Abstract
Cationic polymers are used for non-viral gene delivery, but current materials lack the functionality to address the multiple barriers involved in gene delivery. Here we describe the rational design and synthesis of a new family of quaterpolymers with unprecedented multifunctionality: acid sensitivity, low cationic charge, high hydrophobicity, and biodegradability, all of which are essential for efficient and safe gene delivery. The polymers were synthesized via lipase-catalyzed polymerization of ortho ester diester, lactone, dialkyl diester, and amino diol monomers. Polymers containing ortho ester groups exhibited acid-sensitive degradation at endosomal pH (4~5), facilitated efficient endosomal escape and unpackaging of the genes, and were efficient in delivering genetic materials to HEK293 cells, human glioma cells, primary mouse melanoma cells, and human umbilical vein endothelial cells (HUVECs). We also developed a highly efficient lyophilized formulation of the nanoparticles, which could be stored for a month without loss of efficiency.
Keywords: Non-viral gene delivery, multifunctional polymer, ortho ester, lipase catalysis, DNA, siRNA
Graphical abstract
1. Introduction
Gene therapy with DNA is a promising approach for the treatment of cancer and other diseases 1–2. However, its success is dependent on the development of efficient gene delivery vectors, since unprotected DNA will be degraded by endogenous nucleases and has low cell uptake efficiency. An efficient DNA delivery vector must be multifunctional so that it can address the multiple barriers for gene delivery, such as gene packaging, transport through cell membrane, endosomal escape, and gene unpackaging 3. Viral vectors are highly efficient because they have evolved multiple functions to address these barriers, such as cell uptake, endosomal escape, and nuclear targeting, but they suffer from serious safety issues 3–4. Two groups of non-viral vectors are widely studied: cationic lipids and cationic polymers 5–8. Cationic lipids suffer from difficulties in fabrication and modification, which prevent the incorporation of versatile functionalities. In contrast, synthetic cationic polymers are promising because of their versatile chemistry.
Many cationic polymers for gene transfection have been reported, such as polyethylenimine (PEI) and poly (β-amino ester) (PBAE) 3, 9–10. Recently, a family of poly (amine-co-ester) (PACE) terpolymers was synthesized through lipase catalysis, which featured low cationic density and high hydrophobicity 11–12. These polymers are among the most efficient and safe gene vectors ever reported. However, there is still room for improvement. For example, it is potentially helpful to enhance the escape of the loaded genes from the acidic endolysosomes, which is a major limiting step for gene delivery. This is especially important for PACE because it has low nitrogen content and thus a lesser ability to take advantage of the proton sponge effect 13. The unpackaging of genes from PACE might also be enhanced: while high hydrophobicity can contribute to polyplex stability, it can also diminish the unpackaging of genes in cytosol and lower the transfection efficiency 3, 8.
One way to enhance both endosomal escape and gene unpackaging is to introduce acid-sensitive groups in the polymer backbone. Once taken up by cells through endocytosis, acid-triggered breakdown of polymers in the endosome could produce intra-endosomal osmotic swelling that could rupture the endosomes and lead to escape of plasmids 14–18. The unpackaging of genes from the nanoparticles might also be enhanced after the degradation of acid-sensitive polymer, making the genes accessible for expression 14, 16, 19.
Ortho ester is an ideal group to provide acid-sensitivity, because it exhibits pH-dependent hydrolysis. While it is stable in neutral and basic environments, it undergoes rapid hydrolysis in slightly acidic environment. Compared to other pH-sensitive groups, such as ester 20, acetal 21, ketal 22, and vinyl ether 23, the ortho ester bond hydrolyzes more quickly in response to mildly acidic conditions 24. Poly (ortho esters) (POEs) were developed in the 1970s 25–26 and have been widely studied for drug and gene delivery 25–26. They have been used to fabricate solid drug release device, injectable gel-like materials, and micelles that could encapsulate therapeutics for many diseases, such as cancer and post-operative pain. Several of these products have been studied in clinical trials 27. POEs have also been used for gene delivery. For example, a group of cationic POEs was prepared via the copolymerization of diol, amino-diol, and diketene acetal 28. Microspheres were prepared with these materials as vectors for DNA delivery, which had low toxicity and could release genes in response to acid. However, these particles are huge (>5um), and their chemistry does not give enough flexibility to allow the fine tuning of the physicochemical properties of the polymer. In another studies, poly(ortho ester amidine) copolymers were prepared in multiple steps 29. The polymers exhibited pH-dependent hydrolysis and DNA release. However, these materials were toxic and exhibited low transfection efficiency.
Because of the high acid sensitivity and biocompatibility of ortho ester, we chose to incorporate ortho ester groups into the main chain of PACE molecules, to make them acid-sensitive. However, chemical synthesis to incorporate ortho ester into PACE polymer is challenging because distinctly different polymerization methods are required for poly (ortho ester) and PACE. PACE was synthesized with lipase catalysis; however, the use of ortho ester groups in lipase catalysis has never been reported. To the best of our knowledge, there are no polymerization methods currently available to readily incorporate ortho ester moieties into functional polyester chains with the desirable amine and lactone structures that are essential for efficient and safe gene delivery.
In this paper, we report a novel lipase-catalyzed synthetic method that allows the preparation of a new family of poly (amine-co-ester-co-ortho ester) (oPACE) polymers. The oPACEs have unprecedented multi-functionality: acid sensitivity, low cationic charge, high hydrophobicity, and biodegradability. These polymers are acid-sensitive and efficiently facilitate endosomal escape and gene unpackaging. These polymers are also highly efficient and safe in transfecting genetic materials into different cells, including difficult-to-transfect primary cells.
2. Materials and methods
2.1. