Abstract
Protein acetylation plays important roles in many biological processes. Malate dehydrogenase (MDH), a key enzyme in the tricarboxylic acid (TCA) cycle, has been identified to be acetylated in bacteria by proteomic studies, but no further characterization has been reported. One challenge for studying protein acetylation is to get purely acetylated proteins at specific positions. Here we applied the genetic code expansion strategy to site-specifically incorporate Nε-acetyllysine into MDH. The acetylation of lysine residues in MDH could enhance its enzyme activity. The Escherichia coli deacetylase CobB could deacetylate acetylated MDH, while the E. coli acetyltransferase YfiQ cannot acetylate MDH efficiently. Our results also demonstrated that acetyl-CoA or acetyl-phosphate could acetylate MDH chemically in vitro. Furthermore, the acetylation level of MDH was shown to be affected by carbon sources in the growth medium.
Keywords: post-translational modification, acetyltransferase, deacetylase, genetic code expansion, protein acetylation
Graphical abstract
INTRODUCTION
The acetylation of lysine residues is one of the most common post-translational modifications of proteins that regulates diverse cellular functions including DNA-protein interaction, transcription activity, protein stability, stress response, apoptosis, cellular differentiation, and energy metabolism [1–13]. The abnormality of acetylation modifications is associated with diabetes, cardiovascular diseases, cancers, and neurodegenerative disorders [14–24]. During the last decade, proteomic studies have identified lysine acetylation in thousands of eukaryotic proteins which significantly expanded our knowledge of protein acetylation [25–27].
Interestingly, protein acetylation in bacteria starts to attract attentions in the last five years [28–35]. A series of proteomic studies have identified hundreds of bacterial proteins with lysine acetylation, including metabolic enzymes, stress response proteins, regulator of chemotaxis, chaperones, as well as transcription and translation factors [36–47]. However, few studies have been performed to further characterize these acetylated proteins. Take E. coli as an example, only six proteins with lysine acetylation were studied, including acetyl-CoA synthetase [48–51]; CheY, the regulator of bacterial chemotaxis [52–54]; RcsB, the regulator of capsule synthesis [55–57]; RNase R [58], N-hydroxyarylamine O-acetyltransferase [59], and α-subunit of RNA polymerase [60, 61]. One challenge for studying lysine acetylation is that it is difficult to synthesize purely acetylated proteins at specific sites by most classic methods. To solve this problem, the genetic code expansion strategy has been applied. Rather than adding acetyl group after protein translation, this approach uses an orthogonal pair of an engineered pyrrolysyl-tRNA synthetase variant and its cognate tRNA from Methanosarcinaceae species to co-translationally direct the incorporation of Nε-acetyllysine (AcK) in response to a stop codon at desired positions in target proteins [62–65].
MDH, a widely distributed enzyme catalyzing the conversion of oxaloacetate and malate, plays key roles in many important metabolic pathways including the tricarboxylic acid (TCA) cycle, glyoxylate bypass, amino acid synthesis, gluconeogenesis, and the exchange of metabolites between cytoplasm and subcellular organelles [66, 67]. Previous studies have shown that the acetylation of lysine residues in mammalian MDHs is involved in the cross-talk mechanisms between adipogenesis and the intracellular energy level [68–70]. However, the acetylation of bacterial MDH has not been characterized before. Here, we applied the genetic code expansion strategy to study the lysine acetylation of MDH in E. coli. We also used the same strategy to study human MDH as comparison.
RESULTS
Selecting acetylation sites in MDHs
Recently, several proteomic studies have identified lysine acetylation in E. coli MDH (eMDH), and lysine residues K99 and K140 were identified to be acetylated in all of these reports [37–39, 46, 47]. So we chose these two positions to incorporate AcK. In human cells, there are two MDH isozymes, the cytosol MDH1 and mitochondrial MDH2 [71]. Human MDH2 (hMDH2) has higher homology to eMDH than hMDH1 does [72]. Previous proteomic studies showed that hMDH2 has four lysine residues acetylated at positions K185, K301, K307, and K314 [73]. So we also chose these four positions to incorporate AcK as comparison. Interestingly, the alignment of eMDH and hMDH2 showed different patterns: the acetylation sites in eMDH are at the middle part of the primary sequence, while those in hMDH2 are mainly at the C-terminus (Figure S1). The acetylation site K185 in hMDH2 has its counterpart in eMDH at position K162 which was also selected to incorporate AcK as a control.
Site-specifically incorporating lysine acetylation
We incorporated AcK at selected positions mentioned above in both eMDH and hMDH2 by our recently optimized AcK incorporation system which has an optimized tRNAPyl with better binding with E. coli elongation factor (EF-Tu), thus increasing the incorporation efficiency [65]. The AcK was genetically encoded by an amber stop codon (TAG) which was introduced by site-directed mutagenesis. Previous studies showed that the K12-derived strains have substantially higher acetylation than B-strain-derived BL21 cells during growth [39]. To lower the background of non-specific acetylation at other lysine residues, we used BL21 (DE3) strain as the expression host. The incorporation of AcK was confirmed by both western blotting (Figure 1A) and mass spectrometry (MS) (Figure S2–S8).
Figure 1. AcK incorporation into MDHs.
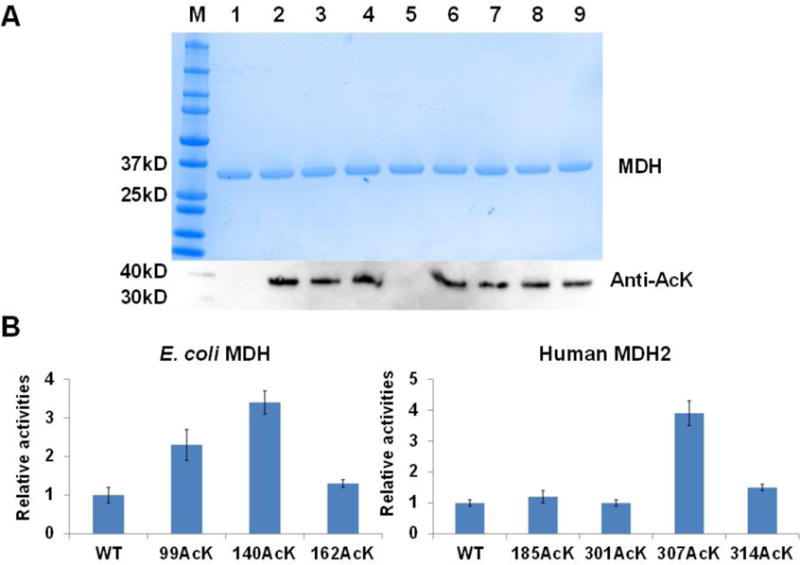
A) SDS-PAGE and western blotting analyses of purified MDHs and their variants. Lane 1, wild-type eMDH; lane 2, eMDH 99AcK; lane 3, eMDH 140AcK; lane 4, eMDH 162AcK; lane 5; wild-type hMDH2; lane 6, hMDH2 185 AcK; lane 7, hMDH2 301AcK; lane 8, hMDH2 307AcK; lane 9, hMDH2 314AcK. The same amounts of proteins were loaded. B) The enzyme activities of MDHs and their acetylated variants. The activities of wild-type E. coli MDH and human MDH2 were set as 1, respectively. The mean values and standard errors were calculated based on three replicates.
