Sickle cell anemia (SCA) is one of the commonest severe inherited disorders affecting millions worldwide. Complications are extensive although severity varies markedly. Renal damage [or sickle cell nephropathy (SCN)] occurs in approximately one-third of SCA children1,2 and a significant number develop renal failure as adults.3 It is not yet possible to predict which children will develop SCN and would, therefore, benefit from earlier, more aggressive management.
Patients have the abnormal hemoglobin (Hb) HbS in their red blood cells (RBCs). HbS has a single amino acid change compared to the normal adult HbA, valine replacing glutamic acid. Deoxygenated HbS molecules may aggregate into polymers.4 These cause RBC shape change (sickling), altered rheology, increased fragility, and other deleterious sequelae which produce chronic anemia, progressive organ damage, and acute ischemia. Life expectancy is reduced, and whilst better medical care has extended lifespan, more patients progress to chronic organ damage, such as SCN.
Biomarkers for SCN include urea, creatinine, cystatin C, and experimental biomarkers such as kidney injury molecule-1 and kallikrein,5 whilst albuminuria, hypertension and persistent nocturnal enuresis indicate renal damage.6 However, there are no biomarkers predicting early nephropathy, and there is no clear strategy to identify children who will develop SCN, nor any prognostic indicators on which to base patient management.
One important manifestation of nephropathy is persistence of nocturnal enuresis in older children and, sometimes, in adults. Enuresis causes significant problems, including sleep impairment, social isolation, and increased expenditure related to washing and replacing bedding. Over 40% of 7-year olds report this to be a significant problem.7 Reduced renal concentrating ability occurs (hypothesnuria), whilst bladder instability, decreased bladder capacity, and sleep-disordered breathing may also contribute. Although desmopressin may be beneficial, treatment is largely ineffectual. A better understanding of the pathophysiology may suggest new, more efficacious therapies.8
HbS polymerization initiates the symptoms of SCA, although progression to organ damage may be complex and indirect. More closely associated with HbS polymerization is altered RBC phenotype, particularly abnormal cation permeability. Three transporters participate:9 the KCl co-transporter, with coupled K+ and Cl− movement; the Gardos channel, a Ca2+-activated K+ channel, for rapid conductive K+ loss with Cl− following through separate anion channels; and Psickle, a deoxygenation-induced non-specific cation conductance. Solute loss via these transport systems causes RBC dehydration and elevation of intracellular concentration of HbS. Higher concentrations of HbS markedly encourages sickling through reduction in the time lag to polymerization following deoxygenation, which is inversely proportional to HbS.4 Repeated sickling damages the RBC membrane with lifespan reduced to approximately one-tenth that of normal RBCs. Ca2+ entry may also activate lipid scrambling with externalization of phosphatidylserine (PS), making RBCs sticky and prothrombotic.
The renal medulla is notably hypoxic and acidic with sluggish blood flow, which encourages HbS polymerization, sickling and K+ loss, RBC dehydration and PS exposure, increasing the vulnerability of this organ to damage. Microalbuminuria and hyperfiltration, indices of renal damage, associate with the more hemolytic SCA phenotypes,2–3,10–12 and may also follow from HbS polymerization and RBC shrinkage.
Small inherited variations in RBC cation permeability, maximized on passage through the renal medulla, may manifest as renal complications. We, therefore, postulated that certain RBC characteristics (sickling, K+ transport, hemolysis and PS exposure), which may be inherited independently of the HbS mutation, correlate with renal pathology, and, importantly, may occur in advance of damage, thereby providing prognostic markers to inform patient management.
One hundred and twelve HbSS children (>4 years old) with SCA attending the Pediatric Hematology clinic at King’s College Hospital, London, UK, were recruited for the study. Patients transfused in the preceding four months or taking medications known to alter RBC permeability (e.g. dipyridamole and Ca2+ channel blockers) were excluded, but the study included those on hydroxyurea. All patients were in the steady state, and had been without acute symptoms for at least seven days.
Standard laboratory parameters, together with age, height, weight, and blood pressure were recorded. Enuresis was defined as being dry for less than five nights a week. Patients were divided into two groups: those who stopped wetting their bed before the age of five years and those who were still enuretic at five years of age, as reported by parents or family members retrospectively using a standardized questionnaire.
Laboratory analyses included: measurement of red cell K+ permeabilities using 86Rb+ as a congener, sickling, exposure of phosphatidylserine (PS) and non-electrolyte hemolysis (see Hannemann et al.13 for references to methodologies). RBC permeabilities and sickling were measured at 100 mmHg, 35 mmHg, 15 mmHg and 0 mmHg O2, because HbS polymerization, the initial event in pathogenesis, begins as Hb deoxygenates (P50 c.25–35 mmHg). Enuresis was analyzed as a binary category (dry before 5 years of age, enuretic aged 5 years or over). RBC phenotype (KCC, Psickle, Gardos channel, PS exposure, hemolysis and sickling) and conventional laboratory indices were compared in the two groups. Statistical significance of normally distributed variables were analyzed by Student t- and χ2 tests, and non-normally distributed ones by Mann-Whitney U-tests, using Microsoft-Excel, Seattle or IBM-SPSS, New York, USA.
