ABSTRACT
Fecal microbiota transplantation (FMT) is a highly effective treatment of recurrent and recalcitrant Clostridium difficile infection (rCDI). In a recent study oral-delivery of encapsulated, freeze-dried donor material, resulted in comparable rates of cure to colonoscopic approaches. Here we characterize shifts in the fecal bacterial community structure of patients treated for rCDI using encapsulated donor material. Prior to FMT, patient fecal samples showed declines in diversity and abundance of Firmicutes and Bacteroidetes, with concurrent increases in members of the Proteobacteria, specifically Enterobacteriaceae. Moreover, patients who experienced recurrence of CDI within the 2-month clinical follow-up had greater abundances of Enterobacteriaceae and did not show resolution of dysbioses. Despite resolution of rCDI following oral-administration of encapsulated fecal microbiota, community composition was slow to return to a normal donor-like assemblage. Post-FMT taxa within the Firmicutes showed rapid increases in relative abundance and did not vary significantly over time. Conversely, Bacteroidetes taxa only showed significant increases in abundance after one month post-FMT, corresponding to significant increases in the community attributable to the donors. Changes in the associations among dominant OTUs were observed at days, weeks, and months post-FMT, suggesting shifts in community dynamics may be related to the timing of increases in abundance of specific taxa. Administration of encapsulated, freeze-dried, fecal microbiota to rCDI patients resulted in restoration of bacterial diversity and resolution of dysbiosis. However, shifts in the fecal microbiome were incremental rather than immediate, and may be driven by changes in community dynamics reflecting changes in the host environment.
KEYWORDS: Capsule-delivered FMT, Clostridium difficile, cure, fecal microbial transplantation, 16S rRNA, next-generation sequencing, microbial community structure
Background
Fecal microbiota transplantation (FMT) is an effective, alternative method to treat recurrent Clostridium difficile infection (rCDI),1 which is now incorporated into standard treatment guidelines in both the United States and Europe. Randomized clinical trials (RCTs) have reported that colonoscopic- and nasogastric-delivered FMT resulted in > 80% cure,1-4 superior to any antibiotic therapies. Unlike standard antibiotic therapy, which only worsens gut dysbiosis and leaves patients even more vulnerable to reinfection, FMT restores the diversity and functionality of gut microbial communities. Until recently, the procedure generally involved infusions of liquid suspensions prepared from healthy stool into the patient's gastrointestinal tract via colonoscopy, nasogastric tube, or fecal enemas.5 The efficacy of using frozen and thawed donor material has been shown to resolve rCDI symptoms6 and was recently shown to be similar in effectiveness to fresh donor material in a RCT,7 with cure rates > 92%. Furthermore, FMT administered orally using frozen, encapsulated donor material has shown similar results to other methods of administration.8
Characterization of the gut microbiome of pre-FMT rCDI patients has consistently revealed, in multiple studies, reduced relative abundances of taxa within the Bacteroidetes and Firmicutes, concurrent with increased relative abundances of Enterobacteriaceae and Bacilli, relative to healthy individuals.9-11 Following FMT, the abundances of several prominent genera increase, including Bacteroides as well as Blautia, Coprococcus, Faecalibacterium, Papillibacter, and Roseburia, in the class Clostridia (Firmicutes), similar to what is seen in healthy donors.10 Concurrently, the relative abundances of Enterobacteriaceae, including Enterobacter, Escherichia, and Raoultella, as well as Lactobacillus and Veillonella decrease following FMT.10,11 Interestingly, after colonoscopic FMT the gut microbiome of patients resembled that of the donor inoculum within a day, after which the patient microbial communities fluctuated in their composition, likely reflective of the normal dynamic behavior of gut microbiota.11
The mechanism(s) of success of FMT are beginning to be elucidated,12 and in addition to restoring gut microbial ecology, several studies have shown a direct association between success of FMT and the restoration of normal bile acid metabolism in the colon.13,14 Recently, Buffie and colleagues14 applied mathematical modeling to identify taxa associated with resistance to rCDI using both patient samples and mouse models. A consortium of 4 of these taxa was used to treat C. difficile infection in mice, resulting in a resolution of the infection.14 However, further investigation revealed that C. scindens was the critical member of the consortium, and the remaining 3 species alone could not abrogate symptoms.14 The authors suggested that C. scindens plays a critical role in restoration of secondary bile acid biosynthesis. Therefore, complete engraftment (successful and sustained transfer) of donor communities may not be essential for the success of FMT in resolving CDI, but is likely necessary for rapid reestablishment of gut microbial community structure and function.
A preliminary feasibility study recently showed that orally-administered capsules containing frozen donor material, which we term “capsule FMT,” resulted in resolution of rCDI in 70% of patients after an initial course and 90% after follow-up courses among patients who relapsed.8 The authors noted that the time to resolution of symptoms by capsule FMT was significantly longer than that observed for administration via colonoscopy or nasogastric tube FMT (P = 0.03). The authors also suggested that, although further validation was necessary, capsule FMT may represent a more cost-effective, less-invasive treatment of rCDI. However, investigation into the mechanism(s) of success of capsule FMT was not pursued and shifts in the microbiome following capsule FMT were not examined. Thus, it is possible that the delay in resolution of symptoms may reflect slower shifts in the microbiome and this remains to be explored.
In the current study, we describe microbiome analysis from an evolving case series in which freeze-dried and encapsulated donor fecal material was used to treat patients with multiply recurrent C. difficile infection.15 Bacterial communities from fecal samples were characterized using Illumina next-generation sequencing of the V5-V6 hypervariable regions of the 16S rRNA gene, and bile acid composition of fecal samples was measured by using liquid chromatography-mass spectrometry (LC-MS). To evaluate the succession of bacterial communities, patient fecal samples were collected before capsule administration and up to 195 d post-FMT. We hypothesized that bacterial community shifts associated with FMT using capsule-administered fecal microbiota would result in potentially slower, more gradual shifts in community composition, since time to resolution of symptoms was previously observed to take longer in the initial feasibility study.8 Bacterial communities, however, were anticipated to resemble donor communities, similar to standard FMT, after the first week post-treatment and maintain levels of engraftment over time. This study provides an assessment of bacterial community succession following treatment of rCDI using a novel preparation of freeze-dried, encapsulated donor material and provides insights into those taxa critical to rapid resolution of rCDI symptoms.
