Abstract
Introduction:
While nicotine has been established as the primary addictive drug that promotes tobacco use, recent peer-reviewed studies suggest that tobacco smoke contains additional chemical constituents that may have addictive potential. Additional research is necessary to determine the addictive potential of these tobacco constituents individually and in combination with tobacco smoke condensate; however, the behaviorally effective constituent doses necessary to conduct such studies are unclear. The primary objective of this study was to conduct behavioral studies in adult rats to determine the relevant behaviorally effective doses of the tobacco constituents, cotinine, myosmine, and anatabine to be used in future studies assessing the addictive potential of these compounds.
Methods:
Separate groups of adult male Sprague Dawley rats were treated with vehicle, nicotine, or various doses of cotinine, mysomine, or anatabine. Effects on locomotor activity were measured in 10-min bins for 60min.
Results:
Nicotine (0.8mg/kg) produced a biphasic effect on locomotor activity, with hypoactivity during the first 10min and hyperactivity at 40–50min. In contrast, cotinine (0.1mg/kg) and myosmine (10–50mg/kg) decreased activity without a later increase. Anatabine significantly increased locomotor activity at 1mg/kg, but decreased it at 10mg/kg. Prominent effects on overt behavior were observed at anatabine doses of 10mg/kg and above.
Conclusion:
Nicotine, cotinine, myosmine, and anatabine produced distinct time- and dose-dependent patterns of effects on locomotor activity. Results from the study will aid in the selection of relevant doses for future studies assessing the addictive potential of these non-nicotine tobacco constituents.
Introduction
Tobacco use continues to be the leading preventable cause of premature deaths in the United States, with over 400,000 deaths per year attributed to cigarette smoking.1 While nicotine is the primary tobacco constituent responsible for addiction, many of the other over 8,400 identified tobacco constituents,2 including alkaloids, flavor additives, β-carbolines, and monoamine oxidase inhibitors, may also affect use and dependence.3 Given the sparse knowledge about the in vivo pharmacology of most of these constituents, the objective of this study was to examine the effects on locomotor activity of anatabine and myosmine (two alkaloids that are present in larger quantities in tobacco products, including e-cigarettes; Etter, Zäther, and Svensson4; Jacob et al.5), cotinine (common tobacco constituent and a metabolite of nicotine; Jacob et al.5) and nicotine (for comparison purposes). Nicotine doses (0.1–0.8mg/kg, subcutaneous [s.c.]) were chosen based upon literature review, which showed that acute doses within this range affected locomotor activity, produced discriminative stimulus effects, and increased dopamine overflow in brain reward areas (for review, see Matta et al.6). Because of the exploratory nature of this study, a broad range of doses was chosen for each non-nicotine alkaloid.
Methods
Adult male Sprague-Dawley rats (250–350g) [Harlan Laboratories] were pair-housed in polycarbonate cages. The studies were carried out in accordance with regulatory guidelines,7 and were approved by the appropriate IACUCs.
(−)-Cotinine, myosmine, and (−)-nicotine hydrogen tartrate salt (Sigma-Aldrich) were dissolved in physiological saline. Anatabine (Matrix Scientific) was mixed in a vehicle of 7.8% Polysorbate 80 (Spectrum Laboratory Products) and 92.2% saline. Doses of all compounds are expressed as mg/kg of the base and were injected s.c. at a volume of 1ml/kg.
Locomotor activity was assessed in clear Plexiglas activity chambers (47cm × 25.5cm × 22cm). Each chamber was surrounded by two arrays of 4×8 photocell infrared beams, interfaced with software for automated data collection (San Diego Instruments). Streaming video allowed the technician to observe the rats during the session.
Rats (n = 10/group) were randomly assigned to receive a single dose (or vehicle) of one of the four test compounds: nicotine (0.1–0.8mg/kg), cotinine (0.1–50mg/kg), myosmine (0.1–50mg/kg) or anatabine (0.1–25mg/kg). On test day, each rat was weighed and placed individually into an activity chamber for a 30-min habituation session. Within 5min after habituation, rats were removed from the chambers and injected with their assigned dose or vehicle and immediately placed into the chambers for a 60-min test session. Number of photocell beam breaks was tallied in 10-min bins.
