Abstract
Context
Evidence has suggested that physical and sexual activity might be triggers of acute cardiac events.
Objective
To assess the effect of episodic physical and sexual activity on acute cardiac events using data from case-crossover studies.
Data Sources
MEDLINE and EMBASE (through February 2, 2011) and Web of Science (through October 6, 2010).
Study Selection
Case-crossover studies investigating the association between episodic physical or sexual activity and myocardial infarction (MI) or sudden cardiac death (SCD).
Data Extraction
Two reviewers extracted descriptive and quantitative information from each study. We calculated summary relative risks (RRs) using random-effects metaanalysis and absolute event rates based on US data for the incidence of MI and SCD. We used the Fisher P value synthesis method to test whether habitual physical activity levels modify the triggering effect and meta-regression to quantify the interaction between habitual levels of physical activity and the triggering effect.
Results
We identified 10 studies investigating episodic physical activity, 3 studies investigating sexual activity, and 1 study investigating both exposures. The outcomes of interest were MI (10 studies), acute coronary syndrome (1 study), and SCD (3 studies). Episodic physical and sexual activity were associated with an increase in the risk of MI (RR=3.45; 95% confidence interval [CI], 2.33-5.13, and RR=2.70; 95% CI, 1.48-4.91, respectively). Episodic physical activity was associated with SCD (RR=4.98; 95% CI, 1.47-16.91). The effect of triggers on the absolute rate of events was limited because exposure to physical and sexual activity is infrequent and their effect is transient; the absolute risk increase associated with 1 hour of additional physical or sexual activity per week was estimated as 2 to 3 per 10000 person-years for MI and 1 per 10000 person-years for SCD. Habitual activity levels significantly affected the association of episodic physical activity and MI (P<.001), episodic physical activity and SCD (P<.001), and sexual activity and MI (P=.04); in all cases, individuals with lower habitual activity levels had an increased RR for the triggering effect. For every additional time per week an individual was habitually exposed to physical activity, the RR for MI decreased by approximately 45%, and the RR for SCD decreased by 30%.
Conclusion
Acute cardiac events were significantly associated with episodic physical and sexual activity; this association was attenuated among persons with high levels of habitual physical activity.
Acute cardiac events are a major cause of morbidity and mortality, with as many as a million acute myocardial infarctions (MIs) and 300 000 cardiac arrests occurring in the United States each year.1,2 Regular physical activity has been identified as strongly associated with a decreased risk of cardiovascular disease and related mortality.3 Despite the well-established benefits of regular physical activity, anecdotal evidence has suggested that physical activity, as well as other acute exposures, such as sexual activity and psychological stress, can act as triggers of acute cardiac events.4-7 In fact, in the original description of MI, Obraztsov and Strazhesko8 observed that the acute event is often precipitated by exposure to physical or mental stressors.9
Traditional epidemiological designs, such as case-control and cohort studies, are not particularly suitable for identifying acute triggers (proximal causes) of cardiac events, primarily because short-term exposures close to the time of event occurrence are likely to be confounded by patient-level factors. In the early 1990s, the case-crossover design was developed specifically to address the problem of identifying triggers of acute events.10,11 A case-crossover study is based on the identification of patients who have experienced the event of interest and requires the assessment of exposure during a relatively brief period preceding the event of interest (the hazard period) and during period(s) when the event did not occur (the control periods). As such, the design allows the individual's exposure status to be assessed during both the hazard and control period(s), in effect serving as his or her own control. Given this unique form of matching, case-crossover studies are not susceptible to confounding by patient characteristics that remain constant over time or to biases in control selection that can compromise case-control studies.
Several case-crossover studies have investigated the association of episodic physical and sexual activity with acute cardiac events, including MI and sudden cardiac death (SCD).12-14 These studies tend to produce wide confidence intervals (CIs), indicating considerable uncertainty around the relative risk (RR) estimates. In addition, although it is hypothesized that the triggering effect of episodic physical and sexual activity varies by habitual levels of physical activity, the consistency of this effect modification across studies as well as the magnitude of the potential modification of risk remains unclear.
We conducted a systematic review and meta-analysis of the literature to provide an overview of case-crossover studies investigating episodic physical and sexual activity as triggers of acute cardiac events and to investigate the interaction of habitual physical activity levels with the triggering effect of these exposures.
Methods
Literature Search and Eligibility Criteria
We searched the MEDLINE and EMBASE databases (through February 2, 2011) to identify studies using a case-crossover design and investigating the association of episodic physical or sexual activity with acute coronary syndromes (including MI) and SCD. We used combinations of keywords related to the exposures (exertion, exercise, physical activity, sexual activity), outcomes (myocardial infarction,acute coronary syndrome, sudden [cardiac] death), and the study design of interest (case-cross-over). The full search strategy is available in the eAppendix (available at http://www.jama.com). To increase the yield of our search, we also searched the reference lists of eligible studies and relevant narrative reviews. We also used the Institute of Scientific Information Web of Science database (through October 6, 2010) to identify all studies citing the studies we considered eligible and reviewed their titles and abstracts to determine eligibility.
One reviewer screened all abstracts (I.J.D.) to identify potentially eligible studies, and 2 reviewers (I.J.D. and J.K.P.) screened all potentially eligible studies in full text to determine eligibility for the review. Eligible studies had to have a case-crossover design, as described by Maclure,10 and had to examine the effect of episodic physical or sexual activity (exposures) on the risk of acute coronary syndrome (including MI) and SCD (outcomes). Given that we expected studies conducted over a period of several years to be eligible for this analysis, we considered the definitions and diagnostic criteria used in the primary studies for the outcomes of interest. We excluded studies with an experimental design, ie, studies where exposure status was assigned by the investigators, including studies of induced exertion. Finally, we excluded observational studies not using a case-crossover design, including those with ecological, case-control, and cohort designs. We did not use any language restrictions, and we did not consider abstracts presented at scientific meetings, since they are frequently not peer reviewed.
