1/3 of patients; case fatality rate is >50% and median time to death is ~4 wk | Prompt diagnosis, early discontinuation of the drug, and supportive treatment may improve the outcome |
| Streptozotocin | Fanconi syndrome, CKD, glomerular toxicity and kidney failure | Discontinuation of the drug (continued treatment generally results in irreversible injury) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; SIADH: syndrome of inappropriate secretion of antidiuretic hormone; GFR, glomerular filtration rate; TMA, Thrombotic microangiopathy; sCr, serum creatinine; IL-2, interleukin 2; IV, intravenous
The most frequent nephrotoxic drug reaction is AKI, characterized by a rapidly increasing serum creatinine. Each drug has its own pattern of injury. If glomerular injury predominates, proteinuria level may be in the nephrotic range or at lower levels in association with microscopic hematuria. Hypertension predominates in vascular or glomerular syndromes. Signs of allergic reaction are absent in most patients.
Platinum salts
Cisplatin and its analogs carboplatin and oxaliplatin are widely used. Early trials with cisplatin reported that more than 70% of patients developed dose-related AKI. At high cisplatin doses, 42% of the treated patients had nephrotoxic injury. In a meta-analysis of randomized phase II and III clinical trials comparing first-line platinum-based chemotherapy to the same regimen without platinum, platinum was associated with a significant increase in nephrotoxicity (18 trials; 4,384 patients; OR 3.09; 95% CI, 1.88–5.06; P < 0.0001). Carboplatin is considered to be less nephrotoxic than cisplatin. Only rare cases of acute tubular necrosis induced by oxaliplatin have been reported. Clinical practice guidelines have been published on the prevention of cisplatin-induced kidney injury. They include correction of pre-existing volume depletion, appropriate provision of parenteral saline during drug administration and the following days, and prevention of chemotherapy-induced nausea and vomiting.
Cisplatin has also been associated with hemolytic uremic syndrome (HUS), either alone or in combination with bleomycin and vinblastine. Signs of HUS occur 1 to 4 months after the last dose of cisplatin. Interventions with aspirin, dipyridamole, fresh frozen plasma, plasma exchange, and red blood cell transfusions have shown variable efficacies.
A significant issue with carboplatin is the calculation of its dosage using the Calvert formula: total dose of carboplatin (mg) = (AUC)×(GFR + 25), where AUC (area under the curve) is the serum concentration being targeted for the drug. The Calvert formula was established using measured GFR using radiolabeled chromium-EDTA as a filtration marker. However in clinical practice, creatinine clearance (calculated by the Cockcroft-Gault formula) or eGFR (calculated by the MDRD Study or CKD-EPI equation) are routinely used. There are only sparse data evaluting the accuracy of the Calvert formula with these estimates.
Methotrexate
A report in the 1970s, reported AKI in 30% to 50% of patients after high-dose methotrexate therapy with leucovorin rescue. In the same era, a single-center study of 64 patients recorded 3 deaths secondary to AKI, and an analysis of 498 patients treated according to National Cancer Institute protocols found that 29 died after high-dose methotrexate therapy, establishing a mortality rate of 6%. Yet in a report published in 2004, only 1.8% of patients with osteosarcoma who were treated with high-dose methotrexate developed significant nephrotoxicity.
Methotrexate is renally excreted both intact and as 7-hydroxymethotrexate, a more insoluble metabolite,. In acid urine (pH <5.5), both compounds precipitate, whereas solubility is 10-fold greater at neutral pH. Furthermore, a dramatic rise in the methotrexate-creatinine clearance ratio is observed when urinary pH is increased from 5.5 to 8.4.
The variable incidence of high-dose methotrexate–induced AKI may be genetically determined. Anionic drugs such as methotrexate can be eliminated by multidrug resistance protein 2 (MRP2) transporter, which is expressed at the luminal side of renal proximal tubular cells. A heterozygous mutation of MRP2 is associated with reduced methotrexate excretion and increased nephrotoxicity; as a result, candidates for methotrexate therapy might benefit from MRP2 functional testing.
High-dose methotrexate-induced nephrotoxicity is managed with parenteral crystalloid and alkalinization (to provide adequate urine output), high-dose leucovorin, dialysis-based methods of methotrexate removal, and thymidine. For patients with delayed methotrexate excretion and high plasma concentrations, use of the recombinant enzyme carboxypeptidase-G2 (CPDG2) cleaves methotrexate to inactive metabolites, potentially lowering plasma methotrexate concentrations.
Gemcitabine
Gemcitabine has been associated with thrombotic microangiopathy (TMA), which is characterized by thrombocytopenia, microangiopathic hemolytic anemia, and various ischemic end organ injuries, has been reported with gemcitabine. In terms of pathology, TMA is marked by endothelial cell swelling, vessel wall thickening, intraluminal platelet thrombi, and microvascular occlusion. TMA may present as HUS, or in some cases has predominant involvement of the central nervous system, presenting as thrombotic thrombocytopenic purpura, demonstrating. Based on adverse events reporting through 1997, the manufacturer of gemcitabine estimated the incidence of TMA with gemcitabine use as 0.015% (reports range from 0.02% to 0.3%). Although underreporting is possible, TMA with gemcitabine remains rare in comparison to incidence rates of 2.6–13.0% for either malignancy-induced or chemotherapy-induced HUS. Signs and symptoms of TMA usually develop within 1–2 months of the last gemcitabine dose, and the outcome with TMA is poor: mortality rates range from 10% to 70%.