The lysine acetylation of MDH increases its enzyme activities
The enzyme activities of eMDH and hMDH2 as well as their acetylated variants were measured (Figure 1B). For eMDH, the acetylation at positions K99 and K140 increased the enzyme activity by 2.3 and 3.4 folds, individually, which is consistent with our previous study [65], while the acetylation at the position K162 had little effect. Our previous study also showed that doubly acetylated eMDH at both positions K99 and K140 had 6-fold higher enzyme activity than that of wild-type eMDH [65]. For hMDH2, only the acetylation at the position K307 increased the enzyme activity by 3.9 folds, while others had no obvious effects.
Steady-state kinetic analyses were performed with wild-type MDHs and their acetylated variants (Table 1). The lysine acetylation appeared not to affect the KM values of both substrates, NAD+ and malate, indicating that the increase of enzyme activities results mainly from the improvement of the overall turnover.
Table 1.
Kinetic analyses of MDHs and acetylated variantsa
| kcat (s−1) | KM, NAD+(mM) | KM, malate (mM) | kcat/KM, NAD+ (S−1 mM−1) | kcat/KM, malate (S−1 mM−1) | |
|---|---|---|---|---|---|
| eMDH WT | 20.2 ± 0.4 | 0.23 ± 0.02 | 2.61 ± 0.20 | 87.8 | 7.74 |
| eMDH 99AcK | 51.2 ± 1.3 | 0.25 ± 0.07 | 2.32 ± 0.32 | 204.8 | 22.07 |
| eMDH 140AcK | 74.3 ± 1.1 | 0.26 ± 0.06 | 2.54 ± 0.64 | 285.8 | 29.25 |
| hMDH2 WT | 69.6 ± 2.9 | 0.16 ± 0.01 | 0.24 ± 0.08 | 435.0 | 290.0 |
| hMDH2 307AcK | 178.9 ± 3.1 | 0.12 ± 0.04 | 0.20 ± 0.12 | 1490.8 | 894.5 |
The mean values and standard errors were calculated based on three replicates. The parameters were determined by nonlinear regression with software GraFit (Erithacus Software).
CobB can deacetylate acetylated MDHs at specific positions
Lysine deacetylases remove the N-acetyl amide moieties, and can be broadly divided into two families according to their reaction mechanism: hydrolytic deacetylases and NAD+-dependent deacetylases which are also named as sirtuins [32, 74]. The CobB protein, a sirtuin family member, was found in many bacteria including E. coli and Salmonella [48, 51]. Recently, YcgC has been confirmed to be a hydrolytic deacetylase and target a distinct set of substrates from E. coli CobB, representing a novel family of prokaryotic deacetylases [75].
To determine the deacetylation activity of the CobB protein on acetylated MDHs, K12-derived E. coli TOP10 strain with higher acetylation levels was used for in vivo tests [39]. The genes of eMDH and hMDH2 with C-terminal His6 tags were expressed and purified in wild-type or ΔcobB cells, individually. Western blotting was used for detecting the acetylation (Figure 2A). The deletion of cobB gene increased the acetylation levels of both eMDH and hMDH2, indicating that CobB could deacetylate their lysine acetylation in vivo. We also measured the enzyme activities of MDHs from wild-type or ΔcobB cells (Figure 2B). The MDHs purified from ΔcobB cells had higher enzyme activities, which was consistent with the western blotting results as lysine acetylation could increase MDH activities.
Figure 2. CobB deacetylates MDHs.
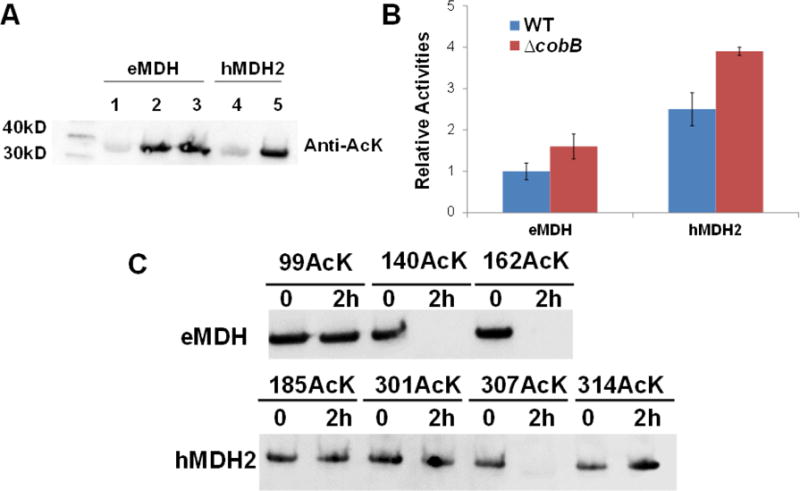
A) Western blotting of purified MDHs from TOP10 and TOP10 ΔcobB cells. Lane 1 and 4 were from wild-type TOP10 cells. Lane 2 and 5 were from TOP10 ΔcobB cells. Lane 3 was from TOP10 ΔcobB ΔyfiQ cells. The same amounts of proteins were loaded. B) The enzyme activities of purified MDHs from TOP10 and TOP10 ΔcobB cells. The activity of eMDH purified from wild-type TOP10 cells was set as 1. The mean values and standard errors were calculated based on three replicates. C) Western blotting of acetylated MDH variants treated with the CobB protein. The acetylation levels of MDH variants after 2-hour incubation were compared with those without CobB treatment. The same amounts of proteins were loaded.
