Abstract
Introduction:
To date, several concerns have been raised on the purity of ingredients employed in the manufacturing processes of refill fluids and cartridges, the device functionality, and the quality control of electronic cigarettes. This article reviews analytical methods so far described for the analysis of liquids to detect their chemical components and to investigate the presence of toxicants and carcinogens that can potentially occur as impurities of ingredients or as a consequence of their degradation.
Results and Discussion:
Based on the scientific literature, high-performance liquid chromatography with diode-array detection (HPLC/DAD) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) are most appropriate for determining nicotine and related compounds in fluids and cartridges, whereas LC-MS/MS has been successfully used to determine nitrosamines. Content analyses of glycols have been performed using gas chromatography equipped with flame ionization detector or gas chromatography/mass spectrometry (GC/MS), whereas carbonyl and other volatile organic compounds determinations have been performed by HPLC/DAD and GC/MS, respectively. Content analyses of heavy metals have been performed by inductively coupled plasma optical emission spectroscopy or inductively coupled plasma mass spectrometry. Since new potentially toxic substances may be created during heating, it is also necessary to investigate the chemical composition of generated aerosol. In this case, similar methods applied for tobacco smoke can be adopted.
Conclusions:
A broad range of analytical techniques are available for the detection of constituents and toxicants in e-liquids and cartridges. Analyses of liquids have been performed with pharmacopeia procedures and methods (International Organization for Standardization, Environmental Protection Agency, and American Public Health Association) developed for other matrices but applicable to e-liquids. Because new potentially harmful substances may be produced during heating process, analyses of aerosol are needed to correlate its composition to the chemical components of liquids.
Introduction
Electronic cigarettes (ECs) are battery-powered devices that simulate tobacco cigarettes by converting liquid into an inhalable aerosol. The liquid (“e-liquid) can be contained in a disposable cartridge or in a tank within the EC device that can be refilled by the users. Because ECs do not burn tobacco, they do not produce the numerous chemicals found in conventional tobacco smoke, and hence they have been proposed as potential products for tobacco harm reduction.1–9 Their safety and efficacy for smoking cessation is controversial, but available studies seem to suggest the potential of ECs to assist smokers to quit or to reduce smoking. Over the last few years, concerns about quality control in the manufacture of these products have been raised. Since ECs are not manufactured according to standards like those imposed on medications or drug delivery devices, cartridges and refill liquids could not comply with the label and could contain impurities or toxic substances.7,10
Liquids containing nicotine consist of nicotine (at different concentrations) in a mixture of vegetable glycerine (VG) and/or propylene glycol (PG) with water.11 PG and VG are used as humectants creating aerosol when heated by the atomizer. A huge variety of chemicals are added to the mixture to produce aromas and flavor.12,13 The chemical components of aerosol can be different from those found in liquids. Using ECs requires the heating of the liquid and under such conditions, chemical reactions may result in the formation of new potentially harmful compounds.11
First tests14 on chemical composition of EC cartridge liquids and aerosol were performed in 2008 by Health New Zealand Ltd to asses safety of Ruyan EC cartridges, the first patented and launched cartridges.1 One year later, the Food and Drug Administration (FDA) was asked to quantitate the amount of nicotine and impurities in two brands of EC cartridges. The study highlighted the presence of low amounts of nicotine in some bottles of liquid labeled “no nicotine.” The same report showed the presence of toxic compounds as impurities of some ingredients. In particular, diethylene glycol (DEG) was found in one cartridge as impurity of PG. The same report showed the presence of tobacco-specific nitrosamines (TSNAs), strong human carcinogens15 in 50% of the tested samples and the presence of nicotine-related compounds (suspected of being harmful) in the majority of them. To date, different studies10–12,16–18 have reported small amounts of formaldehyde, acetaldehyde, and acrolein in the aerosol as results of heating of PG and VG. Low levels of formaldehyde and acetaldehyde have been also found in some cartridges.10,14 In some reports,10,11,17 chemical analysis revealed small amounts of other volatile organic compounds (VOCs) like benzene, toluene, xylene, and styrene in both liquid and aerosol. Because ECs contain various metal components, metals can be also found in fluids from the fibrous pad of “cartomizer” (the new EC model of cartridge where atomizer and cartridge have been combined into a single unit) and in the aerosol.
Recent data10,19 have shown that the differences between content of nicotine and labels are smaller than previously reported, suggesting an improvement in the manufacturing process over the years. Some American industries are currently adopting improved manufacturing standards.2,20 In Europe, some quality standards for refill liquids will be introduced by the revision of Tobacco Product Directive (2001/37/EC), issued by the European Commission.7,21 According to the revised Directive, a maximum threshold of nicotine is set at 20mg/ml for ECs classified as consumer products. Refill liquids with higher concentration of nicotine may be sold only if approved by the pharmaceutical regulation.7
This review is aimed at focusing the role of various analytical techniques in the assay of quality and safety of e-liquids and cartridges and giving a thorough literature survey of the methods used for analysis. In particular, this article reviews the analytical methods so far described for the analysis of cartridges and refill liquids for detection of their chemical components and for investigation of toxic and carcinogenic compounds that can potentially occur. The methods herein reported, summarized in Table 1, allow to determine the content of the following classes of compounds: nicotine and nicotine-related compounds, TSNAs, glycols, carbonyls and other VOCs, and heavy metals.
Table 1.