Results
Fecal samples were collected from a cohort of 39 patients, in total, who received capsule FMT (Supplementary Table S1). Of these, 32 (82%) showed no recurrence of diarrheal symptoms, or detection of C. difficile toxin, throughout the entire follow-up period (up to 195 days). The remaining 7 patients showed recurrences either within the clinical 2-month follow-up period or at later time points. More detailed clinical findings regarding the efficacy of capsule FMT are described elsewhere.15 The frequency of patient sample collection varied for each patient as a result of clinical instruction as the case series evolved. Samples were grouped as: i) pre-FMT, ii) days following FMT, iii) weeks following FMT, iv) months following FMT, and v) longer-term samples (described more thoroughly in methods). This was done to account for differences in the timing and amounts of samples and time periods during which samples were collected.
Effect of prior FMT on diversity
Samples were initially evaluated to determine if differences in clinical outcome (cure: resolution of diarrheal symptoms and/or C.-difficile-toxin negative for 2 months; or recurrence: symptoms recurred and toxin detected) or prior colonoscopic FMT affected communities characterized at time points following FMT (Table S1). No differences in α diversity were observed among post-FMT samples, based on either the Shannon or ACE indices (P ≥ 0.149), as a result of either clinical outcome (i.e. cure or recurrence; Fig. 1A) or prior colonoscopic FMT experience (data not shown). Therefore, patient samples were not grouped separately based on prior FMT intervention. Similar to α diversity, prior intervention via colonoscopically-administered FMT did not significantly affect community composition, as assessed by analysis of similarity (ANOSIM P ≥ 0.067).
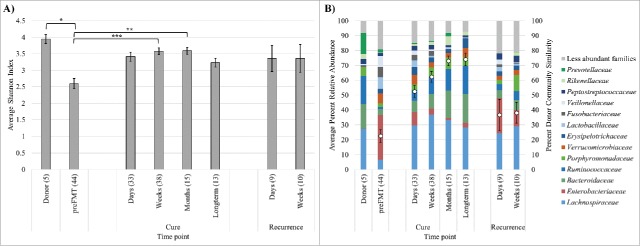
Figure 1.
Shannon indices (A) and distributions of abundant families (B) among sample groups. Families shown are those present at mean >2.0 % among all samples, and bars indicate averages of family abundances for each sample group. The percent donor similarity, determined by SourceTracker, is shown within the stacked column (mean ± standard error). Significance *P < 0.05, **P < 0.01, *** P < 0.0001 by Tukey's post-hoc test. Numbers in parentheses indicate numbers of samples averaged.
Bacterial community α diversity and composition
Among all donor and patient samples who received capsule FMT a mean Good's community coverage of 99.1 ± 0.1 % (mean ± standard error) was achieved via sequencing, with an average of 283 ± 25 operational taxonomic units (OTUs) observed in a single sample. Diversity, as measured by the Shannon index (Fig. 1A), was significantly greater among donor samples and patients who were cured via capsule FMT than their pre-FMT samples (Fisher's F < 0.0001; Tukey's post-hoc tests shown in Fig. 1A). However, Shannon indices among samples from patients who experienced a recurrence within the first month post-FMT did not differ significantly from donors by post-hoc test (P ≥ 0.863). The abundance-based coverage estimate (ACE) of richness ranged from 35 – 2,273 for individual samples, but differences in ACE richness did not differ significantly among the sample groupings shown in Figure 1 (P = 0.628).
Donor communities were primarily comprised of members of the families Lachnospiraceae and Ruminococcaceae, among the Firmicutes, and Bacteroidaceae, Porphyromonadaceae, and Prevotellaceae, among the Bacteroidetes (Fig. 1B). In contrast, pre-FMT samples showed lower relative abundances within these 2 phyla and greater abundances of Enterobacteriaceae in the phylum Proteobacteria. Fecal samples from patients who were cured following treatment with encapsulated freeze-dried microbiota showed increased relative abundances of the Firmicutes, primarily Lachnospiraceae, within the first weeks post-FMT. In contrast, Bacteroidaceae (among other Bacteroidetes families) did not show marked increases in abundance until after the first month post-FMT. Samples from patients who had a recurrence of infection, within the first weeks, showed generally similar trends but also harbored greater relative abundances of Enterobacteriaceae (Proteobacteria) than did samples from cured patients and lower relative abundances within the Firmicutes and Bacteroidetes.
β diversity analysis of donor and patient communities
Ordination of Bray-Curtis dissimilarity matrices among samples by PCoA (Fig. 2) showed significant separation of pre-FMT from donor samples along the x-axis (P < 0.0001), as evaluated by analysis of molecular variance (AMOVA). Furthermore, significant, independent clustering of donors from all other patient sample groups was observed by AMOVA (P ≤ 0.001). Independent clustering of pre-FMT samples from patient groups was also significant (P < 0.001). Analysis of β diversity (community composition) by ANOSIM showed the same pattern for pre-FMT samples as did AMOVA analyses. Donor community composition, however, only differed significantly from pre-FMT samples (P < 0.001) from patients in whom rCDI recurred within 6 d (P = 0.022), and long-term samples collected >2 months post-FMT (P = 0.034).
Figure 2.
