Abstract
In the GIMEMA LAL 0904 protocol, adult Philadelphia positive acute lymphoblastic leukemia patients were treated with chemotherapy for induction and consolidation, followed by maintenance with imatinib. The protocol was subsequently amended and imatinib was incorporated in the induction and post-remission phase together with chemotherapy. Due to the toxicity of this combined approach, the protocol was further amended to a sequential scheme based on imatinib plus steroids as induction, followed by consolidation with chemotherapy plus imatinib and, when applicable, by a hematopoietic stem cell transplant. Fifty-one patients (median age 45.9 years) were enrolled in the final sequential protocol. At the end of induction (day +50), 96% of evaluable patients (n=49) achieved a complete hematologic remission; after consolidation, all were in complete hematologic remission. No deaths in induction were recorded. Overall survival and disease-free survival at 60 months are 48.8% and 45.8%, respectively. At day +50 (end of imatinib induction), a more than 1.3 log-reduction of BCR-ABL1 levels was associated with a significantly longer disease-free survival (55.6%, 95%CI: 39.0–79.3 vs. 20%, 95%CI: 5.8–69.1; P=0.03), overall survival (59.1%, 95%CI: 42.3–82.6 vs. 20%, 95%CI: 5.8–69.1; P=0.02) and lower incidence of relapse (20.5%, 95%CI: 7.2–38.6 vs. 60.0%, 95%CI: 21.6–84.3; P=0.01). Mean BCR-ABL1 levels remained significantly higher in patients who subsequently relapsed. Finally, BCR-ABL1p190 patients showed a significantly faster molecular response than BCR-ABL1p210 patients (P=0.023). Though the study was not powered to evaluate the role of allogeneic stem cell transplant, allografting positively impacted on both overall and disease-free survival. In conclusion, a sequential approach with imatinib alone in induction, consolidated by chemotherapy plus imatinib followed by a stem cell transplant is a feasible, well-tolerated and effective strategy for adult Philadelphia positive acute lymphoblastic leukemia, leading to the best long-term survival rates so far reported. (clinicaltrials.gov identifier: 00458848).
Introduction
The Philadelphia (Ph) chromosome represents the most frequent cytogenetic alteration in adult acute lymphoblastic leukemia (ALL). The incidence of the Philadelphia positive (Ph+) ALL increases with age, from approximately 2%–5% in children/adolescents, to 22% among adult patients aged 21–50 years, and to over 50% in patients over 50 years of age.1–4 The presence of the Ph chromosome has historically defined a subgroup of ALL with a particularly unfavorable prognosis. The advent of tyrosine kinase inhibitors (TKI) profoundly changed the management and prognosis of this high-risk group of patients, and the management of Ph+ ALL is described as pre- or post the TKI era.5–21 Prior to the introduction of TKI, prognosis was very poor, with virtually no adult patients (<5%) cured with standard chemotherapy; median survival was 8–10 months unless an allogeneic hematopoietic stem cell transplant (allo-SCT), the only potentially curative strategy, could be performed.22–25 Today, treatment with TKI, with5–10,12–15,18–21 or without11,16,17 systemic chemotherapy, represents the most appropriate first-line management of patients with Ph+ ALL in terms of rates of complete hematologic remission (CHR) and disease-free survival (DFS). Imatinib has been incorporated into different schedules either in induction5–7,10,12–14,18–21 or following induction8,10 in cohorts including also elderly patients,8 with CHR rates varying from 72% to 96%. Moreover, the use of imatinib can also act as a “bridge” to an allo-SCT for those patients eligible.26–30
In the first TKI-based GIMEMA protocol (LAL 0201), imatinib alone (plus steroids) was used as induction treatment for elderly (>60 years) Ph+ ALL patients.11 The results showed for the first time that a treatment strategy based on a TKI alone [plus steroids and central nervous system (CNS) prophylaxis] and no systemic chemotherapy was associated with a CHR in virtually all elderly patients with no deaths in induction;11 some patients are alive ten years later (S Chiaretti, personal data, 2016). These data provided the starting point for the design of the subsequent GIMEMA LAL 1205 protocol, based on the use of the second-generation TKI dasatinib alone for 12 weeks as first-line induction treatment for all Ph+ ALL over 18 years of age, with no upper age limit; all evaluable patients obtained a CHR with an overall good compliance and no deaths and relapses during induction.16 In the GIMEMA LAL 1205 protocol, post-induction therapy was left to the investigator’s choice. The issue that still remained after this study was how best to consolidate patients who were in CHR following TKI induction, and this is being addressed in the current dasatinib-based GIMEMA 1509 total therapy protocol.17
The GIMEMA LAL 0904 trial was initially designed for both Philadelphia negative (Ph−) and Ph+ ALL cases. When available, imatinib was added for Ph+ ALL patients. The protocol underwent different amendments (see Methods). In the last amended protocol (3rd amendment), imatinib plus steroids was used as induction without systemic chemotherapy, followed by a uniform intensive consolidation chemotherapy plus imatinib and a subsequent hematopoietic SCT [allo-SCT or autologous (auto)-SCT if a donor was not available]. We report the final results of the 3rd amendment for the management of adult Ph+ ALL.
Methods
Study design and therapy
Between October 2004 and April 2010, 100 patients with de novo Ph+ ALL, aged 15–60 years, were enrolled in the GIMEMA 0904 protocol. In the first version of the protocol, Ph+ patients received chemotherapy as induction and consolidation. When imatinib became available, it was administered as maintenance. The protocol underwent a first (1st) amendment based on the use of a novel asparaginase formulation. The protocol was subsequently amended a second time (2nd) and imatinib was incorporated into the induction and post-remission phase. This scheme was associated with an unacceptably high rate of induction deaths: 2 deaths were recorded (due to fungal infection and liver toxicity, respectively) among the first 9 patients enrolled. The protocol underwent a final amendment (3rd) and imatinib (600 mg/day for 50 days) plus steroids (60 mg/m2/day) were used as induction, followed by a HAM cycle plus imatinib (Figure 1 and Online Supplementary Appendix) and a hematopoietic SCT, either allogeneic or autologous if no donor was available. A donor search was carried out first among family members, and if a related donor was not available an alternative donor search was made according to the policy of each center.