Although effective strategies for preventing or reducing the severity of gemcitabine-associated TMA have not been identified, several have been tested: exchange transfusion, hemodialysis, fresh frozen plasma transfusion, immunoadsorption, plasmapheresis, immunosuppressive therapies (azathioprine, corticosteroids, or vincristine), and antiplatelet/anticoagulant therapies (antiplatelet drugs, heparin, prostacyclin, or splenectomy).,. If the drug is still being given when gemcitabine-associated TMA is identified, it must be discontinued. Kidney function could recover completely, but delayed diagnosis is associated with CKD, progression to end-stage renal disease, and death due to progressive disease.
Tyrosine Kinase Inhibitors
Among tyrosine kinase inhibitors, the pattern of kidney injury is glomerular for those targeted to vascular endothelial growth factor (VEGF) receptors. These drugs (sunitinib, sorafenib, axitinib, cediranib) are used to treat kidney cancer because of its high VEGF expression, whether sporadic or from the VHL gene mutation. VEGF is important in maintaining glomerular podocyte and endothelial function. Its blockade leads to proteinuria in over half of patients treated; in less than 10% of cases, to nephrotic syndrome. Rarer still is TMA caused by endothelial injury, which is an indication to stop treatment. Hypertension occurs in 30% or more of treated patients and might be a marker of drug efficacy but is not an indication to stop VEGFantagonists. Similar toxicities are described for anti-angiogenic antibodies, including bevacizumab and ramucirumab.
Other tyrosine kinase inhibitors such as crizotinib and vemurafenib, which act on ALK and BRAF, were not associated with any kidney injury in development trials. However, kidney effects have occurred in practice, with cases of AKI, sometimes severe, rapidly reported in the literature. Tyrosine kinase inhibitors also may cause interstitial nephritis. The BCR-ABL inhibitor imatinib causes phosphaturia, as mentioned in the fluid and electrolyte section of this article.
Suggested readings
-
»
Labaye J, Sarret D, Duvic C, Hérody M, Didelot F, Nédélec G, et al. Renal toxicity of oxaliplatin. Nephrol Dial Transplant. 2005 Jun;20(6):1275–6.
-
»
Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008 May;61(6):903–9.
-
»
van der Heijden M, Ackland SP, Deveridge S. Haemolytic uraemic syndrome associated with bleomycin, epirubicin and cisplatin chemotherapy–a case report and review of the literature. Acta Oncol. 1998;37(1):107–9.
-
»
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989 Nov;7(11):1748–56.
-
»
Abelson HT, Fosburg MT, Beardsley GP, Goorin AM, Gorka C, Link M, et al. Methotrexate-induced renal impairment: clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol. 1983 Mar;1(3):208–16.
-
»
Von Hoff DD, Penta JS, Helman LJ, Slavik M. Incidence of drug-related deaths secondary to high-dose methotrexate and citrovorum factor administration. Cancer Treat Rep. 1977 Jul;61(4):745–8.
-
»
Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, et al. Highdose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004 May 15;100(10):2222–32.
-
»
Sand TE, Jacobsen S. Effect of urine pH and flow on renal clearance of methotrexate. Eur J Clin Pharmacol. 1981;19(6):453–6.
-
»
Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, et al. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005 May;15(5):277–85.
-
»
Flombaum CD, Mouradian JA, Casper ES, Erlandson RA, Benedetti F. Thrombotic microangiopathy as a complication of long-term therapy with gemcitabine. Am J Kidney Dis. 1999 Mar;33(3):555–62.
-
»
Sheldon R, Slaughter D. A syndrome of microangiopathic hemolytic anemia, renal impairment, and pulmonary edema in chemotherapy-treated patients with adenocarcinoma. Cancer. 1986 Oct 1;58(7):1428–36.
-
»
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J,et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008 Mar 13;358(11):1129–36.
-
»
Launay-Vacher V, Zimner-Rapuch S, Poulalhon N, Fraisse T, Garrigue V, Gosselin M, et al. Acute renal failure associated with the new BRAF inhibitor vemurafenib: a case series of 8 patients. Cancer. 2014 Jul 15;120(14):2158–63.
-
»
Airy M, Raghavan R, Truong LD, Eknoyan G. Tubulointerstitial nephritis and cancer chemotherapy: update on a neglected clinical entity. Nephrol Dial Transplant. 2013 Oct;28(10):2502–9.
PARANEOPLASTIC GLOMERULAR DISEASES
Paraneoplastic glomerular diseases are manifestations caused by products secreted by cancer cells such as tumor antigens, hormones, growth factors, or cytokines. Unexplained proteinuria can be an indication to a search for malignancy, especially in older patients.
Epidemiology
Table 4 shows the glomerular lesions linked to cancer. The most common lesion in solid cancers is membranous nephropathy (MN). In patients with MN, the prevalence of solid tumors ranges from 1% to 22%; for instance, a prevalence of 10% was found in a large retrospective study of 240 individuals with biopsy-proven MN. At the time of biopsy, only 50% of the patients may have symptoms related to their cancer. The majority are diagnosed with malignancy within a year of detection of MN; however, the risk of finding cancer could persist for over 10 years from the time of the biopsy. In one report of 21 cases of minimal change disease and Hodgkin lymphoma, nephrotic syndrome appeared in 38%, 19%, and 43% of patients before, with, and after the cancer diagnosis, respectively. In one report of glomerulonephritis and chronic lymphocytic leukemia, the B-cell proliferation and glomerulopathy were simultaneously diagnosed in 7 of 13 (5%) patients.
