Abstract
In hematological malignancies complex interactions exist between the immune system, microorganisms and malignant cells. On one hand, microorganisms can induce cancer, as illustrated by specific infection-induced lymphoproliferative diseases such as Helicobacter pylori-associated gastric mucosa-associated lymphoid tissue lymphoma. On the other hand, malignant cells create an immunosuppressive environment for their own benefit, but this also results in an increased risk of infections. Disrupted innate immunity contributes to the neoplastic transformation of blood cells by several mechanisms, including the uncontrolled clearance of microbial and autoantigens resulting in chronic immune stimulation and proliferation, chronic inflammation, and defective immune surveillance and anti-cancer immunity. Restoring dysfunction or enhancing responsiveness of the innate immune system might therefore represent a new angle for the prevention and treatment of hematological malignancies, in particular lymphoid malignancies and associated infections. Recently, it has been shown that cells of the innate immune system, such as monocytes/macrophages and natural killer cells, harbor features of immunological memory and display enhanced functionality long-term after stimulation with certain microorganisms and vaccines. These functional changes rely on epigenetic reprogramming and have been termed ‘trained immunity’. In this review the concept of ‘trained immunity’ is discussed in the setting of lymphoid malignancies. Amelioration of infectious complications and hematological disease progression can be envisioned to result from the induction of trained immunity, but future studies are required to prove this exciting new hypothesis.
Introduction
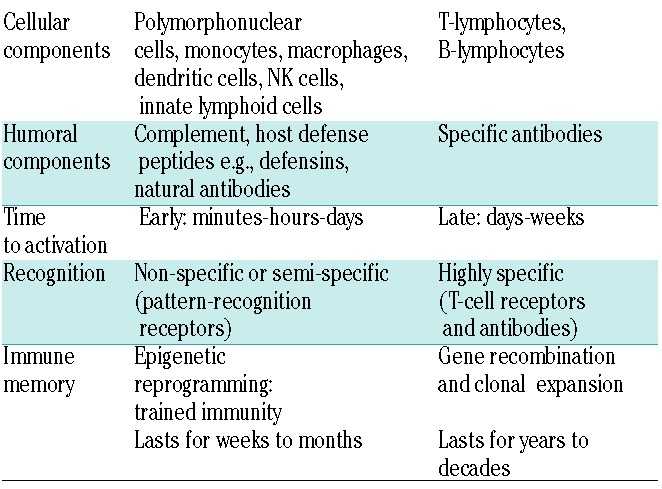
In order to combat infections and cancer the human body is equipped with an innate and adaptive immune system. The two systems are highly intertwined and closely collaborate with a bridging role for antigen-presenting cells e.g., dendritic cells. Their differences and commonalities are depicted in Table 1 (for more detailed reviews see references1–3). After birth, the immune system evolves from and is shaped by exposure to foreign antigens, which for the most part come from microbes belonging to the gut microbiota.4 Gradually, an immune armamentarium is build which, by virtue of memory properties, acts increasingly specifically, rapidly and efficiently. Immune memory has in the past been attributed solely to the adaptive immune system, but recently the paradigm has shifted with evidence showing that the innate immune system possesses the capacity for immunological memory, designated ‘trained immunity’.5 This feature is crucial for organisms that have no adaptive immune response, e.g., plants and invertebrates, but it also exists in humans, where it might be of special benefit in neonates that have yet to develop a mature adaptive immune repertoire.6 Importantly, ‘training’ of the innate immune system by the use of vaccination, resulting in increased immune activation, has been shown feasible. Hopefully these insights can be exploited in the near future for the design of new treatments for immunodeficiencies, infections and cancer.7
Table 1.
Characteristics of innate and adaptive immune responses.
Currently, immunotherapies used for the treatment of hematological malignancies have focused on the adaptive immune system, mostly T and B lymphocyte responses. Examples are numerous and include the use of allogeneic stem cell transplantation (SCT), monoclonal antibodies, immune checkpoint inhibitors and cellular therapies (e.g., adoptive T cell transfer).8–10 However, accumulating evidence has confirmed the significant impact of deregulated interactions between host innate immune cells (e.g., monocytes, dendritic cells, and NK cells) and microbes (e.g., chronic infections and dysbiosis) in the pathogenesis of cancer.2,11–13 This seems especially true for lymphoid malignancies, where antigenic stimulation by microbes and chronic inflammation drive lymphoproliferation and hence tumor progression.14 Moreover, the immunosuppressive environment that develops in many hematological malignancies, consists of a considerable part of functionally altered innate immune cells, including myeloid-derived suppressor cells (MDSC), that cause immune evasion, progression and dissemination of neoplastic cells.15
Therefore, considering the pivotal role of the innate immune system in the initiation and progression of hematological malignancies, it might prove a valuable target for prevention and treatment. One option would be exploiting the recently discovered feature of innate immune memory which can be induced, for instance, by a Bacillus Calmette-Guérin (BCG) vaccination.5 By enhancing and restoring the function of innate immune cells, clearance of microbial and neoantigens can be achieved and the immunosuppressive tumor microenvironment reversed. In addition, by innate immune training, the infection incidence might be reduced in the high-risk setting of cancer therapy.
In the review herein we summarize the current knowledge on the concept of ‘trained immunity’, and hypothesize ways of extending this concept to the field of lymphoid malignancies.
