Abstract
Quitting smoking significantly reduces the risk of tobacco-related morbidity and mortality, yet there is a high rate of relapse amongst smokers who try to quit. Phenotypic biomarkers have the potential to improve smoking cessation outcomes by identifying the best available treatment for an individual smoker. In this review, we introduce the nicotine metabolite ratio (NMR) as a reliable and stable phenotypic measure of nicotine metabolism that can guide smoking cessation treatment among smokers who wish to quit. We address how the NMR accounts for sources of variation in nicotine metabolism including genotype and other biological and environmental factors such as estrogen levels, alcohol use, body mass index, or menthol exposure. Then, we highlight clinical trials that validate the NMR as a biomarker to predict therapeutic response to different pharmacotherapies for smoking cessation. Current evidence supports the use of nicotine replacement therapy for slow metabolizers, and non-nicotine treatments such as varenicline for normal metabolizers. Finally, we discuss future research directions to elucidate mechanisms underlying NMR associations with treatment response, and facilitate the implementation of the NMR as biomarker in clinical practice to guide smoking cessation.
Keywords: Nicotine, nicotine addiction, nicotine metabolite ratio, smoking cessation
I. Introduction
Tobacco smoking is responsible for over six million deaths worldwide each year, and the World Health Organization predicts that this number will rise to eight million per year by 2030 (World Health Organization 2013). Tobacco-related morbidity and mortality cost the world an estimated US$500 billion per year in terms of direct health care costs and lost productivity (Shafey et al. 2009; World Health Organization 2008). Quitting smoking significantly reduces the risk of tobacco-related morbidity and mortality (U.S. Department of Health and Human Services 1990), yet the addictive properties of tobacco result in high rates of relapse among smokers who try to quit (Centers for Disease Control and Prevention 2010).
The primary addictive component in tobacco is nicotine, a stimulant which exerts its rewarding effects through the release of dopamine and other neurotransmitters in the brain (Centers for Disease Control and Prevention 2010). The DSM-V defines tobacco use disorder as a problematic pattern of tobacco use leading to clinically significant impairment or distress, as manifested by at least two of the symptoms listed in Table 1 occurring within a 12-month period (American Psychiatric Association 2013). Nicotine addiction is a chronic, relapsing disorder; many smokers attempt to quit smoking each year, but of these smokers, only 4–7% are able to quit successfully (Fiore et al. 2008).
Table 1. Criteria for the Diagnosis of Nicotine Addiction.
Criteria for the diagnosis of tobacco use disorder according to the DSM-V (American Psychiatric Association, 2013).
The DSM-V defines tobacco use disorder as a problematic pattern of tobacco use leading to clinically significant impairment or distress, as manifested by at least two of the following occurring within a 12-month period:
|
Currently, there are only three approaches to pharmacological treatment approved in the United States and European Union for smoking cessation: nicotine replacement therapies, bupropion, and varenicline (Cahill et al. 2013). The success of these treatments at 1 year range from approximately 7% to 30% (Bauld et al. 2010; Hughes et al. 2003; National Institute for Clinical Excellence 2002; Silagy et al. 2004). Varenicline, an α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist, and bupropion, a dopamine and norepinephrine transporter inhibitor, are non-nicotine treatments which are intended to mitigate cravings and withdrawal symptoms through direct or indirect actions on dopamine levels in the brain (Cahill et al. 2013). Varenicline is thought to also act as an antagonist at α4β2 nAChRs to block the reinforcing effects of nicotine during a quit attempt (Cahill et al. 2012). A randomized, placebo-controlled trial of varenicline and bupropion for smoking cessation found that 23% of participants treated with varenicline and 14.6% of those treated with buproprion were continuously abstinent for one year following treatment, compared to 10.3% of those treated with placebo (Jorenby et al. 2006). Nicotine replacement therapy (NRT) aims to replace nicotine from cigarettes by delivering it slowly via gum, nasal spray, or transdermal patches. A meta-analysis of studies examining NRT for smoking cessation found higher cessation rates one year after treatment with active NRT (12.2%) compared to placebo (7.0%) (Etter and Stapleton 2006).
The application of precision medicine, which tailors treatment to an individual based on genetic and lifestyle factors, has the potential to improve smoking cessation outcomes by identifying the best available treatment for each smoker who wants to quit (Bough et al. 2013; Collins and Varmus 2015; National Research Council 2011). Identifying and understanding factors that contribute to individual variability in treatment response is a key step to the development of personalized smoking cessation treatment. In this article, we review the discovery and validation of a genetically-informed biomarker of smoking cessation treatment outcomes: the nicotine metabolite ratio, or NMR.
II. The nicotine metabolite ratio as a biomarker of nicotine clearance
Nicotine Metabolism and the Reliability of the NMR
Nicotine is metabolized primarily by cytochrome p450 (CYP) 2A6, and weakly by CYP2B6, CYP2D6, and CYP2E1 enzymes (Messina et al. 1997; Nakajima et al. 1996; Yamanaka et al. 2005; Yamazaki et al. 1999). The primary metabolite of CYP2A6-mediated metabolism of nicotine is cotinine, which is further metabolized to 3′-hydroxycotinine (3HC). This pathway accounts for 70–80% of nicotine metabolism, with cotinine metabolites comprising most of the urinary metabolites (Benowitz et al. 1995; Hukkanen et al. 2005). The half-life of cotinine is approximately 13–19 hours, which is much longer than the half-life of either nicotine (1–2 hours) or 3HC (approximately 5 hours) (Malaiyandi et al. 2006). Due to its long half-life, cotinine concentrations in the blood and urine of smokers are relatively stable throughout the day; however, they are still somewhat dependent on the time since last cigarette (Benowitz et al. 1999; Benowitz et al. 2003). Because 3HC concentrations are dependent on CYP2A6-mediated cotinine metabolism (Benowitz and Jacob 2001; Benowitz et al. 2003), the ratio of 3HC to cotinine is a stable measure of CYP2A6 activity that is not dependent on the timing of last nicotine intake.
The ratio of 3HC to cotinine, or nicotine metabolite ratio (NMR), is a validated phenotypic measure of nicotine metabolism; larger ratios indicate faster nicotine clearance. The NMR can be measured reliably in saliva or plasma, has minimal diurnal variation and is independent of smoking patterns or time since last cigarette in smokers who smoke more than 5 cigarettes per day (Dempsey et al. 2004; Lea et al. 2006; Levi et al. 2007). NMR values obtained from saliva or urine are highly correlated with plasma NMR measurements (r=.7) and can be used as proxy measures for plasma NMR (St Helen et al. 2012; Swan et al. 2005). Test and retest reliability of the NMR has been demonstrated in studies with treatment-seeking and non-treatment seeking smokers (Hamilton et al. 2015; St Helen et al. 2012). In a study of ad-libitum smokers over a 44 week period, the NMR was reliable across repeated measurements (reliability coefficient=.70; (St Helen et al. 2012). In plasma samples taken 2–3 weeks apart, short-term reliability was high for NMR quartile assignment (weighted k=.72, 95% CI=.64 to .83%). Test/retest reliability of classification of slow (quartile 1, NMR≤0.24) versus normal/fast metabolizers (quartiles 2–4, NMR >0.24) was comparable to that observed for raw NMR values and NMR quartile assignment (k=.89; 95% CI= .77–1.00), with consistent classification as slow versus normal across assessments for 96% of the sample (Hamilton et al. 2015).