Data Extraction
Two reviewers (I.J.D. and J.K.P.) independently reviewed all eligible studies and extracted data; discrepancies were resolved by consensus. From each eligible study, we extracted the following information: first author, year and country of publication, case selection methods, criteria used for the diagnosis of the outcome, definitions and measurement methods for ascertaining exposure, duration of hazard and control periods, ascertainment of exposure during control periods, and the RR and its variance for the triggering effect of episodic physical or sexual activity for the outcomes of interest.
For studies that explored effect modification of the triggering effect by habitual physical activity levels (ie, the interaction between habitual activity levels and episodic exposures on the outcomes), we extracted information on the methods of assessing habitual activity levels, the RR with its corresponding variance for each habitual activity stratum, and the midpoint of the exposure levels. For open-ended categories, we assigned a value equal to the lower bound of the category plus half the length of the adjacent category. For example, for a study where exposure was categorized as 1 time/week or less, 2 to 3 times/week, and 4 or more times/week, we assigned values of 0.5, 2.5, and 4.5 times/week, respectively. We performed sensitivity analyses to evaluate the impact of alternative assignment methods.
Assessment of Validity
Although case-crossover studies are protected from confounding from factors that remain stable within an individual over time, there exist other threats to their validity, including potential biases in case selection, determination of hazard and control periods, and confounding due to factors that vary over time.10,15 To our knowledge, there are no standard criteria for the assessment of validity of case-crossover studies.16 Instead, based on general epidemiological principles and the particular features of the case-crossover de-sign,10,15,17,18 we attempted to assess the validity of the eligible studies by reviewing the following information: the sampling frame used in each study, whether the study was conducted in a single center or multiple centers, the participation rate, whether definitions for outcomes were clearly reported, whether the hazard and control periods were clearly defined, and whether the selection of hazard and control period duration was justified (for example, whether they were chosen based on external evidence or were determined empirically by a sensitivity analysis).10,15,19
Evidence Synthesis
To assess between-study heterogeneity for each outcome of interest, we used the RR estimates reported from each study and their variance to calculate the Cochran Q statistic.20 Heterogeneity was considered statistically significant at P<.10. Between-study inconsistency was quantified with the I2 index.21 I2 indicates the proportion of between-study heterogeneity that is beyond chance and ranges between 0% and 100%; higher I2 values indicate higher amounts of heterogeneity. We estimated summary RRs with their 95% CIs for each outcome of interest using inverse variance methods with a random-effects model.22,23
Absolute Event Rate Estimates
To demonstrate the association of the triggering effect with an individual's absolute rate of MI or SCD, we used the following methods described in Muller et al.14 We first obtained estimates of the absolute rate per 100 person-years from the Framingham Heart Study24 (for MI, using data from 1980 to 2003) and the US vital statistics mortality data25 (for SCD, using data from 1989 from 1998) for male and female individuals in the 55- to 64-year age bracket. We then applied the meta-analysis estimates for the RR of the triggering effect to calculate the absolute event rate, assuming an individual increased his or her exposure (to episodic physical or sexual activity) by 1 hour once per week.14
Effect Modification by Levels of Habitual Physical Activity
It has been hypothesized that the triggering effect of physical and sexual activity on cardiovascular disease is dependent on the individual's habitual activity level, ie, that habitual physical activity interacts with episodic activity. For studies that used the same scale (times/week) to quantify habitual activity, we used random-effects meta-regression to estimate the change in the RR of a cardiac event for a unit change in habitual exertion frequency. Because not all studies used the same scale to quantify habitual activity levels (for example, some used hours/week or ordinal classifications based on subjective questions), it was not possible to include all studies in the same meta-regression. To overcome this limitation, we used the following approach to test the null hypothesis that habitual activity levels do not influence the RR of adverse cardiac outcomes: for each study, we calculated the P value for the null hypothesis that habitual activity does not influence the triggering effect and then synthesized the 1-sided P values using the Fisher P value synthesis method.26
Sensitivity Analyses and Assessment of Bias
Given the large time span of the eligible studies, we explored trends over time using random-effects meta-regression with the year of publication as the explanatory variable.27 To explore whether studies that estimated the RR with greater precision (ie, smaller standard errors) provided different estimates compared with less precise ones, we used the Egger regression-based test for “small study effects”28 (commonly referred to as a “publication bias” test).29 Also, to ensure the robustness of the dose-response meta-analysis to different value assignment methods for the highest (open-ended) category in each study, we performed sensitivity analysis by assigning to those categories: (1) the lower bound of the category and (2) a value equal to the lower bound of the category plus the length of the adjacent category.30 This range of values is broad enough (ie, the assigned values are substantially different) that the robustness of the results of this sensitivity analysis provides convincing evidence for the dose-response relationship.
Software
Statistical analyses were conducted with Stata version SE/11.1 (StataCorp, College Station, Texas) and Meta-Analyst version 3.0 beta (Tufts Medical Center, Boston, Massachusetts).31 For all comparisons, except those for heterogeneity, statistical significance was defined as P<.05, and all tests were 2-sided.