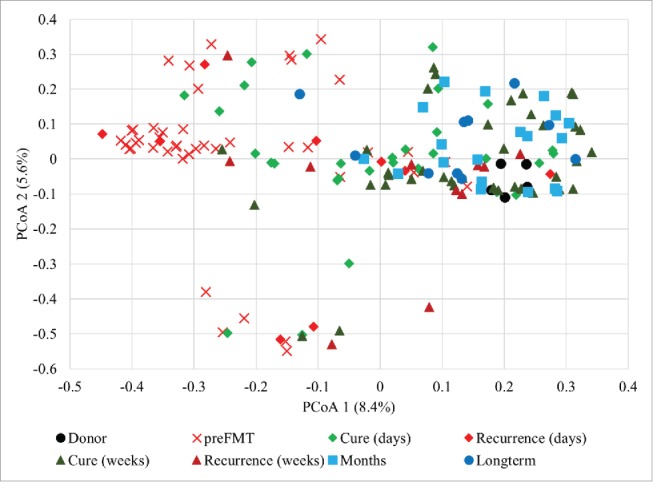
Principal coordinate analysis of Bray-Curtis dissimilarity matrices constructed from OTU tables of donor and patient samples (r2 = 0.341).
Based on both ANOSIM and AMOVA, samples collected within the first days post-FMT clustered closely and had similar community composition among themselves, regardless of clinical outcome (P = 0.079 and 0.220 for ANOSIM and AMOVA, respectively). Similarly, samples collected 1 to 3 weeks post-FMT were not significantly differentiated by clinical outcome by either analysis (P = 0.062 and 0.091). Among the cured patients, the “days” and “weeks” time points were significantly different (P ≤ 0.012), while this was not the case for patients who showed recurrence (P ≥ 0.542). Fecal microbiota in samples collected 1 to 2 months post-FMT were significantly differentiated from those obtained from patients who showed recurrence (P ≤ 0.006), but were similar to samples taken from cured patients after one week post-FMT or samples collected >2 months post-FMT (P ≥ 0.127). Microbiota in samples collected >2 months post-FMT showed significant differences in β diversity from the donor, pre-FMT, and recurrent patient samples collected within days (P ≤ 0.034).
Engraftment of donor communities
Donor engraftment, measured as the percent of the community attributable to donor OTUs using SourceTracker,16 was positively correlated with time (number of days) post-FMT (Fig. 1; Spearman's ρ = 0.547, P < 0.0001). Prior to FMT, samples showed low similarity to donor communities (22.6 ± 4.4%), and the proportion of donor-attributable microbiota in these samples, relative to those of patients who had recurrence of infection, was not significantly different by post-hoc test (P = 0.599 and 0.480 for days and weeks, respectively). Donor similarity in samples from cured patients at any time post-FMT, as measured by SourceTracker, was significantly greater than pre-FMT samples (P ≤ 0.006). In addition, samples from cured patients taken at least one week post-FMT showed significantly greater donor similarity than that observed in samples from patients who had recurrence (P ≤ 0.050). Among cured patients, the percentage of donor similarity among microbiota was significantly greater after at least one month post-FMT than that observed within days (P ≤ 0.016), but was similar to that observed after a week (P ≥ 0.207).
The small proportion of the microbial community attributable to donors in pre-FMT samples was comprised primarily of the genera Escherichia/Shigella, Bacteroides, and Akkermansia (Fig. 3). These genera, along with several others within the Firmicutes and Bacteroidetes, accounted for increases in donor community similarity in samples obtained days and weeks post-FMT, regardless of clinical outcome. However, among cured patient samples, the relative abundances of the genera Bacteroides, Parabacteroides, and Alistipes were also greater after months post-FMT, relative to earlier time points. Moreover, since a small proportion of microbiota were attributed to donors before FMT, genera were further investigated among post-FMT samples from cured patients and those collected ≥ 1 month post-FMT. This was done to determine if abundances of genera correlated with the number of days following FMT (Table 1). Results of this analysis indicated that abundances of several of the genera identified, including Bacteroides, Roseburia, and clade Clostridium IV, trended positively with time, although the Escherichia/Shigella and Clostridium XI showed negative relationships.
Figure 3.
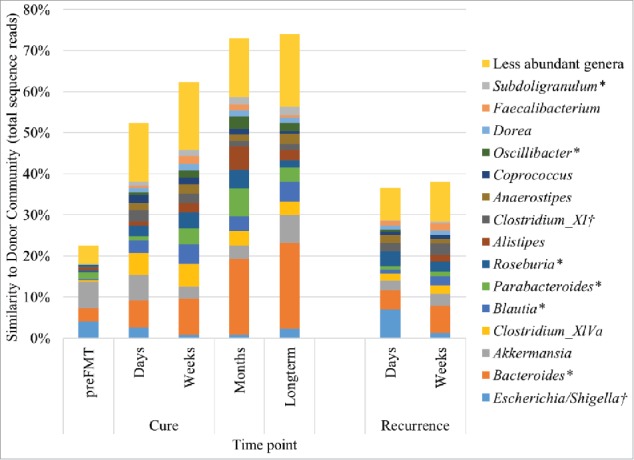
Distribution of genera associated with donor engraftment by SourceTracker, determined as percent similarity to donor communities. The abundances of several genera were also determined to be significantly positively (*) or negatively (†) correlated with time (days) post-FMT among cured patient samples and those collected ≥ 1 month post-FMT (Table 1).
Table 1.