Data for the four vehicle groups were averaged for the purposes of data analysis and to facilitate comparison among compounds. Binned data on total counts were analyzed via a separate mixed model (bin X dose) ANOVA for each compound. When ANOVA revealed significant effects, Tukey-Kramer post hoc tests were used to compare the relevant individual means. Significance level was set at α = 0.05.
Results
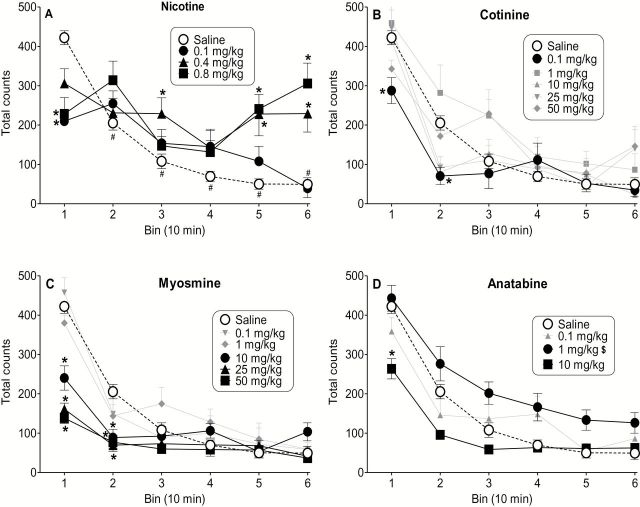
Figure 1 shows total activity counts during 10-min bins for nicotine (Figure 1A), cotinine (Figure 1B), myosmine (Figure 1C), and anatabine (Figure 1D). In the vehicle group, rats showed initially high levels of activity during the first 10-min bin followed by rapid habituation over the course of the session [Figure 1A; F(15,330) = 11.96, p < .05], with significant reductions (compared to bin 1) during all subsequent bins. Nicotine’s effect on activity was biphasic, with decreased activity (0.1 and 0.8mg/kg) during the first 10-min bin and increased activity (0.4 and 0.8mg/kg) during the last 20min of the session [Figure 1A; F(15,330) = 11.96, p < .05]. In contrast, cotinine (0.1mg/kg; Figure 1B) and myosmine (10–50mg/kg; Figure 1C) produced only decreases in activity early in the session [F(25,420) = 3.19, p < .05 and F(25,420) = 7.84, p < .05, respectively]. Decreased activity at the 10–50mg/kg myosmine doses was accompanied by overt signs often associated with malaise, including piloerection, ptosis, and diarrhea. Anatabine also produced prominent effects on overt behavior at higher doses. The 10mg/kg dose of anatabine produced flattened body posture, ptosis, and intermittent tremors approximately 20–25min post-injection. In addition, binned data revealed that the 10mg/kg dose produced significant reductions in total activity during the first 10min of the session [Figure 1D; interaction F(15,330) = 2.50, p < .05] whereas the 1mg/kg dose produced an overall increase in activity across the session [dose main effect F(3,66) = 6.72, p < .05]. At 25mg/kg anatabine, multiple full body seizures were observed 10min after injection in at least 4 of the 10 rats and in all rats by 25–30min post-injection.
Figure 1.
Effects of nicotine (panel A), cotinine (panel B), myosmine (panel C), and anatabine (panel D) on total locomotor activity in rats presented in 10-min bins. Each point represents the mean (± SEM) of 10 rats. Vehicle and doses that significantly affected activity during one or more bins are highlighted by larger and darker symbols. * denotes p < .05 for dose X bin interaction and follow-up Tukey post-hoc tests. $ indicates p < .05 for main effect of anatabine dose and follow-up Tukey post-hoc tests. These comparisons are to vehicle (unfilled circles). In panel A, # signifies p < .05 for dose X bin interaction and follow-up Tukey tests, compared to bin 1 (i.e., habituation). To emphasize dose differences, habituation of the vehicle group is shown only for nicotine, but occurred for all compounds.
