Abstract
Changes in maternal innate immunity during healthy human pregnancy are not well understood. Whether basal immune status in vivo is largely unaffected by pregnancy, is constitutively biased towards an inflammatory phenotype (transiently enhancing host defense) or exhibits anti-inflammatory bias (reducing potential responsiveness to the fetus) is unclear. Here, in a longitudinal study of healthy women who gave birth to healthy infants following uncomplicated pregnancies within the Canadian Healthy Infant Longitudinal Development (CHILD) cohort, we test the hypothesis that a progressively altered bias in resting innate immune status develops. Women were examined during pregnancy and again, one and/or three years postpartum. Most pro-inflammatory cytokine expression, including CCL2, CXCL10, IL-18 and TNFα, was reduced in vivo during pregnancy (20–57%, p<0.0001). Anti-inflammatory biomarkers (sTNF-RI, sTNF-RII, and IL-1Ra) were elevated by ~50–100% (p<0.0001). Systemic IL-10 levels were unaltered during vs. post-pregnancy. Kinetic studies demonstrate that while decreased pro-inflammatory biomarker expression (CCL2, CXCL10, IL-18, and TNFα) was constant, anti-inflammatory expression increased progressively with increasing gestational age (p<0.0001). We conclude that healthy resting maternal immune status is characterized by an increasingly pronounced bias towards a systemic anti-inflammatory innate phenotype during the last two trimesters of pregnancy. This is resolved by one year postpartum in the absence of repeat pregnancy. The findings provide enhanced understanding of immunological changes that occur in vivo during healthy human pregnancy.
Introduction
For healthy pregnancy to proceed to term, changes need to occur to prevent immune mediated rejection of the semi-allogenic fetus. At the same time, the immune system must maintain, or enhance, protection of mother and fetus from external pathogens. There is extensive literature concerning immunity at the maternal-fetal interface and its role in progression of fetal development [1–5]. Similarly, many studies have examined pathologic conditions that can arise during pregnancy (i.e. preeclampsia, infection, hypoxia), often with small, cross-sectional, healthy control groups for comparison.
Surprisingly, healthy pregnancy that leads to healthy infants has not been a major research focus and is not well understood. Publications [6] and recent NIH workshops [7, 8] identify the need for better insight into the biology of normal pregnancy. Attention needs to be given to (i) understanding maternal adaptations and (ii) creating a biological definition of an optimal pregnancy phenotype from fetal, maternal and paternal standpoints. Understanding putative changes in women’s in vivo innate immune status during normal pregnancy–the healthy phenotype–will strengthen efforts to understand linkages between in vivo maternal status and the subsequent development of healthy vs. chronic inflammatory phenotypes such as asthma or autoimmunity in children, or their mothers, later in life [9–13].
The changes, if any, that occur in innate immune status during a healthy pregnancy are controversial. Existing evidence supports several mutually exclusive conclusions. Some data are consistent with the concept that basal maternal systemic immunity exhibits a mild bias towards inflammatory phenotypes (hence, transiently enhancing host defense). Others support the notion that immunosuppressed phenotypes (reducing potential responses to the fetus) are normally dominant during pregnancy [14]. A third school of thought argues that innate immune function is largely unchanged in pregnant and non-pregnant women [3, 15].
Interest in healthy pregnancy is driven by at least three other rationales. Exclusion of pregnant women from clinical research, while well intentioned, can be counterproductive [7]. Their inclusion requires better understanding of maternal health norms during healthy pregnancy. Secondly, identifying and understanding differences in basal innate immune status in vivo during healthy pregnancy will provide better understanding of mechanisms that underlie difficult pregnancies [15]. Finally, with extensive efforts to link systemic innate immunity in vivo and clinical outcomes later in life for both mother and fetus [16, 17], we need to better define immunity in healthy human pregnancy as the entry point to childhood.
The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort was initiated to study the development of allergy and asthma, with a strong focus on clinical, immunologic and environmental assessments of infants and parents. The 3,624 participating infants and families are predominantly from urban centres; over 80% of the Canadian population is urban. The cohort is multicultural and ethnically varied. Its value is enhanced by extensive phenotyping of both children and parents, characterization of their environments and an extensive repository of biological samples [18].
Here we test the hypothesis that resting systemic pro-/anti-inflammatory bias in vivo is transiently shifted during pregnancy. In this longitudinal study of 251 randomly selected healthy women who gave birth to healthy infants, pairwise comparisons were used to assess innate immune biomarker levels in vivo during the second/third trimester then again at one and three years postpartum. An extensive panel of pro-inflammatory cytokines that are constitutively present in most healthy individuals (CCL2, CXCL10, CXCL8, IL-18, IL-6, and TNFα) was examined. While studies of inflammatory processes often include few or no anti-inflammatory regulators, endogenous levels of a broad panel of anti-inflammatory cytokines (IL-10, IL-1Ra, sTNF-RI, and sTNF-RII) were incorporated to provide a better immune signature of women’s health during successful pregnancy. The data reveal that extensive changes occur in vivo. Healthy pregnancy is linked to development of a constitutive systemic anti-inflammatory maternal bias that becomes increasingly intense with increasing gestational age, then self-resolves by one year postpartum.
