ABSTRACT
Severe and severe-complicated Clostridium difficile infection (CDI) is associated with high morbidity and mortality. Colectomy is standard of care; however, post-surgical mortality rates approach 50%. Case reports suggest fecal microbiota transplant (FMT) is a promising treatment of severe and severe-complicated disease but there is a paucity of data. Here, we present a single center experience with a novel sequential FMT protocol for patients refractory to maximal medical therapy. This approach consists of at least one FMT delivered via colonoscopy with criteria for repeat FMT and continued vancomycin therapy based on clinical response and pseudomembranes. Our cohort included 57 consecutive inpatients diagnosed with severe or severe-complicated CDI and treated with FMT. Overall, 91% (52/57) experienced clinical cure at 1 month with a 100% cure rate among severe CDI (n = 19) patients and an 87% cure rate for severe-complicated CDI (n = 33) patients. For the cohort, the survival rate was 94.7% at 1 month and 78.6% at 3 months. There were no serious adverse events related to FMT including no procedure-related complications or perforation. There was no difference in outcome between fresh or frozen fecal material. Sequential FMT for inpatients with severe or severe-complicated CDI is promising and may be preferred over colectomy in certain patients.
KEYWORDS: Fecal microbiota transplant, FMT, fulminant C. difficile, pseudomembranes, severe and complicated C. difficile infection, severe C. difficile infection
Introduction
Clostridium difficile infection (CDI) is a major public health threat with the Centers for Disease Control and Prevention (CDC) placing the pathogen into the top threat category (“urgent”) in its report on antimicrobial resistance.1 CDI is the leading healthcare-associated infection and its prevalence, severity and mortality have dramatically increased.2 Up to 8% of patients progress to severe, complicated or “fulminant” CDI, often culminating in toxic megacolon, multi-organ failure and death.3 The management of life threatening CDI is challenging, frequently resulting in prolonged intensive care unit (ICU) admissions and urgent colectomy.4 Although early total abdominal colectomy with end-ileostomy improves survival in critically ill CDI patients compared with continued medical management, colectomy is linked to poor clinical outcomes with a 30-day mortality between 35–57%.4-7 Loop ileostomy and colonic lavage as a surgical alternative to colectomy reported by Neal and colleagues resulted in a reduced but still high mortality rate of 19% and the technique has not been widely adopted.8 Additionally, many patients with severe or complicated CDI are not considered surgical candidates as they have conditions that predict poor post-surgical outcomes such as acute respiratory failure with intubation, shock requiring vasopressors, age greater than 80 years, dialysis-dependent renal failure, chronic obstructive pulmonary disease, thrombocytopenia and coagulopathy.9
Fecal microbiota transplantation (FMT) is the process of introducing colonic microbial communities from a healthy individual into a patient by colonoscopy, enema, nasoenteric tube, or capsules.10 CDI is associated with altered intestinal microbiota characterized by decreased α diversity and altered metabolic profiles including short chain fatty acids and bile salts.11,12 The introduction of a healthy microbial community by FMT ameliorates CDI-related dysbiosis and metabolic derangements thereby re-establishing resistance to colonization and relieving CDI associated clinical symptoms.13,14
FMT has emerged as the best treatment of multiply recurrent CDI15,16 and recommended in both American, Canadian and European guidelines.15-17 A large number of studies including several randomized controlled trials18-21 support the efficacy and safety of FMT in patients with recurrent CDI.10 However, there has been a paucity of data for the use of FMT in severe or complicated CDI. In 1958, the first published medical report of FMT described rapid recovery of 4 patients with fulminant pseudomembranous colitis, after given stool enemas by Eiseman and colleagues.22 Since then, only a few case reports,23-30 and 2 small albeit impressive case series have been published: a retrospective multicenter experience of 17 patients with 88% cure rate31 and a single center study of 14 patients with 79% success rate following administration of a single FMT.32
In contrast to the remarkable case series, there have been 4 examples where a single FMT in severe CDI was only transiently effective with implications that holding the anti-CDI antibiotic may have led to a poor clinical outcome. First, Weingarden and Khoruts33 reported a dramatic, but unsustained symptomatic and laboratory improvement following a single FMT in a patient with fulminant CDI who ultimately declined and succumbed to the disease. They suggested that reinitiation of anti-CDI antibiotics and repeat FMT might be needed for cure in some cases of severe FMT as has been reported in non-responsive recurrent CDI.34 Second, in a similar severe CDI case treated with FMT, despite transient improvement, the patient developed toxic megacolon and died shortly after the FMT.35 The authors speculated that withholding anti-CDI antibiotics following FMT may have contributed to the outcome. Third, in a randomized trial of FMT versus vancomycin for recurrent CDI, 2 of the 7 patients with pseudomembranous colitis, suggesting severe CDI, died despite a temporary response following a single FMT.20 Lastly, in our early experience, we noted that patients with severe or fulminant CDI and extensive pseudomembranes at endoscopy tended to respond poorly to a single FMT when anti-CDI antibiotics were held. Therefore, we developed and previously published a treatment protocol for severe and complicated CDI refractory to anti-CDI antibiotics comprising an initial FMT by colonoscopy, continued vancomycin therapy in patients with extensive pseudomembranes at the time of FMT, and repeat FMT(s) for patients without clinical response.36 In this report, we describe our extended experience of 57 consecutive patients treated with this novel protocol.
Methods
Definitions
CDI severity was defined based upon the 2013 American College of Gastroenterology (ACG) guidelines.15 Diagnosis of CDI was made in cases of diarrhea (≥ 3 loose stools/day) and positive stool C. difficile PCR. Severe CDI was defined as a serum albumin < 3 g/dl and white blood count (WBC) ≥ 15,000 cells/mm3 or abdominal tenderness. We diagnosed severe and complicated CDI if any of the following attributable to CDI were present: ICU admission for CDI, hypotension with or without required use of vasopressors, fever ≥ 38.5°C, ileus, significant abdominal distention, mental status changes, WBC ≥ 35,000 cells/mm3 or <2,000 cells/mm3, serum lactate levels >2.2mmol/l, and end organ failure (e.g. mechanical ventilation, renal failure).15 The Charlson co-morbidity index (age adjusted) was calculated to assess disease burden from co-morbid conditions and the likelihood of dying within 1 y.37 We defined treatment success as complete resolution of diarrhea, no further need of anti-CDI therapy, avoidance of colectomy, and discharge from the hospital.
Patients
Patient with severe or severe-complicated CDI unresponsive to ACG guideline-directed15 antimicrobial therapy (oral vancomycin 500–2000 mg/day or fidaxomicin 400mg/day and rectal vancomycin 2000 mg/day in patients with ileus, ± IV metronidazole 1500 mg/day administered at least for 5 days) were offered FMT in lieu of colectomy at a tertiary care center between July 2013 and March 2016. All patients were under evaluation of a multidisciplinary team consisting of a gastroenterologist, internist, infectious disease specialist and a colorectal surgeon, and were offered the opportunity to receive FMT. Patients with precipitous clinical deterioration defined as likely fatal outcome in 48 hours at the discretion of the treating physician” before 5-day minimum of anti-CDI antibiotic therapy were also offered FMT.
FMT treatment protocol
We used a previously published sequential FMT protocol in combination with oral vancomycin when appropriate (Fig. 1).36 Fresh stool obtained from either a screened patient-selected donor or universal donor within 6 hours of the procedure was used for the first 29 consecutive patients as described elsewhere.36 Donor selection, screening for relevant communicable diseases, and stool processing were performed as outlined by the Fecal Microbiota Transplantation Working Group.38 Frozen stool sourced from OpenBiome stool bank (Somerville, MA, USA) was administered to the remainder of patients in the cohort (N = 28). The stool bank uses a robust FMT donor screening system.39 Prospective donors are assessed clinically for both infectious and microbiome-mediated diseases by a nurse or physician, and also undergo 30 CLIA-approved laboratory tests including infectious pathogens, hepatic panel and complete blood count which has been previously reported.40 All patients were followed on a daily basis by our inpatient gastroenterology team while hospitalized. Post-discharge, as per our FMT program protocol, patients were followed-up via phone at 24–48 hours, week 1, 4, 8 and 12 and in clinic at 6 months. If a patient could not be reached via phone, a chart review was performed and/or primary care provider was contacted. In the case of the submitted cohort of 57 patients, the follow-up was 100%. The FMT procedure protocol was approved by Indiana University Hospital. Baseline data and outcomes were prospectively captured using a research database that had been approved by the Indiana University Institutional Review Board.
