Abstract
Sodium alginate is an effective biomaterial for tissue engineering applications. Non-purified alginate is contaminated with protein, lipopolysaccharide, DNA, and RNA, which could elicit adverse immunological reactions. We developed a purification protocol to generate biocompatible alginate based on (a) activated charcoal treatment, (b) use of hydrophobic membrane filtration (we used hydrophobic polyvinylidene difluoride membranes to remove organic contaminants), (c) dialysis, and finally (d) ethanol precipitation. Using this approach, we could omit pre-treatment with chloroform and significantly reduce the quantities of reagents used. Purification resulted in reduction of residual protein by 70% down to 0.315 mg/g, DNA by 62% down to 1.28 μg/g, and RNA by 61% down to less than 10 μg/g, respectively. Lipopolysaccharide levels were reduced by >90% to less than 125 EU/g. Purified alginate did not induce splenocyte proliferation in vitro. Three-dimensional scaffolds generated from purified alginate did not elicit a significant foreign body reaction, fibrotic overgrowth, or macrophage infiltration 4 weeks after implantation. This study describes a simplified and economical alginate purification method that results in alginate purity, which meets clinically useful criteria.
Keywords: Alginate, purification, scaffolds, bioengineering, transplantation
Introduction
Sodium alginate is a naturally occurring polysaccharide extracted from marine plants (algae kelp).1 It has been widely used for cell encapsulation and tissue engineering applications.2–8 Our group has recently described the use of a pro-angiogenic 3D alginate scaffold that was pre-vascularized for 2 weeks under the abdominal rodent rectus muscles, and subsequently used successfully as a carrier for pancreatic islets isografts in an extrahepatic, easily accessible site.9
It is well known that non-purified sodium alginate is contaminated with various proteins, lipopolysaccharide (LPS), DNA, and RNA, each of which is capable of inducing host immune responses following implantation.10 Since impurities can induce fibrotic overgrowth that interferes with the diffusion of nutrients and oxygen,11 the use of alginate scaffolds or capsules has not yet achieved clinical application. The production of cytokines by attracted inflammatory cells leads to destruction of transplanted cells residing within the alginate matrix,12 and pancreatic islet viability has been shown to be directly related to the purity of the alginate used for implantation.13
To minimize immune responses that interfere with the clinical usefulness of alginate scaffolds, several methods for alginate purification have been tested previously.14,15 Industrial purification methods suffer from inefficiencies such as the need to use large quantities of organic solvents and/or time consuming extraction steps, and/or use of potentially harmful chemicals such as chloroform.16–18 Previous studies purified alginate with activated charcoal followed by dialysis and ethanol precipitation to remove gross contamination.19 These methods resulted in preparations which were still unacceptable due to the remaining levels of residual LPS and protein contamination that would continue to elicit immunological reactions.14
To avoid the above pitfalls, we therefore evaluated materials that specifically bind organic contaminants without binding alginate. Several bioactive resins and filter membranes are known that meet these requirements,20 for example, aldehyde-based21 or anhydride-based22 chemistry. Unfortunately, these materials need to be activated using hazardous chemicals such as cya-nuric chloride. Moreover, reactive aldehyde or anhydride groups on these materials are rapidly deactivated in water, making them less suitable for large volume treatment of aqueous solutions.
Polyvinylidene difluoride (PVDF) does not suffer from the previously mentioned disadvantages. PVDF is a hydrophobic polymer with high affinity for proteins via both hydrophobic and electrostatic interactions.23,24 Hydrophobic PVDF membranes such as Immobilon-P (Millipore, Temecula, CA) have already been used for protein and LPS analyses.25,26 These membranes have a thickness of 100–130 μm, a pore size of 0.45 μm, and bind 100–200 μg of protein per square centimeter. We hypothesized that the use of a membrane filtration step would enhance the removal of contaminants from alginate by specifically binding residual contaminants. Using our optimal PVDF-based protocol, we could effectively increase the removal of protein, LPS, DNA, and RNA to levels that are clinically acceptable, while omitting pre-treatment with chloroform, which can be toxic to the cells. We could also significantly reduce the total quantities of reagents used compared with methods described earlier17 (reference added).
Materials
Ultrapure water for dialysis was obtained from a Hydro Picopure (Millipore, Temecula, CA) purification system. Low viscosity alginate (MW <35 kD) obtained from brown algae which consists of straight-chain, hydrophilic, colloidal, and polyuronic acid, which is composed primarily of anhydro-β-D-mannuronic and α-L-guluronic acid residues with 1→4 linkage (A0682) and a viscosity of 4–12 cP, 1% at 25°C, activated charcoal (C9157), monosodium phosphate monohydrate (71507), disodium phosphate heptahydrate (431478), hydrochloric acid (H1758), and sodium hydroxide (S8045) were all purchased from Sigma-Aldrich (St Louis, MO). Molecular biology grade water (Hyclone SH30538), neutralized carbon (C170), 50,000 MWCO dialysis tubing (Spectra/Por7, Spectrum Laboratories, Inc., Rancho Dominguez, CA, part # 132130), 35 mm standard closures (Spectrum Laboratories, Inc., Rancho Dominguez, CA, part # 132736), 90 mm Büchner funnel filter setup (Kontes Ultraware, Kimble-Chase, Vineland, NJ), micro-BCA kit (part # 23235), and molecular biology grade ethanol 100% (BP2818-4) were purchased from Thermo Fisher Scientific (Fairlawn, NJ). Hydrophobic Immobilon P membranes (IPVH 00010) with 0.45 μm pore size, 90 mm glass membrane pre-filters (type AP15), and 0.45 μm Stericup filter flasks (SCHVU05RE) were purchased from Millipore (Temecula, CA). Alpha minimum essential medium (MEM), fetal calf serum (FCS), and L-glutamine (GlutaMax®) were purchased from Invitrogen/Gibco (Carlsbad, CA). Primocin was purchased from Invivogen (San Diego, CA).