Published Analytical Methods
| Analytes or classes of analytes | Matrices | Analytical techniques | References |
|---|---|---|---|
| Nicotine | Refill liquid | GC/FID | Schober and Szendrei 12 |
| HPLC/DAD | Davis and Dang 19 | ||
| Cartridgea | GC/FID | Cheah and Chong 22 | |
| HPLC-UV | Westenberger 23 | ||
| Cartridge, aerosol | GC-TSD | Goniewicz and Haiek 24 | |
| Nicotine and nicotine-related compounds | Cartridge | HSGC-MS | Westenberger 23 |
| Cartridgea, refill liquid, aerosol | HPLC/DAD | Trehy and Ye 25 | |
| Tobacco-specific nitrosamines | Cartridgea | LC-MS/MS | Laugesen 14 ; Westenberger 23 |
| Refill liquid | LC-MS/MS | Schober and Szendrea 12 ; Kim and Shin 26 | |
| Diethylene glycol | Cartridgea | GC/MS (1H-NMRb) | Westenberger 23 |
| Propylene glycol | Refill liquid | GC/FID (GC/MSb) | Etter and Zäther 10 |
| Glycerin | Refill liquid | GC/FID (enzymatic analysisb) | Schober and Szendrei 12 |
| VOCs | Refill liquid | GC/MS | Schober and Szendrei 12 |
| Carbonyl compounds and other VOCs | Cartridge | HS-SPME GC-MS | Laugesen 14 |
| Carbonyl compounds | Refill liquid | HS-SPME GC-MSc | Lim and Shi 27 |
| Aerosol | HPLC/DADc | Goniewicz and Knysak 11 ; Kosmider and Spbczak 28 ; Uchiyama and Ohta 18 ; Schripp and Markewitz 16 | |
| Heavy metals | Cartridgea | ICP-MS | Laugesen 14 |
| Aerosol | ICP-MS | Goniewicz and Knysak 11 | |
| ICP-OES | Williams and Villarreal 29 |
aIt requires extraction procedures with organic solvent.
bConfirmatory method.
cDerivatization step previously.
Analytical Methods for Determination of Nicotine and Nicotine-Related Compounds
Nicotine is a highly addictive central and peripheral nervous system stimulant with a lethal dose of 0.8–1.0mg/kg of body weight (acute minimum lethal oral dose) in adult nonsmokers.8,30 Because of both toxic and addictive nicotine activities, it is important that its content in refill liquids and cartridges complies with the label.19 According to the tolerance level suggested by the American E-liquid Manufacturing Standards Association guidelines, nicotine content should be ±10% of the concentration on the label.19,20
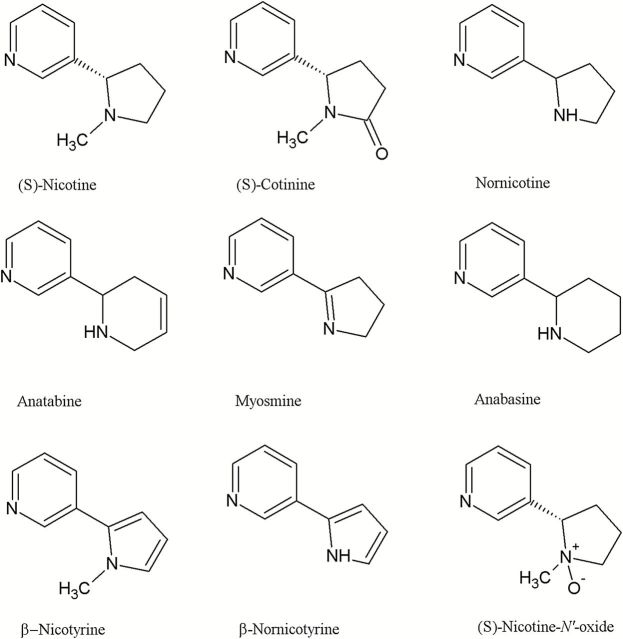
Nicotine used for e-liquid production is extracted from tobacco, and this extraction process may produce some tobacco-specific impurities, suspected of being harmful.17 These impurities are minor alkaloids like nornicotine, anatabine, anabasine, myosmine, cotinine, nicotine-N′-oxides (cis and trans isomers), β-nicotyrine, and β-nornicotyrine (Figure 1.) and are thought to arise by bacterial activity or oxidation during tobacco processing.10 Nicotine and cotinine in tobacco are largely present as the levorotary (S)-isomers (only 0.1%–0.6% of total nicotine content is (R)-nicotine), whereas anabasine, anatabine, and nornicotine in tobacco exist as mixture of enantiomers. Oxidative degradation of nicotine can also occur during the manufacturing processes of e-liquids, and high amounts of nicotine-related substances can indicate an inadequate handling and storage. Unstable formulation or interactions with packaging materials could also enhance nicotine degradation in the final products. For example, chemicals added as flavoring agents are known to affect product stability.10 An FDA report23 showed the presence of tobacco-specific impurities in the majority of the tested samples. These substances are less potent and toxic than nicotine itself, but toxicology studies are needed to demonstrate that high levels of these degradation products do not convey any additional risk to the EC users.10
Figure 1.
Structures for nicotine and nicotine-related compounds.
