Abstract
P450 activity is required to metabolically activate many chemical carcinogens, rendering them highly genotoxic. CYP3A4 is the most abundantly expressed P450 enzyme in the liver, accounting for most drug metabolism and constituting 50% of all hepatic P450 activity. CYP3A4 is also expressed in extrahepatic tissues, including the intestine. However, the role of CYP3A4 in activating chemical carcinogens into potent genotoxins is unclear. To facilitate efforts to determine whether CYP3A4, per se, can activate carcinogens into potent genotoxins, we expressed human CYP3A4 in the DNA-repair mutant (rad4 rad51) strain of budding yeast Saccharomyces cerevisiae and tested the novel, recombinant yeast strain for ability to report CYP3A4-mediated genotoxicity of a well-known genotoxin, aflatoxin B1 (AFB1). Yeast microsomes containing human CYP3A4, but not those that do not contain CYP3A4, were active in hydroxylation of diclofenac (DCF), a known CYP3A4 substrate drug, a result confirming CYP3A4 activity in the recombinant yeast strain. In cells exposed to AFB1, the expression of CYP3A4 supported DNA adduct formation, chromosome rearrangements, cell death, and expression of the large subunit of ribonucleotide reductase, Rnr3, a marker of DNA damage. Expression of CYP3A4 also conferred sensitivity in rad4 rad51 mutants exposed to colon carcinogen, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). These data confirm the ability of human CYP3A4 to mediate the genotoxicity of AFB1, and illustrate the usefulness of the CYP3A4-expressing, DNA-repair mutant yeast strain for screening other chemical compounds that are CYP3A4 substrates, for potential genotoxicity.
Keywords: CYP3A4, budding yeast, Aflatoxin B1, DNA damage
INTRODUCTION
The incidence of liver cancer strongly correlates with exposure to chemical carcinogens, such as aflatoxin B1 (AFB1) (Wild et al., 2009; McGlynn et al., 2011). The correlation between carcinogenic potential and genotoxicity is underscored by observations that a signature p53 mutation is often found in primary liver cancer biopsies from individuals exposed to high levels of AFB1 (Hsu et al., 1991; Wogan, 1992; Shen and Hong, 1996). These studies indicate that many potent hepatocarcinogens are also potent genotoxins. AFB1 requires metabolic activation to become a potent genotoxin (Smela et al., 2000). The direct genotoxic AFB1 metabolite is an epoxy derivative, aflatoxin B1–8,9-epoxide (AFBO), that reacts with DNA to generate 8,9-dihydro-8-(N7-guanyl)-9-hydroxy AFB1 (AFB1 N7-Guanine) adducts, which spontaneously convert to a AFB1-formamidopyrimidine (FAPY) derivative and abasic sites (Smela et al., 2000). These DNA adducts are either directly mutagenic or can trigger DNA polymerase arrest, potentially causing chromosomal breakage and subsequent rearrangements. The full genotoxic potential of these DNA adducts is unknown.
Among the 57 human CYPs, 3A4, 2C9, 2C8, 2E1, and 1A2 are well-expressed in the liver, while 3A5, 2A6, 2D6, 2B6, and 2C19 are less abundant (Zanger et al., 2013; Yang et al., 2010). These genes are highly polymorphic and their expression is highly variable among individuals (for review, see Zanger and Schwab, 2013). Both epidemiological and biochemical studies have focused on identifying which CYP450 genes associate with primary liver cancer; however epidemiological studies are inconclusive (Zeigler-Johnson et al., 2004). In vitro experiments have been performed to identify the genotoxic products generated by specific P450 enzymes. For example, CYP1A1-mediated and CY3A4-mediated metabolism of AFB1 yields the hydroxylated and less toxic derivatives, aflatoxin M1 (AFM1) and aflatoxin Q (AFQ), respectively, while CYP1A2-mediated metabolism of AFB1 yields a higher proportion of the highly reactive epoxy derivative, AFBO (Neal et al., 1998; Gallagher et al., 1996). CYP1A2 has been alleged to be a major contributor of carcinogen-associated liver cancer because of its higher affinity for AFB1 (Landi et al., 1999). Other CYPs which metabolize AFB1 include CYP3A5, CYP2A6, and CYP3A4 (Crespi et al., 1991).
CYP3A4 constitutes 50% of the P450 activity in the human liver (Yang et al., 2010) and is expressed in other tissues exposed to food carcinogens, such as the small intestine (Ding et al., 2003; Gundert-Remy et al., 2014). While CYP3A4 metabolizes many pharmaceuticals, its substrate binding site is wider (Sevrioukova et al., 2015; Guengerich, 2016), rendering a lower affinity for particular compounds. Epidemiological studies are unclear whether CYP3A4 polymorphisms are linked to higher rates of xenobiotic-associated cancer (Klein and Zanger, 2013; Zang and Schwab, 2013). Several studies expressing CYP3A4 and CYP1A2 in lymphocytes or epithelial cells have suggested that CYP3A4-mediated activation of AFB1 is less than that of CYP1A2, as measured by p53 mutations or DNA adduct formation (Crespi et al., 1991; Gallagher et al., 1994, Mace’ et al., 1997). Considering that AFB1 is also a potent recombinagen (Sengstag et al., 1994; Sengstag et al., 1996, Keller-Seitz et al., 2004) and can induce a strong DNA damage response (Fasullo et al., 2008), it is important to measure other genotoxic endpoints in measuring the metabolic activation of liver carcinogens.
While both CYP1A2 and CYP3A4 have been expressed in bacteria to measure carcinogen-associated mutations (Kranendonk et al., 1999, Palma et al., 2013), budding yeast are particularly advantageous for measuring multiple genotoxic endpoints, such as checkpoint activation, recombination, mutation, and chromosome missegregation. The one endogenous P450 in budding yeast is insufficient to activate AFB1 into a potent genotoxin thus allowing for ectopic expression of individual human P450 enzymes (Paladino et al., 1999; Sengstag et al., 1996, Fasullo et al., 2014). Both CYP1A2 (Keller-Seitz et al., 2004) and CYP3A4 (Li et al., 2009) have been individually expressed in yeast; however, mammalian CYP3A4 is particularly prone to proteolysis (Liao et al., 2006). In this study, we individually measured CYP3A4 and CYP1A2-mediated activation of AFB1 using recombination, growth curves, DNA adducts and Rnr3-GFP assays. Our data indicate that CYP3A4 expression is sufficient to activate AFB1, resulting in significant lethality in a rad4 rad51 mutant, defective in both nucleotide excision and recombinational repair. CYP3A4-mediated activation of AFB1 also stimulates recombination that can result in translocated chromosomes, consistent with the idea that multiple P450 genes can convert AFB1 into a recombinagen.