Glassware was cleaned and detoxified by 24 h treatment with 0.5% hypochlorite followed by washing and baking for at least 120 min at 240°C. LPS was measured using the Pyrosate kit according to the manufacturer’s instructions (Cape Cod Inc., East Falmouth, MA). DNA and RNA were determined using the Qubit Quantitation Platform (Invitrogen, Carlsbad, CA). Spectrophotometric absorption was measured on a Shimadzu UV1700 PharmaSpec (Shimadzu Scientific Instruments, Somerset, NJ). Controls used for purification efficiency consisted of non-purified alginate (Sigma-Aldrich 0682) and pharmaceutical grade alginates; low viscosity, high mannuronic acid Pronova UP LVM (approx. MW 75–200 kD, apparent viscosity 20–200 cP), and low viscosity, high guluronic acid, Pronova UP LVG (approx. MW 75–200 kD, apparent viscosity 20–200 cP) (batch numbers FP-106-1 and FP-305-01, respectively, both were obtained from FMC Biopolymer/Novamatrix, Sandvika, Norway). Lyophilization was achieved using a BOC/Edwards 8 vacuum pump and cold trap (Tewkbury, MA) at 0.2 torr. pHs were determined using a digital pH meter (Accumet AB15+, Cole-Parmer, Vernon Hills, IL) calibrated with commercially available buffers (Thermo Fisher Scientific, Fairlawn, NJ).
Methods
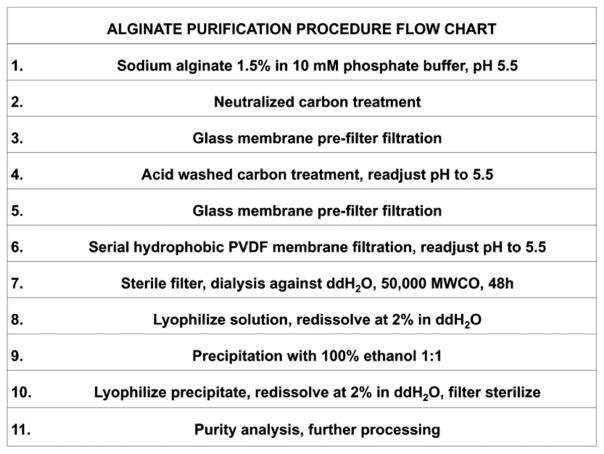
Alginate purification protocol (Chart 1)
Chart 1.
Flow chart showing step-wise purification of raw alginate. Complete description is found in the ‘‘Materials’’ and ‘‘Methods’’ sections.
We investigated several conditions to optimize removal of mitogens and pyrogens by filtration. We employed a minimum pH of 5.5 due to precipitation of alginate at lower pHs, which would result in unacceptable filtration rates. To determine the influence of ionic strength on degree of filtration and purification, we used sodium phosphate buffer concentrations of 10 mM and 50 mM. In total, we produced 17 batches of purified alginate. Comparisons of residual organic contaminants are shown within the final most optimized batch.
Charcoal treatment
After dissolving 1.5% alginate (w/w) in purified water without or with either 10 mM or 50 mM sodium phosphate, 1.5% neutralized carbon was added to the solution and pH was adjusted to 5.5 using 37% hydrochloric acid at room temperature. The solution was subsequently stirred for 20 h at 50°C to prevent potential microbial growth and was then filtered through a glass pre-filter to remove charcoal and any particulate matter prior to collection in a 2 L depyrogenized Erlenmeyer flask. Following filtration, 1.5% of acidic activated charcoal was added to the suspension while stirring and pH adjusted at 5.5 at room temperature, followed by an additional 20 h of stirring at 50°C. A second filtration step through a glass pre-filter to remove charcoal resulted in a filtrate with a yellowish gray color.
Hydrophobic PVDF treatment
Sheets (90 mm × 90 mm) of PVDF were cut prior to their use in the 90 mm Büchner funnel setup. Membrane filters were immersed in methanol for 15 s to remove air, washed in ultrapure H2O, and kept in sterile phosphate buffer solution at pH 5.5. Filtration was performed using a Büchner funnel setup and two alternating Erlenmeyer vacuum flasks. Prior to filtration, the pH of alginate solution was adjusted to 5.5. Using the in-house vacuum system in a laminar flow hood, the complete volume of alginate solution was filtered, the membrane filter was then replaced with a fresh filter and this step was repeated 20 times using a total of 20 filter membranes for 1 L of alginate solution. The price per single PVDF sheet used in this protocol was between US$3 and US$4.
Dialysis
After filtration, alginate solution was sterile filtered using 0.45 μm Stericup filter flasks (Millipore, Temacula, CA). Filtered solution was transferred to pre-washed dialysis tubes (50,000 MWCO), closed with double clamps on each side, and dialyzed for 48 h against ultrapure H2O (1:100 volume) with one change of water after 24 h at room temperature. Following dialysis, the resulting purified solution was collected in 50 mL conical tubes (part # 352070, BD Biosciences, Franklin Lakes, NJ), frozen at −20°C, lyophilized at 0.2 torr, and redissolved in ultrapure H2O at 2% weight/volume prior to use.
Ethanol precipitation
To precipitate 20 mL alginate solution in 50 mL conical tubes, we used equal volumes of 100% ethanol and 2% alginate solution. After adding 20 mL ethanol, the solution was vortexed for 1 min at 3000 rpm and subsequently spun at 4000 rpm for 30 min. Supernatants were removed and pellets dried at 0.2 torr. After drying, pellets were redissolved at 2% in ultrapure H2O and sterile filtered using 0.45 μm Stericup filter flasks (Millipore, Temacula, CA) before analyzing the product for purity.
Determination of alginate purity and immunogenicity
Protein quantification
In preparation for protein assay, alginate solutions were diluted 1:10 to 0.2% in ultra-pure water, followed by 60 min incubation with BCA reagent according to the manufacturer’s instructions. Protein concentrations were compared with standard curves obtained with bovine serum albumin included in the BCA kit. Absorption was read in 200 μL plastic cuvettes at 562 nm. Resulting protein content was converted to milligrams of protein per gram of dry alginate. Measurements were performed in triplicate.