The concept of trained immunity: a novel type of immune memory
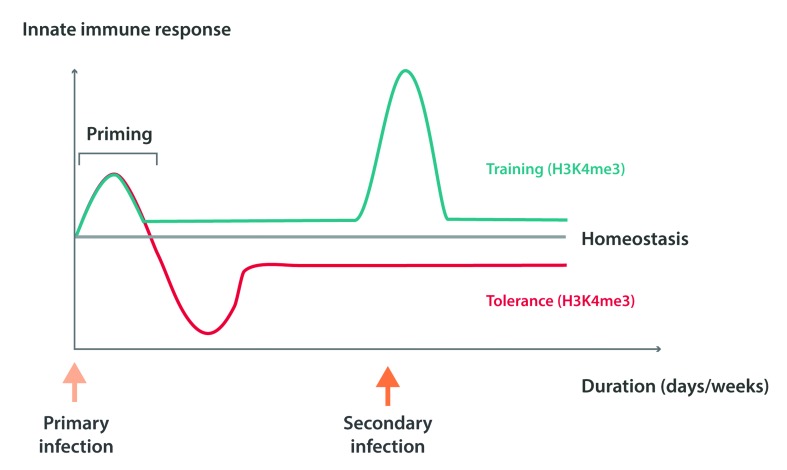
For decades the prevailing assumption has been that immunological memory was a feature characterizing only the acquired immune system. However, recent studies have shown that the mammalian innate immune system also exhibits adaptive properties compatible with immunological memory, for which the term ‘trained immunity’ has been proposed.5 On reinfection or rechallenge with microbial ligands, prototypical innate immune cells such as monocytes/macrophages exhibit enhanced functionality with the release of pro-inflammatory cytokines and effector functions e.g., phagocytosis (Figure 1).16,17 The training of monocytes/macrophages is mediated by the activation of pattern-recognition receptors by microbe-associated molecular patterns (MAMPs) from bacteria and fungi; for example dectin-1 by β-glucan, and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) by components of the BCG vaccine.16–19 Several mechanisms are involved in the development of innate immune memory, among which epigenetic histone modifications (e.g., histone methylation and acetylation), and autophagy play a central role. Monocytes are functionally reprogrammed for either enhanced (training) or decreased (tolerance) cytokine production, depending on the type and concentration of MAMPs they encountered17,20–22 (Figure 1). The epigenetic reprogramming of monocytes after MAMP stimulation results in functional as well as morphological changes, including cell surface marker modifications such as upregulation of TLR expression.17,20 Autophagy contributes to the process of ‘trained immunity’ induced by a BCG vaccination, as pharmacological or genetic (autophagy gene polymorphisms) inhibition blocks the epigenetic programming of monocytes from occuring.23
Figure 1.
Epigenetic regulation of memory-like activity of innate immune cells. The biological phenomena of endotoxin tolerance and trained immunity are depicted in the illustration. Stimulation of monocytes and/or macrophages via pattern recognition receptors (PRRs), such as Toll-like receptor 4 (TLR4) or dectin-1, leads to increased expression of pro-inflammatory genes. Decreased or increased responsiveness of these cells to subsequent PRR stimulation may then occur, depending on the initial type of stimulus that the cell received. TLR4 stimulation with lipopolysaccharide (LPS) can induce a state of endotoxin tolerance (represented by the red line), whereas the stimulation of dectin-1 with β glucan from Candida albicans leads to a state of trained immunity (represented by the blue line). Initial PRR stimulation leads to trimethylation of histone H3K4 (H3K4me3) and global acetylation of histone H4 on promoters of pro-inflammatory genes. In the case of endotoxin tolerance, the removal of the stimulus results in the loss of activating marks and gene expression returns to basal levels. Following a second encounter with the stimulus, ‘tolerized’ genes will not regain the H3K4me3 mark or acetylation, and will remain silent to stimulation. In the case of ‘trained immunity’, pro-inflammatory genes will retain enhancers marked with monomethylation of histone H3K4. A second stimulus will induce transcription factors to bind to the enhancers and promoters of these genes, thereby promoting an increase in H3K4me3 and thus the expression of ‘trained’ genes.22
Natural killer (NK) cells belonging to the innate immune system also display memory functions, mainly during viral infections. Expression of the Ly49H receptor and chemokine receptor CXCR6 seem to be of importance for the memory characteristics of NK cells.24,25 In addition, epigenetic modifications with changes in promoter methylation status are a hallmark of adaptive NK cells.26,27 The adaptive immune features of NK cells result in their long-term activation and protection from infection or reinfection with both herpesviruses and also influenza.24–26,28 NK cell training can also be achieved by BCG vaccination with the induction of non-specific immune memory, as has been shown in healthy volunteers.29
‘Trained immunity’ of innate immune cells provides protection against reinfection in a direct T/B-cell-independent manner. In addition, a more or less indirect effect on the acquired immune system may also occur as monocytes/macrophages and NK cells influence cells of the adaptive immune system.1 For instance, after BCG vaccination complex cytokine profiles are induced that include elevated T-helper (Th) 1 cytokines like IFNγ and also interleukin (IL)-4 (Th2), IL-17 (Th17), and IL-10.30–33 These responses belong to the adaptive immune responses that facilitate and contribute to infection control and vaccination efficacy.
