ABSTRACT
The history of fecal microbiota transplantation (FMT) dates back even to ancient China. Recently, scientific studies have been looking into FMT as a promising treatment of various diseases, while in the process teaching us about the interaction between the human host and its resident microbial communities. Current research focuses mainly on Clostridium difficile infections, however interest is rising in other areas such as inflammatory bowel disease (IBD) and the metabolic syndrome.
With regard to the latter, the intestinal microbiota might be causally related to the progression of insulin resistance and diabetes. FMT in metabolic syndrome has proven to be an intriguing method to study the role of the gut microbiota and open the way to new therapies by dissecting in whom insulin resistance is driven by microbiota. In this article we review the history of FMT, the present evidence on its role in the pathophysiology of metabolic syndrome and its efficacy, limitations and future prospects.
KEYWORDS: microbiota, fecal transplantation, metabolic syndrome
The history of fecal microbiota transplantation
The recent scientific upsurge in the field of gut microbiota has firmly established its role in contemporary clinical medicine. However, the history of fecal infusions in medicine is much longer. Today we know that fecal microbiota transplantation (FMT) or ‘bacteriotherapy’ may also transfer host phenotype. This section will provide a historical overview of FMT indications and applications, and how it has evolved into its current use.
Historical applications of fecal transplantation (300 AD –1950 AD)
The first records of fecal transplantation date back to 4th century China, where “yellow soup” was applied in cases of severe food poisoning and diarrhea.1 Subsequent records reverently speak of “golden syrup.”2 By the 16th century, the Chinese had developed a variety of feces-derived products for gastrointestinal complaints as well as systemic symptoms such as fever and pain.1 Meanwhile, Bedouin groups were said to have consumed the stools of their camels as a remedy for bacterial dysentery.3 Italian anatomist and surgeon Acquapendente (1537–1619) further extended this to a concept he coined “transfaunation,” the transfer of gastrointestinal content from a healthy to a sick animal, which has since been applied extensively in the field of veterinary medicine.4 Interestingly, many animal species are found to naturally practice coprophagia, leading to a greater diversity of microorganisms in their intestines, enabling them to digest a greater number of food sources.5
Slowly these ideas began to spark interest in 18th century European physicians. German born Christian Paullini (1643–1712) was the first to outline the therapeutic potential of human excretions in his work the “Heilsame Dreck-Apotheke” (literally: healing mud pharmacy).4 The fundamental discovery by Antoni van Leeuwenhoek that his stool contained microbes – “God's smallest creatures”6 – as well as observations from the Russian zoologist Metchnikoff (1845–1916), laid an early foundation for the modern field of microbiota study.7 Inspired by the reports of longevity in Bulgarian farmers despite their poor living conditions, Metchnikoff introduced fermented products in his diet and noted improvements in his general health. He hypothesized this to be due to an altered balance in colonic microbes, with an increase in lactic acid bacteria (still called “Lactobacillus bulgaricus”) protecting against senescence-accelerating toxins. Metchnikoff's bacteria captured public interest and were successfully marketed during his lifetime. His concept of increasing the number of beneficial microbes in the gut in an attempt to improve human health, clearly shows a historic use of probiotics avant la lettre. In a similar light, German bacteriologist and physician Alfred Nissle isolated an Escherichia coli strain that to date bears his name. Initially, the microorganism was found protective against Shigella outgrowth and subsequent gastroenteritis, but its impact in human health was later extended to include chronic inflammatory conditions.8
Soon gut bacteria would also be found valuable in recovering from infectious gastroenteritis. When German soldiers of the ‘Afrikakorps’ were dying of locally contracted dysentery in the early 1940s, Nazi scientists were determined to find a cause and cure. Observations of better-faring locals who would take in fresh camel stools upon the first signs of illness led them to analyze the feces and isolate Bacillus subtilis. Subsequent culturing and administration of the bacterium resolved the disease in many.9
By now, the numerous examples of microbes influencing human health have set the stage for a more thorough examination of its applications, and the first controlled studies using therapeutic fecal suspensions have emerged.
FMT in modern science (1950s-2000)
Antibiotics, since their discovery generously prescribed, have ended an era in which infectious diseases were the most common cause of death. But antibiotics also came with side-effects and antimicrobial resistance. In an attempt to ameliorate collateral damage on commensal microbes, bacteriologist Stanley Falkow sampled fecal material from surgical patients before starting them on pre-procedural antibiotics (Fig. 1).10 After converting the stools into pill form, he prescribed their daily intake to half of the group during post-surgical recovery, an idea so repelling at the time it got him fired when the administrative board found out. Anecdotal evidence from this study in the early 1950s describes better outcomes in the treatment group, but the data of the unofficial ‘Ersatz’10 trial were never published.
Figure 1.
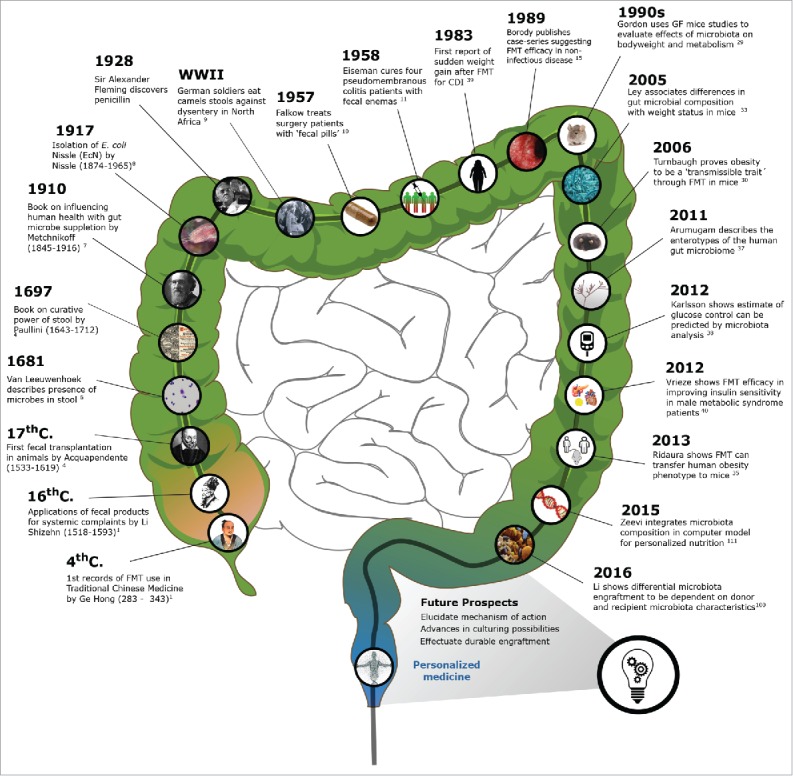
Timeline: Key contributions to FMT development and research.