According to European Pharmacopeia,31 nicotine of pharmaceutical grade may, as raw material, contain up to 0.3% of each of specified nicotine impurities. However, since the e-liquid manufacturing process is not strictly controlled, some products can show levels of impurities above these acceptance limits for pharmaceutical products.10
Sample Preparation of Refill Fluids and Cartridges
Analysis of refill liquids is carried out after dilution with mobile phase or other solvents and subsequent injection into the chromatographic systems.10,12,19,25 e-Liquids are oily and highly viscous, making difficult to pipette and disperse the exact volume. Therefore, sample preparation can result in nonhomogenous samples, leading to differences between assay determinations of duplicates.25
Sample preparation for content analysis of EC cartridges needs a previous step of extraction with an organic solvent. In the cartomizers, removal of the plug allows to access to the cartridge’s PG solution of nicotine adhering to the fibrous pad. After breaking the wires running to the heating element, the pad can be removed with tweezers or unwound. Then, the pad is transferred into the flask together with the plug and the cartridge, and they are extracted with solvent. The results are expressed as milligrams of nicotine per cartridge. Trehy et al.25 used 50ml of methanol for cartridge extraction (stirring for 90min). In the FDA report, cartridges were extracted using two different procedures: extraction with methanol and extraction with a mixture of 10% acetonitrile and 1% phosphoric acid in water. Both extractions provided similar results for the majority of analyzed samples. Cheah et al.22 used the extraction procedure described by Trehy et al.25 and verified the completeness of the analyte extraction, with a recovery between 80% and 90%. The extraction procedure developed by Goniewicz et al.24,32 required 50ml of ethyl acetate and 100 µl of an Internal Standard solution (quinoline, 50mg/ml in methanol) using an ultrasound bath for 30min.
Gas Chromatographic Methods
To date, gas chromatographic (GC) and liquid chromatographic methods have been developed and used for the determination of nicotine in a variety of matrices. The same methods can be suitable for e-liquid and aerosol analysis. Since nicotine is relatively volatile and thermally stable, GC generally offers an appropriate analytical method to quantify nicotine. Schober et al.12 developed and validated a method in gas chromatography equipped with flame ionization detector (GC/FID) using a 5% phenylmethyl-polysiloxane capillary column. They reported a limit of detection (LOD) of 0.1% (w/w) for nicotine. They also confirmed their results by performing analysis on a second GC-column. Cheah et al.22 determined nicotine content in cartridges using an organic solvent extraction followed by detection by GC/FID using a capillary column coated with polyethylene glycol. They reported a LOD value of 0.02mg per cartridge. Goniewicz et al.11,24 developed and validated a method based on gas chromatography with Thermionic Specific Detector (GC-TSD) for determination of nicotine in liquids and cartridges and also in the aerosol generated by ECs. The limits of quantification (LOQ) for nicotine were 0.1mg/cartridge and 0.05 µg/ml for cartridges and aerosol, respectively. In the FDA report,23 a simulated use of cartridges was reported for detection of nicotine and tobacco specific impurities volatilized during use by Head-Space GC-MS (HSGC-MS) technique. Compounds were identified by comparison with a mass spectral library and their relative abundance.
Liquid Chromatographic Methods
While gas chromatography/mass spectrometry (GC/MS) offers highly sensitive and selective methods for the determination of nicotine, it requires the analytes to be volatilized, whereas liquid chromatography only requires the analyte to be soluble in a solvent before their separation. Although nicotine and cotinine can be analyzed directly by GC, several important nicotine-related compounds such as the N′-oxides are not thermally stable and cannot pass intact through GC columns. They need to be derivatized, but a direct method of analysis would be preferred. Thus, LC can be considered a much more appropriate technique for the analysis of nicotine and many of its degradation products and metabolites.33
In the FDA report dated 2009, nicotine content determination was performed by liquid chromatograph equipped with UV detector (HPLC-UV) following the United States Pharmacopeia (USP) assay for “Uniformity of Dosage Units” reported in the “Nicotine Transdermal System Assay” section.34 Similar methods to the USP, one were developed and validated by Trehy et al.25 and by Davis et al.19 Davis’s procedure allowed to achieve LOQ values for nicotine 10-fold lower than those reported by Trehy (10.0 instead of 104.4 µg/ml). However, the USP method is not suitable for nicotine related compounds. As reported by Trehy et al.,25 using the chromatographic conditions reported in the USP procedure, cotinine was poorly retained, and anabasine was not completely resolved from nicotine. An alternative method was then developed and validated using gradient elution.25 Under these improved conditions, all the components were chromatographically resolved. LOD and LOQ values were determined according to ICH Q2B guideline, “Validation of Analytical Procedures: Methodology” (1996). LOD and LOQ values for nicotine, cotinine, anabasine, anatabine, myosmine and β-nycotirine in solutions were in the interval of 0.10–0.40 µg/ml and of 0.25–1.30 µg/ml, respectively.
Among the LC techniques, liquid chromatography-tandem mass spectrometry (LC-MS/MS) provides a rapid, sensitive and selective alternative for the determination of nicotine and nicotine related compounds. A number of reports35–41 on determination of nicotine and related alkaloids by LC-MS/MS in different matrices (especially in biological fluids, like urine, plasma, saliva) have been published. However, these methods can be also suitable for e-liquids and aerosol from ECs, but their applicability to these matrices has to be verified.