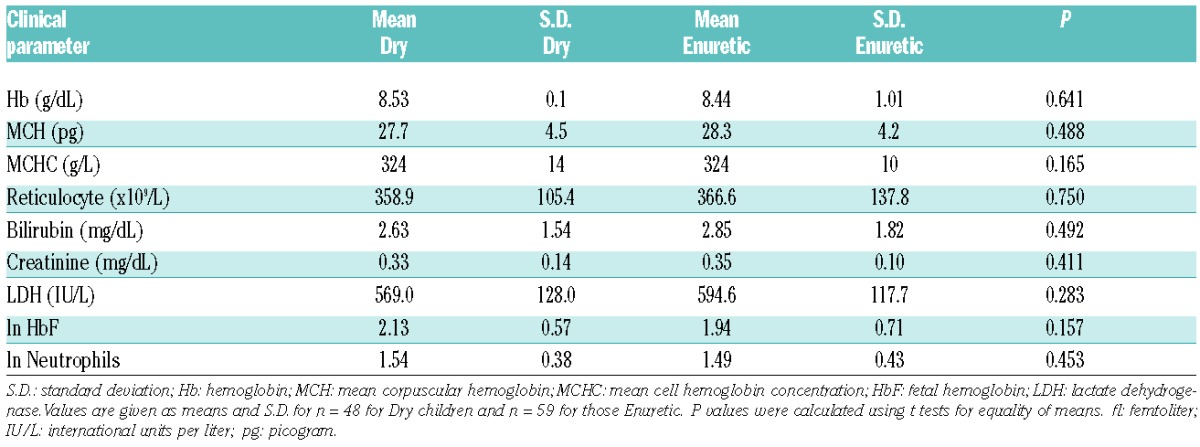
The clinical profile of the patients is summarized in Table 1A. Five children still wetting the bed more than twice per week were under five years of age and were excluded from analysis. Of the remaining 107, 48 were dry before five years of age, and 59 were still enuretic aged five years or over. Their RBC transport activities are given in Table 2 and Figure 1. Activity of the Gardos channel was significantly greater in RBCs from children still enuretic at five years of age at all hypoxic O2 tensions. Psickle activity also showed significant increased levels in those still enuretic aged five years or over, but only in fully deoxygenated RBCs (Table 2). There were no significant differences in percentage sickling, activity of KCC, PS exposure or hemolysis in isosmotic deoxygenated non-electrolyte solutions between the enuretic groups (data not shown). In previous work, the activity of one transport system, KCC, was observed to correlate significantly with age.14 This correlation was confirmed in the present work but was not the explanation for our findings, as enuresis in all children was recorded at five years of age. In contrast to the transport pathways, no significant association between routine laboratory parameters and enuresis was found (Table 1B). The association between children enuretic after reaching five years of age and administration of hydroxyurea was not significant (χ2=0.028; P=0.866) (Figure 1A), suggesting that enuresis is not linked to SCA symptoms used to select patients for this therapy.15
Table 1A.
Clinical profile of patients with sickle cell anemia.
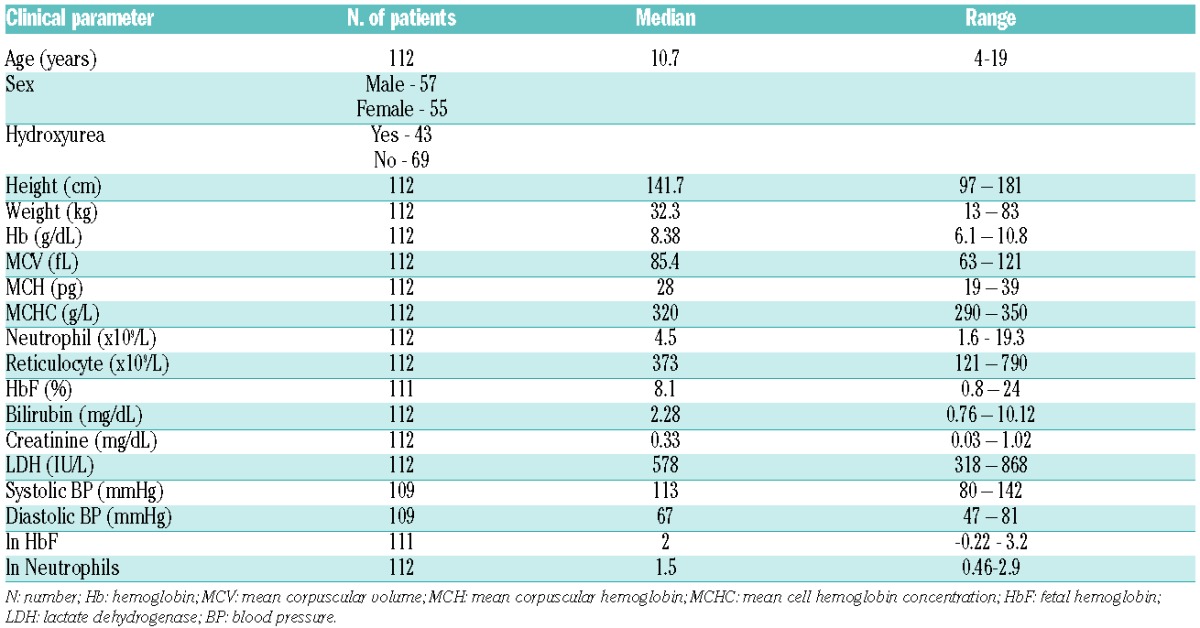
Table 2.
