Myelofibrosis (MF), including primary myelofibrosis (PMF) and MF secondary to essential thrombocythemia (ET) or polycythemia vera (PV), is a chronic Philadelphia chromosome–negative myeloproliferative neoplasm associated with progressive bone marrow fibrosis.1 Many patients with MF experience new or worsening anemia during disease progression. Varying from study to study, 35% to 54% of patients with PMF have been reported to have anemia (i.e., hemoglobin <10 g/dL) at the time of diagnosis.2–5 Anemia adversely affects overall survival (OS), and is included as a key negative prognostic factor in validated prognostic scoring systems for patients with PMF, which were developed before the introduction of Janus kinase (JAK) inhibitor therapy.2,3,5 Ruxolitinib, a JAK1/JAK2 inhibitor, improved OS compared with placebo and best available therapy in patients with intermediate-2 or high-risk MF5 in the phase 3 COntrolled MyeloFibrosis Study With ORal JAK Inhibitor Treatment (COMFORT) studies.6–10 However, in these studies, ruxolitinib treatment was also associated with dose-dependent anemia and thrombocytopenia.6,7 Typically, these events occurred in the first 12 weeks of therapy and were managed with dose adjustments and/or red blood cell (RBC) transfusions. Mean hemoglobin levels reached a nadir at 8–12 weeks and recovered to near baseline levels by week 24 of treatment.6,7 Likewise, the proportion of patients requiring RBC transfusions in the ruxolitinib arm of COMFORT-I increased during the first 8–12 weeks and subsequently decreased to levels similar to those in the placebo arm at approximately week 24.6 We asked whether ruxolitinib-associated anemia has the same adverse prognostic impact on OS as disease-related anemia. To address this question, we conducted exploratory analyses of pooled data from the COMFORT studies, designed to differentiate the effects of disease-related anemia and anemia associated with ruxolitinib therapy on OS in patients with MF.
In COMFORT-I (clinicaltrials.gov identifier:00952289) and COMFORT-II (clinicaltrials.gov identifier:00934544), patients were randomized to receive ruxolitinib or placebo, and ruxolitinib or best available therapy, respectively.6,7 Eligible patients were ≥18 years of age, had PMF, post-PV MF, or post-ET MF, intermediate-2 or high-risk disease [per International Prognostic Scoring System (IPSS)], platelet count ≥100×109/L, and palpable splenomegaly ≥5 cm below the left costal margin. The starting dose of ruxolitinib was 15 mg twice-daily (BID) for patients with a baseline platelet count 100–200×109/L, and 20 mg BID for patients with a baseline platelet count >200×109/L. Protocol-specified dose reductions were mandated for patients who developed neutropenia or thrombocytopenia, but not for those who developed anemia. RBC transfusions were permitted for the management of anemia.
For this analysis, baseline anemia was defined as hemoglobin <10 g/dL at baseline or the requirement for any units of RBC transfusions within 12 weeks prior to day 1 of the study medication. Postbaseline anemia, regardless of RBC transfusion status at baseline, was defined as new or worsening anemia within the first 12 weeks after the initiation of ruxolitinib therapy that was grade ≥2 (per Common Terminology Criteria for Adverse Events v3.0) and a higher grade than that at baseline. COMFORT-I and COMFORT-II 3-year data were pooled for patients who survived at least 12 weeks after day 1. Patients randomized to placebo or best available therapy were combined into 1 control group. Kaplan-Meier analyses of OS were performed for patients in the control group based on baseline anemia status, and for ruxolitinib-treated patients based on baseline and postbaseline anemia status. Patients were assessed for OS using a Cox model, stratified by study and baseline anemia status. All analyses are exploratory and P-values are nominal.