Figure 1.
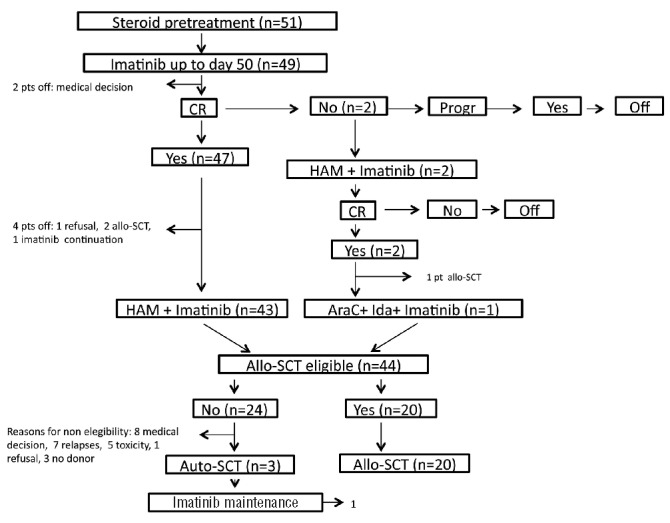
Schematic representation of the GIMEMA 0904 3rd amendment. Steroid pre-phase: oral prednisone at increasing doses (10–60 mg/m2/day) for seven days. Induction therapy: oral imatinib at a dose of 600 mg daily for 50 days; prednisone (60 mg/m2/day) until day +24, then tapered and stopped at day +32; intrathecal methotrexate (15 mg) on days +21 and +35. Consolidation treatment: HAM regimen plus oral imatinib at a dose of 600 mg daily. Post consolidation: a hematopoietic SCT, either allogeneic or autologous (if no donor was available) was offered; otherwise, patients continued treatment with imatinib. Patient flow-chart is also provided. pts: patients; CR: complete remission; Progr: progression; allo-SCT: allogeneic stem cell transplantation; Auto-SCT: autologous stem cell transplantation; AraC: cytarabine.
The enrollment periods, centers, overall survival (OS) and DFS of the 3 amendments are detailed in the Online Supplementary Appendix, while the results presented here refer to the patients enrolled in the 3rd amendment only. The objective of this study was to evaluate the role of imatinib as induction treatment followed by a uniform consolidation treatment based on the HAM regimen and a transplant procedure, when possible.
The study was approved by the Ethics Committees of all participating centers and all patients gave their written informed consent in accordance with the Declaration of Helsinki.
Response assessment
Bone marrow (BM) evaluations and molecular monitoring were performed at baseline, at day +35, +50 (end of induction) and post consolidation. Patients were considered in CHR in the presence of 5% or less BM blasts, absence of blasts in the peripheral blood (PB), no extramedullary involvement and a full PB count recovery (i.e. polymorphonucleates >1.5×109/L and platelets >100×109/L).
Steroid response was based on the PB blast reduction (threshold ≥75%) after the steroid pre-phase. Hematologic relapse was defined as the presence of blasts in the PB or any non-hematologic site, or 5% or more BM blasts.
Molecular diagnosis and minimal residual disease monitoring
Molecular analyses were performed at “La Sapienza” University of Rome, Italy. Total RNA was extracted from BM samples using the TRizol reagent or the Qiagen extraction kit. A reverse transcriptase multiplex polymerase chain reaction (RT-PCR-multiplex)31 was performed to detect the p190 or p210 forms of the BCR-ABL1 fusion product within the 7-day steroid pre-phase. Minimal residual disease (MRD) monitoring was performed by quantitative real-time PCR (Q-RT-PCR).32,33 BCR-ABL1 transcript levels were normalized to the number of the ABL1 control gene and expressed as BCR-ABL1/ABL1 ×100; the level of BCR-ABL1 expression was then converted into a base 10 logarithmic scale. A complete molecular response was defined as a BCR-ABL1/ABL1 ratio equal to zero.
Statistical analysis
Overall survival and DFS were estimated using the Kaplan-Meier method. Cumulative incidence of relapse (CIR) was calculated using the cumulative incidence method (Online Supplementary Appendix).
A 1.3 log-reduction cut off was chosen on the martingale residual analysis on the univariate Cox model; an increasing smoothed martingale residual plot indicated a prognostic effect of log BCR-ABL1 reduction levels on DFS. The statistical significance for reduction of BCR-ABL1 levels was assessed using the Mann-Whitney test.
The log-rank test was used to compare risk-factor categories for the Kaplan-Meier curves and the Gray test for the incidence curves. Multivariate analysis was performed by the Cox model; results were expressed as Hazard Ratios (HR) ± 95% confidence intervals (95%CI). The role of transplant was evaluated in a Cox model with a time-dependent covariate. All tests were two-sided; P<0.05 was considered significant. Analyses were performed using SAS v.9.4 software (SAS Institute, Cary, NC, USA).
Results
Patients
From July 2007 to April 2010, 51 adult Ph+ ALL were enrolled in the 3rd amended protocol; 28 were females and 23 males. Median age was 45.9 years (range 16.9–59.7) and median white blood cell (WBC) count was 28.0×109/L (range 1.4–597.0). Thirty-nine patients had the p190 form of BCR-ABL1, 7 the p210 form, and 5 both p190 and p210 (for all analyses, these latter two groups were considered together).