Spearman correlations relating relative abundances of genera with time (days) post-FMT*.
| Genus† | Family | Spearman's ρ | P value‡ |
|---|---|---|---|
| Bacteroides | Bacteroidaceae | 0.289 | 0.004 |
| Blautia | Bacteroidaceae | 0.238 | 0.018 |
| Parabacteroides | Porphyromonadaceae | 0.257 | 0.011 |
| Escherichia/Shigella | Enterobacteriaceae | −0.261 | 0.009 |
| Roseburia | Lachnospiraceae | 0.205 | 0.042 |
| Fusobacterium | Fusobacteriaceae | −0.399 | <0.0001 |
| Lactobacillus | Lactobacillaceae | −0.610 | <0.0001 |
| Klebsiella | Enterobacteriaceae | −0.301 | 0.003 |
| Clostridium XI | Peptostreptococcaceae | −0.251 | 0.012 |
| Raoultella | Enterobacteriaceae | −0.459 | <0.0001 |
| Veillonella | Veillonellaceae | −0.407 | <0.0001 |
| Clostridium sensu stricto | Clostridiaceae 1 | −0.453 | <0.0001 |
| Oscillibacter | Oscillospiraceae | 0.357 | <0.001 |
| Subdoligranulum | Ruminococcaceae | 0.260 | 0.009 |
| Clostridium IV | Ruminococcaceae | 0.336 | 0.001 |
Note.
Only post-FMT samples from cured patients and those collected ≥ 1 month post-FMT were included in the analysis, and only genera with a mean abundance >1.0 % (n = 27) were included.
Genera are listed in order of abundance.
Values shown in bold were significant after Bonferroni correction for multiple comparisons (α = 0.002).
Evaluation of community dynamics
Correlations among the dominant OTUs, defined as those present at a mean >1.0 % among cured patient samples (n = 20), were assessed within days, weeks, and ≥ 1 month post-FMT, using the SparCC method. This method reduces detection of spurious relationships for large data sets containing relative abundance data.17 These analyses were used to evaluate community dynamics among individual time points to evaluate how these dynamics may be associated with shifts in the microbiome following ingestion of encapsulated, freeze-dried microbiota (Fig. 4). The proportion of positive:negative intra- and inter-phyla associations did not vary significantly by time point among samples collected from patients who were cured at days, weeks, or months following FMT (χ2, P ≥ 0.209). The number of significant associations was greatest, though, among samples collected weeks post-FMT (89, compared with 69 within days and 41 among samples collected >1 month post-FMT). Intra-phyla relationships were predominantly positive, while inter-phyla associations were typically negative at all 3 time points investigated. Among the samples collected within days, the strongest relationships were mainly observed among the Firmicutes (median r = 0.52 compared with r = 0.41 within Bacteroidetes), with stronger negative correlations between the Firmicutes and Bacteroidetes (median r = −0.18). Within weeks post-FMT, associations within the Bacteroidetes were slightly stronger than those among Firmicutes (median r = 0.39 versus r = 0.33), and negative relationships between these phyla were slightly weaker (median r = −0.16). Among samples collected after a month post-FMT, few relationships were observed between phyla, relative to earlier time points.
Figure 4.
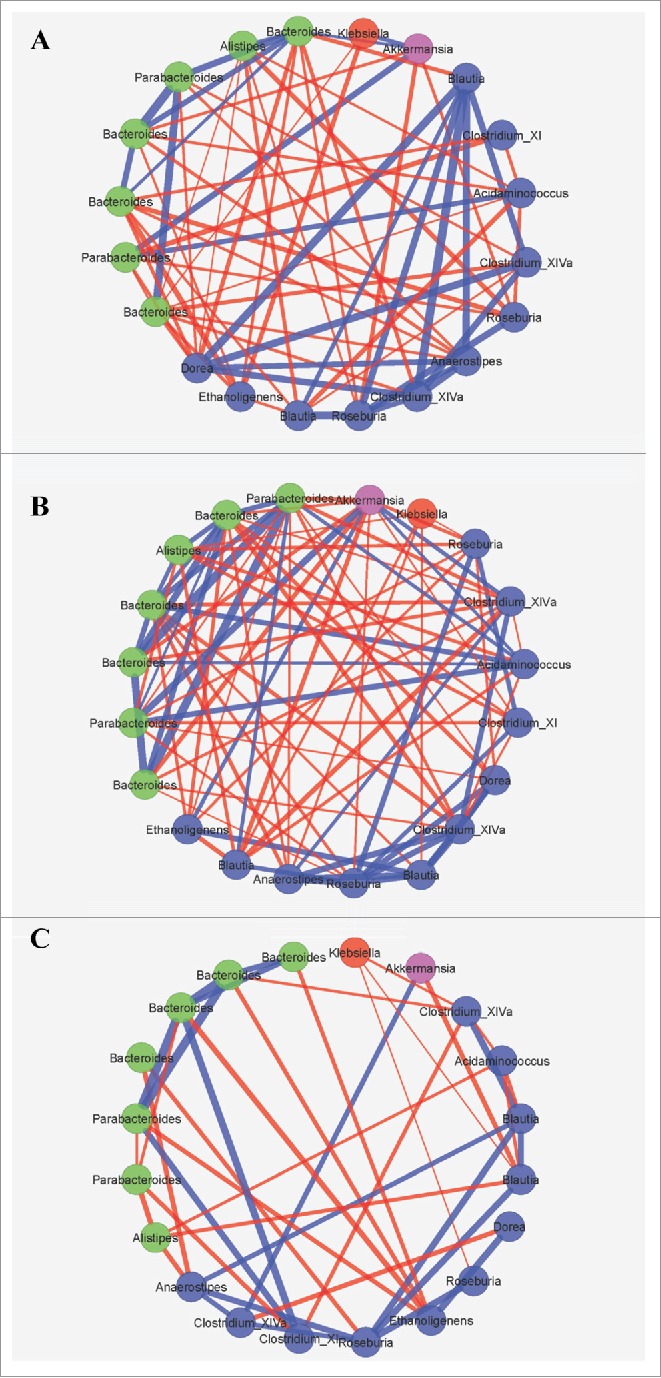
SparCC significant correlations (P < 0.05) of abundant OTUs in cured patient samples at (A) days, (B) weeks, and (C) months post-FMT. Symbols reflect phyla where blue symbols are Firmicutes, green symbols at Bacteroidetes, red are Proteobacteria, and pink are Verrucomicrobia. Line thickness reflects the strength of the correlation where blue line indicate positive and red lines indicate negative correlations.