MATERIALS AND METHODS
Strains, Media, Chemicals
Yeast strains are listed in Table 1. The human oxidoreductase (hOR) had been introduced in the TRP1 locus (trp1::hOR) as previously described (Fasullo et al., 2014). By genetic crosses, strains were constructed that containing trp1::hOR and his3 recombinational substrates on chromosomes II and IV to measure translocation events (Figure 1; Fasullo and Davis, 1987). The rad4 strain, defective in nucleotide excision repair (NER), and the rad4 rad51 strain, defective in both NER and recombinational repair, were also constructed by genetic crosses to contain trp1::hOR.
Table I.
Yeast Strains
| Strain (Synonym) | Genotype | Autonomous plasmid | Reference (Source) |
|---|---|---|---|
| YA101 (MCY726) | MATa ura3-52 his3-Δ200 ade2-101 lys2-801 | M. Carlson | |
| BY4741 | MATa ura3Δ 0 leu2Δ 0 his3Δ1 LYS2 met15Δ0 | wiki.yeastgenome.org/index.php/Commonly_used_strains | |
| YB225 | MATa-inc ura3 rad4::KanMX | This laboratory | |
| YA283 | MATa ura3 Δ0 leu2 Δ0 his3 Δ1 LYS2 met15Δ0 RNR3-GFP | wiki.yeastgenome.org/index.php/Commonly_used_strains, derived from BY4741 | |
| YB226 | MATa-inc trp1 ura3 his3 ade2 rad4::KanMX rad51::URA3 | This laboratory | |
| YB400 | MATa-inc trp1 ura3 his3 ade2 rad4::KanMX rad51 | Derived from YB226 | |
| YB404 | MATa-inc trp1 ura3 his3 ade2 rad4::KanMX rad51 | pCS316 | This laboratory, Ura+ transformant of YB400 |
| YB318 | MATα ura3-52 his3-Δ200 ade2-n trp1-Δ1 gal3− leu2-3, 112 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs | This Laboratory | |
| YB407 | MATα ura3-52, trp1::hOR his3-Δ200,ade2-101 lys2-801, leu2 | This Laboratory | |
| YB425 | MATα ura3-52 his3-Δ200 ade2-n trp1-Δ1 gal3− leu2-3,112 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs rad4::kanMX | Derived from cross of YB318 and YB225 | |
| YB426 | MATa ura3-52, trp1::hOR his3-Δ200,ade2-101 lys2-801 rad4 ::KanMX | Derived from cross of YB407 and YB225 | |
| YB427 | MATa/MATα ura3-52/- his3-Δ200/- ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs rad4::kanMX/rad4 kanMX | Diploid cross of YB425 and YB426 | |
| YB428 | MATa/MATα ura3-52/- his3-Δ200/- ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs rad4::kanMX/rad4 kanMX | pRS424 CYP1A2 | Trp+ transformant of YB427 |
| YB437 | MATa/MATα ura3-52/- his3-Δ200/- ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs rad4::kanMX/rad4 kanMX | pMA91-CYP3A4 | Leu+ transformant of YB427 |
| YB438 | MATα ura3 leu2 trp1::hOR rad4::KanMX rad51 his3-Δ200 | Derived from cross of YB400 and YB407 | |
| YB439 | MATα ura3 leu2 trp1::hOR rad4::KanMX rad51 | pMA91-CYP3A4 | Leu+ transformant of YB438 |
| YB440 | MATa/MATα ura3Δ0/ura3-52 leu2Δ0/leu2 trp1::hOR/+ his3Δ1/his3-Δ200 met15Δ0/MET RNR3-GFP | pMA91-CYP3A4 | Derived from cross of YB439 and YA283 |
Figure 1.
Configuration of his3 fragments in yeast strains to measure translocations. Figure on the left is a model of the recombination assay and the figure on the right is a pulse field gel of chromosomal DNA obtained from AFB1-associated His+ recombinants. The oval represents a centromere and the line represents the chromosome; the left arm of the chromosome is not shown for simplicity. The his3 fragment is shown with arrow and feathers. The similar shaded areas represent shared homology; the tightly-spaced diagonals represent 146 bp of shared DNA sequence and speckles represent 300 bp of shared DNA sequence. An “X” denotes where a cross-over event would occur. The product of the recombination event is shown below where CEN2 is linked to the long arm of chromosome IV (CEN2::IV) and CEN4 is linked to the long arm of chromosome II (CEN4::II). The translocated chromosome CEN4::II is identified as a novel band on the pulse field gel, while the translocated chromosome CEN2::IV, IV, and XII often comigrate. Lanes A and B are His+ recombinants; Lane C is the His− parent. Chromosomal sizes indicated on the left are based on New England Biolab yeast chromosome pulse field standards.
Standard media were used for the culture of yeast and bacterial strains. LB (Luria broth) and LB-AMP (LB containing 100 μg/ml ampicillin) were used for the culture of the bacterial strain DH1 and the DH1 strain containing the vector pMA91-CYP3A4 (Li et al., 2009). Media used for the culture of yeast cells included YPD (yeast extract, peptone, dextrose), SC (synthetic complete, dextrose), SC-LEU (SC lacking leucine), SC-TRP (SC lacking tryptophan), SC-URA (SC lacking uracil), and fluoroorotic acid (FOA) media, as described by Burke et al. (2000). AFB1 was purchased from Sigma Co., and a 10 mM solution was made in dimethyl sulfoxide (DMSO). The heterocyclic aromatic amine 2-amino-3-methylimidazo[4,5-f]quinolone (IQ) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline ( MeIQx) were purchased from Santa Cruz Biotechnology and dissolved in methanol and DMSO, respectively.
Plasmids
We obtained the pMA91-CYP3A4 from a yeast strain INV-3A4, described in Li et al (2010). We first extracted total yeast DNA and re-introduced the plasmid into the bacterial strain DH1 by selecting for AmpR transformants (Ausubel et al, 2000). We confirmed the presence of the CYP3A4 gene by PCR and by mapping DNA endonuclease restriction sites. The forward oligo used to identify CYP3A4 was 5′TGCCCAGTATGGAGATGTGTTGGT3′ and the reverse oligo was 5′TGCCCAGTATGGAGATGTGTTGGT3′. The pMA91-CYP3A4 plasmids were introduced into yeast strains by selecting for Leu+ transformants.
