Abstract
Importance
Sublingual immunotherapy and subcutaneous immunotherapy are effective in seasonal allergic rhinitis. Three years of continuous treatment with subcutaneous immunotherapy and sublingual immunotherapy has been shown to improve symptoms for at least two years following discontinuation of treatment.
Objective
To assess whether 2 years of treatment with grass pollen sublingual immunotherapy compared with placebo provides improved nasal response to allergen challenge at 3 year follow-up.
Design, Setting, Participants
A randomized double-blind, placebo-controlled, 3-parallel group study performed in a single academic centre, Imperial College London, including adult patients with moderate-to-severe seasonal allergic rhinitis (interfering with usual daily activities or sleep). First enrolment was March 2011, last follow-up February 2015.
Intervention
Thirty-six participants received 2 years sublingual immunotherapy (daily tablets containing 15 microgram of major allergen Phleum p 5 and monthly placebo injections), 36 received subcutaneous immunotherapy (monthly injections containing 20 micrograms of Phleum p 5 and daily placebo tablets) and 34 received matched double-placebo. Nasal allergen challenge was performed before treatment, at 1 and 2 years and at 3 years (1 year after treatment discontinuation).
Main outcomes and measures
Total nasal symptom scores (TNSS, range 0 (best) to 12 (worst) were recorded during 0–10 hours after challenge. The minimum clinically important difference for change in TNSS within an individual is 1.08. The primary outcome was TNSS comparing sublingual immunotherapy to placebo at year 3. Subcutaneous immunotherapy was included as a positive control. The study was not powered to compare sublingual immunotherapy with subcutaneous immunotherapy.
Results
Among 106 participants who were randomized (mean age 33.5 years, 32.1% female), 92 completed the study at 3 years. Imputed TNSS scores [mean (95% confidence intervals)] pre-treatment and at 3 years for the sublingual immunotherapy group were 6.36 (5.76, 6.96) and 4.73 (3.97, 5.48) and for the placebo group, 6.06 (5.23, 6.88) and 4.81 (3.97, 5.65), respectively. The between-group difference (adjusted for baseline) (95% CIs) was −0.18 (−1.25, 0.90), p=0.75.
Conclusion
Among patients with moderate-to-severe seasonal allergic rhinitis, two years of sublingual grass pollen immunotherapy was not significantly different than placebo in improving the nasal response to allergen challenge at 3 year follow-up.
INTRODUCTION
The prevalence of allergic rhinitis in the United States has been estimated as 15% based on physician diagnosis and as 30% on the basis of self-reported nasal symptoms.1,2 Rhinitis has major effects on quality of life, sleep and work/school performance.3 Whereas antihistamines and topical nasal corticosteroids are effective,4 community surveys suggest that approximately 60% of patients with allergic rhinitis do not respond adequately to these measures.1 When avoidance of allergens is not feasible and patients have inadequate response to anti-allergic medications or bothersome adverse effects, allergen immunotherapy is a reasonable choice for treatment.5 Subcutaneous immunotherapy is highly effective.5,6 The sublingual route has emerged as an alternative treatment for seasonal allergic rhinitis.7,8 Three years of continuous treatment with immunotherapy via either delivery method modifies the underlying course of the disease with long-term remission of symptoms for several years after stopping treatment.9–11 It is unknown whether a shorter course of immunotherapy would provide long-term benefits, while reducing overall costs, patient inconvenience and adverse events.
The purpose of this study was to explore whether 2 years of immunotherapy with a grass pollen allergen sublingual tablet of proven efficacy induced persistent benefit 1 year after discontinuation (clinical tolerance).
METHODS
Study Design
This was a randomised, double-blind, placebo-controlled single-centre trial conducted over 4 years, March 2011–March 2015. The study was approved by the National Research Ethics Committee. All participants provided written informed consent. Inclusion criteria included age 18 to 65 years, a minimum 2 year clinical history of moderate-to-severe grass-pollen induced allergic rhinitis (that interfered with usual daily activities or sleep3), a positive skin prick test (wheal diameter ≥3mm), elevated serum specific IgE (≥0.7kU/L) and a positive nasal grass allergen challenge (total nasal symptom score (TNSS) ≥7/12 points). Exclusion criteria included a history of moderate-to-severe symptoms on exposure to other overlapping seasonal or perennial allergens, a history of moderate-to-severe or uncontrolled asthma, severe anaphylaxis due to any cause, chronic sinusitis, other diseases of the immune system and current smoking (see eMethods 1.1). At screening, we collected demographic data that included self-reported race (according to fixed categories), as per National Institutes of Health requirements. Eligible participants (Figure 1) were randomized 1:1:1 to receive either sublingual allergen tablet immunotherapy with placebo injections, subcutaneous injection immunotherapy with placebo tablets or double-placebo tablets and injections. Subcutaneous immunotherapy was included as a positive control. Treatment assignment was by use of a central automated web-based randomization system (RhoRAND™) that helped provide remote network backup and 24-hour support (eMethods1.2.1). Clinical surrogate endpoints were collected at baseline, 1 and 2 years on treatment, and at 3 years, 1 year after treatment discontinuation. Double-blinding was maintained for all participants and clinical and laboratory staff throughout the entire duration of the study. (eMethods1.2.2). The study protocol is provided in the Supplement.
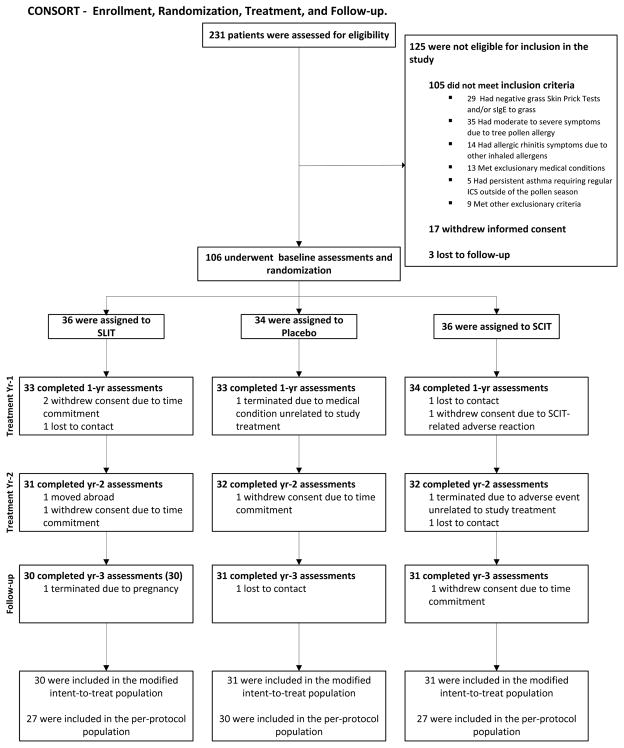
Figure 1.