Results
Our initial searches identified 4914 citations in MEDLINE and 717 citations in EMBASE. After screening titles and abstracts, 70 MEDLINE citations and 19 EMBASE citations were considered potentially eligible and were retrieved in full text; after removing duplicates, 78 articles were retrieved for full text review. Of these, 66 were excluded (30 were editorials, reviews, or letters to the editor containing no primary data; 19 did not have a case-crossover design; 11 did not investigate outcomes of interest; 4 did not investigate exposures of interest; 1 was an experimental study; and 1 was a translation of an article published in English), and 12 were considered eligible for this systematic review. One additional article was identified by perusal of reference lists, thus we considered 13 articles in total.32 In addition, we screened 1017 citations from Web of Science, of which 42 were considered potentially eligible. Of these, 14 did not report primary data; 14 had been identified by the MEDLINE or EMBASE searches, 13 did not have a case-crossover design, and 1 was a case report. Thus, no additional eligible articles were identified through the Web of Science search. Figure 1 presents the overall search flow. One article reported information from 2 separate populations (geographic regions); when available, estimates from these populations were considered as independent strata in our analyses, resulting in a total of 14 studies reported in 13 articles included in the meta-analysis.13
Figure 1. Search Strategy Flowchart.
One publication provided results from 2 separate populations, which were considered as independent strata in our analyses. ISI indicates Institute of Scientific Information.
Table 1 summarizes the characteristics of the eligible studies. Additional details about study design and outcome definitions appear in eTable 1. Interrater agreement for data extraction was high (κ>0.9 both for qualitative and quantitative extraction items). Ten studies provided data on episodic physical activity,12,13,32,33,35-39 3 on sexual activity,14,40,41 and 1 study for both exposures of interest.34 Of the studies that assessed episodic physical activity, 7 studies enrolled patients with MI (1 of the articles reported on 2 independent patient populations that were analyzed separately),12,13,32-35 3 enrolled patients with SCD,36-38 and 1 enrolled patients with mixed diagnoses of acute coronary syndrome,39 the majority of whom had MI. All of the studies assessing sexual activity as the exposure of interest enrolled only patients with MI.14,34,40,41
Table 1. Characteristics of Eligible Studies.
| Source (Location) | Participant Selection | Recruitment Period | No. of Patients | Age, Mean (SD), y | Male Sex, % | Activity Type and Intensity | Hazard Period | Control Exposure and Control Period(s)a |
|---|---|---|---|---|---|---|---|---|
| Episodic Physical Activity: Myocardial Infarction | ||||||||
| Mittleman et al,12 1993 (United States) | Acute MI casesidentified from CCU records | 1989-1992 | 1228 | 62 (13) | 68 | Peak exertion of ≥6 METs | 1 h | Usual frequency of PA in past year; PA in same 1-h period of previous day |
|
| ||||||||
| Willich et al,13 1993 (Berlin, Germany) | Primary diagnosis of acute MI from single center | 1989-1991 | 224 | 63 (13) | 64 | Exertion of ≥6 METs | 1 h | Usual frequency of physical exertion over past year |
|
| ||||||||
| Willich et al,13 1993 (Augsburg, Germany) | Routine monitoring of MI hospitalizations in 26 hospitals | 1989-1991 | 970 | 60 (8) | 77 | Exertion of ≥6 METs | 1 h | Usual frequency of physical exertion over past year |
|
| ||||||||
| Giri et al,32 1999 (United States) | MI patients hospitalized within 12 h of symptom onset | 1995-1998 | 640 | 61 (14) | 70 | Exertion of >6 METs | 1 h | Usual frequency of heavy exertion over past year |
|
| ||||||||
| Hallqvist et al,33 2000 (Sweden) | Patients with first MI from 10 hospitals | 1993-1994 | 660 | NR (45-70)b | 57 | Exertion of ≥6 METs | 1 h | Usual annual frequency of heavy PA; PA in same 1-h period of previous day |
|
| ||||||||
| Baylin et al,34 2007 (Costa Rica) | Incident cases of nonfatal acute first MI from 3 hospitals | 1995-1998 | 480 | 57 (11)c | 74 | Exertion of >6 METs | 1 h | Habitual frequency of heavy PA in last year |
|
| ||||||||
| von Klot et al,35 2008 (Germany) | 24-h survivors of acute MI from population-based registry | 1999-2003 | 1301 | 61 (25-74)d | 77 | Exertion of ≥5 METs | 2h | Two control periods, 24 and 48 h before symptom onset |
|
| ||||||||
| Episodic Physical Activity: Sudden Cardiac Death | ||||||||
| Whang et al,36 2006 (United States) | Sudden cardiac deaths from prospective Nurses' Health Study | 1980-2004 | 288 | NR (NR) | 0 | Exertion of ≥5 METs | “At the time of sudden cardiac death” | Usual amount of exertion over past year, as reported most recentlye |
|
| ||||||||
| Albert et al,37 2000 (United States) | Sudden cardiac deaths from prospective Physicians' Health Study | 1982-1994 | 122 | 61 (10) | 100 | Exertion of ≥6 METs | 1 h | Usual annual frequency of exertion, from baseline questionnaire; sensitivity analysis on usual duration of exercise |
|
| ||||||||
| Selb Semerl and Kenda,38 2003 (Slovenia) | Sudden cardiac deaths outside hospital among residents aged 20-65 y from registry | 2000-2001 | 206 | NR (NR) | 81 | Exertion of ≥6 METs | 1 h | Usual frequency and duration of PA in year before death |
|
| ||||||||
| Episodic Physical Activity: Acute Coronary Syndrome | ||||||||
| Strike et al,39 2006 (United Kingdom) | Patients aged 18-90 y with acute coronary syndrome admitted to 4 hospitals | 2001-2004 | 295 | 60 (12) | 78 | Exertion of ≥6 METs | 1 h | 24 h before symptom onset; usual frequency and duration of exertion over previous 6 mo |
|
| ||||||||
| Sexual Activity: Myocardial Infarction | ||||||||
| Muller et al,14 1996 (United States) | Nonfatal MI among patients with prior coronary artery disease from CCU records | 1989-1993 | 1633 | 63 (12) | 70 | SA | 2h | Average SA over past year; SA in same 2-h period 1 day before MI |
|
| ||||||||
| Möller et al,40 2001 (Sweden) | Patients with first nonfatal MI admitted to CCUs | 1993-1994 | 659f | NR (45-70)b | 50 | SA | 2hg | Usual annual frequency of SA |
|
| ||||||||
| Baylin et al,34 2007 (Costa Rica) | Incident cases of nonfatal acute first MI from 3 hospitals | 1995-1998 | 470 | 57 (11) | 74 | SA | 2h | Usual frequency of SA in last year |
|
| ||||||||
| Masoomi et al,41 2010 (Iran) | Cases with first acute MI from a single center | January-July 2009 | 198 | 60 (12) | 62 | SA | 12 h | NR |
Abbreviations: CCU, coronary care unit; METs, metabolic equivalents; MI, myocardial infarction; NR, not reported; PA, physical activity; SA, sexual activity.
