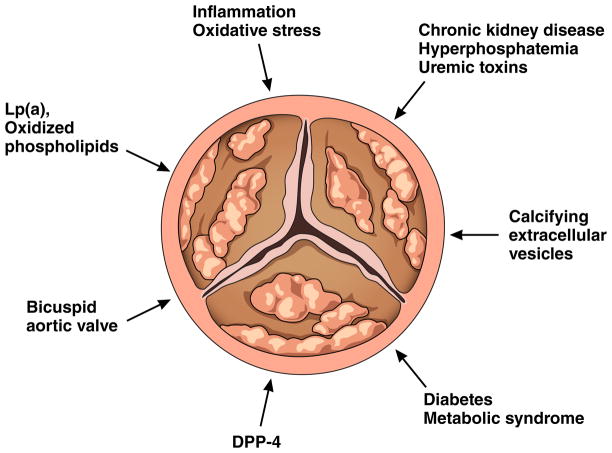
Don’t call it “degenerative!”
Non-rheumatic aortic stenosis has assumed center stage in contemporary cardiology due to the aging of the population and the prodigious advances in percutaneous approaches to its treatment. Many refer to this disease dismissively as “degenerative.” That term implies an inevitable, ineluctably progressive, and inherent process that defies slowing or regressing. Use of the term “degenerative” cloaks our ignorance regarding mechanisms, much as do the designations “idiopathic” cardiomyopathy or “essential” hypertension. Yet, in step with the remarkable advances in addressing the mechanical aspects of aortic stenosis, researchers continue to stride toward better mechanistic understanding of the pathophysiology of this condition. Rather than “degenerative” aortic stenosis, we advocate use of the terms “fibrocalcific” or “sclerocalcific” aortic valve disease: nomenclature that captures the hardening of the valve tissue and its mineralization. We now recognize a number of active biological mechanisms that pave the pathway to alterations in the structure and function of the aortic valve (Figure 1).
Figure 1.
Multifactorial mechanisms contribute to fibrocalcific aortic valve disease.
New insights into the mechanisms of aortic stenosis
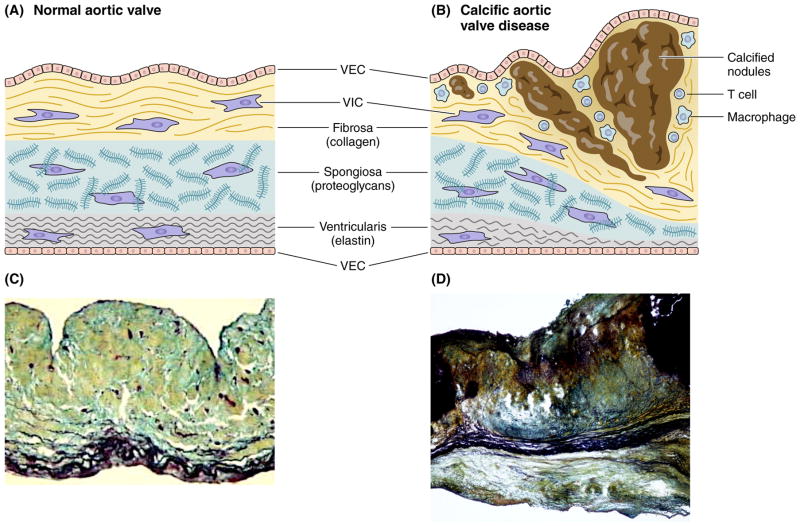
A healthy aortic valve maintains its physiologic functions by means of highly organized tissue architecture. The aortic valve leaflets contain three distinct extracellular matrix layers: a collagen-rich fibrosa, an elastin-rich ventricularis, and a spongiosa, a middle layer rich in glycosaminoglycans (Figure 2A). This tri-laminar structure determines the biomechanical properties of the valve leaflets. Disease progression involves simultaneous thickening and stiffening of the valve leaflets due to fibrosis and formation of calcific nodules that originate on the base of the fibrosa/aortic aspect of the leaflet (Figure 2B).
Figure 2.
Structure of a normal and diseased aortic valve. (2A) A healthy aortic valve leaflet contains VECs and quiescent fibroblast-like VICs, and three distinct extracellular matrix layers: a collagen-rich fibrosa, a glycosaminoglycan-enriched spongiosa, and an elastin-rich ventricularis. (2B) Disease progression involves VIC activation, recruitment of immune cells, and subsequent thickening and stiffening of the valve leaflets due to fibrosis and formation of calcific nodules that originate on the fibrosal surface of the leaflet. Histological staining of normal (C) and diseased (D) valve leaflets. Movat’s staining; collagen – yellow; proteoglycans – blue-green; elastin and calcium – black. (C) Modified from Aikawa E. Circulation 2006.2
The resident cell populations responsible for maintenance of valve homeostasis and structural integrity include valvular endothelial cells (VECs) and valvular interstitial cells (VICs). Healthy adult cardiac valves contain mostly VICs that resemble quiescent fibroblasts. During disease progression, these cells can undergo activation and become myofibroblast–like cells that express metalloproteinases (e.g., MMP-1, MMP-2, MMP-9, MMP-13) and pro-inflammatory cytokines (e.g., IL-1β), mediators that can promote tissue remodeling1 and fetal valve development.2 The extent of VICs heterogeneity (including progenitor cells,) and how VIC subpopulations might contribute to aortic stenosis requires further investigation.
