Abstract
Ibrutinib, a Bruton’s tyrosine kinase inhibitor is approved for relapsed/refractory and del(17p)/TP53 mutated chronic lymphocytic leukemia. Discrepancies between clinical trials and routine health-care are commonly observed in oncology. Herein we report real-world results for 95 poor prognosis Swedish patients treated with ibrutinib in a compassionate use program. Ninety-five consecutive patients (93 chronic lymphocytic leukemia, 2 small lymphocytic leukemia) were included in the study between May 2014 and May 2015. The median age was 69 years. 63% had del(17p)/TP53 mutation, 65% had Rai stage III/IV, 28% had lymphadenopathy ≥10cm. Patients received ibrutinib 420 mg once daily until progression. At a median follow-up of 10.2 months, the overall response rate was 84% (consistent among subgroups) and 77% remained progression-free. Progression-free survival and overall survival were significantly shorter in patients with del(17p)/TP53 mutation (P=0.017 and P=0.027, log-rank test); no other factor was significant in Cox proportional regression hazards model. Ibrutinib was well tolerated. Hematomas occurred in 46% of patients without any major bleeding. Seven patients had Richter’s transformation. This real-world analysis on consecutive chronic lymphocytic leukemia patients from a well-defined geographical region shows the efficacy and safety of ibrutinib to be similar to that of pivotal trials. Yet, del(17p)/TP53 mutation remains a therapeutic challenge. Since not more than half of our patients would have qualified for the pivotal ibrutinib trial (RESONATE), our study emphasizes that real-world results should be carefully considered in future with regards to new agents and new indications in chronic lymphocytic leukemia.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in adults, is characterized by a clonal expansion of CD5+ and CD23+ B-lymphocytes which accumulate in blood, bone marrow and lymphoid tissues. Chemoimmunotherapy is the standard first-line treatment, but patients with del(17p) or TP53 gene mutation have a poor prognosis and inferior clinical outcomes with such regimens.1,2 Patients who are refractory to multi-agents have a very poor prognosis.3,4
The CLL cell receives survival and proliferation signals from the microenvironment, and the B-cell receptor (BCR) is a key factor in this interaction. Brutós tyrosine kinase (BTK) is a non-receptor tyrosine kinase and plays a crucial role in BCR signalling. Ibrutinib is an oral, selective and irreversible inhibitor of BTK. It binds to the cysteine-481 amino acid of the BTK enzyme.5 Next-generation BTK inhibitors are under clinical development with promising early results.6
In the first phase 2 study of ibrutinib, 71% of patients with relapsed or refractory CLL achieved a partial response (PR) according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2008 criteria,7 and an additional 18% of patients had PR with lymphocytosis (PR-L).8 The response was independent of clinical and genomic risk factors including del(17p). The 3-year follow-up of this study reported no late occurring toxicity, and progression was uncommon even though a shorter progression-free survival (PFS) for patients with del(17p) was found.9 In a pivotal phase 3 study (RESONATE), patients with previously treated CLL were randomized between treatment with ibrutinib and ofatumumab with a significantly longer PFS and overall survival (OS) in favor of ibrutinib.10 As a consequence of these results, ibrutinib received US Food and Drug Administration approval in February 2014 for patients with CLL who received at least one prior therapy, and approval in July 2014 for all patients with del(17p). Approval by The European Medicines Agency for patients who had received at least one prior therapy, or for all patients with del(17p) or TP53 mutation was granted in October 2014. It was recently shown in a phase 3 study that ibrutinib was superior to chlorambucil in previously untreated patients with CLL, regarding PFS, OS, overall response rate (ORR), and improvement in hematologic variables.11 This might result in a broader indication for ibrutinib therapy.
However, there is often a discrepancy between data obtained from patients strictly included in clinical trials and those obtained outside trials in routine health care, or in trials conducted in a community-based setting, as shown in CLL patients with the fludarabine, cyclophosphamide and rituximab (FCR) regimen.1,12 Trials in CLL generally enrolled younger patients with fewer comorbidities than in actual clinical practice.13 Thus, knowledge about real-world results in hematology14,15 becomes increasingly important for the optimal usage of new agents in various disorders, including CLL, especially if such agents are to be used continuously until progression. We report herein real -world results for 95 consecutive Swedish patients with poor prognosis CLL who received ibrutinib treatment in a compassionate use program (CUP), outside the setting of clinical trials.
Methods
Study design and participants
This retrospective analysis was conducted at 27 Swedish hospitals that included at least one CLL patient in the CUP, which was open for inclusion between May 15, 2014 and May 31, 2015. The program offered free drug access for patients with CLL until ibrutinib was generally available on the market, after which patients were prescribed ibrutinib capsules according to normal Swedish health care regulations. All patients included in the CUP could be identified. Data was extracted from each patient’s individual medical file and entered into case record forms (CRF) by each treating physician for statistical analysis. The monitoring of data from individual patient files was performed by the academic study team and cross-checked vs. CRFs. The procedure was approved by the regional ethics committee and conducted in accordance with the Declaration of Helsinki.
