ABSTRACT
The advent of fecal microbiota transplantation (FMT) and the prospect of other types of microbiota transplants (MT), e.g. vaginal, skin, oral and nasal, are challenging regulatory agencies. Although FDA is regulating FMT (as a biologic), there is currently no widely accepted or agreed upon scientific or legal definition of FMT or MT. The authors report on discussions regarding a definition of MT that took place among a working group of stakeholders convened under a National Institutes for Allergies and Infectious Diseases grant to address the regulation of MT. In arriving at a definition, the group considered the 1) nature of the material being transplanted; 2) degree of manipulation of the transferred materials prior to implantation; 3) ability to characterize the transplanted product using external techniques; and 4) origin of the stool product (single vs multiple donors).
Keywords: definition, fecal, microbiota transplantation, regulation
The advent of fecal microbiota transplantation (FMT) and the prospect of other types of microbiota transplants (MT), e.g. vaginal, skin, oral and nasal, are challenging regulatory agencies. In the US, the FDA has modified its position on regulating FMT several times and has asked for comments on its most recent draft industry guidance.1-3 While FDA has, at least for now, classified FMT as a drug (a live biotherapeutic product), it is currently exercising enforcement discretion, i.e. not requiring an Investigational New Drug Application (IND) for physicians performing the procedure and stool banks providing fecal matter for individuals with Clostridium difficile infection (CDI) not responding to standard therapies. Although the focus of FDA's industry guidance has been on whether to require an IND in certain situations, the agency has proceeded in its regulatory discussion without defining what it is regulating or considering whether FMT is unique in ways that call out for treating it differently from drugs. An alternative regulatory pathway would be one that treated it as both a product and a procedure by combining, for example, aspects of the ways in which drugs and blood and human tissue for transfusion and transplant are treated4,5 or treating the procedure as “the practice of medicine.”
A number of authors have commented on the difference between MT and more traditional drugs. For example, Petrof and Khoruts argue that “[a]lthough the FDA considers the fecal microbiota to be a drug, like any agent used to treat, mitigate, or prevent disease, it is certainly not like any other drug. It is incredibly complex, comprising hundreds of species of microorganisms …. The composition of the microbiota varies among individuals and within individuals at different times. The microbiota is alive, metabolically active, and highly dynamic in response to multiple environmental factors such as diet. Most of its microorganisms cannot be cultured in vitro, and currently, the therapeutic potential of microbiota can only be adequately tested in patients.”5
The regulation of MT has significant import both for their availability to patients and for the development of new therapies that target the microbiome. This is particularly true for FMT regulation which is the focus of considerable controversy. At its most basic, FMT consists of a fresh stool product diluted with a liquid, like saline, and then delivered into the intestinal tract of another individual. The procedure can be done via enema, colonoscopy, sigmoidoscopy, or nasogastric tube. However, several entities have developed or are in the process of developing stool products that can be packaged, transported, commercialized and/or more easily administered by physicians or consumed by patients. These range from the most basic, e.g., frozen stool, to freeze-dried stool, to more sophisticated products such as capsules containing synthetic stool grown in culture and assembled. If we think of the products on a spectrum from the most basic to the most processed, at one end of the spectrum are stool banks, such as OpenBiome and Advancing Bio, that provide hospitals with screened frozen fecal material ready for clinical use. Not too much further along the continuum are products such as RBX2660, cryopreserved filtered microbiota derived from the stool of screened donors and administered via an off-the-shelf enema delivery system,6 developed by Rebiotix. Also at this end of the spectrum is a lyophilized powder that can be reconstituted for rectal infusion developed by CIPAC Therapeutics.7-8
Moving toward the other end of the spectrum, several pharmaceutical companies and biotechnology start-ups are developing various stool-based products to treat CDI and other conditions.7 These companies are taking “a more targeted approach.” They are identifying “groups of specific bacterial strains (a consortia)”9 purified from stool samples and aimed at treating a particular disease or condition. The approach “enables greater specificity and quality control.”9 For example, Massachusetts-based Seres Therapeutics has several products in various stages of development and clinical testing including a drug product to treat recurrent CDI called SER-109. The substance, an “ecobiotic,” comprises bacterial spores enriched and purified from stool donated by healthy and screened individuals, packaged in capsules and designed to restructure a dysbiotic (unhealthy) microbiome to a healthy microbiome.10,11 Moving closer to the typical biologic drug development process, Vedanta Biosciences is identifying and culturing “bacterial strains that have been shown to suppress inflammation and are decreased or missing in patients with chronic gut inflammation.”9 Similarly, researchers in Canada have developed a “microbial assemblage” derived from stool and grown in culture called RePOOPulate to treat recurrent CDI made from purified intestinal bacterial cultures derived from a single healthy donor and grown in a laboratory.12
The different formulations and processing levels of these materials arguably could have implications for their regulation.13 The variety of products under development to treat gut-related illnesses and conditions derived from fecal matter calls for clarification of what constitutes an MT. There is currently no widely accepted or agreed upon scientific or legal definition of the term. As a result, it is not clear where one would draw the line on the spectrum of products currently in use or now in development as to which constitute an MT and which do not.