In the succeeding year, a group of Colorado-based surgeons led by Dr. Eiseman performed an experiment along the same rationale: that restoration of a healthy gut microbial balance can improve patients' health. After numerous other therapies had failed to effectuate recovery, they treated 4 patients critically ill with pseudomembranous colitis with fecal enemas from healthy donors. Results were impressive, with rapid and complete recovery in all subjects.11 In the following 2 decades, 16 more cases were selected to undergo the same procedure. The attained 94% success rate despite the poor prognosis in this therapy-refractory patient group proved promising.12
By this time, the cause of this potentially life-threatening condition had been identified as infection by the gram-positive anaerobe spore forming bacterium Clostridium difficile, often provoked by antibiotic use.13 Because of clinical similarities between infectious and non-infectious types of colitis, physicians started to speculate whether FMT could also be of help in inflammatory bowel disease and irritable bowel syndrome (IBD and IBS).14 The earliest record of FMT for a non-infectious disease concerns a 45-year old male having refractory ulcerative colitis (UC), showing full and lasting clinical recovery upon “an exchange of bowel flora.”15 Numerous subsequent case studies focused on a multitude of gastrointestinal complaints.15-17 In the majority of cases, patients were found to “[positively] respond to manipulation”17 of their microbiome following the colonic administration of a mixture of microflora from unaffected individuals.
By the turn of the century, new insights connecting gut microbes to the pathophysiology of extra-intestinal diseases would broaden the applications of FMT.
FMT in extra-intestinal disease (2000-current)
Over 700 patients are reported to have been treated with FMT for recurrent Clostridium difficile infection (CDI) since Eiseman's experiment.4 Three recent randomized controlled trials have reported cure rates of 90% or higher.18-20 In general, the treatment effect is lasting,21,22 and safe, with no related side-effects or newly acquired medical conditions during follow-up, even when performed in vulnerable patient groups.18,21,23-25
As the burden shifted from infectious to non-communicable disorders, the range of FMT applications extended. Recent case-reports even include incidental findings of post-FMT remission in extra-gastrointestinal conditions like multiple sclerosis,14 Parkinson,26 idiopathic thrombocytopenic purpura14 and chronic fatigue.14 Another microbiota-associated disease is kwashiorkor (severe malnutrition), which Smith and colleagues studied in 2013 in children in Malawi. They investigated 317 twin pairs for 3 y (from birth until 3 y of age). During these 3 years, 50% of the twin pairs stayed well nourished, but 7% manifested concordance for acute malnutrition and 43% became discordant for malnutrition.27 Hereafter, they transplanted fecal microbiota from discordant pairs into germ-free mice. Mice that received gut microbiota from kwashiorkor children showed significant weight loss, along with dysregulation of carbohydrate and amino acid metabolism.28
Strategies attempting to tackle the other end of the metabolic spectrum, the obesity epidemic, have failed to generate satisfactory results.29,30 However, much recent animal and human evidence points toward a possible role for gut microbiome manipulation in reestablishing energy homeostasis. The following section will briefly review how the microbiome has become connected to the pathophysiology of metabolic syndrome and how FMT may aid future therapy.
Animal FMT studies in metabolic syndrome
Experimental evidence in animals connecting the intestinal microbiota to the metabolic syndrome is widely available. Owing to the creation of germ-free (GF) mice by prof. Jeffrey Gordon and technological advances in sequencing methods, scientists were able to prove causality of microbial involvement in weight management and glucose and lipid metabolism.
For example, Bäckhed and colleagues induced weight gain and increased insulin resistance in GF (C56BL/6) mice upon oral administration of fecal material from their conventional counterparts, despite a simultaneous reduction of food intake.31 Researchers attributed this to a more effective carbohydrate uptake (and subsequent lipolysis leading to increased body fat content) due to processing of nutrients by the microorganisms present.31 Conventionalization of GF mice also amplified the weight gain after putting these mice on a Western or high-fat diet (HFD).29,32 Next, the recipient GF mice of feces from obese (ob/ob) donor mice put on significantly more weight compared with recipients of feces from lean donor mice.30
Ley et al. (2005) went on to show that under similar dietary circumstances, ob/ob mice carry significantly less Bacteroidetes and more Firmicutes in their guts compared with lean ob/+ and wild-type mice.33
A recent study with Sprague-Dawley rats confirmed this microbial signature, where fecal transplants from rats on a control diet restored a rise in plasma fatty acid and glucose intolerance after fructose-induced metabolic syndrome.34 Interestingly, glucose tolerance improved significantly through the administration of an antibiotic mix of ampicillin and neomycin. Ridaura and colleagues (2013) were the first to transplant human feces into GF (C57BL/6J) mice and confirmed increased weight gain upon transfer of fecal material derived from an obese adult compared with that of her lean twin.35 Moreover, co-housing the obese sample recipients with lean-donor animals showed a decrease of obese phenotype acquisition.
Finally, a recent study by Liou et al. (2013) suggests that the positive metabolic effects of Roux-en-Y gastric bypass (RYGB) surgery is partly be due to an altered composition of the gut microbiota.36 They have performed FMT in GF-mice from patients that had undergone RYGB surgery. This procedure resulted in significant loss of weight and fat mass compared with the GF-mice that had received gut microbiota from mice that had undergone a sham procedure. Data validating these effects in humans are eagerly awaited.
FMT in human metabolic syndrome
In the same period, further emphasis was placed on the role of gut microbiota in metabolic disease in humans and on human microbiota variation. Of note, Arumugam described different human enterotypes37 and Karlsson showed that analysis gut microbiota composition can predict metabolic status.38
Interestingly, the first record of sudden weight gain following FMT dates from 1983, as an incidental finding after resolution of recurrent CDI in an adult female patient. Another such case of “new-onset obesity” was published recently, warranting caution in considering the use of obese donor material for fecal transplantations.39
Concerning the treatment of metabolic syndrome with FMT, the only human study to date was performed by Vrieze et al. (2012), which suggests that FMT from lean unaffected donors temporarily increases peripheral insulin sensitivity (with a similar yet not statistically significant trend toward improved hepatic insulin resistance).40 In line with results obtained from the animal models, these changes were found to be positively correlated with an increase in the number of butyrate-producing bacteria in the gut.
In conclusion, previous findings suggest a causal relation between the gut microbiota and metabolic syndrome, but the (patho)physiologic pathways remain to be elucidated. In the next section we will first discuss the pathophysiology of metabolic syndrome and then the role that gut microbiota may play.
The role of gut microbiota in the pathophysiology of the metabolic syndrome
The prevalence of metabolic syndrome and its sequelae has risen to epidemic proportions to become one of the most pressing global health problems of our time. The metabolic syndrome is a cluster of symptoms, defined by insulin resistance, dyslipidemia, high blood pressure and increased abdominal girth, which are strongly associated with the development of type 2 diabetes and cardiovascular disease. The hepatic manifestation of metabolic syndrome, though not part of its criteria, is non-alcoholic fatty liver disease (NAFLD) and its vascular manifestation is atherosclerosis.