We also performed in vitro deacetylation experiments. The optimized AcK incorporation system mentioned above was used to generate site-specially acetylated eMDH at positions K99, K140, and K162, individually, and acetylated hMDH2s at positions K185, K301, K307, and K314, respectively. All these MDH variants were treated with CobB protein separately. Western blotting was used for detecting the acetylation (Figure 2C). Our results showed that CobB was specific for positions K140 and K162 of eMDH and the position K307 of hMDH2. After 2-hour treatment of CobB, no detectable acetylation was observed for these three positions by western blotting, while the acetylation at other positions were not changed obviously. The insensitivity of acetyl-K99 in eMDH against CobB supports the conclusion from previous studies that although CobB is the predominate deacetylase in E. coli, it could not deacetylate the majority of acetylation at lysine residues [39, 46, 76]. For hMDH2 acetylated variants, we also tested them with the counterpart of CobB in human, SIRT3, which is the major protein deacetylase in mitochondria [77]. Similarly, SIRT3 is only specific for acetyl-K307 of hMDH2 (Figure S9).
The acetylation of MDHs
The acetylation of lysine residues is catalyzed by acetyltransferases which can be categorized into five families: the Gcn5-related N-acetyltransferase (GNAT) family, the MYST family, the CBP/p300 co-activators, the SRC family of co-activators, and the TAFII group of transcription factors [78–81]. YfiQ, a member of GNAT family, is the only known acetyltransferase in E. coli, required for glucose-dependent acetylation of several lysine residues within RNA polymerase [58] and for the acetylation of Lys544 in RNase R [61].
To determine the acetylation activity of the YfiQ protein on eMDH, TOP10 strain (a K12-derivated strain with a higher acetylation level) was used for in vivo tests. The gene of eMDH with a C-terminal His6 tag was expressed in TOP10 ΔcobB ΔyfiQ cells and purified. Western blotting was used for detecting the acetylation. Compared with the eMDH which was expressed from TOP10 ΔcobB cells (Figure 2A), the deletion of yfiQ gene did not decrease the acetylation level of eMDH with the ΔcobB background, indicating that the YfiQ protein is not the major acetyltransferase for eMDH in vivo. We also measured the enzyme activity of eMDH from TOP10 ΔcobB ΔyfiQ cells (Figure 3A). eMDH purified from ΔcobB ΔYfiQ cells had a similar enzyme activity with eMDH purified from ΔcobB cells, indicating they had similar levels of acetylation which was consistent with the western blotting results.
Figure 3. Acetylation of E. coli MDH.
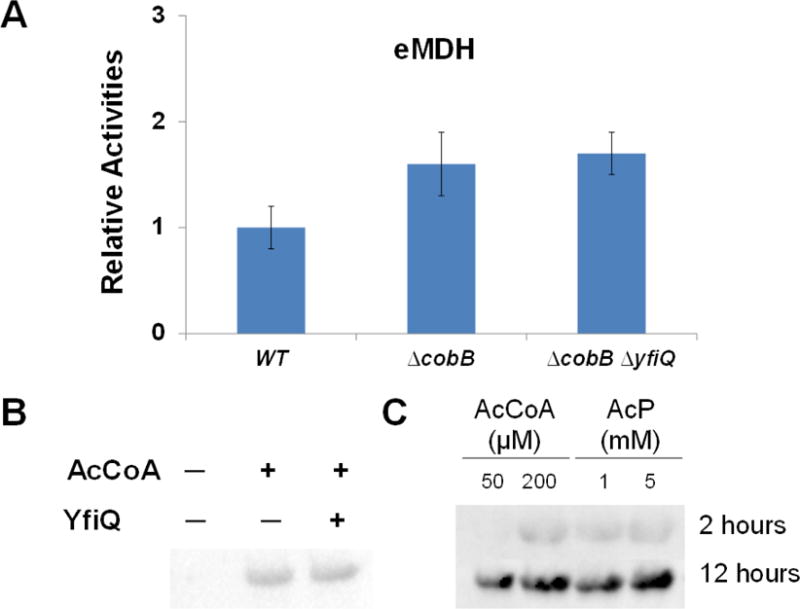
A) The enzyme activities of purified eMDHs from TOP10, TOP10 ΔcobB, and TOP10 ΔcobB ΔyfiQ cells. The activity of eMDH purified from TOP10 cells was set as 1. The mean values and standard errors were calculated based on three replicates. B) Western blotting of purified eMDH from BL21(DE3) treated with the YfiQ protein and acetyl-CoA for 2 hours. The same amounts of proteins were loaded. C) B) Western blotting of purified eMDH from BL21(DE3) treated with acetyl-CoA (AcCoA) or acetyl-phosphate (AcP) for 2 hours and 12 hours, respectively. The same amounts of proteins were loaded.
We also performed in vitro acetylation experiments. The wild-type eMDH expressed and purified from BL21 (DE3) cells which had no detectable acetylation with western blotting (Figure 1B) was treated with purified YfiQ protein and the acetylation donor, acetyl-CoA. Incubated with YfiQ and acetyl-CoA for 2 hours, the acetylation level of eMDH had no obvious difference with that incubated with acetyl-CoA only, indicating that YfiQ cannot acetylate eMDH efficiently in vitro (Figure 3B).
Besides YfiQ, there are 24 members of GNAT family acetyltransferases in E. coli, 12 with known substrates: ArgA, AstA, CitC, MnaT, PanM, PhnO, RimI, RimJ, RimL, SpeG, TmcA, and WecD; the other 12 proteins with unknown functions: ElaA, YafP, YedL, YhbS, YhhY, YiaC, YiiD, YjaB, YjdJ, YjgM, YjhQ, and YpeA [33]. We tested their functions in acetylating eMDH in vitro. Similarly, incubated with individual candidate acetyltransferase and acetyl-CoA for 2 hours, the acetylation level of eMDH had no obvious difference with that incubated with acetyl-CoA only (Figure S10).
