Abstract
Following the principles of care recommended in the 2006 Consensus Statement on Disorders of Sex Development (DSD), along with input from representatives of peer support and advocacy groups, this study surveyed DSD clinical management practices at healthcare facilities in the United States. DSD are congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical. Facilities providing care for patients with DSD were targeted for participation. Specialty providers completed a survey with questions in six broad categories: Institution Information, Nomenclature and Care Guidelines, Interdisciplinary Services, Staff and Community Education, DSD Management, and Research. Twenty-two of 36 targeted sites (61%) participated. Differences were observed between sites with regard to what conditions were considered to be DSD. All sites reported some degree of involvement of pediatric urology and/or surgery and pediatric endocrinology in the care of DSD patients. Gynecology and neonatology were most frequently not represented. Wide variation was observed across sites in continuing education standards, obtaining informed consent for clinical procedures, and in specific clinical management practices. This survey is the first to assess DSD clinical management practices in the United States. The findings establish a baseline of current practices against which providers delivering care to these patients and their families can benchmark their efforts. Such surveys also provide a practical framework for collaboration in identifying opportunities for change that enhance health and quality of life outcomes for patients and families affected by DSD.
Keywords: survey, disorders of sex development, quality improvement, intersex
INTRODUCTION
A consensus conference on nomenclature and clinical management of intersex conditions was convened in 2005 under the auspices of the Lawson Wilkins Pediatric Endocrine Society (LWPES) and the European Society for Paediatric Endocrinology (ESPE). Participants included 50 international experts and patient advocate representatives. The Consensus Statement on Management of Intersex – hereafter referred to as the “consensus statement” – was published the following year and adopted as a policy statement by the American Academy of Pediatrics [Lee et al., 2006]. The consensus statement outlines the principles guiding care, but does not constitute “practice guidelines.”
At the time of the consensus conference, experience suggested that the term “intersex” was perceived as imprecise, confusing to providers and families, and potentially experienced as stigmatizing. In response to these concerns, together with the recognition that medical terminology should reflect current understandings of etiology and be sufficiently flexible to evolve with extended discovery, the consensus statement substituted Disorders of Sex Development (DSD) as a new umbrella term for “intersex.” DSD was defined as “congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical” [Lee et al., 2006]. In addition to the introduction of DSD, and an accompanying nomenclature reflecting genetic etiology of the specific condition, key topics covered in the consensus statement included concepts of optimal care, composition of the healthcare team, diagnostic evaluation, medical/surgical and psychosocial management, and an overview of treatment outcomes. Anticipating the changes to come, the (now bygone) Intersex Society of North America – a patient advocacy organization – published its “Clinical Guidelines for the Management of Disorders of Sex Development” immediately preceding publication of the consensus statement [Consortium on the Management of Disorders of Sex Development, 2006]. These “Clinical Guidelines” were characterized in the consensus statement as reflecting “optimal clinical management” of people affected by DSD and their families [Lee et al., 2006].
The objective of family- and patient-centered care has been broadly accepted in the U.S. and in Europe [Ahmed et al., 2011; Brain et al., 2010; Lee et al., 2006]. Agreement at the level of principles of DSD care notwithstanding, uncertainty and controversy remains regarding the comparative effectiveness of treatment options in delivering somatic health and positive quality of life outcomes. Moreover, there are multiple systemic barriers to fielding a comprehensive and integrated healthcare team for DSD that goes beyond coordinating visits to specialists to reduce family burden, i.e., “one-stop shopping.” The type of “team” created – multidisciplinary, interdisciplinary or transdisciplinary – implies different degrees of collaboration and professional autonomy [Lee et al., 2016].
Quality improvement (QI) involves methodical and uninterrupted activities leading to measurable improvement in healthcare services and the health status of targeted patient groups. Ideally, the metrics used to track QI would indicate how well current systems are working, consequences of recommended changes, and achievement of specific goals [U.S. Department of Health and Human Services, Health Resources and Services Administration, 2011]. In the field of DSD, principles outlined in the consensus statement and aforementioned “Clinical Guidelines” provide potential goals against which healthcare practices for this unique population can be tracked and benchmarked following a QI process. Along these lines, a 2010 survey of European pediatric endocrinologists from 60 centers in 23 different European countries focused on assessing DSD clinical management practices. They found that the majority of centers surveyed implemented practices with regard to team composition, nomenclature, and surgical and psychosexual management that aligned with the 2006 consensus statement [Pasterski et al., 2010]. Another study surveyed members of the ESPE about testicular or ovotesticular DSD management practices and found significant regional variations in care, suggesting that clinical guidelines may require flexibility to account for contextual factors of institutions, such as variation in resources [Josso et al., 2011]. Most recently, an international survey of DSD care was conducted to explore current models of practice in delivering specialist care for children with DSD [Kyriakou et al., 2016]. Responses were received from 78 clinicians (endocrinologists representing 90% of respondents) from 75 centers in 38 countries, but only two of the centers (7%) were from North America. To date, no study has focused on DSD management practices, with input of all specialists, at institutions in the United States.
The DSD-Translational Research Network (DSD-TRN), initiated in late 2011, is a hybrid learning collaborative and DSD patient registry [Sandberg et al., 2015]. The network is designed to capture the “process” of ongoing care using a comprehensive combination of prospectively applied genetic, biochemical, phenotyping, and psychosocial approaches to inform the diagnosis and clinical management of the individual patient and family. This study incorporated input from patient advocates through the Advocacy Advisory Network convened by Accord Alliance (www.accordalliance.org). From its inception, the DSD-TRN has worked to operationalize the principles of care articulated in the 2006 consensus statement. At the time of data collection for this study, the DSD-TRN comprised seven U.S. medical centers [Disorders of Sex Development – Translational Research Network, 2016]. It is a reasonable expectation that care delivered at a center which is part of a network designed to improve adherence to a model of care would achieve this to a higher degree than unaffiliated sites. To date, this hypothesis has not been tested with regard to clinical practice in DSD.