Important hallmarks of ‘trained immunity’ are the nonspecific nature and long duration of the enhanced immune responses. Trained monocytes have been shown to circulate in the peripheral blood for up to 3 months after BCG vaccination, and NK responses, e.g., release of IFNγ, are also enhanced for months.16,29 Immune training of both monocytes and NK cells induces protection from infectious agents other than the primary stimulus. In vitro studies demonstrated an increased innate immune response against a plethora of pathogens after BCG vaccination, including Mycobacterium tuberculosis, Candida albicans, and Staphylococcus aureus.16,29 Moreover, in vivo data exist from large vaccination trials. In low birth weight children from Guinea-Bissau, vaccination with BCG resulted in reduced neonatal mortality, which was the result of a composite effect consisting of reduced neonatal sepsis, respiratory infections and fever.34 However, this protective effect did not result in overall reduced infant mortality, suggesting that it was most pronounced in neonates.6 An alternative explanation, however, might be the fact that the longevity of the training effect is in the order of months and probably not years, although data on the exact duration are lacking. In Danish cohorts similar results were also achieved with BCG and smallpox vaccinations, significantly reducing the risk of hospitalization for most subgroups of infectious diseases, especially respiratory tract infections.35,36 Intriguingly, non-specific protection might go beyond infections, as the BCG vaccination has been shown to be effective in treating carcinomas (e.g., bladder cancer or melanoma).37
Considering the protective effects of ‘trained immunity’ against infections, the concept might be exploited to improve care for cancer patients who suffer from infections. The concept might even be more important in malignancies whose pathogenesis entails exposure to microorganisms (chronic antigenic stimulation) and impaired innate and adaptive immune responses, hence hematological, but in particular, lymphoid malignancies.
Role for microbes in lymphoid malignancies
Complex interactions between microorganisms, immune cells and cells exhibiting features of neoplastic transformation eventually determine the development of overt malignant disease. This intriguing interrelatedness of biological events is especially encountered in those circumstances where immune cells themselves are the cells at risk for malignant transformation, thus in the setting of lymphoid malignancies including multiple myeloma (MM), chronic lymphocytic leukemia (CLL) and malignant lymphomas. These diseases constitute ‘exemplary models’ in which the role of ‘trained immunity’ can be explored.
Infections in patients with lymphoid malignancies
Patients with cancer suffer from an increased risk for common and opportunistic infections that result from disease intrinsic and anti-cancer therapy-induced immune deficiencies. The severity of these deficiencies and the specific nature of the defects determine which infections can be expected to occur in individual patients. An elaborate review of this topic is beyond the scope of this article and an overview can be found in a recent publication.38
Malignant lymphomas and CLL significantly increase the risk for serious infections.39,40 The cause of this increased risk for infections is multifactorial. Early on, the humoral immune abnormalities, resulting from B-lymphocyte dysfunction often accompanied by the emergence of hypogammaglobulinemia, dominate, and infections are caused mainly by viruses and encapsulated bacteria. With the progression of the lymphoid malignancies T cell dysfunction occurs, which is often therapy-related, but not exclusively, as early after diagnosis T cell defects/alterations are already present.41,42 Malignant B lymphocytes produce anti-inflammatory cytokines, like IL-10 and TGF-β, and indoleamine 2,3-dioxygenase that skew the balance from a Th1/Th17 towards a regulatory (FOXP3) T cell phenotype.43,44 Moreover, tumor cells express immunosuppressive ligands like programmed death-ligand 1 (PD-L1) and PD-L2 that inhibit proliferation and activation of effector memory T cells.41,45 In addition, the recruitment of tumor supporting innate myeloid cells; including nurse-like cells, tolerogenic DCs, tumor-associated macrophages (M2-polarized) and MDSCs; to the tumor micro-environment occurs, which enhance the state of tolerance.43,46–49 Through these mechanisms, malignant B cells, besides inhibiting anti-tumor immunity, also impair immune responses that are crucial for the control of both common and opportunistic pathogens. Treatment with anti-neoplastic drugs, including purine analogs, and monoclonal antibodies, further impair specific cellular immunity with an increased risk of opportunistic infections.
Infection-induced lymphoid malignancies
The role of microorganisms in the pathogenesis of cancer is increasingly appreciated. Several classical pathogens have been implicated in the origin of specific hematologic malignancies. The Epstein-Barr virus is exemplary. It is responsible for the disease infectious mononucleosis, which has also been associated with a diverse range of malignant lymphomas in immunocompetent and immunocompromised patients e.g., Burkitt lymphoma, and post-transplant lymphoproliferative disorder. Other well-known examples are Helicobacter pylori, Borelia burgdorferi, Coxiella burnetii and the hepatitis C virus (HCV), which have been associated with marginal zone lymphoma (MZL) and diffuse large B-cell lymphomas (DLBCL).14,50 Some of these diseases can initially be treated with antimicrobial agents with a considerable chance for cure, despite the fact that they can be considered monoclonal lymphoproliferative disorders.51,52
Beyond specific pathogens, it has been shown that experiencing common community-acquired viral and bacterial infections early in life increases the risk for developing lymphoid malignancies later in life. Large epidemiological studies have shown clear associations between infections and monoclonal B-cell lymphocytosis (MBL) and CLL, non-Hodgkin lymphoma (NHL), and MM.53–55 More recently, the commensal bacterial flora of the gut (microbiota) has also been implicated in lymphomagenesis.12
The expression of restricted immunoglobulin gene repertoires/B-cell receptors (BCR) in CLL and malignant lymphomas underscores a key role of an antigenic drive in the initiation and perpetuation of lymphoproliferation; mainly microbial antigens but also self-antigens released on cell apoptosis.56–59 In CLL, over 30% of cases can be grouped together based on the expression of stereotypic BCRs with characteristic complementarity determining region 3 (CDR3) amino acid sequences. As CDR3s are most decisive for the antigen specificity of immunoglobulins (Igs), this strongly suggests that distinctive antigens are involved in the development of subsets of CLL. It was recently shown that a newly identified subset of CLL with mutated IgVH heavy chain, expresses stereotypic BCRs highly specific for β-(1,6)-glucan, a major antigenic determinant of yeast and molds.60 The clonal B-cells of these patients were shown to proliferate in response to β-(1,6)-glucan, suggesting that the fungal microbiota can deliver functional ligands in the process of CLL.