Patients with CLL were eligible for the CUP if they had: a confirmed diagnosis of CLL or small lymphocytic lymphoma (SLL) according to the IWCLL criteria,7 a need for treatment, and a high-risk disease that did not respond to a chemoimmunotherapy regimen or that progressed within 24 months after completion of the regimen. Also included in the CUP were patients with del(17p) or TP53 mutation, who could be included at any time point, patients with an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2, neutrophil count ≥ 0.5×109/L, platelet count ≥ 30×109/L, serum creatinine ≤ 2 times, liver enzymes ≤ 3 times and total bilirubin ≤ 1.5 times the upper limit of normal. Key exclusion criteria were: treatment with a strong CYP3A inhibitor or warfarin, allogeneic stem cell transplantation (SCT) within the past 6 months, ongoing active infection, uncontrolled autoimmune hemolytic anemia or immune-mediated thrombocytopenia, Richter’s transformation (RT), and other active malignancies or uncontrolled cardiovascular disease.
Treatment
Patients received 420 mg oral ibrutinib once daily until progression or occurrence of unacceptable side effects. Individual dose modifications were allowed as recommended by the manufacturer and as decided by the treating physician.
Assessments
Endpoints were ORR, which included PR, PR-L and complete remission (CR) rate, as well as PFS, OS and the safety of ibrutinib.
Treatment response was classified according to the IWCLL response criteria of 2008,7 with the exception that lymphocytosis was not the sole criterion for disease progression.10 Response evaluation in patients with SLL was based on the 2007 International Working Group Criteria for non-Hodgkin lymphoma.16 Lymph node response was evaluated clinically and/or by CT scan, as decided by the physician. Evaluation of bone marrow response was done at the discretion of the physician. CR required evaluation by CT scan and bone marrow examination. Cumulative Illness Rating Scale (CIRS) was used to define comorbidities at baseline.17,18
Treatment toxicity was evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v3.0 except for anemia, thrombocytopenia and neutropenia, which were graded according to the IWCLL grading scale for hematologic toxicity in studies.7 Adverse events (AE) of grade 3 or higher were generally recorded, whereas any grade of AE was reported for hemorrhage, diarrhea, arthralgia, atrial fibrillation and blood counts.