Induction treatment response and toxicity
Of the 51 patients enrolled, 49 were evaluable for response, while 2 discontinued treatment for medical decision. After the steroid pre-phase, 79% of patients showed a PB blast reduction of 75% or over and 21% less than 75%. At the end of the induction treatment with imatinib plus steroids, 47 of 49 evaluable patients (96%) achieved a CHR; of the remaining 2 patients, 1 had a partial response and the other was refractory to induction and both were rescued with the HAM chemotherapy scheme and achieved a CHR.
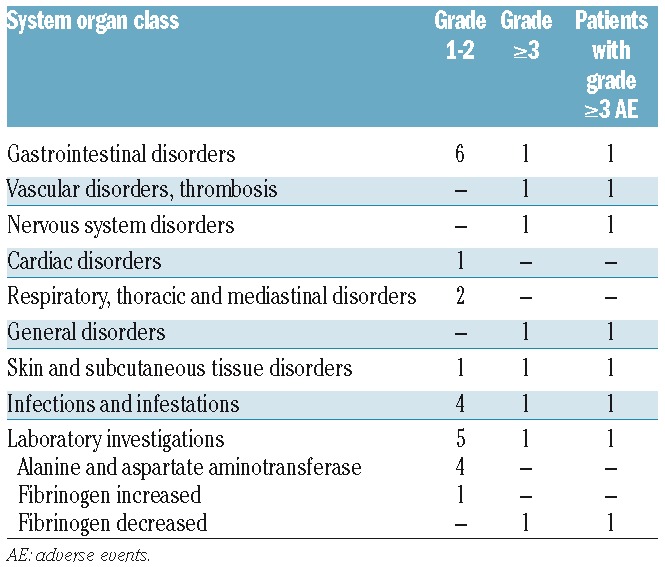
Overall, treatment was well tolerated; adverse events (AE) were recorded in 24 patients and were grade 3 or over only in 7 (Table 1). The most frequent AE were represented by grade 1–2 gastrointestinal disorders (n=6) and increase in alanine/aspartate aminotransferase levels (n=4). No deaths were recorded during the induction phase.
Table 1.
Adverse events in induction.
Post-remission treatment
Of the 47 patients in CHR after the induction phase with only imatinib plus steroids, 43 performed the planned consolidation therapy with the HAM regimen. Of the remaining 4 patients, 1 refused further treatment and 3 did not undergo the treatment scheduled by medical decision: 2 of 3 these patients directly underwent an allo-SCT without additional chemotherapy and the other continued treatment with imatinib.
Both patients who achieved a CHR after HAM underwent an allo-SCT; one after receiving a consolidation cycle with high-dose cytarabine (HD-ARA-C) and idarubicin, as per protocol guidelines, while the other proceeded directly to transplant.
After the planned consolidation chemotherapy, 20 patients underwent an allo-SCT with a myeloablative conditioning regimen [8 siblings, 10 matched unrelated donor (MUD), 2 haploidentical]. Twenty-four patients did not receive a transplant for the following reasons: medical decision in 8 patients, relapse in 7, toxicity in 5, refusal in 1, and no donor in 3. An auto-SCT was performed in the 3 patients for whom no donor was available.
The induction and post-remission results are shown in Figure 1.
Considering the entire cohort, including also the patients (n=5) who did not perform the planned therapy, 9 patients have died in CHR, either due to complications following chemotherapy (6 of 26, 23.07%) or allo-SCT (3 of 23, 13.04%). Among patients who received only chemotherapy, 3 experienced a fatal infection: 1 had a hemorrhage and 1 a fatal neurotoxicity, while cause of death is unknown for the other. Among allografted patients, 1 experienced multiorgan failure, 1 died of infection and 1 of widespread graft-versus-host disease.
Minimal residual disease monitoring
BCR-ABL1 transcript levels decreased during the imatinib plus steroids induction therapy. The highest reduction was observed between the onset and day +35 of therapy (P<0.0001), while reduction did not reach statistical significance (P=0.393) between days +35 and +50, thus indicating that the greatest activity of treatment in terms of MRD reduction is observed during the first four weeks of treatment. Consolidation chemotherapy (HAM regimen) induced a further reduction of the BCR-ABL1 levels compared to those obtained at the end of induction, even if the log-reduction was not statistically significant (P=0.103) (Figure 2). In particular, a disease reduction greater than 1.3 log was observed in 72% of patients at day +35, in 72% of patients at day +50, and in 90% of patients after the post-consolidation therapy; 3% of patients achieved a complete molecular remission at day +50 of the current protocol.
Figure 2.
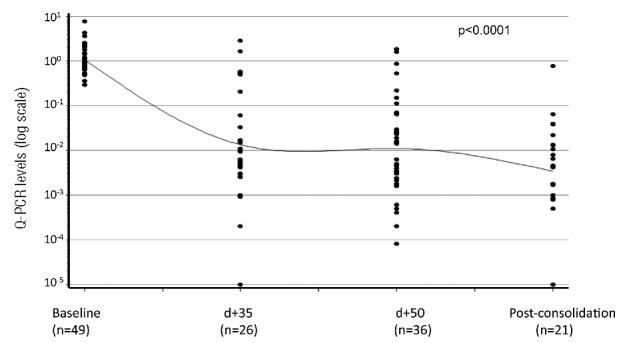
Minimum residual disease (MRD) monitoring during treatment. MRD monitoring was performed by quantitative real-time PCR (Q-RT-PCR) and BCR-ABL1 expression levels converted into a logarithmic (base 10) scale. A highly significant (P<0.0001) disease reduction was observed between the onset and day (d) +35, and an additional decrease [P=not significant (ns)] between days +35 and +50. Consolidation chemotherapy induced a further reduction (P=ns) of the BCR-ABL1 levels compared to those obtained at the end of induction.
Mean BCR-ABL1 levels remained significantly higher in patients who subsequently relapsed compared with those who remained free from relapse at the last follow up (P=0.038). BCR-ABLp190 patients showed a more rapid molecular response compared to BCR-ABLp210 patients and this difference was statistically significant at day +50 (end of induction) (P=0.023).