Mechanisms involved in the neoplastic transformation of lymphocytes by microorganisms include: the release of genotoxic metabolites, antigen-driven lymphoproliferation, induction of chronic inflammation, impaired apoptosis, inactivation of the tumor suppressor gene p53, disrupted DNA repair, mitochondrial dysfunction, and oxidative stress.12,14,61–63 Antigen-driven lymphoproliferation is implicated in the pathogenesis of CLL and NHL, including MZL, mantle cell lymphoma (MCL), follicular lymphoma, and DLBCL (Figure 2).64 However, B-cells express many other (surface) receptors involved in microbial antigen recognition in addition to the BCR, including CD5 and CD6 (CLL) and pattern recognition receptors like TLR1, TLR2, TLR6, TLR7, TLR9, NOD1 and NOD2 (CLL, NHL).65,66 Signaling through these receptors is exploited by the malignant cells for their own survival, precisely as shown for BCR activation. The TLRs expressed by CLL cells, for instance, are functional, as upon stimulation, the nuclear factor-κB signaling pathway becomes activated, protecting CLL cells from spontaneous apoptosis.65
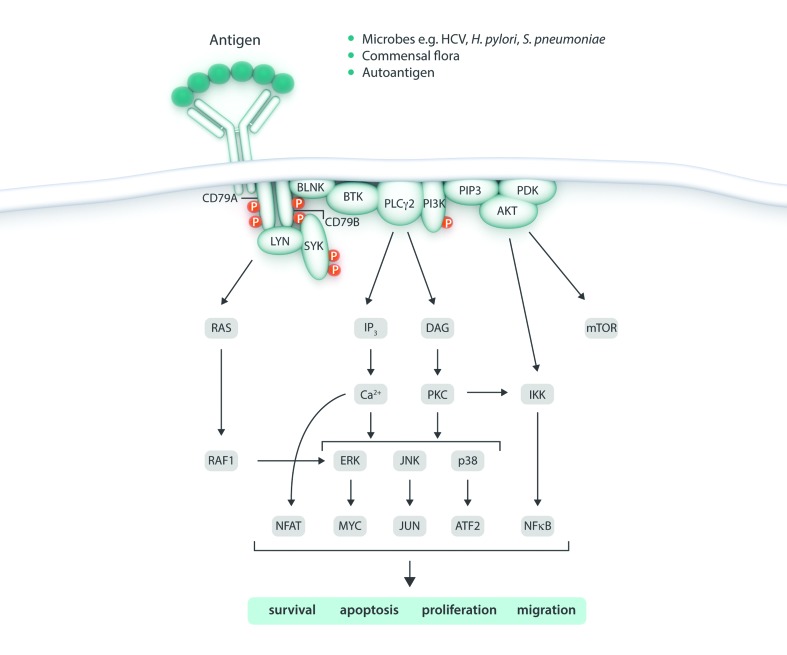
Figure 2.
Antigen-driven B cell receptor (BCR) signaling in lymphoproliferative diseases. Chronic stimulation of the BCR by microbial antigens and autoantigens results in the activation of several intracellular signaling pathways resulting in lymphoproliferation, reduced apoptosis, prolonged cell survival and disrupted cell migration that can contribute to the emergence of lymphoid malignancies. HCV: Hepatitis C virus; H. pylori: helicobacter pylori; S. pneumoniae: streptococcus pneumoniae; mTOR: mechanistic target of rapamycin; BLNK: B cell linker protein; BTK: Bruton’s tyrosine kinase; PLCγ2: phospholipase C Gamma 2; PI3K: phosphatidylinositol 3-kinase; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; IP3: inositol 1,4,5-trisphosphate; DAG: diacylglycerol; PDK: protein kinase D; Ca: calcium; JNK: Jun N-Terminal kinases; IKK: IκB kinase; PKC: protein kinase C; NFAT: nuclear factor of activated T-cells; NF-κB: nuclear factor kappa B; ATF2: activating transcription factor 2.