The historical overview has explained how we came to use FMT in the context of metabolic syndrome. In this section we will first address the pathophysiology of the metabolic syndrome in general with the role of low-grade inflammatory changes particular and then continue by focusing on the role of the gut microbiota and and the possible mechanisms through which they influence host metabolism.
Inflammation and insulin resistance
Studies in the early 20th century already showed that high-dose salicylates attenuate glycosuria in patients, providing early clues that insulin resistance is secondary to inflammation. This was largely ignored until the 1990s, when it was shown that insulin resistance can be provoked by pro-inflammatory cytokines such as tumor necrosis factor α (TNFα).41 A review by Shoelson et al. in 2006 provides an excellent summary on the inflammatory mechanisms of insulin resistance.42 One important pathway is through c-Jun amino-terminal kinases (JNK), activated by inflammatory cytokines and free fatty acids. JNK is increased in obese individuals and absence of JNK results in improved insulin sensitivity.43 Inflammatory pathways (such as JNK and NF-κB) are also induced by obesity-related endoplasmic reticulum (ER) stress.44 JNK changes the function of insulin receptor substrate 1 (IRS-1), which regulates insulin and insulin-like growth factor pathways.45 These pathways play an important role in age-related disease and life span in animal models.46,47 Also, inflammation goes hand-in-hand with lipid accumulation in the vessel wall as an early sign of atherosclerosis, demonstrating the link between lipid accumulation, atherosclerosis and inflammation as hallmark of the metabolic syndrome.42
Another important mechanism that plays a role in insulin sensitivity and obesity is mediated through nuclear peroxisome proliferator-activated receptors (PPARs), of which PPARγ is best characterized. PPARγ is expressed in many tissues including liver, muscle and adipose tissue and its activation attenuates hyperglycemia and hyperlipidemia by regulation of metabolic genes.48 Thiazolidinediones, a class of synthetic oral glucose lowering drugs, are strong agonists of PPARγ. Macrophages highly express PPARγ, further intertwining inflammation and insulin resistance.48 In vitro studies have shown that statins also induce PPARγ-mediated transcriptional activity in macrophages, beside other anti-inflammatory effects like inhibiting lipopolysaccharide-induced TNFα transcription and the NF-κB pathway,49 implying the effects of statins in metabolic syndrome may in fact be partly anti-inflammatory and mediated through PPARy.
In conclusion, there is a firm base of evidence linking the spectrum of metabolic dysfunction seen in metabolic syndrome to inflammation. In the next paragraph we present evidence that this inflammation may derive from the gut.
“Leaky gut”
In the last decades, a solid base of evidence has linked the inflammatory state in metabolic syndrome to impaired gut barrier function and leakage of bacteria and/or bacterial components into the system.50,51 Resulting low grade endotoxemia (and possibly bacteremia) chronically activates inflammatory pathways.52 Bacterial components may also migrate to target organs,53,54 leading to an influx of macrophages that contribute to local as well as systemic low grade inflammation and insulin resistance.52 The intestinal barrier consists of many components each contributing to its function in unique ways: an epithelial lining conjoined by junction proteins, thick (‘outer’ and ‘inner’) mucus layers, a bacteria deterring glycocalix,55 luminal immunoactive components such as IgA, cytokines and mast cell proteases and gut-associated lymphoid tissue trained to discriminate commensals from pathogens.56 Malfunction of any component can be described as impaired gut barrier function and may or may not lead to bacterial translocation depending on the defect. These different components of the gut barrier can be assessed in different indirect ways, each with its own limitations, as elegantly reviewed by Grootjans et al.57 Several clues that the gut becomes permeable to bacteria in metabolic syndrome have been uncovered, but it should be noted that direct evidence of actual live bacterial translocation in humans in the context of metabolic syndrome has not yet been delivered. The first studies linking gut barrier disruption to metabolic derangement have shown increased paracellular transport and impaired tight junction function using oral ingestion of substances, such as lactulose and mannitol, sucralose, polyethylene glycols (PEG) or 51Cr-EDTA and measuring their urinary excretion.58 It is unlikely however that large molecules, let alone bacteria, are transported paracellularly.59 In contrast, bacteria may enter the body by endocytosis, which has been observed in the absence of tight junctional damage,59 when the epithelial cell layer is compromised by apoptosis, cell damage, during physiologic cell shedding, or when the mucus layer is impaired. Incidentally, a recent study emphasized the importance of dietary fibers in maintaining the mucus layer by showing that fiber-deprived microbiota use the colonic mucus layer as an alternative food source.60
A different approach to demonstrate impaired intestinal barrier function is by looking for bacterial signatures in the circulation. Many diseases, including metabolic syndrome, have been linked to endotoxemia, i.e. the presence of lipopolysaccharide (LPS) in the blood, a supposed proxy for translocation of gram-negative bacteria. LPS infusion in rodents showed an increase in insulin resistance to a similar extent as a high-fat diet.61 However, in humans this is still not proven due to reliability issues of endotoxin assays, conflicting study outcomes and the question whether LPS found in metabolic syndrome subjects is actually bioactive.62 Also, transportation of endotoxin and bacteria in metabolic syndrome occurs mainly by route of the lymphatic system and the portal vein.63,64 In both cases, it will first pass a target organ (the liver or via the thoracic lymph duct and the arterial circulation to a different tissue), before it can be measured in a venous blood sample, complicating adequate measurement in humans.
Finally, data in various disease states support the hypothesis of microbiota translocation to the blood stream51 and target organs such as the vessel wall in atherosclerosis,53 the liver in non-alcoholic steatohepatitis65 and mesenteric adipose tissue in inflammatory bowel disease.54 It would be very interesting to investigate whether presence of gut bacteria can also be demonstrated in the blood, the mesenteric lymph nodes or other peripheral tissues of human metabolic syndrome subjects, e.g. by taking tissue samples during elective surgery.
Ways in which the gut microbiota contribute to the metabolic syndrome
We have already described the inflammatory mechanisms through which gut microbes might affect host metabolism. Another important mechanism is the production of signaling molecules by gut microbes. These can affect gut integrity, the immune system and satiety and may impact host metabolic phenotype. Short-chain fatty acids (SCFAs) provide the most well-studied example. SCFAs, such as acetate, propionate and butyrate, are small molecules metabolized by gut microbes from dietary fibers, for which receptors are ubiquitous in the body, resulting in numerous complex effects, reviewed by Den Besten et al. in 201366 and by Canfora et al.67 in 2015 in more detail. Their effects include upregulation of tight junction proteins such as claudin-1 (butyrate),68 epigenetic regulation of immune cells through HDAC-inhibition (butyrate),69 altering of intestinal gluconeogenesis,70 increasing plasma incretin hormones, reduction of TNFα (acetate)71,66 and changing lipid and glucose metabolism (propionate, butyrate).72,73
Bile acids (BA) are another class of molecules that play a role in microbiota-host communication, mediated by nuclear receptor farnesoid X receptor (FXR). This interaction seems an important determinant of metabolic health, as FXR-deficient mice that are fed a high-fat diet or those that are genetically predisposed toward obesity have better glucose regulation than control mice with normally functioning FXR.74 Evidence from animal studies is solid, but human studies on this topic are still lacking. Current evidence on microbiota-BA interaction and the receptors involved was well-reviewed recently by Wahlström et al.74
Finally, metagenomic studies in humans have identified various specific gut microbiota changes in individuals with metabolic syndrome38 including malevolent microbes that contribute to insulin resistance, such as Prevotella copri and Bacteroides vulgatus,75 but also beneficial species such as Akkermansia municiphila,76,77 and Faecalibacterium prausnitzii,78 which are associated with increased insulin sensitivity. Of note, a newly discovered mechanism of action of the widely used glucose lowering drug metformin was shown in diet-induced obese mice, where it improved glucose homeostasis by increasing the population of Akkermansia species.76 Several other examples of specific interactions of gut microbes with the host are discussed in the following paragraphs by reviewing the literature on the involvement of gut microbes in several other aspects of the metabolic syndrome: atherosclerosis, hepatic steatosis and elevated blood pressure.