Previous studies showed chemical acetylation of proteins both in vitro and in vivo [39, 46, 82–85]. We also noticed that eMDH was acetylated after incubation with only acetyl-CoA for 2 hours (Figure 3B). So we determined the chemical acetylation of eMDH by acetyl-CoA or acetyl-phosphate, respectively. eMDH expressed and purified from BL21(DE3) cells which has no detectable acetylation was used. Western blotting was used to detect the acetylation (Figure 3C). The results showed that both acetyl-CoA and acetyl-phosphate could chemically acetylate eMDH at a dose-dependent manner. And the acetylation level of eMDH increased with the incubation time, which is consistent with the previous study on protein acetylation dynamics [47]. Such acetylation accumulation may also result from a carbon-to-nitrogen or a carbon-to-magnesium imbalance in vivo [39, 86].
Acetylation of eMDH with different carbon sources
Previous studies showed that the acetylation level of metabolic enzymes is higher when cells grow with glucose [73]. So we determined the acetylation level of eMDH purified from TOP10 cells grown with different carbon sources. Clearly, glucose could increase the acetylation level of eMDH with a dose-dependent manner (Figure 4A). We also measured the enzyme activities of eMDH from cells with different carbon sources, and it was consistent with the western blotting results (Figure 4B). These results are consistence with previous proteomic studies [47], indicating the possible role of enzyme acetylation in mediating cellular adaptation to different environments.
Figure 4. Acetylation of E. coli MDH with different carbon sources.
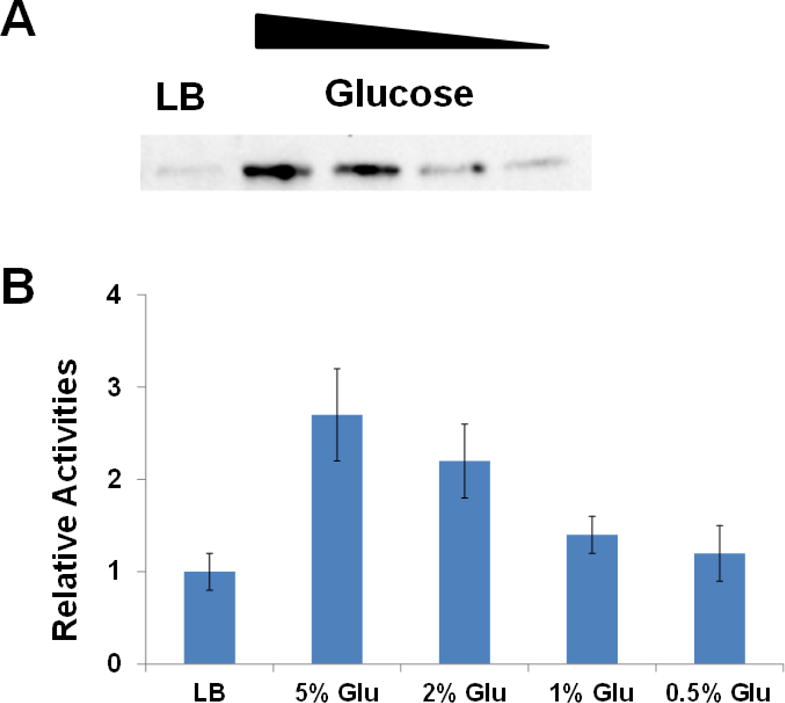
A) Western blotting of purified eMDHs from TOP10 cells grown with different carbon sources. LB medium, minimal medium with different glucose (Glu) concentrations (5%, 2%, 1%, or 0.5%) were used. The same amounts of proteins were loaded. B) The enzyme activities of purified eMDHs from TOP10 cells grown with different carbon sources. The activity of eMDH purified from TOP10 cells grown with LB medium was set as 1. The mean values and standard errors were calculated based on three replicates.
DISCUSSION
Classic approaches use amino acid substitutions to map functional lysine acetylation sites. The substitution with arginine retains a positive charge which is often utilized as a non-acetylated mimic, while the substitution with glutamine abolishes the positive charge which can act as a surrogate of acetylation [87–89]. However, such strategies sometimes do not reveal the real effects of lysine acetylation [90]. Here, we synthesized homogenously acetylated proteins at specific sites by the genetic code expansion strategy to determine the effects of acetylation directly, overcoming the potential problems with the substitution approach.
Our kinetic analysis of MDH variants indicated that the catalytic efficiency of acetylated MDHs increased 2.3 to 3.4 folds (Table 1). The KM values of both substrates, NAD+ and malate, did not change obviously, indicating that acetylation at these positions do not affect the substrate binding. The results also showed that the human enzyme has higher catalytic efficiency and much better binding of malate than those of the E. coli enzyme.
Based on the crystal structures on eMDH (PDB ID: 1EMD) and hMDH2 (PDB ID: 2DFD) [91], we mapped the selected acetylated residues used in this study (Figure 5). None of these acetylated sites are at substrate binding sites. It is consistent with our kinetic analyses which indicated that the lysine acetylation does not affect the substrates binding (Table 1). Our results also showed that CobB deacetylase was specific for positions K140 and K162 of eMDH and the position K307 of hMDH2 (Figure 2C). From the structures of MDHs (Figure 5), we found that these three residues are located at coiled structures. Lysine residues at position K99 in eMDH, positions K301 and K314 in hMDH2 are within either helical structures or sheet structures. These results suggested CobB favors unstructured protein domains which is consistent with previous studies [39, 46, 76, 92]. Interestingly, as a counterpart of eMDH position K162, the lysine acetylation at position K185 (at a coiled structure) in hMDH2 is resistant to the deacetylase (Figure 2C), indicating that the specificity of the deacetylase may also depend on primary sequence contexts, which has been proposed in the previous study [76]. And this difference may be an ideal target for antibacterial agent development.
Figure 5. Mapping of acetylation sites on the crystal structures of MDHs.
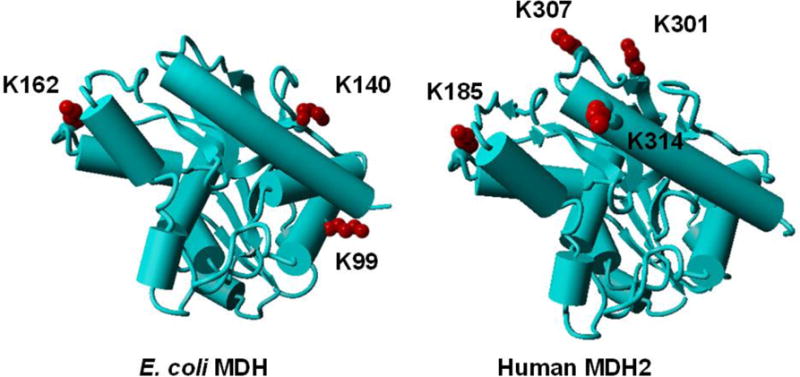
The crystal structures of eMDH (PDB ID: 1EMD) and hMDH2 (PDB ID: 2DFD) were demonstrated with the selected acetylation sites in this study labelled.