Discussion
Despite prior exposure to the locomotor chambers, inspection of the results for the vehicle groups shows that intrasession habituation (i.e., decreased activity across time) occurred during test sessions for each dose, suggesting that 30min habituation may not have been long enough for full habituation to develop and/or that the injection process may have temporarily stimulated activity (e.g., due to stress). Effects of the compound are super-imposed upon this changing baseline and must be interpreted with respect to changes across bin, particularly since statistical comparisons refer to differences from the vehicle control. Hence, one way that a compound may have affected locomotor behavior was to disrupt the process of intrasession habituation.
The results of this study revealed that nicotine and each of three tobacco constituents affected locomotor activity at specific doses and time periods, with behavioral profiles differing among the compounds. Analysis of the binned data for nicotine revealed a biphasic pattern of activity changes over time. Hypoactivity was prominent early in the session, which is consistent with previous studies where the initial effect of nicotine was to decrease activity in rodents.8,9 Hyperactivity was observed at 0.4–0.8mg/kg nicotine during the last 20min of the session. Although nicotine-induced hyperactivity also has been reported, it most notably occurs in habituated rodents,10 providing further support for the idea that rats in the present study were not fully habituated. In the present study, hyperactivity was observed at the 0.4 and 0.8mg/kg doses of nicotine during the last 20min of the session. Interestingly, this hyperactivity was most prominently related to an increase in fine movements rather than increased ambulation (data not shown). These results suggest that the increased activity of rats that received higher doses of nicotine consisted of self-directed behavior (e.g., grooming), a pattern that is consistent with the concomitant observation of slight tremors and minor ataxia in this dose group. These results also suggest that the rats are starting to recover from the sedative effects observed early in the study. A final characteristic of nicotine-induced activity pattern is that habituation that was observed in the vehicle group was slower or absent in the rats that received nicotine, an effect that seemed only partly related to the lower baseline activity at bin 1 in the latter groups. Of the four alkaloids tested herein, nicotine was the only one to alter habituation in this manner. O’Neill and colleagues10 reported a similar attenuation of habituation with nicotine in rats unfamiliar with the test environment.
Unlike nicotine’s biphasic effect on activity over time and dose, the predominant effects of cotinine and myosmine on activity were depressive and occurred during the first 20min of the session, albeit habituation-induced floor effects may have prevented observation of suppression during later bins. Much of the previous research has focused on cotinine as an indicator of nicotine use and/or metabolism,11–13 although scattered exceptions have appeared in the empirical literature (e.g., for reviews, see Hoffman and Evans3; Crooks and Dwoskin14). Previous in vivo research with myosmine is notably absent, with the exception of a couple of recent studies. Clemens et al.15 reported that low intravenous doses of cotinine (0.09 µg/kg) and myosmine (0.09 µg/kg), alone and as part of an alkaloid cocktail, enhanced nicotine-induced stimulation of locomotor activity in rats. Neither compound was tested in the absence of nicotine. In an earlier study, cotinine substituted for nicotine in a nicotine discrimination paradigm in rats, but only at a high dose (100mg/kg), at which the compound may have contained nicotine as an impurity.16 A similar caution was applied to a study from the same lab, in which high doses of cotinine elicited responding on the nicotine-associated lever in squirrel monkeys trained to discriminate nicotine from vehicle.17 Myosmine itself has not been evaluated for self-administration; however, myosmine doses of 0.02–2mg/kg did not affect nicotine self-administration.18 The present results demonstrate that, while the effects of cotinine and myosmine on locomotor activity are more similar to each other than to those of nicotine, the pharmacological profiles also differ, in that higher doses of myosmine (10–50mg/kg) produced signs of malaise (e.g., flattened body posture, ptosis) whereas cotinine at doses up to 50mg/kg did not alter overt behavior.