Microbiota and atherosclerosis
The connection between gut microbiota and atherosclerosis was already described in 1999, when endotoxin levels, following bacterial translocation, were found to be independently correlated with cardiovascular outcome and carotid atherosclerosis measured by duplex ultrasound.79 Traditional risk factors explain about half of the atherosclerotic burden in linear regression. Genetics are believed to explain another 10 percent. Microbiota and their many metabolic products may largely account for the rest.80 For example, DNA of oral microbiota Veillonella and Streptococcus was found in plaques of individuals with atherosclerosis and their abundance correlated with the abundance of these species in the oral cavity.53 As for gut microbiota, Karlsson et al. found in 2012 that atherosclerosis is associated with a different gut metagenome.81 One mechanism of microbiota-mediated atherosclerosis induction that has been elucidated is through L-carnitine and phosphatidylcholine (from red meat, cheese and eggs). These food components are first converted by the microbiota to TMA, then by the liver into TMAO, which increases atherosclerotic burden82 and promotes a prothrombotic phenotype.83 Microbiota can also protect from atherosclerosis, as recently shown when Akkermansia municiphila reversed Western diet-induced atherosclerosis and endotoxemia in ApoE-knockout mice.77 Another recent study in ApoE-KO mice showed that probiotic mixture VSL#3 can protect from atherosclerosis.84 Furthermore, germ free mice showed attenuation of vascular leukocyte infiltration and adhesion and of monocyte attractant protein MCP-1 and proinflammatory cytokine IL-17 in response to an angiotensin II challenge.85
Microbiota and non-alcoholic fatty liver disease and -steatohepatitis
Several human studies show microbiota differences in people with non-alcoholic steatohepatitis (NASH)86 or non-alcoholic fatty liver disease (NAFLD),87,88 compared with healthy controls. Also, people with NALFD have increased LPS-binding peptide levels in the plasma.89 In individuals with NASH levels are even higher and correlate to TNF-α mRNA expression in liver tissue, pointing toward a role for endotoxemia in inflammatory liver steatosis.89 Furthermore, apart from increased endotoxemia, people with NAFLD have increased intestinal permeability, which correlates with bacterial overgrowth in the small intestine and severity of steatosis.90 No human studies on FMT in NASH or NAFLD have been performed yet, but in mice it has been shown that antibiotics can reduce portal endotoxin levels and hepatic accumulation of lipids.91 Furthermore, adding Bifidobacterium pseudocatenulatum to a high-fat diet (HFD) decreased insulin resistance and liver steatosis when compared with only a HFD in C57BL-6 mice.92 Also, microbiota transfer of inflammasome-deficient mice to wild-type mice exacerbated hepatic steatosis and portal endotoxin influx.93 In line, microbiota transfer from a mouse with a HFD-induced phenotype of insulin-resistance and inflammation reproduced this phenotype in recipient mice, whereas the microbiota from a mouse fed the similar HFD that did not develop insulin resistance or inflammation failed to do so.94 These studies all show microbiota-mediated effects on liver phenotype in mice. A human intervention trial, albeit not placebo controlled, showed a decrease in intrahepatic triglyceride content measured by magnetic resonance spectroscopy upon addition of probiotics to usual care.95 Finally, since decades, increased intestinal permeability has been linked to alcohol (ab)use.96 Recently however, studies point toward a role for ethanol-producing microbes in the etiology of NASH and NAFLD,97 bridging alcoholic and ‘non-alcoholic’ causes of steatohepatitis through the microbiome. A randomized clinical trial is currently being performed at our department to see if the effects of fecal microbiota transfer from healthy donors can attenuate NAFLD and NASH severity (Dutch trial register, http://www.trialregister.nl/NTR4339).
Microbiota and blood pressure
Elevated blood pressure is a common feature of metabolic syndrome, but literature on gut microbial involvement is still modest. Several interesting discoveries have been made that suggest a more direct influence than merely through an increase of atherosclerotic burden. First of all, a decreased microbiota diversity and a decreased Firmicutes/Bacteroidetes ratio in humans and in 2 rat models for hypertension was found. More importantly, the blood pressure in these rat models could be corrected by increasing the Firmicutes/Bacteroidetes ratio with antibiotics.98
A possible mechanism through which alterations in the gut microbiota can induce hypertension is through SCFAs, as it was recently found that SCFA-receptor olfactory receptor 78 (Olfr78) is expressed in the kidney and mediates renin production in response to propionate.99 Moreover, this receptor and G protein-coupled receptor 41 (GRP41) were found in the smooth muscle cells of blood vessels, where they increase blood pressure in response to microbiota-derived SCFAs.99
Future perspectives: Microbiota as therapy
Now that we have outlined current evidence on gut microbial involvement in the metabolic syndrome, we will speculate in this final section on the therapeutic possibilities offered by this promising new field of gut microbiota.
Although only one small placebo-controlled RCT has altered the gut microbiota through FMT and showed causality in the interaction between gut microbiota and metabolic syndrome in humans,40 many studies have shown that gut microbiota play a role in all aspects of the metabolic syndrome, including insulin resistance, dyslipidemia, atherosclerosis, hepatic steatosis and elevated blood pressure. Now is the time to further investigate these claims using well-designed randomized placebo-controlled studies featuring FMT that focus on unraveling the mechanisms through which gut bacteria interact with host metabolism, by monitoring microbiota changes and engraftment of beneficial and pathogenic bacterial strains in the intestinal microbiome over time,100 recording changes in metabolites (e.g., short-chain fatty acids, bile acids, incretin hormones), investigating genetic and epigenetic effects (e.g., on the immune system), all while taking dietary habits into account, to ultimately develop novel, more attractive and personalized strategies to manipulate the microbiota.101
As mentioned earlier, the most widely accepted explanation for the origin of metabolic syndrome is translocation of endotoxin (LPS) or direct translocation of gram-negative microbes, causing low grade inflammation. However, data on this phenomenon are still inconclusive. Current research should look extensively into this topic, since it is a key component that will lead to insight and hopefully effective treatment modalities in the future.