Comparison of activities of Psickle and the Gardos channel in children with sickle cell anemia dry before five years of age (Dry) and those remaining enuretic when aged five years or older (Enuretic).
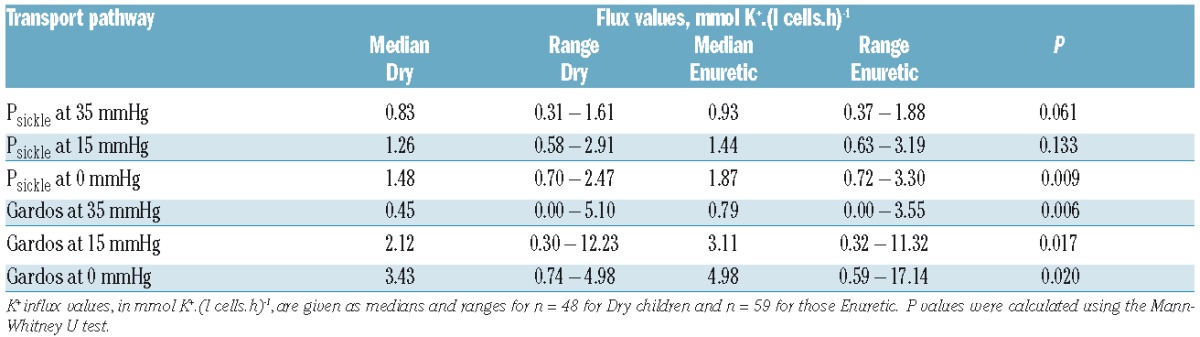
Figure 1.
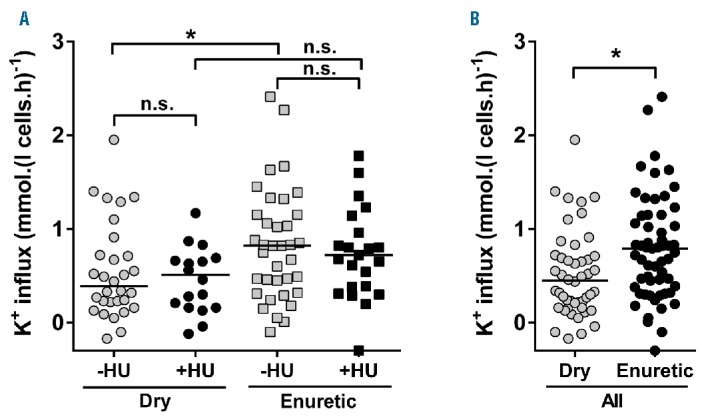
Representative scatter plot of Gardos channel activity in children with sickle cell anemia dry before five years of age (Dry) and those remaining enuretic when aged five years or over (Enuretic). Oxygen tension was 35 mmHg. K+ influx values are given in mmol. (l cells.h)−1. (A) Data from children on hydroxyurea (HU) and those not receiving this medication. (B) Data from all children together. In each case, Dry and Enuretic children are separated. Gardos channel activities correlated with reticulocyte percentage (P<0.001 for both Dry and Enuretic children; Pearson correlation), and the same was observed at O2 tension of 0 and 15 mmHg. However, reticulocytes were not associated with enuresis (P=0.75) (Table 1B). Statistical analysis was carried out using Mann-Whitney U-tests with median values indicated. *P<0.03; n.s.: not significant (P>0.05).
Table 1B.
Relationship between laboratory parameters and enuresis in children with sickle cell anemia dry before five years of age (Dry) and those remaining enuretic when aged five or older (Enuretic).