In a study conducted by Tanner et al (2015), plasma and urine samples were sent to eight different laboratories that used different analytical methods to measure NMR. Measures of plasma NMR were highly correlated between analytical methods; urine metabolite measurements were more variable but still in good agreement (Tanner et al. 2015). The NMR is not affected by sampling time of day or storage temperature; measurements of the NMR in whole blood are stable at 4°C over a 72-hour period, and in plasma and saliva at room temperature over 14 days (Lea et al. 2006; St Helen et al. 2012). The NMR is thus robust to differences in measurement protocols as well as laboratory site.
NMR measurements are consistent within smokers over time despite different patterns or quantity of smoking (Levi et al. 2007). Of particular interest are those who are reducing their nicotine intake over time (St Helen et al. 2013). In a study conducted in 30 participants who decreased plasma cotinine levels by 50% over 24 weeks, NMR assessments were reproducible across 4 separate time points. Plasma NMR showed an absolute change of 28.5%, which was not significant with or without controlling for the effects of age, body mass index, gender, and race (St Helen et al. 2013). This change in plasma NMR is comparable to that of variability in ad-libitum smokers (St Helen et al. 2012). Further evidence for the stability of NMR during nicotine reduction periods was demonstrated by measurements of urine NMR during 12 weeks of nicotine reduction where nicotine replacement therapy was used as desired (Mooney et al. 2008).
Sources of inter-individual variation in nicotine metabolism
Studies have shown the NMR to be highly correlated with CYP2A6 activity (Dempsey et al. 2004; Hamilton et al. 2015; Johnstone et al. 2006; Malaiyandi et al. 2006). This is a key advantage of a phenotypic measure such as the NMR because individual nicotine metabolism rates are influenced by biological and environmental factors as well as genotype. Genetic variation in CYP2A6 contributes to differences in CYP2A6-mediated metabolism; however, there are over 30 known CYP2A6 variations (Nakajima et al. 2002; Oscarson 2001; Xu et al. 2002; http://www.cypalleles.ki.se). Overall, 67% of the variability of the NMR in plasma is attributable to genetic effects, and twin studies suggest that there are additional unknown genetic factors (Swan et al. 2009). A genome-wide association study conducted by Loukola et al (2015) in three large Finnish cohorts (total n=1518) identified novel gene variants influencing the NMR, confirming that genetic effects are a major determinant of inter-individual variance in NMR. This study found the strongest association with NMR in the CYP2A6 gene region. Three independent novel signals combined in CYP2A6 were found to account for a total of 31% of variance in NMR in the study sample. The known CYP2A6 polymorphisms can be associated with increased, reduced, or null activity. For example, CYP2A6 *9 and *12 are reduced function variants and CYP2A6 *2 and *4 are loss of function variants which have been associated with slower plasma clearance of nicotine and cotinine (Benowitz et al. 2006b). CYP2A6*4 homozygous subjects demonstrate low plasma cotinine levels and urinary excretion of cotinine and 3HC after smoking or nicotine administration (Kitagawa et al. 1999; Nakajima et al. 2000; Xu et al. 2002; Zhang et al. 2002). On the other hand, individuals with three functional CYP2A6 genes resulting from gene duplication (CYP2A6*1X2/CYP2A6*1) have higher metabolic capacity and lower nicotine to cotinine ratio (Rao et al. 2000). Plasma NMR correlates with the predicted activity of CYP2A6 based on genotype (Malaiyandi et al. 2006); carriers of reduced function or loss of function such as CYP2A6 alleles *2, *4, *9, or *12 have lower NMR values than those who are homozygous wild-type carriers, indicating slower nicotine metabolism (Dempsey et al. 2004; Johnstone et al. 2006; Malaiyandi et al. 2006).
Observed ethnic differences in nicotine clearance may stem in part from population variability in CYP2A6 alleles. For example, African-Americans have higher frequencies of reduced function variants and higher cotinine levels for a given tobacco exposure than Caucasian smokers (50% versus 20%, respectively) (Zhu et al. 2013). In Japanese and Korean populations, the combined frequencies of null and reduced activity alleles are 53% and 40%, and in Chinese-Americans the combined frequency of null and reduced activity alleles is 31% (Ariyoshi et al. 2002; Benowitz et al. 2002; Pitarque et al. 2001; Yoshida et al. 2003; Yoshida et al. 2002). Distributions of reduced function/null alleles are listed in Table 2 with corresponding mean NMR values. Typically, Caucasians have higher rates of nicotine metabolism than Black and African-American populations, while Asians have the slowest rates of metabolism and Hispanics are not significantly different than whites (Rubinstein et al. 2013b). Overall, relative NMR distributions parallel distributions of reduced function and null alleles (Table 2).
Table 2.
Population distribution of mean NMR and frequency of reduced function/null CYP2A6 alleles.
| Population | Plasmab | NMRa Salivac |
Urined | Frequency of reduced function/null alleles (*4, *5, *7, *9, *10)e |
|---|---|---|---|---|
| White | 0.41 (0.20) | 0.20 (.10) | 5.48 (4.5) | 5.2–12.5 |
| Black/African American | 0.33 (0.21) | 0.14 (.07) | 4.18 (3.1) | 6.6–10.4* |
| Asian | -- | 0.11 (.07) | 3.29 (3.9) | 23.4–60.2** |
| Hispanic/Latino | -- | 0.19 (.08) | 4.87 (2.4) | -- |
Values shown are mean (SD).
Standard deviations shown here were calculated based on reported sample sizes and confidence intervals (Kandel et al. 2007).
Numbers in columns represent allele frequency ranges, as percentage of total alleles, in previously published studies (Liu et al. 2011).