Analytical Methods for Determination of TSNAs
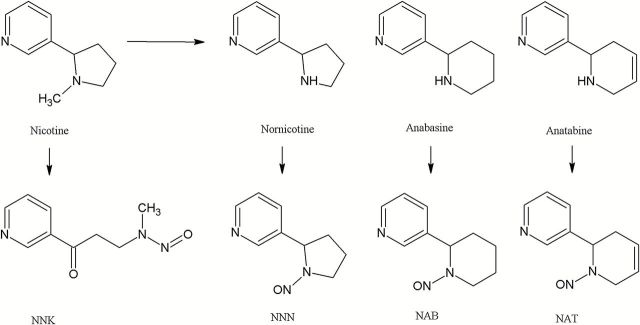
N′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N′-nitrosoanabasine (NAB), and N′-nitrosoanatabine (NAT) are TSNAs, the strong carcinogens identified in tobacco and tobacco smoke. Their presence in refill liquids is mostly due to the extraction processes of nicotine from tobacco leaves. These compounds are formed from their alkaloid precursors and from nitrite or nitrate predominantly during tobacco curing, fermentation and ageing.15 NNN, NAB, and NAT are formed primarily from their corresponding secondary amines (nornicotine, anatabine, and anabasine) in the early stages of tobacco curing and processing whereas, the majority of NNK is formed from the tertiary amine nicotine at the later stages of tobacco curing and fermentation (Figure 2).15 Nitrosation reactions of corresponding amines can occur in liquids,26 especially during an inadequate storage or during manufacturing processes (e.g., an inadequate storage is supposed to increase the levels of NNN as consequence of nitrosation of nornicotine converted from nicotine in liquids).
Figure 2.
Structures for tobacco-specific N-nitrosamines.
To date, many analytical methods for the determination of TSNAs have been published for studies on tobacco and mainstream cigarette smoke. The gas chromatography thermal energy analyzer is a widely used technique, specific for nitroso-compounds and it has been successfully used for many years for measuring NNN and NNK in tobacco smoke. In recent years, LC-MS/MS has also been used showing very high sensitivity and reproducibility to TSNAs.26
The first study14 on TSNA content in cartridge liquids of ECs was dated 2008. In the report, tests were performed by using a LC-MS/MS method developed for determination of TSNAs in whole tobacco and modified for the application on EC cartridges. Results were reported in nanogram per cartridge, but either details on extraction procedure or details on conditions used for chromatographic separation were not reported. In 2009, FDA detected very low levels of TSNAs in 50% of tested cartridges, otherwise this study did not give the exact analytical data. TSNAs were analyzed using a revision of the method based on LC-MS/MS developed by Wu et al.42 for the analysis of TSNAs in cigarette tobacco and mainstream cigarette smoke. The extraction procedure for TSNA content in cartridges was similar to that reported for nicotine (see “Sample preparation of refill fluids and cartridges”). They reported LOQ values higher than those reported in the reference method42: LOQs of NAB, NAT, NNK and NNN were 21, 21, 75, and 24 µg/L respectively, compared to LOQ values of approximately 40 pg/ml. A possible explanation for the different LOQ values was the different kind of analyzed matrices. The method used by Wu et al. was developed for either finely ground tobacco or smoke captured on glass fiber filter pads, whereas the modified method was applied to a PG matrix supported on a fibrous material within a plastic housing. The TSNAs in the extracts were quantified using deuterated IS. Two ion transitions were recorded in MRM mode for each TSNA. TSNA content was reported as weight of TSNA per weight of cartridge (ng/g).
Analysis of refill liquids can be carried out using direct injection into the chromatographic system. Schober et al.12 reported a method for determination of TSNAs using a direct injection into LC-MS/MS. According to their procedure, a 1-mL-aliquot of e-liquid was spiked with IS (NNK-d4). NNN, NNK, NAB, and NAT were analyzed using MRM mode. The LOD ranged from 0.22ng/ml (NAB) to 0.38ng/ml (NNK). Since TSNAs are present in traces in refill liquid, pre-concentration could be necessary for sample preparation. Kim et al.26 developed and validated a LC-MS/MS method using a previous step of purification and pre-concentration for simultaneous determination of the four TSNAs in e-liquids. In this study, preliminary experiments were performed by testing different extraction procedures (solid-phase extraction and liquid-liquid extraction procedures) in order to achieve the best recovery. The liquid-liquid extraction with methylene chloride yielded the best recovery (% recovery > 70% for each analyte) among the tested procedures. Improved recoveries (75%–83%) for TSNAs were observed at basic conditions (pH 9). Analytes were separated by using a C18 column with a binary gradient. Detection was performed in electrospray ionization, setting a positive ionization mode. For each analyte, three ion transition pairs were monitored in MRM mode. The protonated molecular ions [M+H]+ and the product ions formed by the loss of a NO molecule from the precursor ions were characteristic. LOD and LOQ values of TSNAs were 0.01–0.02 µg/L and 0.04–0.06 µg/L, respectively. Accuracy was in the range of 89%–109% and precision less than 10%.
Analytical Methods for Determination of Glycols
E-liquids contain PG or VG or a mix of both. These two glycols are used to generate aerosol in the ECs. Generally, PG is the main component (up to 90% of the liquid, in some products).14 Both PG and VG are classified by FDA as GRAS, “generally recognized as safe,” and are approved solvents for pharmaceutical products. VG is a glycerol derived from plant oils, whereas PG is prepared by hydrolysis of propylene oxide under pressure at high temperature. PG is used in pharmaceuticals as a drug vehicle for asthma inhalers and nebulizer and preservative. Unlike inhalers or nebulizers, the EC devices consist of a heating component and the heating of glycols can generate various potentially toxic carbonyl compounds. Moreover, DEG could be present as an impurity of PG in some EC liquids. The U.S. FDA detected DEG in one cartridge at approximately 1%,23 but no other study conducted to date has shown the presence of this toxic chemical in other liquids. DEG has similar physical and chemical properties to PG and VG and it has been involved in numerous worldwide epidemic poisonings, resulting from the addition of DEG to pharmaceutical products instead of more expensive but non-toxic, glycols, or VG constituents.43
In the USP (National Formulary monographs for PG), one of the tests for the PG identification in pharmaceutical products requires the use of a GC/FID method, and the reported chromatographic parameters can be also used to perform PG content determination on e-liquids.10 This method is also used to determine the presence of DEG and ethylene glycol (another PG impurity) with the aim to verify that these two compounds do not exceed the specific limits of 0.1%. Chromatographic separation was achieved using a column coated with 14% cyanopropylphenyl-86% methylpolysiloxane stationary phase using 2,2,2-trichloroethanol as IS. Moreover, in the “Assay procedure” section,44 another GC method is published but it requires a thermal conductivity detector. In the FDA report, analysis on EC cartridges for DEG determination was performed using a GC-MS method. An aliquot of methanol extract used for nicotine extraction (see “Sample preparation of refill fluids and cartridges”) is injected into the chromatographic system. Samples were screened operating initially in full scan mode and then in selected ion monitoring (SIM) mode using the chromatographic parameters from USP monograph procedure.45 The presence of DEG was confirmed by proton nuclear magnetic resonance spectroscopy (1H-NMR).