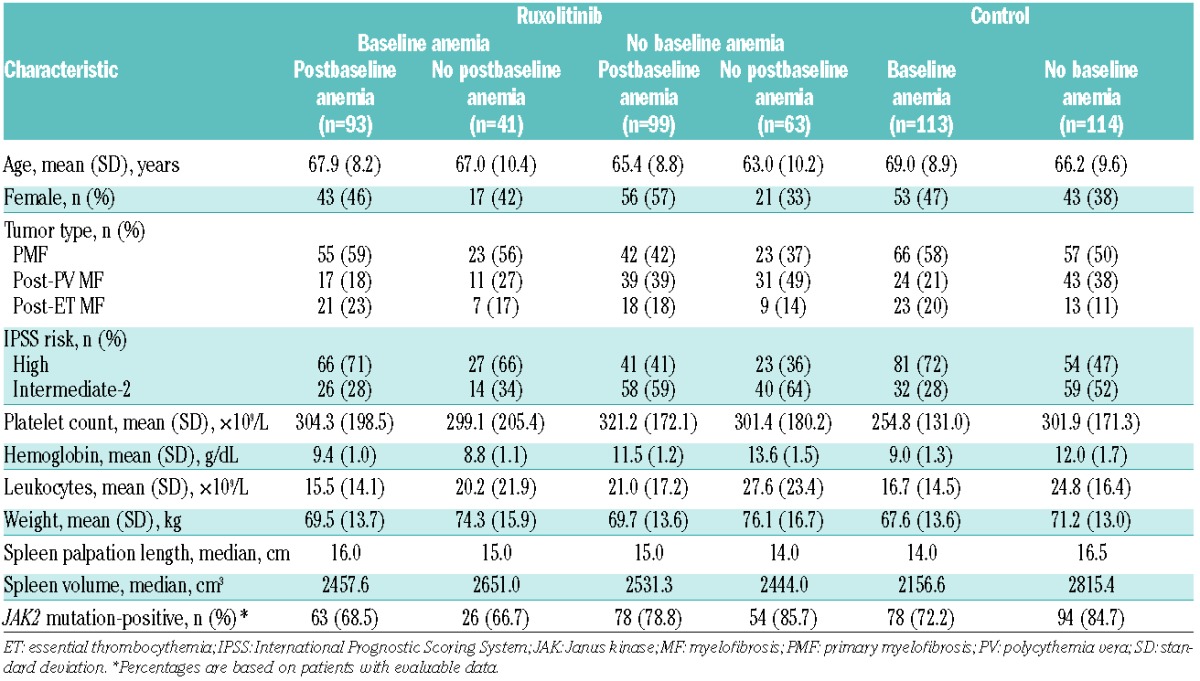
Of 296 patients randomized to ruxolitinib who qualified for this analysis, 162 (54.7%) patients had no baseline anemia and 134 (45.3%) patients had baseline anemia. In the control arm, 113 out of 227 (49.8%) patients had baseline anemia. Among patients with anemia at baseline, the proportions of those with PMF (vs. other MF subtypes) and those with IPSS high-risk (vs. intermediate-2-risk) disease were higher than they were among patients without anemia at baseline (Table 1). Eighty-five percent of new or worsening grade ≥2 anemia events (compared with baseline) in the ruxolitinib arm occurred during the first 12 weeks of therapy. Of the patients in the ruxolitinib group who had no baseline anemia, 61% (99 out of 162) developed postbaseline anemia, and of those with baseline anemia, 69% (93 out of 134) experienced worsening anemia.
Table 1.
Baseline characteristics in patients stratified by baseline anemia (ruxolitinib and control arm) and postbaseline anemia status (ruxolitinib arm only).
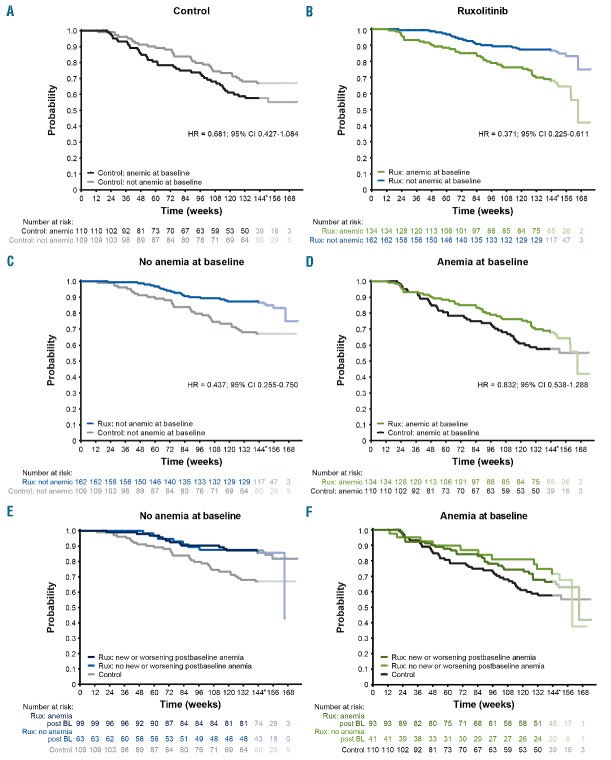
Kaplan-Meier estimates of OS among patients stratified by baseline anemia status within the ruxolitinib and control groups suggest that baseline anemia was associated with inferior survival, regardless of treatment (Figure 1A,B). However, similar to previously published data,10 patients randomized to ruxolitinib in COMFORT-I and COMFORT-II studies had improved OS compared with the control group [hazard ratio (HR) = 0.644; 95% confidence interval (CI) 0.459–0.903; P=0.0102 for ruxolitinib vs. control]. Importantly, improved survival associated with ruxolitinib versus control treatment was seen both among patients with anemia at baseline and among those without anemia at baseline (Figure 1C,D). OS probability at 3 years for ruxolitinib versus control was 0.66 versus 0.57 among patients with baseline anemia, and 0.87 versus 0.66 among patients without baseline anemia. In addition, within the subgroups of patients with and without baseline anemia, new or worsening postbaseline anemia did not affect OS during ruxolitinib therapy (Figure 1E,F; HR = 1.030; 95% CI 0.615–1.725 for no postbaseline anemia vs. postbaseline anemia in the ruxolitinib group).