Table 4.
Classification of paraneoplastic glomerulopathies
| Rank | Solid cancer | Hematological cancer |
|---|---|---|
| 1 | Membranous nephropathy (lung, gastrointestinal, kidney cancer) | Minimal change disease (Hodgkin lymphoma, thymoma) |
| 2 | Minimal change disease (lung, kidney, colorectal cancer) | Membranoproliferative glomerulonephritis (chronic lymphocytic leukemia, non-Hodgkin lymphoma) |
| 3 | Crescentic glomerulonephritis (kidney, gastric cancer) | Membranous Nephropathy (chronic lymphocytic leukemia, non-Hodgkin lymphoma) |
| 4 | Membranoproliferative glomerulonephritis (lung cancer, melanoma, kidney cancer) | Crescentic glomerulonephritis (chronic lymphocytic leukemia, Hodgkin and non-Hodgkin lymphoma) |
| 5 | IgA nephropathy (kidney cancer) | IgA nephropathy (non-Hodgkin lymphoma) |
| 6 | Focal Segmental Glomerulosclerosis (kidney cancer) | Focal Segmental Glomerulosclerosis (Hodgkin lymphoma) |
| 7 | AA Amyloidosis (kidney cancer) | AA Amyloidosis (Hodgkin and non-Hodgkin lymphoma) |
Ranking of most to least frequent glomerulopathies associated with solid (first column) and hematologic (second column) cancers. For each glomerulopathy, the specific cancers that are most commonly associated are given in parentheses. Adapted from Bacchetta J et al (Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70:39–58) and Jhaveri et al (Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013;84:34–44)
Diagnosis
Box 1 shows features that may point to paraneoplastic membranous nephropathy. Confirmation of paraneoplastic glomerular diseases is based on remission of the symptoms and histologic lesions after cure or remission of the cancer, relapse of kidney disease if the cancer recurs, and existence of a pathophysiologic link between cancer and the glomerulopathy (eg, cancer antigen trapped in the glomerular filtration barrier).
Box 1. Characteristics suggestive of paraneoplastic membranous nephropathy.
-
-
Age greater than 65
-
-
Smoking history in excess 20 pack-years
-
-
Absence of anti-phospholipase A2 receptor (PLA2R1) antibodies
-
-
Presence of high IgG1 and IgG2 subtype deposits and more than eight inflammatory cells per glomerulus observed in kidney biopsy specimen
-
-
Regression of glomerular lesions and proteinuria upon remission of the cancer
-
-
No obvious other cause
Abbreviation: Ig, immunoglobulin.
Glomerular disease, notably a nephrotic syndrome related to MN, could also be noted in patients following HSC transplantation. In the case of MCD detection, it seems prudent to look for recurrence of the primary hematologic malignancy.
There is a temporal relationship between cessation of immunosuppressive drugs and nephrotic syndrome, concomitantly with the development of graft-vs-host disease, especially after allogenic HSC transplantation.
Treatment of Paraneoplastic Glomerular Diseases
Cure or remission of the cancer is the primary goal. It is not known if treatments such as cyclophosphamide or rituximab for membranous nephropathy will be effective for its paraneoplastic variant. It is prudent to also use nephroprotective therapies, including low salt diet and appropriate antihypertensive therapy, to try to slow progression to end-stage renal disease.
Suggested Readings
-
»
Bacchetta J, Juillard L, Cochat P, Droz JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70: 39–58.
-
»
Lefaucheur C, Stengel B, Nochy D, et al.; GN-PROGRESS Study Group. Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70: 1510–1517.
-
»
Bjørneklett R, Vikse BE, Svarstad E, et al. Long-term risk of cancer in membranous nephropathy patients. Am J Kidney Dis. 2007;50: 396–403.
-
»
Audard V, Larousserie F, Grimbert P, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: Report of 21 cases and review of the literature. Kidney Int. 2006; 69: 2251–2260.
-
»
Moulin B, Ronco P, Mougenot B et al. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992; 42: 127–135.
-
»
Debiec H, Ronco P. Nephrotic syndrome: A new specific test for idiopathic membranous nephropathy. Nat Rev Nephrol. 2011;7: 496–498.
-
»
Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19: 574–579.
-
»
Cambier JF, Ronco P. Onco-Nephrology: Glomerular Diseases with Cancer. Clin J Am Soc Nephrol. 2012;7: 1701–1712.
-
»
Jhaveri KD, Shah HH, Calderon K, Campenot ES and Radhakrishnan J. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013; 84: 34–44.
PARAPROTEIN-RELATED KIDNEY DISEASE
Paraproteins are directly nephrotoxic, and a wide and diverse range of kidney diseases is associated with them (figure 2). Multiple myeloma, which has a yearly incidence of about 60 per million general population, is the most common cause of paraprotein-induced kidney disease, At the time of diagnosis, half of patients with multiple myeloma will have reduced kidney function and 10% will require dialysis. CKD stage 5 is a poor prognostic factor in this setting, and its appearance is a medical emergency, as recovery of kidney function is unlikely in multiple myeloma patients unless therapy is initiated promptly. Although autologous stem cell transplantation remains the treatment of choice, there are only limited data available in multiple myeloma patients with kidney disease. With the introduction of novel chemotherapeutics such as bortezomib, the kidney outcomes of patients with multiple myeloma have improved significantly. Data regarding plasmapheresis in treating cast nephropathy are limited and do not allow for strong recommendation of this treatment. Recently, dialysis using membranes having a high molecular weight cut-off has been advocated for treating cast nephropathy, but there are no definite data on this question.
