ABSTRACT
The inflammatory response protects the human body against infection and injury. However, uncontrolled and unresolved inflammation can lead to tissue damage and chronic inflammatory diseases. Therefore, active resolution of inflammation is essential to restore tissue homeostasis. This review focuses on the pro-resolving molecule annexin A1 (ANXA1) and its derived peptides. Mechanisms instructed by ANXA1 are multidisciplinary and affect leukocytes as well as endothelial cells and tissue resident cells like macrophages and mast cells. ANXA1 has an outstanding role in limiting leukocyte recruitment and different aspects of ANXA1 as modulator of the leukocyte adhesion cascade are discussed here. Additionally, this review details the therapeutic relevance of ANXA1 and its derived peptides in cardiovascular diseases since atherosclerosis stands out as a chronic inflammatory disease with impaired resolution and continuous leukocyte recruitment.
KEYWORDS: Ac2–26, annexin A1, atherosclerosis, cardiovascular diseases, leukocyte recruitment, myocardial infarction, stroke, resolution of inflammation
Introduction
Atherosclerosis is a common cause of cardiovascular diseases and resulting in a considerable socioeconomic burden to western societies.1 The disease is triggered by hypercholesterolemia causing the retention of lipoproteins in the vessel walls, which initiates an inflammatory response. As a consequence, atherosclerotic plaques develop at specific sites of the arterial tree where the blood flow is disturbed. Continued hypercholesterolemia and inflammation aggravate atherosclerotic plaque progression over time and this inevitably results in luminal narrowing or thrombi that obstruct or block the blood flow. This can lead to life-threatening events such as myocardial infarction and stroke (reviewed in ref. 2).
Current therapies are mainly focused on reducing the level of plasma cholesterol by HMG-CoA reductase inhibitors, also known as statins, often administered in combination with Ezetemibe which reduces the absorption of plasma cholesterol by the small intestine. Moreover, an inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9) has recently been proven to be beneficial in reducing plasma cholesterol (reviewed in ref. 3). Conventionally, PCSK9 binds the receptor of low-density lipoprotein (LDL) which is subsequently internalized and broken down. By blocking PCSK9 the LDL-receptor is kept positioned on the cells surface and is given the opportunity to scavenge and remove excessive cholesterol from the plasma. Presently, cholesterol-lowering is the most effective way to treat cardiovascular diseases and this approach has reduced age-adjusted mortality (reviewed in ref. 4). However, atherosclerotic vascular diseases remain a chronic health concern.1 This is partly because not all patients tolerate statins properly, and in addition, some patients suffer from cardiovascular events in the absence of hypercholesterolemia. Therefore, a different view of atherosclerosis as an inflammatory disease has emerged and targeting inflammation has become a promising direction to improve and complement current approaches (reviewed in ref. 5).
Inflammation is intimately involved in all stages of atherosclerosis.6 Initially, tissue resident macrophages and mast cells sense the presence of the noxious insult (e.g. oxidized low-density lipoprotein) by their pattern recognition receptors. Subsequently, those effector cells start to release pro-inflammatory cytokines, chemokines, and vasoactive mediators to instigate and amplify the immune response. Hereby, endothelial cells are activated and vascular permeability is enhanced resulting in immune cell recruitment. Newly recruited neutrophils augment the immune response with the release of granule proteins (e.g., human cathelicidin LL-37 or mouse cathelicidin-related antimicrobial peptide (CRAMP), azurocidin, cathepsin G, and α-defensins), which aggravate endothelial dysfunction and additional immune cell entry.7-9 Circulating monocytes attracted into the vascular wall transform into lipid-laden foam cells that accumulate in the atherosclerotic plaque.
Atherosclerosis is recognized as a chronic inflammatory disease in which the resolution phase is overwhelmed and fails to succeed to annihilate the inflammation (reviewed in ref. 10). A controlled resolution process is essential to prevent excessive or chronic inflammation (reviewed in ref. 11). The process of resolution includes the termination of inflammatory cell recruitment, the removal of effector cells by the induction of apoptosis and a contained clearance of apoptotic cells by macrophages; a process called efferocytosis. Furthermore, to support tissue regeneration macrophages are polarized toward an anti-inflammatory phenotype. Promoting the resolution of inflammation might be a suitable therapeutic approach to reduce atherosclerotic plaque development and prevention from secondary cardiovascular events.
Annexin A1 (ANXA1), a 37 kDa pro-resolving protein, is part of the annexin superfamily of Ca2+ dependent phospholipid binding proteins. Annexins share a common structure involving 2 distinct regions: an annexin core and an amino (N)-terminus. The annexin core region is greatly conserved between subfamilies, but all proteins have a unique N-terminal sequence exploring specific functions (reviewed in ref. 12). ANXA1 is a molecule promoting the termination of inflammation engaging various important pro-resolution properties and could therefore be an attractive protein to treat several inflammatory diseases as well as cardiovascular complications.
In this review we discuss cells expressing ANXA1 and the different pathways of ANXA1 externalization and secretion. Thereafter, the formyl-peptide receptors (FPRs), which recognize ANXA1 and its derived peptides, will be introduced. The main focus of this review, however, is the role of ANXA1 in the resolution of inflammation and more specifically its effects on leukocyte recruitment and its potential in preventing and curing cardiovascular diseases.
The expression and externalization of ANXA1
Nearly four decades ago ANXA1 was first described as a steroid-induced inhibitor of phospholipase A2 and prostaglandin biosynthesis.13 Subsequently, various studies pointed at ANXA1 as a second messenger of glucocorticoids (reviewed in ref. 14). Upon administration of hydrocortisone circulating leukocytes from healthy volunteers, for example, showed an increased ANXA1 expression level.15 Presently, other stimuli are recognized to induce ANXA1 expression. For instance, interleukin (IL)6 upregulated ANXA1 in human adenocarcinomic alveolar epithelial cells.16
ANXA1 is expressed by multiple cell types including leukocytes, endothelial cells and mast cells.17-19 Endogenous ANXA1, functioning as a scaffolding protein, is imperative in membrane organization and trafficking.12 More specifically, ANXA1 has been shown to ameliorate inward vesiculation in multivesicular endosomes and to act as a functional linker between actin filaments and phagosomes in the presence of Ca2+ during phagocytosis.20,21 Importantly, ANXA1 exerts anti-inflammatory properties outside the cell and hence the protein needs to be externalized to the cell membrane or secreted into the extracellular fluids. Exogenous ANXA1 is cleaved by proteolytic enzymes, including human proteinase and neutrophil elastase leading to the release and presence of ANXA1 and ANXA1-derived peptides such as Ac2–26.22,23 Indeed, ANXA1 and ANXA1-derived peptides are detectable in extracellular fluids such as human plasma and serum under inflammatory conditions.24-26 Interestingly, ANXA1 has no hydrophobic signal sequences and can therefore not be secreted via the classical route through the endoplasmic reticulum and Golgi apparatus.25 As a result, several alternative pathways have been revealed to be responsible for ANXA1 externalization and secretion.