Relapses
At the last follow up (median 51.8 months, range 2.7–75.3), 17 relapses had occurred [11 hematologic, 5 central nervous system (CNS) and 1 not defined], all after completing the induction phase. In those patients who received the planned therapy, 5 relapses occurred in the 20 allo-SCT patients (25%), 11 in the 21 non-transplanted group of patients (52.4%), and an additional relapse was recorded among the 4 patients (25%) who did not undergo consolidation therapy. Overall, the median time to relapse from achievement of 1st CHR was 7.6 months (range 2.1–32.9): this was 3.5 months (range 2.1–32.9) in non-transplanted patients, while in transplanted cases it was 8.6 months (range 5.7–12.2). In both subgroups, no relapse was observed later than 33 months.
Among relapsed patients, there was a statistically significant difference in median WBC count at diagnosis between relapsed versus non-relapsed cases (59.2×109/L vs. 19.5×109/L; P=0.01). Furthermore, relapse was associated with female sex (5 men and 12 women in relapsed cases vs. 18 men and 14 women among non-relapsed cases), although this was not significant (P=0.07). Finally, fewer relapses [not significant (n.s.)] occurred in patients with the BCR-ABL1p190 form (12 of 39, 30.7%) than in patients with the BCR-ABL1p210 form (5 of 12, 41.7%). We observed no differences in age among the two groups (median age 43.0 vs. 46.3 years, respectively).
We also analyzed the impact of MRD in relation to relapse and observed a significant difference in terms of CIR between patients who had reached MRD levels below 1.3 log at day +50 and those who did not (60.0%, 95%CI: 21.6–84.3 vs. 20.5%, 95%CI: 7.2–38.6, respectively; P=0.01) (Figure 3).
Figure 3.

Cumulative incidence of relapse (CIR) on the basis of reduction in minimum residual disease (MRD). Patients were stratified according to the 1.3 log reduction at day +50 (cut-point 1.3 log). CIR was significantly lower in patients with an MRD reduction of 1.3 log or over (continuous line) at day +50 vs. those with an MRD reduction less than 1.3 log (dashed line) (60.0%, 95%CI: 21.6–84.3 vs. 20.5%, 95%CI: 7.2–38.6, respectively; P=0.01). CR: complete remission.
Disease-free survival and overall survival
Median follow up of the study is 51.8 months (range 2.7–75.3). DFS at 60 months is 45.8% (95%CI: 33.6–62.5), with a median DFS of 40.1 months (Figure 4A). OS at 60 months is 48.8% (95%CI: 36.4–65.3), with a median OS of 48.8 months (Figure 4B).
Figure 4.
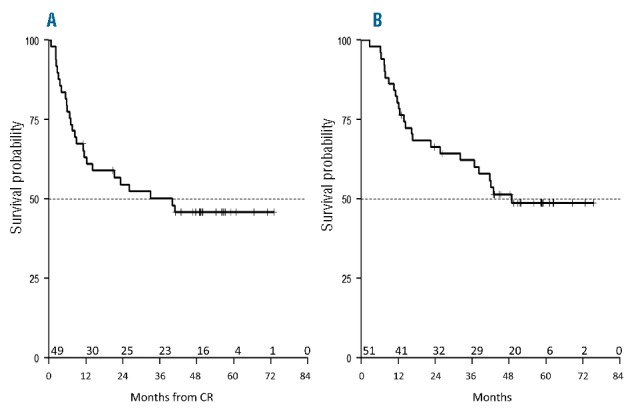
Survival of the whole study population. Median follow up is 51.8 months. (A) Disease-free survival (DFS) at 60 months is 45.8% (95%CI: 33.6–62.5), with a median DFS of 40.1 months. (B) Overall survival (OS) at 60 months is 48.8% (95%CI: 36.4–65.3), with a median OS of 48.8 months. CR: complete remission.
Overall survival and DFS were analyzed taking into account MRD levels and post-consolidation treatment (i.e. allo-SCT vs. no allo-SCT). With regard to MRD, DFS was analyzed according to the molecular response at day +50, i.e. the induction end point: estimations at 60 months were 20% (95%CI: 5.8–69.1) for patients with BCR-ABL1 log reduction levels less than 1.3 log and 55.6% (95%CI: 39.0–79.3) for patients with log reduction levels of 1.3 log or over (P=0.03) (Figure 5A). Accordingly, OS was significantly worse (P=0.02) for patients with BCR-ABL1 log reduction levels less than 1.3 log (20%, 95%CI: 5.8–69.1) than for patients with log reduction levels 1.3 log or over (59.1%, 95%CI: 42.3–82.6) (Figure 5B).
Figure 5.