Figure 2.
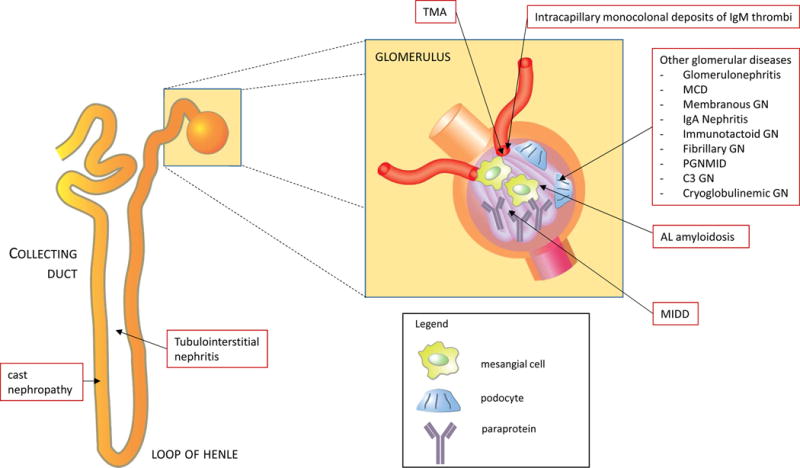
Paraprotein-related kidney injury. A growing number of kidney diseases have been associated with paraproteinemia. Abbreviations: GN, glomerulonephritis; Ig, immunoglobulin; MCD minimal change disease; MIDD, monoclonal immunoglobulin deposition disease; PGNMID, proliferative glomerulonephritis with monoclonal immune deposits; TMA, thrombotic microangiopathy.
Diagnosis of kidney diseases associated with plasma cell malignancies has improved with the advent of the serum free light chain assay and laser microdissection–mass spectrometry. The free light chain assay, in combination with serum protein electrophoresis and immunofixation electrophoresis, is advocated for screening and monitoring of monoclonal gammopathies; normal κ:λ ratios are 0.37 to 3.1 and 0.26 to 1.65 in patients with and without CKD, respectively. With laser microdissection-mass spectrometry, selective isolation of amyloid material from kidney biopsy and subsequent protein analysis using mass spectrometry allows for more precise diagnosis of paraprotein kidney diseases. The most common kidney diseases associated with plasma cell dyscrasia are multiple myeloma and AL amyloidosis. However several other glomerular diseases have been reported to be paraprotein related, and the number is growing: light and heavy chain deposition disease, immunotactoid glomerulonephritis, fibrillary glomerulonephritis, and proliferative glomerulonephritis with monoclonal immunoglobulin deposits Monoclonal gammopathy of undetermined significance is present in 2% to 4% of people 50 years or older and has an annual rate of progression to multiple myeloma of 0.5–1.5%. Recently the term monoclonal gammopathy with renal significance was proposed to distinguish monoclonal gammopathies resulting in the development of kidney diseases in patients with B-cell clones who do not meet the definition of multiple myeloma or lymphoma.
Treatment
The optimal treatment of multiple myeloma and paraprotein-related kidney disease is evolving rapidly with the availability of novel and more targeted treatments. However, high-dose chemotherapy in combination with stem cell transplantation remains the standard of care for eligible patients. Hematologists will guide the treatment but nephrologists will often be consulted because many myeloma patients may have reduced kidney function at some point during their illness.
Suggested readings
-
»
Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma–a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol 1994;53:207–212.
-
»
Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000;65:175– 181.
-
»
Bird JM, Fuge R, Sirohi B et al. The clinical outcome and toxicity of high-dose chemotherapy and autologous stem cell transplantation in patients with myeloma or amyloid and severe renal impairment: a British Society of Blood and Marrow Transplantation study. Br J Haematol 2006;134:385–390.
-
»
Hutchison CA, Heyne N, Airia P et al. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 2012;27:3823–3828.
-
»
Hutchison CA, Basnayake K, Cockwell P. Serum free light chain assessment in monoclonal gammopathy and kidney disease. Nat Rev Nephrol 2009;5:621–628.
-
»
Sethi S, Vrana JA, Theis JD, Dogan A. Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 2013;22:273–280.
-
»
Leung N, Bridoux F, Hutchison CA et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood 2012;120:4292–4295.
-
»
Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol 2012;9:135–143.
URINARY TRACT OBSTRUCTION
Occurrence and Prognosis
Urinary tract obstruction is an ominous development in adults with cancer. It can occur as a presenting feature, in instances of prostate or bladder cancer, or of non-urologic cancers, such as lymphomas or gynecologic cancers. The median survival of these patients is about 100 days. Survival can be further predicted by assessment of metastatic events, degree of hydronephrosis, and serum albumin level. Thus, 3 or more metastases, lesser degrees of hydronephrosis, and a serum albumin < 3 mg/dl predicts a 2% 6-month survival, whereas none of these risk factors predicts a 70% survival over the same period. This assessment is important in deciding on interventions to relieve obstruction.
In children with urinary tract obstruction caused by cancer, survival is much higher: 80% live 5 years post presentation. Survival rate differences between adults and children could relate to the better ability to treat cancers that cause the condition in children (eg, neuroblastoma).