Bile acid composition
Concentrations of the tauro-conjugated primary bile acids taurocholic acid (TCA) and taurochenodeoxycholic acid (TCDCA), as well as the deconjugated primary bile acid cholic acid (CA), tended to be higher in pre-FMT samples and those from patients who had rCDI recurrence (Fisher's F = 0.005, 0.049, and 0.045; Fig. 5). However, Tukey's post-hoc test did not reveal any significant pairwise differences in concentrations (P > 0.05). In contrast, the concentration of the secondary bile acid lithocholic acid (LCA) tended to be greater among samples from patients who were cured (Fisher's F = 0.015), but differences were not significant by post-hoc test. Similarly, concentrations of TCA (Spearman's ρ = −0.280, P = 0.001), CA (ρ = −0.293, P = 0.001), CDCA (ρ = −0.260, P = 0.003) were all significantly negatively correlated with the number of days post-FMT, among all samples from which bile acids were analyzed, following Bonferroni correction for multiple comparisons. The concentration of LCA was also significantly positively correlated with time post-FMT (ρ = 0.542, P = < 0.0001).
Figure 5.
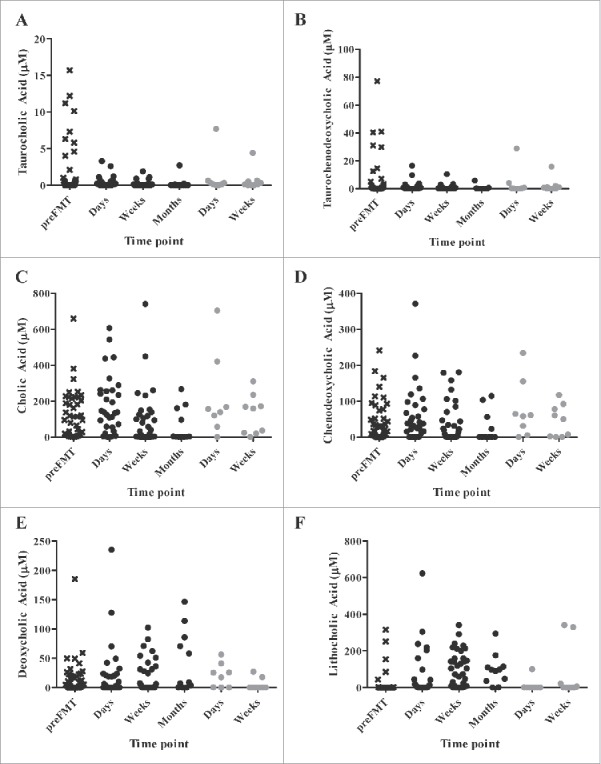
Concentrations of (A) taurocholic, (B) taurochenodeoxycholic, (C) cholic, (D) chenodeoxycholic, (E) deoxycholic, and (F) lithocholic acids in patient sample groups. Samples from cured patients are black and patients who had recurrence are gray.
Discussion
In this study, FMT that was done via orally-administered capsules containing freeze-dried donor fecal microbiota resulted in a similar restoration of α diversity as did that observed via colonoscopic administration.4 However, recovery of donor taxa, especially within the Bacteroidetes, was delayed compared with that seen following colonoscopic FMT. Previously, colonoscopic administration was shown to result in a return to donor-like assemblage within days following FMT.10,11 In contrast, in patients receiving capsules, approximately one month was required before Bacteroides reached donor-like proportions and maximal similarity to donor fecal microbial community structure, determined by SourceTracker, was achieved. Furthermore, while the microbiome of capsule FMT patients showed significant, incremental shifts in community composition, particularly between the days and weeks following FMT, it eventually had a donor-like structure at one month, followed by divergence from donors in longer-term samples. This longer-term divergence, as well as temporal variation within healthy individuals, has also been observed following colonoscopic administration among patient samples.11
The seemingly incremental shift in community composition among time points following capsule FMT may be related to the slower resolution of symptoms observed in the previous feasibility study.8 The microbiome of patients in the days following FMT remained somewhat dysbiotic, with elevated relative abundances of Enterobacteriaceae and Verrucomicrobiaceae, and was not indicative of clinical outcome at this stage. However, patients who were cured of rCDI showed a significant shift after one week, with reductions in these families that were not observed in patients who had a recurrence. This may suggest that these bacterial families serve as markers for unsuccessful engraftment or of underlying susceptibility to recurrence.
Analysis of bile acid composition among cured patients showed a significant decline in primary bile acids at weeks and months post-FMT and suggested an increase in secondary bile acid metabolism, as seen by the increase in LCA. Patients with rCDI, while taking vancomycin, have previously been shown to be deficient in secondary bile acids, which are restored following colonoscopic FMT.13 The role of bile acids in preventing germination and growth of C. difficile spores is complex. Unconjugated CDCA and DCA have been shown to inhibit spore germination in vitro,18 and restoration of bile acid metabolism has been suggested to be a mechanism by which cure occurs.12 In mouse models, antibiotic disruption of the colonic microbiota resulted in higher proportions of primary bile acids relative to secondary bile acids and this shift was correlated with greater colony formation from spores ex vivo.19,20
Stepwise changes in the microbiota are potentially related to changes in bile acid metabolism that correspond with the delayed expansion of Bacteroidetes. The abundances of bile-acid metabolizing Firmicutes, including Clostridium XIVa, Blautia, and Clostridium XI,21 were found to engraft quickly, and likely contributed to inhibition of C. difficile through the production of secondary bile acids.13 Increases in members of the Bacteroidetes were positively correlated with time post-FMT, and these data may suggest that Firmicutes may initially capitalize on the primary-bile-acid-rich, dysbiotic environment,22,21 which later becomes favorable to the expansion of Bacteroidetes upon resumption of normal bile acid metabolism.23 However, Firmicutes may also be more resistant to the stresses of material preparation and FMT due to the formation of spores, and thus more easily establish quickly following capsule FMT. The decline in associations between OTUs after one month post-FMT likely indicates the eventual establishment of a dynamic equilibrium of taxa that closely resembles, but is distinct, from the donor community and is resistant to re-infection.