Based on the bioinformatical analyses, there are 25 putative GNAT family acetyltransferases in E. coli [78]. Some members perform Nα-acetylation such as RimL [93], while some members are specific for free amino acids like ArgA [94]. There are reports to describe the functions of only 13 GNAT members, leaving the other 12 members completely unknown [95]. Although some works have been done to identify the protein motifs which are recognized by protein acetyltransferases [95, 96], the difference between GNAT members may be important for their specificities for different protein substrates, thus it is necessary to characterize those 25 GNAT members for protein acetylation studies as we did in this study (Figure S10).
MATERIALS AND METHODS
General molecular biology
The amino acids in this study were purchased from Sigma-Aldrich or ChemImpex. TOP10 cells (Life Technologies) were used for general cloning. All the cloning experiments were performed by using the Gibson Assembly kit (New England Biolabs). The mutations of stop codons in MDH genes were made by the QuikChange II mutagenesis kit (Agilent Technologies). The strains and plasmids used in this study is listed in Table S1. Western Blots: The purified MDHs and their variants were fractionated by SDS-PAGE and transferred onto a PVDF membrane (Bio-Rad). The membrane was incubated at room temperature with gentle shacking in TTBS and 5% Milk blocking buffer for 60 min. The primary antibody, HRP-conjugated-Acetylated-Lysine (Ac-K2-100) Rabbit mAb (Cell Signaling Technology), was diluted 1:1000 and soaked the membranes overnight at 4 degree. The membrane was prepared for detection using Western Lighting Plus-ECL (PerkinElmer, Inc.). The E. coil proteins including CobB and 25 GNAT family acetyltransferases were purified from the ASKA strain collection by following the purification protocols [97]. The human SIRT3 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The gene deletion in E. coli was performed by recombination as previous protocols [98].
Expression and purification of AcK-containing MDH variants
The genes of MDHs and their variants were cloned into the pET15b or pBAD plasmid with a C-terminal His6-tag and transformed into BL21 (DE3) or Top 10 cells together with the pTech plasmids harboring the AcK incorporation system for expression. For human MDH2 expression, the predicted mitochondrial targeting sequence (residues 2–24) was removed. The expression strain was grown on 1 L of LB medium supplemented with 100 μg/ml ampicillin and 50 μg/ml chloramphenicol at 37°C to an absorbance of 0.6–0.8 at 600 nm and protein expression was induced by the addition of 1 mM IPTG and supplemented with 5 mM AcK and 20 mM nicotinamine (NAM, deacetylases inhibitor). Cells were incubated at 30°C for an additional 8 h and harvested by centrifugation at 5000 × g for 10 min at 4 °C. The cell paste was suspended in 15 ml of lysis buffer (50 mM Tris (pH 7.5), 300 mM NaCl, 20 mM imidazole, 20 mM NAM) and broken by sonication. The crude extract was centrifuged at 20,000 × g for 30 min at 4°C. The soluble fraction was loaded onto a column containing 2 ml of Ni-NTA resin (Qiagen) previously equilibrated with 20 ml lysis buffer. The column was washed with 50 ml lysis buffer. The protein bound to the column was then eluted with 2 ml of 50 mM Tris (pH 7.5), 300 mM NaCl, 150 mM imidazole. The purified protein was dialyzed with 50 mM Tris (pH 7.5), 50 mM NaCl, 1mM DTT and 50% glycerol, and stored at −80°C for further studies.
MDH enzyme assays
The assays were performed by following the instruction of the EnzyChromTM Malate Dehydrogenase Assay Kit (EMDH-100) from BioAssay Systems. This non-radioactive, colorimetric MDH assay is based on the reduction of the tetrazolium salt MTT in a NADH-coupled enzymatic reaction to a reduced form of MTT which exhibits an absorption maximum at 565 nm. The increase in absorbance at 565 nm is proportional to the enzyme activity.
LC-MS/MS analyses
The proteins were trypsin digested by a standard in-gel digestion protocol, and analyzed by LC-MS/MS on an LTQ Orbitrap XL (Thermo Scientific) equipped with a nanoACQUITY UPLC system (Waters). A Symmetry C18 trap column (180 μm × 20 mm; Waters) and a nanoACQUITY UPLC column (1.7 μm, 100 μm × 250 mm, 35°C) were used for peptide separation. Trapping was done at 15 μL min−1, 99% buffer A (water with 0.1% formic acid) for 1 min. Peptide separation was performed at 300 nL min−1 with buffer A and buffer B (CH3CN containing 0.1% formic acid). The linear gradient (51 min) was from 5% buffer B to 50% B at 50 min, to 85% B at 51 min. MS data were acquired in the Orbitrap with one microscan, and a maximum inject time of 900 ms followed by data-dependent MS/MS acquisitions in the ion trap (through collision induced dissociation, CID). The Mascot search algorithm was used to search for the appropriate noncanonical substitution (Matrix Science, Boston, MA).
In vitro acetylation
The reaction was performed in the buffer containing 50 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, 10% glycerol, 1 mM DTT and 10 mM sodium butyrate. The acetylation was carried out by adding 10 μg MDHs, 10 μg of candidate acetyltransferase, and 0.2 mM acetyl-CoA in a volume of 100 μl. Reaction mixtures were completely mixed and incubated at 37 °C.
CobB-mediated in vitro deacetylation
The reaction was performed in buffer contains 40 mM HEPES (pH 7.0), 6 mM MgCl2, 1.0 mM NAD+, 1 mM DTT and 10 % glycerol. 10 μg MDHs, 10 μg CobB, and the reaction buffer were incubated at 37 °C in a total volume of 100 μl.
Supplementary Material
Highlights.
Acetyllysine was site-specifically incorporated into the malate dehydrogenase.
The acetylation of malate dehydrogenase enhanced enzyme activity.
CobB could deacetylate acetylated malate dehydrogenase.
Malate dehydrogenase could be acetylated chemically.