CONSORT diagram. Participant numbers at enrolment, randomization, treatment, follow-up, and for analysis of the primary endpoint (nasal challenge). Eligibility and baseline assessments were completed from September 2011 to January 2012. The last participant completed the study in February 2015. Reasons for drop-outs are indicated.
The Intent-to-treat (ITT) sample was defined as all randomized participants. If participants dropped out post-randomization, they were invited to complete study assessments throughout the duration of the trial. The modified ITT population included all randomized participants with an evaluable outcome.
The Per-protocol (PP) sample was defined as ITT sample participants who remained in the study for at least 3 years and in whom the primary endpoint was assessed. Participants in the PP sample had to be compliant with study medication, defined as taking 50% or more of their study medication for the duration of the study.
Endpoints
The primary endpoint was the nasal response to allergen challenge between sublingual immunotherapy and placebo at 3 year follow-up, one year after discontinuation of treatment.12 This was defined as the equally weighted average of the TNSS/hour measured as the area under the curve (AUC) during the early response (0–1 hour) and late response (1–10 hours) after challenge (eMethods1.3,1.4).12,13 Secondary, exploratory endpoints included change in PNIF after challenge, (eMethods1.4.1, 1.4.2), seasonal weekly visual analogue scale (VAS),14 seasonal weekly rhinitis quality of life (MiniRQLQ),15 end of season global rhinitis severity scores (eMethods1.6), seasonal medication use (eMethods1.7) and early and late skin responses to intradermal allergen (eMethods1.5),16. Before the pollen season participants received a pre-specified package containing tablets (desloratadine), nasal sprays (fluticasone propionate) and eye drops (olopatadine). Both used and unused medication was returned to the investigators who measured ‘use’ of these medications by the amount that was returned. A composite rescue medication score was derived using an algorithm for each prescribed medication and the mean composite score in each treatment group was computed and compared (eMethods1.7). For clinical outcomes where anchor-based methods for determining the minimum clinically important difference (MCID) were not available we used a distribution-based method17 based on 50% of the standard deviation of baseline values from all randomized participants. For TNSS the range was 0 [best] to 12 [worst] and the MCID was 1.08 17 (eMethods1.3). For the change in PNIF after challenge, the observed range was −388 to 26.7L/min and the MCID was 33.9L/min17 (eMethods 1.4.1). For the global evaluation of seasonal symptoms, the range was 0–18 and the MCID was 1.417. For those clinical endpoints for which an MCID was available from published anchor-based methods, the values were as follows: Mini-RQLQ the range was 0–6 and the MCID was 0.715; seasonal weekly visual analogue scale the range 0–10cm and the MCID was 1.014.
Intervention
Subcutaneous alum-adsorbed grass pollen immunotherapy (Alutard SQ Grass Pollen® (ALK, Horsholm, Denmark)18 or matched placebo subcutaneous injections were given weekly for 15 weeks followed by monthly maintenance injections until 2 years. Freeze-dried grass pollen (Phleum Pratense) sublingual tablets (Grazax®, ALK, Horsholm, Denmark)10 or matched placebo sublingual tablets were self-administered daily for 2 years. For immunotherapy protocols see eMethods1.8. Nasal allergen challenge was performed before treatment, at 1 and 2 years and at 3 years (1 year after treatment discontinuation).
Nasal and Intradermal Allergen Challenge
Nasal challenge was performed12 at 9 am using Aquagen® (ALK) Phleum Pratense (Timothy grass) extract and participants were followed for 10 hours (eMethods1.4). Intradermal allergen challenge16 was performed 1 hour after nasal challenge. The early skin response was recorded at 15 minutes and the late response at 8 hours (eMethods1.5).
Serum Immunoglobulins
Timothy grass pollen-specific IgE and specific IgG4 were quantified using the CAP FEIA system (Phadia, Uppsala, Sweden).19
Adverse Event Recording
Adverse events were classified according to the Medical Dictionary for Regulatory Activities v14.0 (MedDRA).20 In view of the known frequent local symptoms that occur after both subcutaneous immunotherapy and sublingual immunotherapy, such symptoms were recorded as adverse events only if they were considered ‘bothersome’ by participants (interfering with usual daily activities or sleep) or as described in eMethods1.9. Observed immediate systemic allergic reactions to immunotherapy injections (active or placebo) were recorded by study clinicians according to the World Allergy Organization (WAO) grading system for subcutaneous immunotherapy(eMethods1.9.2).21 The same system was applied to adverse reactions to sublingual immunotherapy (active or placebo) reported by participants at routine clinic visits. Observed responses to the first sublingual tablet taken were recorded by study clinicians.22
Statistical Analysis
The Intent-to-treat (ITT) population included all randomized participants. The modified ITT population included all randomized participants with an evaluable endpoint. The per-protocol (PP) population included participants who were compliant with study medications, defined as taking 50% or more of their study medication for the duration of the study, and who had an evaluable primary endpoint (eMethods1.10). The primary analysis compared nasal challenge-induced TNSS AUC in sublingual immunotherapy to placebo at 3 years using an ANCOVA model adjusted for baseline values. Participants in the ITT population with a missing primary endpoint had their data imputed using a regression model based on those participants within their randomized treatment group who had available values for TNSS AUC values (eMethods1.3). Subcutaneous immunotherapy vs. placebo was analysed as a positive control. Subcutaneous immunotherapy vs. sublingual immunotherapy was a secondary exploratory analysis, but the study was not powered on this comparison. Secondary outcomes were assessed using ANCOVA or non-parametric methods where appropriate. Allergen-specific immunoglobulin data were assessed in the PP population using linear mixed models adjusted for baseline. The study was powered at 90% to detect a standardized mean difference between sublingual immunotherapy vs. placebo groups of approximately 40% with 15% dropout (eMethods1.11). The threshold for significance was p<0.05 (two-sided). Secondary outcomes were considered exploratory and not adjusted for multiple comparisons. Analyses were performed with JMP V11 and SAS Version 9.3 (SAS Institute Inc., Cary, NC) and R version 3.2.4 (R Foundation for Statistical Computing). Analysis of data was performed at the end of the study with no interim analyses. The statistical analysis plan is provided in the Supplement. Analysis datasets are available through ITN TrialShare, a public website of the Immune Tolerance Network (https://www.itntrialshare.org/GRASS_Primary.url)
RESULTS
Participant Characteristics, Progression and Adherence with Trial Medication
One hundred and six participants, (mean age 33.5 years, 33.1% female, 73.6% white) were enrolled and 92 (87%) completed the primary endpoint evaluation at 3 years (Figure 1 and Table 1). The three groups were similar in age gender, and race. Adherence to injections was recorded by study staff: 100% of completed participants received >50% of their injections, 95% received >75% and 82% received >90% throughout the 2 year treatment period. Adherence to sublingual tablets was assessed by counting returned tablets: 91.3% of completed participants took >50% study tablets (protocol compliant), 75.0% took >75% and 46.7% took >90% tablets.