LPS quantification
LPS levels were determined using the Pyrosate kit, which is based on the limulus amoebocyte lysate (LAL) assay. This kit has a sensitivity of 0.25 EU LPS/mL. Alginate solutions were tested at 2% and 0.2% weight/volume ultrapure H2O after filtration through 0.45 μm syringe filters (Millex-HV, 33 mm diameter, Millipore, Temecula, CA). In brief, according to the manufacturer’s instructions, 0.5 mL LAL reagent water and 0.5 ml alginate solution were added to sample tubes, mixed, and dissolved, followed by transfer of 0.25 mL of each solution to the positive control tubes in duplicate. All tubes were incubated at 37°C for the time specified by the manufacturer. Formation of a clot was determined by visual inspection and inversion of tubes; test was considered valid if sample and positive controls showed clot formation and all negative controls absence of clots.
DNA and RNA quantification
DNA and RNA concentrations in alginate solutions were determined using the Qubit Quant-iT quantitation system using the Qubit Fluorometer (Q32857) with the Quant-iT dsDNA HS (Q32854) and RNA HS (Q32852) assays (Invitrogen, Carlsbad, CA). In brief, non-purified and purified alginate (Sigma-Aldrich 0682) and Pronova LVM and LVG solutions were diluted to 0.2% in working solution as provided by the manufacturer. DNA and RNA concentrations were determined fluorometrically according to the manufacturer’s instructions. Results are presented in micrograms DNA or RNA per gram dry alginate.
Assay for immunogenic activity
Rat splenocyte proliferation was used as an indicator for alginate immunogenicity, based on a modified protocol as previously described.17 Briefly, rat spleens were removed under sterile conditions and collected in alpha MEM supplemented culture medium containing 10% fetal calf serum (FCS), 2 mM L-glutamine, and 100 μg/mL Primocin, minced and passed through a 100 μm cell strainer (part # 352360, BD Biosciences, Franklin Lakes, NJ) into 50 mL conical tubes. In all, 105 splenocytes were incubated in 200 μL alpha MEM and 10% FCS including various alginate solutions in 96-well flat bottom tissue culture plates (part # 353072, BD Biosciences, Franklin Lakes, NJ). Splenocyte proliferation was serially measured at 48 h after incubation at 37°C and 5% CO2. WST-1 (20 μL) (Roche Applied Science, Indianapolis, IN) was added 2 h prior to reading at 450–650 nm in a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA) to determine light absorption which directly correlates with the number of metabolically active cells in the culture. Obtained values were corrected for background absorbance, which was determined by measuring absorption in the medium without the presence of alginate or cells.
Alginate viscosity
Following purification, alginate viscosity may increase to unpractical levels after processing due to excessive removal of short chain alginate molecules.14 Viscosity of purified alginate solution was measured using a stress controlled MCR 501 rheometer (Anton Paar GmbH, Graz, Austria) at 25°C using a maximum of 41 increasing shear rates at minimum torque of 1.0 × 10−7nM at 2.5% and 1.2% w/w solutions. Measurements were done in triplicate and compared with previously reported data.14
Alginate biocompatibility in vivo
Implantation of alginate scaffolds
All animal studies were reviewed and approved by the Columbia University Institutional Animal Care and Use Committee (IACUC). Male Lewis rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and weighed between 200 and 250 g. Three-dimensional scaffolds were fabricated by adding 400 μL alginate to a 4.2 cm2 cell culture insert with transparent 0.4 μm polyethylene terephthalate membranes (part # 353090, BD Falcon, BD Biosciences, Franklin Lakes, NJ). Transparent membranes were found to generate optimal scaffolds with even surfaces. Loaded inserts were placed in six-well tissue culture plates (part # 353046, BD Falcon, BD Biosciences, Franklin Lakes, NJ) and 3 mL of 4.3% calcium gluconate was added to each bottom well. Plates were maintained at 4°C for 24 h to solidify the alginate by diffusion of calcium ions through the insert membrane. Following solidification, scaffolds were removed from the culture inserts and washed 3 times in 3 mL of sterile ultrapure water to remove excess calcium gluconate and to keep them moist in sterile ultrapure water until implantation. Scaffolds were inserted between abdominal muscle layers of Lewis rats (n = 5) following anesthesia with isoflurane gas (1–5%). After shaving and sterilizing the rat’s ventral surface, skin was incised in the midline and the underlying muscle was then carefully dissected just lateral to the midline down to the ventral fascia over the peritoneum. By blunt dissection, a pocket was created within the rectus muscle. The pocket was made large enough to accommodate a scaffold ~10 mm in diameter. The pocket and skin were closed in separate layers after successful scaffold implantation. Four weeks after implantation, the animals were euthanized by exsanguation and the rectus muscles containing scaffolds were harvested. Tissues were fixed in 4% paraformaldehyde in PBS for 24 h, transferred to 70% ethanol and sectioned. Sections (5 μm) were stained with hematoxylin/eosin for general histological analyses, and Masson’s trichrome for collagen and fibrosis. We examined all sections for the well described foreign body reaction, which consists of fusion of macrophages to multinucleated giant cells in response to immunogenic material.27 We quantitated numbers of multinucleated giant cells as number of cells per high power field (400 × magnification) under a light microscope (Olympus BX41, Center Valley, PA) in five serial sections per animal. As a positive control for multinucleated giant cell formation, we used biodegradable polyglycolic acid sutures (VICRYL®, Ethicon, Somerville, NJ) made from polyglycolic acid (PGA), a biodegradable material, which is known to induce a significant giant cell reaction.28 Suture material was placed at a different site at the time of scaffold implantation in the abdominal rectus muscle and that tissue was harvested at the same time as scaffold explantation to use as comparison control for the quantification of macrophage and giant cell reaction in the alginate scaffold material.
Statistical analyses
Student’s t-test (unequal variance, two-tailed) was used to compare the results. A p value <0.05 was considered to be statistically significant. Results are presented as means ± standard error of the mean (SEM).