One surprising result of this study was that complete donor engraftment, as determined by SourceTracker and supported by taxonomic characterization, proved to be unnecessary for the resolution of symptoms, as has been previously suggested.14,24 The percentage of engraftment through later time points corresponded with increases in the members of the Bacteroidetes, including members of the genera Bacteroides, Parabacteroides, and Alistipes, which were initially found at low abundances. Thus, it is likely that the majority of the donor assemblage was transferred at the time of the initial capsule FMT, but taxa were maintained at lower abundance due to difficulty resuscitating, perhaps as a result of gastrointestinal transit; injury of strict anaerobic microbiota during the preparation, freeze-drying, the encapsulation processes; or other host-associated factors. Previous studies have shown no difference in the efficacy or engraftment success using fresh or frozen donor material via colonoscopic administration for FMT,6,7 suggesting impairment of resuscitation is less restrictive using these preparations and procedures.
Conclusions
Results of this study indicated that FMT administered via capsules containing freeze-dried fecal microbiota resulted in restoration of colonic bacterial diversity, eventual resolution of dysbiosis, and cure of rCDI, but the shifts in microbiome were incremental rather than immediate, as was observed previously with colonoscopically-administered FMT. The succession of the community was characterized by an initial increase in Firmicutes, potentially due to exploitation of elevated concentrations of primary bile acids, or resistance to preparation and transfer, that concomitantly influenced early expansion of Bacteroidetes. Within a month following FMT, however, patient communities closely resembled those of the donor and showed normal temporal variability. This was also previously shown in long-term samples obtained from patients following colonoscopic FMT. This study provides a detailed characterization of the changes in the gut microbiome following restoration of gut microbiota by administration of encapsulated, freeze-dried microbiota, and provides important insights into the bacterial taxa predominantly responsible for suppression and resistance to rCDI.
Methods
Preparation of capsules
Donor fecal samples were collected as part of the University of Minnesota donor program, described previously.6 Five different lots from 3 donors [Nos. 20, 41 (3 lots), and 44] were used for capsule preparation. Each course of capsules was prepared from a single donor lot. To prepare capsules, fecal material was homogenized in a blender under N2 gas, sieved to remove larger particles, and concentrated by centrifugation as described previously.6 The fecal preparation was amended with 5% trehalose, rather than glycerol, freeze-dried, and encapsulated, as described in detail elsewhere.15 Capsules were maintained at −80°C until used. Administration of capsules, including bacterial concentration, number of capsules, and duration of therapy, varied during the case series as the clinical experience evolved, and is described in detail elsewhere.15 The total bacterial concentration administered varied from 2.1 × 1011 to 2.5 × 1012 cells, regardless of the number of capsules administered. DNA sequence analysis was performed on source donor fecal samples as described below, which were maintained at −80°C until processed.
Collection of patient samples
Detailed clinical information regarding the patient cohort is described in detail elsewhere,15 and information related to potential capsule FMT success and microbiota succession is summarized in Supplementary Table S1. Briefly, the cohort consisted of 39 patients who had experienced 2 spontaneous recurrences of C. difficile infection following the initial occurrence, failed to respond to an extended course of antibiotics, and were positive for C. difficile infection by stool testing within 3 months. Stool testing was performed by PCR for C. difficile toxin B.15 Patients were treated with a course of oral vancomycin that was discontinued 2 d before administration of capsules. Samples were collected by the patients in single-use specimen collector pans and transferred to 30 ml polystyrene fecal specimen containers (Globe Scientific, Inc., Paramus, NJ, USA). Samples were maintained in the patients' freezers before collection and were transported to the laboratory on dry ice and stored at −80°C until DNA extraction.
Throughout the course of the clinical experience, the number and frequency at which patients were asked to collected fecal samples varied (Supplementary Table S1). Initially, as a result of clinical success,15 defined as the resolution of diarrheal symptoms and negative stool testing for C. difficile toxin B within 2 months of administration, patients were only asked to collect samples through the first weeks of treatment. As microbiota data began to indicate a differential pattern of engraftment following encapsulated FMT from that observed with colonoscopic FMT i.e., donor-like taxonomic composition within days,10,11 the duration of sample collection was extended. Patients were instructed to collect their own fecal samples up to one week before taking capsules and at 3, 7, 14, and 30 d following administration of capsules. Longer-term samples were also requested at 90 d and one year following capsule administration. Furthermore, while several patients remained free of C. difficile and diarrheal symptoms through a 2-month follow-up,15 later relapses occurred, so these longer-term samples were also evaluated to determine if signatures for recurrence could be identified. However, only samples collected from patients who experienced recurrence within 2 months of FMT were considered members of a recurrence group for microbiota analyses.
Due to the uneven nature of sample collection, and collection of longer-term samples from patients who eventually experienced recurrence, samples were grouped for analysis by the time period in which they were collected. This allows for better evaluation of potentially punctuated shifts in microbiota composition following capsule FMT. Based on the range of days post-FMT on which samples were collected and observed patterns in the microbiota, samples were grouped as follows: pre-FMT – before receiving capsule FMT; days – 1 to 7 d following FMT; weeks – 7 to 21 d following FMT; months – 22 to 60 d following FMT; and longer-term – 61+ days following FMT.
DNA extraction and sequencing
DNA was extracted from 250–300 mg of fecal samples using the MoBio PowerSoil® DNA Isolation Kit (MoBio Laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer's instructions. The V5-V6 hypervariable region was amplified from DNA using the BSF784/1046R primer set,25,26 and all PCR reactions included a negative (sterile water) control, which did not amplify. Amplicons were indexed, gel purified, and paired-end sequenced at a read length of 300 nt on the Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA), as described previously.22 Amplification and sequencing were performed by the University of Minnesota Genomics Center (Minneapolis, MN, USA).