Delivering patient- and family-centered care in DSD is often complex and challenging, requiring the input of multiple providers and families. In recent years, substantial focus has been placed on the development of clinical management guidelines in an effort to optimize care. Creation of the DSD-TRN provided the impetus for a structured assessment to characterize standards of care both within and outside the network. The objective of this study was to perform a systematic assessment of clinical management practices for DSD at institutions across the United States. The data presented here – a subset from a larger survey – focus on practices in relation to principles of DSD care described in the 2006 consensus statement, a quality care indicator checklist published by Accord Alliance, and updated with ongoing input from patient advocacy stakeholders. With these and related data, we aim to benchmark the current state of DSD practices in the United States and identify areas for ongoing improvement.
METHODS
Participants and Procedures
Healthcare institutions providing clinical services for patients with DSD were nominated for inclusion by the authors in conjunction with additional clinician, researcher, and patient advocate members of the DSD-TRN. Inclusion criteria were as follows: U.S.-based healthcare institutions providing coordinated care to pediatric patients diagnosed with DSD (one site in Canada was included due to frequent nominations related to their long history as a comprehensive care center for DSD). Institutions surveyed did not require the presence of a dedicated “team,” but did require the participation of multiple specialists who coordinated services, as evidenced by direct knowledge by the nominating individual and/or descriptions of care at the institution’s website. Institutional contacts for the survey were identified by the nominating individual and/or results of web searches. Contacts were emailed a description of the study; non-responders received multiple follow-up emails. Recruitment materials specified that survey responses would not be anonymous; however, any data selected for publication would not identify sites, nor respondents, unless consent was obtained permitting it. [All data in this report are anonymized.] Sites agreeing to participate received a unique link to the online survey, a downloadable PDF that mirrored the online survey, and a worksheet for notes about ideas generated in the process of completing the survey which, in turn, could potentially be used by the site for future modifications of their services. The PDF was also provided so that the institutional contact could collect responses from multiple providers before entering collated responses online. The study was reviewed by the Institutional Review Board at the University of Michigan and categorized as “Not Regulated” (i.e., Research on Organizations).
Of 36 total institutions targeted, 22 (61%) completed and three (8%) began, but did not complete, the survey; three (8%) declined participation; and eight (22%) did not respond to the invitation. Data are reported for the 22 institutions that completed at least 80% of survey items. Twenty (91%) of these 22 institutions completed the attestation that responses reflected the input of the entire group of DSD providers at their institution. Half (n=11) of the sites exclusively served the pediatric population and half served both pediatric and adult populations. Fourteen institutions (64%) provided primary (i.e., providers who act as point of first contact in the healthcare system), secondary (i.e., specialty healthcare services), and tertiary (i.e., specialized and consultative healthcare equipped to conduct in-depth medical investigations, usually involving inpatient services and/or referrals from primary or secondary services) care services. One institution provided only primary and secondary care services; one institution provided only secondary and tertiary care services; and the remaining six institutions provided only tertiary care services. Categorizing participating sites according to the Census Regions of the United States [Census Regions and Divisions of the United States, 2017], seven (32%) sites were located in the West Region, six (27%) in the Midwest Region, five (23%) in the South Region, and four (18%) were located in the Northeast Region. A minority (n=7, 32%) were members of the DSD-TRN at the time of survey administration (June 2014 to January 2015).
Measures
Survey items were generated following a literature review that heavily weighted the 2006 consensus statement [Lee et al., 2006], the “Clinical Guidelines” [Consortium on the Management of Disorders of Sex Development, 2006] and a 2010 survey designed to assess clinical management of DSD across Europe subsequent to the 2006 consensus statement recommendations [Pasterski et al., 2010]. Additionally, items were adapted from the Accord Alliance document describing Quality Care Indicators (QCIs) for the care of children with DSD [Sorenson., 2011]. The survey was beta-tested and piloted at three DSD-TRN sites prior to finalization. The final survey included a total of 137 possible questions divided into six sections: Institution Information, Nomenclature and Care Guidelines, Interdisciplinary Services, Staff and Community Education, DSD Management, and Research. The survey branched based on responses to stem questions.
The survey also requested that participating institutions upload documents integral to routine care such as a mission statement related to DSD care, bibliography/reading list for DSD providers, written protocols/policies, consent forms, etc. Lastly, the person completing the online survey was asked to attest that the responses were reviewed with and reflected the opinions of the entire group of DSD providers at their institution.
Data Analysis Plan
To serve as a benchmark of contemporary clinical service delivery, response frequencies were calculated for a subset of individual survey items. Composite scores were generated for several areas emphasized by the consensus statement and QCIs: DSD Management, Specialist Representation, Informed Consent, Continuing Education, and Research. For composite scores to be generated, respondents needed to complete at least 80% of questions in that scale. Each item was scored on a 0 to 1 (with 1 representing the ideal) scale with the possibility for fractional scoring of individual items. Means of individual items were calculated to generate scale scores, unless as otherwise specified for certain categories. Additional details of scoring algorithms are described within respective results subsections.
Finally, in addition to characterizing the total range of practices across sites, results of the seven DSD-TRN sites were compared to the other participating institutions to examine whether a network structure delivered a higher degree of match with consensus recommendations and QCIs.
RESULTS
DSD Clinical Management
Institutions reported providing clinical services for a median of 48 patients with DSD in the 12 months prior to survey completion (mean = 78; range = 5 to 280). The presenting diagnoses/phenotypes considered to fall under the DSD umbrella varied across sites. Of 24 discrete diagnoses (e.g., 46,XX/46,XY chimera, ovotestes) or phenotypes (e.g., distal/mid-shaft hypospadias) listed in the survey, there was complete agreement on less than half (n = 10; 42%) on whether or not they constituted a DSD (Table I). The area of greatest disagreement (i.e., 50:50 split) was over whether or not “proximal hypospadias with descended testes” were considered a DSD.
Table I.