One might also ask whether MT is distinguishable from probiotic products on the market or being developed for the market. While probiotics do not have a legal definition, there is a commonly accepted definition of the term that is attributed to the work of a Joint FDA/WHO Expert Consultation. The joint report defines probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.” Under this broad definition it appears that there is likely some overlap, i.e., that some MT products are probiotics or that all MT materials come under the broad umbrella of probiotics. One difference that may distinguish the 2 is that traditional probiotics generally do not engraft for the duration observed and expected with MT. They typically need to be taken continuously because they are generally not adapted to the gut environment although evidence is emerging that some bacterial species absent in the gut microbiome of individual humans and administered as a probiotic over several weeks can become resident under certain conditions. 14 Further, the fact that traditional probiotics are defined and grown in pure culture significantly limits the safety risk posed by probiotics, as the risk of introducing pathogens can be more tightly controlled.
In the marketplace, most items labeled probiotics are foods or dietary supplements that have a small number of well-defined microbial strains of bacteria added to them. While none have yet been approved, several probiotics are going through the clinical trial phase for drug approval.
MTs, as currently conceived, would not be considered foods. Nor are they likely to be classified as dietary supplements.15 However, “the public and many physicians may not distinguish” the approach to wellness taken by MT from that of probiotics or dietary supplements.5 Yet, as Petrof and Khoruts have argued, FMTs, which result in “large-scale alteration of the intestinal microbiota,” work very differently from probiotic and dietary supplement products on the market and “require a different set of definitions” and considerations for regulation than these products.5
The question of how MT should be defined was posed to a working group assembled as part of an NIH-funded study16 to address the regulation of MT. The working group included microbiome experts and microbiota transplantation researchers, physicians performing FMT, food and drug law attorneys, patient advocates, bioethicists, industry representatives, legal academics with expertise in health and/or food and drug law and experts in the regulation of blood and human tissues and cells.*
At a meeting in December 2015 the co-investigators of the study asked working group members to consider the following definition of microbiota transplantation: “microorganisms transplanted from one human to another.” While participants agreed this was a good starting point for discussion they also agreed it left out several critical aspects of the product/procedure.
The first critical omission identified was the characterization of the material that was being transplanted. Working group members noted that it is important to incorporate in the definition the fact that what is being transplanted is not simply microorganisms but rather a group of microorganisms in a biologic matrix. To distinguish MTs from therapies that use only a single organism or small number of organisms as the active agent(s), participants suggested the following language to describe the transplanted material:
“Set of organisms existing at a human site”
“Community of organisms” (noting that “community” has connotations of interaction)
“System of organisms” (noting that “system” implies interaction with the host)
“Community of interacting microbes in a defined environment”
“Assemblage of interacting microbes”
The group believed that this concept was essential to the definition of MT because in addition to distinguishing MTs from most conventional probiotics, it distinguishes MTs, which are designed to change an entire ecosystem, from most drugs that aim at single targets like an enzyme or receptor.13 In addition, the group considered the non-microbial components of the material being transplanted and their possible importance in the success of the transplant.