Time to response was defined as the time from start of ibrutinib until the date of fulfilling criteria for PR-L or PR. PFS was defined as the time from start of treatment to progression, the start of new anti-cancer treatment or death from any cause. Two patients proceeded to allogeneic SCT after having responded to ibrutinib and were excluded from PFS analysis. OS was defined as the time from start of ibrutinib treatment to death or latest follow-up.
Statistical analysis
Response rates are presented with 95% confidence intervals (CI). Survival (PFS, OS) was estimated by the Kaplan-Meier method. Univariate and multivariate analyses on time to failure were estimated using the Cox proportional regression hazards model. Results are presented as hazard ratios (HR) and 95% CI. All statistics were performed with IBM SPSS, version 23.0.
Results
Patients
One hundred and one patients were enrolled between May 15, 2014 and May 31, 2015. Four patients died early or were diagnosed upfront with RT; they never received ibrutinib and were excluded from analysis. Two additional CLL patients were excluded as they were diagnosed with acute myeloid leukemia (AML) at the time of inclusion.
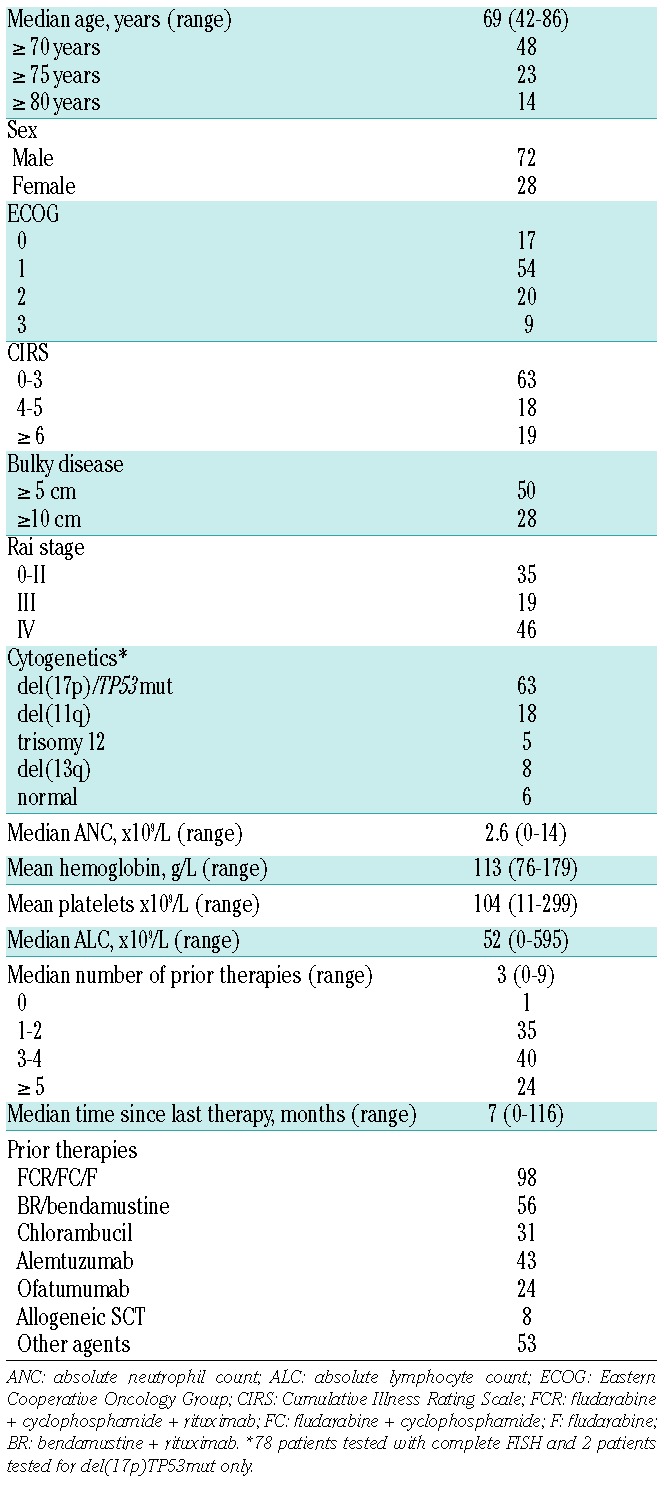
Ninety-five patients (93 with CLL, 2 with SLL) were included in the analysis. Baseline characteristics are shown in Table 1. The median age was 69 years and 23% were 75 years of age or older. Del(17p) and/or TP53 mutation tests were performed in 80 patients and 63% were positive. Del(11q) without del(17p) was present in 18% of the patients. Sixty-five per cent had Rai stage III or IV. Twenty-eight per cent had lymph nodes ≥10 cm. Twenty-nine per cent had ECOG performance status grade 2 or 3 and 19% had CIRS ≥6. A median of 3 prior therapies (range 0–9) was reported.
Table 1.
Patient characteristics (%) prior to start of ibrutinib (n=95).
Efficacy and safety
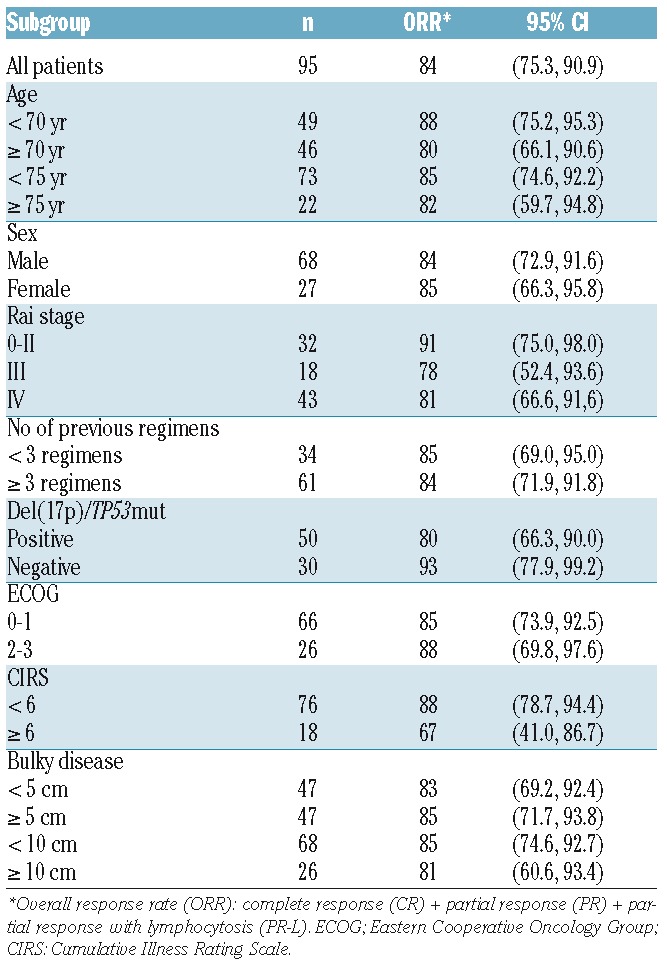
Response rates are shown in Table 2. ORR was 84% (61% PR, 20% PR-L, 3% CR). The ORR among the 42 patients who performed a CT scan as part of their response evaluation was 83%. Responses were consistent across subgroups (Table 2). All 8 patients who had a previous allogeneic SCT responded to ibrutinib.
Table 2.
Response rates (%) according to subgroup.