Diagnosis
Kidney ultrasound or computed tomography (CT) will identify most cases of urinary tract obstruction. A few patients will develop kidney failure due to obstruction with little or no dilation of the urinary tract, which can be due to encasement of the ureters by metastatic cancer in the retroperitoneum. Unilateral or bilateral obstruction may cause pain and predispose to infection. Bilateral obstruction will cause collecting duct malfunction, resulting in the inability to concentrate the urine and variable polyuria. It also will impair potassium excretion, leading to hyperkalemia.
Treatment and prognosis
Retrograde or antegrade stenting have been used to relieve pain and improve kidney function. It is possible that percutaneous nephrostomy tubes and antegrade ureteric stenting have a better outcome as compared to retrograde approaches. Tube dislodgement and infection may complicate both. In 2 recent case series, patients treated with percutaneous nephrostomy and/or ureteric stents reportedly spent 25% to 30% of their remaining lifetime in the hospital. A palliative care consultation should be sought when an adult patient with cancer develops urinary tract obstruction.
Suggested Readings
-
»
Ishioka J, Kageyama Y, Inoue M, Higashi Y, Kihara K. Prognostic model for predicting survival after palliative urinary diversion for ureteral obstruction: analysis of 140 cases. J Urol 2008 Aug;180(2):618–21; discussion 621.
-
»
Lienert A, Ing A, Mark S. Prognostic factors in malignant ureteric obstruction. BJU Int 2009 Oct;104(7):938–941.
-
»
Alexander A, Weber B, Lorenzo A, Keays M, El-Ghazaly T, Bagli DJ, et al. Hydronephrosis in children with abdominal and pelvic neoplasms: outcome and survival analysis of a single center pediatric oncology series. J Urol 2011 Oct;186(4 Suppl):1705– 1709.
-
»
Batlle DC, Arruda JA, Kurtzman NA. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med 1981 Feb 12;304(7):373–380.
-
»
Chitale SV, Scott-Barrett S, Ho ET, Burgess NA. The management of ureteric obstruction secondary to malignant pelvic disease. Clin Radiol 2002 Dec;57(12): 1118– 1121.
-
»
Misra S, Coker C, Richenberg J. Percutaneous nephrostomy for ureteric obstruction due to advanced pelvic malignancy: have we got the balance right? Int Urol Nephrol 2013 Jun;45(3):627–632.
CANCER IN CKD, DIALYSIS, AND TRANSPLANT PATIENTS
General
Epidemiology and Screening
Population studies show a significant excess of cancer in people with ESRD and earlier stages of CKD. In CKD patients who are not dependent on renal replacement therapy, there is significant risk for lip, Kaposi, thyroid, and especially kidney and urinary tract cancer. The pattern is similar for patients treated with long-term dialysis. In kidney transplant recipients, skin cancer and lymphoma become the dominant cancers; in addition, the standardized incidence ratio is well above the expected value for kidney and urinary tract cancers. This raises the question of surveillance and screening to make earlier diagnoses of these cancers and improve treatment outcomes. However, most cancer treatment trials exclude the CKD population. Thus, the treatment benefit that is achieved by screening and early diagnosis is unknown for people with CKD who develop cancer. In an analysis using known dialysis mortality rates and that assumed equivalent results of treatment, the benefit of screening dialysis patients for usual cancers like breast, prostate, and colon was found to be insignificant. This lack of benefit occurs because the major causes of death in these patients are cardiovascular and infectious in nature, so death from cancer is less meaningful as a clinical concern. However, cancer is a feared complication of kidney transplantation. Farrugia et al reported significant cancer-related mortality in people with functioning kidney transplants and advised heightened surveillance. But screening for common cancers such as breast, prostate, and colon is not beneficial in kidney transplant recipients.
Diagnosis and Treatment
Delayed diagnosis of cancer in people with CKD can occur (for instance, pulmonary congestion might obscure a thoracic cancer). However, except for in prostate cancer, the stage of cancer at time of diagnosis is not different in dialysis patients compared to the general population. There are no data on this question for patients with non–dialysis-dependent CKD or people with functioning kidney transplants.
Treating cancer in people with CKD may be less effective and lead to higher mortality rates than when treating age-matched people without CKD. This may reflect altered characteristics of cancer in patients with CKD or may occur because treatment is more difficult to administer on schedule at effective doses. Morbidity and mortality from surgery or chemotherapy are likely to be higher in people with versus without CKD. There are highly effective chemotherapy drugs such as cisplatin that either are not used at all or are very cumbersome to administer in patients with CKD. There are no evidence-based standards for cancer treatment for patients with non– dialysis-dependent CKD, receiving dialysis, or those with a functioning kidney transplant. The nephrotoxicities and dose adjustments of cancer chemotherapy in CKD patients are discussed in the “Cancer Chemotherapy Nephrotoxicity” section of this article.
Kidney-specific Cancer
Acquired Cysts and Cancer
Cyst formation in non-cystic failing kidneys may be complicated by cancer. At a rate about 4 times that of the general population, kidney cancers occur in people with dialysis- or non–dialysis-dependent CKD and in individuals with functioning kidney transplants. These cancers can be papillary or clear cell type and can be asymptomatic, identified radiologically, or present with pain or hematuria. Screening is probably not beneficial or cost-effective. There is no evidence-based guideline on whether uni- or bi-nephrectomy is preferable. Because CKD and its duration correlate with cyst formation and CKD progression can be markedly slowed with newer treatments, it is likely that acquired cysts and their associated cancers will increase in prevalence in the foreseeable future.
Suggested Readings
-
»
Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA 2006 Dec 20;296(23):2823–2831.