Cancer-induced immune-suppression; cancer progression and infections
Many cancers facilitate their preservation and progression by protecting themselves against the host’s immune surveillance armamentarium by inducing an immunosuppressive environment. The acquired immune system seems most affected with pronounced deficits occurring in anti-tumor T cell responses resulting from several interrelated mechanisms, as described above, with an important role for the accumulation of immunosuppressive innate myeloid cells (e.g., MDSC).15 T cells from CLL patients exhibit deviant T cell subset distributions, and have functional defects, including impaired ability to form immunological synapses, decreased proliferative capacity and an impaired effector function.67,68 These functional defects coincide with an increased expression of CD244, CD160, and PD-1 on CLL-derived T cells, a phenotype that is similar to the phenotype of exhausted T cells in chronic viral infections.69 Targeting these immune checkpoints has been a new approach in the treatment of malignant lymphomas, and is now being explored in large clinical studies.70
Also relevant to anti-tumor immune responses are immune cells belonging to the innate immune systems, such as NK cells and monocytes/macrophages. Defects in these immune effectors that contribute to cancer initiation and progression have been increasingly described in lymphoid malignancies. For instance, NK cells found in the circulation of CLL patients appear to have several functional defects, including impaired cytotoxic activity, possibly because of the defective expression of the NKG2D co-receptor.71 Moreover, the monocytic population of CLL patients has an altered composition and monocytes exhibit deregulation of genes that are involved in phagocytosis and inflammation.72 Higher numbers of non-classical CD14+CD16++ monocytes are present that are known to have immune suppressive features opposed to the classical CD14++CD16− counterparts. Intriguingly, monocytes from CLL patients exhibit the primary features of ‘endotoxin tolerance’, including low cytokine production, high phagocytic activity and impaired antigen presentation.73 The refractory state of these cells prohibits sufficient inflammatory responses to occur after pathogens and cancer cells (‘tumor tolerance’). This introduces a new mechanism of innate immune failure that contributes to the susceptibility of CLL patients to infections and tumor progression.73
Trained immunity in hematological malignancies
Evidence for ‘trained immunity’ in cancer therapy
Training the innate immune system seems feasible and effective, as for decades BCG vaccination has been successfully applied in the treatment of urothelial cell carcinomas and melanomas.37 Data from large epidemiological studies and vaccination trials reveal circumstantial evidence for a similar potential in hematological malignancies. In a previous Danish case-cohort study it was shown that BCG vaccination during infancy significantly reduces the risk of developing lymphoma’s (HR 0.49 (95% CI: 0.26–0.93)).74 Mechanisms proposed, attempting to explain this beneficial result, included the stimulating effect of vaccination on immune surveillance of cancer and the decreased incidence of infectious diseases involved in lymphoma pathogenesis. Older studies have tested the concept of the induction of anti-tumour immunity in patients with AML by vaccination.75,76 However, these studies were small, and although some small benefits were shown, these data preclude drawing definite conclusions. At least BCG vaccination in these patients did not result in untoward complications.
The use of β-glucan as an immune adjuvant in the treatment of solid and hematological malignancies has evoked considerable interest for many years, as the MAMP shows promising activity both in vitro and in vivo. The anti-tumor activity directly relates to signaling through dectin-1,77,78 suggesting that the innate and acquired immunity elicited by β-glucan could be of therapeutic value. In a small phase I/II study, twenty patients with advanced malignancies who were receiving chemotherapy were additionally treated with a β-(1,3)/(1,6)-D-glucan preparation. Preliminary results showed that β-glucan was well tolerated in these patients and suggested a beneficial effect on hematopoiesis. Moreover, one patient with a chemotherapy-refractory malignant lymphoma achieved a partial response.79
Mechanisms of action
The concept of ‘trained immunity’ can be explored as a new modality of cancer prevention and therapy, as well as in infection control. The concept seems appropriate to consider in the setting of lymphoid malignancies, and three major mechanisms can be envisioned to contribute to the effects of innate immune enhancement in this setting (Figure 3).
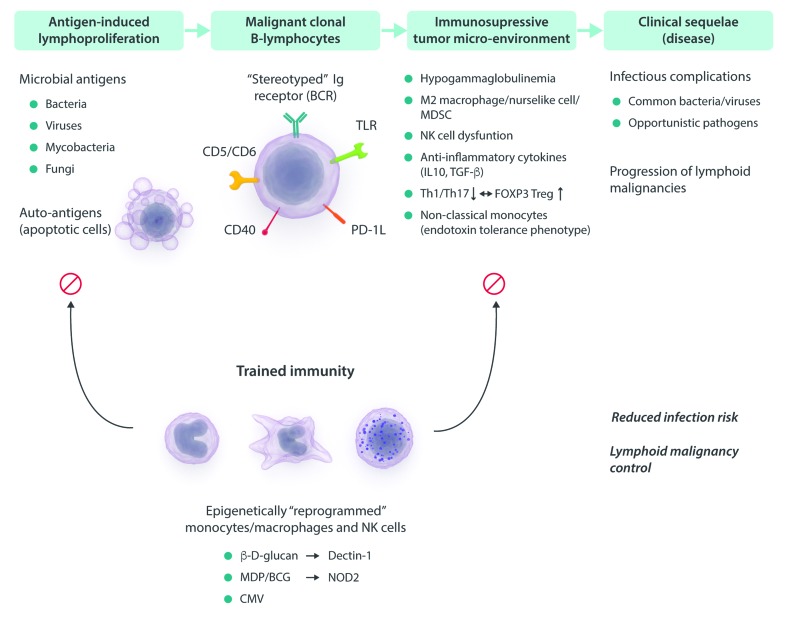
Figure 3.