The median time to PR-L and PR was 1.5 (range 0.3–13.2) and 3.9 (range 0.9–10.9) months, respectively.
Hematologic recovery in patients with pre-existing cytopenias is shown in Figure 1. A gradual improvement of anemia (Figure 1A) and thrombocytopenia (Figure 1B) was observed during treatment. The mean neutrophil count showed a rapid increase early during therapy and thereafter stabilized (data not shown). The response in the bone marrow was analyzed in 26 patients who underwent a second bone marrow biopsy after a median treatment time of 9.5 months (range 2.1–16.4). A >50% reduction of CLL cells in the bone marrow was achieved in 62% of the patients (16/26, including 2 CRs). The ORR in the same 26 individuals was 88% (23/26).
Figure 1.
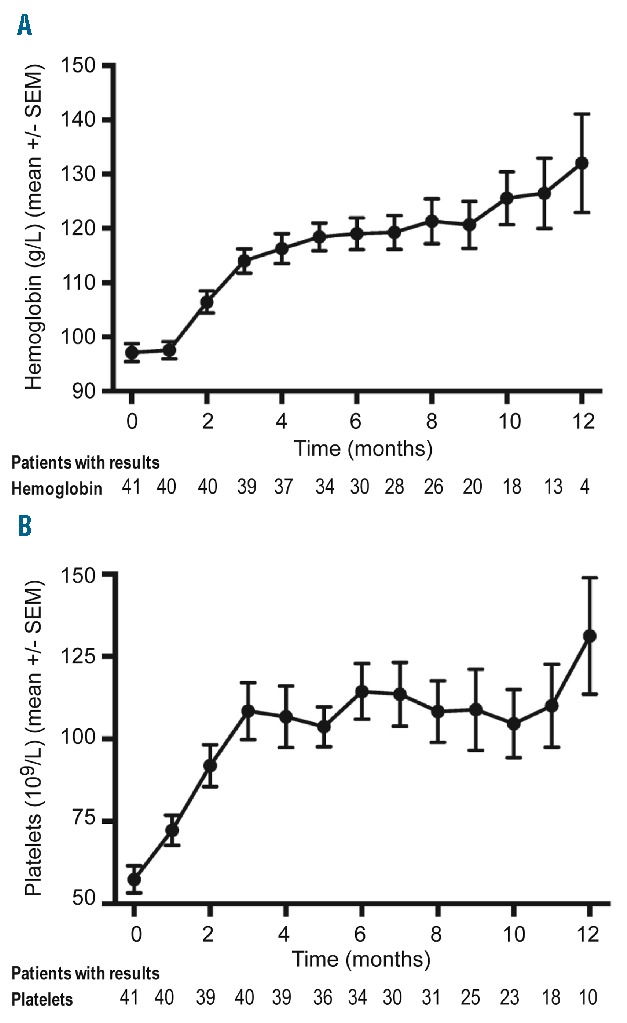
Time kinetics of recovery of blood counts. Hemoglobin concentration (A) and platelet count (B) during ibrutinib therapy in patients with baseline cytopenias. Mean +/− SEM at each timepoint is shown.
Blood lymphocyte counts reached their peak value after a median time of 4 weeks (range: 2 days to 5.2 months) and thereafter declined slowly. Four patients developed a lymphocyte count of > 50×109/L; two of them developed symptoms of hyperviscosity (at a lymphocyte count of 899 and 917×109/L, respectively) which resolved following leukapheresis.
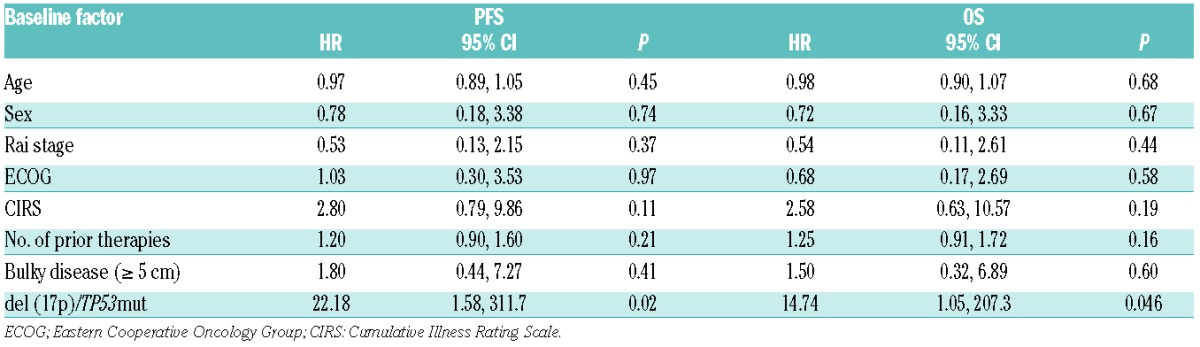
At a median follow-up time of 10.2 months, the median PFS has not yet been reached. The estimated PFS rate at 10 months was 77% (Figure 2A). PFS was significantly shorter in patients with del(17p)/TP53mut (71% at 10 months) than in those without this cytogenetic aberration (93% at 10 months) (P=0.017, log-rank test) (Figure 2B). CIRS ≥6 was also associated with a significantly shorter PFS (P=0.001, log-rank test). A Cox proportional regression hazards model was performed to check whether baseline characteristics (age, sex, Rai stage, ECOG, number of prior therapies, CIRS, bulky disease ≥5 cm or del(17p)/TP53mut) had an impact on PFS. In this analysis, only del(17p)/TP53mut reached statistical significance (HR 22.18, 95% CI 1.58–311.7, P=0.022) while all other baseline factors were statistically non-significant (Table 3).