Measuring DNA Damage-Associated Recombination
To measure AFB1-associated genotoxic events, log phase yeast cells (A600 = 0.5–1) were concentrated ten-fold in phosphate-buffered saline (PBS) and exposed to indicated doses of AFB1, dissolved in DMSO. Cells were maintained in synthetic medium (SC-URA) during the 3h carcinogen exposure. After exposure, cells were washed twice, and an undiluted aliquot was inoculated on SC-HIS to select for His+ recombinants. An appropriate dilution was inoculated on YPD to measure viability (Keller-Seitz et al, 2004). Generally, 100–200 colonies are counted to assess viability while 1–20 colonies are counted on SC-HIS plates. Statistical significance was determined by ANOVA and Dunnett’s test (Zar, 1996).
Electrophoretic Karyotype of His+ Recombinants
Intact yeast chromosomes were prepared as previously described (Fasullo and Davis, 1988). Chromosomal DNA was resolved using a CHEF Mapper XA Chiller clamped homogeneous electrical field (CHEF) electrophoresis system (Bio-Rad). The gels were run at a 60° angle for 18 h with pulse times of 40–75 sec and then for 8 h with pulse times of 110–130 sec. Chromosomal DNA was detected by fluorescence after ethidium bromide staining.
Measuring DNA Adducts
To measure the AFB1-associated DNA adducts in yeast, we used liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS) (Egner et al., 2006). Log phase cultures of yeast expressing human CYP3A4 (pMA91) were exposed to 50 μM AFB1 for 3 h. Because standard protocols for isolating yeast DNA involve alkaline buffers, rendering the highly unstable AFB1 N7-Gua DNA adducts labile, we have modified the “smash-and-grab” protocol (Hoffman and Winston, 1987) using a neutral buffer containing 10 mM Tris–HCl, 1 mM EDTA, 100 mM NaCl, 2% Triton X-100, 1% SDS, pH 7 (Fasullo et al., 2008). DNA was then isolated from two independent samples of yeast cells. The DNA adducts were identified and measured by high performance liquid chromatography and tandem mass spectroscopy (LC-ESI/MS/MS) after acid hydrolysis.
Growth Assays in 96 Well Plates to Measure AFB1 Sensitivity
In brief, individual saturated cultures were prepared for each yeast strain. Cell density was adjusted to ~0.8 × 107 cells/ml for all cultures. We maintained the cells in selective medium (synthetic dextrose lacking leucine, tryptophan, or uracil). In each microtiter well, 95 μl of media and 5 μl of cells (4 × 106 cells) were aliquoted in triplicate for blank, control and experimental samples. For experimental samples, we added AFB1, dissolved in DMSO, for a final concentration of 100 nM and 10 μM. The microtiter dish was placed in a plate reader that is capable of both agitating and incubating the plate at 30°C, as previously described (Fasullo et al., 2010). We measured the OD (A660) at 10 min intervals, for a total period for 22 h, 145 readings. Data at 1h intervals was then plotted. To avoid evaporation during the incubation, the microtiter dishes were sealed with clear optical tape (Fasullo et al., 2010).
Diclofenac (DCF) Activity and Preparation of Yeast Microsomes
Strains were grown to saturation in 20 mL SC-LEU medium. The cells were pelleted, resuspended in 5 ml 50 mM Tris 1 mM EDTA (TE pH 7.4) buffer, incubated at room temperature for 5 min, and suspended in 0.6 M Sorbitol 50 mM Tris (pH 7.4). The cells were lysed by agitation in a bead beater at 4°C, and centrifuged at 10000 × g for 20 min. The supernatant from each sample was made 0.15 M in NaCl, and 10% in polyethylene glycol (MW 3350), and incubated on ice for at least one hour. Each tube was centrifuged at 10000 × g for 10 min. The supernatant was removed, and the resulting pellet was suspended in TE and made 20% in glycerol; supernatants were stored in a −80°C freezer. Microsomal extracts were added to 100 μM diclofenac (DCF), 100 mM KPO4, and 1 mM NADPH (Ngui et al., 2000). The assay mixes were then placed in 37°C water bath for 30 min, with minimal light. After the addition of the standards, 0.4 ng DCF-D4 and 0.4 ng 4′-OH-DCF-13C4, 400 μl acetonitrile was then added. The samples were centrifuged twice and the supernatants were then analyzed using Ultra High Performance Liquid Chromatography (UPLC), as described (Ngui et al., 2000).
UPLC-MS/MS analysis of the DCF CYP3A4 metabolite, 5′OH-DCF, was conducted using an Agilent 1290 Infinity liquid chromatography system coupled to an AB SCIEX 6500 QTrap mass spectrometer. Chromatographic separation was performed with an Agilent Eclipse Plus C18 RRHD column (2.1×50 mm; 1.8 μm) using gradient elution and a 5μL sample injection volume. Mobile phase A (A) consisted of 94.9% H2O, 5% acetonitrile, and 0.1% formic acid and mobile phase B (B) consisted of 94.9% acetonitrile, 5% H2O, and 0.1% formic acid. The total run time was 6.5 min, with a flow rate of 0.2mL/min, and used the following elution gradient: 95%A for 0.2 min, followed by a linear increase from 5%B to 90%B between 0.2 and 2.0 min, the gradient remained at 90%B until 2.5 min and then returned to 95%A for re-equilibration of the column. The mass spectrometer was operated in the positive mode using electrospray ionization and the following parameters: Curtain gas 30.0, CAD gas medium, ionspray voltage 5500.0, source temperature 375.0°C, source gas 1 35.0, source gas 2 27.0, declustering potential 50.0, exit potential 10.0, collision energy 21.0, collision cell exit potential 11.0. The analyte, 5′OH-DCF, was detected with multiple reaction monitoring (MRM) using 312.0/230.0 m/z (dwell time: 150 msec) as the parent/product ion transition, 312.0/265.0 m/z was used as a confirmatory parent/product ion transition. Enhanced Product Ion (EPI) scanning was used to generate MS/MS spectra of the CYP3A4 metabolite in order to confirm its identity as 5′OH-DCF.