Figure 1.
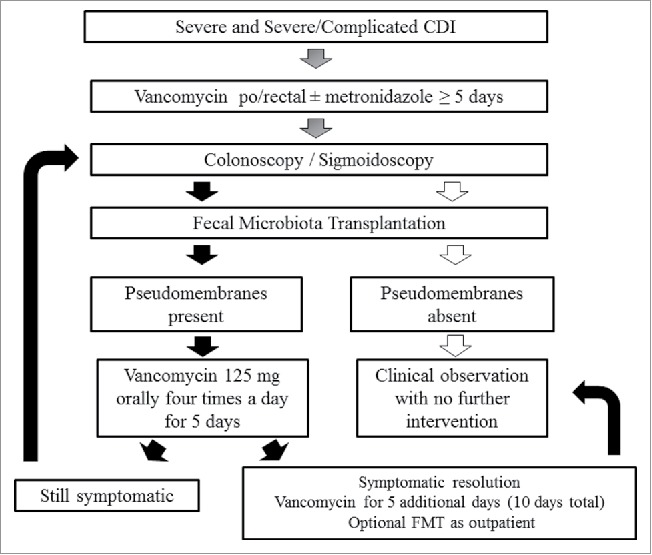
Sequential fecal microbiota transplant protocol.
Patient consent
A detailed discussion with the patients and family prior deciding for FMT was performed. An FMT is being offered under an FDA Guidance for CDI refractory to antimicrobial therapy and it is considered to be an investigational therapy. While there is abundance of data on FMT effectiveness in recurrent CDI, limited evidence exists on its use in severe or severe-complicated cases. In our experience, the success rate of sequential FMT in combination with vancomycin is >90% even in this critically ill population. Notably, a large portion of patients needed more than 1 FMT to achieve cure. Approximately, one half of our patients were cured following a single FMT, about one third needed 2 FMTs, while only 10% required a third or more FMTs.
If the FMT protocol fails or the patient's condition deteriorates, alternatives include urgent colectomy (loop ileostomy with vancomycin lavage is not offered at our institution) deemed necessary by the surgeon. Colectomy in this setting is associated with high mortality up to 50%. Another alternative would be continued antimicrobial therapy.
Regarding stool source and FMT safety, the following was discussed: The stool donor can be patient-directed or universal. In either case, the donor is screened for history of exposure to communicable infections agents and has undergone blood and feces testing for infections pathogens within 2 weeks of donation. The donor may have an unknown disease or infection not found during the screening and stool testing that could be potentially transmitted. FMT can be safely performed via colonoscopy in the hands of an experienced endoscopist even in the setting of severe colitis. Rare complications of colonoscopy include perforation and sedation related adverse events.
Statistical analysis
Baseline patient characteristics and treatment outcomes were summarized using median, interquartile range with 25th and 75th percentiles, and range values for continuous variables due to the skewed distributions and frequencies and proportions for categorical variables. Comparisons between patients with severe and severe-complicated CDI were performed using the Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables. Difference in length of hospital stay for patients with severe and severe-complicated CDI was also summarized using median and interquartile range (IQR) due to the skewness of the data and evaluated using the Wilcoxon rank sum test. Comparisons between patients who received fresh and frozen stool were performed using the Wilcoxon rank sum test for the number of FMTs patients received and using the Fisher's exact for the cure rate at one month.
To examine the potential risk factors for repeat FMT, we performed a univariate analysis where patients with and without repeat FMT were compared using Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables. These risk factors included patients' demographic, clinical, and laboratory variables including age, sex, CDI severity, number of CDI episodes, use of non-CDI antibiotic during the same hospitalization, WBC, serum albumin concentration, ICU admission, presence of pseudomembranes, toxic megacolon, acute renal failure, dialysis, shock, vasopressor use, mechanical ventilation, immunosuppression, Charlson comorbidity index, and source and type of stool. Due to the large number of potential risk factors, we used a forward stepwise procedure to select important risk factors. Time to death was analyzed using the Kaplan-Meier approach. All statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, N.C.).
Results
Patient characteristics
Our cohort included 57 inpatients diagnosed with severe or severe and complicated CDI and treated with FMT. Salient baseline characteristics of these patients are depicted in Table 1. Among severe CDI patients 3 (16%) were admitted to the ICU. In contrast, 16 (42%) severe-complicated patients were admitted to the ICU at the time of FMT; of these patients, 7 presented with toxic megacolon (cecal diameter> 12 cm or rectosigmoid diameter > 6.5 cm), 12 with acute renal failure with 3 requiring dialysis, 10 with hypovolemic or septic shock, 8 required vasopressors, 7 with mental status changes, and 4 patients required mechanical ventilation. In terms of medical history, 10 patients had inflammatory bowel disease (5 with Crohn's and 5 with ulcerative colitis) and 10 patients were on immunosuppressive medications. A history of recurrent CDI was present in 80.7% (46/57) with a median number of prior CDI episodes of 3 (IQR 2–4) and 84% (48/57) were hospitalized with CDI in the past. The severe CDI episode was triggered by an antibiotic in 56% (32/57). A total of 27 patients (47%) were treated with a non-CDI antibiotic during hospital admission. Among these, 11 patients had a documented source of infection including 7 patients with UTI, 1 with empyema, 1 with cellulitis, 1 with post-surgical complications of plastic surgery, 1 for SBP (spontaneous bacterial peritonitis). The remaining patients (N = 18) received broad-spectrum antibiotic coverage for sepsis and critical condition. None of the patients received FMT before hospital admission. Detailed individual patient characteristics including demographics, clinical and laboratory data, number of previous CDI episodes, ICU stay, comorbid conditions, number of FMTs received, length of hospital stay and outcome at 1 and 3 months are shown in Table 2.
Table 1.
Selected baseline demographics, clinical characteristics, and laboratory data of 57 patients (median, interquartile range with 25th and 75th percentiles, and range presented for continuous variables and frequencies and proportions for categorical variables) who underwent fecal microbiota transplantation for severe or severe-complicated Clostridium difficile infection (CDI).
| Total (N = 57) | Severe CDI (N = 19) | Severe/Complicated CDI (N = 38) | P Value | |
|---|---|---|---|---|
| Age (year) | 72 (60 – 79; 25 – 99) | 68 (51 – 77; 26 – 87) | 74 (66 – 81; 25 – 99) | 0.111 |
| Women | 34 (59.6%) | 10 (52.6%) | 24 (63.2%) | 0.569 |
| WBC (k/mm3) | 17 (12.9 – 25; 5.2 – 64.7) | 15.8 (10 – 23.1; 5.5 – 28.3) | 18.6 (14 – 27.4; 5.2 – 64.7) | 0.160 |
| Albumin (g/dL) | 2.5 (2.2 – 2.8; 1.5 – 3.7) | 2.5 (2.2 – 2.6; 1.5 – 3.7) | 2.6 (2.1 – 2.8; 1.7 – 3.1) | 0.518 |
| Number of CDI episodes | 3 (2 – 4; 1–12) | 3 (3 – 4; 1 – 6) | 3 (2 – 4; 1 – 12) | 0.518 |
| ICU stay | 19 (33.3%) | 3 (15.8%) | 16 (42.1%) | 0.073 |
| Pseudomembranes at first FMT | 35 (61.4%) | 14 (73.7%) | 21 (55.3%) | 0.251 |
| Charlson Co-morbidity Index | 6 (4 – 8; 0–11) | 6 (2 – 9; 0 – 11) | 6 (4 – 8; 0 – 11) | 0.945 |
| Immunosuppression | 10 (17.5%) | 5 (26.3%) | 5 (13.2%) | 0.275 |
| Use of non-anti CDI antibiotics during admission | 27 (47.4%) | 8 (42.1%) | 19 (50%) | 0.779 |
| Presence of toxic megacolon | 9 (15.8%) | 0 | 9 (23.7%) | 0.022 |
| Acute renal failure | 26 (45.6%) | 1 (5.3%) | 25 (65.8%) | <0.001 |
| Abdominal pain | 41 (71.9%) | 12 (63.2%) | 29 (76.3%) | 0.356 |
| Shock | 12 (21.1%) | 1 (5.3%) | 11 (29%) | 0.045 |
| Vasopressor use | 11 (19.3%) | 2 (10.5%) | 9 (23.7%) | 0.304 |
| Mechanical ventilation | 4 (7%) | 0 | 4 (10.5%) | 0.290 |
| Dialysis | 6 (10.5%) | 1 (5.3%) | 5 (13.2%) | 0.652 |
| Frozen stool | 28 (49.1%) | 9 (47.4%) | 19 (50%) | 1.000 |
| Use of stool from universal donor | 46 (80.7%) | 12 (63.2%) | 34 (89.5%) | 0.031 |
Table 2.