Frequency of institutions classifying discrete diagnoses/phenotypes as DSD.
| Diagnoses/Phenotypes | Classified as DSD | |
|---|---|---|
| n | % | |
| 45,X/46,XY MGD, ovotestes | 22 | 100 |
| 46,XX/46,XY chimera, ovotestes | 22 | 100 |
| Ovotestes | 22 | 100 |
| 46,XX with male phenotype | 22 | 100 |
| Androgen biosynthesis defect (e.g., 5α reductase deficiency) | 22 | 100 |
| Androgen excess in 46,XX due to fetal (e.g., 21-hydroxylase deficiency), fetoplacental (e.g., aromatase deficiency), or maternal (e.g., luteoma) causes | 22 | 100 |
| Defect in androgen action (e.g., complete androgen insensitivity syndrome (CAIS); partial androgen insensitivity syndrome (PAIS)) | 22 | 100 |
| Complete gonadal dysgenesis | 22 | 100 |
| Partial gonadal dysgenesis | 22 | 100 |
| Disorders of AMH and AMH receptor (persistent Müllerian duct syndrome) | 20 | 91 |
| LH receptor defects (e.g., Leydig cell hypoplasia, aplasia) | 17 | 81* |
| 45,X Turner syndrome and variants | 16 | 73 |
| Proximal hypospadias with uni-/bilateral undescended teste(s) | 14 | 70† |
| 47,XXY Klinefelter and variants | 15 | 68 |
| Gonadal regression (anorchia) | 15 | 68 |
| Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome/Müllerian duct aplasia, renal aplasia, and cervicothoracic somite dysplasia (MURCS) | 14 | 67* |
| 46,XY bladder/cloacal anomalies | 13 | 62* |
| 46,XX bladder/cloacal anomalies | 13 | 62* |
| Vaginal atresia | 13 | 62 |
| Isolated micropenis | 12 | 57* |
| Proximal hypospadias with descended testes | 10 | 50† |
| Distal/mid-shaft hypospadias | 6 | 30† |
| Undescended teste(s) | 6 | 27 |
| Trauma | 0 | 0 |
one site selected “prefer not to answer” and therefore was not included in calculations
two sites selected “prefer not to answer” and therefore were not included in calculations
Note: Diagnoses/Phenotypes are listed identically to how they were displayed on the survey. Abbreviations not included above are as follows: MGD = Mixed Gonadal Dysgenesis; AMH = Anti- Müllerian Hormone; LH = Luteinizing Hormone
Composite scale scores for six areas of clinical management identified in the Consensus recommendations and QCIs (i.e., uniform care plan, follow-up programs, transitional care, genital exams/medical photography, data tracking, and patient/family outreach and/or education & access to support groups) were calculated (Table II). Mean scores were closest to the ideal (1.0) on the availability of follow-up programs (m = .71, sd = .29) and furthest from ideal on Data Tracking (m = .48, sd = .29) and Patient and Family Outreach/Education (m = .48, sd = .32). Scale scores evidenced variability across institutions (Figure 1).
Table II.
Clinical management practices.
| Composite Scales Scale Items |
Sites (n) | Yes (Ideal) | Institutional Score | |||
|---|---|---|---|---|---|---|
| n | % | Mean | SD | Min – Max | ||
| Uniform Care Plan | ||||||
| Routinely assess genital anatomy in comparison to published norms | 22 | 17 | 77 | |||
| Use decision-making algorithms in first line testing for newborns | 19 | 5 | 26 | |||
| Are specialists available for gender dissatisfaction/dysphoria | 19 | 17 | 90 | |||
| Behavioral health provider available in cases of ambiguous genitalia at birth | 21 | 18 | 86 | |||
| Uniform Care Plan subscale composite score | 15 | .68 | .24 | .25 – 1.0 | ||
| Follow-up Programs | ||||||
| Follow a schedule for following up when there is no ongoing problem | 22 | 14 | 64 | |||
| Gender identity routinely assessed | 22 | 19 | 86 | |||
| What behavioral health services are provided* | 21 | 10 | 48 | |||
| Follow-up Programs subscale composite score | 21 | .71 | .29 | .08 – 1.0 | ||
| Transitional Care | ||||||
| Have a separate transitional/adolescent/adult clinic | 20 | 2 | 10 | |||
| Opportunity for adolescents to speak confidentially with provider† | 21 | 17 | 81 | |||
| Transitional Care subscale composite score | 20 | .69 | .27 | .25 – 1.0 | ||
| Genital Exams | ||||||
| Set a max number of providers present during genital exams | 22 | 7 | 32 | |||
| Set a max number of learners/trainees during genital exams | 21 | 9 | 43 | |||
| How often are genital exams (not under anesthesia) performed primarily for education‡ | 21 | 15 | 71 | |||
| Genital Exams subscale composite score | 20 | .52 | .35 | 0 – 1.0 | ||
| Data Tracking | ||||||
| Maintain a local database | 22 | 12 | 54 | |||
| Systematically record procedures | 12 | 3 | 25 | |||
| Systematically record clinical outcomes for procedures | 12 | 2 | 17 | |||
| Systematically record clinical status beyond the period that providers are actively involved in patient’s care | 12 | 4 | 33 | |||
| Affiliated with a multi-site registry | 12 | 8 | 67 | |||
| Data Tracking subscale composite score | 12 | .48 | .29 | .20 – 1.0 | ||
| Patient and Family Outreach/Education | ||||||
| Provide opportunities for patients/families to meet others | 22 | 12 | 54 | |||
| Host workshops/family days | 22 | 6 | 27 | |||
| Attend national support group/advocacy conferences | 22 | 16 | 73 | |||
| Working directly with support/advocacy group members at conferences | 22 | 12 | 54 | |||
| Develop resources/materials with support/advocacy groups | 22 | 6 | 27 | |||
| Invite support and advocacy groups or patient/parent reps to speak to providers | 22 | 10 | 46 | |||
| Other§ | 22 | 2 | 9 | |||
| Patient and Family Outreach/Education subscale composite score | 22 | .48 | .32 | 0 – 1.0 | ||
| Total Scale | 18 | .56 | .22 | .12 – 1.0 | ||
All items are scored yes = 1 (ideal); no = 0; unless as noted:
1 = routinely at initial consultation and at follow-up; .5 = routinely at initial or follow-up; .25 = referral only; 0 = not provided
1 = always; .75 = usually; .5 = sometimes; .25 = rarely; 0 = never
1 = never; 0.5 = rarely; 0 = sometimes, usually, or always
“Other” only contributed to mean subscale score if answered “Yes”
Composite scores are calculated for institutions completing at least 80% of items comprising each scale
Figure 1.