Some studies used multiple control periods.
Range.
Data extracted for the total study population (n=520). Only 480 individuals had information on “heavy physical exertion.”
Median (range).
Different questionnaires were administered between 1980 and 2004. All questionnaires asked about usual exertion over the previous year.
Of 699 interviewed patients, 40 were excluded because of missing, unreliable, or inaccurate information regarding time of disease onset or exposure.
The study reported results for multiple hazard periods. For consistency with the other studies investigating the sexual activity–MI association, we used the results of analyses based on a 2-hour hazard period.
Mean or median age was older than 60 years in 9 of 10 studies that reported relevant information. The majority of participants were male in 12 studies that enrolled individuals of both sexes. One study enrolled exclusively men37 and 1 study exclusively female participants36; both these studies investigated the association of physical activity and SCD.
All 10 studies of episodic physical activity quantified the intensity of the exposure based on multiples of metabolic equivalents (METs). Moderate activity in all studies was fairly uniformly defined as exertion of at least 5 to 6 METs.
Study Validity
Most studies had well-defined sampling frames and reported the diagnostic criteria for the events of interest (eTable 2). Participation rate was variable but was at least 80% in 8 studies and at least 70% in all 13 studies that reported such information. Only 1 study36 did not report the duration of the hazard period used in the primary analysis. Eight of 14 studies either performed sensitivity analyses to identify an “optimal” hazard period or explicitly stated that the choice of hazard period was based on previously published studies. In all studies of MI and in the single study of acute coronary syndrome, exposure ascertainment was performed through interviews, and the majority of studies provided details on the training of personnel collecting the exposure information and the quality control measures that were implemented. Studies of SCD relied on medical records and third-person interviews. All studies but 141 reported the duration of the control period; of 13 studies that reported relevant information, 7 studies used the habitual exposure to physical or sexual activity over long periods of time (typically 1 year preceding the event of interest) to derive the usual frequency of exposure, 1 study35 only used proximal time periods (24 and 48 hours prior to the event), and 5 studies used both approaches.
Episodic Physical Activity and MI
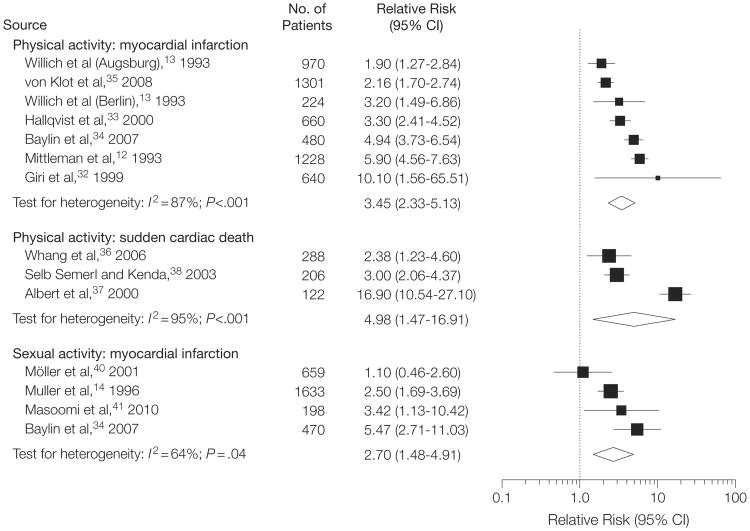
The 7 studies (in 6 articles) investigating the effect of episodic physical activity on the risk of acute MI enrolled a total of 5503 patients and were published between 1993 and 2008.12,13,32-35 Overall the studies suggested a strong association between episodic physical activity and MI (RR = 3.45; 95% CI, 2.33-5.13; P<.001), with substantial between-study heterogeneity (I2=87%; Q statistic P<.001). Figure 2 presents a forest plot of all outcome-exposure associations assessed in this review.
Figure 2. Forest Plot of Studies Assessing the Association of Episodic Physical and Sexual Activity With Myocardial Infarction and Sudden Cardiac Death.