Results
To prepare 3–4 g of purified alginate from 15 g of non-purified alginate, we used a total volume of 105 L of water, mostly due to alginate dialysis steps against water 1:100. We further used a total volume of 500 mL of ethanol and 500 mL of methanol, a significant reduction when compared with other methods such as that of Klöck et al.17,29 Following purification, aforementioned methods yielded 1–2 g of purified alginate from 18 g raw starting material.
Quantification of residual alginate contamination
After purification of low molecular weight alginate (Sigma-Aldrich 0682) using our customized method, protein, DNA, RNA, and LPS contents were determined as described above. Controls included non-purified alginate and ultrapure LVM and LVG alginates (FMC Biopolymer/Novamatrix, Sandvika, Norway).
Protein contamination
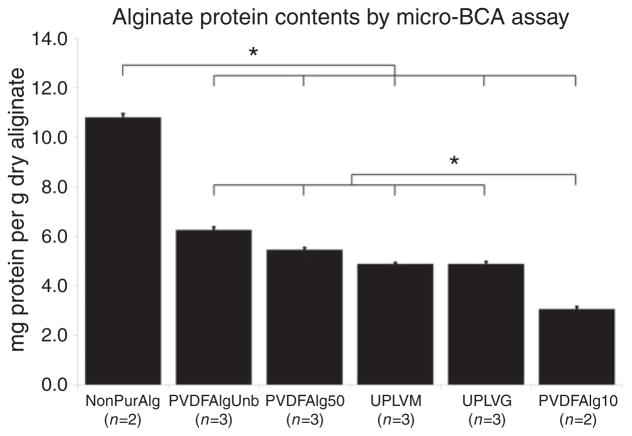
We found filtration through hydrophobic PVDF membranes in 10 mM phosphate buffer at pH 5.5 (PVDFAlg10) to be optimal. As determined by the micro-BCA method, we could reduce protein contamination from 10.8 ± 0.2 mg/g found in non-purified alginate to 3.15 ± 0.1 mg/g following filtration, a 72% reduction (p <0.05) (Figure 1). This reduction was significantly less than when filtration was performed in unbuffered alginate solution (PVDFAlgUnb) or in 50 mM phosphate buffer (PVDFAlg50). Control alginates Pronova Ultrapure LVM (UPLVM) and LVG (UPLVG) alginates both contained 4.9 ± 0.1 mg/g protein, a 38% difference as compared with the alginate we purified (p <0.05). These results are summarized in Figure 1.
Figure 1.
Quantitative evaluation of protein contamination in several different sodium alginate preparations. Results are presented in milligrams of protein per gram of dried alginate ± SEM. Micro-BCA assay sensitivity is 0.5 μg/mL. *p <0.05.
NonPurAlg: non purified Sigma A0682 alginate; PVDFAlgUnb: PVDF purified Sigma A0682 alginate in unbuffered solution; PVDFAlg50: PVDF purified Sigma A0682 alginate in 50 mM phosphate buffer; UPLVM: Novamatrix Ultrapure low viscosity mannuronic acid; UPLVG: Novamatrix Ultrapure low viscosity guluronic acid; PVDFAlg10: PVDF purified Sigma A0682 alginate in 10 mM phosphate buffer.
DNA and RNA contamination
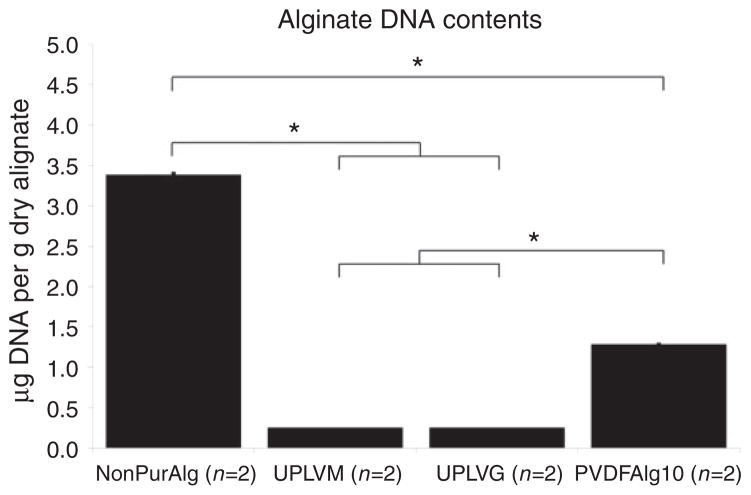
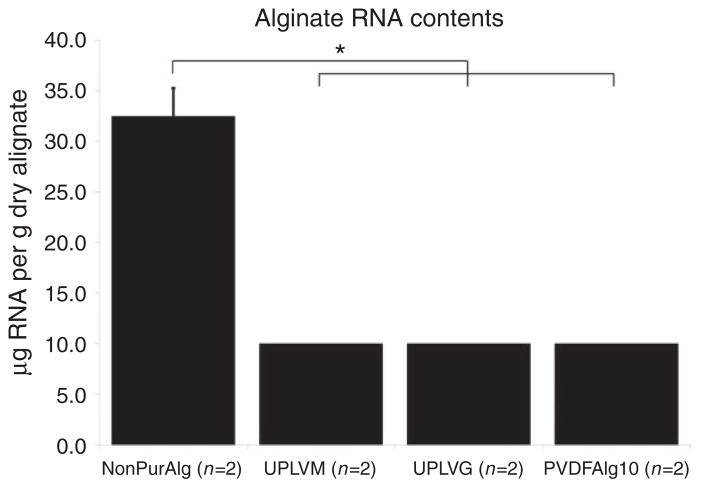
Residual DNA and RNA contamination were determined after optimizing protein purification using PVDF membrane filtration. Non-purified alginate (Sigma-Aldrich 0682) contained 3.39 ± 0.02 μg DNA per gram of dry alginate and 32.48 ± 2.78 μg of RNA per gram of dry alginate. Following purification, the residual amount of DNA was reduced to 1.28 ± 0.01 μg DNA per gram of dry alginate, a reduction by 62% (p <0.01) (Figure 2). RNA content was too low following filtration to be detected using the Qubit system, which indicates that there was less than 10 μg RNA per gram of dry alginate, a reduction by at least 69% (Figure 3). Pronova LVM and LVG alginates contained less than 0.25 μg DNA per gram of dry alginate and less than 10 μg RNA per gram of dry alginate as shown in Figures 2 and 3.