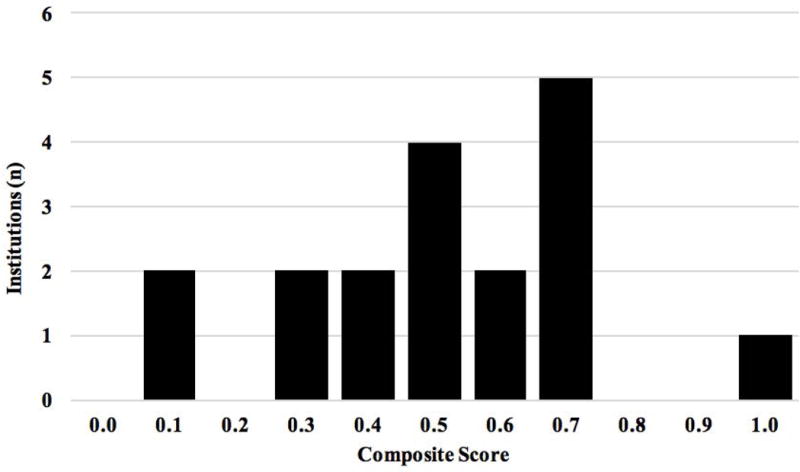
Mean score of institutions on elements of clinical management practices.
Composite score of 1 = ideal score. Composite scores are calculated for institutions completing at least 80% of items comprising the scale.
DSD Specialist Representation
Both consensus statement recommendations and QCIs indicated optimal care requires team care [Lee et al., 2006; Sorenson, 2011]. The consensus statement listed pediatric subspecialists in endocrinology, surgery and/or urology, psychology/psychiatry, gynecology, genetics, and neonatology as “ideal” members of the team. No site reported that all six subspecialists were routinely involved in the delivery of DSD services; distributions of subspecialist involvement differed by site (Figure 2). While urologists/surgeons were “Always” involved; endocrinologists, geneticists, and psychologists/psychiatrists were either “Always” involved or involved on a “Referral/Consult Basis.” Two subspecialties were not represented at all at some sites: gynecology was unavailable at six (27%) sites; neonatology was unavailable at five (23%) sites.
Figure 2.
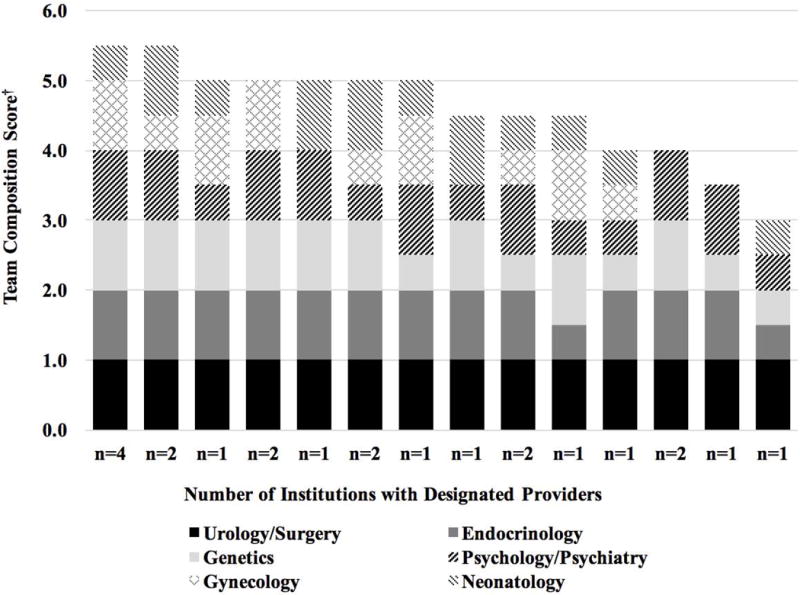
Pediatric specialist availability.
†1 pt = “always involved”; 0.5 pt = “referral/consult involvement only”
Informed Consent
In addition to discussion and documentation related to informed consent for surgical procedures, patient advocates encouraged including specific processes and points of discussion and documentation for any interventions/procedures related to DSD. Universal (100%) agreement occurred on the practice of providing children of assenting age the opportunity to assent or withhold assent for medical procedures including surgery, laparoscopy, or any other non-life-saving measures. Approximately half of sites (n = 12, 55%) reported imposing an interval between discussion of treatment options and patient/family decision (known as a “Thinking Period”). Less than half (n = 6, 40%) of the 15 sites responding to the question routinely provided a legal/bioethics consultation in the context of the clinical informed consent process prior to any plan to remove non-dysgenetic ovaries/testes.
Institutions (n = 19 who completed at least 80% of items in this section) reported wide variation in elements of consent routinely incorporated in discussion and documentation. Although composite scores for information about procedures and risks/benefits (.54 and .59, respectively; Table III) suggested that while nearly all elements were either verbally discussed or incorporated into written informed consent, few institutions routinely included many of the specific elements proposed by patient advocates in both verbal discussion and written documentation (Figure 3). Using both discussion and written documentation as the standard, the most frequently reported informational item delivered to patients and/or their families in this way (n = 3, 14%) was: “in the future the child’s gender identity may not match the surgically reinforced gender; possibility of gender transition.” With regard to risks and benefits of surgical procedures, the items most frequently provided to patients and their families in both verbal and written forms (n = 6, 32%) were: “potential surgical complications and possible need for additional procedures” and “genital anomalies may take more than one procedure to correct and may in fact involve multiple procedures.”
Table III.