-
»
Chertow GM, Paltiel AD, Owen WF,Jr, Lazarus JM. Cost-effectiveness of cancer screening in end-stage renal disease. Arch Intern Med 1996 Jun 24;156(12):1345–1350.
-
»
Farrugia D, Mahboob S, Cheshire J, Begaj I, Khosla S, Ray D, et al. Malignancy-related mortality following kidney transplantation is common. Kidney Int 2014 Jun;85(6):1395– 1403.
-
»
Kiberd BA, Keough-Ryan T, Clase CM. Screening for prostate, breast and colorectal cancer in renal transplant recipients. Am J Transplant 2003 May;3(5):619–625.
-
»
Taneja S, Mandayam S, Kayani ZZ, Kuo YF, Shahinian VB. Comparison of stage at diagnosis of cancer in patients who are on dialysis versus the general population. Clin J Am Soc Nephrol 2007 Sep;2(5):1008–1013.
-
»
Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014 Jan;63(1):23–30.
-
»
Magee C. Kidney disease and death from cancer. Am J Kidney Dis 2014 Jan;63(1):7–9.
-
»
Matson MA, Cohen EP. Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. Medicine (Baltimore) 1990 Jul;69(4):217–226.
-
»
Russo P. Oncological and renal medical importance of kidney-sparing surgery. Nat Rev Urol 2013 May;10(5):292–299.
-
»
Sarasin FP, Wong JB, Levey AS, Meyer KB. Screening for acquired cystic kidney disease: a decision analytic perspective. Kidney Int 1995 Jul;48(1):207–219.
-
»
Wong G, Howard K, Webster AC, Chapman JR, Craig JC. Screening for renal cancer in recipients of kidney transplants. Nephrol Dial Transplant 2011 May;26(5):1729–1739.
KIDNEY CANCER
Overview
Kidney cancer is no longer just an issue for urologists. This has become clear as experience with acquired cysts and their associated cancers has grown. Kidney cancer is more common in people with CKD, and surgery for kidney cancer may result in AKI, CKD, progression of preexisting CKD, or development of end-stage renal disease.
Diagnosis
Diagnosis of kidney cancer in CKD is based on symptoms, though incidental diagnosis by imaging is increasing. Urinary and serum markers may help in diagnosis: urinary kidney injury marker 1 (KIM-1) appears promising but is not in general use. CT scanning remains the standard for diagnosis and pre-operative staging. New magnetic resonance imaging techniques may enable pre-operative histologic diagnosis. Kidney biopsy of small kidney masses appears to be safe and may show benign lesions, which avoids the need for surgery.
CKD After Kidney Cancer
CKD after nephrectomy is associated with increased mortality, especially due to cardiovascular causes. Patients who have a pre-operative serum creatinine of 2 mg/dl or greater appear at increased risk of these adverse outcomes. Many studies report that radical nephrectomy is associated with a lesser patient survival when compared to partial nephrectomy, although a prospective trial comparing partial to radical nephrectomy could not confirm this effect.
Size matters when managing kidney masses. For lesions smaller than 3.5 cm, surveillance may be preferable to surgery. For cancers of up to 7 cm in diameter, partial nephrectomy is safe and may cause less subsequent kidney disease than radical nephrectomy. Some larger cancers may be suitably treated by partial rather than radical nephrectomy. Surgical improvements, including reduced clamp time, as well as laparoscopic and robotic techniques, appear to reduce acute and chronic complications. Nonetheless, cancers greater than 7 cm in diameter have a higher risk of cancer-related morbidity and mortality. Fear of worsening kidney function should not force the surgeon to do a partial nephrectomy when it is more appropriate to remove the entire kidney.
When CKD occurs after surgery for kidney cancer, management is the same as that for any cause of CKD. A patient who has only a fraction of normal kidney tissue remaining after cancer surgery faces a physiologic situation in some ways similar to that of the rat remnant kidney model of progressive loss of kidney function. In this model, low protein diet and/or ACE inhibition may slow the loss of kidney function. Perhaps this explains why patients with remnant kidneys may survive long-term and do not necessarily experience progressive kidney disease leading to the need for dialysis. The principles of care for these patients should be the same as for any cause of CKD: control of blood pressure, use of ACE inhibitors, and relief of metabolic acidosis. Reliable markers of cancer recurrence need to be developed.
Treating metastatic kidney cancer now includes agents that are truly effective and prolong life yet have definite toxicities to the kidney and other organs (see Cancer Chemotherapy Nephrotoxicity section).
Suggested Readings
-
»
Lowrance WT, Ordonez J, Udaltsova N, Russo P, Go AS. CKD and the risk of incident cancer. J Am Soc Nephrol 2014 Oct;25(10):2327–2334.
-
»
Russo P. Oncological and renal medical importance of kidney-sparing surgery. Nat Rev Urol 2013 May;10(5):292–299.
-
»
Zhang PL, Mashni JW, Sabbisetti VS, Schworer CM, Wilson GD, Wolforth SC, et al. Urine kidney injury molecule-1: a potential non-invasive biomarker for patients with renal cell carcinoma. Int Urol Nephrol 2014 Feb;46(2):379–388.
-
»
Lanzman RS, Robson PM, Sun MR, Patel AD, Mentore K, Wagner AA, et al. Arterial spin-labeling MR imaging of renal masses: correlation with histopathologic findings. Radiology 2012 Dec;265(3):799–808.
-
»
Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol 2007 Aug;178(2):379–386.
-
»
Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010 Apr;183(4):1317–1323.
-
»
Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011 Apr;59(4):543–552.