Another critical issue that participants felt was missing from the proposed definition was reference to the degree of manipulation of the transferred materials before implantation into the recipient. The consensus was that anything more than “minimal manipulation” of the human material that is transferred from one person to another would not constitute MT, but would be considered a probiotic or other product that FDA would likely regulate as a drug. However, many noted that some degree of manipulation is necessary or inevitable but could not agree on whether there is - or should be - a well-defined limit on the degree of manipulation that would be appropriate to include within the definition of MT. Prior to conducting an FMT, feces are typically liquefied and filtered. This alone might be considered minimal manipulation. But, if part of the harvested material is killed during the liquefaction process or other step, the procedure could be considered more than minimal manipulation. Participants suggested including the following language about the transplanted material in the definition to indicate that MT involves minimal manipulation:
“Organisms in native form with minimal manipulation” where native means organisms that occur naturally in the site or are present in the harvested material;
“Uncultivated materials,” i.e., materials that have not been cultivated in the laboratory in a growth environment or medium;
Material “maintaining the integrity of the microbial consortium of organisms.”
The concept of “maintaining the integrity of the microbial consortium of organisms” grew out of the concept of minimal manipulation used in regulations governing the transplantation of human cells and tissues (HCT/P). Minimal manipulation is defined by FDA for cells or nonstructural tissues as “processing that does not alter the relevant biologic characteristics of cells or tissues.”17 Figure 1 depicts different levels of manipulation used in preparing fecal material for use in FMT. Although Figure 1 indicates equal spacing between the products on the continuum, this does not necessarily reflect the magnitude of manipulation between the different products.
Figure 1.
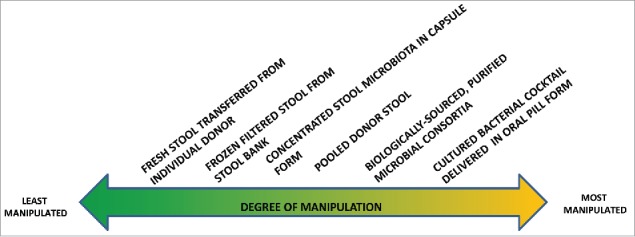
Range of Fecal Material Manipulation for CDI Treatment.
Other concepts discussed that could potentially distinguish the product/procedures on the right of the spectrum from those on the left and that could be relevant for regulatory purposes are (1) the inability of the stool product to be characterized, and (2) whether the stool is obtained from a single individual. By inability to characterize we mean that external techniques cannot be used to probe into the internal structure and properties of the material and document those structures and materials. Characterization of live biotherapeutic products (a category of drugs) is required in the regulatory process.18 Those characterization requirements would be extremely difficult to meet for fecal material used in FMT. It would be both scientifically infeasible and prohibitively expensive to use current whole community genome sequencing to identify and characterize to current standards all the microbes in the fecal material. In addition, because stool differs from person to person, the chemical and biologic components of the stool product would “vary from batch to batch” and would violate the regulatory “requirement for consistency in product composition.”13,19
The use of stool from a single donor whose stool is given to a single or small number of recipients may also be an important characteristic to consider in the definition of FMT. This criterion describes FMT performed by physicians who do not utilize a stool bank but rely on the patient to provide the stool from a friend or relative. This factor clearly distinguishes among the different potential types of FMT and may be a key distinction for regulatory purposes. In fact, in its most recent draft industry guidance document on regulating FMT, FDA seems to be making this distinction stating that it will exercise enforcement discretion when “the stool donor and stool are qualified by screening and testing performed under the direction of the licensed health care provider for the purpose of providing the FMT product for treatment of the patient.”1 The statement appears to contemplate a single donor and a single recipient, which would limit public health concerns compared with universal donors or pooled stool, both of which could negatively affect the health of a large number of MT recipients. Another regulatory consideration for pooled donor stool is the unknown interactions between different microbial communities and the increased risk for infection that pooling raises.