Schober et al.12 developed and validated a GC-FID method for detection of VG and 1,2-propanediol (another humectant to produce mist) in e-liquids. According to their procedure, 0.1g of IS (1,3-propanediol) were added to 0.3g of each liquid. This mixture was dissolved in 5ml of isopropanol and diluted 1:5 with isopropanol. LOD value for 1,2-propanediol was 0.5% (w/w). The results were confirmed by analysis on a second GC-column and in the case of VG by an enzymatic analysis.
Analytical Methods for Determination of Carbonyls and Other VOCs
VOCs include a variety of chemicals (carbonyls, aliphatic and aromatic hydrocarbons, etc.), some of which may have short- and long-term adverse health effects. In general, VOCs have high vapor pressures, low-to-medium water solubilities, and low molecular weights.46 Published results14,18,27 demonstrated the presence of small amounts of formaldehyde and acetaldehyde in some cartridge liquids. Acetaldehyde may occur in some liquids because of the intentional addition as flavor compound. Formaldehyde is classified as carcinogenic to humans (group 1 by International Agency for Research on Cancer [IARC]), acetaldehyde as possibly carcinogenic to humans (group 2B by IARC), and acrolein can cause irritation to the nasal cavity, and damage to the lining of the lungs. Benzene (group 1 by IARC) and other solvents (toluene, xylenes, and styrene) could be present in e-liquids because of their use as solvents for nicotine extraction from tobacco leaves.10 A wide variety of other VOCs in the liquid phase produce aromas and flavor through heating.13
For VOC analysis, Environmental Protection Agency (EPA) method 826047 can be used. This method allows to determine VOCs by GC-MS in a variety of solid waste matrices but it is applicable to nearly all types of samples, including aqueous samples. The most appropriate technique of introducing these compounds into the GC-MS system is Purge-and-Trap by EPA method 5030,48 previously dissolving the e-liquids in a solvent or mixture of solvents (e.g., water or methanol in water). Compounds that can be determined by EPA method 8260 include BTEX (benzene, toluene, ethylbenzene, xylene), styrene, and some halogenated compounds. Since these analytes well dissolve in PG, their extraction from e-liquids should be further studied. Lim et al.23 developed an analytical method for detection of formaldehyde, acetaldehyde, and acrolein in refill fluids by head space solid-phase micro-extraction (HS-SPME) and GC-MS after derivatization with 2,2,2-trifluoroethylhydrazine (TFEH). They reported that the optimal conditions for derivatization of carbonyl compounds in e-liquids were different from those used for water samples. Derivatization was performed in a headspace vial under continuous shaking and adding 40mg of TFEH and 0.05 µg of acetaldehyde-d6 (IS) to 0.5-ml-sample of e-liquid. The derivatives were adsorbed exposing a 65 µm-polydimethylsiloxane-divinylbenzene (PDMS-DVB) SPME fiber in the headspace. Separation was carried out using a HP-INNOWax capillary column. Analysis was performed in SIM mode. LOD and LOQ values ranged 3.1 (formaldehyde) to 6.3 (acetaldehyde) µg/ml and 9.8 (formaldehyde) to 19.0 µg/ml (acetaldehyde), respectively. For other organic compounds, liquids can be also diluted and directly injected into the GC system. Schober et al.12 reported a GC-MS method for determination of compounds used to flavor liquids. According to this procedure, a 1-ml-aliquot of each liquid was diluted with 10ml of chloroform and submitted to analysis. Samples were analyzed in SIM mode. Among the detected analytes, the presence of benzylalcohol, menthol, vanillin, and l-limonene (all known contact allergens) were reported in liquids.
In the Ruyan EC cartridge report, a method is reported to determine the presence of VOCs using HS-SPME technique coupled to GC-MS. The limit of this published method is that samples were incubated at 30 °C because the aim of analysis was to determine VOCs that can be elicited at room temperature in the just opened EC cartridges. Moreover, the analytes were only identified by comparison with a mass spectral library and their relative abundances. The column was a Restek Rtx-WAX fused silica capillary column coupled in series with a Restek Rtx-1ms fused silica capillary column. Samples were incubated for 60min at 37 °C with their enclosed headspace exposed to a 2cm long DVB/CAR/PDMS combination SPME fiber. The investigated analytes were: acetaldehyde, acrolein, acrylonitrile, benzene, 1,3-butadiene, cresols (m-, o-, p), ethylene oxide, styrene, and xylenes.