Table 1. Participant Demographic and Baseline Characteristics.
In the United Kingdom, where the study took place, Participants who self-report as black are predominantly African-Caribbean or African. Thus the black population in the current study was not identical to the American black population. Those who self-reported as Asian were predominantly from the Indian subcontinent.
| Sublingual Immunotherapy N=36) | Placebo (N=34) | Subcutaneous Immunotherapy (N=36) | |
|---|---|---|---|
| Age (years), mean (SD) | 34.1 (9.9) | 32.8 (8.1) | 33.7 (9.1) |
| Male sex, No. (%) | 26 (72.2%) | 23 (67.6%) | 23 (63.9%) |
| Race, No. (%) | |||
| Asian | 5 (13.9%) | 4 (11.8%) | 2 (5.6%) |
| Black | 3 (8.3%) | 4 (11.8%) | 3 (8.3%) |
| Chinese | 0 (0.0%) | 1 (2.9%) | 1 (2.8%) |
| Middle Eastern | 1 (2.8%) | 0 (0.0%) | 0 (0.0%) |
| Mixed | 3 (8.3%) | 1 (2.9%) | 0 (0.0%) |
| White | 24 (66.7%) | 24 (70.6%) | 30 (83.3%) |
| Grass Skin Prick Test (mm), mean (95% CI) | 10.4 (9.31, 11.52) | 8.9 (7.80, 10.09) | 8.6 (7.43, 9.85) |
| Grass sIgE (kU/L), median (Q1, Q3) | 16.8 (5.71, 54.00) | 18.6 (5.94, 35.50) | 10.8 (4.04, 42.85) |
| Grass Allergy History (years), mean (95% CI) | 19.4 (15.62, 23.27) | 16.4 (13.14, 19.65) | 15.9 (12.63, 19.15) |
Primary outcome
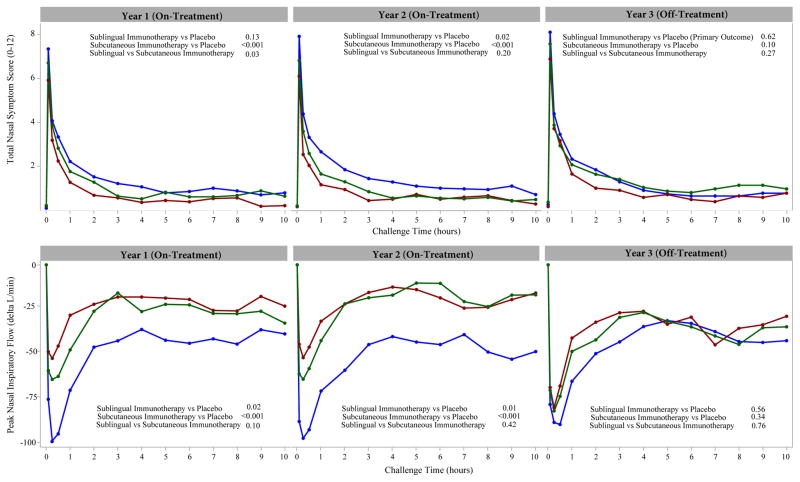
At three year follow-up, 1 year off-treatment, nasal allergen-induced TNSS in the sublingual immunotherapy group did not differ from placebo. In the ITT population, the TNSS AUC (95%CI) at year 3 was as follows: for sublingual immunotherapy 4.73 (3.97, 5.48), for placebo 4.81 (3.97, 5.65). The adjusted mean difference was −0.18 (−1.25, 0.90), equivalent to −1.7% (p=0.75) compared to placebo (Table 2). In the modified ITT population the mean TNSS AUC (95% CI) for sublingual immunotherapy was 4.55 (3.67, 5.43) and for placebo 4.82 (3.90, 5.74); their difference adjusted for baseline was −0.30 (−1.52, 0.92), equivalent to −5.6% compared to placebo (p=0.62) (Figure 2 and Table 2). Subcutaneous immunotherapy, the study’s positive control, had a mean TNSS AUC (95% CI) of 3.96 (3.21, 4.71), a difference from placebo of −0.90 (−1.96, 0.16) equivalent to −17.8% compared to placebo (p=0.10). Baseline (pre-treatment) TNSS AUC values for all 3 groups were as follows: sublingual immunotherapy, 6.36 (5.76, 6.96); placebo, 6.06 (5.23, 6.88); subcutaneous immunotherapy, 6.10 (5.32, 6.89).
Table 2. Total Nasal Symptom Score Weighted 10 Hour Area Under the Curve.
P-values, 95% CIs, and Mean Differences were calculated using an analysis of covariance model (ANCOVA) model adjusted for pre-treatment baseline total nasal symptom score (TNSS, scale 0 [best] to 12 [worst]) area under the curve (AUC) at the 0.05 level of significance. Mean differences were calculated as follows: Sublingual immunotherapy– Placebo; Subcutaneous immunotherapy – Placebo; Sublingual immunotherapy – Subcutaneous immunotherapy. The weighted 10 hour AUC was calculated as the (early phase response (0–1 hour)/1) + (late phase response (1–10 hours)/9). Participants in the ITT population with missing primary endpoint data had their data imputed. Imputation was performed within treatment group using participants who had available TNSS AUC values. Specifically, a linear regression line and 95% confidence bands were fit where Year 3 values were regressed on TNSS AUC values at time t (t = Baseline, Year 1 or Year 2). Within each treatment arm, a missing Year 3 TNSS AUC value was imputed as the value predicted from the linear regression line. The primary endpoint was also calculated in the modified ITT population.