Working group members also discussed whether the definition of MT should include the concept of “restoration” or “restoration to balance” or, like the definition of probiotics, include the concept that MT “confers a health benefit to the host.” In other words, should the definition of MT indicate that the transferred materials must restore a “normal” or “health” microbiome and/or restore the recipient to health? Working group members noted that this is complicated for several reasons. First, the microbiome of the recipient before the MT may never have been in balance. Also, it is impossible to measure restoration of the microbiome - other human health endpoints are used instead. Some argued that implying a health benefit (a claim that the transplanted material would treat, mitigate, cure or prevent disease thus rendering the material a drug having to go through the IND process)20 would require clinical studies to demonstrate the health benefit, thus limiting the use of the definition to MTs with established clinical effectiveness. Others suggested that the definition should not include therapeutic intent, a hallmark characteristic of products regulated as drugs or biologics by FDA.
In written comments reacting to a definition that included reference to conferring a health benefit, working group members expressed different opinions. Those against inclusion of language referring to a health benefit noted that “there are numerous cosmetic applications for a microbiota transplant that would not be covered under a definition that included “health benefit” and that “if the definition includes the concept of conferring a health benefit it will not include ‘the still experimental’ for which there is not yet evidence of efficacy.” One participant who recommended against inclusion of health benefit language noted, “[s]uppose I want to say ‘This microbiota transplant failed to confer a health benefit.’ By definition, I would be unable to do so if conferring a health benefit is part of the definition of microbiota transplant.”
Some working group participants felt strongly that health benefit language should be included and noted the following:
The transplant is always going from a healthy human donor to a sick human recipient. Whether the definition references a “health benefit” or not, the reality is that's the only reason FMT gets done and ignoring that feels incomplete.
Clinicians should not perform a procedure, without the expectation that in so doing the benefits of the procedure and/or product outweigh their risks. Therefore, it is implicit that a microbiota transplantation would be administered for the clinical benefit of a patient with an acceptable risk of both the product and the procedure. It would be unethical to administer a microbiota transplant without the expectation of a clinical benefit that outweighs the known and potential risks.
Many agreed that MT will almost always be used to confer a health benefit, but others reiterated their concern that stating so explicitly in the definition will raise regulatory issues that could limit the use of MT prematurely from a regulatory perspective, i.e., could push it permanently into the drug/biologic category. Ultimately, the group agreed to omit the concept of a health benefit and refer instead to the transplant affecting the recipient's microbiota.
A separate definitional issue was raised regarding whether the definition as written would cover autologous transplantations, i.e., transplanting material derived from the same individual as might happen if a person banked their own stool before surgery to treat potential sequelae of the surgery or hospital stay. Participants agreed that autologous use should explicitly be included in the definition because it presents a unique set of regulatory issues. Autologous MTs in which biologic material is removed and reintroduced to an individual without manipulation of the microbiota may be the safest form of MT, while an autologous MT that included some form of microbiota manipulation before reintroduction could raise many of the same regulatory (safety and efficacy) issues as donor material.
Based on the considerations above, the Working Group adopted the following definition of MT:
A microbiota transplantation is the transfer of biologic material containing a minimally manipulated** community of microorganisms from a human donor to a human recipient (including autologous use) with the intent of affecting the microbiota of the recipient.
Conclusion
The current array of stool based products/procedures being used, or under development, to treat patients with CDI and other conditions calls for the establishment of a definition which will help determine which among them should be “labeled” FMT. Such a definition would arguably be a useful consideration in the development of regulations governing their use. While the definition and other considerations discussed in this Commentary do not lead to a “bright line” rule cutting across the spectrum of stool-based products delineated in Figure 1, they do highlight potential dividing lines where to the left are products/procedures that would be considered FMTs and to the right are products that would be other types of live biotherapeutic products. While this paper has focused on FMT, the proposed definition was developed to cover other types of MTs being contemplated, including vaginal, skin, nares, and oral. A regulatory definition that defines the scope of MT generally will be useful as microbiome-based treatments are developed for other human microbiome sites beyond the gut.
Footnotes
A list of working group members can be found at the following link: https://www.law.umaryland.edu/programs/health/events/microbiota/documents/WG1_members.pdf
Minimal manipulation is processing that does not alter the original relevant characteristics of the transferred community of microorganisms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health.
Funding
Support for this article was provided by Grant Number: NIAID R21 AI119633–01 from the National Institute for Allergies and Infectious Diseases.