Of the four compounds, anatabine produced the most prominent effects on overt behavior. The 10mg/kg anatabine dose produced flattened body posture, ptosis, and intermittent tremors, and 25mg/kg produced severe seizures in all rats. These results are consistent with those of a recent study in which intraperitoneal injection of similarly high doses of anatabine produced severe tremors and death in mice.19 At a non-toxic dose of 1mg/kg, anatabine increased activity across the entire session; however, the 10mg/kg dose produced significant reductions in activity during the first 10min of the session. When combined with the observations of overt behavior, the locomotor data suggest that behaviorally effective doses of anatabine may be relatively close to doses with acute toxicity (i.e., a narrow “therapeutic” index). Although previous in vivo studies with anatabine are relatively scarce, the compound has received considerable research attention recently. Similar to cotinine and myosmine, anatabine (0.09 µg/kg, intravenous [i.v.]) enhanced nicotine-induced stimulation of locomotor activity in rats and acted synergistically with other tobacco plant alkaloids to increase activity.15 In mice trained to discriminate nicotine from vehicle, anatabine substituted for nicotine, but also partially attenuated nicotine’s discriminative stimulus effects,19 an effect attributed to its ability to serve as a full agonist at α4β2 nicotinic acetylcholine receptors.19 In both rats and rhesus monkeys, anatabine attenuated the reinforcing effects of nicotine in i.v. self-administration procedures, but was not reinforcing itself.18–20
In summary, nicotine, cotinine, myosmine and anatabine produced distinctive patterns of effects on locomotor activity that were time- and dose-dependent. Nicotine and cotinine proved to be the most potent constituents (with lowest effective doses of 0.1mg/kg) whereas the lowest effective doses of anatabine and myosmine were 1mg/kg and 10mg/kg, respectively. Since concentrations of these minor alkaloids in tobacco products are often at least 10-fold less than nicotine concentrations,4,5 the doses used herein may be much higher than exposure doses for human tobacco users. Nevertheless, several issues need to be investigated before concluding that these alkaloids are unlikely to produce behavioral disruption in humans. These issues include pharmacokinetics, possible synergistic effects with nicotine, and delineation of any species differences. Further research is needed to address these issues, particularly given the sparse extant research on these constituents coupled with widespread human exposure.
Funding
Research supported by a contract awarded to RTI by the Food and Drug Administration (Center for Tobacco Products).
Declaration of Interests
None declared.
Acknowledgments
The authors thank K. Antonazzo for excellent technical assistance.
References
- 1. Centers for Disease Control. Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2. Rodgman A, Perfetti TA. The chemical constituents of tobacco and tobacco smoke. New York: CRC Press; 2008. [Google Scholar]
- 3. Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. [DOI] [PubMed] [Google Scholar]
- 4. Etter JF, Zäther E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. [DOI] [PubMed] [Google Scholar]
- 5. Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007;190:269–319. [DOI] [PubMed] [Google Scholar]
- 7. National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 8. Damaj MI, Martin BR. Is the dopaminergic system involved in the central effects of nicotine in mice? Psychopharmacology (Berl). 1993;111:106–108. [DOI] [PubMed] [Google Scholar]
- 9. Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl). 1999;145:442–451. [DOI] [PubMed] [Google Scholar]
- 10. O’Neill MF, Dourish CT, Iversen SD. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology (Berl). 1991;104:343–350. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham CS, Javors MA, McMahon LR. Pharmacologic characterization of a nicotine-discriminative stimulus in rhesus monkeys. J Pharmacol Exp Ther. 2012;341:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pehrson AL, Philibin SD, Gross D, et al. The effects of acute and repeated nicotine doses on spontaneous activity in male and female Sprague Dawley rats: analysis of brain area epibatidine binding and cotinine levels. Pharmacol Biochem Behav. 2008;89:424–431. [DOI] [PubMed] [Google Scholar]
- 13. Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl). 1999;143:318–321. [DOI] [PubMed] [Google Scholar]
- 14. Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. [DOI] [PubMed] [Google Scholar]
- 15. Clemens KJ, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12:1355–1366. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl). 1989;97:295–302. [DOI] [PubMed] [Google Scholar]
- 17. Takada K, Swedberg MD, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous l-nicotine and nicotine analogs or metabolites in squirrel monkeys. Psychopharmacology (Berl). 1989;99:208–212. [DOI] [PubMed] [Google Scholar]
- 18. Hall BJ, Wells C, Allenby C, et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav. 2014;120:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol. 2014;22:9–22. [DOI] [PubMed] [Google Scholar]
- 20. Mello NK, Fivel PA, Kohut SJ, Caine SB. Anatabine significantly decreases nicotine self-administration. Exp Clin Psychopharmacol. 2014;22:1–8. [DOI] [PubMed] [Google Scholar]