The acetylation level of malate dehydrogenase was affected by carbon sources.
Acknowledgments
This work was supported by the NIH grant AI119813 to C.F., and the startup fund from the University of Arkansas.
Abbreviations
- AcK
Nε-acetyllysine
- MDH
malate dehydrogenase
- eMDH
Escherichia coli malate dehydrogenase
- hMDH2
human malate dehydrogenase isozyme 2
- TCA
tricarboxylic acid
- MS
mass spectrometry
- GNAT
Gcn5-related N-acetyltransferase
- NAM
nicotinamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen T, Yao TP. AcK-knowledge reversible acetylation. Sci STKE. 2004;2004:pe42. doi: 10.1126/stke.2452004pe42. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta. 2016;1864:1372–1401. doi: 10.1016/j.bbapap.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Menzies KJ, Zhang H, Katsyuba E, Auwerx J. Protein acetylation in metabolism - metabolites and cofactors. Nat Rev Endocrinol. 2016;12:43–60. doi: 10.1038/nrendo.2015.181. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Wei W, Zhou DX. Histone Acetylation Enzymes Coordinate Metabolism and Gene Expression. Trends Plant Sci. 2015;20:614–621. doi: 10.1016/j.tplants.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Koprinarova M, Schnekenburger M, Diederich M. Role of Histone Acetylation in Cell Cycle Regulation. Curr Top Med Chem. 2016;16:732–744. doi: 10.2174/1568026615666150825140822. [DOI] [PubMed] [Google Scholar]
- 8.Verdin E, Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- 10.Banreti A, Sass M, Graba Y. The emerging role of acetylation in the regulation of autophagy. Autophagy. 2013;9:819–829. doi: 10.4161/auto.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott I. Regulation of cellular homoeostasis by reversible lysine acetylation. Essays Biochem. 2012;52:13–22. doi: 10.1042/bse0520013. [DOI] [PubMed] [Google Scholar]
- 12.Arif M, Selvi BR, Kundu TK. Lysine acetylation: the tale of a modification from transcription regulation to metabolism. Chembiochem. 2010;11:1501–1504. doi: 10.1002/cbic.201000292. [DOI] [PubMed] [Google Scholar]
- 13.Close P, Creppe C, Gillard M, Ladang A, Chapelle JP, Nguyen L, et al. The emerging role of lysine acetylation of non-nuclear proteins. Cell Mol Life Sci. 2010;67:1255–1264. doi: 10.1007/s00018-009-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil J, Ramirez-Torres A, Encarnacion-Guevara S. Lysine acetylation and cancer: A proteomics perspective. J Proteomics. 2017;150:297–309. doi: 10.1016/j.jprot.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Gong F, Chiu LY, Miller KM. Acetylation Reader Proteins: Linking Acetylation Signaling to Genome Maintenance and Cancer. PLoS Genet. 2016;12:e1006272. doi: 10.1371/journal.pgen.1006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nat Rev Cancer. 2016;16:694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- 17.Al-Haddad R, Karnib N, Assaad RA, Bilen Y, Emmanuel N, Ghanem A, et al. Epigenetic changes in diabetes. Neurosci Lett. 2016;625:64–69. doi: 10.1016/j.neulet.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima A, Lopaschuk GD. Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta. 2016;1862:2211–2220. doi: 10.1016/j.bbadis.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Bonnaud EM, Suberbielle E, Malnou CE. Histone acetylation in neuronal (dys)function. Biomol Concepts. 2016;7:103–116. doi: 10.1515/bmc-2016-0002. [DOI] [PubMed] [Google Scholar]
- 20.Kaypee S, Sudarshan D, Shanmugam MK, Mukherjee D, Sethi G, Kundu TK. Aberrant lysine acetylation in tumorigenesis: Implications in the development of therapeutics. Pharmacol Ther. 2016;162:98–119. doi: 10.1016/j.pharmthera.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Wang L, Yu C, Yu D, Yu G. Histone Acetylation Modifiers in the Pathogenesis of Alzheimer’s Disease. Front Cell Neurosci. 2015;9:226. doi: 10.3389/fncel.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auburger G, Gispert S, Jendrach M. Mitochondrial acetylation and genetic models of Parkinson’s disease. Prog Mol Biol Transl Sci. 2014;127:155–182. doi: 10.1016/B978-0-12-394625-6.00006-4. [DOI] [PubMed] [Google Scholar]
- 23.You L, Nie J, Sun WJ, Zheng ZQ, Yang XJ. Lysine acetylation: enzymes, bromodomains and links to different diseases. Essays Biochem. 2012;52:1–12. doi: 10.1042/bse0520001. [DOI] [PubMed] [Google Scholar]
- 24.Iyer A, Fairlie DP, Brown L. Lysine acetylation in obesity, diabetes and metabolic disease. Immunol Cell Biol. 2012;90:39–46. doi: 10.1038/icb.2011.99. [DOI] [PubMed] [Google Scholar]
- 25.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 27.Iwabata H, Yoshida M, Komatsu Y. Proteomic analysis of organ-specific post-translational lysine-acetylation and -methylation in mice by use of anti-acetyllysine and -methyllysine mouse monoclonal antibodies. Proteomics. 2005;5:4653–4664. doi: 10.1002/pmic.200500042. [DOI] [PubMed] [Google Scholar]
- 28.Bernal V, Castano-Cerezo S, Gallego-Jara J, Ecija-Conesa A, de Diego T, Iborra JL, et al. Regulation of bacterial physiology by lysine acetylation of proteins. N Biotechnol. 2014;31:586–595. doi: 10.1016/j.nbt.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Jones JD, O’Connor CD. Protein acetylation in prokaryotes. Proteomics. 2011;11:3012–3022. doi: 10.1002/pmic.201000812. [DOI] [PubMed] [Google Scholar]
- 30.Kim GW, Yang XJ. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem Sci. 2011;36:211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Thao S, Escalante-Semerena JC. Control of protein function by reversible Nvarepsilon-lysine acetylation in bacteria. Curr Opin Microbiol. 2011;14:200–204. doi: 10.1016/j.mib.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu LI, Lima BP, Wolfe AJ. Bacterial protein acetylation: the dawning of a new age. Mol Microbiol. 2010;77:15–21. doi: 10.1111/j.1365-2958.2010.07204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hentchel KL, Escalante-Semerena JC. Acylation of Biomolecules in Prokaryotes: a Widespread Strategy for the Control of Biological Function and Metabolic Stress. Microbiol Mol Biol Rev. 2015;79:321–346. doi: 10.1128/MMBR.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe AJ. Bacterial protein acetylation: new discoveries unanswered questions. Curr Genet. 2016;62:335–341. doi: 10.1007/s00294-015-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouidir T, Kentache T, Hardouin J. Protein lysine acetylation in bacteria: Current state of the art. Proteomics. 2016;16:301–309. doi: 10.1002/pmic.201500258. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, et al. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 38.Zhang K, Zheng S, Yang JS, Chen Y, Cheng Z. Comprehensive profiling of protein lysine acetylation in Escherichia coli. J Proteome Res. 2013;12:844–851. doi: 10.1021/pr300912q. [DOI] [PubMed] [Google Scholar]