Chemical composition of aerosol can be different from liquid: Using EC requires heating the liquid and under such conditions, chemical reactions may result in formation of new compounds. In some models, the temperature measured in the centre of heating coil can be notably high (≥ 350 °C) promoting pyrolysis reactions of EC liquid chemical components.28 Many studies report that short-chain aldehydes such as formaldehyde, acetaldehyde and acrolein are produced during the EC heating.10,12,14 Uchiyama et al.18 demonstrated that the 70% of examined EC brands generated formaldehyde, acetaldehyde and acrolein with maximum concentrations of 260, 210, and 73mg/m3, respectively. They also detected two additional harmful carbonyl compounds that to date have not been detected in the mainstream smoke from conventional cigarettes: glyoxal and methylglyoxal. Carbonyl compounds are the results of dehydration and fragmentation of VG. Although these processes can only occur at relatively high temperatures like in pyrolysis or combustion, some specific conditions allow lower dehydration temperatures.10 Also PG can be oxidized to formaldehyde and acetaldehyde during e-liquid heating.18 Generation of carbonyl compounds seems to be increased when liquids incidentally touch the heated nichrome wire in the atomizer as suggested by the change in the color around the wire18 reported in some EC devices. Thus, although carbonyl compounds can be present in the refill liquids, heating can enhance the concentrations of these compounds in the aerosol. Therefore it is necessary to develop analytical methods for detection of toxicants in the aerosol generated by ECs.
Goniewicz et al.,11,24 analyzed carbonyl compounds in aerosol on the basis of the U.S. Environment Protection Agency TO11 standard method for carbonyl determination in air. Analysis required derivatization of carbonyls with 2,4-dinitrophenylhydrazine (DNPH). Aerosol was generated using a smoking machine. Carbonyl compounds were trapped from aerosol using silica gel cartridges coated with DNPH and were analyzed by HPLC/DAD. Compounds were separated using Zorbax Eclipse column (Agilent Technologies) with a gradient elution. The method allowed selective determination of acetone, butanol, formaldehyde, acetaldehyde, acrolein, and other 10 aldehyde compounds. The LOD and LOQ values per one EC (per 150 inhalations or puffs) ranged 0.01–0.1 µg and 0.02–0.29 µg, respectively. The precision of the method was 18% with accuracy of 89%. A similar procedure was used by Kosmider et al. to study the effects of solvent and battery output voltage on carbonyl compound generation.28 The International Organization for Standardization (ISO) 16000-3 “Determination of formaldehyde and other carbonyl compounds in indoor air and test chamber air” was the reference procedure chosen by Schripp et al.16 to study carbonyl levels in aerosol. Uchiyama et al.18 developed a similar method to Goniewicz’s one but using coupled silica cartridges impregnated with hydroquinone (for the inhibition of acrolein polymerization) and DNPH.
Analytical Methods for Determination of Heavy Metals
To date, studies that attempted to quantify metals in refill liquids seem to show that there is no evidence of contamination with heavy metals that warrants a health concern.49 In Ruyan report,14 cartridge liquids were tested for heavy metals but none of them was found at concentration higher than 0.1–0.2 ppm. In some cases, metals can be also found in the fluid from the inner and outer fibers of “cartomizers.” In a study performed on 22 cartomizers from a leading manufacturer, William et al.29 demonstrated that cartomizer fluid from inner and outer fibers contained particles that were shown to be tin by electron dispersion spectroscopy (ESD) microanalysis.
Since ECs contain various metal components, metals can migrate to the generated aerosol constituting a health risk to users and by-standers. William et al.29 showed that small particles comprised of various elements (Sn, other metals, semimetals, and silicate) passed through cartomizer fibers and were present in the aerosol of ECs. A total of 22 elements were identified in EC aerosol including Pb, Ni, and Cr. Pb and Cr concentration in aerosol were within the range of conventional cigarettes, while Ni concentration was about 2–100 times higher in EC aerosol than in Marlboro brand cigarettes. Ni particles likely originated from the nichrome wire. Significant amount of Sn, other metals and silicate beads escaped into aerosol and would result in human exposure, in some cases probably greater than those observed in a conventional cigarette.
In Ruyan report, cartridge14 liquids were also tested for heavy metals by using inductively coupled plasma mass spectrometry (ICP-MS) following a modified American Public Health Association (APHA) 21st edition 3125 method. Liquids were tested for the presence of As, Sb, Cd, Cr, Co, Cu, Pb, Mn, and Ni. LOD values were below 0.01 ppm for Cd, below 0.1 ppm for As and Pb and below 0.2 ppm for Cr and Ni. Goniewicz et al.11,24 analyzed metals in the EC aerosol by using a method similar to that applied for tobacco smoke. Since metals are characterized by lower volatility, they cannot be trapped in tubes packed with solid adsorbent. Thus, they were extracted dissolving the aerosol in a solvent. Williams et al.29, allowed aerosol to fully dissolve in a solution of 10% nitric acid, 3% hydrocloridric acid, and 87% deionized water prior inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis. Goniewicz et al.11,24 extracted metals from aerosol adsorbing it in two gas washing bottles with methanol (50ml in each bottle). 10ml of solution was then collected, and condensed on vacuum evaporator. The samples were acidified with 0.5ml of 70% nitric acid and heated at a temperature of 120 °C (8h). Then 10ml of deionized water and a solution of 10ng/ml of Rh (IS) was added and samples were analyzed by using the ICP-MS technique. The method developed allowed to determine Co, Ni, Cu, Zn, Cd, Pb, As, Cr, Se, Mn, Ba, Rb, Sr, Ag, Tl, and V. LODs and LOQs of analyzed elements were below 0.01 μg. The average precision of the method was 23% and its average accuracy was 84%.