| Unadjusted | Model Adjusted | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sublingual Immunotherapy | Placebo | Subcutaneous Immunotherapy | Sublingual Immunotherapy vs Placebo | Subcutaneous Immunotherapy vsPlacebo | Sublingual Immunotherapy vs Subcutaneous Immunotherapy | ||
| Baseline | |||||||
| n | 36 | 34 | 36 | ||||
| Mean | 6.36 | 6.06 | 6.10 | ||||
| 95% CI | (5.76, 6.96) | (5.23, 6.88) | (5.32, 6.89) | ||||
| Median | 6.39 | 5.95 | 6.38 | ||||
| Min, Max | 2.8, 9.8 | 2.4, 11.5 | 1.9, 10.1 | ||||
|
| |||||||
| Year 1 (Exploratory) | p-value | 0.13 | <0.001 | 0.03 | |||
| n | 33 | 33 | 34 | ||||
| Mean | 3.94 | 4.63 | 3.05 | Mean Difference | −0.75 | −1.60 | 0.84 |
| 95% CI | (3.31, 4.58) | (3.84, 5.42) | (2.50, 3.60) | 95% CI | (−1.71, 0.22) | (−2.49, −0.71) | (0.09, 1.60) |
| Median | 3.47 | 3.97 | 2.70 | ||||
| Min, Max | 0.8, 8.2 | 0.9, 10.4 | 0.9, 6.9 | ||||
|
| |||||||
| Year 2 (Exploratory) | p-value | 0.02 | <0.001 | 0.20 | |||
| n | 31 | 32 | 32 | ||||
| Mean | 3.70 | 5.07 | 2.96 | Mean Difference | −1.42 | −2.11 | 0.68 |
| 95% CI | (2.85, 4.56) | (4.16, 5.97) | (2.21, 3.71) | 95% CI | (−2.61, −0.22) | (−3.22, −1.01) | (−0.36, 1.73) |
| Median | 2.92 | 5.01 | 1.99 | ||||
| Min, Max | 0.7, 8.1 | 0.6, 9.8 | 0.5, 9.2 | ||||
|
| |||||||
| Year 3 (Primary Outcome ITT) | p-value | 0.75 | 0.053 | 0.11 | |||
| n | 36 | 34 | 36 | ||||
| Mean | 4.73 | 4.81 | 3.89 | Mean Difference | −0.18 | −0.94 | 0.73 |
| 95% CI | (3.97, 5.48) | (3.97, 5.65) | (3.25, 4.54) | 95% CI | (−1.25, 0.90) | (−1.88, 0.01) | (−0.17, 1.62) |
| Median | 4.74 | 4.71 | 3.71 | ||||
| Min, Max | 1.1, 11.0 | 0.8, 11.2 | 0.9, 10.6 | ||||
|
| |||||||
| Year 3 (Primary Outcome Modified ITT) | p-value | 0.62 | 0.10 | 0.27 | |||
| n | 30 | 31 | 31 | ||||
| Mean | 4.55 | 4.82 | 3.96 | Mean Difference | −0.30 | −0.90 | 0.58 |
| 95% CI | (3.67, 5.43) | (3.90, 5.74) | (3.21, 4.71) | 95% CI | (−1.52, 0.92) | (−1.96, 0.16) | (−0.46, 1.63) |
| Median | 4.44 | 4.57 | 3.76 | ||||
| Min, Max | 1.1, 11.0 | 0.8, 11.2 | 0.9, 10.6 | ||||
Figure 2.
Time-course of changes after nasal allergen challenge for total nasal symptom scores (TNSS scale 0–12, top panel) and peak nasal inspiratory flow (PNIF, lower panel). Data are mean values for participants treated with Sublingual immunotherapy (green), Subcutaneous immunotherapy (red), and Placebo (blue) during years 1 and 2 (on treatment) and year 3 (1 year follow up). TNSS and PNIF were analyzed in the modified Intent-To-Treat Population comprising: 34 Sublingual immunotherapy participants, 33 Placebo participants, and 33 Subcutaneous immunotherapy participants at year 1; 31 Sublingual immunotherapy participants, 32 Placebo participants, and 32 Subcutaneous immunotherapy participants at year 2; 30 Sublingual immunotherapy participants, 31 Placebo participants, and 31 Subcutaneous immunotherapy participants at year 3.
Top Panel: A higher total nasal symptom score (TNSS) indicates a higher burden of symptoms during the nasal challenge. Mean (95% Confidence intervals) scores for the TNSS for sublingual immunotherapy, placebo and subcutaneous immunotherapy groups, respectively, at baseline and years 1, 2 and 3 were as follows: Baseline: 6.36(5.76, 6.96), 6.06(5.23, 6.88) and 6.10(5.32, 6.89); Year 1: 3.94(3.31, 4.58), 4.63(3.84, 5.42) and 3.05(2.50, 3.60); Year 2: 3.70(2.85, 4.56), 5.07 (4.16, 5.97) and 2.96(2.21, 3.71); Year 3: 4.55(3.67, 5.43), 4.82(3.90, 5.74) and 3.96(3.21, 4.71) (Table 2). The p-values reported compare the TNSS area under the curve (AUC) between treatment groups and were calculated using an analysis of covariance (ANCOVA) model at the 0.05 level of significance adjusted for pre-treatment baseline AUC measures. The minimal clinically important difference for this measure within a participant is 1.08. 17(Table 2).
Bottom Panel: A larger change in peak nasal inspiratory flow (PNIF) indicated a higher burden of symptoms during the nasal challenge. Change (litres/minute) was defined relative to the 0 time point in the challenge. Mean (95% Confidence intervals) values for the change in PNIF for sublingual immunotherapy, placebo and subcutaneous immunotherapy groups, respectively, at Baseline and Years 1, 2 and 3 were as follows: Baseline: −110.75(−129.97, −91.54), −121.63(−145.82, −97.43) and −110.62(−136.75, −84.49); Year 1: −80.36(−98.32, −62.41), −123.51(−149.79, −97.22) and −61.05(−76.50, −45.59); Year 2: −70.65(−92.41, −48.89), −128.63 (−159.87, −97.39) and −59.60(−76.66, −42.54); Year 3: −99.71(−122.89, −76.53), −116.59(−144.89, −88.30) and −91.97(−113.97, −69.97). The p-values reported compared the delta PNIF area under the curve (AUC) between treatment groups and were calculated using an analysis of covariance (ANCOVA) model at the 0.05 level of significance adjusted for pre-treatment baseline AUC measures. The minimal clinically important difference for this measure within a subject is 33.917(eTable1).