Materials and methods
Participants
The Canadian Healthy Infant Longitudinal Development (CHILD) Study is a prospective national population-based longitudinal birth cohort of 3,624 neonates (http://www.canadianchildstudy.ca/knowledge.html) [18]. Following written, informed consent, non-fasting venous blood was obtained at the University of Manitoba recruitment site to yield plasma samples from 499 women. Median age at recruitment was 31.1 years. Women were recruited between conception and delivery, as possible, with most recruitment taking place during the second and third trimester. Inclusion criteria included uneventful pregnancy without documented concerns about hypertension, proteinuria or gestational diabetes that resulted in a healthy singleton baby. Exclusion criteria included in vitro fertilization (IVF), twins, miscarriage, intrauterine growth restriction (IUGR), clinically discernible upper respiratory tract (URT) or gastrointestinal (GI) infection within a week of study visit or repeat pregnancy evident at or within two months following one year and three year postpartum visits. This study was approved by the University of Manitoba Human Research Ethics Board.
To increase power, rather than incorporating unrelated non-pregnant female controls, a longitudinal study design was used where a woman’s status during pregnancy was compared with her own at subsequent times. Samples were obtained once at initial recruitment (a visit between gestational weeks 10–38) and again one year postpartum. Major clinical characteristics of the women studied are provided in Table 1.
Table 1. Demographic and clinical characteristics of study population.
| No. of Women in Study | 251 |
| Maternal Age at Delivery (years) | 31.1 (18.4–43.2) |
| Gestational Age at PN Visit (weeks) | 27.0 (9.9–38.4) |
| Gestational Age at Delivery (weeks) | 38.0 (35.0–42.1) |
| Maternal Tobacco Use | 5% |
| Preeclampsia | 0% (excluded) |
| Gestational Diabetes Mellitus (GDM) | 0% (excluded) |
Values are presented as median (range) or as a percentage of the total population.
251 of the mothers for whom paired plasma samples were available from prenatal (PN) and one year post-partum (1YPP) time points (n = 331) were randomly selected for analysis of in vivo pro- and anti-inflammatory cytokine expression (CCL2 and sTNF-RI). These biomarkers were chosen because published, and our own preliminary data, demonstrated that readily quantified levels are evident in >95% of healthy individuals (cf. IL-6 or IL-10 where a substantial proportion of healthy individuals exhibit sub pg/ml plasma levels). For more extensive analyses, 8 additional in vivo biomarkers were determined using approximately every second individual (n = 120) from the original study group. Investigators were blind to any immunological data at the time of randomization and sample selection.
Among women who provided plasma samples at both one and three years post-partum, (and who did not experience pregnancy in the interim) 32 were randomly selected to further compare potential changes in selected plasma biomarker levels post-pregnancy (see Results).
Immunological assays
Samples were processed as described [19]. Briefly, whole blood samples were kept at room temperature during transport and prior to processing later that day. Whole blood plasma was collected from 10 mL sodium heparin Vacutainer tubes (BD, Mississauga, Canada) by centrifugation at 500g for 10 minutes, aliquoted and stored at -20°C. Immediately prior to use in immunological assays, thoroughly mixed plasma was subjected to a quick spin (500g, 1 minute) to remove protein/lipid precipitate. Samples for pregnant and one year postpartum, or one and three years postpartum, were analyzed as pairs on the same assay plates.
Meso Scale Discovery (MSD, Rockville, Maryland) singleplex assays were used to quantify analyte concentrations following the manufacturer`s instructions. Catalog numbers for the reagent kits used are provided at Table 2.
Table 2. Catalog numbers for Meso Scale Discovery kits used in this publication.
| Cytokine | MSD Catalog Number |
|---|---|
| CCL2 | K151AYB-2 |
| CXCL8 | K151ANB-2 |
| CXCL10 | K151NVD-2 |
| IL-6 | K151AKB-2 |
| IL-10 | K151AOB-2 |
| IL-18 | K151MCD-2 |
| IL-1Ra | N45ZA-1 |
| sTNF-RI | K151BIC-2 |
| sTNF-RII | K151BJB-2 |
| TNFα | K151BHB-2 |
For all assays, concentrations were determined based on standard curves created using serial dilutions of fresh aliquots of constant recombinant lab standards prepared in culture medium and stored at -80°C in individual 400 uL aliquots (Cedarlane, Burlington, Canada; PeproTech, Quebec, Canada; R&D Systems, Minneapolis, Minnesota). Longitudinal sample sets from each individual were always paired on the same assay plates. Median intra-assay variation was typically below 5%.
Statistics
Group results are presented as medians, with each point representing the average (mean) value obtained from duplicate or triplicate analyses of an individual woman’s plasma at that timepoint. Supporting information S1 File contains the raw duplicates or triplicates used to calculate the mean values for each cytokine or chemokine, for each woman, at each visit, under each condition examined, that were then used to create the figures presented. Data for each population were analyzed using GraphPad Prism (La Jolla, California). Pairwise comparisons (Wilcoxon Matched Pairs/Signed Rank tests) were used for most data sets. The Spearman rank order correlation coefficient (non-parametric) was used to assess potential associations between plasma biomarker levels and increasing gestational age. Differences were considered significant at the 95% confidence level (two-tailed p<0.05).
Results
Alterations in basal innate immune balance are characteristic of healthy human pregnancy
This longitudinal study aimed to determine whether the constitutive immune status of healthy pregnant women exhibits a pro- or anti-inflammatory bias and whether it changes during pregnancy. As described in detail at Material and Methods, plasma samples were obtained at a single time point during pregnancy, again one year postpartum, and for a randomly obtained subset of individuals who did not experience further pregnancies in the interim, at three years postpartum. Two representative biomarkers of pro- and anti-inflammatory responses were assessed initially (Fig 1). Pro-inflammatory chemokine CCL2 was readily detectible in all individuals. Among the 251 women examined, systemic CCL2 was found to be reduced by 40% during pregnancy compared to levels one year postpartum (p<0.0001, Wilcoxon matched pairs, medians 110 vs. 154 pg/mL). Conversely, sTNF-RI was 38% elevated during pregnancy (medians 1,266 vs. 913, p<0.0001). Thus, the median ratio of these anti-inflammatory:pro-inflammatory innate biomarkers (sTNF-RI:CCL2) changed from 11.4 during pregnancy to 5.9 a year following delivery. This suggests that constitutive maternal status may incline towards an anti-inflammatory balance over the course of pregnancy.