Characteristics of 57 patients who underwent FMT.
| Patient no. | Age | Sex | CDI severity | # CDI episodes | WBC | Albumin | Abdo-minal pain | ICU | Pseudo- membranes on colonoscopy with 1st FMT | Complications due to CDI | Comorbidities | Charlson score | FMTs no. | Length of hospital stay post 1st FMT (days) | Outcome 1 mo resolution | Outcome 3 mo resolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 81 | M | S | 5 | 22.0 | 2.2 | no | no | yes | none | Parkinson, polycythemia vera, CAD, COPD, HTN, empyema, skin CA, GERD, dementia, CVA | 10 | 1 | 7 | yes | Death unrelated |
| 2 | 68 | M | S | 6 | 23.8 | 2.6 | no | no | yes | none | Prostate CA, DM, Ileal conduit, recurrent UTI | 11 | 2 | 5 | yes | Recurrence CDI related death |
| 3 | 87 | F | S | 2 | 5.5 | 2.8 | yes | no | no | none | Right leg BKA,CAD, CHF, COPD, rectal CA | 10 | 1 | 1 | yes | Yes |
| 4 | 77 | F | S | 3 | 8.4 | 2 | yes | yes | yes | none | Pulm fibrosis, Myasthenia Gravis, Afib, DVT, CVA, recurrent UTIs, COPD | 7 | 2 | 16 | yes | Death unrelated |
| 5 | 63 | F | S | 4 | 24.5 | 2.3 | yes | no | no | none | ESRD s/p renal transplant, muscular dystrophy, DM | 6 | 1 | 13 | Yes | Yes |
| 6 | 43 | F | S | 5 | 10 | 2.6 | yes | no | yes | none | UC | 0 | 1 | 4 | Yes | Yes |
| 7 | 31 | F | S | 1 | 28.3 | 1.5 | yes | no | yes | none | RA, asthma, iritis, UC | 2 | 1 | 3 | Yes | Yes |
| 8 | 26 | M | S | 3 | 15.8 | 2.8 | yes | no | yes | none | Ileocolonic Crohn's | 0 | 2 | 20 | Yes | Yes |
| 9 | 60 | F | S | 1 | 19.1 | 2.6 | yes | no | yes | none | ESRD on HD, epilepsy | 1 | 1 | 2 | Yes | Yes |
| 10 | 72 | F | S | 1 | 11.1 | 2.5 | yes | no | yes | none | CVA, DM, Afib, CAD | 9 | 1 | 13 | Yes | Yes |
| 11 | 25 | F | SC | 3 | 34.2 | 2 | yes | yes | yes | ileus, fever | Colonic Crohn's | 1 | 2 | 7 | Yes | Yes |
| 12 | 79 | M | SC | 4 | 8.2 | 2.7 | yes | no | no | fever, hypotension, AMS | CAD, ischemic CM, PVD, MRSA necrotizing fasciitis | 8 | 1 | 1 | Yes | Yes |
| 13 | 56 | M | SC | 1 | 14 | 2.2 | yes | yes | yes | ARF, fever, septic shock on pressors, dialysis, ileus, mechanical ventilation | liver transplant, DM | 11 | 3 | 65 | Yes | Recurrence CDI related death |
| 14 | 76 | F | SC | 3 | 10.3 | 2.6 | yes | yes | yes | ARF, septic shock on pressors, toxic megacolon, mechanical ventilation | CHF, DM, HTN, UTI | 5 | 3 | 25 | Yes | Yes |
| 15 | 60 | F | SC | 2 | 29 | 1.9 | yes | no | yes | ARF, hypovolemic shock | Non-ischemic CM, HTN, morbid obesity | 6 | 2 | 9 | Yes | Yes |
| 16 | 73 | F | SC | 6 | 21.7 | 2 | no | no | yes | ARF, acidosis | COPD, ARF on CKD stage IV, CAD, Dementia, CHF, AFib | 10 | 2 | 11 | Yes | Death unrelated |
| 17 | 61 | M | SC | 1 | 16.5 | 2.6 | yes | yes | yes | Fever, ileus, toxic megacolon | CAD, Afib, NSTEMI, VSD on aortic balloon pump | 4 | 1 | 28 | Yes | Yes |
| 18 | 86 | F | SC | 1 | 27.4 | 2.6 | no | yes | yes | ARF, toxic megacolon, septic shock | UTI, Alzheimer, CVA | 6 | 2 | 12 | Yes | Yes |
| 19 | 78 | M | SC | 3 | 40.2 | 2 | yes | yes | yes | ARF on CVVH, fever, septic shock on pressors, AMS | HTN, memory loss, CHF, CAD, Afib | 8 | 1 | 22 | Yes | Yes |
| 20 | 51 | M | SC | 1 | 19.2 | 2.5 | yes | no | yes | sepsis, toxic megacolon | Cerebral palsy, hypothyroidism, scoliosis | 3 | 2 | 7 | Yes | Yes |
| 21 | 66 | M | SC | 1 | 18.1 | 1.7 | yes | yes | yes | ARF, fever, septic shock on pressors, toxic megacolon, AMS, mechanical ventilation | Injury of external genitalia morbid obesity | 2 | 1 | 0 | CDI related death | n/a |
| 22 | 40 | M | SC | 3 | 14.3 | 2.8 | yes | no | no | ARF, sepsis, fever | Crohns colitis | 0 | 1 | 3 | Yes | Yes |
| 23 | 77 | F | SC | 1 | 26 | 2.5 | yes | yes | yes | ARF, fever, hypovolemia, ileus | Afib, DM, COPD, OSA | 6 | 2 | 7 | Yes | Yes |
| 24 | 77 | M | SC | 4 | 39.1 | 2 | yes | yes | yes | ARF, acidosis | CKD, afib, OSA, hypogammaglobulinemia, dementia | 6 | 1 | 7 | Yes | Yes |
| 25 | 84 | F | SC | 3 | 9 | 2.9 | yes | no | no | ARF, hypotension | DM, CKD, CAD | 8 | 1 | 5 | Yes | Yes |
| 26 | 55 | F | SC | 2 | 17 | 2.3 | yes | yes | no | ARF on dialysis, septic shock on pressors, ileus | ESLD, ascites, CKD | 6 | 1 | 28 | Yes | Death unrelated |
| 27 | 92 | F | SC | 4 | 16.5 | 2.1 | no | no | no | ARF, AMS, ileus | dementia | 6 | 2 | 5 | Yes | Yes |
| 28 | 73 | F | SC | 12 | 56 | 2.3 | yes | yes | yes | ARF, septic shock on pressors, toxic megacolon | seizures, dementia, HTN, asthma, | 4 | 1 | 22 | Yes | Yes |
| 29 | 76 | M | SC | 7 | 25 | 1.9 | yes | yes | yes | hypotension, sepsis, ileus | CAD, Afib, CHF, recurrent UTI, COPD | 5 | 2 | 15 | Yes | Yes |
| 30 | 84 | M | SC | 3 | 23.2 | 1.9 | yes | no | yes | Fever, hypotension, sepsis, AMS | Afib, HTN, Parkinson, CAD, CVA | 6 | 1 | 13 | Yes | Yes |
| 31 | 58 | M | S | 4 | 23.1 | 2.2 | no | no | no | none | DM, CHF, Total artificial heart implantation | 4 | 1 | 61 | Yes | Death Unrelated |
| 32 | 85 | F | SC | 4 | 31.3 | 2.9 | no | no | no | hypotension | CAD, COPD, DM, HTN, afib, dementia | 8 | 2 | 4 | Recurrence | Yes |
| 33 | 69 | M | SC | 6 | 14.9 | 2.5 | yes | no | no | ARF on dialysis, sepsis | DM, HTN, cirrhosis | 9 | 1 | 6 | Yes | Death Unrelated |
| 34 | 81 | F | SC | 1 | 23.3 | 2.2 | yes | yes | NA, enema | ARF, septic shock on pressors, mechanical ventilation, toxic megacolon, AMS | Afib, asthma, CHF | 5 | 2 | 2 | CDI related death | CDI related death |
| 35 | 75 | F | S | 4 | 21.8 | 1.8 | yes | no | yes | none | Kidney Transplant | 5 | 4 | 35 | Yes | Yes |
| 36 | 28 | M | S | 4 | 15.1 | 2.5 | no | no | yes | none | Crohn's disease | 0 | 1 | 11 | Yes | Yes |