Black-African and African-American
Chinese, Japanese and Korean
Additional Environmental and Biological Factors
Environmental and biological factors such as estrogen levels, alcohol use, body mass index (BMI), and menthol exposure may also contribute to individual variations in nicotine metabolism. Although men typically have higher plasma cotinine levels compared with women, nicotine clearance is significantly higher in women compared to men [mean NMR of 0.37 (SD 0.20) in women vs 0.41 (SD 0.22) in men]; higher in women who use oral contraceptives (mean 0.49, SD 0.24) compared to women who do not (mean 0.41, SD 0.22); and higher during pregnancy compared to postpartum (Benowitz and Dempsey 2004; Benowitz et al. 2006a; Benowitz et al. 1999; Dempsey et al. 2002; Gan et al. 2008; Prather et al. 1993). In pregnant women, NMR was significantly higher at 18–22 weeks (26% higher, 95% CI 12% to 38%) and 32–36 weeks (23% higher, 95% CI 9% to 35%) of pregnancy compared to NMR at 12 weeks post-partum (Bowker et al. 2015). These findings suggest that estrogen induces CYP2A6 activity. Indeed, other studies have shown a dose-response relationship between estrogen and CYP2A6 activity, with the highest degree of CYP2A6 induction observed during pregnancy (Benowitz and Dempsey, 2004; Benowitz et al. 2006a; Hukkanen et al. 2005). Nicotine metabolism among oral contraceptive users was shown to be higher among users taking combined and estrogen-only contraceptives but not progesterone-only contraceptive (Benowitz et al. 2006a). Body mass index is negatively associated with NMR after controlling for smoking levels, sex, and ethnicity (rho=−.14, p<.001) (Binnington et al. 2012; Ho et al. 2009a; Mooney et al. 2008; Swan et al. 2009). It is possible that increased adipose levels associated with higher BMI may alter the activity of enzymes that are involved in nicotine metabolism, but this remains to be tested. Menthol inhibits CYP2A6 activity in vitro by interacting with the heme iron of P450 2A6 and inhibiting the microsomal oxidation of nicotine to cotinine (MacDougall et al. 2003). Benowitz and colleagues (2004) demonstrated that smoking menthol cigarettes reduced nicotine clearance by ~11%. In a multiethnic sample of young adult daily smokers, the NMR was found to be significantly lower among menthol compared with nonmenthol smokers after adjusting for race/ethnicity, gender, BMI, and cigarettes smoked per day (0.19 vs. 0.24, p=.03; (Fagan et al. 2015). Alcohol use is positively associated with NMR (Chenoweth et al. 2014) but the mechanism underlying this association is yet to be determined. However, as predictors in a linear regression model, race (Caucasian vs. African-American), sex, estrogen, alcohol use, and cigarette consumption contribute less than 8% to total NMR variation with each individual factor accounting for less than or equal to 2% (Chenoweth et al. 2014), suggesting that the NMR also reflects currently unknown influences on nicotine metabolism rate. Loukola et al (2015) found similar results in three Finnish cohorts, where age, sex, and BMI accounted for up to 8.9% of variation in NMR.
Given the diverse genetic, biological and environmental influences on nicotine metabolism, a genetically informed phenotypic measure such as the NMR may be a more useful biomarker of CYP2A6-mediated nicotine metabolism than genotype alone (Bough et al. 2013). Furthermore, more than 30 CYP2A6 variants have been identified (http://www.cypalleles.ki.se/), and specific reduced function or null alleles may have a low frequency (Mwenifumbo and Tyndale 2007; Piliguian et al. 2014; Wassenaar et al. 2011). Due to the large number of CYP2A6 alleles, genotyping to characterize inherited differences in nicotine metabolism can be much more costly than testing for the NMR, which can be determined from blood or saliva for approximately US$50 per sample (Lerman et al. 2015). Lastly, primary care physicians may be less inclined to offer a genetic test compared to a phenotypic biomarker; these concerns may relate in part to lack of knowledge about genetics and concerns about the sensitivity of genetic information (Levy et al. 2007; Shields et al. 2008).
II. Associations of the NMR with smoking behavior
Heaviness of smoking
The NMR has been associated with smoking quantity and smoking behavior in a number of studies of adult smokers. Faster metabolizers, who clear nicotine more quickly, may need to smoke more frequently to maintain desired nicotine concentrations (Dempsey et al. 2004; Gambier et al. 2005). Indeed, in a cohort of 545 continuing smokers who were contacted eight years after participating in a placebo-controlled smoking cessation program using NRT, the NMR was positively associated with cigarette consumption (Johnstone et al. 2006). Although the difference is modest, it is consistent: a systematic review (West et al. 2011) found that 9 out of 15 studies observed a positive association between number of cigarettes smoked per day (CPD) and NMR. In a study of 1030 participants of European ancestry, normal metabolizers (NMR≥0.27) smoked about one additional cigarette per day than slow metabolizers (NMR<0.27) (Falcone et al. 2011). This is similar to results found in a recent study of 834 normal metabolizers (NMR >0.35) and 838 slow metabolizers (NMR ≤.350); slow metabolizers smoked on average 17.9 (SD 6.8) and normal metabolizers smoked on average 19.5 (SD 8.1) cigarettes per day (p<.001). Genetic studies demonstrate similar results; for example, one study found that CYP2A6 variants associated with reduced protein function smoked fewer cigarettes per day (20 CPD, compared to 24 CPD in those without these variants) (Malaiyandi et al. 2006), and another study found that two single nucleotide polymorphisms (rs4803381 and rs1137115) associated with reduced CYP2A6 protein levels and activity were associated with reduced cigarette consumption (0.99 and 0.88 fewer cigarettes per day, respectively) (Bergen et al. 2015). Although some studies have not found associations between the NMR and CPD, this may be due to differences in sample size and methods of NMR determination. A few of these studies utilized smaller sample sizes, which may have been underpowered to detect a modest effect (Tang et al. 2012, n=31; Lea et al. 2006, n=6; Malaiyandi et al. 2006, n=152). Other studies measure NMR in urine rather than blood or saliva, which may be less predictive (Kandel et al. 2007; St Helen et al. 2012).
In addition to smoking more cigarettes throughout the day, normal metabolizers may also smoke more intensely than slow metabolizers. In a laboratory topography study, faster metabolizers (those in the third and fourth quartiles of NMR) took larger puff volumes while smoking their preferred brand than those in the first quartile (the slowest metabolizers). Puff volume increased by approximately 23% and 28% with each increasing quartile and the NMR explained 51% of the variance in total puff volume (Strasser et al. 2011). This is consistent with findings showing that smokers carrying CYP2A6 variants associated with reduced or null function took smaller puffs than those without these variants (Strasser et al. 2007). This suggests that faster metabolizers may inhale more deeply to increase nicotine exposure per cigarette while slow metabolizers reduce their inhalation volume. An important consequence of the association between nicotine metabolism and smoking behavior is carcinogen exposure. The increased total puff volume exhibited by smokers who are faster metabolizers is associated with increased total levels of the nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a biomarker of carcinogen exposure (Strasser et al. 2011), which could result in increased cancer risk among normal metabolizers.