Fig 1. Among healthy women, a systemic bias towards a resting anti-inflammatory phenotype is evident in vivo during pregnancy.
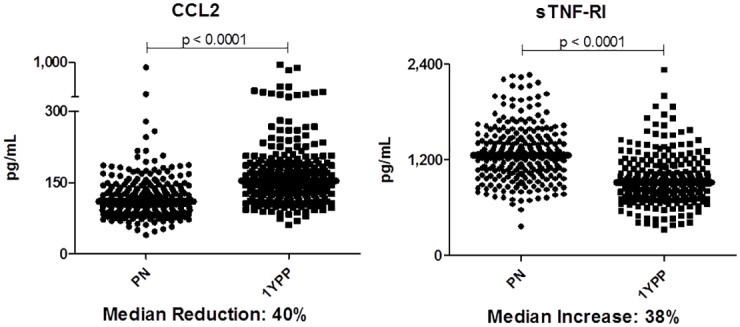
Paired plasma samples from 251 women are assessed during and one year following healthy pregnancy. Data are presented as pg/mL. Bars represent median values of each data set. Wilcoxon paired analyses are shown.
Given the large number of pro- and anti-inflammatory cytokines and inhibitors involved in human immune regulation, this analysis was extended in approximately every second woman (n = 120) to a panel of five additional pro-inflammatory biomarkers (CXCL10, TNFα, IL-18, IL-6 and CXCL8) as well as three more anti-inflammatory biomarkers (IL-10, sTNF-RII and IL-1Ra). The cytokines selected were chosen on the basis of being (i) routinely present in the plasma of healthy humans and (ii) having been extensively implicated in immune regulation of inflammation in murine and human studies. These large panels of biomarkers were used in preference to relying upon a single biomarker (CRP or IL-6 or IL-10, as is commonly done) in order to strengthen the capacity to draw conclusions about pro- vs. anti- inflammatory bias in vivo during pregnancy. A brief overview of the major cellular sources and biological functions of the cytokines, chemokines and receptors studied is provided in Table 3.
Table 3. Cytokine, chemokine and soluble receptor biomarkers examined in this publication.
| Cytokine | Major Sources | Major Functions | Major role as a Pro- or Anti-Inflammatory immune response modifier |
|---|---|---|---|
| CCL2 (MCP-1) |
Macrophages, monocytes, endothelial cells, fibroblasts, epithelial cells, smooth muscle cells, mesangial cells, astrocytic cells, monocytic and microglial cells [20]. | CCL2 recruits monocytes, memory T cells, and dendritic cells to sites of inflammation [21–23]. It has been Implicated in pathogenesis or exacerbation of many inflammatory diseases including asthma, atherosclerosis, gestational diabetes mellitus, hypertension and rheumatoid arthritis [24, 25]. | Pro-Inflammatory |
| CXCL8 (IL-8) |
Macrophages, monocytes, epithelial cells, airway smooth muscle cells and endothelial cells [26, 27]. | CXCL8 is an inflammatory chemokine involved in neutrophil (and other granulocyte) recruitment/chemotaxis towards sites of infection and subsequent phagocytosis/ degranulation [27]. It is also a potent promoter of angiogenesis [28–30]. | Pro-Inflammatory |
| CXCL10 (IP-10) |
Macrophages, monocytes, endothelial cells and fibroblasts [31]. | CXCL10 is involved in chemoattraction for monocytes/ macrophages, T cells, NK cells, and dendritic cells [32, 33]. It is also involved in the promotion of T cell adhesion to endothelial cells, enhances Th1 activity and has antitumor activity [32–35]. | Pro-Inflammatory |
| IL-6 | Macrophages, monocytes, T cells, endothelial cells, placental cells and adipocytes [20, 35, 36]. | In response to infection (e.g. viruses, bacteria) or trauma (e.g. surgery, burns, tissue damage), IL-6 is secreted and stimulates inflammation [37]. It is an important mediator of fever, the acute phase response, neutrophil production in bone marrow, supports B cell growth and is antagonistic to regulatory T cells [35]. Clinically, it stimulates inflammatory processes in host defense and many diseases including diabetes [38], atherosclerosis [39], depression [40], Alzheimer’s [41], systemic lupus erythematosus [42], multiple myeloma [43], prostate cancer [44], and rheumatoid arthritis [45]. | Pro-Inflammatory |
| IL-10 | Macrophages, monocytes, T cells, B cells, NK cells, mastocytes and adipocytes [20, 46, 47]. | IL-10 inhibits T cell derived cytokine production, MHC class II and co-stimulatory molecule expression, and enhances B cell survival, proliferation, and antibody production [48–52]. It inhibits TLR mediated induction of multiple pro-inflammatory cytokines including TNFα, IL-1β, IL-12, and IFNγ [46, 47]. | Anti-Inflammatory |
| IL-18 | Macrophages, monocytes, dendritic cells, endothelial cells, keratinocytes and intestinal epithelial cells [53, 54]. | IL-18 induces cell-mediated immunity following infection with microbial products (e.g. LPS) [53]. IL-18 stimulation of NK and T cells stimulates IFNγ release, activating macrophages and other cells [55–57]. | Pro-Inflammatory |
| IL-1Ra | Macrophages, monocytes, neutrophils, mast cells, epithelial cells and adipocytes [46, 54, 58–60]. | IL-1Ra inhibits the activities of pro-inflammatory cytokines IL-1α and IL-1β, modulating IL-1 related immune and inflammatory responses [58, 61–63]. | Anti-Inflammatory |
| sTNF-RI | Macrophages, monocytes, T cells, B cells, NK cells, dendritic cells, endothelial cells and epithelial cells [46, 54, 64]. | sTNF-RI is generated by the shedding of its membrane-expressed counterpart (TNF-RI) [65]. Thus, sTNF-RI has inherent anti-inflammatory activity, as it competes with membrane-associated receptors for the binding of free cytokines, confining the pro-inflammatory cytokine activity to the local site of inflammation [65, 66]. | Anti-Inflammatory |
| sTNF-RII | Macrophages, monocytes, T cells, B cells, NK cells, dendritic cells, endothelial cells and epithelial cells [46, 54, 64]. | sTNF-RII is generated via shedding of a membrane-expressed counterpart (TNF-RII) [65]. As for sTNF-RI, it competes with membrane-associated signaling receptors for the TNF binding, confining the pro-inflammatory cytokine activity to the local site of inflammation [65, 66], it has inherent anti-inflammatory activity. | Anti-Inflammatory |