Secondary (exploratory) outcomes
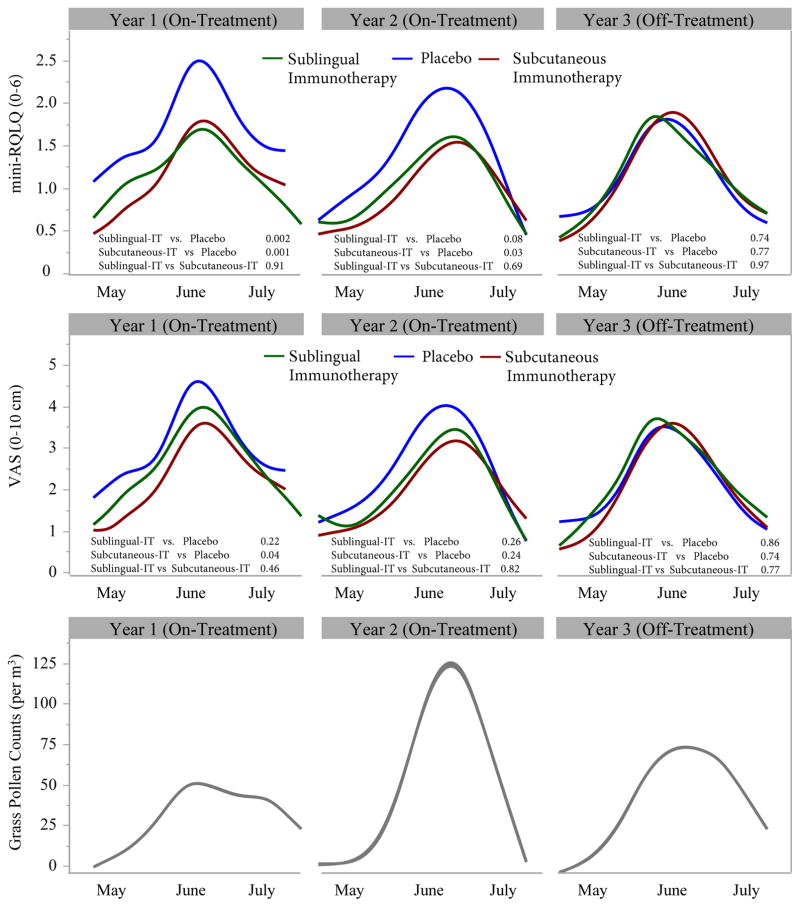
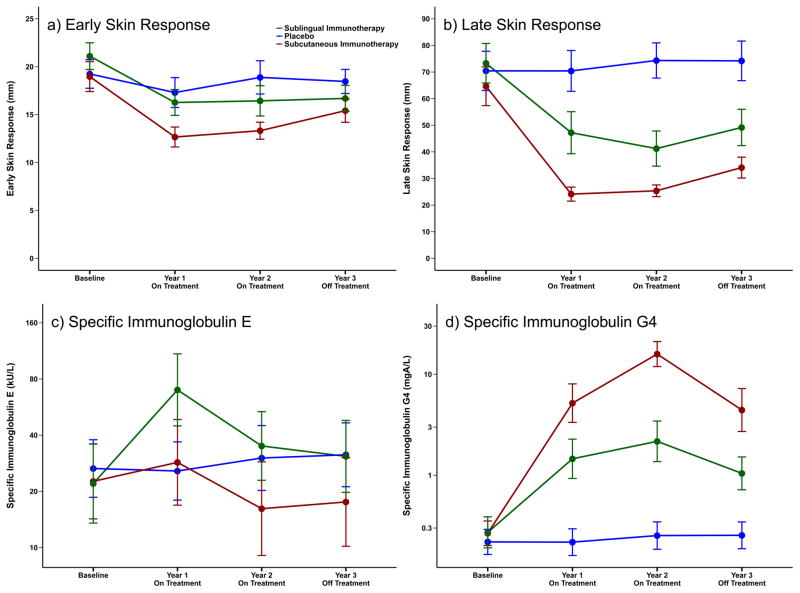
At year 3, 1 year after discontinuation of treatment, allergen-induced reduction from pre-challenge baseline in PNIF (Figure 2, etable 1), expressed as the 0–10 hour AUC, did not differ from placebo with either form of immunotherapy. Similarly at year 3, no benefits of either form of immunotherapy was observed in the weekly seasonal mini-RQLQ and VAS symptom scores (Figure 3, eTable2a,b) or in global evaluations of rhinitis severity compared to placebo. (eFigure1, eTable2c). Pollen season medication use was assessed by returned used and unused packages. Approximately 90% of participants returned some medication, whereas complete returns were obtained from 47% to 70% of participants throughout the 3 years (eTable3a). No significant differences in total rescue medication scores between the 3 groups were observed at year 3 after 1 year off-therapy (eTable3b). In contrast, at year 3, 1 year off-therapy, both sublingual and subcutaneous immunotherapy had lower early and late skin responses to allergen than placebo (Figure 4, eTable4a,b). Serum allergen-specific IgE did not differ between sublingual immunotherapy and placebo at year 3, but the subcutaneous immunotherapy group was significantly lower than the other two groups (Figure 4, eTable5a). Allergen-specific IgG4 in serum was significantly higher with both forms of immunotherapy compared to placebo at year 3 (Figure 4, eTable5b).
Figure 3.
Time-course of weekly seasonal rhinitis quality of life scores (top panel) and rhinitis severity scores (visual analogue 0–10 cm) during May-July (middle panel). Data are mean weekly values for participants treated with Sublingual immunotherapy (green) Subcutaneous immunotherapy (red), and Placebo (blue) during years 1 and 2 (on treatment) and year 3 (off treatment). The curves in Figure 3 have been smoothed using a cubic spline smoothing function (The cubic spline method uses a set of third-degree polynomials spliced together such that the resulting curve is continuous and smooth at the splices (knot points). The estimation is done by minimizing an objective function that is a combination of the sum of squares error and a penalty for curvature integrated over the curve extent.35 The p-values reported compare the average values between treatment groups and were calculated using an analysis of covariance (ANCOVA) model at the 0.05 level of significance adjusted for pre-treatment baseline measures. Mini-Rhinoconjunctivitis quality of life questionnaire (mini-RQLQ) and Visual analogue scale (VAS) were analyzed in the modified Intent-to-Treat population comprising: 33 Sublingual immunotherapy participants, 33 Placebo participants, and 34 Subcutaneous immunotherapy participants at year 1; 28 Sublingual immunotherapy participants, 31 Placebo participants, and 30 Subcutaneous immunotherapy participants at year 2; 27 Sublingual immunotherapy participants, 30 Placebo participants, and 29 Subcutaneous immunotherapy participants at year 3.
Top Panel: Mini-RQLQ values range from 0–6. Higher RQLQ values reflect subjects who experienced more troublesome nose, eye, and other symptoms effecting regular activities resulting in a lower quality of life. The minimal clinically important difference for this measure within a subject is 0.715(etable 2a).
Middle Panel: VAS values range from 0–10 cm. Higher VAS values reflect subjects who experienced worse hay fever symptoms. The minimal clinically important difference for this measure within a subject is 1.0 cm.14 (etable 2b).
Bottom Panel: Average weekly grass pollen counts/cubic meter from a single site in Islington, London, were provided by the Met Office (UK’s national weather service).
Figure 4.
Time-course of early (15 min) and late (8 hour) skin responses to intradermal allergen (a, b), changes in serum grass pollen allergen-specific Immunoglobulin E (c), and Immunoglobulin G4 (d). Data are once-yearly mean and 95% confidence intervals for participants treated with Sublingual immunotherapy (green), Subcutaneous immunotherapy (red), and Placebo (blue) at baseline, and years 1 and 2 (on treatment) and year 3 (off treatment).
Skin responses were analyzed using ANCOVA with adjustment for baseline values in the modified Intent-To-Treat Population comprising: 33 Sublingual immunotherapy participants, 33 Placebo participants, and 34 Subcutaneous immunotherapy participants at year 1; 31 Sublingual immunotherapy participants, 32 Placebo participants, and 32 Subcutaneous immunotherapy participants at year 2; 30 Sublingual immunotherapy participants, 31 Placebo participants, and 31 Subcutaneous immunotherapy participants at year 3. See etable 4a and etable 4b.