Figure 2.
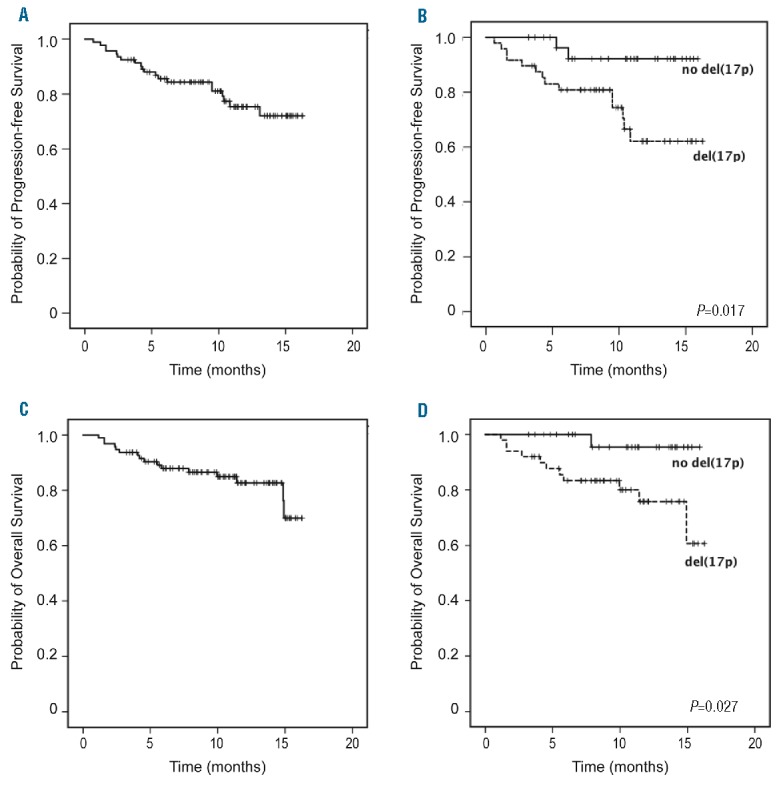
Overall survival (OS) and progression-free survival (PFS). Kaplan-Meier curves of PFS in (A) all patients and (B) patients with or without del(17p)/TP53 mutation and OS in (C) all patients and (D) patients with or without del(17p)/TP53 mutation.
Table 3.
Cox proportional regression hazards model of factors on PFS and OS.
Ten patients had progressive disease (PD) during ibrutinib therapy; 7 had RT and 3 had CLL progression. RT occurred after a median time of 9.5 months (range 3.8–13.1). Six out of 7 patients with RT had baseline del(17p)/TP53mut and 4 patients had bulky disease ≥10 cm.
Median OS was not reached, with a 10-month OS rate of 83% (Figure 2C). OS was significantly shorter in patients with del(17p)/TP53mut (78% were alive at 10 months) than in non-del(17p) patients (97% were alive at 10 months) (P=0.027, log-rank test) (Figure 2D). Similar to the PFS analysis, CIRS ≥6 was associated with a significantly shorter OS (P=0.006, log-rank test), but del(17p)/TP53mut was the only significant factor in the Cox proportional regression hazards model (HR 14.74, 95% CI 1.05–207.3, P=0.046) (Table 3).
Twenty-three patients (24%) discontinued ibrutinib therapy due to PD (n=10), AE (n=10) or due to other reasons (n=3). Reasons for withdrawal other than PD were infection (n=4), secondary malignancy (n=2), generalized exanthema or blisters (n=2), sudden death of unknown origin (n=1), allogeneic SCT (n=1), diarrhea (n=1), need for strong antithrombotic therapy (n=1) and patient decision (n=1). Two patients were switched to treatment with idelalisib; one due to the need for strong antithrombotic therapy who developed cutaneous toxicity on idelalisib and was switched back to ibrutinib with a maintained response. The second patient discontinued ibrutinib after 3 weeks due to cutaneous toxicity, and thereafter reached a response on idelalisib + rituximab therapy.
Sixteen patients have died. The reasons for death were infection (n=6), RT (n=5), CLL progression (n=2), secondary malignancy (n=2) and sudden death of unknown origin (n=1).