Summary results for each exposure-outcome subgroup are presented separately. Within each subgroup, studies are arranged by the point estimate of relative risk. Values greater than 1 indicate that exposure is associated with increased risk of the outcome. Squares are proportional to the weight of each study in the meta-analysis. CI indicates confidence interval.
One study39 of episodic physical activity included patients with any diagnosis of acute coronary syndrome of whom the majority had MI (67.8% of participants had an ST-elevation MI and the remainder had a non–ST-elevation MI or unstable angina). Inclusion of this study in the metaanalysis did not substantially affect the summary estimate (RR=3.93; 95% CI, 2.63-5.89), again with substantial heterogeneity (I2 = 89%; Q statistic P< .001).
Episodic Physical Activity and SCD
Three studies (616 SCD events) assessed the potential of episodic physical activity to trigger SCD.36-38 One study was nested within the Physicians' Health Study and enrolled 122 male patients.37 The other study was nested within the Nurses' Health Study and enrolled 288 female patients.36 The third study enrolled a mixed, predominantly male (81%) population.38 Overall there was evidence of an increase in the risk of SCD triggered by episodic physical exertion (RR=4.98; 95% CI, 1.47-16.91; P=.01); however, there was substantial between-study heterogeneity (I2 = 95%; Q statistic P<.001) (Figure 2).
Sexual Activity and MI
Four studies (2960 patients) investigated the association between sexual activity and triggering of MI.14,34,41,42 Overall, sexual activity was associated with an increased risk of infarction (RR=2.70; 95% CI, 1.48-4.91; P=.001) with moderate heterogeneity (I2=64%; Q statistic P = .04) (Figure 2).
Table 2 presents a summary of the meta-analysis results for all exposure-outcome associations we investigated.
Table 2. Meta-analysis Results for the Association of Physical and Sexual Activity With the Triggering of Myocardial Infarction and Sudden Cardiac Death.
| Exposure | Outcome | Studies (Patients), No. | Test for Heterogeneitya | RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Physical activity | MI | 7 (5503) | I2 = 87%; P < .001 | 3.45 (2.33-5.13) | <.001 |
| Physical activity | SCD | 3 (616) | I2 = 95%; P < .001 | 4.98 (1.47-16.91) | .01 |
| Sexual activity | MI | 4 (2960) | I2 = 64%; P = .04 | 2.70 (1.48-4.91) | .001 |
Abbreviations: CI, confidence interval; MI, myocardial infarction; RR, relative risk; SCD, sudden cardiac death
P values from Cochran Q statistic. Heterogeneity was considered statistically significant at P< .10
Absolute Event Rate Estimates
Although the RR estimates indicate a large and highly statistically significant increase in the risk of MI and SCD during periods of episodic physical and sexual activity, because these exposures are infrequent and their effect on the outcomes of interest is transient, their impact on an individual's absolute event rate is small. The absolute risk increase associated with an hour of additional physical or sexual activity per week was estimated as 2 to 3 per 10 000 person-years for MI and 1 per 10 000 person-years for SCD.
Effect Modification by Habitual Activity Levels
All studies provided some measure of the effect of habitual physical activity on the triggering effect of episodic exertion (eTable 3). Overall, subgroups of patients with higher habitual activity levels tended to be less susceptible to the triggering effect of episodic physical activity. However, studies used heterogeneous scales to quantify habitual activity levels. In groups with the lowest habitual activity, the RR for the triggering effect of episodic physical activity ranged from 4.47 to 107 for MI, indicating a very substantial increase in risk during or following exertion. The corresponding range in the highest habitual activity groups was 0.86 to 3.3, indicating much smaller increases in risk. Similar patterns were observed for the associations of sexual activity with MI and episodic physical activity with SCD, although the differences were less pronounced and fewer studies contributed data.
Three studies12,13,33 (1897 MI patients) classified habitual exertion based on weekly frequency. We estimated that the RR of MI triggered by episodic physical activity was decreased by approximately 45% for each additional time per week a person was habitually exposed to physical activity (relative RR=0.53; 95% CI, 0.41-0.69; P=.001) (Figure 3). Similarly, 2 studies37,38 (328 SCD cases) quantified habitual physical activity by weekly frequency. The RR of SCD triggered by episodic physical activity was decreased by approximately 30% for each additional time per week a person was habitually exposed to physical activity (relative RR=0.70; 95% CI, 0.50-0.99; P=.05) (Figure 3). Studies of sexual activity did not provide adequate data for dose-response analyses.
Figure 3. Meta-regression Graph of Triggering Relative Risks for Myocardial Infarction and Sudden Cardiac Death Over Habitual Physical Activity Levels.
Meta-regression graph of relative risks (RRs) for myocardial infarction is based on 3 studies that used the same scale (weekly frequency) to report habitual activity levels12,13,33; graph for sudden cardiac death is based on 2 studies that used the same scale.37,38 Subgroup estimates are depicted as circles proportional to their precision (inverse of the variance of the log[RR]). The solid line indicates fitted values by random-effects meta-regression. rRR indicates relative RR calculated from the meta-regression.
To further examine the effect of habitual activity by taking into account all available studies (eTable 2), we first performed interaction tests within each study and used the Fisher P value synthesis method to combine the interaction P values across studies. The combined P value tests the null hypothesis that habitual activity levels (regardless of measurement scale) do not affect the triggering RR. The combined P values were P = 6.81 × 10−20 for the episodic physical activity–MI association, P=1.58 × 10−4 for the episodic physical activity–SCD association, and P = .04 for the sexual activity–MI association, in all cases suggesting that higher levels of habitual physical activity reduce the acute triggering effect of episodic physical or sexual activity.