Detecting CYP3A4 Expression by Western blots
Expression of CYP3A4 was determined by Western blots. Cells containing CYP3A4 were inoculated in SC-LEU medium. Log phase cells (A600 = 0.5–1) were lysed by agitation with glass beads and protein extracts were concentrated by tichloroacetic acid (TCA) precipitation, as previously described by Foiani et al. (1994). Proteins were separated on 10% acrylamide/0.266% bis-acrylamide gels, and transferred to nitrocellulose membranes. Human CYP3A4 was detected by Western blots using 1:1000 diluted mouse monoclonal anti-CYP3A4 (Invitrogen LSC169171), and a 1:10,000 diluted secondary goat anti-mouse antibody conjugated with horse radish peroxidase (Santa Cruz Biotechnology). Signal was detected by chemiluminescence using ThermoFischer SuperSignal™ West Femto Maximum Sensitivity Substrate.
Measuring RNR3 Induction by Rnr3-GFP Fluorescence
Cells expressing CYP3A4 were inoculated in SC-LEU medium. Log phase cells (A600 =0.5–1) were exposed to 50 μM AFB1 for 3 h, and washed twice in PBS, and then concentrated tenfold. To measure RNR3-GFP expression after radiation exposure, we used an X-ray radiation source purchased from Faxitron, Inc. (Wheeling, IL), and the dose rate was 100 rad/min. Approximately 10e4 cells were then analyzed in the Amnis Image Stream. Cell shape and GFP fluorescence was measured with both a visible light and a 495 nM laser, using the image stream software to calibrate cell shape and fluorescence. Untreated cells were used as controls.
RESULTS
CYP3A4 Activity and Protein Can Be Detected in Yeast
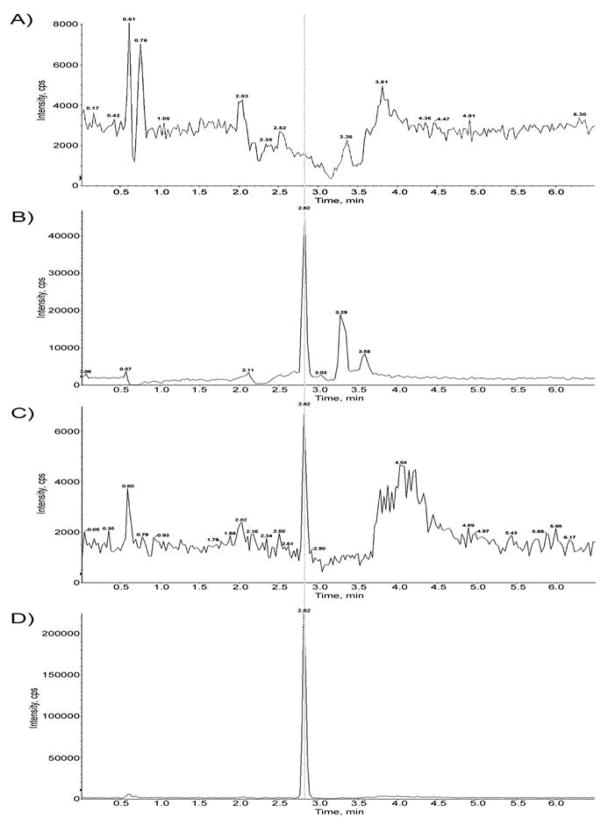
To confirm that the recombinant yeast strain expresses functional human CYP3A4, we performed CYP3A4 activity assays using DCF as the substrate. Microsomal preparations from a strain (YB226) expressing CYP3A4 were compared to a strain not expressing CYP3A4. The results showed CYP3A4-dependent formation of 4′-OH-DCF, a known DCF metabolite produced by CYP3A4 metabolism (Figure 2, panels A, C). In positive control experiments, 4′-OH-DCF was also detected in reactions containing rat liver post-mitochondrial S9 fraction as a source of P450 enzymes (Figure 2B). Formation of the 5′OH-DCF metabolite using the yeast microsomes is 0.88 pmol/min*mg protein, N = 3. These results confirm that YB226 cells express functional CYP3A4 enzyme.
Figure 2.
Results of DCF assays. Reaction mixtures contained (A) microsomal extracts from rad4 rad51 strain expressing no CYPs (YB226, 0.5 mg protein/ml), (B) rat liver S9 fraction (0.5 mg protein/ml), or (C) microsomal extracts from rad4 rad51 expressing CYP3A4 (YB439, 0.5 mg protein/ml); 100 μM DCF; 100 mM KPO4 buffer, pH 7.4; and 1 mM NADPH, in 1.0 ml final volume. The incubation was performed at 37°C for 30 min, and terminated by the addition of 400 μl of acetonitrile, containing internal standards. Equal amounts (200 μl) of the extracted samples (from A–C) were analyzed by LC-MS, as described in Methods. (D), LC-MS chromatogram of standard 4′-OH-DCF-13C6 showing retention time of 2.82 min, the same as shown for the metabolite peak in B and C (arrow).
We detected CYP3A4 protein in yeast cells (YB226) by Western blot using a mouse monoclonal antibody made against rat CYP3A4 (Supplemental Figure). Cell extracts were obtained from control cells lacking the pMA91-CYP3A4 expression vector and cells expressing CYP3A4. Protein was extracted, TCA-precipitated, resolved on SDS-PAGE gels, and blotted on nitrocellulose paper. Yeast protein was visualized by Coomassie staining, and CYP3A4 was identified by 50 kDa protein standard, consistent with the size reported by the commercial supplier (Invitrogen).