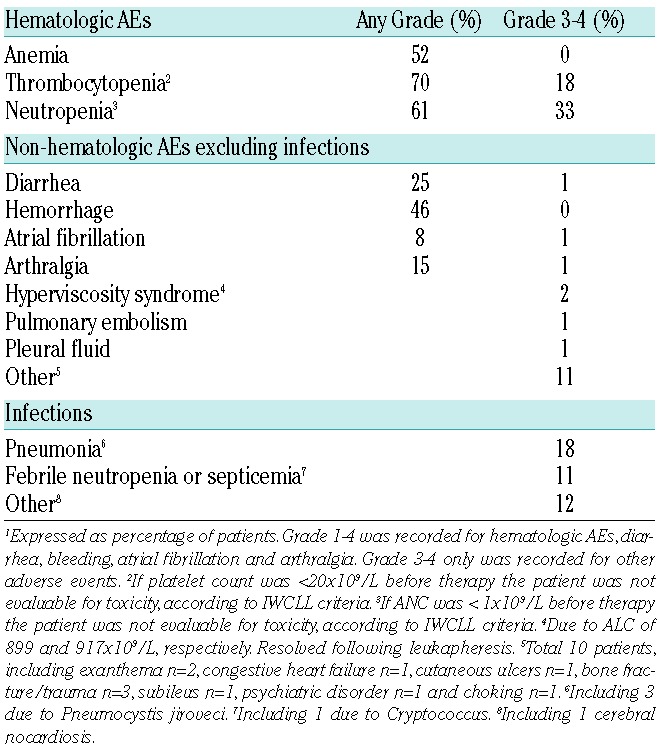
All included patients (n=95) were assessed for safety. AEs during ibrutinib therapy are listed in Table 4. Ibrutinib was generally well tolerated. The most common non-hematologic grade 3–4 AE was infection, which occurred in 39% of the patients. Diarrhea was reported in 25%; only 1 patient had grade 3. Arthralgia or muscle pain was reported in 15%, all but one were grade 1–2. Hemorrhage occurred in 46%; all were grade 1–2 and no major bleeding was observed. Nineteen patients received concomitant low molecular heparin and 5 patients received aspirin or clopidogrel. There was no increased incidence of bleeding in these patients. Atrial fibrillation occurred in 8 patients (8%) during ibrutinib treatment.
Table 4.
Adverse Events.1
Six patients developed a secondary malignancy during therapy: 4 had squamous cell carcinoma of the skin, one had generalized adenocarcinoma, and one had a suspicion of myelodysplastic syndrome (MDS), which was not confirmed by cytogenetics.
Fifty-three patients (56%) were hospitalized at least once; the major reason was infection (32%). In the remaining patients the reasons for hospitalization varied.
Hematologic toxicity is shown in Table 4. Thirteen percent of the patients had grade 4 neutropenia and 18% had grade 3–4 thrombocytopenia. Eighteen patients (19%) received treatment with granulocyte-colony stimulating factor (G-CSF).
A dose reduction of ibrutinib was conducted in 21 patients; 11 were dose-reduced by one level and 10 by two levels to 140 mg. The reasons were: neutropenia (n=4), hematoma (n=4), infection (n=3), thrombocytopenia (n=2), diarrhea (n=2), the need of a strong CYP inhibitor (n=1), anemia (n=1), cutaneous AE (n=1), arthralgia (n=1), increasing serum creatinine (n=1), and aggravation of atrial fibrillation with fluid retention (n=1). Cytopenias generally resolved or improved following dose reduction. To check whether dose reduction may impact on the outcome we compared PFS and OS in patients who had a dose reduction lasting longer than 3 months vs. patients with no or only a short, temporary dose reduction. No differences in PFS and OS were observed (data not shown).
Discussion
This real-world report on ibrutinib was based on consecutively identified CLL patients from a geographically well-defined region. Ibrutinib was provided through the CUP (from May 2014 to May 2015) and thereafter prescribed as licensed indication. The size of the study group (n=95), in relation to the length of the recruitment period (12 months), is likely to be representative for a general population of advanced phase CLL patients in Sweden. Nineteen of the 27 participating centers were non-university hospitals, and together our centers covered all health care and geographical regions in the country. Thirty-four of the 95 patients were treated at non-university hospitals. Furthermore, practically all Swedish CLL patients are followed and treated at hospital-based clinics within the public health care sector, whereas private and office-based hematology is almost absent in our country. The active, nationwide network established within the Swedish CLL Study Group further reduced the risk of missing patients eligible for the CUP. Quality assurance of data was made by the on-site monitoring of individual patient files which were cross-checked. Taken together, we assume that our study represents a real-world population of CLL patients.