Furthermore, until recently the majority of intestinal microbes could not be cultured. However recent endeavors in culturing these mostly anaerobic microbiota proved successful and challenged the notion that most microbiota is unculturable.102 Browne and colleagues used fresh fecal samples to test a novel method based on targeted phenotypic culturing, using broad-range growth medium called YCFA. Overall, 137 distinct species were isolated, 90 of which were on the Human Microbiome Project's “most wanted” list of previously uncultured and unsequenced microbes.102 These inspiring results will hopefully lead to further insights on the function and interactions between various gut microbes and help improve understanding which cases will respond to FMT and which will not, which remains a vital question. Moreover, it is not only the question which microbes should be infused but also how many species or strains are needed to alter the gut microbiota effectively.103 Studies to date have mainly been limited to genus- and species-comparisons and have not clarified to which extent donor microbiota colonizes a recipient.104 Previous findings in non-FMT settings found that newly introduced (non-pathological) strains are not able to persevere in an established gut ecosystem, particularly if the species were already present.105,106 We have recently found that effective colonization by donor fecal bacteria (engraftment) is partly driven by the gut microbial composition of the recipient and differs between metabolic syndrome subjects.104 The next step will be a more precise manipulation of the gut microbiota, for example by introducing specific microbes to outcompete undesirable strains.
Finally, FMT has logistical challenges and is associated with several risks (e.g., infections), undesirable outcomes (e.g., increased risk of microbiota-associated diseases, such as new-onset obesity) and distaste by the recipients and doctors, the so called ‘yuck’factor.107 Isolating specific bacterial strains for the production of novel probiotics could be a safer and more elegant treatment.108 This process raises 2 major challenges that preclude rapid translation into clinical practice. First of all, regulation of FMT can be a major hurdle. The US Food and Drug Administration (FDA) has determined that FMT constitutes a biologic product and drug and therefore maintained that the FMT process (e.g., donor eligibility, screening and stool processing) falls under FDA jurisdiction.109 Because safety and efficacy has not yet been proven with large RCTs, investigational new drug application (IND) is required for fecal transplantation in the US. In Europe however, FMT has no such status, which makes it easier to use FMT in clinical trials while at the same time studying pathophysiological mechanisms. In this phase we deem it critical to characterize donors in detail, constantly adjust donor screening to the most recent insights and to closely monitor long-term effects in recipients. After beneficial strains have identified using FMT, another hurdle concerns the culturing and storage of therapeutically interesting bacterial strains under good manufacturing practice, which are 2 essential, but also time consuming factors.108
In conclusion, FMT will allow us to study the pathophysiology of metabolic syndrome and help us to identify novel therapeutic targets. In this endeavor, we should study specific host genetic, epigenetic, dietary and microbial characteristics to be able to predict therapeutic efficacy of these novel nutritional, pre-, pro- and post-biotic interventions,110,111 which will enable us to modulate disease phenotype through manipulation of the gut microbiota using a personalized approach.110
Disclosure of potential conflicts of interest
M. Nieuwdorp is on the Scientific Advisory Board of Caelus Health.
References
- [1].Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol [Internet] 2012. [cited 2015February13]; 107:1755; author reply p.1755-p.6. Available from: http://dx.doi.org/ 10.1038/ajg.2012.251; PMID:23160295; http://dx.doi.org/ 10.1038/ajg.2012.251 [DOI] [PubMed] [Google Scholar]
- [2].Gerke H. “Fecal Transplantation.” Health at Iowa. University of Iowa Health Care [Internet] 2014; Available from: http://medcom.uiowa.edu/health/fecal-transplantation/ [Google Scholar]
- [3].Uwlfoh SS, Dgglwlrqdo RU, Derxw L, Duwlfoh W, Lewin RA. More on Merde. 2016 [Google Scholar]
- [4].Tetro JA. Applications of Fecal Microbiota Transplantation - The Human Microbiome Handbook. 2016 [Google Scholar]
- [5].Merde: Excursions in Scientific, Cultural, and Socio-Historical Coprology: Ralph A. Lewin: 9780812992519: Amazon.com: Books [Internet]. [cited 2015February15]; Available from: http://www.amazon.com/Merde-Excursions-Scientific-Socio-Historical-Coprology/dp/0812992512 [Google Scholar]
- [6].Shanahan F. The Ungloved Gut Hektoen International - A Journal of Medical Humanities - Hektoen Institute of Medicine. 2016 [Google Scholar]
- [7].Anukam K, Reid G. Probiotics: 100 years ( 1907–2007 ) after Elie Metchnikoff's Observation. Commun Curr Res Educ Top trends Appl Microbiol 2007; 466-74 [Google Scholar]
- [8].Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis [Internet] 2008. [cited 2017January9]; 14:1012-8. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00054725-200807000-00017; PMID:18240278; http://dx.doi.org/ 10.1002/ibd.20377 [DOI] [PubMed] [Google Scholar]
- [9].DeSalle R. The Great Camel Dung Mystery - welcome to the microbiome. 2015 [Google Scholar]
- [10].Yong E. Sham Poo Washes Out - The Atlantic - Atlantic Media Company. 2016 [Google Scholar]
- [11].Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery [Internet] 1958. [cited 2016September16]; 44:854-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13592638 PMID: 135926387224366 [PubMed] [Google Scholar]
- [12].Bowden TA, Mansberger AR, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg [Internet] 1981. [cited 2016September16]; 47:178-83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7224366; PMID:7224366 [PubMed] [Google Scholar]
- [13].Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med [Internet] 1978. [cited 2016September16]; 298:531-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/625309; PMID:625309; http://dx.doi.org/ 10.1056/NEJM197803092981003 [DOI] [PubMed] [Google Scholar]
- [14].Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr Gastroenterol Rep 2013; 15:1-7; http://dx.doi.org/ 10.1007/s11894-013-0337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust [Internet] 1989. [cited 2016September12]; 150:604. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2783214; PMID:2783214 [DOI] [PubMed] [Google Scholar]
- [16].Bennet J, Brinkman M. Treatment of Ulcerative Colitis By Implantation of Normal Colonic Flora. Lancet 1989; 333:164; http://dx.doi.org/ 10.1016/S0140-6736(89)91183-5 [DOI] [PubMed] [Google Scholar]
- [17].Andrews PJ, Borody TJ. “Putting back the bugs:” Bacterial treatment relieves chronic constipation and symptoms of irritable bowel syndrome [7].Med. J Aust 1993; 159:633-4 [DOI] [PubMed] [Google Scholar]
- [18].Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al.. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: A randomized trial. Ann Intern Med 2016; 165(9):609-616:1–9; PMID:27547925; http://dx.doi.org/ 10.7326/M16-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, Sanguinetti M, Gasbarrini A. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41(9):835-43; PMID:25728808; http://dx.doi.org/ 10.1111/apt.13144 [DOI] [PubMed] [Google Scholar]
- [20].van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, et al.. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med [Internet] 2013; 368:407-15. Available from: http://dx.doi.org/ 10.1056/NEJMoa1205037; PMID:23323867; http://dx.doi.org/ 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- [21].Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol 2010; 44:551-61; PMID:20716985; http://dx.doi.org/ 10.1097/MCG.0b013e3181e5d06b [DOI] [PubMed] [Google Scholar]
- [22].Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079-87; PMID:22450732; http://dx.doi.org/ 10.1038/ajg.2012.60 [DOI] [PubMed] [Google Scholar]