The adenosintriphosphat (ATP)-binding cassette transporters are a large group of transporters with varied roles that include the externalization of proteins. The ATP binding cassette A1 transporter was revealed to be involved in the secretion of ANXA1 derived from various cell types under inflammatory conditions.27,28 Moreover, ANXA1 mobilization has been shown to be dependent on serine-27 phosphorylation.29
The P2X purinoceptor 7 receptor (P2X7R) is an innate immune receptor detecting extracellular ATP. The activation of P2X7R on pro-inflammatory M1 polarized macrophages was shown to induce the assembly of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome resulting in the release of pro-inflammatory cytokines. However, on alternatively activated, anti-inflammatory M2 macrophages, ANXA1 was released upon P2X7R stimulation independent of NLRP3 inflammasome. By this means, ANXA1 release was not dependent on de novo gene transcription since ANXA1 was already detectable after 5 min upon receptor stimulation with ATP. Evidence suggests the translocation of phosphatidylserine (PS) to the outer plasma membrane leaflet upon P2X7R activation. Hereby, ANXA1 bound to plasma membrane phospholipids in the presence of Ca2+ and therefore was accompanying phosphatidylserine by its transfer.30
In neutrophils, ANXA1 was predominantly found within gelatinase granules and in the cytoplasm.31 Circulating neutrophils showed a higher expression of intracellular ANXA1 compare with transmigrated neutrophils upon acute inflammation indicating a loss of ANXA1 during neutrophil cell adhesion and transmigration.32,33 In vitro, human neutrophils interacted with the endothelium, and more specifically with Intercellular Adhesion Molecule 1 (ICAM-1) and Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1). This interaction provoked the externalization of ANXA1 toward the outer leaflet of the plasma membrane.31,32 Moreover, other factors have been shown to provoke ANXA1 mobilization and externalization in neutrophils.34 Examples include, pro-resolving lipid lipoxin A4 (LXA4) and pro-inflammatory ligand N-Formyl-Met-Leu-Phe (fMLF), which induced cytosolic (but not granular) ANXA1 to mobilize to the cell surface. Again, ANXA1 mobilization and externalization was observed to be dependent on phosphorylation of the protein. Following, externalized ANXA1 can exert several anti-inflammatory properties via binding Formyl Peptide Receptors (FPRs) when extracellular concentrations of Ca2+ induce a conformational change, resulting in an active form of the protein.35
Additionally, ANXA1 is found to be present in extracellular vesicles which can be subclassified into exosomes (40 to 60 nm) and microparticles (100 to 1000 nm). Endogenous ANXA1 was shown to be released as a component of exosomes derived from intestinal human epithelial cells and those activated pathways imperative in wound repair.36 Besides, ANXA1 was found to be present in human neutrophil-derived microparticles obtained from activated neutrophils, which interacted with an endothelial monolayer.37,38 In vivo, those neutrophil-derived microparticles showed to enter the cartilage, maintained its integrity, and therefore protected against tissue remodeling in inflammatory arthritis.39
Formyl-peptide receptors recognize ANXA1 and Ac2–26
ANXA1 and its derived peptide Ac2–26 bind FPRs, which are G-protein-coupled receptors expressed by several cell types, but are highly present on leukocytes. There are 3 known human FPRs named FPR1, FPR2 and FPR3 and fpr orthologous are found in mice and rats (reviewed in ref. 40). Full length ANXA1 binds specifically to FPR2. ANXA1 peptides bind with lower affinity to FPR2 and have a lack of receptor specificity because they bind with similar effectiveness to FPR1.41,42
FPRs were initially identified to bind highly chemotactic N-formyl peptides, originated from invading pathogens or derived from disrupted mitochondria43,44 Hence, FPRs, and in particular FPR1, play an important role in both host defense against bacterial infection and in the clearance of damaged cells during sterile inflammations.45,46 It is now acknowledged that N-formyl peptides are not the only ligands known to bind FPRs and numerous pro- and anti-inflammatory ligands are identified to induce cell activation via FPRs. Important pro-inflammatory ligands for FPR2 are fMLF, serum amyloid A (SAA), Aβ42 and cathelicidin (LL37 in humans, CRAMP in mice). On the other hand, LXA4, resolvin D1, ANXA1 and Ac2–26 induce anti-inflammatory signaling via FPR2.
Considerable hypotheses are given and examined to explain the divergent role of FPR2 in inflammation and resolution since both pro- and anti-inflammatory ligands regulate inflammatory and resolving circuits via the same receptor. First, ligand recognition was shown to be dependent on different binding sites.47 Second, FPR2 has been shown to form homodimers and heterodimers with other FPRs dependent on its interaction with a specific ligand and thereby provoking different signaling.48 For instance, ANXA1, but not SAA, was found to activate FPR2 homodimers triggering intracellular changes culminating in the release of anti-inflammatory cytokines such as IL-10. Furthermore, Ac2–26 evoked FPR2/FPR1 heterodimerization resulting in the activation of pro-apoptotic signaling pathways.48
ANXA1 turns off leukocyte recruitment
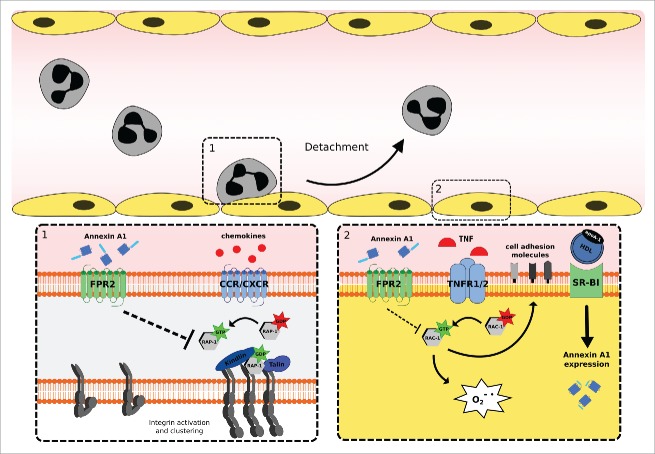
Leukocyte recruitment forms an important constitute to the immune response caused by pathogens as well as sterile provocations. Leukocytes move from the circulation through the endothelium toward the inflammatory insult in a controlled sequence, simply divided in rolling, adhesion, endothelial transmigration, and chemotactic migration (reviewed in ref. 49, 50). To control chronic or excessive leukocyte recruitment, endogenous anti-inflammatory pro-resolving mediators such as lipid mediators (LXA4, resolvins, maresins and protectins) and peptides/proteins (growth differentiation factor (GDF)-15, developmental endothelial locus (Del)-1, melanocortins, galectins, ANXA1 and Ac2–26) released by various cell types or from synthetic orgin interfere with different steps of the leukocyte adhesion cascade and therefore block leukocyte recruitment (reviewed in ref. 51). For instance, GDF-15 blocked neutrophil integrin activation and therefore neutrophil recruitment after myocardial infarction.52 Otherwise Del-1 prevented the interaction between lymphocyte function-associated antigen-1 and ICAM-1 and consequently leukocyte adhesion to the endothelium.53 Similarly, ANXA1 and its derived peptide Ac2–26 were recognized as important modulators of leukocyte recruitment (see Fig. 1).
Figure 1.
Annexin A1 (ANXA1) moderates leukocyte recruitment by instructing leukocytes and endothelial cells to prevent excessive leukocyte adhesion to the vascular wall. (1) ANXA1 interferes with chemokine induced Rap1 activation and consequently with integrin activation and clustering via FPR2. (2) HDL increases endogenous ANXA1 expression in TNF-α activated endothelial cells via SR-B1. Exogenous ANXA1 prohibits TNF-induced Rac1 activation and therefore superoxide production and cell adhesion molecule expression via binding FPR2. Reduced expression of adhesion molecules on the cell surface of endothelial cells limits leukocyte adhesion and recruitment. Abbreviations: FPR2, formyl peptide receptor 2; CCR/CXCR, CC-chemokine receptor/CXC-chemokine receptor; HDL, high density lipoprotein; apoA-1, apolipoprotein A1; SR-B1, scavenger receptor class B type 1; TNF-α, tumor necrosis factor α.
Several studies using animal models of inflammation have demonstrated that the administration of ANXA1 or Ac2–26 moderates neutrophil recruitment.54-58 Early studies showed that both ANXA1 and Ac2–26 inhibited adhesion of human neutrophils to activated endothelial cells under static54,59 and flow conditions60,61 in vitro and thus impairing neutrophil recruitment. Use of an ANXA1 blocking antibody increased the ability of neutrophils to transmigrate through an endothelial monolayer indicating that ANXA1 is able to impair neutrophil transmigration.32,62 The ability of inhibiting neutrophil adhesion and emigration by ANXA1 and its derived peptide Ac2–26 was further supported by intravital microscopy analysis of the mesenteric postcapillary venules, which showed no change in rolling, but reduced adhesion and emigration upon inflammation.63,64 Interestingly, ANXA1 and Ac2–26 detached adherent neutrophils within 2 minutes after administration during on-going inflammation proving a tremendous effect of those proteins.50 Besides, Ac2–26 has been shown to inhibit leukocyte recruitment to the carotid artery under high cholesterol conditions, indicating that the anti-inflammatory actions of ANXA1 are not dependent on the type of blood vessel.65 Likely, administration of ANXA1 or Ac2–26 mimics the effect of endogenous ANXA1 that is externalized upon neutrophil adhesion to the endothelium. Since most studies focused on the effects of ANXA1 on neutrophils, it is important to remark that also monocyte recruitment is inhibited by ANXA1 or its derived peptides.56,65,66 Possibly, these findings can be extrapolated to other leukocyte subsets.