| TNFα | Macrophages, monocytes, T cells, NK cells, neutrophils, mast cells, eosinophils and neurons [20, 67]. | TNFα is a major player in chronic and acute inflammation [67, 68]. It also inhibits tumorigenesis and viral replication [67]. TNFα dysregulation has been implicated in a broad variety of human inflammatory diseases [68, 69]. | Pro-Inflammatory |
Fig 2 demonstrates that during healthy pregnancy, most pro-inflammatory cytokines were indeed reduced relative to levels in the same women one year postpartum. Thus, constitutive CXCL10, TNFα and IL-18 were reduced during pregnancy. Median IL-6 trended lower by 42%, but the difference did not reach statistical significance. There was no evidence of changes in constitutive CXCL8 levels during healthy pregnancy. Note that due to the inherent immunological diversity characteristic of human populations, and to avoid compression of clearly positive but low levels of cytokine expression in some individuals, y-axis breaks are included.
Fig 2. Basal levels of multiple pro-inflammatory innate biomarkers are reduced in vivo during healthy pregnancy.
n = 120 longitudinal sample pairs. Bars represent median values of each data set. Wilcoxon paired analyses are shown.
Anti-inflammatory biomarkers (sTNF-RI, sTNF-RII, and IL-1Ra) demonstrated that unlike the reductions seen in constitutive pro-inflammatory cytokine expression, anti-inflammatory biomarker levels were elevated during pregnancy (Figs 1 and 3, p<0.0001). Interestingly, plasma IL-10, readily quantified in ~85% of the population, did not differ. Taken together, the data suggest that healthy pregnancy is characterized by transiently decreased pro-, and increased anti-inflammatory expression. This self-resolves by one year postpartum.
Fig 3. Constitutive expression of multiple anti-inflammatory biomarkers is increased during pregnancy.
n = 120 longitudinal sample pairs. Bars represent median values of each data set. Wilcoxon paired analyses are shown.
Validity of one year postpartum as a surrogate for the non-pregnant state
It is not logistically feasible to recruit women one year prior to an anticipated conception in order to assess their then basal systemic immune status. We therefore addressed the possibility that one year postpartum does not allow sufficient time for the innate immune system to return to the non-pregnant status, hence is not an appropriate comparison group. 32 randomly selected women who remained non-pregnant over the two years following the one year postpartum visit were examined. At this year three visit, plasma samples were obtained and compared (at the same time, in parallel assays) with plasma obtained from the same women at one year postpartum. One pro- and one anti-inflammatory biomarker that had exhibited differential expression during pregnancy (CCL2 and IL-1Ra) and one pro- and one anti-inflammatory biomarker that had been unchanged during pregnancy (CXCL8 and IL-10) were examined. Fig 4 demonstrates that the level of each cytokine was stable within an individual at one and three years post-pregnancy. This supports the concept that one (or three) year postpartum samples offer an appropriately stable comparator for non-pregnant status in this longitudinal study.
Fig 4. Stability of in vivo pro- and anti-inflammatory plasma biomarkers.
n = 32 longitudinal sample pairs. Wilcoxon paired analyses are shown.
Anti-inflammatory bias is evident at least as early as the second trimester
The comparisons above test the hypothesis that resting constitutive innate immune status is distinct during healthy pregnancy. To better understand the kinetics of this shift in resting immune status, the population was next stratified by trimester of recruitment. With only a small number of women recruited during the first trimester, analysis centred on the second and third trimesters. Fig 5 demonstrates basal (i.e. healthy, resting) innate immune status is skewed towards an anti-inflammatory phenotype at least as early as the second trimester.
Fig 5. Longitudinal analysis of differences in pro- and anti-inflammatory cytokines in second and third trimesters.

Independent panels of volunteers were examined during the second or third trimester and compared with levels one year postpartum. Bars represent median values of each data set. Wilcoxon paired analyses are shown.
The intensity of anti-inflammatory bias increases with increasing gestational age
Finally, to determine if the intensity of anti-inflammatory phenotypic changes are altered with the progression of pregnancy, we assessed relationships between plasma biomarker concentrations and gestational age. sTNF-RI, IL-1Ra (Fig 6) and sTNF-RII (not shown) all exhibited increasingly intense in vivo expression with increased gestational age. Conversely, the decrease in pro-inflammatory cytokine expression (Fig 6: CCL2 and IL-18; data not shown: CXCL8, CXCL10, IL-6 and TNFα) was constant and exhibited no relationship with increasing gestational age. Thus, the shift towards an increasingly anti-inflammatory bias intensified with increasing gestational age during healthy pregnancy.