Multiple mechanisms lead to the evolution of the normal to the diseased valve. Two distinct forms of calcification predominate in calcific aortic valve disease (CAVD): (1) inflammatory/oxidative stress-driven and (2) hyperphosphatemia-related. These processes involve distinct mechanisms and may operate simultaneously in the same tissue.
Inflammation and immunity: Newly recognized drivers of aortic stenosis
In addition to the resident cells, the diseased valve harbors different denizens, including immune cells such as macrophages and CD8-positive T lymphocytes, likely recruited through an abnormal endothelium. Emerging evidence suggests that these immune cells contribute to the formation of osteoclast-like cells, although they remain dysfunctional as they lack calcium resorptive activity.3 During the early pro-inflammatory phases of calcification, activated macrophages and other inflammatory cells likely drive disease progression through pathological matrix remodeling and the release of osteogenic cytokines.4 TNF-α or IL-6 can trigger biomineralization and osteogenic signaling through activation of bone morphogenetic protein family members (BMP) and Wnt signaling. Foci of incipient calcification/microcalcification may then activate macrophages and promote a positive calcification-inflammation feedback loop that drives disease progression. Indeed, chronic inflammation contributes to valve calcification in mice, a phenomenon now established by Fluoride and fluorodeoxyglucose imaging in humans.5
Generation of reactive oxygen species (ROS) often accompanies inflammation, and has particularly pathogenic properties in CAVD.6 ROS activate osteogenic reprogramming in human CAVD and in cultured VICs evident by DNA damage and elevation of the transcriptional factors Runx2 and Msx2. Increased intracellular ROS accumulation causes mitochondrial dysfunction in type 2 diabetes mellitus and the metabolic syndrome, conditions that both associate with CAVD. Therefore, targeting ROS-associated osteogenic signaling cascade provides one potential avenue for modifying the progression of aortic stenosis.
Elevated levels of oxidized LDL associate with heightened fibrocalcific responses in aortic valvular tissue, possibly through pro-inflammatory pathways that involve Toll-like receptors. Strong human genetic evidence implicates Lipoprotein (a) [Lp(a)] as a causal factor in aortic stenosis. Lp(a) has many physiological and pathological actions including transport of oxidized phospholipids and binding to LDL and very low-density lipoprotein receptors. Lp(a) also transports lysophosphatidic acid secreted by VICs and promotes mineralization of aortic valves.7 In view of the strong association between the LPA gene variant (rs10455872) with the increased risk of developing aortic stenosis by >50%, Lp(a)-targeted interventions (including anti-sense oligonucleotide and anti-PCSK9 therapies) warrant further exploration in the context of CAVD.
Renal dysfunction, hyperphosphatemia, and uremic toxins also contribute to aortic stenosis
Approximately 50% of individuals with chronic kidney disease (CKD) die from cardiovascular complications.8 In addition to classic risk factors such as age and dyslipidemia, patients with CKD have hyperphosphatemia, an independent risk factor for cardiovascular death. Recent preclinical studies show that macrophage-derived MMPs or cathepsins degrade elastin fibers, producing biologically active elastin peptides that can initiate calcification of VICs, which together with elevated phosphate concentrations could accelerate valve mineralization. Emerging evidence suggests that high serum concentrations of uremic toxins, including indoxyl sulfate, increase with the progression of CKD, particularly in patients undergoing hemodialysis. In vitro studies using vascular cells further demonstrate that uremic toxins promote cell proliferation, and inflammatory cytokine and ROS production. In addition, administration of indoxyl sulfate to rats induced aortic calcification and fibrosis.
Microvesicles can contribute to calcification
Elevated extracellular calcium and phosphate, as found in patients with CKD, can induce the release of calcifying extracellular vesicles (microvesicles) from cardiovascular cells, including macrophages and vascular smooth muscle cells. Calcifying vesicles can carry cargo that promotes calcification of neighboring cells and extracellular matrix. The content of extracellular vesicles dictates their function. Intracellularly, phosphorylated sortilin transports tissue-nonspecific alkaline phosphatase, a molecule required for calcification, from the Golgi to the intracellular membrane resulting in the formation of calcification-prone vesicles.9 Upon their release these vesicles tend to aggregate, merge, nucleate hydroxyapatite, generate microcalcifications, and thus foster the formation and growth of advanced calcific nodules.10 Proteomic studies have shown that circulating extracellular vesicles contain multiple mediators implicated in cardiovascular calcification (e.g., sortilin, annexins, S100A9, tissue-nonspecific alkaline phosphatase, microRNAs, oxidized LDL.)