Concept of ‘trained immunity’ of the innate immune system in lymphoid malignancies. Training of innate immune cells, including monocytes/macrophages and NK cells, with microbial ligands results in enhanced effector functions of these cells. By facilitating antigen eradication and restoration of tumor surveillance and anti-tumor immunity, the succeeding events that would ultimately result in the development of lymphoid malignancies might be interrupted or at least delayed. In addition, enhanced immunity will also prevent and reduce infectious complications. Ig: immunoglobin; BCR: B cell receptor; TLR: toll-like receptor; MDP: muramyl dipeptide; BCG: Bacillus Calmette-Guérin; CMV: cytomegalovirus; NK: natural killer; PD-1L: programmed death-ligand 1; MDSC: myeloid-derived suppressor cells; Th: T helper; TREG: regulatory T cell; IL-10: interleukin 10; TGF-β: transforming growth factor-beta; NOD2: nucleotide-oligomerization domain-containing protein 2.
1. Decreasing antigen-driven lymphoproliferation:
Many data support the hypothesis that malignant B cells, found in CLL and NHL e.g., MZL and MCL, resemble antigen-activated B cells and that ongoing antigen-induced modulation of cell responses occur in the context of antigen recognition by the BCR and affiliated receptors (Figure 2). Inhibiting this signaling cascade has already proven to be of clinical use with the introduction of potent BCR inhibitors, e.g., ibrutinib, and therefore eradicating B-cell stimulating antigens and infectious antigens and autoantigens may also alter the course of lymphoproliferative diseases. This might be achieved by ‘trained immunity’, as monocytes and macrophages can be activated to prevent many infections that have been implicated in the pathogenesis of lymphoma. Since monocytes and macrophages are also pivotal in apoptotic cell clearance, training of these cells might contribute to autoantigen eradication.80,81
2. Reversing immune tolerance:
Innate immunity
Considering the contribution of monocytes that exhibit ‘endotoxin and tumor tolerance’ to tumor progression and infection risk, the reversal of their refractory state by ‘trained immunity’ might prove clinically beneficial. An increased tolerogenic state of monocytes and macrophages has clearly been implicated in lymphoid malignancies.82 Nevertheless, whether vaccination with BCG or β-glucan can restore the balance and skew tumor associated macrophages from the M2 towards a more M1 phenotype with beneficial anti-tumor characteristics, remains to be proven. However, recently data have shown that another type of immunosuppressive myeloid cell, i.e., MDSC, can be successfully reprogrammed by exposure to β-glucan, resulting in the loss of its immunosuppressive phenotype and gain of enhanced antigen presenting capacities.83
In addition, a therapeutic approach in which NK cell immunity can be enhanced, for instance by BCG vaccination, may lead to an improved function of NK cells, thereupon overcoming cancer-induced NK cell function defects.29,84
Acquired immunity
General T cell dysfunction and exhaustion contribute to tumor tolerance and defective immune surveillance. Training the innate immune system also influences T cell activity and skewing of acquired immune responses towards Th1 and Th17 phenotypes that contribute to anti-tumor immunity.
Generation of adaptive NK cells
NK cells play a pivotal role during the treatment of hematological malignancies, and their activity might be enhanced by ‘training’. The adoptive transfer of ex vivo generated autologous and allogeneic NK cells is being explored for immunotherapy in the setting of lymphoid malignancies.85,86 Interestingly, considerable evidence exists for a close relationship between CMV infection post SCT and relapse of AML, in which NK cells are deemed to be the effectors. Moreover, CMV infection results in a mature phenotype of NK cells which also have memory-like features.87 Hence, the training of NK cells by exposure to BCG or CMV antigens and/or cytokines ex vivo, might be a novel approach for the optimization of NK cell based immunotherapies.26
How to apply “trained immunity” in hematology patients?
The prevention of lymphoid, and maybe, myeloid malignancies on the population might be achieved with immune training. Vaccination programs have underlined the effect of immune training and the impact on cancer development.74 However, additional studies are needed before standard vaccination can be introduced on a larger scale. But what about the individual patient? Can innate immune training alter the course of manifest hematological malignancies¿ Malignancies that rely on antigen-driven proliferation, hence lymphoid malignancies, seem, at least conceptually, the best candidates for innate immune training. The timing of immune training might, however, prove crucial. The efficacy of this approach is probably at the highest level early on in the disease process, when the tumor burden is low and before tumor evolution has resulted in BCR signaling, independent proliferation and profound immune exhaustion. Moreover, applying a live attenuated vaccine such as BCG, which is normally considered safe, might prove to be less safe in patients with severely impaired T cell immunity. Alternatives, such as gamma-irradiated BCG, may prove more desirable.88 Since these severe immune deficits occur in late stage disease, an early initiation of vaccinations must be pursued. Two conditions therefore seem ideal for testing the concept of innate immune training: MBL/early stage CLL and MZL.
Training the immune system might be most feasible by applying the BCG vaccination. Considerable experience exists with this old vaccination strategy and safety is hardly an issue, even in vulnerable patients, including low birth weight children. Currently no standard immune adjuvant, which is based on β-glucan or MDP, exists, and considerable questions remain to be answered about the route of administration, dosage and safety of these antigens.