Discussion and Conclusions
Since their first introduction in the market in 2004,1 first tests on chemical composition of EC cartridge liquids and generated aerosol were dated only in the 2008. Analysis on chemical composition is needed to assess quality of manufactured products and to reveal potentially harmful contaminants and impurity of ingredient impurities. Quantification of specific analytes (e.g., nicotine degradation products) can be useful to indicate if an inadequate handling and storage of EC liquids have been occurred or an unstable formulation has been used.10 Although some contaminants, like DEG, have been detected in products of earlier generations, risk of contamination with toxic substances should be reduced by inducing manufacturers to adopt strictly manufacturing standards.
Sample preparation for content analysis of EC cartridges needs a previous step of extraction with an appropriate solvent, whereas analysis of refill liquids is carried out after dilution with mobile phase or other solvents to the injection concentration. A broad range of analytical techniques used for analysis and detection of EC constituents and toxicants in e-liquids and cartridges are available. Regarding nicotine and relating compounds, HPLC/DAD or LC-MS/MS can be considered more reliable methods than GC/MS because several important nicotine analogues are thermally unstable and cannot pass intact through GC-columns. LC-MS/MS technique has shown very high sensitivity and reproducibility to TSNAs in tobacco mainstream smoke and it has been successfully used for tests on refill fluids and cartridges. Content analyses of glycols, including their contaminants, have been performed using GC/FID or GC/MS methods, whereas carbonyl compounds and other VOC determinations have been performed by GC-MS (or as alternative HS-SPME coupled to GC/MS). In many cases, analyses of liquids have been performed using USP procedures or methods (ISO, EPA or APHA methods) developed for other matrices that can be applicable also for e-liquids. For example, analysis of carbonyl compounds in e-liquids can be performed using a similar method to that applied for aqueous samples. However, the optimal conditions for derivatization of carbonyl compounds are different from those used for water specimens.27
The main drawback in the analysis of e-liquids is that they are oily and highly viscous leading to possible differences between assay determination of duplicates. Analytical determination of nicotine-related compounds is not simple because of the low concentration levels at which the compounds are present in the samples and because it is not simple to obtain a chromatographic separation between nicotine and its isomer anabasine.25 Since some toxicants like TSNAs are present in traces in refill liquids, pre-concentration can be necessary for sample preparation. In this case, extraction of nitrosamines from e-liquids could be a crucial step of the procedure affecting the analytical recoveries.
It should be noted that examination of liquid composition does not assure the safety of generated aerosol after their use due to the production of new substances during heating and vaporization processes. Therefore, analysis on generated aerosol could be useful to correlate its composition with the chemical components of liquid.49 For example, analysis of degradation products in the generated aerosol has highlighted that PG is more susceptible to thermal decomposition than VG, leading to the highest levels of carbonyls.28 Analysis of EC aerosol can be conducted using similar methods like those applied for tobacco smoke.11 The extracting procedure depends on the type of analyte: Volatile compounds like carbonyl compounds can be trapped in tubes packed with solid adsorbent, whereas compounds with a low volatility like metals can be extracted dissolving the aerosol in a solvent.
Over the last few years, some work has been done to study the applicability of developed methods to e-liquids and cartridges. Further investigations may be required to respond to the list of new compounds that could potentially occur in the e-liquids. However, the application of the specific regulation (Revision of Tobacco Product Directive 2001/37/EC) should contribute to reduce possible risks of contamination during the manufacturing process. More data regarding how the EC hardware can influence aerosol composition are needed to predict hazardous exposures for the users. To date, studies have highlighted that the contact between liquids and the heated nichrome, the various temperature ranges reached in the vaporization process and the different battery voltages can affect EC toxicity.18,28 Tests for delivery dose uniformity and aerodynamic particle size distribution should also be required.25 Therefore, only a systematic monitoring of e-liquids quality together with quality control of manufactured devices would contribute to ensure public health and to reassure consumers.
Funding
None declared.
Declaration of Interests
None declared.
Acknowledgments
All of the authors meet the following criteria for authorship: (a) contributing to the conception and design of the work; (b) revising the work critically for important intellectual content; (c) finalizing the version to be published; (d) being accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1. Bullen C, Williman J, Howe C, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health. 2013;13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polosa R, Rodu B, Caponnetto P, Maglia M, Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis. 2013;79:12–19. [DOI] [PubMed] [Google Scholar]
- 4. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:1–12. 10.1371/journal.pone.0066317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abuse. 2013;7:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’ Connor RJ. Non-cigarette tobacco products: what have we learned and where are we headed? Tob Control. 2012;21:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Etter JF, Bullen C, Flouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manzoli L, La Vecchia C, Flacco ME, et al. Multicentric cohort study on the long-term efficacy and safety of electronic cigarettes: study design and methodology. BMC Public Health. 2013;13:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etter JF, Zäther E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. [DOI] [PubMed] [Google Scholar]
- 11. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schober W, Szendrea K, Matzea W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2013;217:628–637. 10.1016/j.ijheh.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 13. Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. [DOI] [PubMed] [Google Scholar]
- 14. Laugesen M. Safety report on the Ruyan® e-cigarette cartridge and inhaled aerosol. Christchurch, New Zealand: Health New Zealand Ltd; 2008. http://www.healthnz.co.nz/2ndSafetyReport_9Apr08.pdf. Accessed September 30, 2014. [Google Scholar]
- 15. IARC. Some tobacco-specific N-nitrosamines. IARC Monogr. 2007;89:421–456. http://monographs.iarc.fr/ENG/Monographs/vol89/mono89-7.pdf. Accessed September 30, 2014. [Google Scholar]
- 16. Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25–31. [DOI] [PubMed] [Google Scholar]
- 17. FDA. Laboratory analysis of electronic cigarettes conducted by FDA 2009. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm173146.htm. Accessed September 30, 2014.