Sensitivity Analysis, Trends Over Time, and Small Study Effects
Effect sizes for the association of episodic physical or sexual activity with MI or episodic physical activity with SCD did not appear to significantly change over time (meta-regression P values, .71, .49, and .35, respectively). There was no evidence that smaller studies produced different results compared with larger studies. The Egger test P values for the association of episodic physical and sexual activity with MI and the association of physical activity with SCD were .76, .97, and .98, respectively.
Three of the studies investigating the association of episodic physical activity and MI, and 1 of the studies investigating the association of sexual activity and MI, reported estimates from analyses using control periods proximal to their hazard period (typically within 24 hours of the hazard period).12,14,33,35 Using these estimates in the respective meta-analyses did not substantially modify the results: the summary RR for MI was 3.40 (95% CI, 2.25-5.15) for physical activity and 2.93 (95% CI, 1.43-6.02) for sexual activity.
In sensitivity analyses, assigning different values of weekly exercise frequency to the highest (open-ended) exposure category in the 5 studies that provided relevant data did not substantially modify our results, and the dose-response relationship remained robust both for the association of episodic physical activity with MI (P=.001 both when the highest category was assigned a value equal to its lower bound and when it was assigned the sum of its lowest bound plus the length of the adjacent category) as well as for the association of episodic physical activity with SCD (P=.03 and .08 under the 2 assignment methods, respectively).
Comment
Several lines of evidence suggest that acute cardiac events, including MI and SCD, may be triggered by short-term exposures, such as episodic physical activity and emotional stress.12-14,43 Multiple studies have established the existence of circadian variation in the incidence of acute cardiovascular events, with the lowest rate being observed during sleep, a peak during the early morning hours, and a steady lower level during the remainder of the day.43-46 Multiple biological parameters, such as blood pressure, heart rate, blood viscosity, platelet aggregability, and adrenergic activity, follow similar circadian patterns, suggesting that exposures that can affect the levels of these factors may exert a triggering effect on cardiovascular events.47,48 The case-crossover study was designed specifically to allow the reliable identification of triggers of acute outcomes.10,17 Our review of 14 case-crossover studies investigating the association of episodic physical and sexual activity on acute MI, acute coronary syndrome, and SCD indicates that both exposures are associated with a statistically significant short-term increase in the risk of the outcomes of interest. In addition, we demonstrate that this increase in risk is strongly modified by habitual activity levels, with individuals with higher activity levels experiencing lower increase (and often no increase) in risk after exposure to triggering activities.
The counterfactual hypothesis of case-crossover studies is distinct from that of case-control studies, making the former suitable for answering the question “Why did the event occur now?” compared with the question “Why did the event occur in this individual?” typically the purview of case-control studies.49 Because of this unique property of the case-crossover design, our results are not incompatible with the well-established beneficial effect of regular physical activity on the risk of acute coronary events3: active individuals are overall at a lower risk of such events compared with inactive individuals; however, during the short time period of acute exposure to physical or sexual activity, an individual's risk of an event is increased compared with unexposed periods of time. This conclusion is supported by our absolute event rate calculations, which demonstrate that the short-term effect of the triggers we evaluated is unlikely to be large in absolute terms. In contrast, regular physical activity may reduce an individual's absolute risk by more than 30%.50
This temporary increase in risk may be modified by baseline characteristics of each individual. To explore whether habitual physical activity levels modify the triggering effect of episodic physical and sexual activity, we used data from studies reporting effect sizes stratified by habitual exertion levels. We found strong evidence to reject this null hypothesis, indicating that habitual activity levels modify the triggering effect, with individuals having the lowest habitual levels of physical activity experiencing the highest triggering risk for all exposures and outcomes. Although the Fisher P value synthesis method allowed us to combine evidence from all studies, it cannot provide an estimate of the magnitude of the interaction between habitual physical activity levels and the triggering effect of episodic exertion. Our estimates of this dose-response relationship, although based on a smaller number of studies (3 for MI and 2 for SCD), indicated that for each additional time an individual is exposed to episodic physical activity per week, the RR of the triggering effect is reduced by approximately 45% and 30%, respectively. This suggests that individuals with high levels of habitual exertion experience a much smaller increase in the risk of MI and SCD during the hazard period of episodic physical exertion compared with individuals with low levels of habitual activity. Clinically, this result suggests that physicians counseling patients regarding their exercise habits may need to tailor their advice to the patients' habitual activity levels: sedentary individuals should be counseled to increase the frequency and intensity of physical activity gradually.
Several limitations should be considered when interpreting our findings. First, there was substantial statistical heterogeneity for all outcomes and exposures of interest, and differential recall (information bias) of exposure status during hazard and control periods may also have affected the estimates of the RR, particularly in studies of SCD. However, studies followed similar protocols and had well-defined hazard and control periods (at least within each trial). In addition, methods for exposure ascertainment were generally reported in detail, and all studies classified physical activity using fairly uniform thresholds (5-6 METs). In studies of MI, exposure ascertainment was based on structured interviews and extensive efforts to standardize the process and perform quality control. In contrast, exposure ascertainment in studies of SCD was based on review of medical records and third-person interviews, suggesting that mis-classification or information bias may have been present. Studies were conducted over a relatively long period of time, over which the diagnostic criteria and diagnostic tests for their verification have substantially changed. Despite this, meta-regression analysis using the publication year as an explanatory variable did not reveal any trends over time. As with all syntheses of observational studies, the estimated average effect across studies and the hypothesis-generating results of our meta-analysis should not be overinterpreted.51 Further prospective studies may be necessary to confirm our findings, particularly for the association between sudden cardiac death and episodic physical activity, for which extreme between-study heterogeneity was observed.