| 37 | 94 | F | SC | 5 | 5.2 | 3 | yes | no | no | ARF | HTN, CAD, basal cell CA, hypothyroidism | 8 | 1 | 7 | Recurrence | Yes |
| 38 | 81 | F | S | 3 | 8 | 2.4 | yes | no | yes | none | HTN, Breast CA, hypothyroidism | 6 | 3 | 14 | Yes | Recurrence |
| 39 | 75 | F | SC | 2 | 14.8 | 2.7 | yes | no | yes | ARF, hypotension, sepsis | CAD, CKD, DM, CHF, HTN | 9 | 3 | 20 | Yes | Death unrelated |
| 40 | 68 | F | SC | 6 | 17 | 2.9 | yes | yes | yes | Septic shock on pressors, toxic megacolon | Lymphoma (DLBCL), DM, HTN, MS, afib | 7 | 3 | 42 | Yes | Yes |
| 41 | 39 | M | SC | 2 | 27 | 3.1 | yes | no | no | ARF on dialysis, hypotension, toxic megacolon, AMS | HTN, alcoholic hepatitis, ESRD | 3 | 1 | 72 | Yes | Yes |
| 42 | 83 | M | S | 5 | 15.4 | 3.7 | no | no | no | none | Afib, CAD, CVA | 6 | 1 | 11 | Yes | Recurrence |
| 43 | 51 | M | S | 3 | 12.5 | 2.5 | yes | yes | yes | hypotension on pressors | Cirrhosis, ESRD, hyperparathyroidism | 6 | 1 | 97 | Yes | Death Unrelated |
| 44 | 71 | F | S | 3 | 17 | 2.3 | no | no | yes | hypotension | DM, HTN, Afib, acquired factor VIII inhibitor | 6 | 2 | 13 | Yes | Yes |
| 45 | 89 | F | SC | 4 | 8.1 | 2.6 | yes | no | no | ARF | Afib, HTN, CHF, CAD | 6 | 1 | 6 | Yes | Yes |
| 46 | 55 | M | SC | 5 | 14.1 | 2.9 | yes | no | no | ARF | PSC, liver transplant, UC, gall bladder adenoCA, PVD | 10 | 1 | 6 | Yes | Yes |
| 47 | 78 | F | SC | 2 | 20.89 | 2.8 | no | no | no | ARF, fever, hypotension | UC | 4 | 1 | 3 | Yes | Yes |
| 48 | 73 | M | S | 4 | 6.7 | 2.8 | no | no | no | none | CVA, HTN, DM, ESRD, COPD, RCC | 10 | 1 | 4 | Yes | Yes |
| 49 | 68 | F | SC | 4 | 12.9 | 2.9 | yes | no | no | ARF, hypotension | AAA, HTN, breast CA | 4 | 1 | 10 | Yes | Yes |
| 50 | 69 | F | SC | 5 | 64.7 | 2.1 | yes | yes | yes | ARF, sepsis, AMS | CHF | 3 | 2 | 28 | Yes | Yes |
| 51 | 81 | F | SC | 2 | 12.3 | 2.9 | yes | no | yes | ARF, hypotension | HTN, DM, colon CA | 7 | 1 | 4 | Recurrence | Yes |
| 52 | 60 | M | S | 3 | 25.4 | 2.4 | yes | yes | yes | ARF, septic shock on pressors, AMS, ileus | Crohn's, diverticulitis, CAD, CHF, asthma, TIA | 6 | 5 | 15 | Yes | Yes |
| 53 | 77 | F | SC | 1 | 14 | 2.8 | no | no | no | ARF | HLD, HTN, Crohn's, SCC, CHF, afib | 5 | 1 | 8 | Yes | Yes |
| 54 | 72 | F | SC | 3 | 9.8 | 3 | yes | no | no | ARF, hypotension | HTN, Asthma, DJD | 4 | 1 | 4 | Recurrence | Yes |
| 55 | 72 | F | SC | 4 | 19.1 | 2.6 | no | yes | yes | ARF, septic shock on pressors | Dementia, CHF, TIA, T2DM | 6 | 1 | 5 | Yes | Yes |
| 56 | 99 | F | SC | 2 | 43.2 | 2 | no | no | no | ARF, hypotension, AMS | HTN, HLD | 5 | 2 | 12 | Recurrence | Yes |
| 57 | 66 | M | SC | 3 | 27.9 | 2.6 | no | no | yes | ARF, sepsis, toxic megacolon | HTN, OA, DJD | 2 | 2 | 8 | Yes | Yes |
Note.
Abbreviations: UC-ulcerative colitis, RA rheumatoid arthritis, CD-Crohn's disease, ESRD-end-stage renal disease, CVA-cerebrovascular accident, DM-diabetes mellitus, afib-atrial fibrillation, CAD-coronary artery disease, CM-cardiomyopathy, PVD-peripheral vascular disease, HTN-hypertension, UTI-urinary tract infection, CA-carcinoma, ARF-acute renal failure, CKD-chronic kidney disease, VSD-ventricular septum defect, AMS-altered mental status, BKA-below the knee amputation, OSA- obstructive sleep apnea, GERD- gastroesophageal reflux disease, COPD-chronic obstructive pulmonary disease, MRSA- methicillin resistant Staphylococcus aureus, DVT-deep venous thrombosis
Treatment effect
Overall, treatment success at 1 month was achieved by 91% (n = 52) of the patients; 100% of severe CDI (n = 19) and 87% of severe-complicated CDI (n = 38) patients. A single FMT was needed in 30 (52.6%) patients, 2 FMTs in 16 (28.1%) patients, 3 FMTs in 4 patients (7%), and 4–5 FMTs in 2 (3.5%) patients to achieve clinical cure (Table 3). The remaining 5 (8.8%) patients had treatment failure at 1 month. The 5 treatment failures were as follows: death from sepsis within 24 hours of first FMT (arterial pH 7.1 at time of procedure) (pt#21), death following colectomy after failing 3 FMTs in a patient who was 6 weeks post-orthotopic liver transplantation (pt#13), withdrawal of care in the setting of marginally improved septic shock after first FMT (pt#34). The 2 remaining patients both recovered after just one FMT and were discharged home, but returned within 1 month due to recurrent CDI; these patients underwent a second FMT with complete resolution of symptoms thereafter. Importantly, no procedure related complication such as perforation, bleeding, aspiration or sedation related adverse event occurred.
Table 3.
Summary of therapy outcome and number of fecal transplants needed to achieve resolution of symptoms in patients with severe and severe-complicated Clostridium difficile infection (CDI).
| Total (N = 57) | Severe CDI (N = 19) | Severe-complicated CDI (N = 38) | P Value | |
|---|---|---|---|---|
| Number of FMT received, n (%) | 0.261 | |||
| 1 FMT | 33 (57.9%) | 12 (63.1%) | 21 (55.3%) | |
| 2 FMTs | 17 (29.8%) | 4 (21%) | 13 (34.2%) | |
| 3 FMTs | 5 (8.8%) | 1 (5.3%) | 4 (10.5%) | |
| 4 FMTs | 1 (1.7%) | 1 (5.3%) | 0 (0%) | |
| 5 FMTs | 1 (1.7%) | 1 (5.3%) | 0 (0%) | |
| Length of hospital stay (days), median (IQR; range) | 11 (6 – 21; 2 – 97) | 13 (6 – 17; 3 – 97) | 9.5 (6 – 23; 2 – 72) | 0.76 |
| Overall success rate at 30 days, n (%) | 52 (91.2%) | 19 (100%) | 33 (86.8%) | 0.158 |
Note.
The length of hospital stay reported above is the total length of stay, not the length of stay after FMT.