Figure 2.
DNA content was determined using the Qubit quantitation system. The amount of DNA is represented in micrograms of DNA per gram of dry alginate ± SEM. dsDNA assay sensitivity is 0.2–100 ng per sample. *p <0.01.
NonPurAlg: non purified Sigma A0682 alginate; UPLVM: Novamatrix Ultrapure low viscosity mannuronic acid; UPLVG: Novamatrix Ultrapure low viscosity guluronic acid; PVDFAlg10: PVDF purified Sigma A0682 alginate in 10 mM phosphate buffer.
Figure 3.
RNA content was determined using the Qubit quantitation system. The amount of RNA is represented in micrograms of RNA per gram of dry alginate ± SEM. RNA assay sensitivity is 5–100 ng per sample. *p <0.01.
NonPurAlg: non purified Sigma A0682 alginate; UPLVM: Novamatrix Ultrapure low viscosity mannuronic acid; UPLVG: Novamatrix Ultrapure low viscosity guluronic acid; PVDFAlg10: PVDF purified Sigma A0682 alginate in 10 mM phosphate buffer.
LPS
The commercial Pyrosate assay to detect LPS has a detection level of 0.25 EU/mL. PVDF purified alginate (Sigma-Aldrich 0682) and control alginates (Pronova UP LVM and UP LVG) at both 2% and 0.2% dilution did not induce gel clot formation, indicating LPS levels of <125 EU/g dry alginate. Testing of non-purified alginate (Sigma-Aldrich 0682) resulted in gel clot formation at both 2% and 0.2% concentration, indicating that there was LPS contamination of >125 EU/g dry unpurified alginate, and that there was at least a 90% reduction of LPS levels after PVDF purification.
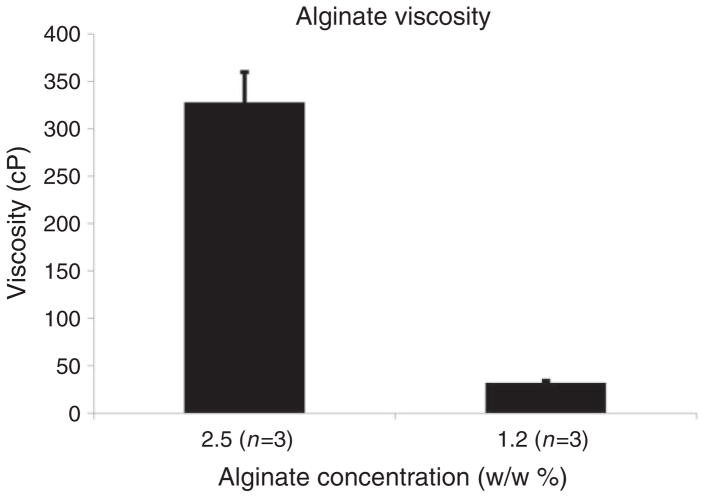
Viscosity
Purified alginate displayed a mean viscosity of 328 ± 55.6 cP at 2.5% w/w solution and 32 ± 2.4 cP at 1.2% w/w solution (Figure 4). These values correspond to previously reported pre- and post-purification viscosities and are within range of the optimal viscosity of 200 cP at 2.0%–2.5% for post-processing (i.e. alginate bead formation) procedures.14
Figure 4.
Alginate viscosity was measured using a rotational rheometer at two dilutions in triplicate ± SEM. Minimum torque is 1 nNm.
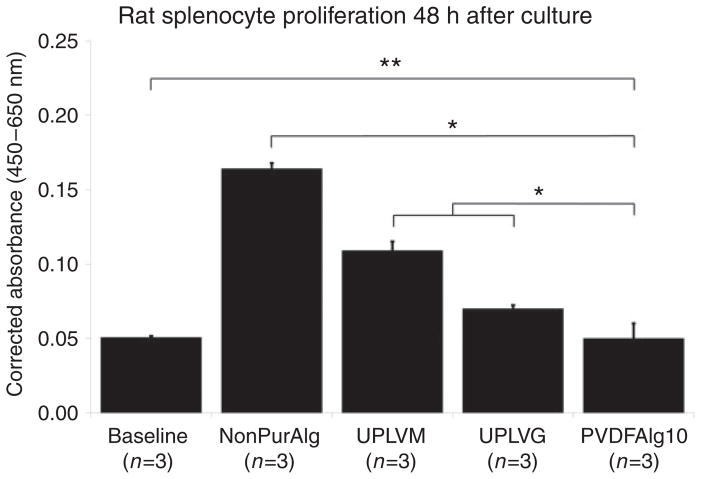
Splenocyte proliferation in vitro
Stimulation with different alginate samples at 0.2% concentration of rat splenocyte proliferation after 48 h of incubation was determined by spectrophotometric assay of colored product in the Roche assay using WST-1 (Figure 5). Non-purified low molecular weight alginate (Sigma-Aldrich 0682) induced the highest degree of proliferation. The control alginates (Pronova Ultrapure LVM and Ultrapure LVG) induced a significantly lower degree of proliferation than non-purified alginate, but significantly higher than baseline, which was determined by unstimulated splenocyte proliferation in growth medium alone. Alginate purified by our newly described protocol did not induce proliferation above that found at unstimulated baseline. This result is consistent with the observation that alginates with a high mannuronic acid ratio (i.e. Pronova Ultrapure LVM) are reportedly more immunogenic than alginates with a high guluronic acid ratio (i.e. Pronova Ultrapure LVG).29 However, since Sigma-Aldrich 0682 contains primarily mannuronic acid residues without induction of proliferation, it is possible that residual protein contamination or other contaminants may be more important for stimulation of immunogenicity of alginates than the composition of alginate itself. Results are presented in arbitrary absorbance units corrected for background absorbance by unstimulated cells at 450–650 nm (Figure 5).
Figure 5.