Case-crossover studies are susceptible to confounding by factors that change over time. The co-occurrence of episodic physical or sexual activity with other exposures that can trigger MI or SCD (for example, smoking, coffee consumption, illicit drug use, or emotional stress) may account for the observed RR estimates. In addition, premonitory symptoms may affect individuals' exposure to physical or sexual activity and thus confound the association with the outcomes of interest; however, such an association would tend to bias the estimates toward the null. Control for such confounding is typically hard to implement and depends on the method of sampling control person-time and the collection of data on potential confounders.10,18,19 The consistency of findings across studies conducted over more than 15 years in diverse populations, as well as the robustness of the estimates in sensitivity analysis using alternative control period durations, provides reassurance that our findings are indicative of a causal association. In addition, some of the studies we considered attempted to adjust for potential confounders, and in all cases the results remained unchanged. However, we caution that additional confounding by unknown (unobserved) time-related factors may also have been present.
In conclusion, based on our review of 14 case-crossover studies of acute cardiac events, we found a significant association between episodic physical and sexual activity and MI and suggestive evidence of an association between episodic physical activity and SCD. Most importantly, these associations appear to be strongly modified by habitual physical activity, with individuals with higher habitual activity levels experiencing much smaller increases in risk compared with individuals with low activity levels. In view of this, as well as the small absolute magnitude of the risk associated with acute exposure to episodic physical or sexual activity, our findings should not be misinterpreted as indicating a net harm of physical or sexual activity; instead they demonstrate that these exposures are associated with a temporary short-term increase in the risk of acute cardiac events.
Supplementary Material
eAppendix. Search Strategies for Bibliographic Databases
eTable 1. Detailed Characteristics of Eligible Studies
eTable 2. Quality Assessment of Eligible Studies
eTable 3. Effect of Physical Exertion on Serious Cardiac Events by Groups of Usual Physical Activity
Acknowledgments
Funding/Support: This work was supported in part by award UL1RR025752 from the National Center for Research Resources.
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Additional Contributions: We thank Thomas Trikalinos, MD, PhD, and George Kitsios, MD, PhD, both at Tufts Medical Center, Boston, Massachusetts, for their comments on a previous version of the manuscript. We also thank Dr Trikalinos for helpful discussions on the statistical methods and Teruhiko Terasawa, MD, PhD (Fujita Health University Nanakuri Sanatorium, Tsu, Mie, Japan), and Monika Schoels, MD (Hietzing Hospital, Vienna, Austria), for help in translating non–English language articles (Japanese and German, respectively). Drs Trikalinos, Kitsios, Terasawa, and Schoels did not receive any compensation for their contributions.
Footnotes
Author Contributions: Dr Dahabreh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahabreh, Paulus.
Acquisition of data: Dahabreh.
Analysis and interpretation of data: Dahabreh, Paulus.
Drafting of the manuscript: Dahabreh.
Critical revision of the manuscript for important intellectual content: Dahabreh, Paulus.
Statistical analysis: Dahabreh.
Obtained funding: Paulus.
Administrative, technical, or material support: Dahabreh.
Study supervision: Paulus.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Online-Only Material: The eAppendix and eTables 1, 2, and 3 are available at http://www.jama.com.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Writing Group Members; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. published correction in Circulation. 2010;121(12):e260. [DOI] [PubMed] [Google Scholar]
- 2.Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction: challenges and opportunities. Arch Intern Med. 2010;170(9):759–764. doi: 10.1001/archinternmed.2010.88. [DOI] [PubMed] [Google Scholar]
- 3.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 4.Gunby P. Snow falls; ischemic heart deaths rise. JAMA. 1979;241(19):1987. doi: 10.1001/jama.241.19.1987. [DOI] [PubMed] [Google Scholar]
- 5.Trichopoulos D, Katsouyanni K, Zavitsanos X, Tzonou A, Dalla-Vorgia P. Psychological stress and fatal heart attack: the Athens (1981) earthquake natural experiment. Lancet. 1983;1(8322):441–444. doi: 10.1016/s0140-6736(83)91439-3. [DOI] [PubMed] [Google Scholar]
- 6.Möller J, Theorell T, de Faire U, Ahlbom A, Hallqvist J. Work related stressful life events and the risk of myocardial infarction: case-control and case-crossover analyses within the Stockholm Heart Epidemiology Programme (SHEEP) J Epidemiol Community Health. 2005;59(1):23–30. doi: 10.1136/jech.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass RI, Zack MM., Jr Increase in deaths from ischaemic heart-disease after blizzards. Lancet. 1979;1(8114):485–487. doi: 10.1016/s0140-6736(79)90835-3. [DOI] [PubMed] [Google Scholar]
- 8.Obraztsov VP, Strazhesko ND. The symptomatology and diagnosis of coronary thrombosis. In: Vorobeva VA, Konchalovski MP, editors. Works of the First Congress of Russian Therapists. 1910. pp. 26–43. [Google Scholar]
- 9.Muller JE. Diagnosis of myocardial infarction: historical notes from the Soviet Union and the United States. Am J Cardiol. 1977;40(2):269–271. doi: 10.1016/0002-9149(77)90018-2. [DOI] [PubMed] [Google Scholar]
- 10.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann U. Epidemiologic assessment of risks of adverse reactions associated with intermittent exposure. Biometrics. 1993;49(2):419–428. [PubMed] [Google Scholar]