- 39.Weinert BT, Iesmantavicius V, Wagner SA, Scholz C, Gummesson B, Beli P, et al. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell. 2013;51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Kim D, Yu BJ, Kim JA, Lee YJ, Choi SG, Kang S, et al. The acetylproteome of Gram-positive model bacterium Bacillus subtilis. Proteomics. 2013;13:1726–1736. doi: 10.1002/pmic.201200001. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Vellaichamy A, Wang D, Zamdborg L, Kelleher L, Huber SC. Differential lysine acetylation profiles of Erwinia amylovora strains revealed by proteomics. J Proteomics. 2013;79:60–71. doi: 10.1016/j.jprot.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DW, Kim D, Lee YJ, Kim JA, Choi JY, Kang S, et al. Proteomic analysis of acetylation in thermophilic Geobacillus kaustophilus. Proteomics. 2013;13:2278–2282. doi: 10.1002/pmic.201200072. [DOI] [PubMed] [Google Scholar]
- 43.Crosby HA, Pelletier DA, Hurst GB, Escalante-Semerena JC. System-wide studies of N-lysine acetylation in Rhodopseudomonas palustris reveal substrate specificity of protein acetyltransferases. J Biol Chem. 2012;287:15590–15601. doi: 10.1074/jbc.M112.352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okanishi H, Kim K, Masui R, Kuramitsu S. Acetylome with structural mapping reveals the significance of lysine acetylation in Thermus thermophilus. J Proteome Res. 2013;12:3952–3968. doi: 10.1021/pr400245k. [DOI] [PubMed] [Google Scholar]
- 45.Baeza J, Dowell JA, Smallegan MJ, Fan J, Amador-Noguez D, Khan Z, et al. Stoichiometry of site-specific lysine acetylation in an entire proteome. J Biol Chem. 2014;289:21326–21338. doi: 10.1074/jbc.M114.581843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, et al. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One. 2014;9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilling B, Christensen D, Davis R, Sahu AK, Hu LI, Walker-Peddakotla A, et al. Protein acetylation dynamics in response to carbon overflow in Escherichia coli. Mol Microbiol. 2015;98:847–863. doi: 10.1111/mmi.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao K, Chai X, Marmorstein R. Structure and substrate binding properties of cobB, a Sir2 homolog protein deacetylase from Escherichia coli. J Mol Biol. 2004;337:731–741. doi: 10.1016/j.jmb.2004.01.060. [DOI] [PubMed] [Google Scholar]
- 49.Castano-Cerezo S, Bernal V, Blanco-Catala J, Iborra JL, Canovas M. cAMP-CRP co-ordinates the expression of the protein acetylation pathway with central metabolism in Escherichia coli. Mol Microbiol. 2011;82:1110–1128. doi: 10.1111/j.1365-2958.2011.07873.x. [DOI] [PubMed] [Google Scholar]
- 50.Starai VJ, Escalante-Semerena JC. Acetyl-coenzyme A synthetase (AMP forming) Cell Mol Life Sci. 2004;61:2020–2030. doi: 10.1007/s00018-004-3448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Gu J, Chen YY, Xiao CL, Wang LW, Zhang ZP, et al. CobB regulates Escherichia coli chemotaxis by deacetylating the response regulator CheY. Mol Microbiol. 2010;76:1162–1174. doi: 10.1111/j.1365-2958.2010.07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barak R, Prasad K, Shainskaya A, Wolfe AJ, Eisenbach M. Acetylation of the chemotaxis response regulator CheY by acetyl-CoA synthetase purified from Escherichia coli. J Mol Biol. 2004;342:383–401. doi: 10.1016/j.jmb.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Liarzi O, Barak R, Bronner V, Dines M, Sagi Y, Shainskaya A, et al. Acetylation represses the binding of CheY to its target proteins. Mol Microbiol. 2010;76:932–943. doi: 10.1111/j.1365-2958.2010.07148.x. [DOI] [PubMed] [Google Scholar]
- 55.Hu LI, Chi BK, Kuhn ML, Filippova EV, Walker-Peddakotla AJ, Basell K, et al. Acetylation of the response regulator RcsB controls transcription from a small RNA promoter. J Bacteriol. 2013;195:4174–4186. doi: 10.1128/JB.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castano-Cerezo S, Bernal V, Post H, Fuhrer T, Cappadona S, Sanchez-Diaz NC, et al. Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol Syst Biol. 2014;10:762. doi: 10.15252/msb.20145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thao S, Chen CS, Zhu H, Escalante-Semerena JC. Nepsilon-lysine acetylation of a bacterial transcription factor inhibits Its DNA-binding activity. PLoS One. 2010;5:e15123. doi: 10.1371/journal.pone.0015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang W, Deutscher MP. Post-translational modification of RNase R is regulated by stress-dependent reduction in the acetylating enzyme Pka (YfiQ) RNA. 2012;18:37–41. doi: 10.1261/rna.030213.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang QF, Gu J, Gong P, Wang XD, Tu S, Bi LJ, et al. Reversibly acetylated lysine residues play important roles in the enzymatic activity of Escherichia coli N-hydroxyarylamine O-acetyltransferase. FEBS J. 2013;280:1966–1979. doi: 10.1111/febs.12216. [DOI] [PubMed] [Google Scholar]