The overall survival rate was 94.7% (95% CI: 89.1% to 100%) at 1 month and 78.6% (95% CI: 68.6% to 90.1%) at 3 months (Fig. 3). Of the 12 deaths at the 3-month mark, only 4 succumbed to CDI-related causes: 3 as previously characterized, in addition to a fourth (pt#2) patient who responded to the initial FMT and was discharged, but succumbed to CDI-related sepsis after being treated for recurrent urinary tract infection 92 d following the first FMT. The remaining 8 patients were successfully treated with sequential FMT therapy and discharged from the hospital, but later succumbed to causes unrelated to CDI.
Figure 3.
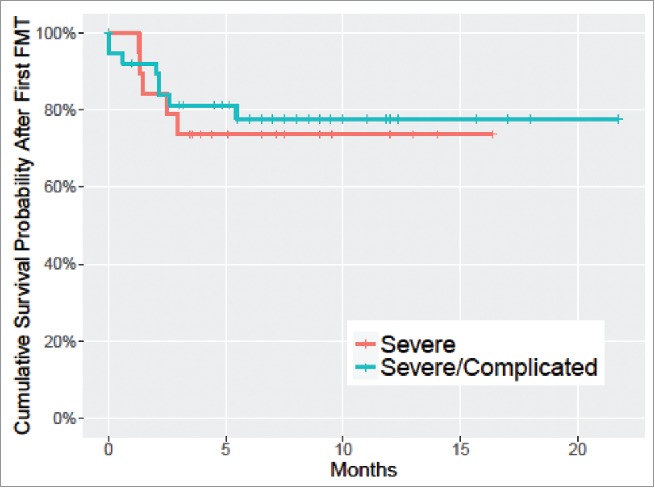
Kaplan-Meier curve of overall survival in severe and severe/complicated CDI patients following the first FMT.
A summary flowchart of patient response to our FMT protocol and outcome is detailed in Figure 2.
Figure 2.
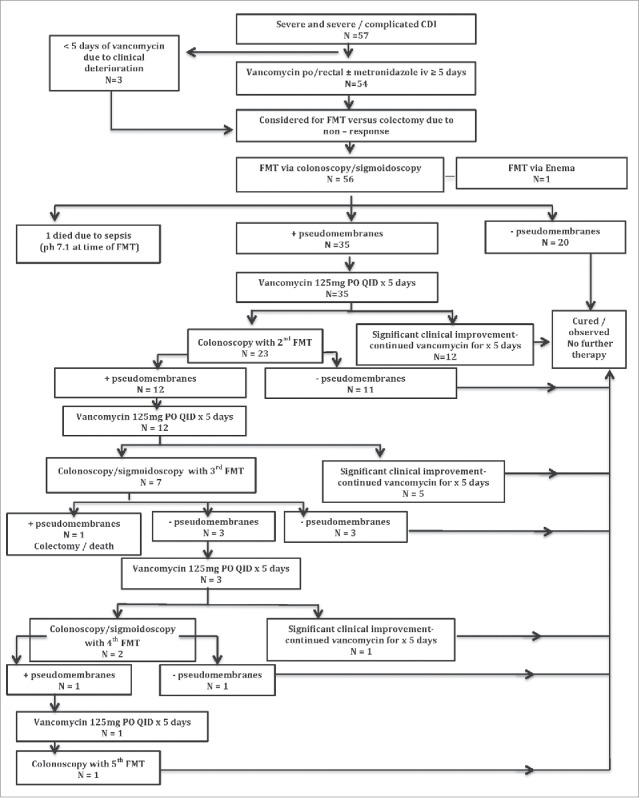
Summary flowchart of patient response to our FMT protocol and outcome.
Impact of FMT source
Overall, a total of 91 FMTs were administered to the severe and severe-complicated CDI cohort. The source of the FMT was screened, patient-directed donor for 16 transplants and universal donor for 75. Fresh stool was the source of 44 FMTs used in a total of 29 patients. Among patients who received fresh stool, 10 patients had severe CDI with 100% resolution of symptoms at 1 month and 19 patients had severe-complicated CDI with 89.5% cure at 1 month. Alternatively, frozen stool was the source of 47 FMTs used in a total of 28 patients. Among patients who received frozen stool, 9 patients had severe CDI with 100% cure at 1 month and 19 patients had severe and complicated disease with 84.2% cure at 1 month. Overall, there was no statistically significant difference in cure rate between fresh or frozen FMT (p = 0.67) or the number of FMTs needed to achieve cure (average number of FMTs is 1.5 for fresh FMT and 1.7 for frozen FMT, p = 0.993)
Predictors of repeat FMT
Univariate assessments of risk factors that predict the need for repeat FMT suggested that patients with a higher white blood cell count (p = 0.02), lower albumin (p = 0.028), presence of pseudomembranes at first FMT(p = 0.006), and use of non-CDI antibiotics during admission (p = 0.017) were more likely to have a repeat FMT (Table 4). Results from the multivariate logistic regression model illustrate the odds of requiring repeat FMT were over 6-fold higher with the presence of pseudomembranes during the first FMT (OR 6.21; 95% CI 1.54–25.12) and over 3-fold higher with use of non-CDI antibiotics during admission (OR 3.56; 95% CI 1.03–12.33). There was a trend toward female sex being associated with repeat FMT after adjusting for the effect of pseudomembranes and the use of non-CDI antibiotics but this risk factor was not statistically significant (OR 3.43; 95% CI 0.92 – 12.83).
Table 4.
Comparison of baseline demographics, clinical characteristics, and laboratory data (median and interquartile range with 25th and 75th percentiles for continuous variables and frequency and proportion for categorical variables) for patients with and without repeat FMT.
| No repeat FMT (N = 33) | Repeat FMT (N = 24) | P Value | |
|---|---|---|---|
| Age (year) | 72 (58 – 79) | 74 (63 – 79) | 0.71 |
| Women | 17 (51.5%) | 17 (70.8%) | 0.178 |
| Severe/complicated CDI | 21 (63.6%) | 17 (70.8%) | 0.777 |
| WBC | 15.1 (11.1 – 22) | 22.6 (16.2 – 27.7) | 0.02 |
| Albumin | 2.6 (2.3 – 2.8) | 2.3 (2 – 2.6) | 0.028 |
| Number of CDI episodes | 3 (2 – 4) | 3 (2 – 4) | 0.699 |
| ICU admission | 8 (24.2%) | 11 (45.8%) | 0.099 |
| Presence of toxic megacolon | 3 (9.1%) | 6 (25%) | 0.146 |
| Acute renal failure | 15 (45.5%) | 11 (45.8%) | 1 |
| Pseudomembranes at first FMT | 15 (45.5%) | 20 (83.3%) | 0.006 |
| Charlson co-morbidity index | 6 (4 – 8) | 6 (5 – 7) | 0.806 |
| Immunosuppression | 5 (15.2%) | 5 (20.8%) | 0.728 |
| Use of non-anti CDI antibiotics during admission | 11 (33.3%) | 16 (66.7%) | 0.017 |
| Abdominal pain | 25 (75.8%) | 16 (66.7%) | 0.554 |
| Shock | 5 (15.2%) | 7 (29.2%) | 0.324 |
| Vasopressor use | 6 (18.2%) | 5 (20.8%) | 1 |
| Mechanical ventilation | 1 (3%) | 3 (12.5%) | 0.3 |
| Dialysis | 5 (15.2%) | 1 (4.2%) | 0.385 |
| Frozen stool | 17 (51.5%) | 11 (45.8%) | 0.79 |
| Use of stool from universal donor | 26 (78.8%) | 20 (83.3%) | 0.745 |
Discussion
To our knowledge, we report the largest experience using FMT for severe or severe-complicated CDI patients. Our study demonstrates a 91% cure rate at 1 month for endoscopic response-guided FMT with vancomycin in selected cases. This minimally invasive approach has benefits over the current standard of care, colectomy, for CDI patients who are clinically deteriorating in the ICU. The Eastern Association for the Surgery of Trauma practice guidelines “strongly recommend that adult patients with CDI undergo early surgery, before the development of shock and the need for vasopressors.”41 This recommendation is based upon a risk reduction of 0.5 with early surgery. However, the morbidity and mortality of colectomy, even when conducted in a timely fashion, is considerable ranging between 35–80%7 with the mean length of hospital stay of 45 d.42 Moreover, in patients who survive colectomy and are discharged from the hospital, the median survival is estimated to be 20 months.43 This is likely reflective of the impact of abdominal surgery on a patient population that typically has multiple, significant co-morbidities and poor nutrition. In our study, the in-hospital mortality rate was 5% and the 3-month survival of discharged patients was 79%, with no serious adverse events attributable to FMT. Accordingly, colectomy may not be the only therapeutic option in this high risk patient population. Indeed, Cammarota and colleagues reported a rapid decrease in CDI-related colectomy rate, from a 1.9–5% annual rate to 0%, following introduction of an inpatient FMT program.44 Beyond clinical care, data suggests sequential FMT approach with interval antibiotics outlined here is more cost-effective than standard colectomy. In a decision analysis model evaluating competing treatment strategies in severe-complicated CDI, the sequential FMT approach was the most cost-effective (ICER 1,973 USD, cost 26,700 USD) compared with total colectomy and ileostomy (ICER 72,493 USD, cost 67,422 USD).45
There may be a role for a synergistic, staggered therapeutic approach with early FMT and follow-up colectomy if there is no clinical response. This concept is orthogonally supported by a recent study from Clanton and colleagues demonstrating that the post-surgical mortality was significantly worse in patients who were taken for colectomy at 2 d vs. 3 d (p < 0.01).46 The authors contend that contrary to traditional teaching, a delay in surgery may be beneficial to allow medical treatment and stabilization before surgery. As an adjunct to therapy, a single FMT may be seamlessly and rapidly administered in the ICU without delaying potential colectomy. Based on our clinical experience, nearly 90% of patients had a clinical response – reduced diarrhea, improved vital signs and physical exam – within 24 to 48 hours of FMT. If a patient does not respond to the FMT, colectomy-associated mortality is unlikely to be substantially affected.