Fig 6. Relationships between gestational age and intensity of anti-inflammatory or pro-inflammatory plasma biomarker expression.
Spearman regression analyses are shown.
Discussion
Although most pregnancies lead to healthy infants, prior research has focused heavily on diseases of pregnancy. Here, maternal health was examined in 251 healthy pregnant women over up to three years. The data reveal that at least as early as the second trimester, women exhibit increasingly strong constitutive expression of anti-inflammatory mediators and reduced expression of many, but not all, cytokines linked to host defense and inflammatory immune capacity. This transient anti-inflammatory phenotype intensifies with increasing gestational age and is self-resolving by one year postpartum.
Our primary focus here is maternal health and the systemic, constitutive innate immune status exhibited by women during pregnancy. This is not an investigation of factors which enable pregnancy to become established, to proceed to term, or to respond to acute viral or bacterial infection. There is a longstanding lack of agreement regarding constitutive innate immune status during a healthy pregnancy. Small cohorts of healthy pregnant women are incorporated in most studies that examine diseases of pregnancy. In those studies, cross-sectional comparisons are drawn with a broad range of difficult pregnancies, rather than explicitly using well-powered longitudinal study designs to examine the status of healthy women during vs. post-pregnancy. Not surprisingly, such studies do not provide a clear consensus. One school argues that systemic immune status in vivo, unlike immune status at the maternal/fetal interface, reflects a generalized mild inflammatory response [70–73]. Others argue for the dominance of anti-inflammatory responses [74, 75]. These apparent contradictions may be due to cross-sectional study designs, cohorts of relatively small size, the resulting impact of subject to subject variability, and reliance on a small number of biomarkers (often one) to assess innate immune bias in vivo. For example, two of the largest longitudinal studies available [76, 77] are composed of 48 and 21 healthy women respectively who are followed through pregnancy and postpartum. Many studies involve different ethnicities: Kraus included a 94% Hispanic/Black population [76], while Szarka utilized a Caucasian population [78]. As pointed out more than two decades ago [79], due to high inter-subject variability inherent to any outbred population, a longitudinal, matched study design involving a large n is logistically challenging, but highly important.
This research has important caveats. Firstly, because a longitudinal, multi-year study design was selected to achieve increased sensitivity, it was not socially or logistically feasible to recruit individuals prior to an anticipated pregnancy. For this reason, pregnant women were compared with their immune status at one year and, for a subset, three years postpartum. Pre-pregnancy immune status could not be directly determined. Secondly, a potential confounder that cannot be ruled out is the impact of specific environmental antigens and pathogens on these findings. Women with complicated pregnancies with known linkages to altered innate immunity (i.e. preeclampsia) are excluded from this study. Among the women studied, clinical assessments at each blood draw excluded women with active transient URT or GI infections. The data reported above, characterizing the constitutive in vivo phenotype of healthy normal pregnancy positions us to better assess the impact of environmental and genetic influences in pregnancy in subsequent studies. A third caveat is that the impact of menstrual stage postpartum at sampling could influence some values obtained. This underlines the need for sufficient power because the impact of such variables is increasingly reduced as the number of individuals examined increases.
A potential confounder to interpretation of the data obtained was day-to-day stability of biomarker expression within a given individual. We found this to have minimal impact because, as shown in Fig 4, and as was previously reported in short-term studies conducted in men and non-pregnant women [76, 80], systemic levels of most biomarkers are remarkably stable across time (days-weeks) in healthy individuals.
The immunological and clinical implications that result from the increasingly pronounced anti-inflammatory phenotype that developed over the course of healthy pregnancy remains speculative. Extensive literature demonstrates associations between pro-inflammatory or type 1 cytokines and recurrent spontaneous miscarriage or preterm delivery. Exogenous TNFα and IFN inhibit the outgrowth of human trophoblasts in vitro and induce apoptosis of human villous trophoblast cells. Murine studies demonstrate that administration of pro-inflammatory cytokines (i.e. TNFα, IFN, IL-2) into pregnant mice causes increased abortion. Conversely, administration of IL-10 prevents fetal wastage in abortion-prone mice [81, 82]. Thus, there is consensus that controls on excessive pro-inflammatory innate responses are essential for successful pregnancy [75].
These data may stand in contrast to in vivo expression of acute phase proteins during healthy pregnancy. Multiple studies have found increased plasma/serum CRP levels vs. non-pregnant controls [83–86]. The extent to which changes in this largely IL-6 and TNFα driven biomarker of inflammation are due to activation of classical innate immune responses or other cells (i.e. adipocytes or necrotic processes associated with placenta ageing) is under active investigation [83, 87, 88]. Interestingly, several groups have found undetectable changes or decreases in CRP over the course of healthy pregnancy [83, 89–92]. This inconsistency, and the many pregnancy independent factors (i.e. obesity) that can influence CRP levels, underlines a need for caution and continued research prior to drawing mechanistic conclusions about the role any of these mediators play in successful conclusion of pregnancy.
In this study, we do not address putative differences in maternal immune responses upon infection in vivo or acute in vitro activation. Studies examining in vitro responses to various pattern recognition receptor (PRR) ligands are currently underway as a complementary approach to understanding changes in maternal health that can occur during pregnancy.
With increasing attention given to defining what constitutes a healthy pregnancy [93], this study provides valuable insight into systemic innate immune changes that result in expression of an increasingly intense anti-inflammatory phenotype in vivo during pregnancy. It underlines the need for further characterization of what constitutes a successful environment for healthy human pregnancy.