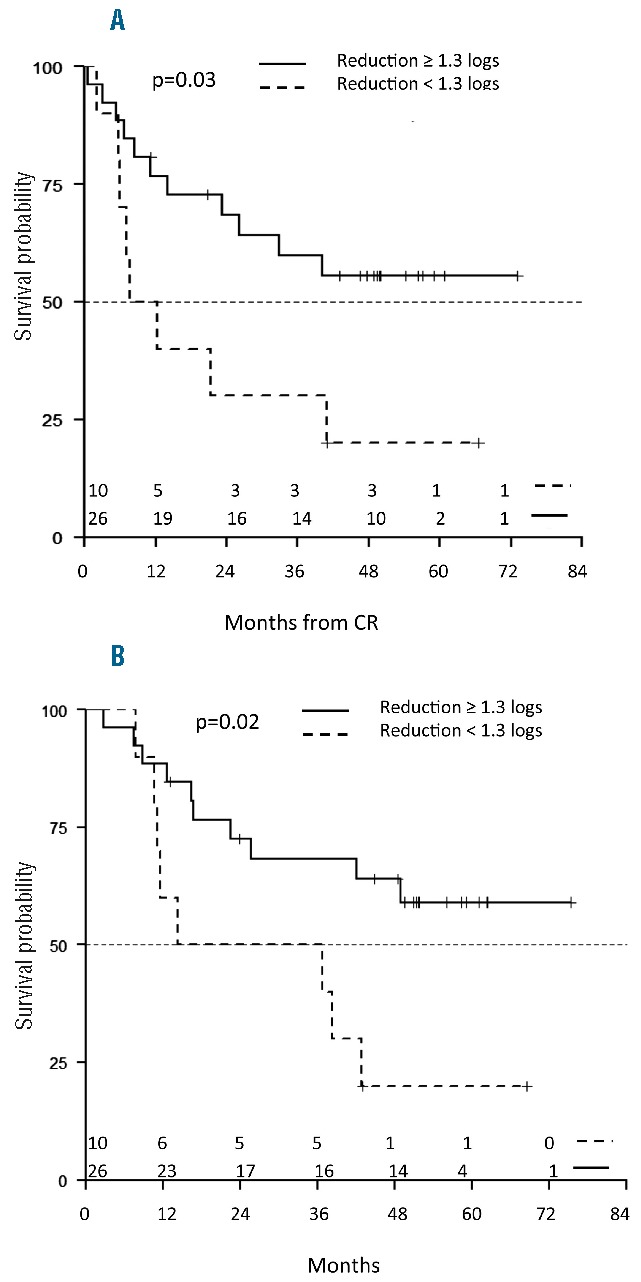
Survival at 60 months on the basis of the reduction in minimal residual disease (MRD). (A) Disease-free survival (DFS) was significantly better for patients with BCR-ABL1 log reduction levels of 1.3 log or over (55.6%, 95%CI: 39.0–79.3, continuous line) than for patients with log reduction levels less than 1.3 log (20.0%, 95%CI: 5.8–69.1, dashed line) (P=0.03). (B) Overall survival (OS) at 60 months is 59.1% (95%CI: 42.3–82.6) for patients with BCR-ABL1 log reduction levels of 1.3 log or over, and 20% for patients with log reduction levels less than 1.3 log (95%CI: 5.8–69.1). CR: complete remission.
Disease-free survival and OS were also analyzed on the basis of the allo-SCT procedure (allo-SCT, n=23; no allo-SCT, n=26). Patients undergoing an auto-SCT (n=3) were not considered given the small number of patients. Allo-SCT, considered as a time-dependent covariate in patients undergoing HAM therapy as consolidation treatment, impacted on all survival end points (DFS, P=0.06; OS, P=0.03; CIR, P=0.06).
Univariate and multivariate DFS analysis
Taking into account in univariate analysis WBC count at diagnosis (as continuous variable), age (as continuous variable), sex, response to the steroid pre-phase, type of BCR-ABL1 transcript, BCR-ABL1 log-reduction at day +35, day +50 and post consolidation, and allo-SCT, a significant correlation was found with DFS for the WBC count (P=0.03), with DFS and OS for response to the steroid pre-phase (P=0.004 and 0.0035, respectively) and BCR-ABL1 1.3 log-reduction at day +50 (P=0.04 and 0.028); concerning OS, a trend towards significance was only observed according to age (P=0.06).
In multivariate analysis, only response to the steroid pre-phase and a BCR-ABL1 1.3 log-reduction at day +50 correlated with DFS (P=0.002 and P=0.05, respectively) and OS (P=0.002 and P=0.008, respectively).
Discussion
We report the final results of the GIMEMA LAL 0904 protocol for adult Ph+ ALL patients. This protocol was initially based on an induction and post-remission phase based on chemotherapy, followed by imatinib administration as maintenance. Subsequently, it was modified to a combined imatinib-chemotherapy induction treatment which, due to toxicity, was amended to a sequential program schedule based on imatinib plus steroids in induction, followed by the HAM chemotherapy regimen and, when applicable, a transplant procedure. The results obtained indicate that not only in the elderly,11 but also in young adult ALL patients (age 15–60 years), imatinib plus steroids alone as induction treatment result in a marked debulking of the neoplastic clone. In fact, at the end of the induction phase, 47 of 49 of the evaluable patients (96%) achieved a CHR and the 2 remaining patients obtained a CHR with the HAM regimen. In terms of CHR, these results compare favorably with those reported by other studies in which imatinib was administered concomitantly to chemotherapy or in various schedules during induction or consolidation, with CHR rates ranging from 72% to 96%.5,6,10,12,13,15,18–21,34,35 Furthermore, our schedule has the advantage that there are fewer deaths during induction treatment, in contrast to the majority of the combination studies in which, with few exceptions,5 toxic deaths were recorded in 2%–7% of cases.6,8,12–14,18–20 Indeed, toxicity was recorded in the initial combination protocol (imatinib+chemotherapy) that led to the final amendment to a sequential strategy. These results indicate that the induction treatment for adult Ph+ ALL can be effectively based on the administration of imatinib plus steroids, without systemic chemotherapy, which enables a CHR to be obtained in virtually all patients with no deaths in induction. The toxicity of the combination of a tyrosine kinase inhibitor (TKI) with conventional chemotherapy has also been reported by the PETHEMA group,19 and more recently by Chalandon et al.36 who have shown that imatinib can be effectively and more safely combined with reduced intensity chemotherapy.