Nicotine Dependence and Withdrawal Symptoms
In contrast to other aspects of smoking behavior, the NMR is not consistently associated with degree of nicotine dependence. Those studies which have found associations indicate that nicotine metabolism rate may influence the physiological aspects of dependence primarily through effects on smoking quantity. Schnoll et al. (2014) found that NMR was most predictive of the Heaviness of Smoking Index (HSI), which includes the two items from the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991) regarding time to first cigarette after waking and smoking quantity. These two items measure the physiological elements of dependence more than the behavioral elements. The study also found that the NMR was predictive of FTND score among men, but not women, which is consistent with prior studies demonstrating that smoking behavior in men is more responsive to physiological dependence, whereas women are more likely to smoke for other reasons (e.g. affect regulation and conditioned responses to non-nicotine cues) (Field and Duka 2004; Perkins et al. 2006; Perkins et al. 2001). However, the majority of studies have not found associations between nicotine metabolism rate and nicotine dependence (Benowitz et al. 2003; Ho et al. 2009b; Johnstone et al. 2006; Kandel et al. 2007; Lerman et al. 2006; Patterson et al. 2008; Schnoll et al. 2009; Strasser et al. 2011). Similarly, associations between the NMR and withdrawal symptoms are inconsistent. Although some studies found modest associations between nicotine metabolism rate and withdrawal symptoms in adolescents (Rubinstein et al. 2008) and more severe cravings during abstinence in adults (Lerman et al. 2006), others found no association between the NMR and withdrawal symptoms during abstinence (Schnoll et al. 2009) or a slower increase in craving during abstinence among faster metabolizers (Hendricks et al. 2014).
IV. The NMR as a biomarker of treatment response
The association between individual nicotine metabolism rate and response to pharmacological treatment for smoking cessation was first noted in an open-label trial of nicotine patch versus nicotine nasal spray in 480 treatment-seeking smokers (Lerman et al. 2006). In the nicotine patch group, there was an almost 30% reduction in the odds of quitting with each increasing quartile of NMR. However, there was no association between the NMR and quitting success for participants who received nicotine nasal spray (Lerman et al. 2006). This may be attributable to titration of self-administration of nasal spray based on nicotine metabolism rate; slow metabolizers used nasal spray less frequently than normal metabolizers in this study.
To validate these findings in an independent sample, Schnoll and colleagues analyzed NMR data from a clinical trial involving 568 treatment-seeking smokers all treated with the nicotine patch (Schnoll et al. 2009). This study found significantly higher quit rates at end of treatment for participants in the first quartile of NMR (the slowest metabolizers) compared to all other quartiles (Schnoll et al. 2009). Similar results were observed among African-American light smokers (<10 CPD) who were randomly assigned to receive either nicotine or placebo gum and counseling (Ho et al. 2009b). There was a trend toward greater quitting success among the slowest metabolizers at the end of treatment, compared to normal or fast metabolizers. However, these differences were observed in both the placebo and active nicotine gum groups suggesting that the NMR did not predict the efficacy of nicotine gum (vs. placebo) in this study (Ho et al. 2009b). In another trial, extended treatment with the nicotine patch (i.e. six months of treatment, compared to standard therapy of 8 weeks) was found to improve quit rates among slow metabolizers but not normal metabolizers (Lerman et al. 2010). Based on these data, one might expect that higher dose nicotine patch would be more effective than standard dose nicotine patch in normal metabolizers. However, data from a proof of concept clinical trial of high dose patch for fast metabolizers do not support this hypothesis (Schnoll et al. 2013).
An alternative strategy for treating normal metabolizers would be use of non-nicotine medications. Thus, the NMR was examined at pre-treatment in another clinical trial involving 414 treatment-seeking smokers randomized smokers to receive 10 weeks of treatment with bupropion or placebo (with counseling). Among those receiving placebo, faster metabolizers displayed lower quit rates at end of treatment compared to slower metabolizers. Quit outcomes for the slowest metabolizers (those in the first quartile) were approximately the same (~32%) in both treatment groups. However, the fastest metabolizers (those in the fourth quartile) significantly benefited from bupropion treatment: end of treatment quit rates on bupropion were approximately 34%, compared to 10% among fast metabolizers who received placebo (Patterson et al. 2008). These data suggest that non-nicotine therapies may be efficacious alternative treatments for normal metabolizers who do not respond well with nicotine replacement.
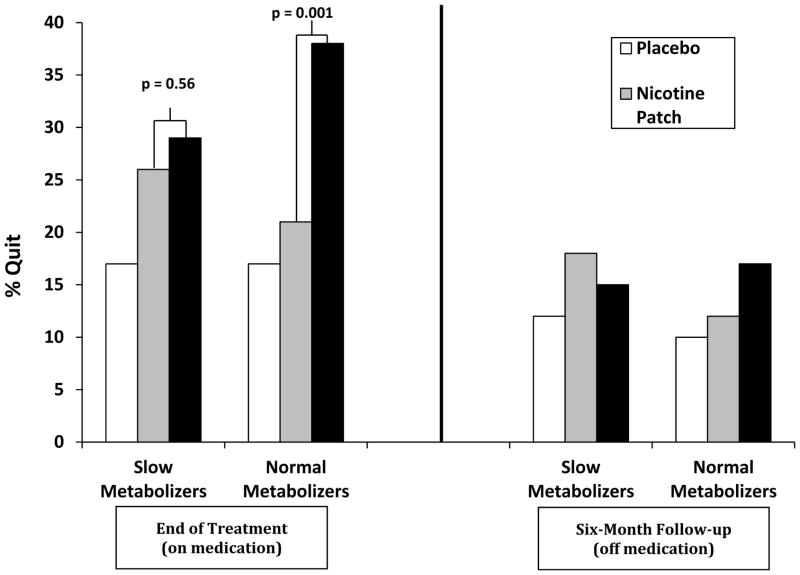
Building on these prior retrospective studies in which the NMR was assessed following study completion, a large multi-site, placebo-controlled clinical trial using prospective NMR stratification was conducted (Lerman et al. 2015). Treatment-seeking smokers (n=1,246) were tested for the NMR and randomly assigned by NMR group to one of three treatment groups: placebo (placebo patch and placebo pill), nicotine patch (active nicotine patch plus placebo pill), or varenicline (placebo patch plus active varenicline pill). Stratification by NMR was based on classification as either slow (plasma NMR < 0.31, approximately first quartile based on one of the prior clinical trials; (Schnoll et al. 2009) versus normal (plasma NMR ≥ 0.31, all other quartiles). Slow metabolizers were oversampled in order to provide approximately equal numbers of slow versus normal metabolizers. Results revealed a significant NMR by treatment arm interaction: among normal metabolizers, varenicline improved quit rates significantly compared to the nicotine patch. However, among slow metabolizers, varenicline was not more efficacious than nicotine patch at promoting cessation (Figure 1). The relative efficacy of varenicline versus nicotine patch in slow and normal metabolizers can be illustrated by the “number needed to treat” (NNT), a standardized measure indicating the average number of patients that must be treated in order to benefit one (Cook and Sackett 1995). Among normal metabolizers, the NNT was 26.0 for nicotine patch and 4.9 for varenicline; among slow metabolizers, the NNT was 10.3 for nicotine patch and 8.1 for varenicline. Importantly, there was also a significant NMR by treatment interaction observed in reported side effects of varenicline (versus placebo): slow metabolizers reported a significant increase in side effects on active pill versus placebo, but there was no increase in side effects for normal metabolizers receiving active varenicline. There was no NMR by treatment interaction effect for side effects of nicotine patch. These results suggest that treating normal metabolizers with varenicline and slow metabolizers with nicotine patch for smoking cessation may optimize quit outcomes while minimizing the risk of side effects. Thus, the NMR could provide a useful biomarker for personalized smoking cessation treatment.