Expression of CYP3A4 in the DNA-repair Mutant, rad4 rad51, Confers Sensitivity to AFB1
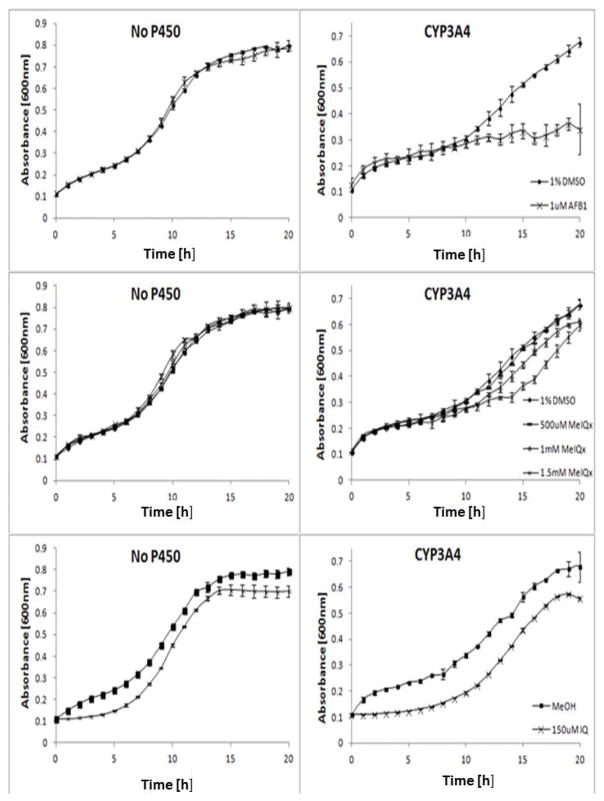
Inhibition of growth in the rad4 rad51 strain is indicative of genotoxicity, since this strain is defective in both nucleotide excision and recombinational repair (Fasullo et al, 2010). We observed that growth of CYP1A2-expressing rad4 rad51 cells was inhibited after exposure to 1 μM AFB1, while exposure to higher concentrations largely blocked growth, consistent with previous studies (Figure 3; Fasullo et al., 2010). Similarly, we exposed CYP3A4-expressing rad4 rad51 cells to AFB1 and observed growth inhibition after exposure to 100 nM AFB1 (Figure 3). Growth inhibition was not evident until 7–10 h, the time at which cells enter exponential phase; previous results indicate that AFB1 elicits a strong DNA damage response as cells enter S phase (Fasullo et al., 2010). By measuring area under the curve, growth was inhibited by (44 ± 4)% and (48 ± 0.4)% when CYP1A2-expressing cells and CYP3A4-expressing cells were exposed to 20 μM AFB1, respectively. The rad4 rad51 cells not expressing any exogenous CYP did not exhibit sensitivity to 1 μM AFB1 (Figure 4). These studies indicate that CYP3A4 metabolically activates AFB1 into a DNA damaging agent.
Figure 3.
Growth curves of the DNA repair rad4 rad51 mutant expressing CYP1A2 (YB404) or CYP3A4 (YB439) after exposure to AFB1. The figure represents one experiment where each data point is the average of two biological duplicates. Approximately 105 cells were inoculated in each well in a 96-well plate platform; yeast growth in each well was monitored in a Tecan Plate reader and over a period of twenty-four hours. Absorbance (A600) is plotted against time (h). The first panel is growth curves of cells expressing CYP1A2 after exposure DMSO or indicated doses of AFB1. The second panel is growth curves of cells expressing CYP3A4 after exposure DMSO or indicated doses of AFB1. Error bars are standard deviations of two biological duplicates.
Figure 4.
Growth curves of the DNA repair rad4 rad51 mutant expressing CYP3A4 (YB439) or no CYP after exposure to AFB1, MeIQx, or IQ. Approximately 105 cells were inoculated in each well in a 96-well plate platform; yeast growth in each well was monitored in a Tecan Plate reader and over a period of twenty-four hours. Absorbance (A600) is plotted against time (h). The first row is growth curves of cells expressing CYP3A4 or no CYP after exposure DMSO or indicated doses of AFB1. The second row is growth curves of cells expressing CYP3A4 or no CYP after exposure DMSO or indicated doses of MeIQx. The third row is growth curves of cells expressing CYP3A4 or no CYP after exposure DMSO or indicated dose of IQ. Error bars are standard deviations of two biological duplicates.
CYP3A4 Expression in Yeast Increases AFB1-associated Recombination
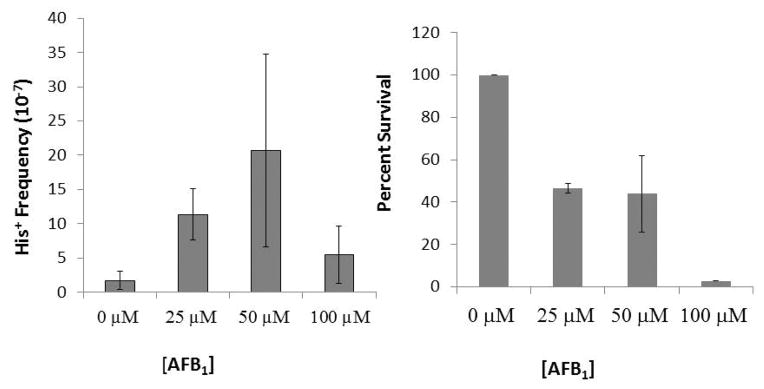
We had previously shown that either CYP1A2 or CYP1A1 expression in yeast is sufficient to metabolically activate AFB1into a potent recombinagen (Sengstag et al., 1996; Keller-Seitz et al., 2004). Considering that growth of a CYP3A4-expressing rad4 rad51 mutant, which is defective in homologous recombination, is inhibited after exposure to AFB1, we hypothesized that expression of CYP3A4 in yeast is also sufficient to activate AFB1 into a recombinagen. We introduced pMA91-CYP3A4 into a rad4 diploid strain that can measure translocation events (Figure 1) and is defective in nucleotide excision repair (NER); NER mutants exhibit enhanced levels of recombination likely due to an inability to remove AFB1-associated DNA adducts (Keller-Seitz et al., 2004). We exposed the CYP3A4-expressing yeast to 25μM, 50 μM, 100 μM AFB1, and measured the frequencies of AFB1-associated recombination (Figure 5). We also compared the frequencies of AFB1-associated recombination in cells expressing either CYP1A2 or CYP3A4 after exposure to 50 μM AFB1 (Table 2). The overall spontaneous frequency of recombination was higher in cells expressing CYP3A4, compared to the cells expressing CYP1A2 (Table 2). Significant levels of AFB1-associated recombination were observed after cells were exposed to 50 μM AFB1 (Figure 5, P < 0.05). In cells exposed to 50 μM AFB1, there was a 56% reduction in viability and a six-fold increase in recombination (N =3). In comparison, in cells expressing CYP1A2 that were exposed to 50 μM AFB1, there was a 49% reduction in viability and more than a twenty-fold increase in recombination; the higher fold increase is due to the lower spontaneous recombination frequency. Significant levels of AFB1-associated recombination was not observed after CYP3A4-expressing cells were exposed 100 μM AFB1, likely due to low viability (< 10%) after exposure. Although a direct comparison of the enzymatic activities of CYP1A2 and CYP3A4 would require additional experiments, we conclude that expression CYP3A4 in yeast is sufficient activate AFB1 into a potent recombinagen.
Figure 5.