Supporting information
(XLSX)
Acknowledgments
We are grateful to all the families who took part in this study and the entire CHILD research team. We also thank Saiful Huq for database assistance and Paul Lopez for editorial assistance. CHILD (Canadian Healthy Infant Longitudinal Development Study, headquartered at McMaster University, Hamilton, Canada) investigators include: MR Sears (Director), McMaster University; P Subbarao (co-Director), The Hospital for Sick Children; R Allen, Simon Fraser University; SS Anand, McMaster University; AB Becker, University of Manitoba; AD Befus, University of Alberta; M Brauer, University of British Columbia; JR Brook, University of Toronto; E Chen, Northwestern University, Chicago; M Cyr, McMaster University; D Daley, University of British Columbia; S Dell, Sick Children’s Hospital; JA Denburg, McMaster University; S Elliott, University of Waterloo; H Grasemann, Sick Children’s Hospital; K HayGlass, University of Manitoba; R Hegele, Sick Children’s Hospital; DL Holness, University of Toronto; WYW Lou, University of Toronto; MS Kobor, University of British Columbia; TR Kollman, University of British Columbia; AL Kozyrskyj, University of Alberta; C Laprise, Universite du Quebec a Chicoutimi; M Larche, McMaster University; J Macri, McMaster University; PM Mandhane, University of Alberta; G Miller, Northwestern University, Chicago; R Moqbel (deceased), University of Manitoba; T Moraes, Sick Children’s Hospital; PD Pare, University of British Columbia; C Ramsey, University of Manitoba; F Ratjen, Sick Children’s Hospital; A Sandford, University of British Columbia; JA Scott, University of Toronto; J Scott, University of Toronto; F Silverman, University of Toronto; T Takaro, Simon Fraser University; P Tang, University of British Columbia; S Tebbutt, University of British Columbia; T To, Sick Children’s Hospital; SE Turvey, University of British Columbia, all in Canada.
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment (AllerGen) Network of Centres of Excellence provided core funding for the CHILD Study. Support for this work has also been provided by Canada Research Chairs, Children's Hospital Research Foundation of Manitoba. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chaouat G, Tranchot Diallo J, Volumenie JL, Menu E, Gras G, Delage G, et al. Immune suppression and Th1/Th2 balance in pregnancy revisited: a (very) personal tribute to Tom Wegmann. Am J Reprod Immunol. 1997. June;37(6):427–34. [DOI] [PubMed] [Google Scholar]
- 2.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, et al. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol. 2016. February 01;196(3):1044–59. 10.4049/jimmunol.1501962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015. April;16(4):328–34. 10.1038/ni.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015. February 15;194(4):1534–44. 10.4049/jimmunol.1401536 [DOI] [PubMed] [Google Scholar]
- 5.Baylis F. Pregnant women deserve better. Nature. 2010. June 10;465(7299):689–90. 10.1038/465689a [DOI] [PubMed] [Google Scholar]
- 6.Catalano PW, MA. Wise, PH. Bianchi, DW. Saade, GR. Scientific Vision Workshop on Pregnancy and Pregnancy Outcomes. Bethesa, Maryland2011 [updated 2011; cited 2016 02/18/2016]; https://www.nichd.nih.gov/vision/vision_themes/pregnancy/Documents/Vision_Pregnancy_WP_042811.pdf.
- 7.Foulkes MA, Grady C, Spong CY, Bates A, Clayton JA. Clinical research enrolling pregnant women: a workshop summary. J Womens Health (Larchmt). 2011. October;20(10):1429–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyborne KS, R M. Reproductive Immunology. Bronson RA, N J. Anderson D. Branch DW. Kutteh WH., editor. Cambridge: Blackwell Science; 1996. [Google Scholar]
- 9.Huang E, Wells CA. The ground state of innate immune responsiveness is determined at the interface of genetic, epigenetic, and environmental influences. J Immunol. 2014. July 1;193(1):13–9. 10.4049/jimmunol.1303410 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, Lamouse-Smith ES. Maternal Antibiotic Treatment Impacts Development of the Neonatal Intestinal Microbiome and Antiviral Immunity. J Immunol. 2016. May 01;196(9):3768–79. 10.4049/jimmunol.1502322 [DOI] [PubMed] [Google Scholar]
- 11.Mendola P, Wallace M, Hwang BS, Liu D, Robledo C, Mannisto T, et al. Preterm birth and air pollution: Critical windows of exposure for women with asthma. J Allergy Clin Immunol. 2016. August;138(2):432–40 e5. 10.1016/j.jaci.2015.12.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Mutius E, Martinez FD. Inconclusive Results of Randomized Trials of Prenatal Vitamin D for Asthma Prevention in Offspring: Curbing the Enthusiasm. JAMA. 2016. January 26;315(4):347–8. 10.1001/jama.2015.18963 [DOI] [PubMed] [Google Scholar]
- 13.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, et al. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA. 2016. March 15;315(11):1129–40. 10.1001/jama.2016.1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006. October;11(5):279–95. 10.1016/j.siny.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007. March;62(3):191–201. 10.1097/01.ogx.0000256779.06275.c4 [DOI] [PubMed] [Google Scholar]
- 16.Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014. June;90(6):129 10.1095/biolreprod.113.116186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawes BL, Stokholm J, Bonnelykke K, Brix S, Bisgaard H. Neonates with reduced neonatal lung function have systemic low-grade inflammation. J Allergy Clin Immunol. 2015. June;135(6):1450–6 e1 10.1016/j.jaci.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015. October;70(10):998–1000. 10.1136/thoraxjnl-2015-207246 [DOI] [PubMed] [Google Scholar]
- 19.Moraes TJ, Lefebvre DL, Chooniedass R, Becker AB, Brook JR, Denburg J, et al. The Canadian healthy infant longitudinal development birth cohort study: biological samples and biobanking. Paediatr Perinat Epidemiol. 2015. January;29(1):84–92. 10.1111/ppe.12161 [DOI] [PubMed] [Google Scholar]
