Up to 20% of patients with multiple myeloma (MM) show signs of peripheral neuropathy (PN) at primary diagnosis.1 Treatment with neurotoxic agents such as bortezomib or thalidomide increases rates of PN in newly diagnosed patients by up to 50%.2 Since subcutaneous (SC) administration reduces rates of bortezomib-induced neuropathy (BiPN),3–5 nowadays bortezomib is mainly given subcutaneously in clinical trials and in general practice. Only limited data are available on risk factors for BiPN in the era of SC bortezomib. The GMMG MM5 phase III trial (Eudract n. 2010-019173-16) (Online Supplementary Appendix) demonstrated non-inferiority of 3 cycles of VCD (bortezomib, cyclophosphamide, dexamethasone) compared to PAd (bortezomib, doxorubicin, dexamethasone) induction therapy for newly diagnosed MM.6 The route of administration for bortezomib was changed from intravenous (IV) to SC after 314 of 604 patients were enrolled due to the improved toxicity profile demonstrated in relapsed MM.3 The first comparison between IV- and SC-treated patients (published in this Journal in 20155) showed a reduction of adverse events (AEs) and no impact on overall response rates (ORR). This current subanalysis aimed to identify risk factors for BiPN in using IV and SC administration routes in MM patients and analyzed potential effects on treatment response.
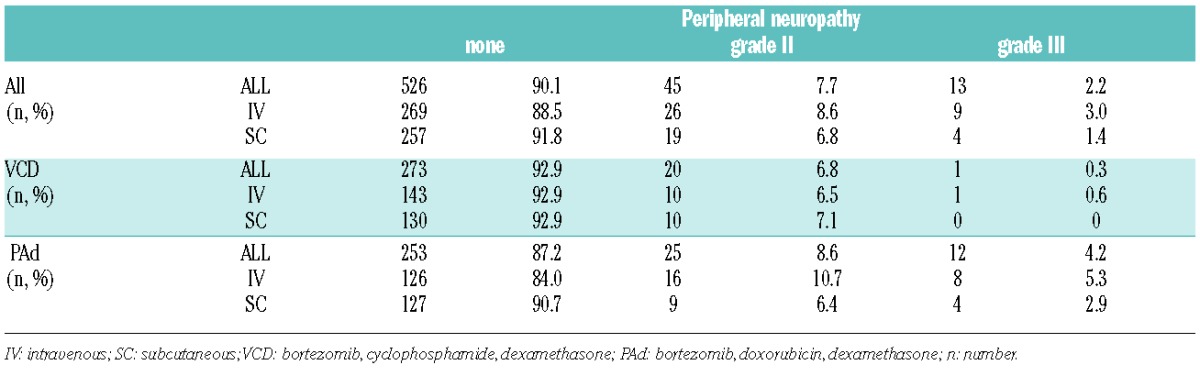
In the GMMG MM5 trial, PN was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), v.4.0. Patients were assessed before and during every cycle on a regular basis by a physician, and this included a physical examination. Peripheral neuropathy of grade II or over within 30 days after end of induction therapy was recorded on a per patient basis. Patients in the PAd arm developed more frequently PN grade I or over compared to VCD-treated patients, regardless of whether they received SC or IV bortezomib (Table 1). No PN grade IV was observed. Bortezomib was discontinued by 13 patients in the PAd arm (IV: n=11; SC: n=2) and by 7 patients in the VCD arm (IV: n=4; SC: n=3). Causal relationship between treatment with bortezomib and occurrence of PN was characterized as related or probably related in 89.2% of cases in the PAd arm (IV: 83.0%; SC: 100%) and 76.2% in the VCD arm (IV: 72.7%; SC: 80.0%). A possible explanation for higher PN rates in the PAd arm might be that inflammation is a major contributor in the pathogenesis of BiPN.7 Due to the different cumulative dexamethasone doses in VCD and PAd (320 mg/cycle vs. 240 mg/cycle), and the immunosuppressive properties of cyclophosphamide, patients in the VCD arm might experience less neuro-inflammation, and ultimately less PN. The different combination partners most likely caused the observed effect since there was no difference in median cumulative bortezomib doses between the PAd and VCD arms (28.8 mg). Furthermore, a recent trial by the Intergroupe Francophone du Myélome (IFM) combined the immunomodulatory drug lenalidomide with bortezomib/dexamethasone and demonstrated lower rates of grade II or over PN compared to already published results from prospective trials of bortezomib/doxorubicin combinations before autologous stem cell transplantation (ASCT).8–10 No grade III or grade IV PN was observed in the IFM trial.8 This underlines the impact of combination partners on bortezomib-induced toxicity and suggests that immunological effects might influence occurrence of PN.