Recombination frequencies and viability after cells expressing CYP3A4 are exposed to AFB1. Approximately 108 cells on SC-HIS plates to select for His+ recombinants, and the appropriate dilution was plated on YPD to measure viability. (Left ) Recombination frequencies are represented in a bar graph for each exposure to AFB1 (0 μM, 25 μM, 50 μM, 100 μM). (Right) Percent viability is represented in a bar graph for each exposure (0 μM, 25 μM, 50 μM, 100 μM). Significant levels of AFB1-associated recombination were observed after cells were exposed to 50 μM AFB1 as determined by ANOVA (P = 0.04) and Dunnett’s test (P < 0.05).
Table II.
AFB1 DNA adducts and translocation frequencies in rad4 mutants expressing CYP1A2 or CYP3A4
| Straina | Plasmid | Spont. Frequency (x 10−7) | Induced Frequencyb (x 10−7) | Viabilityc | Fold inductiond | AFB1 DNA adducts (pmol/mg DNA)e |
|---|---|---|---|---|---|---|
| YB428 | pRS424-CYP1A2 | 0.5 ± 0.2 | 13 ± 7 | 51% | 29 | 3.4 |
| YB437 | pMA91-CYP3A4 | 1.6 ± 1.3 | 21± 14 | 44% | 5.6 | 26.2 |
For complete genotype, see Table I
After exposure to 50 μM AFB1, His+ colonies/Total colony forming unit (CFU), N ≥ 2
CFU after exposure to AFB1 / CFU without exposure × 100%
Frequency after AFB1 exposure (Induced Frequency)/Frequency before exposure (Spont. Frequency)
N = 2
To verify that His+ recombinants contain chromosomal translocations (Figure 1), we characterized the electrophoretic karyotype of ten AFB1-associated His+ recombinants, using pulse field gel electrophoresis. We expected to observe reciprocal translocations, CEN4::II and CEN2::IV, where HIS3 is present on CEN2::IV, as previously documented (Sengstag et al., 1996; Fasullo and Davis, 1988). Reciprocal translocations were present in 3/10 of the recombinants, and we speculate that 7/10 of the recombinants lost CEN4::II, although it is also possible that the altered mobility was due to additional rearrangements. These results confirm that AFB1 can stimulate homologous recombination in CYP3A4-expressing yeast, generating chromosomal rearrangements.
AFB1 N7-Guanine DNA Adducts are Detected in Yeast Cells Expressing CYP3A4
Since AFB1 -associated recombination was detected CYP3A4-expressing yeast cells, we hypothesized that AFB1-associated DNA adducts should also be also detected. Diploid cells expressing either CYP3A4 or CYP1A2 were exposed to 50 μM AFB1, lysed, the DNA extracted, and the AFB1 N7-guanine DNA adducts were measured (Table II). No AFB1 N7-guanine DNA adducts were detected in cells exposed to DMSO. Overall levels of DNA adducts in cells expressing CYP3A4 were similar to that observed previously in cells expressing CYP1A2 after exposure to AFB1 (Fasullo et al, 2008; Fasullo et al., 2014). These results indicate that, similar to CYP1A2, expression of CYP3A4 induces AFB1 N7-guanine DNA adducts
Expression of CYP3A4 in Yeast Stimulates RNR3-GFP Expression After AFB1 Exposure
RNR3 encodes a subunit of ribonucleotide reductase that is DNA damage-inducible and has a very low expression in the absence of DNA damage (Elledge and Davis 1990). Expression generally peaks about three h after exposure. We detected Rnr3-GFP expression in cells using the Amnis flow cytometer in CYP1A2 and CYP3A4-expressing cells after exposure to AFB1. We detected fluorescence in ~30% of the cells expressing CYP1A2 (n=2, ~6,000 cells). As can be seen in Figure 6, among CYP3A4-expressing cells, both small and large-budded (dumb-bell cells) were fluorescent; the number of which were significantly different (P < 0.05) than those treated with DMSO alone (P<0.05). We conclude that CYP3A4-mediated activation of AFB1 triggered a DNA damage response.
Figure 6.
The DNA damage response in CYP3A4-expressing cells (YB440) measured by RNR3 induction. The yeast cells containing RNR3-GFP and expressing CYP3A4 were exposed to either DMSO, 2 Krads X-rays, or 50 μM AFB1. GFP fluorescence of single cells was measured in the Amnis. The percent cells showing significant GFP fluorescence was plotted against each type of exposure. The percent of fluorescent cells is indicated for the single unbudded cells and large budded (G2-arrested) cells.
Activation of heterocyclic aromatic amines (HAAs) by expression of CYP3A4 in yeast
CYP3A4 is also expressed in the colon (Ding and Kaminsky, 2003) and could potentially activate heterocyclic aromatic amines that are present in ingested food. We therefore exposed a CYP3A4-expressing rad4 rad51 strain to IQ and MeIQx, which are potent colon carcinogens. We detected growth inhibition when CYP3A4-expressing cells were exposed to either 150 μM IQ or MeIQx; IQ exposure conferred 21% growth inhibition, while 1.5 mM MeIQx exposure conferred 15% growth inhibition (Figure 4). MeIQx exposure did not inhibit growth in cells that did not express CYP3A4, but some growth inhibition was observed when cells were exposed to IQ.
DISCUSSION
CYP3A4 importance in drug metabolism is well-established. While CYP3A4 can accommodate diverse substrates due to large binding site (Yano et al., 2004), other CYPs, such as CYP1A2, have narrower binding site rendering a greater specificity for particular carcinogens, such as AFB1 and heterocyclic aromatic amines (HAAs) (Guengerich et al., 2016). It is unclear whether CYP3A4-mediated carcinogen metabolism can elicit similar genotoxic responses as that mediated by CYP1A2. We expressed CYP3A4 in budding yeast and measured genotoxic endpoints after AFB1 exposure, including DNA adduct formation, recombination, RNR3-GFP induction and growth inhibition of a DNA repair mutant. The major conclusion of this manuscript is that CYP3A4 expression in yeast is sufficient to activate AFB1 into a genotoxin and recombinagen. We suggest that CYP3A4-mediated activation of AFB1 in humans could also result in a recombinagen.