As expected, at day +50 (end of the induction), the molecular disease was still present at low levels in the majority of patients. Mean BCR-ABL1 levels appeared to be more rapidly reduced in p190+ cases than in p210+ patients, as observed in our previous study based on dasatinib,16 confirming a significantly greater susceptibility of BCR-ABLp190-expressing cells to TKI. In our analysis, patients who reached lower levels of disease at day +50 had a significantly better outcome than those with higher levels of residual disease at the end of induction, both in terms of DFS (20.0% vs. 55.6%; P=0.03), reduced CIR (20.5% vs. 60.0%; P=0.01), and OS (20.0% vs. 64.0%; P=0.02) at 60 months. In particular, patients who maintained their remission state over time had achieved significantly lower levels of MRD at day +50 than patients who subsequently relapsed. Therefore, MRD levels post induction or in consolidation is a significant risk factor for treatment failure. These results confirm the observations made in our previous study based on the use of dasatinib in induction,16 and are in line with the current general knowledge on the prognostic impact of MRD.12,19,37–43 Furthermore, they strengthen the notion that MRD negativity should be regarded as a major goal in Ph+ ALL treatment. Indeed, with few exceptions,20,38 it is now well established that BCR-ABL1 transcript levels correlate with response. Lee et al.37 were able to demonstrate that a 3-log reduction in BCR-ABL1 transcripts after one month of imatinib treatment strongly predicted a reduced risk of relapse and confirmed these results in a subsequent study.40 A correlation between MRD levels and outcome has also been reported by Ravandi et al. in patients treated either with imatinib or dasatinib and chemotherapy.41 Two studies44,45 analyzing the outcome of patients with Ph+ ALL who underwent a transplant showed that the persistent expression of BCR-ABL1 during the first 100 days post transplant was associated with a higher incidence of relapse and a lower DFS. Both studies argue in favor of a maintenance therapy with imatinib after transplant in patients with a positive MRD evaluation, and this has also been suggested in more recent studies.46,47 In contrast, Yanada et al.38 observed no association between rapid achievement of BCR-ABL1 negativity and long-term outcome after an initial imatinib/chemotherapy induction regimen, and the GRAAL group also reported that early MRD evaluation did not significantly influence patient outcome, either in terms of OS or DFS.20
One issue that remains open to discussion concerning the role of MRD is represented by the substantial differences in the way PCR for BCR-ABL1 detection is performed, how results are reported in different laboratories worldwide, and the timing of MRD evaluation. The indications suggested by White et al.48 will hopefully lead to an international standardization of the assessment of the levels of BCR-ABL1. With these premises in mind, the MRD clearance obtained in the current study is inferior to that reported by other groups in which the reported levels of MRD, evaluated at different time points and with different cut-off points, ranged from 26% to 86%.5,7,10,13,18,19 This could be explained by the fact that, in this trial, chemotherapy was not part of the induction treatment. However, in the 2nd amended version of this trial, where both chemotherapy and TKI were simultaneously administered, 2 deaths in induction were recorded in the first 9 treated patients (1 fungal infection and 1 liver toxicity). The protocol was, therefore, discontinued and modified into a sequential strategy. In addition, it should be remembered that imatinib has per se a less profound molecular debulking effect compared to dasatinib, which in vitro has a 325-fold greater potency in inhibiting BCR-ABL1.49 In line with this, in our previous GIMEMA LAL 1205 trial16 based on dasatinib administration and steroids as induction (without a uniform post-induction treatment), we documented a more pronounced MRD clearance at the end of induction; in fact, 16% of patients achieved a complete molecular response at day +57 and 20% at day +85 (end of induction) compared with 3% at day +50 in the current protocol.
One of the aims of the present study was to see whether a uniform post-induction therapy was capable of improving outcome. The comparison between this regimen and the dasatinib study leads to two main conclusions. 1) Dasatinib exerts a more potent anti-leukemic activity compared to imatinib, mostly in terms of MRD clearance. 2) It confirms the importance of a uniform consolidation chemotherapy regimen (HAM) and transplant when possible; in fact, this translated into significantly better DFS and OS rates compared to the LAL 1205 protocol in which the post-consolidation phase was open (Online Supplementary Figure S2).
A formal comparison with other studies, with the limitation that the follow-up period is extremely heterogeneous, shows that our data compare favorably in terms of both short- and, in particular, long-term DFS and OS. In fact, in our study, OS and DFS at 24 months were 66.4% and 54.6%, respectively; these results are in line with studies from the MDACC,5 GRAAL8 and GRAAPH-200310 trials, and with a more recent report from the MDACC14 based on dasatinib administration. With regard to the long-term follow up, our data show that the OS and DFS at 60 months are 48.8% and 45.8%, respectively. In the Italian NILG 09/00,12 Spanish CSTIBES02,13 French GRAAL,20 English UKALLXII/ECOG299321 and MDACC35 studies, the 4–5 year OS and DFS rates were 38% and 39%,12 30% for both,13 52% and 44%,20 38% of OS and 50% at four years of relapse-free survival,21 and 43% for both.35 Thus, our long-term outcome results appear superior; this could be due to the fact that we have had no deaths in induction nor major toxicities associated to chemotherapy, which was delivered to patients (96% of cases) already in hematologic remission. All relapses occurred after completing the imatinib plus steroids induction phase and took place during/after the post-consolidation therapy; in fact, the median time to relapse from achievement of the first CHR was 7.6 months. Five of the 17 relapses were at the CNS level. Notably, no CNS relapses were observed in our previous dasatinib-based study, thus confirming the scarce penetration capability of imatinib;50–52 indeed, Pfeifer et al. reported that treatment with imatinib without CNS prophylaxis is associated with meningeal leukemia in 12% of cases.50 In line with this, Takayama et al.51 showed that the concentration of imatinib in the cerebrospinal fluid is roughly 92-fold lower than that in the blood.51 Finally, Porkka et al. reported that dasatinib is capable of increasing survival in a K562 intracranial chronic myeloid leukemia (CML) mouse model, whereas imatinib is not.52 Overall, these results indicate that a more active CNS prophylaxis must be administered when using imatinib as front-line therapy.
This study was not powered to define the efficacy of allo-SCT but rather the feasibility of a sequential therapeutic strategy that contemplated also a transplant. (allo-SCT was administered to patients with an available donor, mostly siblings, and to those deemed fit to proceed to transplant procedures.) Nevertheless, allo-SCT, that is still the standard curative approach in this subset of patients, was associated with a better DFS, OS or CIR in this imatinib-based protocol. It must be noted that the non-transplanted group included patients who experienced an early relapse, the main reason for not undergoing an allo-SCT, and older patients. It is also worth underlining that in both transplanted and non-transplanted patients relapses were not observed later than 33 months, suggesting that a subgroup of patients might be spared transplant procedures and the related morbidities.