Table 1.
Incidence of grade II or over peripheral neuropathy during and 30 days after induction therapy.
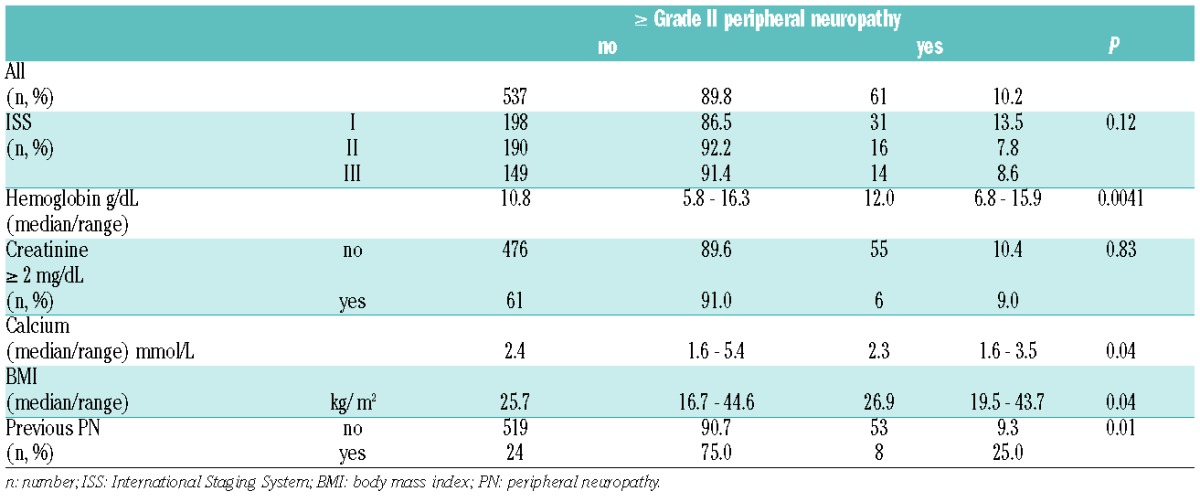
The analysis of baseline characteristics revealed that patients with pre-existing PN [32 of 604 (5.3%) patients] developed more frequently grade II or over PN after induction therapy compared to asymptomatic patients (25.0 vs. 9.3%; P=0.01) (Table 2). This is in line with a subanalysis from the VISTA phase III trial of IV bortezomib in combination with melphalan/prednisone in newly diagnosed MM that identified pre-existing PN as the only consistent risk factor for BiPN.2 In line with the VISTA study, we did not observe that baseline International Staging System (ISS) or creatinine had any effect on the development of PN. However, we observed a trend towards higher baseline body mass index (BMI) in patients with grade II PN or over.2 This might reflect the known association of obesity with neuropathy.11
Table 2.
Differences in baseline characteristics between patients with or without grade II or over peripheral neuropathy.