- 20.Pendeloski KP, Ono E, Torloni MR, Mattar R, Daher S. Maternal obesity and inflammatory mediators: A controversial association. Am J Reprod Immunol. 2017. March 22; [DOI] [PubMed] [Google Scholar]
- 21.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994. April 26;91(9):3652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996. September;60(3):365–71. [DOI] [PubMed] [Google Scholar]
- 23.Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol. 2016. December 13;4443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat. 2009. March;19(3):295–303. 10.1517/13543770902755129 [DOI] [PubMed] [Google Scholar]
- 25.O'Connor T, Borsig L, Heikenwalder M. CCL2-CCR2 Signaling in Disease Pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015. 15(2):105–18. [DOI] [PubMed] [Google Scholar]
- 26.Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol. 2000. July;23(1):86–94. 10.1165/ajrcmb.23.1.4014 [DOI] [PubMed] [Google Scholar]
- 27.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994. November;56(5):559–64. [PubMed] [Google Scholar]
- 28.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989. October;84(4):1045–9. 10.1172/JCI114265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016. February;16(1):45–50. 10.1097/ACI.0000000000000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan SY, Weninger W. Neutrophil migration in inflammation: intercellular signal relay and crosstalk. Curr Opin Immunol. 2016. December 09;4434–42. [DOI] [PubMed] [Google Scholar]
- 31.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985. June 20–26;315(6021):672–6. [DOI] [PubMed] [Google Scholar]
- 32.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002. April 01;168(7):3195–204. [DOI] [PubMed] [Google Scholar]
- 33.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995. July 01;182(1):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell JD, Gangur V, Simons FE, HayGlass KT. Allergic humans are hyporesponsive to a CXCR3 ligand-mediated Th1 immunity-promoting loop. FASEB J. 2004. February;18(2):329–31. 10.1096/fj.02-0908fje [DOI] [PubMed] [Google Scholar]
- 35.Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu Rev Physiol. 2017. February 10;79567–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015. May;16(5):448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 37.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997. August;176(2):439–44. [DOI] [PubMed] [Google Scholar]
- 38.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005. December;54 Suppl 2S114–24. [DOI] [PubMed] [Google Scholar]
- 39.Dubinski A, Zdrojewicz Z. [The role of interleukin-6 in development and progression of atherosclerosis]. Pol Merkur Lekarski. 2007. April;22(130):291–4. [PubMed] [Google Scholar]
- 40.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010. March 01;67(5):446–57. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 41.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010. November 15;68(10):930–41. 10.1016/j.biopsych.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 42.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004. 13(5):339–43. 10.1191/0961203304lu1023oa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gado K, Domjan G, Hegyesi H, Falus A. Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell Biol Int. 2000. 24(4):195–209. 10.1006/cbir.2000.0497 [DOI] [PubMed] [Google Scholar]
- 44.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001. March;12(1):33–40. [DOI] [PubMed] [Google Scholar]
- 45.Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006. May;18(3):277–81. 10.1097/01.bor.0000218949.19860.d1 [DOI] [PubMed] [Google Scholar]
- 46.Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012. October;13(10):925–31. 10.1038/ni.2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrysova L, Howes A, Saraiva M, O'Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014. 380157–90. [DOI] [PubMed] [Google Scholar]
- 48.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008. December;226205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004. 22929–79. [DOI] [PubMed] [Google Scholar]
- 50.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010. March;10(3):170–81. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 51.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991. November 01;174(5):1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001. 19683–765. [DOI] [PubMed] [Google Scholar]
- 53.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013. October 08;4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banchereau R, Cepika AM, Banchereau J, Pascual V. Understanding Human Autoimmunity and Autoinflammation Through Transcriptomics. Annu Rev Immunol. 2017. January 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutinen EM, Pirttila T, Anderson G, Salminen A, Ojala JO. Pro-inflammatory interleukin-18 increases Alzheimer's disease-associated amyloid-beta production in human neuron-like cells. J Neuroinflammation. 2012. August 16;9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995. November 02;378(6552):88–91. 10.1038/378088a0 [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Wang H, Xiao W, Wang C, Liu G, Hong T. Thyrocyte interleukin-18 expression is up-regulated by interferon-gamma and may contribute to thyroid destruction in Hashimoto's thyroiditis. Int J Exp Pathol. 2010. October;91(5):420–5. 10.1111/j.1365-2613.2010.00715.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006. November 27;580(27):6289–94. 10.1016/j.febslet.2006.10.061 [DOI] [PubMed] [Google Scholar]
- 59.Di Paolo NC, Shayakhmetov DM. Interleukin 1alpha and the inflammatory process. Nat Immunol. 2016. July 19;17(8):906–13. 10.1038/ni.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito Y, Kaneko N, Iwasaki T, Morikawa S, Kaneko K, Masumoto J. IL-1 as a target in inflammation. Endocr Metab Immune Disord Drug Targets. 2015. 15(3):206–11. [PubMed] [Google Scholar]