Figure 1. Quit Rates by Treatment Arm and NMR Group.
The NMR predicts treatment outcomes on nicotine replacement therapy and varenicline
Smoking cessation rates by NMR and treatment group. Varenicline treatment significantly improved quit rates compared to the nicotine patch among normal metabolizers; however, among slow metabolizers, varenicline was no better than the nicotine patch at promoting cessation. Adapted from Lerman et al. 2015.
V. Mechanisms
The mechanisms underlying the associations between the NMR and treatment response are not fully understood. Associations between the NMR and treatment response are not likely to be mediated by nicotine dependence or heaviness of smoking, because these associations remain unaltered after controlling for nicotine dependence, subjective craving, or heaviness of smoking in linear regression models (Benowitz et al. 2003; Ho et al. 2009b; Johnstone et al. 2006; Kandel et al. 2007; Lerman et al. 2006; Patterson et al. 2008; Schnoll et al. 2009; Strasser et al. 2011). Studies have also found no association between the NMR and withdrawal symptoms during abstinence (Schnoll et al. 2009).
Potential mechanisms underlying the association between the NMR and treatment response include differences in nicotinic receptor availability, subjective measures of nicotine reward and physiological effects of nicotine, or conditioned responses to smoking cues. Because nicotine exerts its effects by binding to nicotinic acetylcholine receptors, Dubroff et al (2015) assessed the relationship between the NMR and α4β2* nAChR availability using PET imaging with 2-(18)F-fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-(18)F-FA). Results showed a reduction of thalamic α4β2* nAChR availability and a greater reduction of craving in slow nicotine metabolizers compared to normal metabolizers after 18 hours of abstinence.
The NMR has also been associated with subjective measures of nicotine reward and physiological effects of nicotine. In one study (Sofuoglu et al. 2012), smokers received nicotine intravenously at escalating quantities over 30 minutes following overnight abstinence. Higher NMR (i.e. faster metabolism) was associated with greater self-reported craving following overnight abstinence, and higher ratings of nicotine-induced good drug effects, drug liking, and wanting more drug compared to slow metabolizers. Faster metabolizers also had a greater heart rate increase in response to nicotine. This enhanced reward response may explain why faster metabolizers also display greater cue reactivity (a conditioned response to stimuli associated with smoking, such as a lit cigarette, lighter, or ashtray).
Neuroimaging studies have demonstrated that smokers display greater brain activation in areas related to reward, visual attention, and habitual learning, such as the insula, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and midtemporal gyrus, when viewing smoking cues compared to neutral cues (Brody et al. 2002; David et al. 2005; Engelmann et al. 2012; McClernon et al. 2005). A recent functional magnetic resonance imaging (fMRI) study compared cue reactivity in the fastest and slowest nicotine metabolizers (first versus fourth quartile of NMR) (Tang et al. 2012). Participants in this study watched video clips displaying smoking-related and neutral scenes during fMRI scanning. Compared to slow metabolizers, fast metabolizers displayed greater activation in response to smoking cues (versus neutral cues) in the ACC, PCC, and insula when smokers were not deprived of cigarettes. These results were consistent whether fast metabolizers were classified by the NMR or by CYP2A6 genotype. Another recent neuroimaging study found that slow metabolizers showed a significant decrease in brain response to smoking cues in several regions (the inferior frontal gyrus, frontal pole, and caudate) following 24 hours of abstinence (compared to when they were smoking as usual), whereas normal metabolizers showed an increase in cue reactivity during abstinence (Falcone et al. 2015). Cue reactivity is important because it has been linked to relapse (Janes et al. 2010); thus, fast metabolizers who show greater neural responses to smoking cues may experience greater difficulty quitting. Future research examining associations between NMR and cue reactivity in treatment-seeking smokers may offer additional insight into a possible mechanism for associations between nicotine metabolism rates and smoking behavior.
VI Future Directions
To maximize the utility of the NMR for improving public health, there are important lines of research that remain to be conducted. For example, the predictive validity of the NMR for treatment response has largely been examined in otherwise healthy adult populations. Future studies are needed to evaluate associations between NMR and smoking cessation in psychiatric populations, as many psychiatric disorders have a high comorbidity with smoking dependence. Between 21.1% and 31.7% of nicotine dependent individuals have a current alcohol use, mood, or anxiety disorder, and this population consumes 34.2% of all cigarettes smoked in the United States (Grant et al. 2004). In a study of the prevalence of smoking among individuals with schizophrenia or bipolar disorder, 64% of individuals with schizophrenia and 44% of individuals with bipolar disorder reported smoking compared to 19% of individuals without a psychiatric illness (Dickerson et al. 2013).
Associations between nicotine metabolism rates and smoking behavior have been shown to differ for adolescents compared to adults, and it is possible that adolescents may also differ in response to smoking cessation treatment as a function of the NMR (Berlin et al. 2007; Rubinstein et al. 2013a). Additionally, the NMR may be less predictive of smoking behavior in lighter smokers; Ho and colleagues (2009a) found no predictive value of NMR for smoking quantity in light smokers, and relationships with treatment outcomes were less robust. Additional research is necessary to evaluate the utility of the NMR in light and non-daily smokers.
The feasibility of the NMR as a biomarker in clinical practice must also be assessed. Individual NMR values may be obtained from blood or saliva samples collected at a primary care facility and sent to a laboratory for analysis of cotinine and 3HC concentrations using liquid chromatography-tandem mass spectrometry (Jacob et al. 2011). One challenge that must be addressed prior to implementation is determining a precise cut-point to classify slow versus normal metabolizers. Although there is typically consensus on defining slow metabolizers as those in the lowest quartile of NMR (see Table 3), the majority of studies have defined quartiles within each sample, leading to variation in specific cut-points used to define slow versus normal metabolizers. This approach is impractical from a clinical standpoint. After reviewing cut-points used in prior studies and examining the distribution of NMR values within the population screened for their clinical trial, Lerman et al (2015) selected a plasma cut-point of 0.31 to classify slow versus normal metabolizers, and demonstrated significant differences in treatment response using this classification scheme. Based on published correlations between plasma and saliva NMR values, a plasma cut-point of 0.31 corresponds to a saliva cut-point of 0.22. (Chenoweth et al. 2014). For these reasons, we recommend that slow metabolizers be classified as those with a plasma NMR value <0.31 or saliva NMR value <0.22.