Informed consent specific to DSD.
| Composite Scales Scale Items |
Sites (n) | Yes (Ideal) | Institutional Score | |||
|---|---|---|---|---|---|---|
| n | % | Mean | SD | Min – Max | ||
| Consent Procedures | ||||||
|
| ||||||
| Are children of assenting age given the opportunity to assent or withhold assent for medical procedures (including surgery, laparoscopy, or any other non-life-saving measures)? | 21 | 21 | 100 | |||
|
| ||||||
| Is a legal/bioethics consult routinely provided in the context of the clinical informed consent process prior to any irreversible procedures if there is a plan to remove non-dysgenetic ovaries/testes? | 15 | 6 | 40 | |||
|
| ||||||
| Is it the practice of your DSD providers to impose an interval between discussion of treatment options and patient/family decision (known as a “Thinking Period”)? | 19 | 12 | 63 | |||
|
| ||||||
| Consent Procedures subscale composite score | 15 | .67 | .25 | .33 – 1.0 | ||
|
| ||||||
| Informed Consent Elements* | ||||||
|
| ||||||
| Information: | ||||||
|
| ||||||
| Whether procure is medically necessary or elective | 19 | 2 | 11 | |||
|
| ||||||
| Which interventions are reversible/partly reversible/irreversible | 19 | 2 | 11 | |||
|
| ||||||
| In the future the child’s gender identity may not match surgically reinforced gender; possibility of gender transition | 19 | 3 | 16 | |||
|
| ||||||
| Gender assignment is often less stable in individuals with DSD | 19 | 2 | 11 | |||
|
| ||||||
| Information subscale composite score | 19 | .54 | .14 | .38 – 1.0 | ||
|
| ||||||
| Risks/Benefits: | ||||||
|
| ||||||
| Potential effects on fertility | 19 | 4 | 21 | |||
|
| ||||||
| Potential effects on sensitivity of sex organs and sexual function | 19 | 5 | 26 | |||
|
| ||||||
| Potential effects on psychological and social adjustment | 19 | 3 | 16 | |||
|
| ||||||
| Potential effects on continence | 19 | 5 | 26 | |||
|
| ||||||
| Potential effects on other functions | 14 | 1 | 7 | |||
|
| ||||||
| Hormonal consequences of removal of gonads (e.g., induced puberty, need for lifelong hormone replacement) | 19 | 4 | 21 | |||
|
| ||||||
| Potential surgical complications and possible need for additional procedures | 19 | 6 | 32 | |||
|
| ||||||
| Genital anomalies may take more than one procedure to correct and may in fact involve multiple procedures | 19 | 6 | 32 | |||
|
| ||||||
| Risks/Benefits subscale composite score | 19 | .59 | .19 | .44 – 1.0 | ||
|
| ||||||
| Informed Consent Elements subscale composite score | 19 | .57 | .16 | .42 – .92 | ||
|
| ||||||
| Total Score | 18 | .60 | .15 | .40 – .93 | ||
All items are scored yes = 1 (ideal); no = 0 unless otherwise noted:
1 = discussed and included in written informed consent document; .5 = discussed or written only; 0 = neither
Composite scores are calculated for institutions completing at least 80% of items comprising each scale
Figure 3.
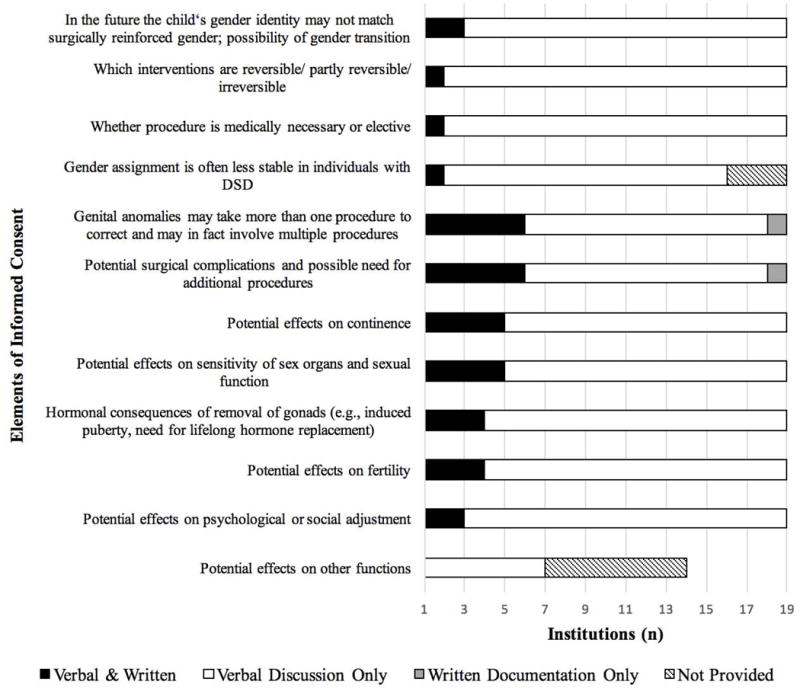
Elements of informed consent: discussion and documentation
Continuing Education
The QCIs included continuing education as a feature of optimal care. Continuing education was operationalized as encompassing activities directed at healthcare providers within the institution (i.e., staff and provider education) and outside the institution (i.e., education of providers in the wider community). Responses to discrete items showed variability across institutions (Table IV; Figure 4). The most highly reported staff education method was attendance at least one DSD-related teaching or conference/symposium during the past year (n = 22, 100%); the least frequently reported were providing protocols/guides for new DSD providers and inviting outside experts to address DSD providers at institution within the past year (n = 4, 18%). The most frequently reported community education was to host educational workshops (n = 9, 41%) and least was to provide in-service training for NICU or labor and delivery nurses or staff at outside hospitals (n = 3, 14%).
Table IV.