In line with these findings ANXA1-deficient mice subjected to various inflammatory stimuli exhibited a pronounced inflammation compare with control mice indicating a protective role of ANXA1.67,70 Additionally, dexamethasone was shown to be less effective in ANXA1 deficient mice67 or when mice where immunized against ANXA156,69,71 showing the importance of ANXA1 in the anti-inflammatory and immunosuppressive effects of glucocorticoids. Again, evaluation of the cremasteric microcirculation by intravital microscopy revealed ANXA1 as a modulator of the leukocyte adhesion cascade since ANXA1 null mice demonstrated increased transendothelial migration.68 Furthermore those mice showed an enhanced adhesion of leukocytes to the carotid artery under high fat diet conditions.65
ANXA1 and Ac2–26 inhibit leukocyte-endothelial interaction by binding FPR2 reducing leukocyte adhesion and detachment.65,72-76 Conforming with previously described research, fpr2-deficient mice suffered from a more severe inflammatory response indicated by enhanced cell adhesion and emigration into the inflamed mesenteric microcirculation and aggravated inflammation in models for paw edema and arthritis.77 Strikingly, endogenous ANXA1 was released as a component of extracellular vesicles such as microparticles, which were also shown to inhibit neutrophil recruitment.38 In vitro those microparticles inhibited the adhesion of naïve neutrophils to human endothelial cells and this effect was abrogated in the presence of a FPR2 blocking antibody.38 Moreover, intravenous administration of ANXA1-containing microparticles inhibited leukocyte recruitment in a mouse model of air pouch inflammation. Curiously, this effect was not observed when microparticles were obtained from ANXA1 null mice.38
On a molecular level, leukocyte adhesion is orchestrated by neutrophil intrinsic molecules (e.g., L-selectin, β1 and β2 integrins) interacting with endothelial cell intrinsic molecules (e.g., e-selectin, vascular cell adhesion molecule (VCAM)-1 and ICAM-1). L-selectin is an adhesion/homing receptor, which recognizes sialylated carbohydrate groups and is mainly involved in tethering and rolling. Several studies showed that administration of glucocorticoids induces L-selectin shedding on neutrophils in humans.78 In addition, glucocorticoid-induced L-selectin shedding was mediated by ANXA1.79,80 Integrins interact with cell adhesion molecules and thereby the overall strength of cellular adhesiveness is governed by the intrinsic affinity of the individual receptor-ligand bonds and their valency. The affinity of integrins to interact with their partner depends on its activation status. Integrin valency is determined by the basal expression of the receptor and its geometric arrangement. Several studies have shown that ANXA1 and Ac2–26 interfere with basal αmß2 integrin expression on leukocytes under inflammatory challenges.64,67,68,81 Additionally, it has been observed that Ac2–26 prevents chemokine-induced ß1 and ß2 integrin activation and clustering via Rap1, a member of the Ras family of GTPases, which has an important role in the regulation of cell migration and adhesion. In line, Ac2–26 effects were absent in leukocytes isolated from fpr2-deficient mice65 (Fig. 1).
Most studies demonstrate a neutrophil intrinsic effect of ANXA1 and its role in leukocyte adhesion and migration toward the inflammatory insult. However, few studies indicate endothelial cells, the second important player in leukocyte recruitment, as a target of ANXA1 or its derived peptides.19,82 At first, Ac2–26 was shown to prohibit tumor necrosis factor α (TNFα)-induced superoxide production; ICAM- 1 and VCAM-1 expression in Human Mammary Epithelial Cells. Thus, Ac2–26 blocked TNFα activation via Rac1 (studied with N17rac1, dominant negative rac1) through binding FPR2. Whether those interesting findings are pathophysiologic relevant has not been confirmed so far82 (Fig. 1).
Accordingly, high density lipoprotein (HDL) increased TNF-α activated aortic endothelial ANXA1 expression in vivo and in vitro, suggesting an important role of this protein in this cell type.19 Subsequently, HDL-induced ANXA1 expression prevented human monocytic (THP-1) cell adhesion to activated endothelial cells. HDL and more specifically apolipoprotein AI, enhanced ANXA1 expression by binding scavenger receptor B1 and inducing extracellular signal–regulated kinase, p38 mitogen-activated protein kinase (P38MAPK), serine/threonine kinase Akt and protein kinase C (PKC) signaling and subsequently reduced endothelial activation indicated by an abridged expression of ICAM-1, VCAM-1, E-selectin, CC-chemokine ligand (CCL)2 and IL-819 (Fig. 1).
Furthermore, platelets play a pivotal role in leukocyte recruitment and can interact with the endothelium or with the leukocyte itself (reviewed in ref. 83). Platelet-endothelial interactions are facilitated by the adhesion receptors P-selectin, glycoprotein (GP)Ib-IX-V, GPVI, GPIIb-IIIa and CD40L expressed by platelets and P-selectin, E-selectin, ICAM-1, VCAM-1 and the blood glycoprotein von Willebrand factor by endothelial cells. Platelets synthesize, store and release inflammatory cytokines as well as chemokines, and thereby activate the endothelium and promote vascular permeability. Besides, platelets directly recruit leukocytes by depositing chemokines (e.g., CCL5) on the endothelium to attract monocytes.84 Platelet-leukocyte interactions are foremost mediated by P-selectin expressed by platelets interacting with P-selectin glycoprotein ligand-1 on the surface of leukocytes. This interaction primes the leukocyte and promotes integrin activation. Interestingly enough, platelet recruitment is likewise supported by circulating leukocytes indicating the existence of a tightly controlled network including platelet-leukocyte crosstalk. Early studies indicated the presence of ANXA1 in human platelets in the cytosolic fraction.85,86 However, in platelets ANXA1 did not appear to be released upon stimulation.71 In a mouse model of cerebral inflammation, ANXA1 was shown to prevent neutrophil-platelet aggregation and therefore limited neutrophil adhesion and recruitment. Interestingly, ANXA1 induced this effect by binding murine fpr2 on neutrophils and not platelets, since neutrophil-platelet aggregation was not altered in mice with fpr2-deficient platelets, but was enhanced in mice with fpr2-deficient neutrophils.72
Additionally, tissue resident effector cells including mast cells and macrophages play an important role in leukocyte recruitment by the release of inflammatory cytokines (e.g., TNFα and IL1β) and chemokines (e.g., chemokine (C-X-C motif) ligand 10 and CCL11), which attract leukocytes and might facilitate their adhesion to the endothelium (reviewed in ref. 87, 88). In contrast, those effector cells release anti-inflammatory mediators (e.g., IL10 and transforming growth factor β) during the resolution phase to terminate the inflammation. Mast cells express ANXA1 abundantly and there is compelling evidence supporting the notion that mast cells regulate leukocyte recruitment by the release of inflammatory mediators.18 In a rat model of pleurisy ANXA1 mimetic peptide Ac2–26 prohibited the release of inflammatory histamine and CCL11 in pleural effluents. Besides, IL-13-evoked CCL11 release was inhibited by Ac2–26 in rat mesothelial cells and full length ANXA1 was shown to inhibit histamine and prostaglandin D2 release from activated human and mouse mast cells.89,90 Simultaneously, macrophages are important effector cells involved in fine-tuning the immune response. In macrophages, ANXA1 or its derived peptide Ac2–26, enhanced IL10 production and secretion. Moreover, ANXA1 or related peptides inhibited nitric oxide release from macrophages.91 Furthermore, a combination of LXA4 and ANXA1 induced macrophages polarization toward a more anti-inflammatory macrophage phenotype that secreted IL10, a mechanism working via FPR2.92
Hence, ANXA1 acts as an anti-inflammatory protein, which is important in fine-tuning the leukocyte recruitment and inflammatory response to protect from aggressive and chronic inflammation.