The importance of real-world results on patient cohorts, treated outside prospective clinical trials, has been increasingly recognized in hematologic malignancies,14,15 in which complex or toxic regimens are frequently used or new agents are introduced. It was reported that CLL patients included and treated in confirmatory community-based trials responded less well and experienced more side effects than reported in the preceding pivotal studies.1,12 Similar findings have been published on patients with advanced phase CLL when monoclonal antibodies were used as single-agent therapy in routine health care.19,20 Patients included in CLL trials are often younger and have less advanced disease than the general CLL population.13 This is also evident from the current real-world report, in which one fourth of the patients were 75 years or older, more than 60% had del(17p)/TP53mut, most had a reduced ECOG performance status and many had large lymph nodes. Patients included in the pivotal clinical trials on ibrutinib were often younger, had a better ECOG and less pronounced lymphadenopathy.8,10
The ORR of 84% reported in our study is similar to that of the multicenter pivotal trials.8,10 despite the fact that our patients had more poor prognosis characteristics. Many of our patients are still in the relatively early phase of ibrutinib treatment and may possibly demonstrate an improved response with time, as reported in the trials.8,9,21 However, one should be aware that response evaluation in our study was made retrospectively and thus should be viewed with some caution.
The PFS rate of 77% and OS rate of 83% at 10 months is also similar to that of prospective clinical trials,8,10 even though a longer follow-up is required to make a more reliable judgment upon the outcome (PFS and OS). Notably, the PFS and OS were significantly shorter in patients with del(17p)/TP53mut than in those without this aberration. CIRS ≥6 showed a similar pattern but was not statistically significant in the Cox proportional regression hazards model. Similar findings in del(17p) patients were recently reported in the 3-year follow-up of a phase 1b-2 multicenter study9 as well in the RESONATE-17 study.22,23 Thus additional therapeutic actions appear to be required in this difficult-to-treat subgroup of patients. The outcome of del(17p) patients was similar among patients with vs. without bulky disease.
Only limited information is available on the bone marrow effects of ibrutinib. A gradual improvement of cytopenias was described by Byrd et al.,9 providing indirect evidence that ibrutinib induces tumor regression in the bone marrow compartment. This is further supported by our results on longitudinal follow-up of blood counts (Figure 1). In our study, the response rate in the bone marrow was 62% after a median treatment time of 9.5 months; more and deeper remissions are likely to occur over time during prolonged therapy, as recently reported in a follow-up of the NIH conducted clinical trial.24
Ibrutinib was well tolerated. AEs were consistent with those reported in prospective clinical trials, with the exception of grade 3–4 infections, which occurred in almost half of our patients. This is likely to be attributable mainly to the advanced disease of our patients, as most infectious AEs appeared early during ibrutinib therapy. The infection rate was similar in patients with or without del(17p) and was unrelated to CIRS. Well-known side effects such as diarrhea, arthralgia and atrial fibrillation were observed at the expected frequency and severity. A longer follow-up is required to obtain a complete picture of toxicity during ibrutinib therapy. It should be noted that patients with severe cardiovascular disease, warfarin treatment as well as severe neutropenia were excluded from participation in the CUP.
RT occurred in 7 patients, but there is currently no evidence to suggest an increased risk of RT during ibrutinib therapy.9,25 Cutaneous hematomas were common, but no patient had a major bleed. When cross-checking individual patient files we often identified uncertainty among physicians on how to deal with anti-coagulative agents (other than warfarin) in various clinical situations and in relation to the platelet counts; guidelines for routine health care are warranted as these situations are not uncommon.
One may argue that the CUP inclusion criteria to some extent may parallel those of clinical trials. We therefore checked whether our patients fulfilled the inclusion criteria of the pivotal phase 3 study (RESONATE).10 Forty-five percent of our patients had one or more exclusion criteria for RESONATE, with poor performance status or cytopenias as the main reasons, further supporting the real-world representativity of our patient material.
In conclusion, our real-world results confirm and extend the data reported in prospective clinical trials on single-agent ibrutinib in high-risk or R/R CLL patients.8,10,22 Good tolerability, a high ORR and a promising PFS were found in our group of consecutive patients, despite the fact that many of them were old and had poor prognostic features. The shorter PFS and OS in patients with del(17p)/TP53mut highlights the need for continued development of new therapeutic principles for this most difficult-to-treat subgroup of CLL patients. Our study emphasizes that real-world results should be carefully considered from now on regarding new agents6,26 and new indications11 in CLL.