Finally, it has recently been proposed12,36,53,54 that auto-SCT might have a role in Ph+ ALL since the introduction of TKI and the precise quantification of MRD. The NILG group12 reported a 5-year cumulative survival of 67% for 9 auto-grafted patients. Chalandon et al.36 showed that, among the 29 autografted patients in major molecular response, there was no statistically significant difference in outcome from that of allografted cases. Similarly, the CALGB Study 10001 (Alliance)53 proved that an auto-SCT, performed in 19 individuals, gave results similar to those obtained with an allo-SCT (n=15). Finally, Giebel et al.54 extensively evaluated the role of auto-SCT in a cohort of 177 adult Ph+ ALL. Patients were subdivided into 3 categories, according to the period in which the procedure was performed: 1996–2001, 2002–2006 (during which sporadic cases received TKI during therapy), and 2007 onwards (when all patients received imatinib). OS and leukemia-free survival (LFS) significantly increased among the 3 categories, being 16% and 11%, 48% and 39%, and 57% and 52%, respectively. In our study, only 3 patients underwent an auto-SCT and it is, therefore, impossible to draw any conclusion on this topic; nevertheless, 2 of 3 patients are in continuous complete remission at 42.8 and 57.2 months, respectively, confirming that this procedure may indeed be effective in this subset of patients. In conclusion, this study shows that in adult Ph+ ALL a sequential strategy based on an induction with imatinib and steroids followed by chemotherapy and, when possible, by a SCT is feasible, well tolerated and provides superior results in terms of CHR achievement, long-term DFS and OS compared to other studies in which imatinib/dasatinib are administered together with chemotherapy during the induction phase or following chemotherapy. Based on a case series of almost 200 adult patients with Ph+ ALL, with no upper age limit, the GIMEMA group has observed that, with the use of a TKI alone (plus steroid and intrathecal treatment), more than 95% of cases can obtain a CHR with no deaths in induction. We continue, therefore, to support a strategy that allows a CHR to be obtained in virtually all patients, including the elderly, based on this chemotherapy-free approach. Furthermore, our study confirms the importance of MRD, the negativity of which should be a major objective in this disease: a higher rate of MRD negativity is likely to be achieved with the inclusion of 2nd- and, more likely, 3rd-generation TKI such as ponatinib, which have been proven to have a greater debulking effect,9,14,16,55 and might possibly be more active in patients with the BCR-ABLp210 form, given the lower MRD clearance observed in this subgroup of patients. As far as ponatinib is concerned, a recent study from the MDACC55 on 37 patients has indeed shown that its use as upfront therapy, combined with chemotherapy, is able to induce extremely promising results with 2-year event-free survival (EFS) and OS of 81% and 80%, respectively, although 6 toxic deaths were recorded.55
Other strategies, based on the use of monoclonal antibodies or other immunologic approaches, need to be tested with the goal of offering an overall chemotherapy-free approach that might eradicate/control residual leukemic clones. This is particularly challenging for the elderly/less fit patients, particularly as the prevalence of Ph+ ALL cases increases with age.4 Finally, an allo-SCT, that is still the only potentially curative strategy, might be offered to patients who have been spared prior chemotherapy. This approach should help to reduce side effects, thus sparing further toxicity.
Supplementary Material
Acknowledgments
The authors wish to thank Sandra De Simone for administrative support and data management.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/12/1544
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) Special Program Molecular Clinical Oncology, 5×1000 (MCO1007), Milan, Italy; Ministero dell’Università e Ricerca (MIUR), Fondo per gli Investimenti della Ricerca di Base (FIRB), Rome, Italy; Progetti di Ateneo Sapienza Università di Roma, 2015 (#C26A15F9WW); Progetto Giovani Ricercatori 2010, Policlinico di Modena (#GR-2010-2313609). The GIMEMA Foundation, sponsor of the study, received unrestricted research grants from Novartis. Finally, we also would like to acknowledge AIL (Associazione Italiana contro le Leucemie-Linfomi e Mielomi).