Continuing education for providers.
| Composite Scales Scale Items |
Sites (n) | Yes (Ideal) | Institutional Score | |||
|---|---|---|---|---|---|---|
| n | % | Mean | SD | Min – Max | ||
| Staff Education | ||||||
|
| ||||||
| Provide protocols/guides for new DSD providers | 22 | 4 | 18 | |||
|
| ||||||
| Provide in-service training for NICU and/or labor & delivery nurses/staff? | 22 | 7 | 32 | |||
|
| ||||||
| Provide printed protocols for services to follow in the event of ascertaining a patient with a potential DSD | 22 | 7 | 32 | |||
|
| ||||||
| Providers attended at least one conference/symposium over the past year* | 22 | 22 | 100 | |||
|
| ||||||
| DSD Journal Club available for providers | 22 | 9 | 41 | |||
|
| ||||||
| Invited outside experts to address DSD providers at institution within the past year | 22 | 4 | 18 | |||
|
| ||||||
| Subscale composite score | 22 | .40 | .24 | .17 – 1.0 | ||
|
| ||||||
| Community Education | ||||||
|
| ||||||
| Provide in-service training for NICU/L&D nurses/staff at outside hospitals | 22 | 3 | 14 | |||
|
| ||||||
| Consultation and assistance to other hospitals/clinics: | ||||||
|
| ||||||
| Host educational workshops | 22 | 9 | 41 | |||
|
| ||||||
| Consultation and assistance to hospitals/clinics unfamiliar with DSD management | 22 | 5 | 23 | |||
|
| ||||||
| Communicating with DSD providers at other institutions about models of care | 22 | 4 | 18 | |||
|
| ||||||
| Other† | 1 | 4 | ||||
|
| ||||||
| Subscale composite score | 22 | .24 | .29 | 0 – 1.0 | ||
|
| ||||||
| Total Score | 22 | .34 | .24 | .10 – 1.0 | ||
All items are scored 1 = yes (ideal); 0 = no, unless otherwise noted:
10 conferences and symposia were listed, a write-in space “other” was also included; 1 = attendance at any; 0 = no attendance
“Other” only contributed to mean subscale score if answered “Yes”
Figure 4.
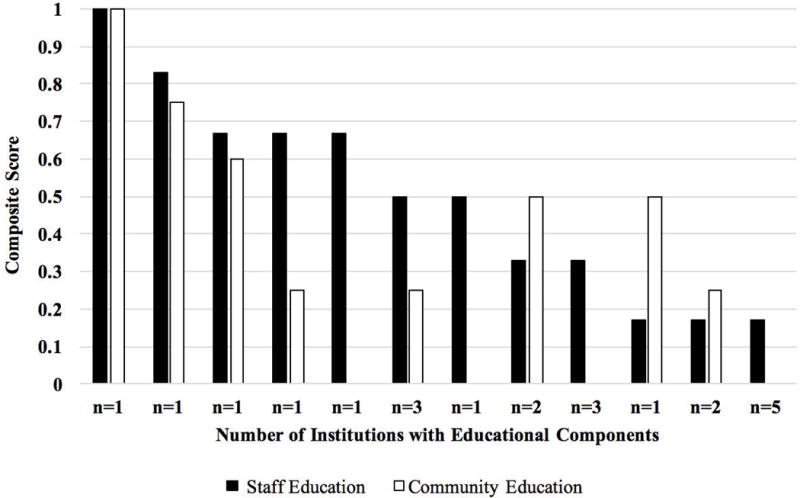
Continuing education for providers.
Composite score of 1 = ideal score
Research
The majority (n = 15, 79%) of 19 sites that responded to questions about research reported that they participate in DSD-related research. Among these sites, 11 (73%) reported on specific research studies (e.g., “surgery and outcomes of hypospadias repair”) and/or affiliation with research groups (e.g., DSD-TRN).
Comparison of DSD-TRN Member Sites with Others
DSD-TRN member sites (n = 7) reported participating in research (m = 1.0, sd = 0.0) more frequently than unaffiliated sites (n = 15) (m = .67, sd = .49), t (11) = 2.34, p = .039. DSD-TRN sites also reported providing more (m = .66, sd=.27) patient and family outreach and educational services than non-participating sites (m = .39, sd = .32). Similarly, DSD-TRN sites reported providing more (m = .41, sd = .33) continuing education for providers outside their home institution than non-participating sites (m = .17, sd = .24). Statistical testing showed these latter two comparisons approached, but did not achieve statistical significance: t (20) = 1.92, p = .069 and t (20) = 1.94, p = .067, respectively. Although not achieving statistical significance, this same pattern – DSD-TRN sites endorsing a greater number of items than unaffiliated sites – held true for all other items, with the exception of follow-up programs and all items related to informed consent (Figure 5).
Figure 5.
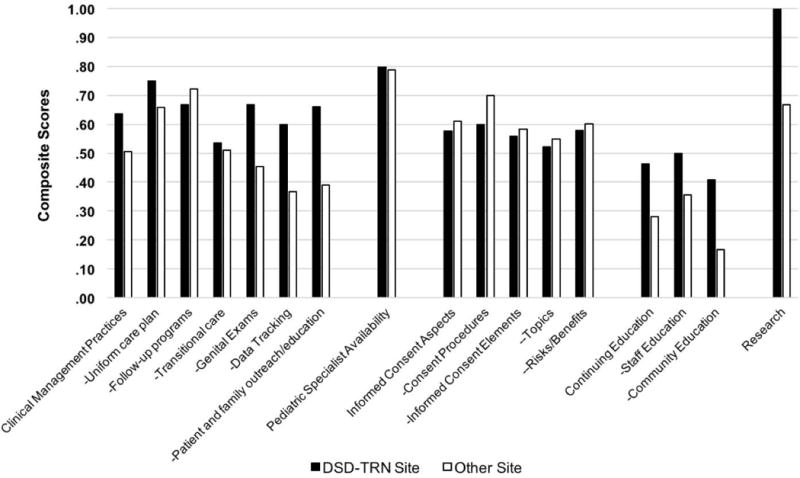
Comparison of institutions participating in the DSD-TRN (n=7) with others (n=15)
Composite score of 1 = ideal score. For details regarding items comprising each composite scale, see Tables II–IV.
DISCUSSION
This survey of U.S. healthcare institutions delivering clinical services to patients with DSD and their families is the first of its kind. Notable strengths of the survey design include the breadth and depth of topics and the requirement, through attestation, that responses reflect the input of the entire group of DSD providers at a given institution. This survey is also the first to systematically involve patient advocacy stakeholders in the planning and formulation of survey items: this important partnership adds to the content validity of a survey assessing patient/family-centered care [Vayena et al., 2016].