Our previous studies indicated that CYP1A2-mediated activation of AFB1 elicits a robust DNA damage response in yeast as measured by AFB1-associated recombination and the growth inhibition of the rad4 rad51 DNA repair double mutant. Although we did not directly compare the intracellular activity of either CYP3A4 or CYP1A2, AFB1-associated genotoxicity phenotypes are as readily detectable in yeast strains that express CYP3A4 as those that express CYP1A2. Here, we reported lower AFB1-associated recombination frequencies and DNA adduct concentrations in cells expressing CYP1A2 than in previous studies (Fasullo et al., 2008, Fasullo et al., 2014). However, in the strains used in this study, hOR was expressed at a chromosomal location and was not over-expressed on a multi-copied vector. Further experiments are in progress to determine whether CYP3A4-mediated activation of AFB1 also triggers other genotoxic endpoints, such as Rad53 activation, which are observed after CYP1A2-mediated activation (Fasullo et al., 2008, Fasullo et al., 2010).
Our data indicate that CYP3A4 expression in yeast is sufficient to activate AFB1 into a DNA damaging agent at concentrations as low as 100 nM. Other biomarkers in CYP3A4-expressing yeast, such as α-amylase inhibition, have been detected by other investigators after exposure to lower AFB1 concentrations (Li et al., 2009). Previous studies indicate that CYP3A4 likely generates ten-times more AFQ than AFBO (Gallagher et al., 1994). Thus it would be important to know whether different AFB1 metabolites affect different cellular functions, such as RNA translation and protein stability.
Although CYP3A4-mediated activation of AFB1 may be most relevant to hepatocarcinogenesis, the expression of CYP3A4 in the human intestine suggests that the gut may be prone to carcinogen-induced toxicity, as induced by AFB1 and HAAs. We did not measure HAA-associated recombination because co-expression of NAT2 is required to obtain detectable levels of carcinogen-associated recombination (Paladino et al., 1999). Studies done in rat indicate that acute exposures of AFB1 result in minimal toxicity, perhaps due to the intestinal microbes that bind AFB1 adducts. Further studies are necessary to know the fate of the AFB1-associated metabolites in the gut (Wang et al., 2016).
In conclusion, we have shown that CYP3A4 expression in yeast is sufficient to induce DNA damage and elicit genotoxic responses in yeast. These studies allow us to determine whether other carcinogens can be activated in yeast by expression of CYP3A4 and open the avenue for studying CYP3A4 polymorphisms.
Supplementary Material
Supplemental Figure. Western blot of protein extracted from yeast cells containing pMA91-CYP3A4. The Coomassie stain is shown on the left and the immunoblot is shown on the right, in which a mouse monoclonal antibody (Invitrogen MA5-17064) to CYP3A4 was used. An arrow indicates the location of 50 kDa marker. Molecular weight markers are shown in the first lane. In the lanes containing protein extracts from cells that do not express CYP3A4 (YB226), 15 μg were loaded; in lanes containing protein extract from cells containing pMA91-CYP3A4, 7 μg were loaded. The bright band that is detected right below the 50 kDa marker is consistent with the position of mammalian CYP3A4, according to the commercial supplier (Invitrogen).
Acknowledgments
We thank Autumn Smith and Alicia Britton for their technical support. We thank Patrick Maxwell for assistance in running pulse field gels. This research was supported by grants R21ES015954 and R15ES023685-01 (MF) and R01CA092596 (XD) from the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
Michael Fasullo and Xinxin Ding designed the study. Patricia Egner measured the DNA adducts from yeast strains expressing P450 genes that were exposed to AFB1. Julian Freedland imaged cells in the Amnis, performed the DCF assays, and assisted with the growth curves. Cinzia Cera verified the presence of CYP3A4 on the expression plasmid using PCR and restriction enzyme digestions. Nick St. John performed the Western blots and growth curves. Matt Hartog performed the HPLC analysis for to detect the CYP3A4 metabolites. All the authors read and approved the study.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. 4. Wiley; New York, NY: 1999. [Google Scholar]
- Burke D, Dawson D, Stearns T. Methods in yeast genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Press; New York, NY: 2000. [Google Scholar]
- Crespi CL, Penman BW, Steimel DT, Gelboin HV, Gonzalez FJ. The development of a human cell line stably expressing human CYP3A4: role in the metabolic activation of aflatoxin B1 and comparison to CYP1A2 and CYP2A3. Carcinogenesis. 1991;12:355–359. doi: 10.1093/carcin/12.2.355. [DOI] [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal Tracts. Ann Rev of Pharmacology and Toxicology. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- Egner PA, Groopman JD, Wang JS, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem Res Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- Elledge SJ, Davis RW. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 1990;4:740–751. doi: 10.1101/gad.4.5.740. [DOI] [PubMed] [Google Scholar]
- Fasullo MT, Davis RW. Recombinational substrates designed to study recombination between unique and repetitive sequences in vivo. Proc Natl Acad Sci U S A. 1987;84:6215–6219. doi: 10.1073/pnas.84.17.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo MT, Davis RW. Direction of chromosomal rearrangements in Saccharomyces cerevisiae by use of his3 recombination substrates. Mol & Cell Bio. 1988;8:4370–4380. doi: 10.1128/mcb.8.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo M, Sun M, Egner P. Stimulation of sister chromatid exchanges and mutation by aflatoxin B1-DNA adducts in Saccharomyces cerevisiae requires MEC1 (ATR), RAD53, and DUN1. Mol Carcinog. 2008;47:608–615. doi: 10.1002/mc.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo M, Chen Y, Bortcosh W, Sun M, Egner PA. Aflatoxin B(1)-associated DNA adducts stall S phase and stimulate Rad51 foci in Saccharomyces cerevisiae. J Nucleic Acids. 2010;1:456–487. doi: 10.4061/2010/456487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo M, Smith A, Egner P, Cera C. Activation of aflatoxin B1 by expression of human CYP1A2 polymorphisms in Saccharomyces cerevisiae. Mutat Res Genet Toxicol Environ Mutagen. 2014;761:18–26. doi: 10.1016/j.mrgentox.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foiani M, Marini F, Gamba D, Lucchini G, Plevani P. The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol. 1994;14:923–933. doi: 10.1128/mcb.14.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Res. 1994;54:101–108. [PubMed] [Google Scholar]