Table 3.
Clinical trials of the NMR as a predictor of treatment response.
| Study | Population | NMR classification | Results |
|---|---|---|---|
| Lerman et al. 2006 | 480 treatment seeking smokers | Slower metabolizers (NMR <0.23) versus normal/faster metabolizers (NMR≥0.23) | Quitting success with nicotine patch decreased significantly as the NMR increased. The NMR did not predict cessation in smokers using nicotine nasal spray. |
| Schnoll et al. 2009 | 568 treatment seeking smokers | Slowest metabolizers NMR<0.26 versus normal/faster metabolizers NMR≥0.26 | Normal/faster metabolizers were significantly less likely to quit with nicotine patch compared to slow metabolizers. |
| Ho et al. 2009b | 646 treatment seeking African-American Smokers | Slowest quartile versus all other quartiles | Individuals in the slowest quartile had higher quitting rates with both placebo and nicotine gum treatments compared to normal/faster metabolizers. |
| Lerman et al. 2010 | 470 treatment seeking Caucasian smokers | Slowest metabolizers <0.26 versus normal metabolizers (NMR ≥0.26) | Extended duration therapy was superior to standard therapy in genotypic or phenotypic slower metabolizers of nicotine, but not in normal metabolizers. |
| Schnoll et al. 2013 | 87 treatment seeking fast metabolizers of nicotine | Faster metabolizers >0.18 | There were no differences in quit rates at the end of treatment in fast metabolizers treated with high dose vs. standard dose patch |
| Patterson et al. 2008 | 414 treatment seeking smokers | Slowest metabolizers <0.26 versus fastest metabolizers >0.54 | Slow metabolizers had equivalent quit rates with placebo or bupropion after 10 weeks of treatment (32%), whereas the fastest metabolizers had low quit rates with placebo (10%) which were significantly increased by bupropion (34%). |
| Lerman et al. 2015 | 1246 treatment seeking smokers | Slow metabolizers (NMR <0.31) versus normal metabolizers (NMR ≥0.31) | Varenicline was more efficacious than nicotine patch in normal metabolizers but not in slow metabolizers. Slow metabolizers reported greater overall side-effect severity with varenicline versus placebo, whereas there were no differences in side effects by treatment group among normal metabolizers. |
Cost-effectiveness data from prospective clinical trials using the NMR will be critical for future implementation of this biomarker (Schnoll and Leone 2011). To illustrate, an analysis of cost-effectiveness of genetic testing to predict treatment outcomes on varenicline compared to bupropion suggested that prior genetic testing may be justified only if the genotype is neither too rare nor common (Heitjan et al. 2008). Because of the population distribution of nicotine metabolism groups, and the low cost of testing, the NMR may be cost effective; however, this is yet to be analyzed formally. Other factors to consider include ease of implementation in a healthcare setting, and whether primary care physicians would be willing to incorporate biomarker assessment into standard treatment (Cummings et al. 1989; Emmons and Goldstein 1992; Heitjan et al. 2008; Shields et al. 2008). Future studies are necessary to evaluate cost effectiveness, optimal implementation in the electronic health record, and potential efficacy in the healthcare settings. This research will give valuable insight into implementing the NMR as a biomarker to maximize successful response to current treatments.
VII Conclusions
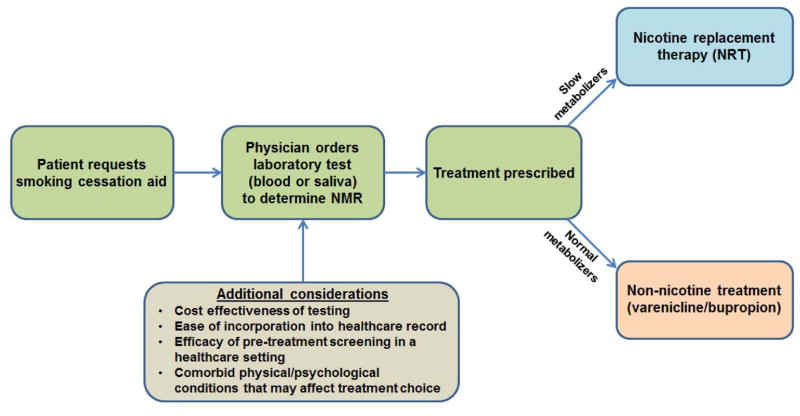
The NMR is a reliable measure of inherited individual differences in nicotine metabolism rate, and a validated biomarker of pharmacological treatment response among smokers who wish to quit. Existing evidence supports recommendation of nicotine replacement therapy for slow metabolizers, and non-nicotine treatments such as varenicline for normal metabolizers (Figure 2). Because it is easy to assess (in saliva as well as blood), stable over time, and not dependent on time of day or time since last cigarette, the NMR is a practical clinical biomarker and could provide useful information to help clinicians guide treatment approach. Although further research is necessary to develop a simple and cost-effective point-of-care assessment to facilitate clinical applications, the NMR may provide a worthwhile approach to personalized medicine for smoking cessation.
Figure 2. Incorporating the NMR to aid in smoking cessation treatment selection.
A proposed model for incorporating the NMR into smoking cessation treatment decision-making.
Acknowledgments
Funding
This work was supported by NIH grants R35-CA197461 and U01-DA020830.
Footnotes
Conflicts of Interest
Dr. Lerman received donated drug and placebo supplies from Pfizer for conducting a randomized clinical trial. The other authors report no conflicts of interest.