The results indicate that all participating sites include representation from pediatric urology and/or surgery and pediatric endocrinology, which supports the call for involvement of these services in the clinical management of all forms of DSD [Ahmed et al., 2011; Brain et al., 2011; Lee et al., 2006]. This finding is consistent with data from a recent international survey of DSD services [Kyriakou et al., 2016]. In the present study, pediatric and adolescent gynecology and neonatology were the most frequently missing specialties, which could reflect limited availability of these providers at some institutions and/or the perception that they are infrequently needed in DSD management. Kyriakou et al. (2016) reported a higher percentage involvement of neonatology (91%); however, involvement of pediatric gynecology was not included in their survey.
Our survey also showed marked variability across institutions in practices surrounding continuing education, informed consent, and clinical management. Previous research suggests that large regional variations in management practices for DSD exist and are purported to be due to differences in resources, medical training, and culture/religious beliefs [Josso et al., 2011; Kyriakou et al., 2016; Pasterski et al., 2010]. Our observed variability regarding which diagnoses and phenotypes are considered to comprise DSD is also important to note in this context as how DSD are defined may affect team composition, mission, and practices. While all of these factors are likely to play a role, specific reasons for variability in practices should be investigated with an eye to establishing which are systematically associated with better patient outcomes.
With regard to areas of greatest concern to patient advocates (practices surrounding genital exams and informed consent), our results again showed marked variability, but also clear areas for focused improvement. Less than half of the sites reported setting a maximum number of providers/trainees to be present during genital exams. Though the majority (71%) of sites reported that they never perform genital exams on awake patients primarily for education, the 29% that did report this practice at least some of the time remains an area of concern. With regard to informed consent, our results show that the majority of the elements were discussed with patients and families; however, only in a minority of situations were the elements also included in documentation.
QI data in other fields suggests that surveys like this are most meaningful if completed at regular intervals [Health Service and Resource Administration, 2011]. The results of this initial survey establish a baseline from which institutions can track progress over time. Furthermore, qualitative feedback to the survey indicates that participation, by itself, potentially contributes to practice enhancements. Comments received included: “this survey gave [us] great ideas and we are currently working on an algorithm for evaluation, guidelines for photography and genital exams, and starting a journal club;” “[the survey was] a helpful way to discuss these issues with the team;” and “[the survey] was useful and informative – there is so much more we need and want to do.”
Survey participation rate was similar to many other clinician surveys [Burns et al., 2008; Farquhar et al., 2002; Kyriakou et al., 2016]. Survey length should be noted in this context, as the number and nature of questions demanded substantial time and investment from multiple providers at each institution. We recognize this likely hindered participation by some sites, but it also highlights our response rate as a positive indicator for the way in which many institutions prioritized participation.
Furthermore, we recognize that despite broad inclusion criteria along with input of multiple providers, researchers, and patient advocates regarding sites to target for participation, it is possible that there are additional institutions that were not approached regarding participation. The most likely possibility would be an institution with newly formed DSD services at the time of study recruitment with limited presence at national conferences and/or limited established web presence. Overall, we expect the number of sites in this category to be quite low with small effect on study results. Future surveys should work to incorporate sites not previously identified.
Self-report is an inherent limitation of these findings, though our results did show large variability amongst sites and floor or ceiling effects were not evident. Regardless, it is important to consider how clinician perceptions of service, as demonstrated in surveys like this, may differ from actual performance and the experience of patients/families and related clinical outcomes. Nevertheless, there is evidence from other fields, such as management of craniofacial abnormalities, that standardized audits are effective in identifying areas for focused improvement and lead to substantially improved clinical outcomes [Hachach-Haram et al., 2012]. Moving forward, effort should be placed on linking reported practices with clinical outcomes in DSD.
Although consensus statement recommendations and other quality indicators help to establish a framework for best practices, the unique context of each institution (the availability of resources, the dynamics and needs of the team in place, etc.) should be considered for optimal quality of care. As such, these results should be used to facilitate collaboration among sites to share ideas, resources, and best practices. Our comparison between DSD-TRN sites and non-TRN sites suggests that participation in a learning collaborative such as this was associated with higher scores on almost all scales. The differences observed are likely to be conservative estimates of the benefit because institutions joined the DSD-TRN at different times, with one site joining soon before launch of this survey. Accordingly, participation in a network may serve to enhance practices as sites work together, share resources and help to fill gaps in care.
All institutions share the goal of optimizing care of patients with DSD. Results of this survey benchmark current clinical management practices in the United States. Large variations in care exist and more work is needed to understand how reported practices relate to realized clinical outcomes. Our results, as well as research in other rare diseases, for example cystic fibrosis [Stevens and Marshall, 2014], suggests that significant gains in the quality of patient outcomes can derive from participating in a network that facilitates the sharing of resources and strategies to improving care.
Acknowledgments
Contributing co-authors include members of the DSD-TRN Advocacy Advisory Network (Arlene Baratz, Lissa Moran, and Miriam Muscarella) and Accord Alliance (Janet Green). This work was supported, in part, through a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD068138; DSD-Translational Research Network). We thank all respondents for their participation in this survey of DSD clinical services.
Biographies
Aimee M. Rolston, M.D., M.S. is a resident physician in Obstetrics and Gynecology at the University of Michigan. She is a member of the University of Michigan Interdisciplinary Disorders/Differences of Sex Development program and is actively involved in DSD research.
Melissa Gardner, M.A. is a Research Area Specialist - Senior in the Department of Pediatrics and the Child Health Evaluation and Research Center at the University of Michigan.
Kathleen van Leeuwen, M.D. is the Director of the Reproductive Anomalies & Disorders of Sexual Development Clinic at Phoenix Children’s Hospital, specializing in Pediatric General Surgery.
Lauren Mohnach, M.S., C.G.C. is a Certified Genetic Counselor at the University of Michigan in Ann Arbor, MI. She is the clinical genetic counselor and coordinator of the University of Michigan Interdisciplinary Disorders/Differences of Sex Development program.
Catherine E. Keegan, M.D., Ph.D. is an Associate Professor of Pediatrics and Human Genetics at the University of Michigan in Ann Arbor, MI. She is trained as a medical geneticist and is the Director of the University of Michigan Interdisciplinary Disorders/Differences of Sex Development program. She has an interest in advancing genetic diagnosis for individuals with DSD to optimize interdisciplinary care.