- 18. Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 2013;29:1219–1222. [DOI] [PubMed] [Google Scholar]
- 19. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2014. 10.1093/ntr/ntu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AEMSA. American e-liquid manufacturing standards association. Version 1.8 2014. http://www.aemsa.org/standards. Accessed September 30, 2014.
- 21.Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC. O J L127/1; 29.4.2014.
- 22. Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014;23:119–125. [DOI] [PubMed] [Google Scholar]
- 23. Westenberger B.J. Evaluation of e-cigarettes; FDA, DPATR-FY-09-232009 (pp. 1–8) 2009. http://www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf. Accessed September 30, 2014.
- 24. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109:500–507. [DOI] [PubMed] [Google Scholar]
- 25. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr Relat Technol. 2011;34:1442–1458. [Google Scholar]
- 26. Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1291:48–55. [DOI] [PubMed] [Google Scholar]
- 27. Lim HH, Shi HS. Measurement of aldehydes in replacement liquids of electronic cigarettes by headspace gas chromatography-mass spectrometry. B Kor Chem Soc. 2013;34:2691–2696. [Google Scholar]
- 28. Kosmider L, Sobczak A, Fik M, et al. Carboyl compounds in electronic cigarette vapors-effect of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;161319–1326. 10.1093/ntr/ntu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004;43:619–632. [DOI] [PubMed] [Google Scholar]
- 31. Nicotine. European Pharmacopeia 6.0, Council of Europe, Strasbourg. 01/2008:1452. 2007:. 2500–2501. [Google Scholar]
- 32. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. [DOI] [PubMed] [Google Scholar]
- 33. Smyth TJ, Ramachandran VN, McGuigan A, Hopps J, Smyth WF. Characterisation of nicotine and related compounds using electrospray ionisation with ion trap mass spectrometry and with quadrupole time-of-flight mass spectrometry and their detection by liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:557–566. [DOI] [PubMed] [Google Scholar]
- 34. United States Pharmacopeia. Nicotine transdermal system/Nicotine, Official Monograph, USP35/NF30. U.S. Pharmacopeial Convention; 2012; 4046–4048. [Google Scholar]
- 35. Vieira-Brock PL, Miller EI, Nielsen SM, Fleckenstein AE, Wilkins DG. Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3465–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller EI, Norris HRK, Rollins DE, et al. Identification and quantification of nicotine biomarkers in human oral fluid from individuals receiving low-dose transdermal nicotine: a preliminary study. J Anal Toxicol. 2010;34:357–366. [DOI] [PubMed] [Google Scholar]
- 37. Miller EI, Norris HR, Rollins DE, Tiffany ST, Wilkins DG. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheidweiler KB, Shakleya DM, Huestis MA. Simultaneous quantification of nicotine, cotinine, trans-3’-hydroxycotinine, norcotinine and mecamylamine in human urine by liquid chromatography-tandem mass spectrometry. Clin Chim Acta. 2012;413:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kataoka H, Inoue R, Yagi K, Saito K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;49:108–114. [DOI] [PubMed] [Google Scholar]
- 40. Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM. Specific detection of anabasine, nicotine, and nicotine metabolites in urine by liquid chromatography-tandem mass spectrometry. Am J Clin Pathol. 2006;126:880–887. [DOI] [PubMed] [Google Scholar]
- 41. Xu X, Iba MM, Weisel CP. Simultaneous and sensitive measurement of anabasine, nicotine, and nicotine metabolites in human urine by liquid chromatography-tandem mass spectrometry. Clin Chem. 2004;50:2323–2330. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Joza P, Sharifi M, Rickert WS, Lauterbach JH. Quantitative method for the analysis of tobacco-specific nitrosamines in cigarette tobacco and mainstream cigarette smoke by use of isotope dilution liquid chromatography tandem mass spectrometry. Anal Chem. 2008;80:1341–1345. [DOI] [PubMed] [Google Scholar]
- 43. Schier JG, Hunt DR, Perala A, et al. Characterizing concentrations of diethylene glycol and suspected metabolites in human serum, urine, and cerebrospinal fluid samples from the Panama DEG mass poisoning. Clin Toxicol (Phila). 2013;51:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. United States Pharmacopeia. Propylene glycol/Official Monograph, USP37/NF32. Rockville, MD: U.S. Pharmacopeial Convention; 2014: 4474–4475. [Google Scholar]
- 45. United States Pharmacopeia. Official NF Monographs: Diethylene glycol monoethyl ether: Assay; USP 37/NF 32. Rockville, MD: U.S. Pharmacopeial Convention; 2014: 5967. [Google Scholar]
- 46. Moran MJ, Hamilton PA, Zogorski JS. Volatile organic compounds in the Nation’s ground water and drinking-water supply wells-A Summary. U.S. Geological Survey Fact Sheet, 6 2006. http://water.usgs.gov/nawqa/vocs/national_assessment/. Accessed September 30, 2014.
- 47. Environmental Protection Agency (EPA). Method 8260B/US, volatile organic compounds by gas chromatography/mass spectrometry (GC/MS). Revision 2 December 1996. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/8260b.pdf. Accessed September 30, 2014. [Google Scholar]
- 48. Environmental Protection Agency (EPA). Method 5030B/US, purge-and-trap for aqueous samples. Revision 2 December 1996. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/5030b.pdf. Accessed September 30, 2014. [Google Scholar]
- 49. Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]