- 60.Lima BP, Thanh Huyen TT, Basell K, Becher D, Antelmann H, Wolfe AJ. Inhibition of acetyl phosphate-dependent transcription by an acetylatable lysine on RNA polymerase. J Biol Chem. 2012;287:32147–32160. doi: 10.1074/jbc.M112.365502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima BP, Antelmann H, Gronau K, Chi BK, Becher D, Brinsmade SR, et al. Involvement of protein acetylation in glucose-induced transcription of a stress-responsive promoter. Mol Microbiol. 2011;81:1190–1204. doi: 10.1111/j.1365-2958.2011.07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neumann H, Peak-Chew SY, Chin JW. Genetically encoding Nε-acetyllysine in recombinant proteins. Nat Chem Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 63.Umehara T, Kim J, Lee S, Guo LT, Söll D, Park HS. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 2012;586:729–733. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 64.Mukai T, Kobayashi T, Hino N, Yanagisawa T, Sakamoto K, Yokoyama S. Adding l-lysine derivatives to the genetic code of mammalian cells with engineered pyrrolysyl-tRNA synthetases. Biochem Biophys Res Commun. 2008;371:818–822. doi: 10.1016/j.bbrc.2008.04.164. [DOI] [PubMed] [Google Scholar]
- 65.Fan C, Xiong H, Reynolds NM, Soll D. Rationally evolving tRNAPyl for efficient incorporation of noncanonical amino acids. Nucleic Acids Res. 2015;43:e156. doi: 10.1093/nar/gkv800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musrati RA, Kollarova M, Mernik N, Mikulasova D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998;17:193–210. [PubMed] [Google Scholar]
- 67.Minarik P, Tomaskova N, Kollarova M, Antalik M. Malate dehydrogenases–structure and function. Gen Physiol Biophys. 2002;21:257–265. [PubMed] [Google Scholar]
- 68.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim EY, Kim WK, Kang HJ, Kim JH, Chung SJ, Seo YS, et al. Acetylation of malate dehydrogenase 1 promotes adipogenic differentiation via activating its enzymatic activity. J Lipid Res. 2012;53:1864–1876. doi: 10.1194/jlr.M026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim EY, Han BS, Kim WK, Lee SC, Bae KH. Acceleration of adipogenic differentiation via acetylation of malate dehydrogenase 2. Biochem Biophys Res Commun. 2013;441:77–82. doi: 10.1016/j.bbrc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Joh T, Takeshima H, Tsuzuki T, Shimada K, Tanase S, Morino Y. Cloning and sequence analysis of cDNAs encoding mammalian mitochondrial malate dehydrogenase. Biochemistry. 1987;26:2515–2520. doi: 10.1021/bi00383a017. [DOI] [PubMed] [Google Scholar]
- 72.Joh T, Takeshima H, Tsuzuki T, Setoyama C, Shimada K, Tanase S, et al. Cloning and sequence analysis of cDNAs encoding mammalian cytosolic malate dehydrogenase. Comparison of the amino acid sequences of mammalian and bacterial malate dehydrogenase. J Biol Chem. 1987;262:15127–15131. [PubMed] [Google Scholar]
- 73.Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 75.Tu S, Guo SJ, Chen CS, Liu CX, Jiang HW, Ge F, et al. YcgC represents a new protein deacetylase family in prokaryotes. Elife. 2015;4 doi: 10.7554/eLife.05322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AbouElfetouh A, Kuhn ML, Hu LI, Scholle MD, Sorensen DJ, Sahu AK, et al. The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites. Microbiologyopen. 2015;4:66–83. doi: 10.1002/mbo3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279–285. doi: 10.1042/BJ20071624. [DOI] [PubMed] [Google Scholar]
- 78.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 79.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 81.Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 82.Ramponi G, Manao G, Camici G. Nonenzymatic acetylation of histones with acetyl phosphate and acetyl adenylate. Biochemistry. 1975;14:2681–2685. doi: 10.1021/bi00683a018. [DOI] [PubMed] [Google Scholar]
- 83.Kuo YM, Andrews AJ. Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS One. 2013;8:e54896. doi: 10.1371/journal.pone.0054896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner GR, Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. 2013;288:29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barak R, Welch M, Yanovsky A, Oosawa K, Eisenbach M. Acetyladenylate or its derivative acetylates the chemotaxis protein CheY in vitro and increases its activity at the flagellar switch. Biochemistry. 1992;31:10099–10107. doi: 10.1021/bi00156a033. [DOI] [PubMed] [Google Scholar]
- 86.Christensen DG, Orr JS, Rao CV, Wolfe AJ. Increasing Growth Yield and Decreasing Acetylation in Escherichia coli by Optimizing the Carbon-to-Magnesium Ratio in Peptide-Based Media. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.03034-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci USA. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albaugh BN, Arnold KM, Lee S, Denu JM. Autoacetylation of the histone acetyltransferase Rtt109. J Biol Chem. 2011;286:24694–24701. doi: 10.1074/jbc.M111.251579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall MD, Banaszak LJ. Crystal structure of a ternary complex of Escherichia coli malate dehydrogenase citrate and NAD at 1.9 A resolution. J Mol Biol. 1993;232:213–222. doi: 10.1006/jmbi.1993.1377. [DOI] [PubMed] [Google Scholar]
- 92.Khan AN, Lewis PN. Unstructured conformations are a substrate requirement for the Sir2 family of NAD-dependent protein deacetylases. J Biol Chem. 2005;280:36073–36078. doi: 10.1074/jbc.M508247200. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka S, Matsushita Y, Yoshikawa A, Isono K. Cloning and molecular characterization of the gene rimL which encodes an enzyme acetylating ribosomal protein L12 of Escherichia coli K12. Mol Gen Genet. 1989;217:289–293. doi: 10.1007/BF02464895. [DOI] [PubMed] [Google Scholar]
- 94.Marvil DK, Leisinger T. N-acetylglutamate synthase of Escherichia coli: purification, characterization, and molecular properties. J Biol Chem. 1977;252:3295–3303. [PubMed] [Google Scholar]
- 95.Escalante-Semerena JC. Nε-acetylation control conserved in all three life domains. Microbe. 2010;5:340–344. doi: 10.1128/microbe.5.340.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crosby HA, Escalante-Semerena JC. The acetylation motif in AMP-forming Acyl coenzyme A synthetases contains residues critical for acetylation and recognition by the protein acetyltransferase pat of Rhodopseudomonas palustris. J Bacteriol. 2014;196:1496–1504. doi: 10.1128/JB.00004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, et al. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 98.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