ANXA1 in cardiovascular disease
ANXA1 in atherosclerosis
Atherosclerosis is characterized by atheroma build up inside the arterial wall, a process mainly triggered by LDL, which is susceptible to oxidative modification by reactive oxygen species (reviewed in ref. 88). In addition, atherosclerosis is characterized by the adherence of circulating leukocytes to vascular endothelial cells and subsequently the migration of those cells to the sub-endothelial space (reviewed in ref. 93). In the sub-endothelial space macrophages, derived from newly recruited monocytes or local proliferation, remove modified lipoproteins to restore tissue functions. However, by a persistent leakage of lipoproteins, this resolving mechanism is overwhelmed and macrophages turn into lipid-laden inflammatory foam cell. Hence, the leukocyte recruitment cascade has shown to be imperative in the development of atherosclerosis and atherosclerosis-related diseases (reviewed in ref. 94, 95). Since ANXA1 has been indicated as an important regulator of leukocyte recruitment by modulating distinct steps of the leukocyte adhesion cascade, it might be a suitable candidate to limit inflammation in atherosclerotic plaque formation and its derived cardiovascular diseases. Besides, ANXA1 has been recognized as a modulator of apoptosis, efferocytosis and macrophage polarization, which are all important facets that are malfunctioning in atherosclerosis.
Efferocytosis of apoptotic cells by macrophages polarizes macrophages toward an anti-inflammatory M2 phenotype. ANXA1 has been shown to promote neutrophil apoptosis by targeting constitutive apoptotic pathways96 or counteracting survival signals from other inflammatory mediators.48,97 The working mechanism of ANXA1 in efferocytosis is multi-functional. ANXA1 was shown to function as a bridging molecule and co-localizes with PS on apoptotic cells to interact with scavenging macrophages.98 Moreover, the protein was released by neutrophils to attract the macrophage99,100 and it has been observed to be externalized by macrophages to facilitate engulfment of apoptotic cells in an autocrine/paracrine fashion.101 Additionally, ANXA1 polarized macrophages toward an anti-inflammatory phenotype,92 all of which are qualities with potential importance to reduce damage and improve resolution in atherosclerosis or related cardiovascular insults.
In human coronary atherosclerotic plaques ANXA1 was found to localize in macrophages and endothelial cells in the tunica intima.65,102 Likewise, ANXA1 was observed to be highly expressed in areas containing apoptotic cells (TUNEL+) indicating that the high expression of ANXA1 by macrophages reflects its importance the phagocytosis of apoptotic cells.102 Two other studies detected ANXA1 expression in plaques obtained from patients with carotid stenosis undergoing carotid endarterectomy. A higher expression of ANXA1 was found in carotid plaques from asymptomatic patients compare with symptomatic patients implying a protective role of ANXA1 in atherosclerosis.103,104
Table 1 summarizes studies on ANXA1 or its derived peptides performed in animal models of cardiovascular diseases. In line with previous findings ANXA1 and Ac2–26 protect from atherogenesis and atheroprogression in mice.61,65,105 Administration of Ac2–26 demonstrated a protective effect in a model of atherogenesis modulating an early stage of plaque development.65 In this model, Ac2–26 reduced the accumulation of leukocytes by inhibiting neutrophil and monocytes adhesion to the inflamed carotid artery. As described previously, Ac2–26 blocked chemokine induced leukocyte adhesion via FPR2 and Rap1.65
Table 1.
Pharmacokinetics and therapeutic potential of Annexin A1 and its derived peptides in cardiovascular disease models.
| Drug | Disease model | Administration | Pharmacokinetics | Outcome | Ref. |
|---|---|---|---|---|---|
| Atherosclerosis | |||||
| Col IV–Ac2–26 NPs | Advanced atherosclerosis | NPs contain 10 μg of Ac2–26 per injection (mouse) 1x/week, 5 weeks (starting after 12 weeks HFD) |
Linear release in 96 hours (in vitro) | Lesion size ↓ Plaque stability ↑ Oxidative stress ↓ Necrotic area ↓ ICAM-1↓ Lesional IL10 ↑ |
105 |
| Ac2–26 | Atherogenesis | 50 μg/injection (mouse) 3x/week, 4 weeks |
Not studied | Lesion size ↓ Leukocyte recruitment ↓ Integrin activation ↓ |
65 |
| h-annexin A1 | Atherogenesis Advanced atherosclerosis | 1 mg/kg (mouse) 3x/week, 6 weeks 3x/week, 6 weeks (starting after 6 weeks HFD) |
Peaks after 50 min in the blood circulation T½ = 6 hours | No effect Lesion size ↓ Necrotic core ↓ |
61 |
| Myocardial infarction | |||||
| Ac2–26 | I/R | 5 or 50 μg/injection (rat) At the start of reperfusion |
Not studied | Infarct area ↓ Myocardial MPO ↓ Myocardial TNF-α ↓ Myocardial CCL3 ↓ Leukocyte adhesion ↓ |
113 |
| Ac2–26 h-annexin A1 | I/R | 0.5 or 1 mg/kg (rat) 0, 30 and 60 min after reperfusion 25 mg/kg (rat) |
Not studied | Infarct area ↓ Myocardial MPO ↓ Myocardial IL-1β ↓ Infarct area ↓ |
111 |
| Ac2–26 | I/R | 1 mg/kg (mouse) At the start of reperfusion |
Not studied | Infarct area ↓ Neutrophil count ↓ Myocardial CXCL1 ↓ |
112 |
| AnxA12–50 or CR-AnxA12–50 | I/R | 5 μg/injection (mouse) 0 and 60 min after reperfusion |
Not studied | Infarct size ↓ Plasma troponin levels ↓ Plasma CCL5 levels ↓ Plasma IL1β levels ↓ Incidence of death (after 24 hr) ↓ |
74 |
| Stroke | |||||
| Annexin A1 fragment (NH2-terminal 1–188 aa) | I/R | 1.2 μg/injection (rat) 10 min after start ischemia |
Not studied | Infarct size ↓ Cerebral edema ↓ |
118 |
| h-annexin A1 Ac2–26 | I/R | 1 μg/injection (mouse) Repetitive injection after 0, 6 and 18 hours 100 μg/injection (mouse) (Repetitive) injection after 0, 6 and/or 18 hours |
Not studied | Infarct volume ↓ Neurological score ↓ Leukocyte adhesion ↓ MPO activity ↓ Infarct volume (best results obtained with an injection after 1 and 18 hours) ↓ Neurological score ↓ Leukocyte adhesion ↓ |
119 |
| Ac2–26 | I/R | 2.5 μg/kg (mouse) At the start of the reperfusion |
Not studied | Leukocyte adhesion ↓ | 75 |
| Ac2–26 | I/R | 100 μg/injection (mouse) At the start of cerebral reperfusion |
Not studied | Infarct volume ↓ Neurological score ↓ Neutrophil-platelet aggregation ↓ Leukocyte adherence ↓ Platelet adherence ↓ |
72 |
Abbreviations: Col, collagen; NPs, nanoparticles; HFD, high fat diet; IL, interleukin; ICAM-1, intracellular adhesion molecule-1; Ac2–26, N-terminal fragment of Annexin A1; h-, human-; I/R, ischemia/reperfusion; CCL, CC chemokine ligand; CXCL, CXC chemokine ligand; CR-AnxA12–50, cleavage resistant annexin A12–50; MPO, Myeloperoxidase.
Similar results were shown in a model of advanced atherosclerosis105 where Apoe−/− mice were subjected to a high cholesterol diet for a period of 12 weeks and treated with collagen IV (Col IV)-targeted nanoparticles (NPs) containing Ac2–26 (Col IV-Ac2–26 NPs) in the last 5 weeks.105 Col IV-Ac2–26 NPs were able to increase cap thickness of atherosclerotic plaques and reduced collagenase production, 2 indicators of atherosclerotic plaque progression. Resembling effects were observed when mice where treated with full length ANXA1.61
Taken together, ANXA1 might represent an innovative strategy to counteract continuous leukocyte recruitment and macrophage activity during atheroprogression.