References
- 1.Mancini M, Scappaticci D, Cimino G, et al. A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood. 2005;105(9):3434–3441. [DOI] [PubMed] [Google Scholar]
- 2.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007; 109(8):3189–3197. [DOI] [PubMed] [Google Scholar]
- 3.Burmeister T, Schwartz S, Bartram CR, et al. Patients’ age and BCR–ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918–919. [DOI] [PubMed] [Google Scholar]
- 4.Chiaretti S, Vitale A, Cazzaniga G, et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. [DOI] [PubMed] [Google Scholar]
- 6.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–466. [DOI] [PubMed] [Google Scholar]
- 7.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL). Blood. 2006;108(5):1469–1477. [DOI] [PubMed] [Google Scholar]
- 8.Delannoy A, Delabesse E, Lhéritier V, et al. Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: results of the GRAALL AFR09 study. Leukemia. 2006;20(9):1526–1532. [DOI] [PubMed] [Google Scholar]
- 9.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. [DOI] [PubMed] [Google Scholar]
- 10.de Labarthe A, Rousselot P, Huguet-Rigal F, et al. Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–1413. [DOI] [PubMed] [Google Scholar]
- 11.Vignetti M, Fazi P, Cimino G, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–3678. [DOI] [PubMed] [Google Scholar]
- 12.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. [DOI] [PubMed] [Google Scholar]
- 13.Ribera JM, Oriol A, Gonzalez M, et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravandi F, O’Brien S, Thomas DA, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome– positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuta S, Matsuo K, Yagasaki F, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25:41–47. [DOI] [PubMed] [Google Scholar]
- 16.Foà R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. [DOI] [PubMed] [Google Scholar]
- 17.Chiaretti S, Vitale A, Elia L, et al. Multicenter total therapy Gimema LAL 1509 protocol for de novo adult Ph+ acute lymphoblastic leukemia (ALL) patients. Updated results and refined genetic-based prognostic stratification. Abstract 81, 57th ASH Annual Meeting & Exposition December 5–8, 2015, Orlando, Florida; USA. [Google Scholar]
- 18.Thyagu S, Minden MD, Gupta V, et al. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia with imatinib combined with a paediatric-based protocol. Br J Haematol. 2012; 158(4):506–514. [DOI] [PubMed] [Google Scholar]
- 19.Ribera JM, García O, Montesinos P, et al. Treatment of young patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia using increased dose of imatinib and deintensified chemotherapy before allogeneic stem cell transplantation. Br J Haematol. 2012; 159(1):78–81. [DOI] [PubMed] [Google Scholar]
- 20.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150–155. [DOI] [PubMed] [Google Scholar]
- 21.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–2366. [DOI] [PubMed] [Google Scholar]
- 23.Laport GG, Alvarnas JC, Palmer JM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood. 2008;112(3):903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in Philadelphia-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2008;41(5):447–453. [DOI] [PubMed] [Google Scholar]
- 25.Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber F, Mustjoki S, Porkka K. Impact of tyrosine kinase inhibitors on patient outcomes in Philadelphia chromosome-positive acute lymphoblastic leukaemia. Br J Haematol. 2009;145(5):581–597. [DOI] [PubMed] [Google Scholar]
- 27.Ottmann OG, Pfeifer H. First-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia in adults. Cur Opin Oncol. 2009;21:S43–S46. [DOI] [PubMed] [Google Scholar]
- 28.Mathisen MS, O’Brien S, Thomas DA, et al. Role of tyrosine kinase inhibitors in the management of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2011;6(3):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu-Dumlao T, Kantarjian H, Thomas DA, et al. Philadelphia-positive acute lymphoblastic leukemia: Current treatment options. Curr Oncol Rep. 2012;14(5):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas X. Philadelphia chromosome-positive leukemia stem cells in acute lymphoblastic leukemia and tyrosine kinase inhibitor therapy. World J Stem Cells. 2012; 26;4(6):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elia L, Mancini M, Moleti ML, et al. A multiplex reverse transcriptase-polymerase chain reaction strategy for the diagnostic molecular screening of chimeric genes: a clinical evaluation on 170 patients with acute lymphoblastic leukemia. Haematologica. 2003;88(3):275–279. [PubMed] [Google Scholar]
- 32.Beillard E, Pallisgaard N, van der Velden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe Against Cancer program. Leukemia. 2003;17(12): 2474–2486. [DOI] [PubMed] [Google Scholar]
- 33.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003; 17(12):2318–2357. [DOI] [PubMed] [Google Scholar]
- 34.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome– positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Kim DW, Kim YJ, et al. Minimal residual disease-based role of imatinib as a first-line interim therapy prior to allogeneic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2003;102(8):3068–3070. [DOI] [PubMed] [Google Scholar]
- 38.Yanada M, Sugiura I, Takeuchi J, et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008; 143(4):503–510. [DOI] [PubMed] [Google Scholar]
- 39.Mizuta S, Matsuo K, Maeda T, et al. Prognostic factors influencing clinical outcome of allogeneic hematopoietic stem cell transplantation following imatinib-based therapy in BCR–ABL-positive ALL. Blood Cancer Journal. 2012;2(5),e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Kim D-W, Cho B-S, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26(11):2367–2374. [DOI] [PubMed] [Google Scholar]
- 41.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome– positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preudhomme C, Henic N, Cazin B, et al. Good correlation between RT-PCR analysis and relapse in Philadelphia (Ph1)-positive acute lymphoblastic leukemia (ALL). Leukemia. 1997;11(2):294–298. [DOI] [PubMed] [Google Scholar]
- 43.Pane F, Cimino G, Izzo B, et al. Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult Philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2005;19(4): 628–635. [DOI] [PubMed] [Google Scholar]
- 44.Radich J, Gehly G, Lee A, et al. Detection of bcr-abl transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation. Blood. 1997;89(7):2602–2609. [PubMed] [Google Scholar]
- 45.Stirewalt DL, Guthrie KA, Beppu L, et al. Predictors of relapse and overall survival in Philadelphia chromosome-positive acute lymphoblastic leukemia after transplantation. Biol Blood Marrow Transplant. 2003;9(3):206–212. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Liu KY, Xu LP, et al. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. J Hematol Oncol. 2012;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1 positive acute lymphoblastic leukemia. Leukemia. 2013;27(6): 1254–1262. [DOI] [PubMed] [Google Scholar]
- 48.White HE, Matejtschuk P, Rigsby P, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood. 2010;116(22):e111–e117. [DOI] [PubMed] [Google Scholar]
- 49.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005; 65(11):4500–4505. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer H, Wassmann B, Hofmann WK, et al. Risk and prognosis of central nervous system leukemia in patients with Philadelphia chromosome-positive acute leukemias treated with imatinib mesylate. Clin Cancer Res. 2003;9(13):4674–4681. [PubMed] [Google Scholar]
- 51.Takayama N, Sato N, O’Brien SG, et al. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid. Br J Haematol. 2002;119(1):106–108. [DOI] [PubMed] [Google Scholar]
- 52.Porkka K, Koskenvesa P, Lundán T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. [DOI] [PubMed] [Google Scholar]
- 53.Wetzler M, Watson D, Stock W, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. 2014;99(1):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giebel S, Labopin M, Gorin NC, et al. Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur J Cancer. 2014; 50(2):411–417. [DOI] [PubMed] [Google Scholar]
- 55.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015; 16(15):1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