Splenocyte proliferation in the presence of different alginate preparations was determined by spectrophotometric assay ± SEM. **p = not significant; *p <0.05.
NonPurAlg: non purified Sigma A0682 alginate; UPLVM: Novamatrix Ultrapure low viscosity mannuronic acid; UPLVG: Novamatrix Ultrapure low viscosity guluronic acid; PVDFAlg10: PVDF purified Sigma A0682 alginate in 10 mM phosphate buffer.
Immune response in vivo
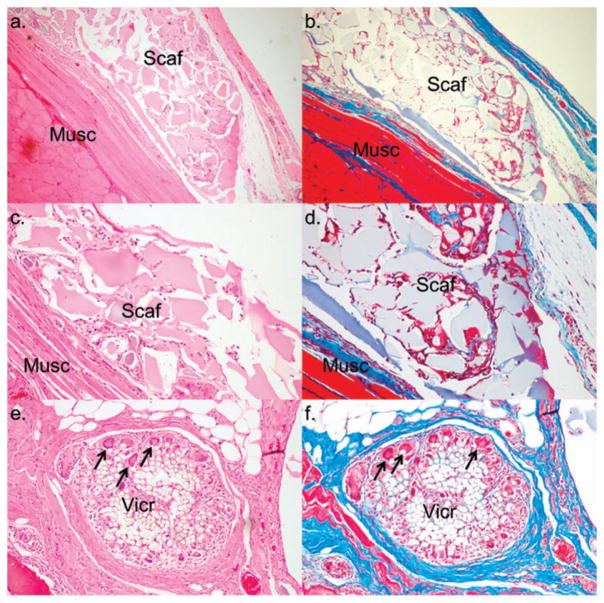
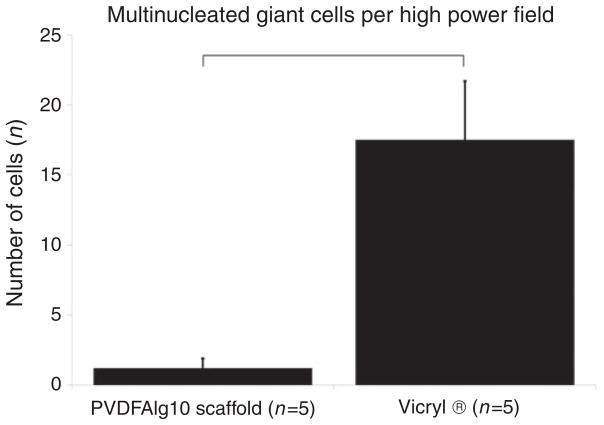
To determine biocompatibility, purified alginate (Sigma-Aldrich 0682) scaffolds were implanted between the rectus muscles of male Lewis rats (n = 5). Scaffolds were well tolerated throughout the duration of all the experiments. One month following implantation, scaffold material was easily recovered with little evidence of adhesion or scarring. Histologically, fibrotic overgrowth as determined by blue staining using Masson’s trichrome was limited when compared with fibrous overgrowth in the area of PGA suture material (Figure 6). Inflammatory reactions, as quantified by the number of multinucleated giant cells, were minimal, and significantly fewer than the number found at the site of PGA sutures (p <0.05) (Figure 7). We did not implant unpurified alginate scaffolds as controls due to expected adverse reactions based on level of impurities and significant splenocyte proliferation in vitro, which could cause unnecessary distress in vivo.
Figure 6.
Scaffold histology 1 month after implantation. Scaffolds (Scaf) were implanted between abdominal muscle layers (Musc) of male Lewis rats (n = 5). H&E shows histological characteristics with cellular invasion into scaffold material (panels a and c) and PGA (panel e). Masson’s trichrome shows little fibrosis (blue) around scaffold material (panels b and d) compared with PGA/VICRYL® (Vic) (panel f), and significantly fewer giant cell phagocytic activity (arrows). This indicates scaffold material did not induce an immunological foreign body response. (a and b at 40×, c and d at 100×, e and f at 200×). We omitted implantation of unpurified scaffolds since in vitro experiments had already shown a significant proliferative response.
Figure 7.
Quantification of giant cell numbers per high power field (400 × magnification) in proximity to scaffold material or polyglycolic/VICRYL® sutures (positive control). Results are presented as number of cells ± SEM. *p <0.05.
PVDFAlg10: PVDF purified Sigma A0682 alginate in 10 mM phosphate buffer.
Discussion
We developed a practical and economical alginate purification protocol aimed at reducing residual protein and LPS contamination, which are typically found in many alginate preparations following various published purification protocols.14 Our method drastically reduced the quantities of organic reagents needed for the purification while being able to provide high levels of alginate purity. The only inorganic component that we employed was sodium phosphate in water (Flow chart 1).
We report here a novel alginate purification protocol, which leads to much less residual organic contamination of the final product when compared with either commercially available ultrapure alginates (i.e. Pronova UPLVM and UPLVG) or alginates purified by other existing purification protocols.14 Our protocol is based on the high affinity of hydrophobic PVDF for organic materials under specific buffer conditions. PVDF binds organic molecules through both hydrophobic and electrostatic interactions; we found that an alginate solution of low ionic strength, which is slightly acidic (10 mM sodium phosphate buffer at pH 5.5) could optimally bind and therefore remove organic contaminants under flow-through conditions. Overall, approximately 70%–80% of alginate was lost, mostly due to charcoal treatment and ethanol precipitation, both of which are generally used for removal of gross organic contamination from non-purified alginates and therefore considered unavoidable loss.