-
»
Patel N, Cranston D, Akhtar MZ, George C, Jones A, Leiblich A, et al. Active surveillance of small renal masses offers short-term oncological efficacy equivalent to radical and partial nephrectomy. BJU Int 2012 Nov;110(9): 1270–1275.
-
»
El-Ghazaly TH, Mason RJ, Rendon RA. Oncological outcomes of partial nephrectomy for tumours larger than 4 cm: A systematic review. Can Urol Assoc J 2014 Jan;8(1–2):61–66.
-
»
Long CJ, Canter DJ, Kutikov A, Li T, Simhan J, Smaldone M, et al. Partial nephrectomy for renal masses >/= 7 cm: technical, oncological and functional outcomes. BJU Int 2012 May;109(10):1450–1456.
-
»
Leslie S, Goh AC, Gill IS. Partial nephrectomy–contemporary indications, techniques and outcomes. Nat Rev Urol 2013 May;10(5):275–283.
-
»
Foster MH, Sant GR, Donohoe JF, Harrington JT. Prolonged survival with a remnant kidney. Am J Kidney Dis 1991 Mar;17(3):261–265.
RADIATION NEPHROPATHY
Epidemiology
Radiation nephropathy is uncommon. Its classical occurrence is after radiotherapy for testicular cancer in which treatments are given over a month or longer in total doses > 20 Gy. It occurs in adults or children undergoing HSC transplantation that is preceded by chemo-irradiation conditioning. It also occurs after radionuclide therapies that deliver radioisotope to kidneys in sufficient doses. However, the doses of diagnostic x-ray, CT, or radionuclide are well below those that cause kidney injury.
Presentation and Management
Radiation nephropathy occurs after the radiotherapy is complete, with a latent period of several months or more. Proteinuria, azotemia, and hypertension are the presenting features. Histologic features include mesangiolysis and tubule-interstitial scarring, with only scant inflammation. Unilateral kidney irradiation may cause renin-dependent hypertension with secondary injury to the non-irradiated kidney. Management is as for any form of CKD. As is seen in experimental models of radiation nephropathy, ACE inhibitors or angiotensin receptor blockers may be particularly effective therapies.
Newer radiotherapy protocols, for instance for pancreatic or gastric cancer, may irradiate sufficient kidney volumes at high-enough doses so as to cause kidney injury. Coordination with radiation oncology teams is important in establishing the diagnosis in such cases.
Suggested Readings
-
»
Luxton RW, Kunkler PB. Radiation Nephritis. Acta Radiol Ther Phys Biol 1964 Jun;2:169–178.
-
»
Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int 2000 Aug;58(2):903–918.
-
»
Cohen EP, Robbins ME. Radiation nephropathy. Semin Nephrol 2003 Sep;23(5):486– 499.
-
»
Cohen EP, Fish BL, Moulder JE. Mitigation of radiation injuries via suppression of the renin-angiotensin system: emphasis on radiation nephropathy. Curr Drug Targets 2010 Nov;11(11):1423–1429.
KIDNEY DISEASE AFTER HSC TRANSPLANTATION
Acute kidney injury
AKI often complicates HSC transplantation, and is more common after myelo-ablative versus non–myelo-ablative HSC transplantation (Figure 3). This might be expected because lower doses of chemotherapy and radiotherapy are used for non–myelo-ablative HSC transplantation. The median time to AKI occurrence is 20 to 30 days after the procedure. Sepsis and use of nephrotoxic antibiotics or calcineurin inhibitors are common causes of AKI, but other etiologies (such as TLS) are possible. AKI after HSC transplantation is associated with a more than 50% increase in mortality post-HSC transplantation and is strongly associated with the development of CKD.
Figure 3.
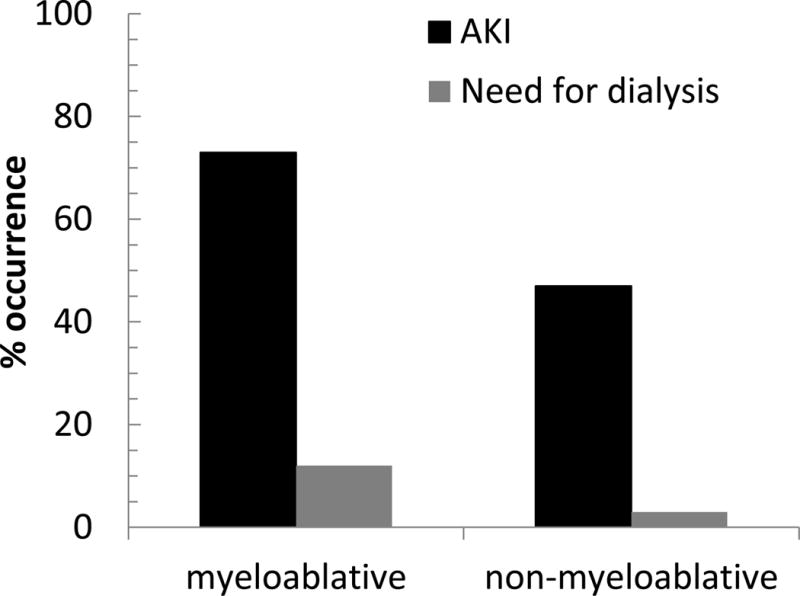
The occurrence of acute kidney injury after HSC transplantation. Acute kidney injury (AKI) is more common after myeloablative HSC transplantation, and more often leads to a requirement for dialysis. AKI is defined here as a more-than-doubling of the serum creatinine. Data from Parikh et al (Comparison of ARF After Myeloablative and Nonmyeloablative Hematopoietic Cell Transplantation. Am J Kidney Dis. 2005;45:502–509).