ANXA1 in myocardial infarction
Myocardial infarction occurs when the blood flow to the heart is abrogated, mostly instigated by a rupture of an atherosclerotic plaque in coronary arteries, causing damage to the heart muscle. Leukocyte infiltration after myocardial infarction has recently become an important focus of research (reviewed in refs. 94, 106) and can be divided in distinct waves. At the start of the ischemia, neutrophil infiltration is initiated and peaks after 24 hours. Its primary role is to amplify the inflammatory response. Neutrophil infiltration is followed by a wave of monocytes of which there are different subsets present in the circulation with functionally different properties (reviewed in ref. 107). The Ly6Chigh monocytes (human equivalent of CD14+CD16− monocytes) are rapidly recruited to sites of inflammation and give rise to monocyte-derived dendritic cells and macrophages and are of importance to scavenge dead cells and debris. Pro-inflammatory ly-6Chigh monocytes appear after 24 hours in the myocardium and their presence peaks at day 3.108 Those monocytes are of high importance in the restoration after myocardial damage, since the depletion of monocytes compromises the hearts to heal.109 Ly6Clow monocytes (human equivalent of CD14lowCD16+ monocytes) or also called patrolling monocytes are associating with the vascular endothelium and coordinate repair. On day 7 anti-inflammatory ly-6Clowmacrophages dominate and are as well important in the resolution of inflammation and tissue repair.108 Reperfusion therapy (e.g., by vasodilatative drug or thrombolytic drugs) is applied to improve the blood supply to the heart; however the restoration of oxygen and nutrients supply to the ischemic area also result in inflammation and tissue damage, called reperfusion injury (reviewed in ref. 110).
The ANXA1 derived peptide Ac2–26 has been described to be protective in mouse and rat models of myocardial infarction, however solely in models of acute damage after reperfusion.111-113 Studies with a larger time frame looking at the effects of ANXA1 in the myocardial repair have not been reported. After an ischemia period of 25 min followed by a reperfusion period of 1–2 hours ANXA1 mimetic peptide Ac2–26 reduced the infarct area. Furthermore, inflammatory cytokine (e.g., TNFα and IL-1ß) and myeloperoxidase (as a marker of neutrophil recruitment) expression was reduced after Ac2–26 treatment.111-113
Myocardial infarction is an acute life-threatening disorder and therefore timing of treatment is indispensable. Treatment potential was indicated by injecting Ac2–26 0, 30 and 60 min after the start of the reperfusion period to mimic the clinical situation of patients which likely cannot be treated directly upon restoration of the blood flow.111 The most prominent effect of Ac2–26 was found when the protein was administered 30 min after the start of the reperfusion. One potential problem in using ANXA1 or Ac2–26 in clinics might be the cleavage and inactivation of those proteins by proteases since externalized and exogenous ANXA1 is cleaved by human proteinase and neutrophil elastase.22,23 Cleavage resistant Annexin A12–50 (CR-AnxA12–50) was designed to overcome this problem.74 Tested in a mouse model of myocardial reperfusion injury, CR-AnxA12–50 reduced infarct area 2 hours after reperfusion. Additionally, CR-AnxA12–50 increased the survival rate 24 hours post-reperfusion of mice exposed to ischemia and reperfusion injury.
Ex vivo studies using isolated but perfused hearts from rat and mouse demonstrated a protective effect of Ac2–26 after ischemia and reperfusion.114 In this model, Ac2–26 administration from the onset of reperfusion restored cardiac function via FPR1 activation. In vitro, Ac2–26 potently prevented from ischemic injury induced by metabolic inhibition in cardiomyocytes, an action dependent on PKC, P38/MAPK and ATP-dependent potassium channels KATP in cardiomyocytes in vitro.115
Patients suffering from myocardial infarction are typically exposed to angioplasty to push open blocked arteries. Next to it, medications are used to induce thrombolysis. Equally, patients are commonly treated with aspirin, known as an anti-platelet drug.2 For this aspect, a combined treatment of LXA4 and ANXA1 could be beneficial and evidence to support this hypothesis was obtained in a murine air pouch model where an additive effect of a combined treatment with aspirin-triggered lipoxins and glucocorticoids-induced ANXA1 was observed.116 Potentially, both ANXA1 and LXA4 interacted with FPR2 and act in concert to downregulate neutrophil recruitment and overcome functional redundancies. However, how far an additive compound can improve ANXA1 therapy in cardiovascular disease models has not been examined.
ANXA1 in stroke
Cerebral ischemia, also called stroke, occurs when the blood supply to the brain is obstructed. This happens when atherosclerosis blocks or narrows the lumen of blood vessels to certain parts of the brain (reviewed in ref. 117). Ischemia activates tissue resident cells (mainly microglia) and promotes the release of inflammatory mediators. As consequence leukocytes and T cells are recruited to the inflamed area.
Four different studies investigated the effect of ANXA1 or its derived peptides in models of ischemic stroke.72,75,118,119 Rats exposed to ischemia and Ac2–26 administration showed reduced infarct sizes and limited cerebral edema after 2 and 24 hours and this effect was most prominent when Ac2–26 was directly given upon reperfusion.118 Correspondingly, Ac2–26 reduced leukocyte adhesion in the cerebral microvasculature though FPR2. Similar protective properties where observed using full length ANXA1.119 Equivalently, Ac2–26 inhibited neutrophil-platelet aggregate formation and in consequence the adhesion of neutrophils to the endothelium again via binding though FPR2 and thus reduced the size of the infarcted area, decreased neurological score and abrogated cytokines secretion.72
Conclusions
Inhibiting inflammation has proven to be beneficial in animal models of atherosclerosis, however translation into clinical practice has failed up to now120,121 probably caused by divergent leukocytes behavior since inflammation is instigated to clear the affected area, but uncontrolled or aggressive inflammation causes tissue damage. Therefore, stimulating resolution of inflammation instead might be an attractive strategy.
Substantial progress has been made in ANXA1 research, exposing ANXA1 as a potential therapeutic drug facilitating a wide range of pro-resolving responses. In cardiovascular diseases the protein has been shown to be beneficial in protecting against inflammation in atherosclerosis, myocardial infarction and stroke. As discussed, ANXA1-instructed mechanisms are multidisciplinary and affect leukocytes as well as endothelial cells and tissue resident cells like macrophages and mast cells. In summary, ANXA1 and its derived peptide Ac2–26 are important modulators of the leukocyte adhesion cascade and limit leukocyte recruitment. Furthermore, ANXA1 promotes apoptosis of neutrophils and subsequently efferocytosis by macrophages. Additionally, it polarizes macrophages toward an anti-inflammatory phenotype with the additional effect of an enhanced anti-inflammatory cytokine secretion and a suppressed secretion of inflammatory mediators. Importantly, much research is focused on pharmaceutical tools to improve pharmacokinetics and drug targeting. For example, polymeric nanoparticles have been developed to deliver ANXA1 directly at the side of inflammation. These strategies will favor the translation of ANXA1 to clinical practice as an attractive protein to halt disease progression and restore homeostasis.
Abbreviations
- ANXA1
annexin A1
- ATP
adenosintriphosphat
- CRAMP
cathelicidin-related antimicrobial peptide
- CCL
CC-chemokine ligand
- Col IV
collagen IV
- CXCL
chemokine (C-X-C motif) ligand
- CR-AnxA12–50
cleavage resistant annexin A12–50
- Del-1
developmental endothelial locus-1
- FPR
formyl peptide receptor
- fMLF
formyl-met-leu-phe
- GDF-15
growth differentiation factor-15
- GP
glycoprotein
- HDL
high density lipoprotein
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- LDL
low density lipoprotein
- LXA4
lipoxin A4
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- NPs
nanoparticles
- P2X7R
P2X purinoceptor 7
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PECAM-1
platelet endothelial cell adhesion molecule 1
- PS
phosphatidylserine
- SAA
serum amyloid A
- TNFα
tumor necrosis factor alfa
- VCAM-1
vascular cell adhesion molecule 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The author's research is supported by the Deutsche Forschungsgemeinschaft (SFB914 TP B08 to O.S., SFB1123 TP A06/B05 to O.S. and M.D.), the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (VIDI project 91712303) to O.S., the Horizon 2020 Program (EVOLUTION ITN) to O.S., the LMUexcellent program to O.S. and the Fritz Thyssen Foundation to G.L.