FMT may be the ideal treatment of patients who are too sick to be taken for surgery. Among our cohort, 5 of the critically ill patients were deemed too unstable to undergo colectomy. However, 80% were successfully treated with FMT. In our experience there are 2 patient phenotypes that are not well suited for FMT. First, patients with multiorgan failure refractory to supportive therapy and severe acidosis (pH < 7.2) are unlikely to have successful outcomes with FMT. Second, patients with toxic megacolon and signs of impending perforation such as intramural air should proceed to colectomy without any delay. Clinicians should be cautious about selecting the most suitable patients for FMT in this context and navigating the risks, benefits and alternatives, including no treatment, in this challenging patient population.
The FMT treatment of severe and severe-complicated hospitalized CDI patients differs from standard outpatient FMT of recurrent CDI. Among outpatients with recurrent CDI, a single FMT by colonoscopy has a 85–91% cure rate with out the need for further CDI antimicrobial therapy.10,47 While a similarly high overall cure was observed in severe and severe-complicated CDI patients using our protocol, they often required repeat FMTs and antibiotics to achieve a successful outcome. Although our protocol did not limit the number of FMTs used, we found the majority of patients were cured with one or 2 FMTs when combined with vancomycin, and only 12% required a 3rd or more. Previously, we reported that severe CDI is a strong, independent predictor of early FMT failure and increases the likelihood of requiring repeat FMT(s) by 6-fold.48 We speculate, that these severely ill patients often fail FMT because the C. difficile burden is too high and a single FMT is insufficient for overall cure, but it restores a microbial scaffolding to enable a response to anti-CDI therapy (vancomycin or fidaxomicin) in an otherwise therapy-refractory patient33,34 Although our choice of anti-CDI antibiotics, vancomycin, was dictated by hospital policy, we believe fidaxomicin is a more desirable option given its narrower antimicrobial spectrum, bactericidal nature and limited disruption on the gut microbiota.49,50 Some argue, that reinitiation of an antibiotic following FMT is counterintuitive and rather FMT, in rapid cycles (daily x5) should be repeated (T. Borody-personal communication); however, there is a paucity of data to support this suggestion. In a randomized trial, Cammarota and colleagues successfully treated 5 patients with pseudomembranous colitis by administering an FMT every 3 d.20 Although the authors report a high success rate (100%), the FMT requirement in their study was nearly double compared with our FMT combined with vancomycin approach (2.8 vs. 1.5).
FMT may be delivered by different routes including colonoscopy, retention enema, naso-enteric tube and capsules.51-53 Although FMT administration by upper route such as nasogastric tube in severe and debilitated CDI patients was advocated by others,32,54 we remain concerned that in the presence of ileus, the transplanted microbiota will not reach the colon. Given the prevalence of critical care ileus, upper gastrointestinal delivery including FMT capsules should not be used in this patient population given the risk of a potentially fatal fecal aspiration.55 Additionally, while non-endoscopic delivery of FMT such as rectal enema would be ideal (low cost, safety profile, lack of sedation),34 we found that most severely ill patients are unable to retain the enema for sufficient length of time due to poor rectal sphincter tone. Enema administration also does not facilitate assessment of mucosal response to treatment and a response-based therapy. We found that pseudomembranes are a useful prognostic marker of disease burden and predictor of needing repeat FMT(s). In our initial experience before implementing the study protocol, CDI patients who did not have complete resolution of pseudomembranes by the 2nd FMT, even a few scattered patches, invariably relapsed without the addition of subsequent antibiotics. In our view, continuation of vancomycin or another anti-CDI antibiotic following FMT appears to be catalytic to cure when pseudomembranes are present but warrants further study. In our opinion, colonoscopy can be performed safely by a skilled endoscopist without complications in patients with severe CDI, even in the setting of toxic megacolon. With gentle CO2 insufflation and avoidance of loop formation, in nearly all cases, the colonoscope can be advanced beyond the splenic flexure without complications. To facilitate repeated FMT delivery to the cecum, Zhang invented a colonic transendoscopic enteral tubing system that remains in place during the initial FMT and can be used for several days to weeks but requires further validation.56
The adoption of FMT by clinicians was hampered by logistics of preparation and complexity as well as cost of donor screening.57 Universal stool banks emerged to ensure universal access to FMT and enable clinicians to treat CDI patients both effectively and safely.58 In a 2,050 patient multi-center cohort, an international stool bank reported an 84.0% clinical cure rate from physician-reported data across all delivery modalities and CDI patient populations (recurrent, refractory, severe/severe-complicated). From a safety perspective, 42 serious adverse events were reported as part of a mandatory safety system; however, no adverse events were determined to be definitely related to FMT, 3 were possibly related to FMT and 39 not related based on NIH criteria.59 A significant advantage of using local or public stool banks using a universal donor is the availability of FMT material for immediate treatment. When implementing a patient-selected donor, donor screening may take 3–7 d and the candidate donor commonly fails screening. However, in this critically ill population, early intervention is crucial to prevent further deterioration and death. Therefore, our FMT program switched to frozen stool sourced from pre-screened universal donors and prepared in a stool bank allowing urgent FMT to occur within 30 minutes. Our analysis suggests there was no statistically significant difference between fresh vs. frozen stool consistent with other publications.60,61
Our study has several limitations. This study was not controlled and outcome assessment was not blinded. The absence of a placebo arm is a significant drawback; however, a significant placebo effect seems unlikely given the severity of CDI symptoms and mortality as outcome metrics. Also, given the severe consequences of a placebo arm (colectomy) and the clear benefit of FMT compared with historical controls, a placebo-controlled trial would be difficult to justify.62 This is also a single center experience with a skilled endoscopist that may not be generalizable. Lastly, there is an absence of microbiome profiling to help navigate mechanistic insights and longer follow-up may be helpful in confirming these promising results. There are several ongoing questions and future studies would be well placed to evaluate: a) role of CDI-antimicrobial therapy, whether it is crucial or if repeat FMTs in short cycles could alleviate the need of antimicrobial therapy; b) type of CDI-antimicrobial therapy if used; c) optimal length of antimicrobial therapy following the first, second or third FMT; d) the utility of endoscopic FMT delivery vs. rectal enema in critically ill patients; e) role of bowel prep; f) role of pseudomembranes as a prognostic marker of disease severity and response to therapy g) timing of discharge after single dose of FMT.
In conclusion, FMT for patients with severe or severe-complicated CDI is a promising treatment and should be advocated early in the disease course to prevent colectomy and fatal consequences. Overall, endoscopic response guided FMT for severe and severe-complicated CDI had a 91% cure rate with no serious adverse events directly attributable to FMT. Given its beneficial results, favorable risk profile, and accessibility through frozen stool specimens, FMT should be considered before colectomy, even among patients that are too ill to be surgical candidates. Further studies are needed to better understand the utility of FMT in this patient population and whether endoscopic delivery is required.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed. ZK is employed by Openbiome.