- 12.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE Determinants of Myocardial Infarction Onset Study Investigators. Triggering of acute myocardial infarction by heavy physical exertion: protection against triggering by regular exertion. N Engl J Med. 1993;329(23):1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 13.Willich SN, Lewis M, Löwel H, Arntz HR, Schubert F, Schröder R. Triggers and Mechanisms of Myocardial Infarction Study Group. Physical exertion as a trigger of acute myocardial infarction. N Engl J Med. 1993;329(23):1684–1690. doi: 10.1056/NEJM199312023292302. [DOI] [PubMed] [Google Scholar]
- 14.Muller JE, Mittleman MA, Maclure M, Sherwood JB, Tofler GH. Determinants of Myocardial Infarction Onset Study Investigators. Triggering myocardial infarction by sexual activity: low absolute risk and prevention by regular physical exertion. JAMA. 1996;275(18):1405–1409. doi: 10.1001/jama.275.18.1405. [DOI] [PubMed] [Google Scholar]
- 15.Redelmeier DA, Tibshirani RJ. Interpretation and bias in case-crossover studies. J Clin Epidemiol. 1997;50(11):1281–1287. doi: 10.1016/s0895-4356(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 17.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 18.Marshall RJ, Jackson RT. Analysis of case-crossover designs. Stat Med. 1993;12(24):2333–2341. doi: 10.1002/sim.4780122409. [DOI] [PubMed] [Google Scholar]
- 19.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142(1):91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 25.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 26.Fisher RA. Statistical Methods for Research Workers. London, UK: Oliver and Boyd; 1932. [Google Scholar]
- 27.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Il'yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C. Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control. 2005;16(4):383–388. doi: 10.1007/s10552-004-5025-x. [DOI] [PubMed] [Google Scholar]
- 31.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giri S, Thompson PD, Kiernan FJ, et al. Clinical and angiographic characteristics of exertion-related acute myocardial infarction. JAMA. 1999;282(18):1731–1736. doi: 10.1001/jama.282.18.1731. [DOI] [PubMed] [Google Scholar]
- 33.Hallqvist J, Möller J, Ahlbom A, Diderichsen F, Reuterwall C, de Faire U. Does heavy physical exertion trigger myocardial infarction? a case-crossover analysis nested in a population-based case-referent study. Am J Epidemiol. 2000;151(5):459–467. doi: 10.1093/oxfordjournals.aje.a010231. [DOI] [PubMed] [Google Scholar]
- 34.Baylin A, Hernandez-Diaz S, Siles X, Kabagambe EK, Campos H. Triggers of nonfatal myocardial infarction in Costa Rica: heavy physical exertion, sexual activity, and infection. Ann Epidemiol. 2007;17(2):112–118. doi: 10.1016/j.annepidem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.von Klot S, Mittleman MA, Dockery DW, et al. Intensity of physical exertion and triggering of myocardial infarction: a case-crossover study. Eur Heart J. 2008;29(15):1881–1888. doi: 10.1093/eurheartj/ehn235. [DOI] [PubMed] [Google Scholar]
- 36.Whang W, Manson JE, Hu FB, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295(12):1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 37.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 38.Selb Semerl J, Kenda MF. Out of hospital sudden cardiac death among physically active and inactive married persons younger than 65 years in Slovenia. J Clinical Basic Cardiology. 2003;6(1-4):63–67. [Google Scholar]
- 39.Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart. 2006;92(8):1035–1040. doi: 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möller J, Ahlbom A, Hulting J, et al. Sexual activity as a trigger of myocardial infarction: a case-crossover analysis in the Stockholm Heart Epidemiology Programme (SHEEP) Heart. 2001;86(4):387–390. doi: 10.1136/heart.86.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masoomi M, Zare J, Kahnooj M, Mirzazadeh A, Sheikhvatan M. Sex differences in potential daily triggers of the onset of acute myocardial infarction: a case-crossover analysis among an Iranian population. J Cardiovasc Med (Hagerstown) 2010;11(10):723–726. doi: 10.2459/JCM.0b013e32833892da. [DOI] [PubMed] [Google Scholar]
- 42.Möller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? a case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP) Psychosom Med. 1999;61(6):842–849. doi: 10.1097/00006842-199911000-00019. [DOI] [PubMed] [Google Scholar]
- 43.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 44.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 45.Tofler GH. Triggering and the pathophysiology of acute coronary syndromes. Am Heart J. 1997;134(5 pt 2):S55–S61. [PubMed] [Google Scholar]
- 46.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114(17):1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 47.Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J Am Coll Cardiol. 1994;23(3):809–813. doi: 10.1016/0735-1097(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 48.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316(24):1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 49.Maclure M. “Why me?” versus “why now?”: differences between operational hypotheses in case-control versus case-crossover studies. Pharmacoepidemiol Drug Saf. 2007;16(8):850–853. doi: 10.1002/pds.1438. [DOI] [PubMed] [Google Scholar]
- 50.Thompson PD, Franklin BA, Balady GJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American College of Sports Medicine Exercise and acute cardiovascular events: placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 51.Egger M, Schneider M, Davey Smith G. Spurious precision? meta-analysis of observational studies. BMJ. 1998;316(7125):140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategies for Bibliographic Databases
eTable 1. Detailed Characteristics of Eligible Studies
eTable 2. Quality Assessment of Eligible Studies
eTable 3. Effect of Physical Exertion on Serious Cardiac Events by Groups of Usual Physical Activity