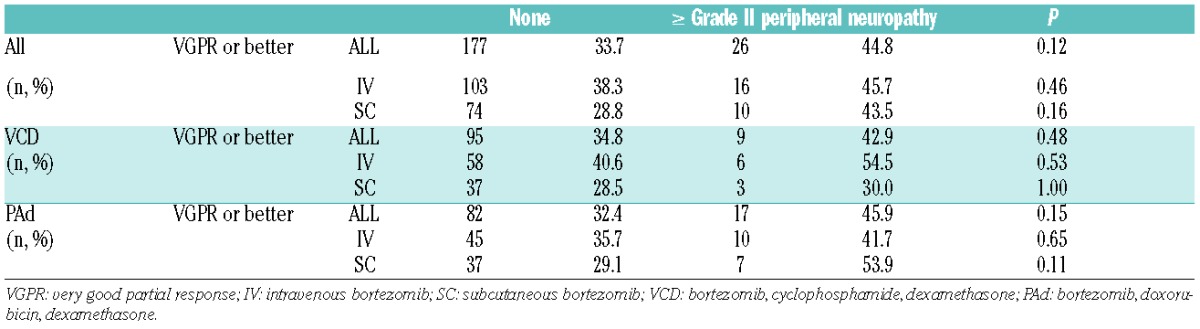
To further characterize risk factors for PN, a multivariate model was fitted that took into consideration the route of administration (SC vs. IV), treatment arm (VCD vs. PAd), and prior PN (yes vs. no). Multivariate analysis confirmed that treatment with VCD instead of PAd was the most important protective factor for grade II or over PN in the GMMG MM5 trial [odds ratio (95% confidence interval): 0.49 (0.28–0.89); P=0.017]. Patients with preexisting PN had the highest risk of developing grade II or over PN [3.56 (1.42–8.21); P=0.004]. The fact that patients with pre-existing PN developed grade II or over PN more often than asymptomatic patients (even if treated subcutaneously) emphasizes the importance of screening for neurological symptoms before the start of therapy. Interestingly, a recent study found that even untreated MM patients without clinically evident PN show decreased peripheral innervation.12 Therefore, early detection and monitoring of PN is important since dose modifications might prevent occurrence of severe PN and symptoms reverse in more than 50% of patients.13 The treatment protocol of the GMMG MM5 trial included dose modification guidelines for BiPN based on previously published data (Online Supplementary Appendix) and approximately 50% of patients in both arms showed improvement of PN during follow up.5 Loss of efficacy due to reduced cumulative doses is a major concern with treatment modifications. A previous publication reported that patients in the GMMG MM5 trial showed high treatment adherence and median cumulative bortezomib doses were higher in SC-treated patients from both arms (VCD: SC 28.8 mg, IV 27.9 mg; PAd: SC 28.9 mg, IV 27.6 mg).5 Response assessment after 3 cycles of induction therapy revealed no differences in rates of very good partial remission (VGPR) or better between patients with or without grade II or over PN. Patients experiencing grade II or over PN tended to achieve VGPR or better more often after induction therapy, irrespective of whether they were treated with VCD or PAd, or whether bortezomib treatment was given via IV or SC administration (Table 3). Similar results were obtained in the VISTA trial.2 The authors suggested that higher cumulative doses of bortezomib are associated with both increased quality of response and occurrence of PN.2 These and our results do not allow us to draw any conclusions as to whether higher susceptibility for PN is associated with increased treatment response.
Table 3.
Differences in response to induction therapy in patients with or without grade II or over peripheral neuropathy.
Multivariate analysis revealed also that patients treated with SC bortezomib had lower risk for grade II or over PN without reaching statistical significance [0.70 (0.40–1.22); P=0.212]. The first interim analysis from the GMMG MM5 trial showed that rates of BiPN were reduced in a dose-dependent fashion since significant differences between SC- and IV-treated patients occurred only in the last cycle of induction therapy.5 Furthermore, our current analysis showed no difference in PN rates between SC- and IV-treated patients in the VCD arm. This is in contrast to the results from Moreau et al. in relapsed MM.3 However, the comparison between both trials is compromised by the fact that we analyzed treatment-naïve, newly diagnosed patients and who received only 3 cycles of a bortezomib-based induction therapy. In the study by Moreau et al., patients might have been already exposed to neurotoxic agents in previous treatment lines and received up to 8 cycles of bortezomib with or without dexamethasone. Furthermore, in contrast to the trial by Moreau et al., we used the up-dated CTCAE catalog version 4.0 instead of version 3.0, which might have caused stage migration effects, especially between grades II and III PN.