Sequence processing
Sequence processing was performed using the mothur (version 1.34.0) program,27 as described previously.28 Briefly, sequences were paired-end joined, trimmed for quality (average quality score of 35 nt over a 50 nt sliding window, ≥ 2 nt mismatches from primers, ≥ 8 nt homopolymers, and 0 ambiguous bases), and aligned against the SILVA database (version 119).29 High-quality sequences were subjected to a 2% pre-clustering step30 and chimeras were identified and removed using UCHIME.31 For unbiased comparisons, samples were rarefied to 11,500 sequence reads per sample by random subsampling.32 Operational taxonomic unit (OTU) binning was performed at a 97% similarity cut-off using the furthest-neighbor algorithm, and taxonomic classifications were made against the version 14 database from the Ribosomal Database Project.33 α and β diversity assessments and statistical analyses were done as described below.
Liquid chromatography-mass spectrometry (LC-MS) quantification of bile acids
Bile acids were quantified as described previously.13 Briefly, fecal samples were extracted by 10 volumes (1:10 wt/vol) of 50% aqueous acetonitrile containing 5 µM 13C-glycocholic acid as an internal standard. After mixing, the suspension was centrifuged twice at 18,000 × g for 10 min and then transferred to a sample vial for LC-MS analysis. The fecal extract (5 μL) was injected into an Acquity UPLC system (Waters, Milford, MA) and separated by an Acquity C18 column (1.7 µm, 2.1 × 50 mm). Mobile phases A and B are 10 mM ammonium acetate (pH 9.0) and 95% aqueous acetonitrile with 10 mM ammonium acetate (pH 9.0), respectively. The mobile phase gradient ranged from 0.5% B to 100% B over a 10-min run. The LC eluant was introduced into a Xevo-G2-S QTOF mass spectrometer (Waters) for metabolite identification and quantification. Capillary and cone voltage for electrospray ionization (ESI) were maintained at −10 V and −5 V for negative-mode detection, respectively. Source temperature and desolvation temperature were 120 °C and 350 °C, respectively. Nitrogen was used as a cone gas (50 L h−1) and desolvation gas (800 L h−1). Mass chromatograms were processed by MassLynx™ software (Waters) in centroided format. The concentrations of various primary and second bile acids in fecal samples were determined using corresponding standard curves and QuanLynx™ software (Waters).
Statistical analysis
α and β diversity, ordination metrics, and SparCC correlations were calculated using the mothur program. Shannon indices and abundance-based coverage (ACE) estimates were used to evaluate parametric and non-parametric α diversity. Differences in β diversity (community composition) were evaluated using Bray-Curtis dissimilarity matrices34 and analysis of similarity (ANOSIM).35 Ordination was performed by principal coordinate analysis (PCoA) of Bray-Curtis distances and significance of clustering was determined by analysis of molecular variance (AMOVA).36 The SparCC program17 was used to determine correlations among OTUs that showed mean relative abundance of at least 1.0% among cured patients. This cutoff was selected to improve computational efficiency. Correlation networks were visualized using CytoScape ver. 3.1.0.37 SourceTracker16 was used to determine engraftment, and donors and patients were treated as source and sink communities, respectively. Jackknife analysis revealed that SourceTracker could identify separate donor lots with 100% accuracy. Engraftment was represented as the percentage of the patient community that could be attributed to the donor community using default settings. Spearman correlations with Bonferroni correction for multiple comparisons and Fisher's F test of ANOVA models with Tukey's post-hoc test were performed using XLSTAT version 2015.1.01 (Addinsoft, Belmont, MA, USA). All statistics were evaluated at α = 0.05.
Consent
Approval for this study was given by the University of Minnesota Institutional Review Board (Protocol Number:0901M56962). All human subjects provided informed consent for participation in the study and collection and analysis of data. All human subjects gave their permission for their information to be published.
Availability of data and material
Data were returned as fastq files that are archived in the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) under accession numbers SRP064361 and SRP070464 for donors and patients, respectively.
Supplementary Material
Abbreviations
- AMOVA
analysis of molecular variance
- ANOSIM
analysis of similarity
- ANOVA
analysis of variance
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- FMT
fecal microbial transplantation
- LCA
lithocholic acid
- LC-MS
liquid chromatography-mass spectrometry
- nt
nucleotide
- OTU
operational taxonomic unit
- PCoA
principal coordinate analysis
- rCDI
recurrent Clostridium difficile infection
- RCT
randomized clinical trial
- TCA
taurocholic acid
- TCDCA
taurochenodeoxycholic acid
Disclosure of potential conflicts of interest
AK, MJH and MJS hold the following patent which relates to the content of this manuscript: Compositions and methods for transplantation of colon microbiota, US Patent 2014/0147417 A1, filed 9 March 2012, accepted 29 May 2014.
Acknowledgments
This work was performed, in part, using the resources of the University of Minnesota's Supercomputing Institute.
Funding
This research was made possible by support by grants from the NIH 1R21-AI114722–01 (AK, MJS), CIPAC Limited (AK, MJS), Achieving Cures Together (AK), and the Hubbard Foundation (AK).
Authors' contributions
CS performed data acquisition and analysis and drafted the manuscript. CTG coordinated with patients, transported patient samples, and maintained patient records. BPV and AK provided clinical supervision for patients and contributed to writing the manuscript. SS, MJH, DY, and CC performed extraction and LC-MS analysis of bile acids. CC, AK and MJS directed research and contributed to writing the manuscript. All authors have read and approved the final manuscript.