Supplementary Material
Appendix
The following centers and physicians recruited and treated patients in the CUP: South Älvsborg Hospital, Borås (P-O Andersson); Central Hospital in Karlstad, Karlstad (B Hedlund, I Nilsson); Falun Hospital, Falun (M Flogegård); Gävle Hospital, Gävle (A Othzén); Hallands Hospital, Halmstad (C Karlsson, N Kuric); Hallands Hospital, Varberg (C Simonson); Kalmar County Hospital, Kalmar (J Häggström); Karolinska University Hospital, Stockholm (A Asklid, L Hansson, C Karlsson, J Lundin, M Machaczka, S Norin, M Palma, M Winqvist, A Österborg); Kristianstad Central Hospital, Kristianstad (E Horney); Ljungby Hospital, Ljungby (A Swinarska-Kluska); Linköping University Hospital, Linköping (A Bergendahl Sandstedt); Mälarsjukhuset, Eskilstuna (F Pasquariello); Norrland University Hospital, Umeå (M Erlanson, K Forsberg, C Isaksson, M Liljeholm,); Oskarshamns Hospital, Oskarshamn (P Hinnen); Sahlgrenska University Hospital, Gothenburg (M Sender); Skaraborgs Hospital, Lidköping (S Erdal); Skaraborg Hospital, Skövde (R Billström); Skåne University Hospital, Lund and Malmö (E Holm, M Jerkeman, G Juliusson, B Kapas, K Karlsson); Sunderby Hospital, Luleå (L Brandefors, M Johansson, C Kämpe Björkvall, B Lauri); Södersjukhuset, Stockholm (G Lärfars); Uddevalla Hospital, Uddevalla (P Johansson); University Hospital Örebro, Örebro (P Kozlowski, B Uggla); Uppsala University Hospital, Uppsala (M Höglund, M Mattsson); Visby Hospital, Visby (A Aldrin, K Ekman); Växjö Central Hospital, Växjö (J Bjereus, MS Carlsson Alle); Östersund Hospital, Östersund (A Asklund, U Sokolowska-Kolacz), Sweden.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/12/1573
Funding
This study was supported by grants from The Swedish Cancer Society (Ref no: 15 0894), The Cancer Society in Stockholm (Ref no: 144142, 151313), King Gustav V Jubilee Fund (Ref no: 144193), The Cancer and Allergy Foundation (Ref no: 150 420, 150 431), StratCan Karolinska Institutet (Proj code: 2201), AFA Insurance (Ref no: 130054) and The Stockholm County Council (Ref no: 20150070), Sweden. We thank Ms Leila Relander for editorial assistance and Dr. Ben King for linguistic corrections.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 2.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29): 4473–4479. [DOI] [PubMed] [Google Scholar]
- 3.Eketorp Sylvan S, Hansson L, Karlsson C, et al. Outcomes of patients with fludarabine-refractory chronic lymphocytic leukemia: a population-based study from a well-defined geographic region. Leuk Lymphoma. 2014;55(8):1774–1780. [DOI] [PubMed] [Google Scholar]
- 4.Tam CS, O’Brien S, Lerner S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma. 2007;48(10):1931–1939. [DOI] [PubMed] [Google Scholar]
- 5.Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2(1):58–61. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds C, Di Bella N, Lyons RM, et al. A Phase III trial of fludarabine, cyclophosphamide, and rituximab vs. pentostatin, cyclophosphamide, and rituximab in B-cell chronic lymphocytic leukemia. Invest New Drugs. 2012;30(3):1232–1240. [DOI] [PubMed] [Google Scholar]
- 13.Terasawa T, Trikalinos NA, Djulbegovic B, Trikalinos TA. Comparative efficacy of first-line therapies for advanced-stage chronic lymphocytic leukemia: a multiple-treatment meta-analysis. Cancer Treat Rev. 2013;39(4): 340–349. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014;124(8):1288–1295. [DOI] [PubMed] [Google Scholar]
- 15.Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570–1577. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goede V, Bahlo J, Chataline V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk Lymphoma. 2015:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. [DOI] [PubMed] [Google Scholar]
- 19.Fiegl M, Falkner A, Hopfinger G, et al. Routine clinical use of alemtuzumab in patients with heavily pretreated B-cell chronic lymphocytic leukemia: a nationwide retrospective study in Austria. Cancer. 2006;107(10):2408–2416. [DOI] [PubMed] [Google Scholar]
- 20.Moreno C, Montillo M, Panayiotidis P, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica. 2015;100(4):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien S, Jones JA, Coutre S, et al. Efficacy and Safety of Ibrutinib in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Leukemia with 17p Deletion: Results from the Phase II RESONATE (TM)-17 Trial. 57th ASH Annual Meeting and Exposition. Orlando, FL, USA. Blood. 2014;124(21). [Google Scholar]
- 23.Stilgenbauer S, Jones JA, Coutre S, et al. Outcome of Ibrutinib Treatment by Baseline Genetic Features in Patients with Relapsed or Refractory CLL/SLL with del17p in the Resonate-17 Study. 57th ASH Annual Meeting and Exposition. Orlando, FL, USA. Blood 2015:Abstract 833. [Google Scholar]
- 24.Farooqui M, Valdez J, Soto S, et al. Single Agent Ibrutinib in CLL/SLL Patients with and without Deletion 17p. 57th ASH Annual Meeting and Exposition. Orlando, FL, USA. Blood 2015:Abstract 2937. [Google Scholar]
- 25.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab (BR) in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): First results from a randomized, double-blind, placebo-controlled, phase III study. ASCO Annual Meeting, Chicago, Illinois, USA 2015: Abstract LBA7005. [Google Scholar]
- 26.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