Serum allergen-specific Immunoglobulin parameters were analyzed in the Per Protocol Population, comprising 27 Sublingual immunotherapy, 30 Placebo, and 27 Subcutaneous immunotherapy participants at all time points, using a linear mixed model with adjustment for baseline values. Serum allergen-specific Immunoglobulin responses (c and d) were plotted after log transformation for normalization of these variables. The per-protocol sample included participants who were compliant with study medications, defined as taking 50% or more of their study medication for the duration of the study, and who had an assessment of the primary endpoint.
All comparisons for skin and serum allergen-specific Immunoglobulin responses between treatment groups at years 1, 2, and 3 have p-values less than 0.01, with the following exceptions: Early skin responses (a) between sublingual immunotherapy and subcutaneous at immunotherapy year 2 and year 3 (p=0.02 and p=0.94 respectfully); Specific Immunoglobulin E (c) between sublingual immunotherapy and placebo at year 2 (p=0.04) and year 3 (p=0.32) and between subcutaneous immunotherapy and placebo at year 1 (p=0.10). See etable 5a and etable 5b.
At the end of the 1st year of treatment, TNSS AUC improved over placebo on subcutaneous immunotherapy, but not on sublingual immunotherapy. At the end of the 2nd year, both forms of immunotherapy performed better than placebo (Figure 2, Table 2). For the seasonal mini-RQLQ and global severity evaluations, both forms of immunotherapy showed improvement over placebo, both after the 1st and 2nd years of treatment (Figures 2, 3 and eFigure1, eTable1, eTable2a,b and eTable2c). Results for other secondary/exploratory outcomes at years 1 and 2 of treatment can be summarized as follows: sublingual immunotherapy but not subcutaneous immunotherapy was associated with decreased use of seasonal rescue medications (eTable3b); both sublingual immunotherapy and subcutaneous immunotherapy resulted in decreased early (15 min) and late (8hr) skin responses to intradermal allergen challenge at 1 and 2 years. (Figure 4, eTable4a,b); for allergen-specific IgE, sublingual immunotherapy resulted in increased values over placebo whereas subcutaneous immunotherapy had the opposite effect (Figure 4, eTable5a); and for IgG4, both forms of treatment resulted in increases over placebo (Figure 4, eTable5b).
Adverse Events
A total of 553 adverse events were recorded, out of which 116 were related to study participation. All adverse events are shown in eTable8. No serious treatment-related adverse events were recorded. Adverse events were numerically higher in the subcutaneous immunotherapy group. Adverse events with significant differences between groups are shown in Table 3. Seventeen participants in the subcutaneous immunotherapy group (47.2%) experienced ‘hypersensitivity’ episodes, following injections, compared to 1 (2.8%) in the sublingual immunotherapy group and 4 (11.8%) in the placebo. Dyspepsia was reported by 8 (22%) participants who received active sublingual immunotherapy compared to none in the subcutaneous immunotherapy group and 2.9% in the placebo group. Episodes of ‘dyspepsia’ comprised mild heartburn or ‘indigestion’, were short-lived and were either not treated or self-treated with antacids or antihistamines. No participant on sublingual immunotherapy withdrew due to adverse events.
Table 3. Adverse Events.
Adverse Events were reported by observing the participant, questioning the participant in an objective manner or receiving an unsolicited complaint from the participant. Any reactions occurring in the clinic due to procedures, administration of subcutaneous immunotherapy or the first administration of sublingual immunotherapy were recorded on related CRFs and entered into the electronic database. Reactions occurring outside the clinic to either subcutaneous or sublingual immunotherapy or other adverse events were assessed by study staff at clinic visits and recorded on related CRFs and entered into the electronic database.
Adverse events for this trial have been coded according to international MedDRA classification. Only system organ classes and preferred terms with a significant p value are displayed. P-values for participant level analyses (percentages) were computed using Fisher’s Exact Tests. P-values for event level analyses (total number of events) were computed using a Poisson regression model comparing the person-year adjusted event rates between each treatment group. ‘Hypersensitivity’ indicates systemic reactions after subcutaneous immunotherapy which included mostly mild reactions such as itchy eyes or nose, blocked nose, runny nose, sneezing, itchiness or rash; two events with shortness of breath and sensation of throat closure resulted in administration of epinephrine. There were no serious treatment-related adverse events.
| System Organ Class Preferred Term |
Sublingual-IT (N=36) | Placebo (N=34) | Subcutaneous-IT (N=36) | Total (N=106) | p-values | ||
|---|---|---|---|---|---|---|---|
| Sublingual-IT vs. Placebo | Subcutaneous-IT vs. Placebo | Subcutaneous-IT vs. Sublingual-IT | |||||
| Total # Adverse Events | 163 | 174 | 216 | 553 | 0.63 | 0.05 | 0.01 |
| Total # Related AEs | 34 | 18 | 64 | 116 | 0.02 | <0.001 | 0.004 |
| # Subjects with ≥ 1 AE | 34 (94.4%) | 33 (97.1%) | 35 (97.2%) | 102 (96.2%) | >0.99 | >0.99 | >0.99 |
| Immune System Disorders | 5 (13.9%) | 9 (26.5%) | 20 (55.6%) | 34 (32.1%) | 0.24 | 0.02 | <0.001 |
| Hypersensitivity | 1 ( 2.8%) | 4 (11.8%) | 17 (47.2%) | 22 (20.8%) | 0.19 | 0.002 | <0.001 |
| Gastrointestinal Disorders | 13 (36.1%) | 8 (23.5%) | 8 (22.2%) | 29 (27.4%) | 0.30 | >0.99 | 0.30 |
| Dyspepsia | 8 (22.2%) | 1 ( 2.9%) | 0 | 9 ( 8.5%) | 0.03 | 0.49 | 0.005 |
Events occurring in the first hour following administration of the first sublingual tablet (active or placebo) under supervision were also recorded (eTable6); none of the 106 participants had a systemic allergic reaction and 11/106 (10 on active sublingual and 1 on subcutaneous immunotherapy) reported a mild local reaction.
Systemic allergic reactions after subcutaneous immunotherapy were graded according to the World Allergy Organization classification (eTable7a).21 A total of 41 systemic reactions after injections occurred in 19 participants. The majority were mild: 33 grade 1, of which 9 were early [0–60minutes], 17 were delayed [after 1 hour], in 7 the timing was undefined; 8 were grade 2 [2 early, 6 delayed] and 2 were grade 3 [1 early, one delayed (at 2 hours)]. In participants with grades 1 and 2, symptoms resolved with no treatment or with oral antihistamines. In grade 3 reactions, adrenaline was used with prompt response. The same classification was used for participant-reported systemic reactions after administration of sublingual immunotherapy tablets (eTable7b). Of 18 reported reactions, 16 were grade 1 and 2 were grade 2. Although these events strictly fulfilled criteria for WAO systemic reactions, they all consisted of local or upper gastro-intestinal symptoms. All were transient and mild and none required adrenaline or resulted in participant withdrawals.