Funding
The study was funded in part by IU GI Division endowment to MF. ZK is in part funded by the Neil & Anna Rasmussen Foundation and Anna-Maria & Stephen Kellen Foundation.
References
- [1].Prevention CfDCa. Antibiotic Resistance Threats in the United States, 2013. Accessed at www.cdc.gov/drugresistance/threat-report-2013). [Google Scholar]
- [2].Lessa FC, Winston LG, McDonald LC. Emerging Infections Program CdST. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369-70; PMID:26061850; http://dx.doi.org/ 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adams SD, Mercer DW. Fulminant Clostridium difficile colitis. Curr Opin Crit Care 2007; 13:450-5; PMID:17599017; http://dx.doi.org/ 10.1097/MCC.0b013e3282638879 [DOI] [PubMed] [Google Scholar]
- [4].Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, Simmons RL. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002; 235:363-72; PMID:11882758; http://dx.doi.org/ 10.1097/00000658-200203000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Seder CW, Villalba MR Jr., Robbins J, Ivascu FA, Carpenter CF, Dietrich M, Villalba MR Sr.. Early colectomy may be associated with improved survival in fulminant Clostridium difficile colitis: an 8-year experience. Am J Surg 2009; 197:302-7; PMID:19245905; http://dx.doi.org/ 10.1016/j.amjsurg.2008.11.001 [DOI] [PubMed] [Google Scholar]
- [6].Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research C. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg 2012; 99:1501-13; PMID:22972525; http://dx.doi.org/ 10.1002/bjs.8868 [DOI] [PubMed] [Google Scholar]
- [7].Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis 2013; 15:798-804; PMID:23350898; http://dx.doi.org/ 10.1111/codi.12134 [DOI] [PubMed] [Google Scholar]
- [8].Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg 2011; 254:423-7; discussion 7-9; PMID:21865943; http://dx.doi.org/ 10.1097/SLA.0b013e31822ade48 [DOI] [PubMed] [Google Scholar]
- [9].Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg 2008; 196:384-8; PMID:18519126; http://dx.doi.org/ 10.1016/j.amjsurg.2007.11.017 [DOI] [PubMed] [Google Scholar]
- [10].Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, Rutks I, Wilt TJ. Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Ann Intern Med 2015; 162:630-8; PMID:25938992; http://dx.doi.org/ 10.7326/M14-2693 [DOI] [PubMed] [Google Scholar]
- [11].Allegretti JR, Kearney S, Li N, Bogart E, Bullock K, Gerber GK, Bry L, Clish CB, Alm E, Korzenik JR. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther 2016; 43:1142-53; PMID:27086647; http://dx.doi.org/ 10.1111/apt.13616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol 2013; 108:177-85; PMID:23318479; http://dx.doi.org/ 10.1038/ajg.2012.450 [DOI] [PubMed] [Google Scholar]
- [13].Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 2016; 13:508-16; PMID:27329806; http://dx.doi.org/ 10.1038/nrgastro.2016.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weingarden AR, Dosa PI, DeWinter E, Steer CJ, Shaughnessy MK, Johnson JR, Khoruts A, Sadowsky MJ. Changes in Colonic Bile Acid Composition following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium difficile Germination and Growth. PLoS One 2016; 11:e0147210; PMID:26789728; http://dx.doi.org/ 10.1371/journal.pone.0147210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478-98; quiz 99; PMID:23439232; http://dx.doi.org/ 10.1038/ajg.2013.4 [DOI] [PubMed] [Google Scholar]
- [16].Moayyedi P, Marshall JK, Yuan Y, Hunt R. Canadian Association of Gastroenterology position statement: fecal microbiota transplant therapy. Can J Gastroenterol Hepatol 2014; 28:66-8; PMID:25232572; http://dx.doi.org/ 10.1155/2014/346590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical M, Infectious D. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20 Suppl 2:1-26; PMID:24118601; http://dx.doi.org/ 10.1111/1469-0691.12418 [DOI] [PubMed] [Google Scholar]
- [18].van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al.. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407-15; PMID:23323867; http://dx.doi.org/ 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- [19].Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 312:1772-8; PMID:25322359; http://dx.doi.org/ 10.1001/jama.2014.13875 [DOI] [PubMed] [Google Scholar]
- [20].Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, Sanguinetti M, Gasbarrini A. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41:835-43; PMID:25728808; http://dx.doi.org/ 10.1111/apt.13144 [DOI] [PubMed] [Google Scholar]
- [21].Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al.. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med 2016; 165:609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854-9; PMID:13592638 [PubMed] [Google Scholar]
- [23].You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med 2008; 148:632-3; PMID:18413636; http://dx.doi.org/ 10.7326/0003-4819-148-8-200804150-00024 [DOI] [PubMed] [Google Scholar]
- [24].Neemann K, Eichele DD, Smith PW, Bociek R, Akhtari M, Freifeld A. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis 2012; 14:E161-5; PMID:23121625; http://dx.doi.org/ 10.1111/tid.12017 [DOI] [PubMed] [Google Scholar]
- [25].Gweon TG, Lee KJ, Kang DH, Park SS, Kim KH, Seong HJ, Ban TH, Moon SJ, Kim JS, Kim SW. A case of toxic megacolon caused by clostridium difficile infection and treated with fecal microbiota transplantation. Gut Liver 2015; 9:247-50; PMID:25721003; http://dx.doi.org/ 10.5009/gnl14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe clostridium difficile infection? J Clin Gastroenterol 2011; 45:655-7; PMID:21716124; http://dx.doi.org/ 10.1097/MCG.0b013e3182257d4f [DOI] [PubMed] [Google Scholar]
- [27].Shin JY, Ko EJ, Lee SH, Shin JB, Kim SI, Kwon KS, Kim HG, Shin YW, Bang BW. Refractory pseudomembranous colitis that was treated successfully with colonoscopic fecal microbial transplantation. Intest Res 2016; 14:83-8; PMID:26884739; http://dx.doi.org/ 10.5217/ir.2016.14.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim JE, Gweon TG, Yeo CD, Cho YS, Kim GJ, Kim JY, Kim JW, Kim H, Lee HW, Lim T, et al.. A case of Clostridium difficile infection complicated by acute respiratory distress syndrome treated with fecal microbiota transplantation. World J Gastroenterol 2014; 20:12687-90; PMID:25253977; http://dx.doi.org/ 10.3748/wjg.v20.i35.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gallegos-Orozco JF, Paskvan-Gawryletz CD, Gurudu SR, Orenstein R. Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex 2012; 77:40-2; PMID:22450020 [PubMed] [Google Scholar]
- [30].Yu S, Abdelkarim A, Nawras A, Hinch BT, Mbaso C, Valavoor S, Safi F, Hammersley J, Tang J, Assaly R. Fecal Transplant for Treatment of Toxic Megacolon Associated With Clostridium Difficile Colitis in a Patient With Duchenne Muscular Dystrophy. Am J Ther 2016; 23:e609-13; PMID:24858336; http://dx.doi.org/ 10.1097/MJT.0000000000000062 [DOI] [PubMed] [Google Scholar]
- [31].Aroniadis OC, Brandt LJ, Greenberg A, Borody T, Kelly CR, Mellow M, Surawicz C, Cagle L, Neshatian L, Stollman N, et al.. Long-term Follow-up Study of Fecal Microbiota Transplantation for Severe and/or Complicated Clostridium difficile Infection: A Multicenter Experience. J Clin Gastroenterol 2016; 50:398-402; PMID:26125460 [DOI] [PubMed] [Google Scholar]
- [32].Zainah H, Hassan M, Shiekh-Sroujieh L, Hassan S, Alangaden G, Ramesh M. Intestinal microbiota transplantation, a simple and effective treatment for severe and refractory Clostridium difficile infection. Dig Dis Sci 2015; 60:181-5; PMID:25052150; http://dx.doi.org/ 10.1007/s10620-014-3296-y [DOI] [PubMed] [Google Scholar]