References
- [1].Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, et al.. Fecal microbiota transplantation for Clostridium difficile infection: a systemic review. Ann. Intern. Med. American College of Physicians 2015; 162:630-8; PMID:25938992; 23323867http://dx.doi.org/10.7326/M14-2693 [DOI] [PubMed] [Google Scholar]
- [2].van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al.. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med 2013; 368:407-15; PMID:23323867; http://dx.doi.org/ 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- [3].Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, Sanguinetti M, Gasbarrini A. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther 2015; 41:835-43; PMID:25728808; http://dx.doi.org/ 10.1111/apt.13144 [DOI] [PubMed] [Google Scholar]
- [4].Kelly C, Khoruts A, Staley C, Sadowsky M, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al.. Fecal microbiota transplant prevents recurrence in multiply recurrent C. difficile. Ann. Intern. Med 2016; 165:609-16; http://dx.doi.org/ 10.7326/M16-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol. 2013; 108:500-8; PMID:23511459; http://dx.doi/org/22290405 10.1038/ajg.2013.59 [DOI] [PubMed] [Google Scholar]
- [6].Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol 2012; 107:761-7; PMID:22290405; http://dx.doi.org/ 10.1038/ajg.2011.482 [DOI] [PubMed] [Google Scholar]
- [7].Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M, et al.. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA 2016; 315:142-9; PMID:26757463; http://dx.doi.org/ 10.1001/jama.2015.18098 [DOI] [PubMed] [Google Scholar]
- [8].Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 312:1772-8; PMID:25322359; http://dx.doi.org/ 10.1001/jama.2014.13875 [DOI] [PubMed] [Google Scholar]
- [9].Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013; 4:125-35; PMID:23333862; http://dx.doi.org/ 10.4161/gmic.23571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shankar V, Hamilton MJ, Khoruts A, Kilburn A, Unno T, Paliy O, Sadowsky MJ. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome 2014; 2:13; PMID:24855561; http://dx.doi.org/ 10.1186/2049-2618-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weingarden A, González A, Vázquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, Knights D, Unno T, Bobr A, Kang J, et al.. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015; 3:10; PMID:25825673; http://dx.doi.org/ 10.1186/s40168-015-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sadowsky MJ, Khoruts A. Faecal microbiota transplantation is promising but not a panacea. Nat. Microbiol. 2016; 1:16015; PMID:27572174; http://dx.doi.org/ 10.1038/nmicrobiol.2016.15 [DOI] [PubMed] [Google Scholar]
- [13].Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. - Gastrointest. Liver Physiol. 2014; 306:G310-9; PMID:24284963; http://dx.doi.org/ 10.1152/ajpgi.00282.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, et al.. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015; 517:205-8; PMID:25337874; http://dx.doi.org/ 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Staley C, Hamilton MJ, Vaughn BP, Graiziger CT, Newman KM, Kabage AJ, Sadowsky MJ, Khoruts A. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated microbiota. Am. J. Gastroenterol 2017; [Epub ahead of print] PMID: 28195180; DOI: 21765408 10.1038/ajg.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 2011; 8:761-U107; PMID:21765408; http://dx.doi.org/ 10.1038/nmeth.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Friedman J, Alm EJ. Inferring correlation networks from genomic survey data. PLoS Comput. Biol 2012; 8:e1002687; PMID:23028285; http://dx.doi.org/ 10.1371/journal.pcbi.1002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J. Bacteriol 2009; 191:1115-7; PMID:19060152; http://dx.doi.org/ 10.1128/JB.01260-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. Ratner AJ, editor. PLoS One 2010; 5:e8740; PMID:20090901; http://dx.doi.org/ 10.1371/journal.pone.0008740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Theriot CM, Bowman AA, Young VB. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 2016; 1:e00045-15; PMID:27239562; http://dx.doi.org/ 10.1128/mSphere.00045-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol 2014; 30:332-8; PMID:24625896; http://dx.doi.org/ 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 2013; 4:382-7; PMID:23851335; http://dx.doi.org/ 10.4161/gmic.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141:1773-81; PMID:21839040; http://dx.doi.org/ 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- [24].Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ. Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. MBio 2016; 7:e01965-16; PMID:27999162; http://dx.doi.org/ 10.1128/mBio.01965-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. U. S. A 2006; 103:12115-20; PMID:16880384; http://dx.doi.org/ 10.1073/pnas.0605127103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Claesson MJ, Wang QO, O'Sullivan O, Greene-Diniz R, Cole JR, Ross RP, O'Toole PW. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res 2010; 38:gkq873; http://dx.doi.org/ 10.1093/nar/gkq873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al.. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol 2009; 75:7537-41; PMID:19801464; http://dx.doi.org/ 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ. Evaluation of water sampling methodologies for amplicon-based characterization of bacterial community structure. J. Microbiol. Methods 2015; 114:43-50; PMID:25956022; http://dx.doi.org/ 10.1016/j.mimet.2015.05.003 [DOI] [PubMed] [Google Scholar]
- [29].Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 2007; 35:7188-96; PMID:17947321; http://dx.doi.org/ 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ. Microbiol 2010; 12:1889-98; PMID:20236171; http://dx.doi.org/ 10.1111/j.1462-2920.2010.02193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011; 27:2194-200; PMID:21700674; http://dx.doi.org/ 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gihring TM, Green SJ, Schadt CW. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ. Microbiol 2012; 14:285-90; PMID:21923700; http://dx.doi.org/ 10.1111/j.1462-2920.2011.02550.x [DOI] [PubMed] [Google Scholar]
- [33].Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, et al.. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37:D141-5; PMID:19004872; http://dx.doi.org/ 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr 1957; 27:325-49; http://dx.doi.org/ 10.2307/1942268 [DOI] [Google Scholar]
- [35].Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol 1993; 18:117-43; http://dx.doi.org/ 10.1111/j.1442-9993.1993.tb00438.x [DOI] [Google Scholar]
- [36].Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes - application to human mitochondrial DNA restriction data. Genetics 1992; 131:479-91; PMID:1644282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13:2498-504; PMID:14597658; http://dx.doi.org/ 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.