References
- Ariyoshi N, Miyamoto M, Umetsu Y, Kunitoh H, Dosaka-Akita H, Sawamura Y, Yokota J, Nemoto N, Sato K, Kamataki T. Genetic polymorphism of CYP2A6 gene and tobacco-induced lung cancer risk in male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:890–894. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Association; Washington, D.C: 2013. [Google Scholar]
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf) 2010;32:71–82. doi: 10.1093/pubmed/fdp074. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Dempsey D. Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob Res. 2004;6(Suppl 2):S189–202. doi: 10.1080/14622200410001669169. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Trans-3′-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol. 2001;51:53–59. doi: 10.1046/j.1365-2125.2001.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Sachs DP. Deficient C-oxidation of nicotine. Clin Pharmacol Ther. 1995;57:590–594. doi: 10.1016/0009-9236(95)90044-6. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006a;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291:1196–1203. [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006b;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Michel M, Nishita D, Krasnow R, Javitz HS, Conneely KN, Lessov-Schlaggar CN, Hops H, Zhu AZ, Baurley JW, McClure JB, Hall SM, Baker TB, Conti DV, Benowitz NL, Lerman C, Tyndale RF, Swan GE Transdisciplinary Research in Cancer of the Lung Research T. Drug Metabolizing Enzyme and Transporter Gene Variation, Nicotine Metabolism, Prospective Abstinence, and Cigarette Consumption. PLoS One. 2015;10:e0126113. doi: 10.1371/journal.pone.0126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Gasior MJ, Moolchan ET. Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine Tob Res. 2007;9:493–498. doi: 10.1080/14622200701243193. [DOI] [PubMed] [Google Scholar]
- Binnington MJ, Zhu AZ, Renner CC, Lanier AP, Hatsukami DK, Benowitz NL, Tyndale RF. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenet Genomics. 2012;22:429–440. doi: 10.1097/FPC.0b013e3283527c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Lerman C, Rose JE, McClernon FJ, Kenny PJ, Tyndale RF, David SP, Stein EA, Uhl GR, Conti DV, Green C, Amur S. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93:526–538. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker K, Lewis S, Coleman T, Cooper S. Changes in the rate of nicotine metabolism across pregnancy: a longitudinal study. Addiction. 2015;110:1827–1832. doi: 10.1111/add.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US); Atlanta, GA: 2010. [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LW, Jr, Schnoll RA, George TP, Cinciripini PM, Lerman C, Tyndale RF. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23:1773–1782. doi: 10.1158/1055-9965.EPI-14-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA. 1989;261:75–79. [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, Yolken RH. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatr Serv. 2013;64:44–50. doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- Dubroff JG, Doot RK, Falcone M, Schnoll RA, Ray R, Tyndale RF, Brody AL, Hou C, Schmitz A, Lerman C. Decreased Nicotinic Receptor Availability in Smokers with Slow Rates of Nicotine Metabolism. J Nucl Med. 2015;56:1724–1729. doi: 10.2967/jnumed.115.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Goldstein MG. Smokers who are hospitalized: a window of opportunity for cessation interventions. Prev Med. 1992;21:262–269. doi: 10.1016/0091-7435(92)90024-c. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control. 2006;15:280–285. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan P, Pokhrel P, Herzog TA, Pagano IS, Franke AA, Clanton MS, Alexander LA, Trinidad DR, Sakuma KL, Johnson CA, Moolchan ET. Nicotine Metabolism in Young Adult Daily Menthol and Nonmenthol Smokers. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Cao W, Bernardo L, Tyndale R, Loughead J, Lerman C. Brain Responses to Smoking Cues Differ Based on Nicotine Metabolism Rate. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, Baldwin D, Tyndale RF, Lerman C, Ray R. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine Tob Res. 2011;13:498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaen C, Baker T, Bailey W, Benowitz N, Curry S, Dorfman S, Froelicher E, Goldstein M, Healton C, Henderson P, Heyman R, Koh H, Kottke T, Lando H, Mecklenburg R, Mermelstein R, Mullen P, Orleans C, Robinson L, Stitzer M, Tommasello A, Villejo L, Wewers M. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- Gambier N, Batt AM, Marie B, Pfister M, Siest G, Visvikis-Siest S. Association of CYP2A6*1B genetic variant with the amount of smoking in French adults from the Stanislas cohort. Pharmacogenomics J. 2005;5:271–275. doi: 10.1038/sj.tpj.6500314. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Cohen SB, Man SF, Sin DD. Sex-related differences in serum cotinine concentrations in daily cigarette smokers. Nicotine Tob Res. 2008;10:1293–1300. doi: 10.1080/14622200802239132. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Mahoney MC, Novalen M, Chenoweth MJ, Heitjan DF, Lerman C, Tyndale RF, Hawk LW., Jr Test-Retest Reliability and Stability of the Nicotine Metabolite Ratio Among Treatment-Seeking Smokers. Nicotine Tob Res. 2015;17:1505–9. doi: 10.1093/ntr/ntv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heitjan DF, Asch DA, Ray R, Rukstalis M, Patterson F, Lerman C. Cost-effectiveness of pharmacogenetic testing to tailor smoking-cessation treatment. Pharmacogenomics J. 2008;8:391–399. doi: 10.1038/sj.tpj.6500492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Benowitz NL, Hall SM. Clinical significance of early smoking withdrawal effects and their relationships with nicotine metabolism: preliminary results from a pilot study. Nicotine Tob Res. 2014;16:615–620. doi: 10.1093/ntr/ntt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Faseru B, Choi WS, Nollen NL, Mayo MS, Thomas JL, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev. 2009a;18:3426–3434. doi: 10.1158/1055-9965.EPI-09-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009b;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2003:CD000031. doi: 10.1002/14651858.CD000031. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, Murphy M, Walton R. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR Varenicline Phase 3 Study G. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol. 2007;165:901–910. doi: 10.1093/aje/kwm010. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Kunugita N, Katoh T, Yang M, Kawamoto T. The significance of the homozygous CYP2A6 deletion on nicotine metabolism: a new genotyping method of CYP2A6 using a single PCR-RFLP. Biochem Biophys Res Commun. 1999;262:146–151. doi: 10.1006/bbrc.1999.1182. [DOI] [PubMed] [Google Scholar]
- Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, Benowitz N, Tyndale RF. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW, Jr, Cinciripini P, George TP, Wileyto EP, Swan GE, Benowitz NL, Heitjan DF, Tyndale RF, Group P-PR. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3:131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Levi M, Dempsey DA, Benowitz NL, Sheiner LB. Prediction methods for nicotine clearance using cotinine and 3-hydroxy-cotinine spot saliva samples II. Model application. J Pharmacokinet Pharmacodyn. 2007;34:23–34. doi: 10.1007/s10928-006-9026-0. [DOI] [PubMed] [Google Scholar]
- Levy DE, Youatt EJ, Shields AE. Primary care physicians’ concerns about offering a genetic test to tailor smoking cessation treatment. Genet Med. 2007;9:842–849. doi: 10.1097/gim.0b013e31815bf953. [DOI] [PubMed] [Google Scholar]