Figure 1.
Effect of disease- and treatment-related anemia on overall survival (OS). OS is shown by baseline anemia status (A, B) in the control (A) and ruxolitinib (B) arms; by treatment group (C, D) in patients with no anemia at baseline (C) and anemia at baseline (D); and by postbaseline anemia status in the ruxolitinib arm (E, F) in patients with (E) and without (F) baseline anemia. BL: baseline; CI: confidence interval; HR: hazard ratio; Rux: ruxolitinib.*After week 144, the interpretation of the curves is affected by low numbers of patients.
MF-related anemia is a negative prognostic factor in the IPSS and related scoring systems that were developed before the advent of JAK inhibitor therapy.2,3,5 As anemia is a common adverse effect of JAK inhibitor therapy, including ruxolitinib,6,7 it is important to assess whether treatment-related anemia has the same adverse prognostic impact as disease-related anemia. In this exploratory analysis of the COMFORT studies, baseline anemia was associated with shortened OS in both the ruxolitinib and control groups, confirming the previously established negative prognostic impact of disease-related anemia in MF.2,3,5 In contrast, new or worsening anemia that occurred during, and possibly as a result of, ruxolitinib therapy, had no effect on OS. Furthermore, although ruxolitinib therapy did not abrogate the survival disadvantage associated with MF-related anemia prior to ruxolitinib therapy, it provided a survival advantage compared with control treatments in patients with and without baseline anemia. Consistent with our findings, a related analysis from the COMFORT studies showed that hemoglobin decreases from baseline of ≤30 g/L versus >30 g/L at week 12 had no significant association with OS (HR = 0.84; 95% CI 0.47–1.52).11 Together, these findings suggest that early-onset ruxolitinib-related anemia may not have the same deleterious prognostic implication for OS as disease-related anemia, and therefore, by itself should not be considered a cause for treatment discontinuations. Of note, experience from the COMFORT studies indicates that ruxolitinib-associated decreases in hemoglobin early during ruxolitinib therapy may often be transient,6,12 can be successfully managed by RBC transfusions, and rarely lead to treatment discontinuations.6,7 In conclusion, ruxolitinib-associated anemia, which occurs predominantly during early therapy, is not predictive of shortened survival. Unlike disease-related anemia, ruxolitinib-related anemia in patients with MF is manageable and does not appear to adversely impact survival.
Supplementary Material
Acknowledgments
The authors would like to thank Beth Burke, PhD, of Evidence Scientific Solutions, Philadelphia, PA, USA, for medical writing support, which was funded by Incyte Corporation.
Footnotes
Funding: The COMFORT-I study (clinicaltrials.gov identifier:00952289) was sponsored by Incyte Corporation; the COMFORT-II study (clinicaltrials.gov identifier:00934544) was sponsored by Novartis Pharmaceuticals. MD Anderson receives a cancer center support grant from the NCI of the National Institutes of Health (P30 CA016672).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Mesa RA, Green A, Barosi G, Verstovsek S, Vardiman J, Gale RP. MPN-associated myelofibrosis (MPN-MF). Leuk Res. 2011;35(1):12–13. [DOI] [PubMed] [Google Scholar]
- 2.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397. [DOI] [PubMed] [Google Scholar]
- 3.Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116(15):2857–2858. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc. 2012;87(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. [DOI] [PubMed] [Google Scholar]
- 6.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. [DOI] [PubMed] [Google Scholar]
- 9.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Ali HK, Stalbovskaya V, Gopalakrishna P, Perez-Ronco J, Foltz L. Impact of ruxolitinib treatment on the hemoglobin dynamics and the negative prognosis of anemia in patients with myelofibrosis. Leuk Lymphoma. 2016;57(10):2464–2467. [DOI] [PubMed] [Google Scholar]
- 12.Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. OncoTargets Ther. 2013;7:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.