DISCUSSION
This study, by use of nasal allergen challenge, demonstrated that in patients with moderate-to-severe seasonal allergic rhinitis, treatment for two years with grass pollen sublingual immunotherapy was not sufficient to achieve an allergic response improvement at 3-year follow-up. Previous randomised placebo-controlled trials demonstrated that three years of continuous therapy with either sublingual immunotherapy or subcutaneous immunotherapy resulted in long-term clinical efficacy with decreases in seasonal symptoms and use of anti-allergic medications that persisted for at least 2 years after discontinuation9–11 (3 years for subcutaneous immunotherapy 11). Subcutaneous immunotherapy has been used for over 100 years,6,23 whereas in recent years the sublingual route has been shown to be an effective and safer alternative.7 International guidelines on immunotherapy recommend a minimum of 3 years treatment7,24 with both delivery methods; had a 2 year regimen demonstrated long-term benefits in addition to efficacy, this could have represented cost savings in terms of clinical resources and improved convenience for the patient. Since this was not observed, clinicians should be advised to follow established guidelines that recommend at least 3 years treatment.
The World Allergy Organisation has recommended (empirically) a 20% difference from placebo as the minimum clinically meaningful difference for seasonal outcomes in immunotherapy trials.25 Previous randomized clinical trials of both the sublingual and subcutaneous allergen immunotherapy used in the current trial demonstrated a 30% difference from placebo in seasonal symptoms.6,18 In a previous cross-sectional study, a 45% reduction in TNSS and 54% improvement in PNIF following allergen challenge was shown in grass pollen immunotherapy-treated compared to untreated patients with seasonal rhinitis.13 Therefore, this trial was powered to detect a difference of 40% between either form of immunotherapy and placebo. There was no significant decrease in the primary outcome, TNSS, one year following withdrawal of treatment (5.6% for sublingual immunotherapy compared to placebo and 17.8% for subcutaneous immunotherapy). Nonetheless, both treatments were superior to placebo as shown by significant reductions for sublingual and subcutaneous immunotherapy, 27.0% and 41.6% respectively, at 2 years (Table 2).
There are limitations to this study. First, daily symptom diary records were not used during the grass pollen season. However, nasal allergen challenge was used as a surrogate12 for seasonal outcomes, thereby allowing reproducible exposure to grass pollen allergen in a controlled environment while avoiding the high season-to-season variability to natural pollen exposure. A previous study showed a significant correlation between TNSS and reductions in PNIF after challenge and seasonal symptoms.13 Second, the study was not designed to compare 2 vs. 3 years sublingual immunotherapy and, therefore, this study cannot determine whether 3 years of therapy would have been sufficient to produce long-term benefits. Given that previous studies have consistently shown long-term benefits when therapy is discontinued after 3 years,9–11,26 this trial was designed to address the question whether 2 years of treatment were adequate. Thirdly, although protocol-defined adherence was >90% for all treatment groups, for sublingual immunotherapy 47% of participants took >90% of doses over the two year period, compared to 82% for subcutaneous immunotherapy. In addition, although both treatments were administered double-blind with both sublingual and subcutaneous placebos, it is possible that the occurrence of local reactions in a proportion of subjects after both forms of immunotherapy may have compromised blinding. For this reason, all nasal challenges and skin tests were performed by one individual (GS) who was not involved in the clinical immunotherapy protocol or in seasonal assessments. There were no differences in drop outs among the three groups.
Secondary exploratory, seasonal, outcomes were in accord with the observed lack of effect of both treatment modalities on nasal challenge at 3 years. Seasonal outcomes and response to nasal challenge were also consistent in showing improvement while on treatment at years 1 and 2. (Figure 3 and eTable2a–c). The comparative efficacy of the two routes of immunotherapy is unknown. Previous systematic reviews have relied on indirect comparisons,27–29 with few head-to-head randomised trials of natural allergen exposure.30 This study was not powered to detect differences between active treatments. However, at year 1, subcutaneous immunotherapy was more effective than sublingual immunotherapy in reducing TNSS after challenge; conversely, the use of seasonal rescue medication was lower for sublingual immunotherapy compared to subcutaneous immunotherapy. These data highlight the need for a head-to-head clinical trial of sublingual and subcutaneous immunotherapy during natural pollen exposure using seasonal outcomes. Sublingual immunotherapy was associated with a transient increase in allergen-specific IgE at year 1, whereas after subcutaneous immunotherapy, specific IgE levels were unchanged at year 1 and decreased during years 2 and 3 compared to placebo. These findings are in agreement with previous studies although the mechanisms are unknown.10,31 Changes in serum allergen-specific IgG432, 16 paralleled suppression of allergen-induced early and late skin responses. These immunologic changes, although reduced, persisted at year 3 (Figure 4). Together with the accompanying suppression of early and late skin responses, these effects could be regarded as early indicators of effective clinical tolerance that has previously been convincingly documented following 3 years immunotherapy via both routes.9–11
Almost all adverse reactions to sublingual immunotherapy were isolated, mild, transient, local oral or upper gastro-intestinal symptoms (eTable7b). None of these reactions required acute medical intervention and none resulted in withdrawal from the trial. While they were recorded as “systemic” according to the WAO grading for subcutaneous immunotherapy eMethods 1.9.2.,21 this may not be appropriate given the proximity of the symptoms to the site of sublingual immunotherapy administration. These results are consistent with the safe self-administration of sublingual immunotherapy as reported in systematic reviews8,28 and large controlled trials.33,34 Subcutaneous immunotherapy was associated with expected systemic reactions21 including two grade 3 reactions requiring adrenaline, (eTable7a). This emphasises the need for close observation in a specialist setting for subcutaneous immunotherapy.
Conclusion
Among patients with moderate-to-severe seasonal allergic rhinitis, two years of sublingual grass pollen immunotherapy was not significantly different than placebo in improving the nasal response to allergen challenge at 3 year follow-up.
Supplementary Material
KEY POINTS.
Question
Does 2 years of grass pollen sublingual immunotherapy reduce symptoms after nasal allergen challenge at 3 year follow-up (1 year after discontinuation of treatment)?
Findings
In this randomized clinical trial that included 106 adults, 2 years of treatment with sublingual immunotherapy compared to placebo did not reduce total nasal symptom scores after challenge at 3 years (mean difference [95% Confidence Intervals], −0.18 (−1.25, 0.90), p=0.75.
Meaning
Among patients with moderate-to-severe seasonal allergic rhinitis, two years of sublingual grass pollen immunotherapy was not significantly different than placebo in improving the nasal response to allergen challenge at 3 year follow-up.