Conclusion
Epigenetic reprogramming of innate immune cells results in enhanced non-specific immunity and immune memory. ‘Training’ of the innate immune system is a novel concept in immunology and infectious disease that may be exploited in hematological malignancies, especially lymphoid malignancies, where antigen-driven lymphoproliferation and immune impairments are the hallmarks of the disease. Amelioration of infectious complications and cancer progression can be envisioned as goals of ‘trained immunity’ in cancer therapy. Acknowledging the current limited data on the effects of ‘trained immunity’, and the hypothetical status, thus far, of the concept in the setting of clinical hematology, further studies are mandatory.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/100/12/1460
Funding
MGN was supported by an ERC Consolidator Grant (#310372).
References
- 1.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Ann Rev Immunol. 2011;29(235–271. [DOI] [PubMed] [Google Scholar]
- 3.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175(3):1373–1381. [DOI] [PubMed] [Google Scholar]
- 4.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011; 9(5):355–361. [DOI] [PubMed] [Google Scholar]
- 6.Levy O, Wynn JL. A prime time for trained immunity: innate immune memory in newborns and infants. Neonatology. 2014; 105(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto ML, Maier I, Dang AT, et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013;73(14):4222–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–287. [DOI] [PubMed] [Google Scholar]
- 14.Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107(8):3034–3044. [DOI] [PubMed] [Google Scholar]
- 15.Senovilla L, Aranda F, Galluzzi L, Kroemer G. Impact of myeloid cells on the efficacy of anticancer chemotherapy. Curr Opin Immunol. 2014;30:24–31. [DOI] [PubMed] [Google Scholar]
- 16.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintin J, Saeed S, Martens JH, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinman DM, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect Immun. 1999;67(11):5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ifrim DC, Joosten LA, Kullberg BJ, et al. Candida albicans primes TLR cytokine responses through a Dectin-1/Raf-1-mediated pathway. J Immunol. 2013; 190(8):4129–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ifrim DC, Quintin J, Joosten LA, et al. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clinical Vaccine Immunol. 2014;21(4):534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014; 345(6204):1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol. 2015;15(1):7–17. [DOI] [PubMed] [Google Scholar]
- 23.Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG-induced traine immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10(10):e1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010; 11(12):1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209(5):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clinical Immunol. 2014; 155(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalor MK, Smith SG, Floyd S, et al. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2010;28(6):1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42(2):364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitt JM, Stavropoulos E, Redford PS, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012; 189(8):4079–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinnijenhuis J, Quintin J, Preijers F, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J innate Immun. 2014;6(2):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–252. [DOI] [PubMed] [Google Scholar]
- 35.Sorup S, Villumsen M, Ravn H, et al. Smallpox vaccination and all-cause infectious disease hospitalization: a Danish register-based cohort study. Int J Epidemiol. 2011;40(4):955–963. [DOI] [PubMed] [Google Scholar]
- 36.de Castro MJ, Pardo-Seco J, Martinon-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015; 60(11):1611–1619. [DOI] [PubMed] [Google Scholar]
- 37.Hersh EM, Gutterman JU, Mavligit GM. BCG as adjuvant immunotherapy for neoplasia. Ann Rev Med. 1977;28:489–515. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly JP, Blijlevens NM, van der Velden WJ. Host impairments in patients with neoplastic diseases. Cancer Treat Res. 2014; 161:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9(5):365–370. [DOI] [PubMed] [Google Scholar]
- 40.Morrison VA. Infections in patients with leukemia and lymphoma. Cancer Treat Res. 2014;161:319–349. [DOI] [PubMed] [Google Scholar]
- 41.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. [DOI] [PubMed] [Google Scholar]
- 42.Riches JC, Gribben JG. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Haematol. 2014; 51(3):228–234. [DOI] [PubMed] [Google Scholar]
- 43.Jitschin R, Braun M, Buttner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–760. [DOI] [PubMed] [Google Scholar]
- 44.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2009;69(13):5522–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClanahan F, Riches JC, Miller S, et al. Mechanisms of PD-L1/PD-1-mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Emicro-TCL1 CLL mouse model. Blood. 2015;126(2):212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99(3):1030–1037. [DOI] [PubMed] [Google Scholar]
- 47.Nam SJ, Go H, Paik JH, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014;55(11):2466–2476. [DOI] [PubMed] [Google Scholar]
- 48.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ysebaert L, Fournie JJ. Genomic and phenotypic characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52(7):1404–1406. [DOI] [PubMed] [Google Scholar]
- 50.Melenotte C, Million M, Audoly G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. 2016;127(1):113–121. [DOI] [PubMed] [Google Scholar]
- 51.Ferreri AJ, Sassone M, Kiesewetter B, et al. High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT): the HD-K phase II trial. Ann Oncol. 2015;26(8):1760–1765. [DOI] [PubMed] [Google Scholar]