- [23].Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol [Internet] 2012. [cited 2015January15]; 107:1079-87. Available from: https://c6-qzr6satum-7fsf.sec.amc.nl/ajg/journal/v107/n7/full/ajg201260a.html; PMID:22450732; http://dx.doi.org/ 10.1038/ajg.2012.60 [DOI] [PubMed] [Google Scholar]
- [24].Agrawal M, Aroniadis OC, Brandt LJ, Kelly C, Freeman S, Surawicz C, Broussard E, Stollman N, Giovanelli A, Smith B, et al.. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol 2016; 50:403-7; PMID:26352106 [DOI] [PubMed] [Google Scholar]
- [25].Mandalia A, Ward A, Tauxe W, Kraft CS, Dhere T. Fecal transplant is as effective and safe in immunocompromised as non-immunocompromised patients for Clostridium difficile. Int J Colorectal Dis [Internet] 2016. [cited 2016September16]; 31:1059-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26410257; PMID:26410257; http://dx.doi.org/ 10.1007/s00384-015-2396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ananthaswamy A. Bugs from your gut to mine. New Sci 2008; 209:8-9; http://dx.doi.org/ 10.1016/S0262-4079(08)62088-6 [DOI] [Google Scholar]
- [27].Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al.. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science [Internet] 2013. [cited 2017January9]; 339:548-54. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1229000; PMID:23363771; http://dx.doi.org/ 10.1126/science.1229000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al.. Gut Microbiomes of Malawian Twin. Science (80-) 2013; 339(6119):548; http://dx.doi.org/ 10.1126/science.1229000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci 2007; 104:979-84; PMID:17210919; http://dx.doi.org/ 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature [Internet] 2006. [cited 2014July10]; 444:1027-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17183312; PMID:17183312; http://dx.doi.org/ 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- [31].Bäckhed F, Ding H, Wang T, Hooper L V, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A [Internet] 2004. [cited 2016August26]; 101:15718-23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15505215; PMID:15505215; http://dx.doi.org/ 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kulecka M, Paziewska A, Zeber-Lubecka N, Ambrozkiewicz F, Kopczynski M, Kuklinska U, Pysniak K, Gajewska M, Mikula M, Ostrowski J. Prolonged transfer of feces from the lean mice modulates gut microbiota in obese mice. Nutr Metab (Lond) 2016; 13:57; PMID:27559357; http://dx.doi.org/ 10.1186/s12986-016-0116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102:11070-5; PMID:16033867; http://dx.doi.org/ 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, Walser JC, Widmer A, Baccigalupi L, Ricca E, Iossa S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One 2015; 10:1-19; http://dx.doi.org/ 10.1371/journal.pone.0134893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al.. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341:1241214; PMID:24009397; http://dx.doi.org/ 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med [Internet] 2013. [cited 2014September30]; 5:178ra41. Available from: http://stm.sciencemag.org/content/5/178/178ra41.long; PMID:23536013; http://dx.doi.org/ 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al.. Enterotypes of the human gut microbiome. Nature [Internet] 2011. [cited 2016September2]; 473:174-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21508958; PMID:21508958; http://dx.doi.org/ 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature [Internet] 2013. [cited 2014July9]; 498:99-103. Available from: https://c6-qzr6satum-7fsf.sec.amc.nl/nature/journal/v498/n7452/full/nature12198.html; PMID:23719380; http://dx.doi.org/ 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- [39].Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open forum Infect Dis [Internet] 2015. [cited 2016September16]; 2:ofv004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26034755; PMID:26034755; http://dx.doi.org/ 10.1093/ofid/ofv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al.. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology [Internet] 2012. [cited 2014July9]; 143:913-6.e7. Available from: http://www.sciencedirect.com/science/article/pii/S001650851200892X; PMID:22728514; http://dx.doi.org/ 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- [41].Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science [Internet] 1993. [cited 2016September5]; 259:87-91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7678183; PMID:7678183; http://dx.doi.org/ 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- [42].Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest [Internet] 2006. [cited 2014November12]; 116:1793-801. Available from: /pmc/articles/PMC1483173/?report=abstract; PMID:16823477; http://dx.doi.org/ 10.1172/JCI29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature [Internet] 2002. [cited 2016September13]; 420:333-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12447443; PMID:12447443; http://dx.doi.org/ 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- [44].Özcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Özdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science (80-) 2004; 306:457-61; http://dx.doi.org/ 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- [45].Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia [Internet] 2012. [cited 2016September15]; 55:2565-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22869320; PMID:22869320; http://dx.doi.org/ 10.1007/s00125-012-2644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science [Internet] 2001. [cited 2016September15]; 292:104-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11292874; PMID:11292874; http://dx.doi.org/ 10.1126/science.1057991 [DOI] [PubMed] [Google Scholar]
- [47].Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, et al.. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J [Internet] 2008. [cited 2016September15]; 22:807-18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17928362; PMID:17928362; http://dx.doi.org/ 10.1096/fj.07-9261com [DOI] [PubMed] [Google Scholar]
- [48].Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARγ. Annu Rev Biochem [Internet] 2008. [cited 2017January2]; 77:289-312. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18518822; PMID:18518822; http://dx.doi.org/ 10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- [49].Zelvyte I, Dominaitiene R, Crisby M, Janciauskiene S. Modulation of inflammatory mediators and pparγand nfκb expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol Res [Internet] 2002. [cited 2017January2]; 45:147-54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11846628; PMID:11846628; http://dx.doi.org/ 10.1006/phrs.2001.0922 [DOI] [PubMed] [Google Scholar]
- [50].Piya MK, Harte AL, McTernan PG. Metabolic endotoxaemia: is it more than just a gut feeling? Curr Opin Lipidol [Internet] 2013. [cited 2016September8]; 24:78-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23298961; PMID:23298961; http://dx.doi.org/ 10.1097/MOL.0b013e32835b4431 [DOI] [PubMed] [Google Scholar]
- [51].Bester J, Soma P, Kell DB, Pretorius E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS). Oncotarget [Internet] 2015. [cited 2016September9]; 6:35284-303. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26462180; PMID:26462180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest [Internet] 2006. [cited 2016September6]; 116:1793-801. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16823477; PMID:16823477; http://dx.doi.org/ 10.1172/JCI29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, et al.. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A [Internet] 2011; 108 [cited 2016September5]:4592-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20937873; PMID:20937873; http://dx.doi.org/ 10.1073/pnas.1011383107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zulian A, Cancello R, Ruocco C, Gentilini D, Di Blasio AM, Danelli P, Micheletto G, Cesana E, Invitti C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study. PLoS One [Internet] 2013. [cited 2016September7]; 8:e78495. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24205244; PMID:24205244; http://dx.doi.org/ 10.1371/journal.pone.0078495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol [Internet] 2011. [cited 2016September7]; 9:265-78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21407243; PMID:21407243; http://dx.doi.org/ 10.1038/nrmicro2538 [DOI] [PubMed] [Google Scholar]