Psickle and the Gardos channel activity are the two RBC transport functions most directly linked to HbS polymerization. Psickle activation is associated with HbS deoxygenation, polymerization and RBC shape change.9 Its most important role is to allow entry of Ca2+. Should Ca2+ accumulate sufficiently, it leads to secondary activation of the Ca2+-activated Gardos channel with high rates of K+ loss, RBC shrinkage, and increased likelihood of sickling. We have previously demonstrated a significant correlation between Psickle and Gardos channel activities.13 The hypoxic renal medulla is a region of the circulation expected to promote HbS polymerization, sickling, and Psickle and Gardos channel activation. The central role for Psickle and Gardos channel in pathogenesis of SCA9 is also present in the case of SCN.
Increased Gardos channel activity was associated with persistence of enuresis in older children at all hypoxic O2 tensions investigated. Psickle activity showed an association only in fully deoxygenated RBCs. Oxygen tensions are unlikely to fall to such profound hypoxic values in vivo such that levels of around 15 and 35 mmHg are probably most relevant. Lack of significant association of Psickle with enuresis at O2 tensions of 15 and 35 mmHg may be because K+ permeability through this pathway is not an ideal marker for Ca2+ as required for Gardos channel activation whilst RBC Ca2+ homeostasis is complex,9 factors which may suggest activity of the Gardos channel is not related to that of Psickle. Variation in cation transport activity in these children with SCA was particularly marked (Table 2 and Figure 1). At least some of this variability is probably inherited and associated with sequence variation in the genes and transcription factors involved in the control of these pathways. Understanding these genetic factors may allow early identification of children at risk of renal complications, including prolonged nocturnal enuresis.
The present findings emphasize the importance of a thorough appreciation of RBC permeability to SCA pathogenesis.13,14 They are particularly exciting because they show that the activity of particular transport pathways, abnormally elevated in SCA patients, are associated with enuresis. Renal pathology may be central to the prolongation of nocturnal enuresis into older childhood, and the early identification of children with increased Gardos channel and Psickle activity may indicate children who would benefit from early treatment for nocturnal enuresis. As changes in RBC permeability are likely to occur before renal damage, these findings represent a potential prognostic test for SCN to inform patient management, whilst treatment targeting Gardos and Psickle activity may be beneficial in preventing enuresis, and, potentially, other forms of SCN.
Supplementary Material
Acknowledgments
We thank Action Medical Research (GN 2030) and Stroke Association for their generous financial support. All participants gave written informed consent.
Footnotes
Funding: the study was approved by the National Research Ethics Committee (reference 13/NW/0141) and conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2008.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Becker AM. Sickle cell nephropathy: challenging the conventional wisdom. Ped Nephrol. 2011;26(12):2099–2109. [DOI] [PubMed] [Google Scholar]
- 2.Scheinman JI. Sickle cell disease and the kidney. Nature Clin Pract Nephrol. 2009;5(2):78–88. [DOI] [PubMed] [Google Scholar]
- 3.Guasch A, Navarette J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228–2235. [DOI] [PubMed] [Google Scholar]
- 4.Eaton JW, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70(5):1245–1266. [PubMed] [Google Scholar]
- 5.Rees DC, Gibson JS. Biomarkers in sickle cell disease. Br J Haematol. 2012:156(4):433–445. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe CC, Thein SL. Sickle cell nephropathy - a practical approach. Br J Haematol. 2011;155(3):287–297. [DOI] [PubMed] [Google Scholar]
- 7.Field JJ, Austin PF, Yan Y, DeBaun MR. Enuresis is a common and per sistent problem among children and young adults with sickle cell anemia. Urology. 2008;72(1):81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf RB, Kassim AA, Goodpaster RL, DeBaun MR. Nocturnal enuresis in sickle cell disease. Exp Rev Hematol. 2014;7(2):245–254. [DOI] [PubMed] [Google Scholar]
- 9.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85(1):179–200. [DOI] [PubMed] [Google Scholar]
- 10.Becton LJ, Kalpatthi RV, Rackoff E, et al. Prevalence and clinical correlates of microalbuminuria in children with sickle cell disease. Ped Nephrol. 2010;25(8):1505–1511. [DOI] [PubMed] [Google Scholar]
- 11.Hayman JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol. 2010;5(5):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day TG, Fulford T, Sharpe CC, Thein SL. Association between hemolysis and albuminuria in adults with sickle cell anemia. Hematologica. 2012;97(2):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannemann A, Rees DC, Tewari S, Gibson JS. Cation homeostasis in red cells from patient with sickle cell disease heterologous for HbS and HbC (HbSC genotype). EBioMedicine. 2015;2(11):1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees DC, Thein SL, Osei A, et al. The clinical significance of KCl cotransport activity in red cells of patients with HbSC disease. Haematologica. 2015;100(5):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees DC. The rationale for using hydroxycarbamide in the treatment of sickle cell disease. Haematologica. 2011;96(4):488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