Acknowledgments
GRASS Study Contributors: Imperial College Nursing Staff: Andrea Goldstone, RN, Ms; Fotini Rozakeas, Rachel Yan, RN, Ms; Imperial College Study management and administration: Natalia Klimowska-Nassar, Mimi Poon. Imperial College Laboratory projects: Delica Kit Cheung, Constance Ito, Janice Layhadi, Elisabeth Lemm, Ellen Macfarlane, Orla MacMahon, Tomokasu Matsuoka, Rebecca Parkin, Amy Switzer. ITN Staff: Adam Asare, PhD (past); Eduard Chani, PhD; Judith Evind; Deborah Phippard PhD (past); Peter Sayre MD, PhD; Maureen Sharkey, MA (past); Don Whitehouse, MS. DAIT-NIAID Staff: Steven Adah, PhD (past); Theresa Allio, PhD; Christine Czarniecki, PhD; Jui Shah, PhD (past). Rho Federal Systems Staff: Travis Mason, Ann Nguyen, Shayala Gibbs, Spencer Childress
We especially thank the patients for their participation in this study
Funding/Support:
The trial was conducted by the Immune Tolerance Network (ITN) with financial support from the Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases (DAIT-NIAID), National Institutes of Health (NIH) under award numbers NO1-AI-15416, UM1AI109565, and UM2AI117870; the following DAIT-NIAID funded groups) Statistical and Clinical Coordinating Centers (contract HHSN272200800029C and grant UM2AI117870), ii) Clinical Site Monitoring Center (Contract HHSN272201200004C) and iii) Regulatory Management Center (Contract HHSN272201200002C) ALK-Abello A/S Horsholm, Denmark supplied Alutard SQ Grass Pollen® and Grazax® and matching placebos used for the GRASS clinical trial to DAIT, NIAID without charge. Dr. Durham served as the Sponsor of the MHRA Clinical Trial Application.
The sponsor for the study was the Immune Tolerance Network under a contract from the National Institute of Allergy and Infectious Diseases. The ITN Clinical Trial Physician and administrative team and ITN scientists liaised closely concerning all aspects of the trial, with the Principal Investigator (Protocol Chair) and the site team at Imperial College London. Similarly, the Medical Monitor from NIAID and the regulatory team at NIAID worked closely with ITN and the protocol chair and study team concerning all aspects of the study. ALK Denmark supplied the allergy vaccines, matched placebos and allergen extracts for skin testing and nasal allergen provocation for the study free of charge. ALK had no input to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript or decision to submit the manuscript for publication.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT01335139, EudraCT Number: 2010-023536-16
Conflicts of Interest Disclosure: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclaimer: The contents of this article are solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions:
Access to Data. Stephen R Durham, MD and Alkis Togias, MD had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Stephen R Durham, MD; Nadia K. Tchao, MD; Alkis Togias, MD.
Data acquisition: Stephen R. Durham, MD; Guy W. Scadding MB BS PhD, Moises A. MD; Calderon MD, PhD.
Data analysis and interpretation: Stephen R. Durham, MD; Alkis Togias, MD; Nadia K. Tchao, MD; Kristina M. Harris, PhD; Michelle L. Sever, PhD; Henry T. Bahnson, M.P.H.; Kaitie Lawson, MS.
Drafting of the manuscript: Stephen R. Durham, MD; Alkis Togias, MD.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Michelle L. Sever, PhD; Henry T. Bahnson, M.P.H.; Kaitie Lawson, MS; Noha Lim, PhD; Tielin Qin, PhD.
Administrative, technical, or material support: Michelle L. Sever, PhD; Kaitie Lawson, MS; Audrey G. Plough RN, MSN; Kristina M. Harris, PhD; Noha Lim, PhD.
Obtained funding: Stephen R. Durham, MD.
Study Supervision: Stephen R. Durham, MD; Nadia K. Tchao, MD; Audrey G. Plough, RN, MSN; Alkis Togias, MD; Joy Laurienzo-Panza, RN, BSN.
References
- 1.Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 Suppl):S43–70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Salo PM, Calatroni A, Gergen PJ, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127(5):1226–1235. e1227. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372(5):456–463. doi: 10.1056/NEJMcp1412282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136(3):556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. Jama. 2013;309(12):1278–1288. doi: 10.1001/jama.2013.2049. [DOI] [PubMed] [Google Scholar]
- 9.Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. doi: 10.1186/s13601-015-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725. e715. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 11.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 12.Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384(1–2):25–32. doi: 10.1016/j.jim.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Scadding GW, Eifan AO, Lao-Araya M, et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy. 2015;70(6):689–696. doi: 10.1111/all.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousquet PJ, Combescure C, Klossek JM, Daures JP, Bousquet J. Change in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol. 2009;123(6):1349–1354. doi: 10.1016/j.jaci.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2000;30(1):132–140. doi: 10.1046/j.1365-2222.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 16.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121(5):1120–1125. e1122. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of clinical epidemiology. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Frew AJ, Powell RJ, Corrigan CJ, Durham SR, Group UIS. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(2):319–325. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Shamji MH, Ljørring C, Francis JN, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed October 17, 2016];Medical Dictionary for Regulatory Activities (MedDRA) 2011 http://www.meddra.org.
- 21.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125(3):569–574. 574 e561–574 e567. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 22.Passalacqua G, Baena-Cagnani CE, Bousquet J, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132(1):93–98. doi: 10.1016/j.jaci.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102(4 Pt 1):558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 24.Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41(9):1177–1200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 25.Canonica GW, Baena-Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62(3):317–324. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 26.Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64(9):1394–1401. doi: 10.1111/j.1398-9995.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of Grass Pollen Allergen Sublingual Immunotherapy Tablets for Seasonal Allergic Rhinoconjunctivitis: A Systematic Review and Meta-analysis. JAMA Intern Med. 2015;175(8):1301–1309. doi: 10.1001/jamainternmed.2015.2840. [DOI] [PubMed] [Google Scholar]
- 28.Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137(2):339–349. e310. doi: 10.1016/j.jaci.2015.12.1298. [DOI] [PubMed] [Google Scholar]
- 29.Lin S, Erekosima N, Suarez-Cuervo C, et al. Allergen-Specific Immunotherapy for the treatment of Allergic Rhinoconjunctivitis and/or asthma: Comparative Effectiveness Review. Rockville, MD, USA: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 30.Khinchi MS, Poulsen LK, Carat F, André C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59(1):45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 31.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70(4):261–271. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 32.Lima MT, Wilson D, Pitkin L, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2002;32(4):507–514. doi: 10.1046/j.0954-7894.2002.01327.x. [DOI] [PubMed] [Google Scholar]
- 33.Maloney J, Bernstein DI, Nelson H, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112(2):146–153. e142. doi: 10.1016/j.anai.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Maloney J, Prenner BM, Bernstein DI, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol. 2016;116(1):59–65. doi: 10.1016/j.anai.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Reinsch CH. Smoothing by spline functions. Numerische Mathematik. 1967;10(3):177–183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.