- [56].Quigley EMM. Leaky gut – concept or clinical entity? Curr Opin Gastroenterol [Internet] 2016. [cited 2016September7]; 32:74-9. Available from: http://content.wkhealth.com/linkback/openurl?sid = WKPTLP:landingpage&an = 00001574-201603000-00004; PMID:26760399; http://dx.doi.org/ 10.1097/MOG.0000000000000243 [DOI] [PubMed] [Google Scholar]
- [57].Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg [Internet] 2010. [cited 2016September7]; 2:61-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21160852; PMID:21160852; http://dx.doi.org/ 10.4240/wjgs.v2.i3.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) [Internet] 1986. [cited 2017January3]; 71:71-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3086024; PMID:3086024; http://dx.doi.org/ 10.1042/cs0710071 [DOI] [PubMed] [Google Scholar]
- [59].Wu L-L, Peng W-H, Kuo W-T, Huang C-Y, Ni Y-H, Lu K-S, Turner JR, Yu LCH. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-γ. Am J Pathol [Internet] 2014. [cited 2016September7]; 184:2260-74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24911373; PMID:24911373; http://dx.doi.org/ 10.1016/j.ajpath.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al.. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell [Internet] 2016. [cited 2016November18]; 167:1339-1353.e21. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867416314647; PMID:27863247; http://dx.doi.org/ 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al.. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes [Internet] 2007. [cited 2016September7]; 56:1761-72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17456850; PMID:17456850; http://dx.doi.org/ 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- [62].Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol [Internet] 2016. [cited 2016September7]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/27418356; PMID:27418356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lu M, Munford RS. The transport and inactivation kinetics of bacterial lipopolysaccharide influence its immunological potency in vivo. J Immunol [Internet] 2011. [cited 2016September7]; 187:3314-20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21849675; PMID:21849675; http://dx.doi.org/ 10.4049/jimmunol.1004087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Olofsson P, Nylander G, Olsson P. Endotoxin: routes of transport in experimental peritonitis. Am J Surg [Internet] 1986. [cited 2016September7]; 151:443-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3963300; PMID:3963300; http://dx.doi.org/ 10.1016/0002-9610(86)90098-X [DOI] [PubMed] [Google Scholar]
- [65].Cindoruk M, Cirak MY, Unal S, Karakan T, Erkan G, Engin D, Dumlu S, Turet S. Identification of Helicobacter species by 16S rDNA PCR and sequence analysis in human liver samples from patients with various etiologies of benign liver diseases. Eur J Gastroenterol Hepatol [Internet] 2008. [cited 2016September8]; 20:33-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18090988; PMID:18090988; http://dx.doi.org/ 10.1097/MEG.0b013e3282efa4f2 [DOI] [PubMed] [Google Scholar]
- [66].den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res [Internet] 2013. [cited 2014November17]; 54:2325-40. Available from: http://www.jlr.org/content/54/9/2325.long; PMID:23821742; http://dx.doi.org/ 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol [Internet] 2015. [cited 2016September13]; 11:577-91. Available from: http://www.nature.com/doifinder/10.1038/nrendo.2015.128; PMID:26260141; http://dx.doi.org/ 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- [68].Wang H-B, Wang P-Y, Wang X, Wan Y-L, Liu Y-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci [Internet] 2012. [cited 2016June6]; 57:3126-35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22684624; PMID:22684624; http://dx.doi.org/ 10.1007/s10620-012-2259-4 [DOI] [PubMed] [Google Scholar]
- [69].Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol [Internet] 2012. [cited 2016September5]; 302:G1405-15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22517765; PMID:22517765; http://dx.doi.org/ 10.1152/ajpgi.00543.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell [Internet] 2014. [cited 2016April15]; 156:84-96. Available from: http://www.sciencedirect.com/science/article/pii/S009286741301550X; PMID:24412651; http://dx.doi.org/ 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- [71].Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr [Internet] 2010. [cited 2016September5]; 103:460-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19818198; PMID:19818198; http://dx.doi.org/ 10.1017/S0007114509991863 [DOI] [PubMed] [Google Scholar]
- [72].Todesco T, Rao A V, Bosello O, Jenkins DJ. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am J Clin Nutr [Internet] 1991. [cited 2016September5]; 54:860-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1951157; PMID:1951157 [DOI] [PubMed] [Google Scholar]
- [73].Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009; 58:1509-17; PMID:19366864; http://dx.doi.org/ 10.2337/db08-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wahlström A, Sayin SI, Marschall H-U, Bäckhed F. Intestinal Crosstalk between Bile acids and microbiota and its impact on host metabolism. Cell Metab 2016; 24(1):41-50; PMID:27320064; http://dx.doi.org/ 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- [75].Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, Forslund K, Hildebrand F, Prifti E, Falony G, et al.. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature [Internet] 2016. [cited 2016September5]; 535:376-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27409811; PMID:27409811; http://dx.doi.org/ 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- [76].Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, Bae J-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut [Internet] 2014. [cited 2015March5]; 63:727-35. Available from: https://c6-axo6gmj-8jj.sec.amc.nl/content/63/5/727.full; PMID:23804561; http://dx.doi.org/ 10.1136/gutjnl-2012-303839 [DOI] [PubMed] [Google Scholar]
- [77].Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− Mice. Circulation [Internet] 2016. [cited 2016September5]; 133:2434-46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27143680; PMID:27143680; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.115.019645 [DOI] [PubMed] [Google Scholar]
- [78].Nadal I, Donat E, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol [Internet] 2007. [cited 2015November14]; 56:1669-74. Available from: http://jmm.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.47410-0; PMID:18033837; http://dx.doi.org/ 10.1099/jmm.0.47410-0 [DOI] [PubMed] [Google Scholar]
- [79].Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol [Internet] 1999. [cited 2016August26]; 34:1975-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10588212; PMID:10588212; http://dx.doi.org/ 10.1016/S0735-1097(99)00448-9 [DOI] [PubMed] [Google Scholar]
- [80].Spence JD. Effects of the Intestinal Microbiome on Constituents of Red Meat and Egg Yolks: A New Window Opens on Nutrition and Cardiovascular Disease. Can J Cardiol 2014; 30:150-1; PMID:24461914; http://dx.doi.org/ 10.1016/j.cjca.2013.11.019 [DOI] [PubMed] [Google Scholar]