One criticism of both trials is the dosing schedule of twice-weekly bortezomib, since AEs can be reduced by once weekly application of bortezomib without loss of efficacy.14 In addition, a recent retrospective analysis by the Czech Myeloma Group did not show any difference in PN rates between SC- and IV-treated patients.15 The majority of patients were treated with weekly instead of twice-weekly bortezomib in the respective study,15 which underlines the fact that not only route of administration but also dose intensity influences development of PN. Although the route of administration might be less important for the occurrence of BiPN in newly diagnosed patients receiving short-term treatment, we still recommend SC administration, since other non-PN AEs occur less frequently.5
Taken together, we confirm that, even in the era of SC bortezomib, pre-existing PN is the most important risk factor for BiPN. Therefore, physicians need to be aware of PN symptoms and dose modification guidelines. Occurrence of PN during therapy has no negative impact on treatment response.
Supplementary Material
Acknowledgments
The authors thank the investigators, the study nurses and all members of the study teams at the participating GMMG trial sites, the teams of the myeloma research laboratory, the FISH laboratory and the central laboratory at the University Hospital Heidelberg, the co-ordination centers for clinical trials (KKS) in Heidelberg and Leipzig, the pharmacies at the trial sites and, most importantly, the participating patients and their families.
Footnotes
Funding: the clinical trial is registered at eudract.ema.europa.eu (n. 2010-019173-16) and at the IRSCTN registry (ISRCTN05622749). Parts of this work were presented at the 57th annual meeting of the 2015 American Society of Hematology, Orlando, FL, USA.
The online version of this letter has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Mateos M-V, Richardson PG, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31. [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 4.Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97(12):1925–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merz M, Salwender H, Haenel M, et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: an interim analysis from the prospective GMMG-MM5 trial. Haematologica. 2015;100(7):964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721–1729. [DOI] [PubMed] [Google Scholar]
- 7.Mangiacavalli S, Corso A, De Amici M, et al. Emergent T-helper 2 profile with high interleukin-6 levels correlates with the appearance of bortezomib-induced neuropathic pain. Br J Haematol. 2010; 149(6):916–918. [DOI] [PubMed] [Google Scholar]
- 8.Roussel M, Lauwers-Cances V, Robillard N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myélome. J Clin Oncol. 2014;32(25):2712–2717. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28(5):800–807. [DOI] [PubMed] [Google Scholar]
- 10.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955. [DOI] [PubMed] [Google Scholar]
- 11.Miscio G, Guastamacchia G, Brunani A, Priano L, Baudo S, Mauro A. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst. 2005;10(4):354–358. [DOI] [PubMed] [Google Scholar]
- 12.Kosturakis AK, He Z, Li Y, et al. Subclinical peripheral neuropathy in patients with multiple myeloma before chemotherapy is correlated with decreased fingertip innervation density. J Clin Oncol. 2014; 32(28):3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. [DOI] [PubMed] [Google Scholar]
- 14.Mateos M-V, Bringhen S, Richardson PG, et al. Bortezomib cumulative dose, efficacy, and tolerability with three different bortezomib-melphalan-prednisone regimens in previously untreated myeloma patients ineligible for high-dose therapy. Haematologica. 2014; 99(6):1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minarik J, Pavlicek P, Pour L, et al. Subcutaneous bortezomib in multiple myeloma patients induces similar therapeutic response rates as intravenous application but it does not reduce the incidence of peripheral neuropathy. PloS One. 2015;10(4):e0123866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