References
- [1].Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, et al.. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38-60; PMID:26673558; https://doi.org/ 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- [2].Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014; 114:1852-66; PMID:24902970; https://doi.org/ 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- [3].Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr., et al.. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40-51; PMID:25915661; https://doi.org/ 10.7326/M14-2957 [DOI] [PubMed] [Google Scholar]
- [4].Pedersen TR. The success story of LDL cholesterol lowering. Circ Res 2016; 118:721-31; PMID:26892969; https://doi.org/ 10.1161/CIRCRESAHA.115.306297 [DOI] [PubMed] [Google Scholar]
- [5].Shapiro MD, Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ Res 2016; 118:732-49; PMID:26892970; https://doi.org/ 10.1161/CIRCRESAHA.115.306471 [DOI] [PubMed] [Google Scholar]
- [6].Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340:115-26; PMID:9887164; https://doi.org/ 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- [7].Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res 2012; 110:875-88; PMID:22427325; https://doi.org/ 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- [8].Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010; 122:1837-45; PMID:20956207; https://doi.org/ 10.1161/CIRCULATIONAHA.110.961714 [DOI] [PubMed] [Google Scholar]
- [9].Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res 2012; 110:1052-6; PMID:22394519; https://doi.org/ 10.1161/CIRCRESAHA.112.265868 [DOI] [PubMed] [Google Scholar]
- [10].Viola J, Soehnlein O. Atherosclerosis - A matter of unresolved inflammation. Semin Immunol 2015; 27:184-93; PMID:25865626; https://doi.org/ 10.1016/j.smim.2015.03.013 [DOI] [PubMed] [Google Scholar]
- [11].Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med 2013; 5:661-74; PMID:23592557; https://doi.org/ 10.1002/emmm.201202382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 2005; 6:449-61; PMID:15928709; https://doi.org/ 10.1038/nrm1661 [DOI] [PubMed] [Google Scholar]
- [13].Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature 1979; 278:456-9; PMID:450050 [DOI] [PubMed] [Google Scholar]
- [14].Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol 2009; 9:62-70; PMID:19104500; https://doi.org/ 10.1038/nri2470 [DOI] [PubMed] [Google Scholar]
- [15].Goulding NJ, Godolphin JL, Sampson MB, Maddison P, Flower RJ. Hydrocortisone induces lipocortin 1 production by peripheral blood mononuclear cells in vivo in man. Biochem Soc Trans 1990; 18:306-7; PMID:2143158 [DOI] [PubMed] [Google Scholar]
- [16].Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine 1998; 10:514-21; PMID:9702415; https://doi.org/ 10.1006/cyto.1997.0325 [DOI] [PubMed] [Google Scholar]
- [17].Morand EF, Hutchinson P, Hargreaves A, Goulding NJ, Boyce NW, Holdsworth SR. Detection of intracellular lipocortin 1 in human leukocyte subsets. Clin Immunol Immunopathol 1995; 76:195-202; PMID:7614738 [DOI] [PubMed] [Google Scholar]
- [18].Oliani SM, Ciocca GA, Pimentel TA, Damazo AS, Gibbs L, Perretti M. Fluctuation of annexin-A1 positive mast cells in chronic granulomatous inflammation. Inflamm Res 2008; 57:450-6; PMID:18827967; https://doi.org/ 10.1007/s00011-008-7222-7 [DOI] [PubMed] [Google Scholar]
- [19].Pan B, Kong J, Jin J, Kong J, He Y, Dong S, Ji L, Liu D, He D, Kong L, et al.. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim Biophys Acta 2016; 1861:501-12; PMID:27012521; PMID:8093248; https://doi.org/ 10.1016/j.bbalip.2016.03.022 [DOI] [PubMed] [Google Scholar]
- [20].Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol 1993; 120:77-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Patal R, Szyper-Kravitz M. [The dilemma regarding treatment of pulmonary embolism among elderly patients]. Harefuah 2011; 150:572–3, 618 [PubMed] [Google Scholar]
- [22].Vong L, D'Acquisto F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, Perretti M. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem 2007; 282:29998-30004; PMID:17681950; https://doi.org/ 10.1074/jbc.M702876200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rescher U, Goebeler V, Wilbers A, Gerke V. Proteolytic cleavage of annexin 1 by human leukocyte elastase. Biochim Biophys Acta 2006; 1763:1320-4; PMID:17023068; https://doi.org/ 10.1016/j.bbamcr.2006.08.041 [DOI] [PubMed] [Google Scholar]
- [24].Uemura K, Inagaki H, Wada Y, Nakanishi K, Asai K, Kato T, Ando Y, Kannagi R. Identification of immuno-reactive lipocortin 1-like molecules in serum and plasma by an enzyme immunoassay for lipocortin 1. Biochim Biophys Acta 1992; 1119:250-5; PMID:1532130 [DOI] [PubMed] [Google Scholar]
- [25].Christmas P, Callaway J, Fallon J, Jones J, Haigler HT. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J Biol Chem 1991; 266:2499-507; PMID:1824943 [PubMed] [Google Scholar]
- [26].Romisch J, Schuler E, Bastian B, Burger T, Dunkel FG, Schwinn A, Hartmann AA, Paques EP. Annexins I to VI: quantitative determination in different human cell types and in plasma after myocardial infarction. Blood Coagul Fibrinolysis 1992; 3:11-7; PMID:1623112 [PubMed] [Google Scholar]
- [27].Wein S, Fauroux M, Laffitte J, de Nadai P, Guaini C, Pons F, Comera C. Mediation of annexin 1 secretion by a probenecid-sensitive ABC-transporter in rat inflamed mucosa. Biochem Pharmacol 2004; 67:1195-202; PMID:15006554; https://doi.org/ 10.1016/j.bcp.2003.11.015 [DOI] [PubMed] [Google Scholar]
- [28].Chapman LP, Epton MJ, Buckingham JC, Morris JF, Christian HC. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology 2003; 144:1062-73; PMID:12586783; https://doi.org/ 10.1210/en.2002-220650 [DOI] [PubMed] [Google Scholar]
- [29].Solito E, Christian HC, Festa M, Mulla A, Tierney T, Flower RJ, Buckingham JC. Post-translational modification plays an essential role in the translocation of annexin A1 from the cytoplasm to the cell surface. FASEB J 2006; 20:1498-500; PMID:16720734; https://doi.org/ 10.1096/fj.05-5319fje [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].de Torre-Minguela C, Barbera-Cremades M, Gomez AI, Martin-Sanchez F, Pelegrin P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep 2016; 6:22586; PMID:26935289; https://doi.org/ 10.1038/srep22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int 2000; 24:163-74; PMID:10772777; PMID:8898757; https://doi.org/ 10.1006/cbir.1999.0468 [DOI] [PubMed] [Google Scholar]
- [32].Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med 1996; 2:1259-62 [DOI] [PubMed] [Google Scholar]
- [33].Oliani SM, Perretti M. Cell localization of the anti-inflammatory protein annexin 1 during experimental inflammatory response. Ital J Anat Embryol 2001; 106:69-77; PMID:11729999 [PubMed] [Google Scholar]
- [34].Brancaleone V, Dalli J, Bena S, Flower RJ, Cirino G, Perretti M. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J Immunol 2011; 186:4905-14; PMID:21398608; PMID:12595246; https://doi.org/ 10.4049/jimmunol.1003145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rosengarth A, Luecke H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J Mol Biol 2003; 326:1317-25 [DOI] [PubMed] [Google Scholar]
- [36].Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et al.. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 2015; 125:1215-27; PMID:25664854; https://doi.org/ 10.1172/JCI76693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dalli J, Montero-Melendez T, Norling LV, Yin X, Hinds C, Haskard D, Mayr M, Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics 2013; 12:2205-19; PMID:23660474; https://doi.org/ 10.1074/mcp.M113.028589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008; 112:2512-9; PMID:18594025; https://doi.org/ 10.1182/blood-2008-02-140533 [DOI] [PubMed] [Google Scholar]