- 61.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994. December;8(15):1314–25. [PubMed] [Google Scholar]
- 62.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000. March 23;404(6776):398–402. 10.1038/35006081 [DOI] [PubMed] [Google Scholar]
- 63.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009. June 04;360(23):2426–37. 10.1056/NEJMoa0807865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willrich MA, Murray DL, Snyder MR. Tumor necrosis factor inhibitors: clinical utility in autoimmune diseases. Transl Res. 2015. February;165(2):270–82. 10.1016/j.trsl.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 65.Ware CF, Santee S, Glass A. Tumor necrosis factor-related ligands and receptors In: Thomson A, editor. The Cytokine Handbook. Third ed San Diego, CA: Academic Press; 1998. p. 549–92. [Google Scholar]
- 66.Wei M, Kuukasjarvi P, Laurikka J, Pehkonen E, Kaukinen S, Laine S, et al. Inflammatory cytokines and soluble receptors after coronary artery bypass grafting. Cytokine. 2001. August 21;15(4):223–8. 10.1006/cyto.2001.0920 [DOI] [PubMed] [Google Scholar]
- 67.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001. February 23;104(4):487–501. [DOI] [PubMed] [Google Scholar]
- 68.Baxter AE, Kaufmann DE. Tumor-necrosis factor is a master of T cell exhaustion. Nat Immunol. 2016. May;17(5):476–8. 10.1038/ni.3436 [DOI] [PubMed] [Google Scholar]
- 69.Green WD, Beck MA. Obesity altered T cell metabolism and the response to infection. Curr Opin Immunol. 2017. March 27;461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009. February;16(2):206–15. 10.1177/1933719108329095 [DOI] [PubMed] [Google Scholar]
- 71.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999. March;20(3):114–8. [DOI] [PubMed] [Google Scholar]
- 72.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998. July;179(1):80–6. [DOI] [PubMed] [Google Scholar]
- 73.Blank V, Hirsch E, Challis JR, Romero R, Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour—a workshop report. Placenta. 2008. March;29 Suppl AS102–4. [DOI] [PubMed] [Google Scholar]
- 74.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998. August 21;281(5380):1191–3. [DOI] [PubMed] [Google Scholar]
- 75.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front Immunol. 2014. 5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010. December;64(6):411–26. 10.1111/j.1600-0897.2010.00889.x [DOI] [PubMed] [Google Scholar]
- 77.Bjorkander S, Bremme K, Persson JO, van Vollenhoven RF, Sverremark-Ekstrom E, Holmlund U. Pregnancy-associated inflammatory markers are elevated in pregnant women with systemic lupus erythematosus. Cytokine. 2012. August;59(2):392–9. 10.1016/j.cyto.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 78.Szarka A, Rigo J Jr., Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010. 1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnstone FD, Thong KJ, Bird AG, Whitelaw J. Lymphocyte subpopulations in early human pregnancy. Obstet Gynecol. 1994. June;83(6):941–6. [DOI] [PubMed] [Google Scholar]
- 80.Campbell JD, Stinson MJ, Simons FE, Rector ES, HayGlass KT. In vivo stability of human chemokine and chemokine receptor expression. Hum Immunol. 2001. July;62(7):668–78. [DOI] [PubMed] [Google Scholar]
- 81.Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, et al. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995. May 01;154(9):4261–8. [PubMed] [Google Scholar]
- 82.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010. June;63(6):482–91. 10.1111/j.1600-0897.2010.00810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, Pereira-Leite L, et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005. November 01;123(1):46–51. 10.1016/j.ejogrb.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 84.Miller EM. Changes in serum immunity during pregnancy. Am J Hum Biol. 2009. May-Jun;21(3):401–3. 10.1002/ajhb.20882 [DOI] [PubMed] [Google Scholar]
- 85.Perucci LO, Carneiro FS, Ferreira CN, Sugimoto MA, Soriani FM, Martins GG, et al. Annexin A1 Is Increased in the Plasma of Preeclamptic Women. PLoS One. 2015. 10(9):e0138475 10.1371/journal.pone.0138475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McDade TW, Borja JB, Largado F, Adair LS, Kuzawa CW. Adiposity and Chronic Inflammation in Young Women Predict Inflammation during Normal Pregnancy in the Philippines. J Nutr. 2016. February;146(2):353–7. 10.3945/jn.115.224279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romem Y, Artal R. C-reactive protein in pregnancy and in the postpartum period. Am J Obstet Gynecol. 1985. February 01;151(3):380–3. [DOI] [PubMed] [Google Scholar]
- 88.Rebelo F, Schlussel MM, Vaz JS, Franco-Sena AB, Pinto TJ, Bastos FI, et al. C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status. J Hypertens. 2013. January;31(1):16–26. 10.1097/HJH.0b013e32835b0556 [DOI] [PubMed] [Google Scholar]
- 89.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014. December;70(2):134–40. 10.1016/j.cyto.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friis CM, Paasche Roland MC, Godang K, Ueland T, Tanbo T, Bollerslev J, et al. Adiposity-related inflammation: effects of pregnancy. Obesity (Silver Spring). 2013. January;21(1):E124–30. [DOI] [PubMed] [Google Scholar]
- 91.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007. March;92(3):969–75. 10.1210/jc.2006-2083 [DOI] [PubMed] [Google Scholar]
- 92.Simavli S, Derbent AU, Uysal S, Turhan NO. Hepcidin, iron status, and inflammation variables among healthy pregnant women in the Turkish population. J Matern Fetal Neonatal Med. 2014. January;27(1):75–9. 10.3109/14767058.2013.804054 [DOI] [PubMed] [Google Scholar]
- 93.NICHD. Pregnancy: For Researchers and Health Care Practitioners 2013; https://www.nichd.nih.gov/health/topics/pregnancy/resources/Pages/providers.aspx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its supporting information files.