- Liu T, David SP, Tyndale RF, Wang H, Zhou Q, Ding P, He YH, Yu XQ, Chen W, Crump C, Wen XZ, Chen WQ. Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction. 2011;106:985–994. doi: 10.1111/j.1360-0443.2010.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukola A, Buchwald J, Gupta R, Palviainen T, Hallfors J, Tikkanen E, Korhonen T, Ollikainen M, Sarin AP, Ripatti S, Lehtimaki T, Raitakari O, Salomaa V, Rose RJ, Tyndale RF, Kaprio J. A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism. PLoS Genet. 2015;11:e1005498. doi: 10.1371/journal.pgen.1005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JM, Fandrick K, Zhang X, Serafin SV, Cashman JR. Inhibition of human liver microsomal (S)-nicotine oxidation by (-)-menthol and analogues. Chem Res Toxicol. 2003;16:988–993. doi: 10.1021/tx0340551. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–1614. [PubMed] [Google Scholar]
- Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1396–1400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Kuroiwa Y, Yokoi T. Interindividual differences in nicotine metabolism and genetic polymorphisms of human CYP2A6. Drug Metab Rev. 2002;34:865–877. doi: 10.1081/dmr-120015696. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamagishi S, Yamamoto H, Yamamoto T, Kuroiwa Y, Yokoi T. Deficient cotinine formation from nicotine is attributed to the whole deletion of the CYP2A6 gene in humans. Clin Pharmacol Ther. 2000;67:57–69. doi: 10.1067/mcp.2000.103957. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Characterization of CYP2A6 involved in 3′-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277:1010–1015. [PubMed] [Google Scholar]
- National Institute for Clinical Excellence. Guidance on the Use of Nicotine Replacement Therapy (NTR) and Bupropion for Smoking Cessation. Washington, DC: National Institute for Clinical Excellence; 2002. Technical appraisal report 39. [Google Scholar]
- National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington (DC): 2011. [PubMed] [Google Scholar]
- Oscarson M. Genetic polymorphisms in the cytochrome P450 2A6 (CYP2A6) gene: implications for interindividual differences in nicotine metabolism. Drug Metab Dispos. 2001;29:91–95. [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, Hawk LW, Tyndale RF, Benowitz N, Lerman C. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl) 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Piliguian M, Zhu AZ, Zhou Q, Benowitz NL, Ahluwalia JS, Sanderson Cox L, Tyndale RF. Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo. Pharmacogenet Genomics. 2014;24:118–128. doi: 10.1097/FPC.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque M, von Richter O, Oke B, Berkkan H, Oscarson M, Ingelman-Sundberg M. Identification of a single nucleotide polymorphism in the TATA box of the CYP2A6 gene: impairment of its promoter activity. Biochem Biophys Res Commun. 2001;284:455–460. doi: 10.1006/bbrc.2001.4990. [DOI] [PubMed] [Google Scholar]
- Prather RD, Tu TG, Rolf CN, Gorsline J. Nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) in women and obese men compared with normal-sized men. J Clin Pharmacol. 1993;33:644–649. doi: 10.1002/j.1552-4604.1993.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, Tyndale RF. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics. 2008;122:e643–647. doi: 10.1542/peds.2007-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction. 2013a;108:406–412. doi: 10.1111/j.1360-0443.2012.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Shiffman S, Rait MA, Benowitz NL. Race, gender, and nicotine metabolism in adolescent smokers. Nicotine Tob Res. 2013b;15:1311–1315. doi: 10.1093/ntr/nts272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl) 2014;231:2515–2523. doi: 10.1007/s00213-013-3421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Leone FT. Biomarkers to optimize the treatment of nicotine dependence. Biomark Med. 2011;5:745–761. doi: 10.2217/bmm.11.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Leone FT, Tyndale RF, Benowitz NL. High dose transdermal nicotine for fast metabolizers of nicotine: a proof of concept placebo-controlled trial. Nicotine Tob Res. 2013;15:348–354. doi: 10.1093/ntr/nts129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafey O, Eriksen M, Ross H, Mackay J. The Tobacco Atlas. 3. American Cancer Society; Bookhouse Group, Inc; Atlanta, GA: 2009. [Google Scholar]
- Shields AE, Levy DE, Blumenthal D, Currivan D, McGinn-Shapiro M, Weiss KB, Yucel R, Lerman C. Primary care physicians’ willingness to offer a new genetic test to tailor smoking treatment, according to test characteristics. Nicotine Tob Res. 2008;10:1037–1045. doi: 10.1080/14622200802087580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Jacob P, 3rd, Benowitz NL. Stability of the nicotine metabolite ratio in smokers of progressively reduced nicotine content cigarettes. Nicotine Tob Res. 2013;15:1939–1942. doi: 10.1093/ntr/ntt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P, 3rd, Aziziyeh A, Wing VC, George TP, Tyndale RF, Benowitz NL. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21:1105–1114. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, Tyndale RF, Lerman CE. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9:511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- Swan GE, Benowitz NL, Lessov CN, Jacob P, 3rd, Tyndale RF, Wilhelmsen K. Nicotine metabolism: the impact of CYP2A6 on estimates of additive genetic influence. Pharmacogenet Genomics. 2005;15:115–125. doi: 10.1097/01213011-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenet Genomics. 2009;19:388–398. doi: 10.1097/FPC.0b013e32832a404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DW, Hello B, Mroziewicz M, Fellows LK, Tyndale RF, Dagher A. Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. NeuroImage. 2012;60:2136–2143. doi: 10.1016/j.neuroimage.2012.01.119. [DOI] [PubMed] [Google Scholar]
- Tanner JA, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J, Kankaanpaa A, Galanti L, Stefan C, George TP, Benowitz NL, Lerman C, Tyndale RF. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiol Biomarkers Prev. 2015;24:1239–1246. doi: 10.1158/1055-9965.EPI-14-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The Health Benefits of Smoking Cessation: a report of the Surgeon General. U.S. Department of Health and Human Services Office on Smoking and Health; 1990. DHHS Publication No. (CDC) YO-K-116. 1990. [Google Scholar]
- Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West O, Hajek P, McRobbie H. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology (Berl) 2011;218:313–322. doi: 10.1007/s00213-011-2341-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic: enforcing bans on tobacco advertising, promotion and sponsorship. Geneva, Switzerland: 2013. [Google Scholar]
- Xu C, Goodz S, Sellers EM, Tyndale RF. CYP2A6 genetic variation and potential consequences. Adv Drug Deliv Rev. 2002;54:1245–1256. doi: 10.1016/s0169-409x(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nakajima M, Katoh M, Kanoh A, Tamura O, Ishibashi H, Yokoi T. Trans-3′-hydroxycotinine O- and N-glucuronidations in human liver microsomes. Drug Metab Dispos. 2005;33:23–30. doi: 10.1124/dmd.104.001701. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inoue K, Hashimoto M, Shimada T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch Toxicol. 1999;73:65–70. doi: 10.1007/s002040050588. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nakajima M, Nishimura K, Tokudome S, Kwon JT, Yokoi T. Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6*9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin Pharmacol Ther. 2003;74:69–76. doi: 10.1016/S0009-9236(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nakajima M, Watanabe Y, Kwon JT, Yokoi T. Genetic polymorphisms in human CYP2A6 gene causing impaired nicotine metabolism. Br J Clin Pharmacol. 2002;54:511–517. doi: 10.1046/j.1365-2125.2002.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ameno K, Ameno S, Kinoshita H, Kubota T, Kumihashi M, Mostofa J, Iwahashi K, Ijiri I. Effects of whole deletion of CYP2A6 on nicotine metabolism in humans. Drug Chem Toxicol. 2002;25:203–213. doi: 10.1081/dct-120003260. [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. Variation in trans-3′-hydroxycotinine glucuronidation does not alter the nicotine metabolite ratio or nicotine intake. PLoS One. 2013;8:e70938. doi: 10.1371/journal.pone.0070938. [DOI] [PMC free article] [PubMed] [Google Scholar]