- [39].Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell'Accio F, Pitzalis C, et al.. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med 2015; 7:315ra190; https://doi.org/ 10.1126/scitranslmed.aac5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 2009; 61:119-61; PMID:19498085; https://doi.org/ 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dalli J, Montero-Melendez T, McArthur S, Perretti M. Annexin A1 N-terminal derived Peptide ac2-26 exerts chemokinetic effects on human neutrophils. Front Pharmacol 2012; 3:28; PMID:22403546; https://doi.org/ 10.3389/fphar.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ernst S, Lange C, Wilbers A, Goebeler V, Gerke V, Rescher U. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol 2004; 172:7669-76; PMID:15187149 [DOI] [PubMed] [Google Scholar]
- [43].Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med 1982; 155:264-75; PMID:6274994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Galper JB. Mitochondrial protein synthesis in HeLa cells. J Cell Biol 1974; 60:755-63; PMID:4824294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464:104-7; PMID:20203610; https://doi.org/ 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010; 330:362-6; PMID:20947763; https://doi.org/ 10.1126/science.1195491 [DOI] [PubMed] [Google Scholar]
- [47].Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev 2006; 58:463-87; PMID:16968948; https://doi.org/ 10.1124/pr.58.3.4 [DOI] [PubMed] [Google Scholar]
- [48].Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 2013; 110:18232-7; PMID:24108355; https://doi.org/ 10.1073/pnas.1308253110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7:678-89; https://doi.org/ 10.1038/nri2156; PMID:17717539 [DOI] [PubMed] [Google Scholar]
- [50].Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 2011; 17:1381-90; PMID:22064428; https://doi.org/ 10.1038/nm.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol 2013; 34:1-6; PMID:22951309; https://doi.org/ 10.1016/j.it.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, et al.. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 2011; 17:581-8; PMID:21516086; https://doi.org/ 10.1038/nm.2354 [DOI] [PubMed] [Google Scholar]
- [53].Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, et al.. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 2008; 322:1101-4; PMID:19008446; https://doi.org/ 10.1126/science.1165218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Perretti M, Ahluwalia A, Harris JG, Goulding NJ, Flower RJ. Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse. A qualitative comparison with an anti-CD11b monoclonal antibody. J Immunol 1993; 151:4306-14; PMID:8409403 [PubMed] [Google Scholar]
- [55].Harris JG, Flower RJ, Perretti M. Alteration of neutrophil trafficking by a lipocortin 1 N-terminus peptide. Eur J Pharmacol 1995; 279:149-57; PMID:7556395 [DOI] [PubMed] [Google Scholar]
- [56].Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol 1997; 120:1075-82; PMID:9134220; https://doi.org/ 10.1038/sj.bjp.0701029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang Y, Hutchinson P, Morand EF. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum 1999; 42:1538-44; PMID:10403283; https://doi.org/ 10.1002/1529-0131(199907)42:73.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- [58].Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am J Pathol 2001; 158:1969-73; PMID:11395373; https://doi.org/ 10.1016/S0002-9440(10)64667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Perretti M, Wheller SK, Choudhury Q, Croxtall JD, Flower RJ. Selective inhibition of neutrophil function by a peptide derived from lipocortin 1 N-terminus. Biochem Pharmacol 1995; 50:1037-42; PMID:7575659 [DOI] [PubMed] [Google Scholar]
- [60].Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood 2006; 107:2123-30; PMID:16278303; https://doi.org/ 10.1182/blood-2005-08-3099 [DOI] [PubMed] [Google Scholar]
- [61].Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA, Perretti M, Schurgers LJ, et al.. Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR−/− Mice on Western Type Diet. PLoS One 2015; 10:e0130484; PMID:26090792; https://doi.org/ 10.1371/journal.pone.0130484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell 2000; 5:831-40; PMID:10882119 [DOI] [PubMed] [Google Scholar]
- [63].Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci U S A 1998; 95:14535-9; PMID:9826735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood 2003; 101:4140-7; PMID:12560218; https://doi.org/ 10.1182/blood-2002-11-3411 [DOI] [PubMed] [Google Scholar]
- [65].Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, et al.. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res 2015; 116:827-35; PMID:10903766; https://doi.org/ 10.1161/CIRCRESAHA.116.305825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Solito E, Romero IA, Marullo S, Russo-Marie F, Weksler BB. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the alpha 4 beta 1 integrin. J Immunol 2000; 165:1573-81 [DOI] [PubMed] [Google Scholar]
- [67].Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, et al.. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J 2003; 17:253-5; PMID:12475898; https://doi.org/ 10.1096/fj.02-0239fje [DOI] [PubMed] [Google Scholar]
- [68].Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol 2005; 78:639-46; PMID:16000391; https://doi.org/ 10.1189/jlb.0405206 [DOI] [PubMed] [Google Scholar]
- [69].Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation 1997; 21:583-96; PMID:9429906 [DOI] [PubMed] [Google Scholar]
- [70].Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al.. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest 2013; 123:443-54; PMID:23241962; https://doi.org/ 10.1172/JCI65831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mancuso F, Flower RJ, Perretti M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule. Involvement of endogenous lipocortin 1. J Immunol 1995; 155:377-86; PMID:7602112 [PubMed] [Google Scholar]
- [72].Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, Gavins FN. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation 2016; 133:2169-79; PMID:27154726; https://doi.org/ 10.1161/CIRCULATIONAHA.115.020633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gavins FN, Hughes EL, Buss NA, Holloway PM, Getting SJ, Buckingham JC. Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB J 2012; 26:4977-89; PMID:22964301; https://doi.org/ 10.1096/fj.12-205971 [DOI] [PubMed] [Google Scholar]
- [74].Dalli J, Consalvo AP, Ray V, Di Filippo C, D'Amico M, Mehta N, Perretti M. Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol 2013; 190:6478-87; PMID:23686496; https://doi.org/ 10.4049/jimmunol.1203000 [DOI] [PubMed] [Google Scholar]
- [75].Smith HK, Gil CD, Oliani SM, Gavins FN. Targeting formyl peptide receptor 2 reduces leukocyte-endothelial interactions in a murine model of stroke. FASEB J 2015; 29:2161-71; PMID:25690650; https://doi.org/ 10.1096/fj.14-263160 [DOI] [PubMed] [Google Scholar]
- [76].Gastardelo TS, Damazo AS, Dalli J, Flower RJ, Perretti M, Oliani SM. Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am J Pathol 2009; 174:177-83; PMID:19095957; https://doi.org/ 10.2353/ajpath.2009.080342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol 2010; 184:2611-9; PMID:20107188; https://doi.org/ 10.4049/jimmunol.0903526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jilma B, Voltmann J, Albinni S, Stohlawetz P, Schwarzinger I, Gleiter CH, Rauch A, Eichler HG, Wagner OF. Dexamethasone down-regulates the expression of L-selectin on the surface of neutrophils and lymphocytes in humans. Clin Pharmacol Ther 1997; 62:562-8; PMID:9390113; https://doi.org/ 10.1016/S0009-9236(97)90052-7 [DOI] [PubMed] [Google Scholar]
- [79].de Coupade C, Solito E, Levine JD. Dexamethasone enhances interaction of endogenous annexin 1 with L-selectin and triggers shedding of L-selectin in the monocytic cell line U-937. Br J Pharmacol 2003; 140:133-45; PMID:12967943; https://doi.org/ 10.1038/sj.bjp.0705413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Strausbaugh HJ, Rosen SD. A potential role for annexin 1 as a physiologic mediator of glucocorticoid-induced L-selectin shedding from myeloid cells. J Immunol 2001; 166:6294-300; PMID:11342653 [DOI] [PubMed] [Google Scholar]
- [81].Zouki C, Ouellet S, Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J 2000; 14:572-80; PMID:10698973 [DOI] [PubMed] [Google Scholar]