Diagnosis and Management
Diagnosis of AKI after HSCT may be compromised by lesser elevations in serum creatinine than expected, because of previous cancer and loss of muscle mass. If dialysis is needed, it is not known whether continuous or intermittent dialysis is preferentially effective.
Chronic Kidney Disease
As stated, AKI after HSC transplantation is associated with the development of CKD. This may occur as it can after any AKI event, related to residual kidney injury and scarring.
Other specific causes of CKD after HSC transplantation are well-known, and include drug toxicities (such as from calcineurin inhibitors), radiation nephropathy, and membranous nephropathy (Figure 4).
Figure 4.
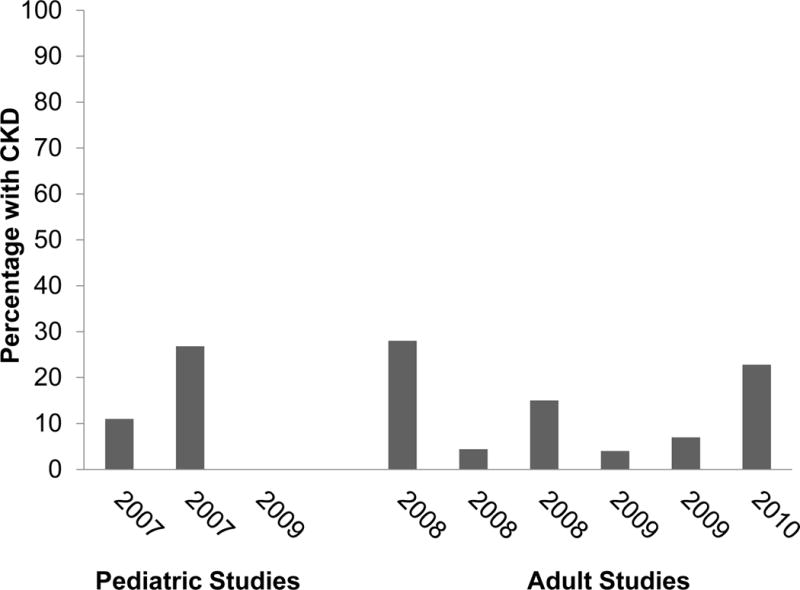
The prevalence of CKD after HSC transplantation in children and adults, based on studies from 2007 onward. The arithmetic average for all studies is 13%. Reproduced from Cohen et al (Chronic Kidney Disease After Hematopoietic Stem Cell Transplantation. Semin Nephrol. 2010;30:627–634) with permission from Elsevier.
Diagnosis and Management
CKD that presents with features of thrombotic microangiopathy within a year after HSC transplantation is likely due to calcineurin toxicity or radiation nephropathy. CKD with nephrotic range proteinuria in a patient with chronic graft-vs-host disease may indicate MCD or MN. A past history of ifosfamide and cis-platinum use in a patient with phosphaturia points to these chemotherapies as culprits. Managing CKD in a patient who received a HSC transplant is complicated by co-morbidities such as cardiotoxicity from previous chemotherapy; however, stabilization of kidney function can be achieved. Unfortunately, end-stage renal disease is much more common after HSC transplantation than in age-matched healthy individuals. Mortality of maintenance dialysis patients is quite high. Kidney transplantation is a well-described option and can be done without immunosuppression if the kidney donor is the same person who donated the HSCs.
Suggested Readings
-
»
Parikh CR, Schrier RW, Storer B, Diaconescu R, Sorror ML, Maris MB, et al. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am J Kidney Dis 2005 Mar;45(3):502–509.
-
»
Singh N, McNeely J, Parikh S, Bhinder A, Rovin BH, Shidham G. Kidney complications of hematopoietic stem cell transplantation. Am J Kidney Dis 2013 May;61(5):809–821.
-
»
Parikh CR, Yarlagadda SG, Storer B, Sorror M, Storb R, Sandmaier B. Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008 Mar;14(3):309–315.
-
»
Shimoi T, Ando M, Munakata W, Kobayashi T, Kakihana K, Ohashi K, et al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant 2013 Jan;48(1):80–84.
-
»
Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens 2014 Mar;23(2): 149–154.
-
»
Cohen EP, Pais P, Moulder JE. Chronic kidney disease after hematopoietic stem cell transplantation. Semin Nephrol 2010 Nov;30(6):627–634.
-
»
Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transplant 2007 May;39(9):571–572.
-
»
Butcher JA, Hariharan S, Adams MB, Johnson CP, Roza AM, Cohen EP. Renal transplantation for end-stage renal disease following bone marrow transplantation: a report of six cases, with and without immunosuppression. Clin Transplant 1999 Aug;13(4):330–335.
Acknowledgments
Support: This work was supported in part by Merit Review Awards 5I01BX002256 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development and 1I01CX000569 from the Clinical Sciences Research and Development, both with Dr Cohen as Principal Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: The authors declare that they have no relevant financial interests.
Contributor Information
Eric P Cohen, Zablocki VAMC, Medical College of Wisconsin, Milwaukee, USA.
Jean-Marie Krzesinski, CHU Université de Liège, Liège, Belgium.
Vincent Launay-Vacher, Hôpital de la Salpetrière, Paris, France.
Ben Sprangers, Katholieke Universiteit Leuven, Leuven, Belgium.