Monogenic disorders contribute to cardiovascular calcification
Bicuspid aortic valve, the most common congenital heart valve disease, occurs in 1–2% of the population. A combination of factors, including genetic susceptibility, abnormal mechanical forces, and environmental risk likely contributes to the pathobiology and earlier onset of symptoms in patients with BAV.11 BAV contributes to 50% of aortic stenosis and associates with structural abnormalities of the aortic wall such as aortic aneurysm and aortic dissection. Genetic variants may promote abnormal expression of proteins regulating extracellular matrix and alter different signal transduction cascades during valvulogenesis. After birth, severe disruption of valve structure can create anomalous blood flow, which in turn may alter cell signaling and tissue remodeling. Mutations in the Notch signaling pathway associate with BAV.12 In addition, studies indicate that eNOS-deficient mice develop BAV and eNOS-derived nitric oxide modulates the Wnt/Lrp5 pathway, which may mediate aortic valve calcification.
Dipeptidyl peptidase-4: A new link between aortic stenosis and pathways involved in diabetes?
In this issue of Circulation Song and colleagues13 propose a novel mechanism that promotes aortic valve calcification, action of the enzyme dipeptidyl peptidase-4 (DPP4), a multifunctional enzyme, found in plasma in the catalytically active soluble form and on the surface of many cells. DDP-4 inhibitors, oral hypoglycemic drugs, enjoy broad use for the treatment of type 2 diabetes mellitus. The authors found that VEC dysfunction gauged by nitric oxide depletion promotes DPP-4 expression in human VICs. DPP-4 in turn induces osteogenic differentiation of VICs through increasing degradation of insulin-like growth factor-1 (IGF-1). Hence, the DPP-4-IGF-1 axis may mediate VEC-VIC interaction. In vitro, the inhibition of DPP-4 enzymatic activity blocked the osteogenic changes in VICs. DPP-4 inhibition reduced calcified lesion formation in eNOS-deficient mice and prevented experimental CAVD in rabbits. This study underscores again a critical role for the endothelium, particularly eNOS, in the initiation of CAVD through paracrine regulation of VICs. These novel findings raise the exciting possibility that currently available DPP-4 inhibitors might mitigate aortic stenosis, an issue ripe for formal investigation.
Therapeutic horizons for medical modification of fibrocalcific aortic valve disease
Pharmacologic modification of valvular heart disease has lagged behind other aspects of cardiovascular medicine. Strong association between lipids and CAVD led to trials of statins in treatment of CAVD. SEAS (the Simvastatin and Ezetimibe in Aortic Stenosis trial) and other clinical studies showed a lack of the therapeutic benefits of statins in CAVD and further emphasized the need to focus on valve-specific therapeutic targets.14 The studies of Song et al.13 indicate the need to evaluate DPP-4 inhibitors in this regard, and examine whether they have adverse effects on bone metabolism or promote heart failure, the latter of particular concern in the CAVD population.15 The recent advances in the mechanistic understanding of CAVD such as the provocative results with DPP-4 presented by Song et al. should reveal more novel targets for non-interventional approaches to modulating valve disease.
Acknowledgments
Source of funding: This manuscript is supported by funding from National Institutes of Health (NIH) (grants R01HL114805 and R01HL109506 to E.A. and R01 HL080472 to P.L). Dr. Libby also received funding from the RRM Cardiovascular Inflammation Research Fund.
The authors acknowledge Michael Creager for his histological assistance and Chelsea Swallom for her editorial expertise.
Footnotes
Disclosures: None declared.
References
- 1.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 3.Nagy E, Lei Y, Martínez-Martínez E, Body SC, Schlotter F, Creager M, Assmann A, Khabbaz K, Libby P, Hansson GK, Aikawa E. Interferon-γ released by activated CD8+ T-lymphocytes impairs the calcium resorption potential of osteoclasts in calcified human aortic valves. Am J Path. 2017 doi: 10.1016/j.ajpath.2017.02.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 5.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 6.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lepine JL, Laflamme MH, Hadji F, Couture C, Trahan S, Page S, Bosse Y, Pibarot P, Scipione CA, Romagnuolo R, Koschinsky ML, Arsenault BJ, Marette A, Mathieu P. Autotaxin Derived From Lipoprotein(a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O’Donnell CJ, D’Agostino RB, Sr, Benjamin EJ. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 9.Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126:1323–36. doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, Libby P, Aikawa M, Weinbaum S, Aikawa E. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–343. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieu P, Bosse Y, Huggins GS, Corte AD, Pibarot P, Michelena HI, Limongelli G, Boulanger MC, Evangelista A, Bedard E, Citro R, Body SC, Nemer M, Schoen FJ. The pathology and pathobiology of bicuspid aortic valve: State of the art and novel research perspectives. The journal of pathology Clinical research. 2015;1:195–206. doi: 10.1002/cjp2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 13.Choi B, Lee S, Kim S-M, Lee E-J, Lee SR, Kim D-H, Jang JY, Kang S-W, Lee K-U, Chang E-J, Song J-K. Dipeptidyl Peptidase-4 Induces Aortic Valve Calcification by Inhibiting Insulin-like Growth Factor-1 Signaling in Valvular Interstitial Cells. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.024270. [DOI] [PubMed] [Google Scholar]
- 14.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 15.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]