- [82].Peshavariya HM, Taylor CJ, Goh C, Liu GS, Jiang F, Chan EC, Dusting GJ. Annexin peptide Ac2-26 suppresses TNFalpha-induced inflammatory responses via inhibition of Rac1-dependent NADPH oxidase in human endothelial cells. PLoS One 2013; 8:e60790; PMID:23637767; https://doi.org/ 10.1371/journal.pone.0060790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ed Rainger G, Chimen M, Harrison MJ, Yates CM, Harrison P, Watson SP, Lordkipanidze M, Nash GB. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets 2015; 26:507-20; PMID:26196409; https://doi.org/ 10.3109/09537104.2015.1064881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 2002; 106:1523-9; PMID:12234959 [DOI] [PubMed] [Google Scholar]
- [85].Murphy CT, Peers SH, Forder RA, Flower RJ, Carey F, Westwick J. Evidence for the presence and location of annexins in human platelets. Biochem Biophys Res Commun 1992; 189:1739-46; PMID:1482379 [DOI] [PubMed] [Google Scholar]
- [86].Eldering JA, Kocher M, Clemetson JM, Clemetson KJ, Frey FJ, Frey BM. Presence of lipocortins I and IV, but not II and VI, in human platelets. FEBS Lett 1993; 318:231-4; PMID:8440377 [DOI] [PubMed] [Google Scholar]
- [87].Bot I, Shi GP, Kovanen PT. Mast cells as effectors in atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35:265-71; PMID:25104798; https://doi.org/ 10.1161/ATVBAHA.114.303570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013; 13:709-21; PMID:23995626; https://doi.org/ 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bandeira-Melo C, Bonavita AG, Diaz BL, PM ES, Carvalho VF, Jose PJ, Flower RJ, Perretti M, Martins MA. A novel effect for annexin 1-derived peptide ac2-26: reduction of allergic inflammation in the rat. J Pharmacol Exp Ther 2005; 313:1416-22; PMID:15784654; https://doi.org/ 10.1124/jpet.104.080473 [DOI] [PubMed] [Google Scholar]
- [90].Yazid S, Sinniah A, Solito E, Calder V, Flower RJ. Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1. PLoS One 2013; 8:e58963; PMID:23527056; https://doi.org/ 10.1371/journal.pone.0058963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ferlazzo V, D'Agostino P, Milano S, Caruso R, Feo S, Cillari E, Parente L. Anti-inflammatory effects of annexin-1: stimulation of IL-10 release and inhibition of nitric oxide synthesis. Int Immunopharmacol 2003; 3:1363-9; PMID:12946433; https://doi.org/ 10.1016/S1567-5769(03)00133-4 [DOI] [PubMed] [Google Scholar]
- [92].Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, et al.. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 2011; 30:3887-99; PMID:21499310; https://doi.org/ 10.1038/onc.2011.112 [DOI] [PubMed] [Google Scholar]
- [93].Drechsler M, Soehnlein O. The complexity of arterial classical monocyte recruitment. J Innate Immun 2013; 5:358-66; PMID:23571485; https://doi.org/ 10.1159/000348795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Swirski FK. Inflammation and repair in the ischaemic myocardium. Hamostaseologie 2015; 35:34-6; PMID:25375277; https://doi.org/ 10.5482/HAMO-14-09-0045 [DOI] [PubMed] [Google Scholar]
- [95].Rodrigues SF, Granger DN. Leukocyte-mediated tissue injury in ischemic stroke. Curr Med Chem 2014; 21:2130-7; PMID:24372215 [DOI] [PubMed] [Google Scholar]
- [96].Solito E, Kamal A, Russo-Marie F, Buckingham JC, Marullo S, Perretti M. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J 2003; 17:1544-6; PMID:12824302; https://doi.org/ 10.1096/fj.02-0941fje [DOI] [PubMed] [Google Scholar]
- [97].El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol 2007; 179:616-22; PMID:17579083 [DOI] [PubMed] [Google Scholar]
- [98].Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 2003; 4:587-98; PMID:12689596 [DOI] [PubMed] [Google Scholar]
- [99].Blume KE, Soeroes S, Keppeler H, Stevanovic S, Kretschmer D, Rautenberg M, Wesselborg S, Lauber K. Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J Immunol 2012; 188:135-45; PMID:22116825; https://doi.org/ 10.4049/jimmunol.1004073 [DOI] [PubMed] [Google Scholar]
- [100].Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, Maderna P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol 2007; 178:4595-605; PMID:17372018 [DOI] [PubMed] [Google Scholar]
- [101].Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2-26). J Immunol 2005; 174:3727-33; PMID:15749912 [DOI] [PubMed] [Google Scholar]
- [102].Bagnato C, Thumar J, Mayya V, Hwang SI, Zebroski H, Claffey KP, Haudenschild C, Eng JK, Lundgren DH, Han DK. Proteomics analysis of human coronary atherosclerotic plaque: a feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics 2007; 6:1088-102; PMID:17339633; https://doi.org/ 10.1074/mcp.M600259-MCP200 [DOI] [PubMed] [Google Scholar]
- [103].Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, Berge RK, Seppala I, Shalhoub J, Franklin IJ, et al.. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol 2013; 54:65-72; PMID:23154128; https://doi.org/ 10.1016/j.yjmcc.2012.11.002 [DOI] [PubMed] [Google Scholar]
- [104].Cheuk BL, Cheng SW. Annexin A1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur J Vasc Endovasc Surg 2011; 41:364-71; PMID:21195640; https://doi.org/ 10.1016/j.ejvs.2010.11.021 [DOI] [PubMed] [Google Scholar]
- [105].Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med 2015; 7:275ra20; PMID:25695999; https://doi.org/ 10.1126/scitranslmed.aaa1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Swirski FK, Nahrendorf M. Leukocyte behavior inatherosclerosis, myocardial infarction, and heart failure. Science 2013; 339:161-6; PMID:23307733; https://doi.org/ 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14:392-404; PMID:24854589; https://doi.org/ 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- [108].Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007; 204:3037-47; PMID:18025128; https://doi.org/ 10.1084/jem.20070885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014; 124:1382-92; PMID:24569380; https://doi.org/ 10.1172/JCI72181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 2011; 17:1391-401; PMID:22064429; https://doi.org/ 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].La M, D'Amico M, Bandiera S, Di Filippo C, Oliani SM, Gavins FN, Flower RJ, Perretti M. Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: analysis of their mechanism of action. FASEB J 2001; 15:2247-56; PMID:11641252; https://doi.org/ 10.1096/fj.01-0196com [DOI] [PubMed] [Google Scholar]
- [112].Gavins FN, Kamal AM, D'Amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J 2005; 19:100-2; PMID:15507472; https://doi.org/ 10.1096/fj.04-2178fje [DOI] [PubMed] [Google Scholar]
- [113].D'Amico M, Di Filippo C, La M, Solito E, McLean PG, Flower RJ, Oliani SM, Perretti M. Lipocortin 1 reduces myocardial ischemia-reperfusion injury by affecting local leukocyte recruitment. FASEB J 2000; 14:1867-9; PMID:11023969; https://doi.org/ 10.1096/fj.99-0602fje [DOI] [PubMed] [Google Scholar]
- [114].Qin C, Buxton KD, Pepe S, Cao AH, Venardos K, Love JE, Kaye DM, Yang YH, Morand EF, Ritchie RH. Reperfusion-induced myocardial dysfunction is prevented by endogenous annexin-A1 and its N-terminal-derived peptide Ac-ANX-A1(2-26). Br J Pharmacol 2013; 168:238-52; PMID:22924634; https://doi.org/ 10.1111/j.1476-5381.2012.02176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ritchie RH, Gordon JM, Woodman OL, Cao AH, Dusting GJ. Annexin-1 peptide Anx-1(2-26) protects adult rat cardiac myocytes from cellular injury induced by simulated ischaemia. Br J Pharmacol 2005; 145:495-502; PMID:15821756; https://doi.org/ 10.1038/sj.bjp.0706211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med 2002; 8:1296-302; PMID:12368905; https://doi.org/ 10.1038/nm786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010; 17:197-218; PMID:20074922; https://doi.org/ 10.1016/j.pathophys.2009.12.001 [DOI] [PubMed] [Google Scholar]
- [118].Relton JK, Strijbos PJ, O'Shaughnessy CT, Carey F, Forder RA, Tilders FJ, Rothwell NJ. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J Exp Med 1991; 174:305-10; PMID:1830327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J 2007; 21:1751-8; PMID:17317721; https://doi.org/ 10.1096/fj.06-7842com [DOI] [PubMed] [Google Scholar]
- [120].Enlimomab Acute Stroke Trial I. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57:1428-34; PMID:11673584 [DOI] [PubMed] [Google Scholar]
- [121].Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin 2002; 18 Suppl 2:s18-22; PMID:12365824 [DOI] [PubMed] [Google Scholar]