- [81].Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun [Internet] 2012. [cited 2016September6]; 3:1245. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23212374; PMID:23212374; http://dx.doi.org/ 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med [Internet] 2013. [cited 2016September5]; 368:1575-84. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1109400; PMID:23614584; http://dx.doi.org/ 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al.. Gut Microbial Metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell [Internet] 2016. [cited 2016September6]; 165:111-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26972052; PMID:26972052; http://dx.doi.org/ 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chan YK, El-Nezami H, Chen Y, Kinnunen K, Kirjavainen P V. Probiotic mixture VSL#3 reduce high fat diet induced vascular inflammation and atherosclerosis in ApoE(−/−) mice. AMB Express [Internet] 2016. [cited 2016September5]; 6:61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27576894; PMID:27576894; http://dx.doi.org/ 10.1186/s13568-016-0229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, et al.. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular Dysfunction. J Am Heart Assoc [Internet] 2016. [cited 2016September6]; 5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27577581; PMID:27577581; http://dx.doi.org/ 10.1161/JAHA.116.003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology [Internet] 2013. [cited 2016September13]; 57:601-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23055155; PMID:23055155; http://dx.doi.org/ 10.1002/hep.26093 [DOI] [PubMed] [Google Scholar]
- [87].Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, et al.. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol [Internet] 2013. [cited 2016September13]; 11:868-75-3 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23454028; http://dx.doi.org/ 10.1016/j.cgh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- [88].Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology [Internet] 2013. [cited 2016September13]; 58:120-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23401313; PMID:23401313; http://dx.doi.org/ 10.1002/hep.26319 [DOI] [PubMed] [Google Scholar]
- [89].Ruiz AG, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, Fernadez-Escalante JC, Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg [Internet] 2007. [cited 2016September6]; 17:1374-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18000721; PMID:18000721; http://dx.doi.org/ 10.1007/s11695-007-9243-7 [DOI] [PubMed] [Google Scholar]
- [90].Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al.. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology [Internet] 2009. [cited 2016September6]; 49:1877-87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19291785; PMID:19291785; http://dx.doi.org/ 10.1002/hep.22848 [DOI] [PubMed] [Google Scholar]
- [91].Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol [Internet] 2008. [cited 2016September6]; 48:983-92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18395289; PMID:18395289; http://dx.doi.org/ 10.1016/j.jhep.2008.01.035 [DOI] [PubMed] [Google Scholar]
- [92].Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) [Internet] 2013. [cited 2016September13]; 21:2310-21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23418126; PMID:23418126; http://dx.doi.org/ 10.1002/oby.20330 [DOI] [PubMed] [Google Scholar]
- [93].Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al.. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature [Internet] 2012. [cited 2016August26]; 482:179-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22297845; PMID:22297845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al.. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut [Internet] 2013. [cited 2016September13]; 62:1787-94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23197411; PMID:23197411; http://dx.doi.org/ 10.1136/gutjnl-2012-303816 [DOI] [PubMed] [Google Scholar]
- [95].Wong VW-S, Won GL-H, Chim AM-L, Chu WC-W, Yeung DK-W, Li KC-T, Chan HL-Y. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol [Internet] [cited 2016September13]; 12:256-62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23396737; PMID:23396737 [PubMed] [Google Scholar]
- [96].Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet (London, England) [Internet] 1984. [cited 2016September6]; 1:179-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6141332; PMID:6141332; http://dx.doi.org/ 10.1016/S0140-6736(84)92109-3 [DOI] [PubMed] [Google Scholar]
- [97].Moschen AR, Kaser S, Tilg H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol Metab [Internet] 2013. [cited 2016September6]; 24:537-45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23827477; PMID:23827477; http://dx.doi.org/ 10.1016/j.tem.2013.05.009 [DOI] [PubMed] [Google Scholar]
- [98].Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al.. Gut dysbiosis is linked to hypertension. Hypertens (Dallas, Tex 1979) [Internet] 2015. [cited 2016September7]; 65:1331-40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25870193; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, et al.. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A [Internet] 2013. [cited 2016September7]; 110:4410-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23401498; PMID:23401498; http://dx.doi.org/ 10.1073/pnas.1215927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojarvi J, Voigt AY, et al.. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science (80-) [Internet] 2016. [cited 2016April29]; 352:586-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27126044; http://dx.doi.org/ 10.1126/science.aad8852 [DOI] [PubMed] [Google Scholar]
- [101].Smits LP, Bouter KEC, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology [Internet] 2013. [cited 2014November18]; 145:946-53. Available from: http://www.sciencedirect.com/science/article/pii/S0016508513012791; PMID:24018052;http://dx.doi.org/ 10.1053/j.gastro.2013.08.058 [DOI] [PubMed] [Google Scholar]
- [102].Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. Culturing of “unculturable” human microbiota reveals novel taxa and extensive sporulation. Nature [Internet] 2016. [cited 2016May4]; 533:543-6. Available from: http://www.nature.com/nature/journal/v533/n7604/full/nature17645.html?WT.ec_id=NATURE-20160526&spMailingID=51458273&spUserID=NTMxMjc2MzUyNDYS1&spJobID =923132954&spReportId =OTIzMTMyOTU0S0; PMID:27144353; http://dx.doi.org/ 10.1038/nature17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].De Vos WM. Fame and future of faecal transplantations - developing next-generation therapies with synthetic microbiomes. Microb Biotechnol 2013; 6:316-25; PMID:23574632; http://dx.doi.org/ 10.1111/1751-7915.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojärvi J, Voigt AY, et al.. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016; 352:586-9; PMID:27126044; http://dx.doi.org/ 10.1126/science.aad8852 [DOI] [PubMed] [Google Scholar]
- [105].Robins-browne RM, Levine M, Path FF. The fate of ingested in the proximal small intestine. Am J Clin Nut 1981; 34(4):514-9 [DOI] [PubMed] [Google Scholar]
- [106].Seedorf H, Griffin NW, Ridaura VK, Reyes A, Cheng J, Rey FE, Smith MI, Simon GM, Scheffrahn RH, Woebken D, et al.. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 2014; 159:253-66; PMID:25284151; http://dx.doi.org/ 10.1016/j.cell.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: Patients are ready, are we? Inflamm Bowel Dis 2012; 18:676-84; PMID:21618362; http://dx.doi.org/ 10.1002/ibd.21775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab [Internet] 2014. [cited 2015January7]; 20:753-60. Available from: http://www.sciencedirect.com/science/article/pii/S1550413114003143; PMID:25176147; http://dx.doi.org/ 10.1016/j.cmet.2014.07.006 [DOI] [PubMed] [Google Scholar]
- [109].Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on fecal microbiota transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015; 149:223-37; PMID:25982290; http://dx.doi.org/ 10.1053/j.gastro.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: Personalized utilization of the human microbiome in health and disease. Cell Host Microbe 2016; 19:12-20; PMID:26764593; http://dx.doi.org/ 10.1016/j.chom.2015.12.016 [DOI] [PubMed] [Google Scholar]
- [111].Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al.. Personalized nutrition by prediction of glycemic responses. Cell [Internet] 2015. [cited 2015November19]; 163:1079-94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26590418; PMID:26590418;http://dx.doi.org/ 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]