- Gallagher EP, Kunze KL, Stapleton PL, Eaton DL. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Waterman MR, Egli M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol Sci. 2016;37:625–640. doi: 10.1016/j.tips.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundert-Remy U, Bernauer U, Blömeke B, Döring B, Fabian E, Goebel C, Hessel S, Jäckh C, Lampen A, Oesch F, Petzinger E, Völkel W, Roos PH. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev. 2014;46:291–324. doi: 10.3109/03602532.2014.900565. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Kranendonk M, Carreira F, Theisen P, Laires A, Fisher CW, Rueff J, Estabrook RW, Vermeulen NP. Escherichia Coli MTC, a human NADPH P450 reductase competent mutagenicity tester strain for the expression of human cytochrome P450 isoforms 1A1, 1A2, 2A6, 3A4, or 3A5: Catalytic activities and mutagenicity studies. Mutat Res. 1999;441:73–83. doi: 10.1016/s1383-5718(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Keller-Seitz M, Certa U, Sengstag C, Wurgler F, Sun M, Fasullo M. Transcriptional response of the yeast to the carcinogen Aflatoxin B1: Recombinational repair involving RAD51 and RAD1. Mol Biol Cell. 2004;15:4321–4336. doi: 10.1091/mbc.E04-05-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K, Zanger UM. Pharmacogenomics of Cytochrome P450 3A4: Recent Progress Toward the “Missing Heritability” Problem. Frontiers in Genetics. 2013;4:12. doi: 10.3389/fgene.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2. In: Ryder W, editor. Metabolic Polymorphisms and Susceptibility to Cancer. Vol. 148. IARC Scientific Publication; Lyon, France: 1999. pp. 173–195. [PubMed] [Google Scholar]
- Li X, Millson S, Coker R, Evans I. A sensitive bioassay for the mycotoxin aflatoxin B(1), which also responds to the mycotoxins aflatoxin G(1) and T-2 toxin, using engineered baker’s yeast. J Microbiol Methods. 2009;77:285–291. doi: 10.1016/j.mimet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Liao M, Faouzi S, Karyakin A, Correia MA. Endoplasmic reticulum-associated degradation of cytochrome P450 CYP3A4 in Saccharomyces cerevisiae: further characterization of cellular participants and structural determinants. Mol Pharmacol. 2006;69:1897–1904. doi: 10.1124/mol.105.021816. [DOI] [PubMed] [Google Scholar]
- Macé K, Aguilar F, Wang JS, Vautravers P, Gómez-Lechón M, Gonzalez FJ, Groopman J, Harris CC, Pfeifer AM. Aflatoxin B1-induced DNA adduct formation and p53 mutations in CYP450-expressing human liver cell lines. Carcinogenesis. 1997;18:1291–1297. doi: 10.1093/carcin/18.7.1291. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–43. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal GE, Eaton DL, Judah DJ, Verma A. Metabolism and toxicity of aflatoxins M1 and B1 in human-derived in Vitro systems. Toxicology and Applied Pharmacology. 1998;151:152–158. doi: 10.1006/taap.1998.8440. http://dx.doi.org/10.1006/taap.1998.8440. [DOI] [PubMed] [Google Scholar]
- Ngui JS, Tang W, Stearns RA, Shou M, Miller RR, Zhang Y, Lin JH, Baillie TA. Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos. 2000;28:1043–1050. [PubMed] [Google Scholar]
- Palma BB, Silva e Sousa M, Urban P, Rueff J, Kranendonk M. Functional characterization of eight human CYP1A2 variants: the role of cytochrome b5. Pharmacogenetics and Genomics. 2013;23:41–52. doi: 10.1097/FPC.0b013e32835c2ddf. [DOI] [PubMed] [Google Scholar]
- Paladino G, Weibel B, Sengstag C. Heterocyclic aromatic amines efficiently induce mitotic recombination in metabolically competent Saccharomyces cerevisiae strains. Carcinogenesis. 1999;20:2143–2152. doi: 10.1093/carcin/20.11.2143. [DOI] [PubMed] [Google Scholar]
- Sengstag C, Würgler FE. DNA recombination induced by aflatoxin B1 activated by cytochrome P450 1A enzymes. Mol Carcinog. 1994;11:227–235. doi: 10.1002/mc.2940110408. [DOI] [PubMed] [Google Scholar]
- Sengstag C, Weibel B, Fasullo M. Genotoxicity of aflatoxin B1: evidence for a recombination-mediated mechanism in Saccharomyces cerevisiae. Cancer Res. 1996;56:5457–5465. [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF, Poulos TL. Current approaches for investigating and predicting Cytochrome P450 3A4-Ligand Interactions. Advances in Experimental Medicine and Biology. 2015;851:83–105. doi: 10.1007/978-3-319-16009-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Ong CN. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat Res Rev Genet Toxicol. 1996;366:23–44. doi: 10.1016/s0165-1110(96)90005-6. [DOI] [PubMed] [Google Scholar]
- Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE. Gut instincts: CYP3A4 and intestinal drug metabolism. The Journal of Clinical Investigation. 2007;117:3173–3176. doi: 10.1172/JCI34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tang L, Glenn TC, Wang JS. Aflatoxin B1 Induced Compositional Changes in Gut Microbial Communities of Male F344 Rats. Toxicol Sci. 2016;150:54–63. doi: 10.1093/toxsci/kfv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24:117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992;52:2114s–2118s. [PubMed] [Google Scholar]
- Yang X, Zhang B, Molony C, Chudin E, Hao K, Zhu J, Gaedigk A, Suver C, Zhong H, Leeder JS, et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Research. 2010;20:1020–1036. doi: 10.1101/gr.103341.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, Johnson EF. The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-A resolution. J Biol Chem. 2004;279:38091–38094. doi: 10.1074/jbc.C400293200. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. 3. New Jersey: Prentice Hall; 1996. p. 221. [Google Scholar]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zeigler-Johnson C, Friebel T, Walker AH, Wang Y, Spangler E, Panossian S, Patacsil M, Aplenc R, Wein AJ, Malkowicz SB, Rebbeck TR. CYP3A4, CYP3A5, and CYP3A4 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 2004;64:8461–8467. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Western blot of protein extracted from yeast cells containing pMA91-CYP3A4. The Coomassie stain is shown on the left and the immunoblot is shown on the right, in which a mouse monoclonal antibody (Invitrogen MA5-17064) to CYP3A4 was used. An arrow indicates the location of 50 kDa marker. Molecular weight markers are shown in the first lane. In the lanes containing protein extracts from cells that do not express CYP3A4 (YB226), 15 μg were loaded; in lanes containing protein extract from cells containing pMA91-CYP3A4, 7 μg were loaded. The bright band that is detected right below the 50 kDa marker is consistent with the position of mammalian CYP3A4, according to the commercial supplier (Invitrogen).