In devising this protocol, we reasoned that serial filtration through protein binding membranes would gradually reduce residual organic contaminants. We evaluated a range of conditions, such as pH and ionic strength of the alginate solution prior to settling on the optimal one for filtration. Alginate’s pKa is approximately 3.2, depending on the proportion of mannuronic and guluronic acid residues. We found that a pH of 5.5 in 10 mM phosphate buffer was optimal when combined with filtration through hydrophobic PVDF membranes. We also tested activated membranes based on aldehyde chemistry (Pall Ultrabind, 66544, Pall Corporation, Ann Arbor, MI) or anhydride chemistry (Pall Immunodyne ABC, NBCHI3R, Pall Corporation, Ann Arbor, MI), which covalently bind organic molecules via amino groups at neutral or basic pHs. However, we were not able to achieve efficient removal of proteins using the latter membranes. It is possible that these activated membranes need longer exposure to protein-containing solution or that proteins prefer to remain bound to alginate under the conditions necessary to optimally bind protein with the filters, i.e. pH 8–9. A more important disadvantage of such membranes is their high sensitivity to aqueous and humid environments, which rapidly inactivate the membrane’s active protein binding groups. In contrast, hydrophobic PVDF membranes are highly stable, have low cost (approximately US$ 0.04–US$ 0.05 per cm2), and can be easily stored in a standard laboratory environment. The influence of ionic strength and pH in our purification method was striking. Using an aqueous unbuffered alginate solution at pH 5.5 or 50 mM phosphate buffer at pH 5.5 or 8.0, protein removal was not efficient. Hydrophobic PVDF interacts with organic molecules under a wide range of conditions through both hydrophobic and electrostatic interactions.24 The exact kinetics of these interactions are not known, however contaminating proteins may carry a different charge, which may favor binding to hydrophobic PVDF when compared with alginate placed in low ionic strength buffers. Of note, residual DNA, while significantly reduced, remained higher compared with Pronova alginates. DNA is known to elicit immune responses and may warrant additional purification steps. Histologically, we compared purified alginate scaffolds to PGA suture material and found significantly more scarring and giant cells around PGA compared with alginate. Foreign body giant cell formation is the end-stage response of the inflammatory and wound healing process following implantation of biomaterials. Little fibrosis and low numbers of giant cells could implicate low immunogenicity or active suppression of an inflammatory response by alginate. We quantified average number of giant cells per high power field at 4 weeks. To get a better understanding of the regenerative process, multiple timepoint measurements may be desirable.
In 1994, Klöck et al. developed an alginate purification process based on free flow electrophoresis (FFE).17 A comparable approach was performed more recently, by using size exclusion chromatography (SEC).30 These methods resulted in highly purified alginate preparations, with residual protein contamination of only 0.05% of dry alginate weight, significantly less than 0.3% that is obtained with the chemical extraction protocol of Klöck et al. or the membrane filtration protocol described here. However, by adding a final dialysis step against saline instead of water in addition to the chemical extraction method of Klöck et al., Menard et al. recently showed that they could reduce protein contamination to 0.05% without the use of FFE or SEC. This accomplishment was probably due to reduction of electrostatic interaction between proteins and alginate in saline during dialysis.30 The addition of dialysis in saline instead of dialysis in water to step 7 (Flow chart 1) of our purification protocol should achieve a similarly high purity of alginate, using significantly lower volumes and number of organic reagents making the process more useful and practical.
Previously used methods such as FFE or SEC have been already shown to result in a product contaminated by residual protein that is responsible for immunogenicity of alginate. However, for large scale application, the disadvantages of such purification methods is high cost of equipment and that these procedures are highly labor-intensive. Whether alginate purity achieved regarding residual protein contamination using current methods is clinically acceptable remains to be determined in further trials, as there are no regulatory limits for residual protein contamination due to the number of variables involved, i.e. quality and quantity of contamination leaves it up to the manufacturer to determine safety. With regards to residual LPS, the Food and Drug Administration (FDA) accepts levels <5 EU/kg for solutions or implantable devices to be used clinically. With a residual level of <125 EU/g dry alginate following PVDF purification and potentially much lower, a quantity of 5 EU/kg in a 80 kg human would allow implantation of at least 3.2 g purified alginate before pyretic effects would ensue. At 2% w/w alginate solution, this would allow a volume of 160 ml, which is more than sufficient to generate relatively large size scaffolds for clinical use. For large scale clinical tissue-engineering applications, a combination of relatively high purity non-immunogenic alginate together with minimal systemic or local immunosuppression appears more desirable than the use of ultrahigh purity alginate, which comes with considerable cost and questionable immunologic value. Application of hydrophobic PVDF-based filtration would allow for significant up scaling of the alginate purification process by using reactors with large surface PVDF membranes or porous PVDF columns. Reconstitution of PVDF by elution of organic contaminants with sodium dodecyl sulfate (SDS)23 would allow reuse of equipment for repeatable purifications.
Other polysaccharide biomaterials like chitosan or xanthan gum may also benefit from PVDF purification. However, several physical and chemical conditions apply to make the process useful. Polymer solutions should be aqueous and viscosity low enough for sufficient flow through. Buffer conditions should enhance affinity of organic contaminants for PVDF, while preventing the binding of polymers. The high chemical stability of PVDF membranes permits their use under different experimental conditions with appropriate polymers and buffers.
Conclusions
Because of its versatile nature and inertness (non-immunogenicity), purified alginate remains one of the most attractive biomaterials for experimental applications in clinical tissue engineering. The development of large scale clinical tissue engineering trials will require standards for alginate purity that should be environmentally, economically, and biologically acceptable. We hereby describe a novel method of alginate preparation and purification based on PVDF membrane filtration that addresses these issues. The use of PVDF membrane filtration is an economical solution, which reduces waste and results in alginate with clinically acceptable purity levels while remaining non-immunogenic. With recent advances in regenerative medicine and tissue engineering, clinically useful and affordable biomaterials are desperately needed and will become of paramount importance for successful clinical progress.
Acknowledgments
We would like to acknowledge Fiona See, PhD, for careful reading and correction of the manuscript.