- [33].Weingarden AR, Hamilton MJ, Sadowsky MJ, Khoruts A. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. J Clin Gastroenterol 2013; 47:735-7; PMID:23632358; http://dx.doi.org/ 10.1097/MCG.0b013e31829004ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee CH, Belanger JE, Kassam Z, Smieja M, Higgins D, Broukhanski G, Kim PT. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis 2014; 33:1425-8; PMID:24627239; http://dx.doi.org/ 10.1007/s10096-014-2088-9 [DOI] [PubMed] [Google Scholar]
- [35].Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered enthusiasm for fecal transplant. Clin Infect Dis 2014; 59:319; PMID:24759832; http://dx.doi.org/ 10.1093/cid/ciu278 [DOI] [PubMed] [Google Scholar]
- [36].Fischer M, Sipe BW, Rogers NA, Cook GK, Robb BW, Vuppalanchi R, Rex DK. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharmacol Ther 2015; 42:470-6; PMID:26096320; http://dx.doi.org/ 10.1111/apt.13290 [DOI] [PubMed] [Google Scholar]
- [37].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987; 40:373-83; PMID:3558716; http://dx.doi.org/ 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- [38].Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, et al.. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044-9; PMID:21871249; http://dx.doi.org/ 10.1016/j.cgh.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dubois N LK, Osman M, Burns L, Mendolia G, Blackler D, Burgess J, Edelstein C, Noh A, Vo E, Alm E, Smith M, Kassam Z. Prospective Assessment of Donor Eligibility for Fecal Microbiota Transplantation at a Public Stool Bank: Results From the Evaluation of 1,387 Candidate Donors. IDWeek 2015. p. 962 Available at https://idsa.confex.com/idsa/2015/webprogram/Paper52979.html [Google Scholar]
- [40].Smith M KZ, Burgess J, Perrotta AR, Burns LJ, Mendolia GM, Dubois N, Edelstein C, Noh A, Alm E. The International Public Stool Bank: A Scalable Model for Standardized Screening and Processing of Donor Stool for Fecal Microbiota Transplantation. Gastroenterology 2015; 148:S211; http://dx.doi.org/ 10.1016/S0016-5085(15)30704-6 [DOI] [Google Scholar]
- [41].Ferrada P, Velopulos CG, Sultan S, Haut ER, Johnson E, Praba-Egge A, Enniss T, Dorion H, Martin ND, Bosarge P, et al.. Timing and type of surgical treatment of Clostridium difficile-associated disease: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2014; 76:1484-93; PMID:24854320; http://dx.doi.org/ 10.1097/TA.0000000000000232 [DOI] [PubMed] [Google Scholar]
- [42].Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg 2008; 143:150-4; discussion 5; PMID:18283139; http://dx.doi.org/ 10.1001/archsurg.2007.46 [DOI] [PubMed] [Google Scholar]
- [43].Dallas KB, Condren A, Divino CM. Life after colectomy for fulminant Clostridium difficile colitis: a 7-year follow up study. Am J Surg 2014; 207:533-9; PMID:24674828; http://dx.doi.org/ 10.1016/j.amjsurg.2013.04.008 [DOI] [PubMed] [Google Scholar]
- [44].Cammarota G, Ianiro G, Magalini S, Gasbarrini A, Gui D. Decrease in Surgery for Clostridium difficile Infection After Starting a Program to Transplant Fecal Microbiota. Ann Intern Med 2015; 163:487-8; PMID:26370022; http://dx.doi.org/ 10.7326/L15-5139 [DOI] [PubMed] [Google Scholar]
- [45].Nguyen L OM, Chiang A, Edelstein C, Fischer M, Ananthakrishnan A, Allegretti J, Smith M, Kassam Z. The Cost-Effectiveness of Competing Strategies for Treating Severe-Complicated Clostridium difficileInfection: Comparing Fecal Microbiota Transplantation With Standard Colectomy. Gastroenterology 2015; 150:S543; http://dx.doi.org/ 10.1016/S0016-5085(16)31862-5 [DOI] [Google Scholar]
- [46].Clanton J, Fawley R, Haller N, Daley T, Porter J, Paranjape C, Bonilla H. Patience is a virtue: an argument for delayed surgical intervention in fulminant Clostridium difficile colitis. Am Surgeon 2014; 80:614-9; PMID:24887802 [PubMed] [Google Scholar]
- [47].Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013; 108:500-8; PMID:23511459; http://dx.doi.org/ 10.1038/ajg.2013.59 [DOI] [PubMed] [Google Scholar]
- [48].Fischer M, Kao D, Mehta SR, Martin T, Dimitry J, Keshteli AH, Cook GK, Phelps E, Sipe BW, Xu H, et al.. Predictors of Early Failure After Fecal Microbiota Transplantation for the Therapy of Clostridium Difficile Infection: A Multicenter Study. Am J Gastroenterol 2016; 111:1024-31; PMID:27185076; http://dx.doi.org/ 10.1038/ajg.2016.180 [DOI] [PubMed] [Google Scholar]
- [49].Pecere S, Sabatelli M, Fantoni M, Ianiro G, Gasbarrini A, Cammarota G. Letter: faecal microbiota transplantation in combination with fidaxomicin to treat severe complicated recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 42:1030; PMID:26374258; http://dx.doi.org/ 10.1111/apt.13362 [DOI] [PubMed] [Google Scholar]
- [50].Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis 2012; 55 Suppl 2:S132-42; PMID:22752862; http://dx.doi.org/ 10.1093/cid/cis338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kassam Z, Lee CH, Hunt RH. Review of the emerging treatment of Clostridium difficile infection with fecal microbiota transplantation and insights into future challenges. Clin Lab Med 2014; 34:787-98; PMID:25439277; http://dx.doi.org/ 10.1016/j.cll.2014.08.007 [DOI] [PubMed] [Google Scholar]
- [52].Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med 2012; 172:191-3; PMID:22271132; http://dx.doi.org/ 10.1001/archinte.172.2.191 [DOI] [PubMed] [Google Scholar]
- [53].Stollman N, Smith M, Giovanelli A, Mendolia G, Burns L, Didyk E, Burgess J, Noh A, Edelstein C, Alm E, et al.. Frozen encapsulated stool in recurrent Clostridium difficile: exploring the role of pills in the treatment hierarchy of fecal microbiota transplant nonresponders. Am J Gastroenterol 2015; 110:600-1; PMID:25853204; http://dx.doi.org/ 10.1038/ajg.2015.81 [DOI] [PubMed] [Google Scholar]
- [54].Gweon TG, Kim J, Lim CH, Park JM, Lee DG, Lee IS, Cho YS, Kim SW, Choi MG. Fecal Microbiota Transplantation Using Upper Gastrointestinal Tract for the Treatment of Refractory or Severe Complicated Clostridium difficile Infection in Elderly Patients in Poor Medical Condition: The First Study in an Asian Country. Gastroenterol Res Pract 2016; 2016:2687605; PMID:27127501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, Yan F, Cao H, Wang B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One 2016; 11:e0161174; PMID:27529553; http://dx.doi.org/ 10.1371/journal.pone.0161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peng Z, Xiang J, He Z, Zhang T, Xu L, Cui B, Li P, Huang G, Ji G, Nie Y, et al.. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc Int Open 2016; 4:E610-3; PMID:27556065; http://dx.doi.org/ 10.1055/s-0042-105205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moore T, Rodriguez A, Bakken JS. Fecal microbiota transplantation: a practical update for the infectious disease specialist. Clin Infect Dis 2014; 58:541-5; PMID:24368622; http://dx.doi.org/ 10.1093/cid/cit950 [DOI] [PubMed] [Google Scholar]
- [58].Smith M, Kassam Z, Edelstein C, Burgess J, Alm E. OpenBiome remains open to serve the medical community. Nat Biotechnol 2014; 32:867; PMID:25203030; http://dx.doi.org/ 10.1038/nbt.3006 [DOI] [PubMed] [Google Scholar]
- [59].Osman M ObK, Stoltzner Z, Ling K, Koelsch E, Dubois N, Khoiri A, Amaratunga K, Smith M, Kassam Z. Safety and efficacy of fecal microbiota transplantation for recurrent Clostridium difficile infection from an international public stool bank: Results from a 2,050 patient multi-center cohort IDWeek; 2016; New Orleans. [Google Scholar]
- [60].Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013; 4:125-35; PMID:23333862; http://dx.doi.org/ 10.4161/gmic.23571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M, et al.. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2016; 315:142-9; PMID:26757463; http://dx.doi.org/ 10.1001/jama.2015.18098 [DOI] [PubMed] [Google Scholar]
- [62].American Medical Association Code of Medical Ethics' opinion on cultural sensitivity and ethnic disparities in care. The virtual mentor : VM 2012; 14:312-3; PMID:23352066 [DOI] [PubMed] [Google Scholar]