Reference
- [1].United States Food and Drug Administration. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies: draft guidance for industry. 2016 Mar [accessed 2016 Sept 14]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM488223.pdf.
- [2]. United States Food and Drug Administration. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. 2013 July [accessed 2016 Sept 14]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM361393.pdf.
- [3]. United States Food and Drug Administration. Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. 2014 Mar [accessed 2016 Sept 14]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM387255.pdf.
- [4]. Sachs R, Edelstein C, Ensuring the safe and effective FDA regulation of fecal microbiota transplantation. J Law Biosci. 2015; 2(2):396–415; PMID:27774199; http://dx.doi.org/10.1093/jlb/lsv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Petrof E, Khoruts A, From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014; 146(6):1573–1582; http://dx.doi.org/10.1053/j.gastro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Rebiotix Frequently asked questions. [accessed 2016 Sept 14]. http://www.rebiotix.com/news-media/rebiotix-faqs/. [Google Scholar]
- [7]. The Power of Poop Innovation. [accessed 2016 Sept 14]. http://thepowerofpoop.com/resources/innovation/. [Google Scholar]
- [8]. Borody T, Fischer M, Mitchell S, Campbell J. Fecal microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev Gastroenterol Hepatol 2015; 9(11):1375–1391; http://dx.doi.org/10.1586/17474124.2015.1086267. [DOI] [PubMed] [Google Scholar]
- [9]. biotechPrimer Blunt approach: fecal transplants. [accessed 2016 Sept 14]. http://biotechprimer.com/blunt-approach-fecal-transplants/. [Google Scholar]
- [10]. Seres Therapeutics Clostridium difficile infection (CDI). [accessed 2016 Sept 14]. http://www.serestherapeutics.com/pipeline/ser-109. [Google Scholar]
- [11]. BusinessWire Seres Therapeutics announces interim results from SER-109 Phase 2 ECOSPOR study in multiply recurrent Clostridium difficile infection. 2016 July 29 [accessed 2016 Sept 14]. http://www.businesswire.com/news/home/20160729005385/en/Seres-Therapeutics-Announces-Interim-Results-SER-109-Phase.
- [12]. Petrof E, Globor G, Vanner S, Weese S, Carter D, Daigneault M, Brown E, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013; 1(1):3; PMID:24467987; http://dx.doi.org/10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Olle B. Medicines from microbiota. Nat Biotechnol 2013; 31(4):309–315; PMID:23563425; http://dx.doi.org/10.1038/nbt.2548. [DOI] [PubMed] [Google Scholar]
- [14]. Maldonado-Gomez M, Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host & Microbe Host & Microbe 2016; 20, 515–526; http://dx.doi.org/10.1016/j.chom.2016.09.001. [DOI] [PubMed] [Google Scholar]
- [15]. United States Food and Drug Administration FDA Basics: what is a dietary supplement? [accessed 2016 Sept 14]. http://www.fda.gov/AboutFDA/Transparency/Basics/ucm195635.htm. [Google Scholar]
- [16]. NIAID Grant R21 AI119633-01, “Microbiota Transplantation: Recommendations for a Regulatory Framework,” Principal Investigator: Diane Hoffmann.
- [17]. United States Food and Drug Administration Draft guidance for industry and Food and Drug Administration staff: minimal manipulation of human cells, tissues, and cellular and tissue-based products. 2014 Dec. [accessed 2016 Sept 14]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM427746.pdf. [Google Scholar]
- [18]. United States Food and Drug Administration Guidance for industry: early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information. 2012 Feb, updated 2016 Jun [accessed 2016 Sept 14]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/General/UCM292704.pdf. [Google Scholar]
- [19]. Hoffmann D, Fraser C, Palumbo F, Ravel J, Rowthorn V, Schwartz J. Probiotics: achieving a better regulatory fit. Food Drug Law J 2014; 69(2):237–272; PMID:25163211 [PMC free article] [PubMed] [Google Scholar]
- [20]. FDCA , Pub. L. No. 75–717, 52 Stat. 1040 (codified at 21 U.S.C. § 321(g)). [Google Scholar]