Funding
NIH SCCOR # 5P50HL077096-02 and NIH T32HL007854-19 (MAH)
Footnotes
Declaration of Conflicting Interests
Hugo P Sondermeijer, Piotr Witkowski, and Mark A Hardy are patent provisional holders of methods described in this manuscript.31
References
- 1.Johnson FA, Craig DQ, Mercer AD. Characterization of the block structure and molecular weight of sodium alginates. J Pharm Pharmacol. 1997;49:639–643. doi: 10.1111/j.2042-7158.1997.tb06085.x. [DOI] [PubMed] [Google Scholar]
- 2.Eiselt P, Yeh J, Latvala RK, et al. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials. 2000;21:1921–1927. doi: 10.1016/s0142-9612(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 3.Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12:1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102:III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 5.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 6.Read TA, Sorensen DR, Mahesparan R, et al. Local endo-statin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 7.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann H, Shirley SG, Zimmermann U. Alginate-based encapsulation of cells: past, present, and future. Curr Diab Rep. 2007;7:314–320. doi: 10.1007/s11892-007-0051-1. [DOI] [PubMed] [Google Scholar]
- 9.Witkowski P, Sondermeijer H, Hardy MA, et al. Islet grafting and imaging in a bioengineered intramuscular space. Transplantation. 2009;88:1065–1074. doi: 10.1097/TP.0b013e3181ba2e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam SK, Dusseault J, Polizu S, et al. Impact of residual contamination on the biofunctional properties of purified alginates used for cell encapsulation. Biomaterials. 2006;27:1296–1305. doi: 10.1016/j.biomaterials.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Orive G, Carcaboso AM, Hernandez RM, et al. Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules. 2005;6:927–931. doi: 10.1021/bm049380x. [DOI] [PubMed] [Google Scholar]
- 12.Robitaille R, Dusseault J, Henley N, et al. Inflammatory response to peritoneal implantation of alginate-poly-L-lysine microcapsules. Biomaterials. 2005;26:4119–41127. doi: 10.1016/j.biomaterials.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Langlois G, Dusseault J, Bilodeau S, et al. Direct effect of alginate purification on the survival of islets immobilized in alginate-based microcapsules. Acta Biomater. 2009;5:3433–3440. doi: 10.1016/j.actbio.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Dusseault J, Tam SK, Menard M, et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A. 2006;76:243–251. doi: 10.1002/jbm.a.30541. [DOI] [PubMed] [Google Scholar]
- 15.Kim AR, Hwang JH, Kim HM, et al. Reduction of inflammatory reaction in the use of purified alginate microcapsules. J Biomater Sci Polym Ed. 2013;24:1084–1098. doi: 10.1080/09205063.2012.735100. [DOI] [PubMed] [Google Scholar]
- 16.De Vos P, De Haan BJ, Wolters GH, et al. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40:262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 17.Klock G, Frank H, Houben R, et al. Production of purified alginates suitable for use in immunoisolated transplantation. Appl Microbiol Biotechnol. 1994;40:638–643. doi: 10.1007/BF00173321. [DOI] [PubMed] [Google Scholar]
- 18.Liao KH, Tan YM, Conolly RB, et al. Bayesian estimation of pharmacokinetic and pharmacodynamic parameters in a mode-of-action-based cancer risk assessment for chloroform. Risk Anal. 2007;27:1535–1551. doi: 10.1111/j.1539-6924.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 19.Prokop A, Wang TG. Purification of polymers used for fabrication of an immunoisolation barrier. Ann N Y Acad Sci. 1997;831:223–231. doi: 10.1111/j.1749-6632.1997.tb52197.x. [DOI] [PubMed] [Google Scholar]
- 20.Castilho LR, Birger Anspach F, Deckwer WD. Comparison of affinity membranes for the purification of immunoglobulins. J Membr Sci. 2002;207:253–264. [Google Scholar]
- 21.Krysteva MA, Shopova BI, Yotova LY, et al. Covalent binding of enzymes to synthetic membranes containing acrylamide units, using formaldehyde. Biotechnol Appl Biochem. 1991;13:106–111. [PubMed] [Google Scholar]
- 22.De Saeger S, Van Peteghem C. Flow-through membrane-based enzyme immunoassay for rapid detection of ochratoxin A in wheat. J Food Protect. 1999;62:65–69. doi: 10.4315/0362-028x-62.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Mozdzanowski J, Speicher DW. Microsequence analysis of electroblotted proteins. I. Comparison of electro-blotting recoveries using different types of PVDF membranes. Anal Biochem. 1992;207:11–18. doi: 10.1016/0003-2697(92)90492-p. [DOI] [PubMed] [Google Scholar]
- 24.Reim DF, Speicher DW. Microsequence analysis of electroblotted proteins. II. Comparison of sequence performance on different types of PVDF membranes. Anal Biochem. 1992;207:19–23. doi: 10.1016/0003-2697(92)90493-q. [DOI] [PubMed] [Google Scholar]
- 25.Choli T, Kapp U, Wittmann-Liebold B. Blotting of proteins onto Immobilon membranes. In situ characterization and comparison with high-performance liquid chromatography. J Chromatogr. 1989;476:59–72. doi: 10.1016/s0021-9673(01)93856-7. [DOI] [PubMed] [Google Scholar]
- 26.Stein MA, McAllister SA, Johnston KH, et al. Detection of lipopolysaccharides blotted to polyvinylidene difluoride membranes. Anal Biochem. 1990;188:285–287. doi: 10.1016/0003-2697(90)90607-b. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakkum EA, Dalmeijer RA, Verdel MJ, et al. Quantitative analysis of the inflammatory reaction surrounding sutures commonly used in operative procedures and the relation to postsurgical adhesion formation. Biomaterials. 1995;16:1283–1289. doi: 10.1016/0142-9612(95)91042-w. [DOI] [PubMed] [Google Scholar]
- 29.Klock G, Pfeffermann A, Ryser C, et al. Biocompatibility of mannuronic acid-rich alginates. Biomaterials. 1997;18:707–713. doi: 10.1016/s0142-9612(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 30.Menard M, Dusseault J, Langlois G, et al. Role of protein contaminants in the immunogenicity of alginates. J Biomed Mater Res B Appl Biomater. 2010;93:333–340. doi: 10.1002/jbm.b.31570. [DOI] [PubMed] [Google Scholar]
- 31.Sondermeijer H, Witkowski P, Hardy MA. Uses of immunologically modified scaffold for tissue prevascularization and cell transplantation. 2009/039185 A1. International publication number WO. 2009 Mar 26;