Emmanuèle Délot, Ph.D. is an Associate Professor of Human Genetics and Pediatrics at the University of California, Los Angeles and the Scientific Coordinator for the Disorders of Sex Differentiation Translational Research Network (DSD-TRN). She is trained as a developmental geneticist.
Eric Vilain, M.D., Ph.D. is a Professor of Human Genetics, Pediatrics and Urology at the University of California, Los Angeles and the Institute for Society and Genetics. He currently is the Chief of the Division of Medical Genetics at UCLA and the Director of the Center for Gender-Based Biology. He is a principal investigator of the NIH-funded DSD – Translational Research Network.
David E. Sandberg, Ph.D. is a Professor of Pediatrics at the University of Michigan in Ann Arbor. He is Director of the Division of Pediatric Psychology and Faculty Investigator in the Child Health Evaluation and Research Center. Dr. Sandberg is a member of the University of Michigan Interdisciplinary Disorders/Differences of Sex Development program and serves as the psychosocial lead. He is also a principal investigator of the NIH-funded DSD – Translational Research Network.
Footnotes
The authors have no conflicts of interest to declare.
References
- Ahmed SF, Achermann JC, Arlt W, Balen AH, Conway G, Edwards ZL, Elford S, Hughes IA, Izatt L, Krone N, Miles HL, O’Toole S, Perry L, Sanders C, Simmonds M, Wallace AM, Watt A, Willis D. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin Endocrinol (Oxf) 2011;75(1):12–26. doi: 10.1111/j.1365-2265.2011.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain CE, Creighton SM, Mushtaq I, Carmichael PA, Barnicoat A, Honour JW, Larcher V, Achermann JC. Holistic management of DSD. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24(2):335–354. doi: 10.1016/j.beem.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KEA, Duffett M, Kho ME, Meade MO, Adhikari NKJ, Sinuff T, Cook DJ, for the ACCADEMY Group A guide for the design and conduct of self-administered surveys of clinicians. Can Med Assoc J. 2008;179(3):245–252. doi: 10.1503/cmaj.080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census Regions and Divisions of the United States. US Department of Commerce Economics and Statistics Administration US Census Bureau. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf.
- Consortium on the Management of Disorders of Sex Development. Clinical Guidelines for the Management of Disorders of Sex Development in Childhood. Whitehouse Station, NJ: Accord Alliance; 2006. [Google Scholar]
- Disorders of Sex Development – Translational Research Network. 2016 https://dsdtrn.genetics.ucla.edu/
- Farquhar CM, Kofa EW, Slutsky JR. Clinicians’ attitudes to clinical practice guidelines: a systematic review. Med J Australia. 2002;177(9):502–506. doi: 10.5694/j.1326-5377.2002.tb04920.x. [DOI] [PubMed] [Google Scholar]
- Hachach-Haram N, Benyon SL, Eccles SJ, Kirkpatrick WNA, Kelly M, Waterhouse N. Facing the World: Audit of activity 2002–2010. J Plast Reconstr Aes. 2012;65:1312–1324. doi: 10.1016/j.bjps.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Health Services and Resources Administration. Quality Improvement Methodologies. 2011 https://www.hrsa.gov/quality/toolbox/methodology/index.html.
- Josso N, Audi L, Shaw G. Regional variations in the management of testicular or ovotesticular disorders of sex development. Sex Dev. 2011;5(5):225–234. doi: 10.1159/000334263. [DOI] [PubMed] [Google Scholar]
- Kyriakou A, Dessens A, Bryce J, Iotova V, Juul A, Krawczynski M, Nordenskjold A, Rozas M, Sanders C, Hiort O, Ahmed S. Current models of care for disorders of sex development – results from an International survey of specialist centres. Orphanet Journal of Rare Diseases. 2016;11:155. doi: 10.1186/s13023-016-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, Houk CP, Ahmed SF, Hughes IA, in collaboration with the participants in the International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology Consensus statement on management of intersex disorders. Pediatrics. 2006;118(2):e488–500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- Lee PA, Nordenstrom A, Houk CP, Ahmed SF, Auchus R, Baratz A, Baratz Dalke K, Liao LM, Lin-Su K, Looijenga LH, 3rd, Mazur T, Meyer-Bahlburg HF, Mouriquand P, Quigley CA, Sandberg DE, Vilain E, Witchel S, Global DSD Update Consortium Global disorders of sex development update since 2006: Perceptions, approach and care. Horm Res Paediat. 2016;85(3):158–180. doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Prentice P, Hughes I. Consequences of the Chicago consensus on disorders of sex development (DSD): Current practices in Europe. Arch Dis Child. 2010;95(8):618–623. doi: 10.1136/adc.2009.163840. [DOI] [PubMed] [Google Scholar]
- Sandberg D, Callens N, Wisniewski A. Disorders of Sex Development (DSD): Networking and standardization considerations. Horm Metab Res. 2015;47(05):387–393. doi: 10.1055/s-0035-1548936. [DOI] [PubMed] [Google Scholar]
- Sorenson C. Quality care indicators for the pediatric management of disorders of sex development. Accord Alliance. 2011 http://www.accordalliance.org/learn-about-dsd/quality-care-indicators/
- Stevens DP, Marshall BC. A decade of healthcare improvement in cystic fibrosis: lessons for other chronic diseases. BMJ Qual Saf. 2014;23(Suppl 1):i1–i2. doi: 10.1136/bmjqs-2014-002871. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Quality Improvement. 2011 Apr; https://www.hrsa.gov/quality/toolbox/508pdfs/qualityimprovement.pdf.
- Vayena E, Brownsword R, Edwards SJ, Greshake B, Kahn JP, Ladher N, Montgomery J, O’Connor D, O’Neill O, Richards MP, Rid A, Sheehan M, Wicks P, Tasioulas J. Research led by participants: a new social contract for a new kind of research. J Med Ethics. 2016;42(4):216–219. doi: 10.1136/medethics-2015-102663. [DOI] [PMC free article] [PubMed] [Google Scholar]