- 52.Ferreri AJ, Govi S, Raderer M, et al. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multi-center phase 2 trial. Blood. 2012;120(18):3858–3860. [DOI] [PubMed] [Google Scholar]
- 53.Anderson LA, Landgren O, Engels EA. Common community acquired infections and subsequent risk of chronic lymphocytic leukaemia. Br J Haematol. 2009;147(4):444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McShane CM, Murray LJ, Engels EA, Anderson LA. Community-acquired infections associated with increased risk of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinaemia. Br J Haematol. 2014;164(5):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McShane CM, Murray LJ, Engels EA, Landgren O, Anderson LA. Common community-acquired infections and subsequent risk of multiple myeloma: a population-based study. Int J Cancer. 2014;134(7):1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanemo Myhrinder A, Hellqvist E, Sidorova E, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111(7):3838–3848. [DOI] [PubMed] [Google Scholar]
- 58.Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118(11):3088–3095. [DOI] [PubMed] [Google Scholar]
- 59.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010; 463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoogeboom R, van Kessel KP, Hochstenbach F, et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med. 2013;210(1):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado AM, Desler C, Boggild S, et al. Helicobacter pylori infection affects mitochondrial function and DNA repair, thus, mediating genetic instability in gastric cells. Mech Ageing Dev. 2013;134(10):460–466. [DOI] [PubMed] [Google Scholar]
- 62.Strickertsson JA, Desler C, Martin-Bertelsen T, et al. Enterococcus faecalis infection causes inflammation, intracellular oxphos-independent ROS production, and DNA damage in human gastric cancer cells. PloS one. 2013;8(4):e63147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegl C, Rudel T. Modulation of p53 during bacterial infections. Nat Rev Microbiol. 2015;13(12):741–748. [DOI] [PubMed] [Google Scholar]
- 64.Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol. 2013;23(6):410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muzio M, Scielzo C, Bertilaccio MT, Frenquelli M, Ghia P, Caligaris-Cappio F. Expression and function of toll like receptors in chronic lymphocytic leukaemia cells. Br J Haematol. 2009;144(4):507–516. [DOI] [PubMed] [Google Scholar]
- 66.Fonte E, Agathangelidis A, Reverberi D, et al. Toll-like receptor stimulation in splenic marginal zone lymphoma can modulate cell signaling, activation and proliferation. Haematologica. 2015;100(11):1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013; 121(9):1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang ZZ, Grote DM, Xiu B, et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin’s lymphoma. Leukemia. 2014;28(9):1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kater AP, van der Windt GJ. PD-L1 blockade: rejuvenating T cells in CLL. Blood. 2015;126(2):126–128. [DOI] [PubMed] [Google Scholar]
- 71.Huergo-Zapico L, Acebes-Huerta A, Gonzalez-Rodriguez AP, et al. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease. PloS one. 2014;9(10):e108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maffei R, Bulgarelli J, Fiorcari S, et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica. 2013;98(7):1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jurado-Camino T, Cordoba R, Esteban-Burgos L, et al. Chronic lymphocytic leukemia: a paradigm of innate immune cross-tolerance. J Immunol. 2015; 194(2):719–727. [DOI] [PubMed] [Google Scholar]
- 74.Villumsen M, Sorup S, Jess T, et al. Risk of lymphoma and leukaemia after bacille Calmette-Guerin and smallpox vaccination: a Danish case-cohort study. Vaccine. 2009; 27(49):6950–6958. [DOI] [PubMed] [Google Scholar]
- 75.Omura GA, Vogler WR, Lefante J, et al. Treatment of acute myelogenous leukemia: influence of three induction regimens and maintenance with chemotherapy or BCG immunotherapy. Cancer. 1982;49(8):1530–1536. [DOI] [PubMed] [Google Scholar]
- 76.Powles RL, Russell JA, Selby PJ, et al. Maintenance of remission in acute myelogenous leukaemia by a mixture of B.C.G. and irradiated leukaemia cells. Lancet. 1977;2(8048):1107–1110. [DOI] [PubMed] [Google Scholar]
- 77.Ikeda Y, Adachi Y, Ishii T, et al. Blocking effect of anti-Dectin-1 antibodies on the anti-tumor activity of 1,3-beta-glucan and the binding of Dectin-1 to 1,3-beta-glucan. Biol Pharm Bull 2007;30(8):1384–1389. [DOI] [PubMed] [Google Scholar]
- 78.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e S. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112(13):4971–4980. [DOI] [PubMed] [Google Scholar]
- 79.Weitberg AB. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J Exp Clin Cancer Res 2008;27(40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanna BS, McClanahan F, Yazdanparast H, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2015; [DOI] [PubMed] [Google Scholar]
- 83.Albeituni SH, Ding C, Liu M, et al. Yeast-derived particulate beta-gluten treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in Cancer. J Immunol. 2016; 196(5):2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shatnyeva OM, Hansen HP, Reiners KS, Sauer M, Vyas M, von Strandmann EP. DNA damage response and evasion from immunosurveillance in CLL: new options for NK cell-based immunotherapies. Front Genet. 2015;6(11)eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz CR, Bollard CM. T-cell and natural killer cell therapies for hematologic malignancies after hematopoietic stem cell transplantation: enhancing the graft-versus-leukemia effect. Haematologica. 2015;100(6):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Della Chiesa M, Falco M, Muccio L, Bertaina A, Locatelli F, Moretta A. Impact of HCMV Infection on NK Cell Development and Function after HSCT. Front Immunol. 2013;4(458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arts RJ, Blok BA, Aaby P, et al. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J Leukoc Biol. 2015;98(6):995–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.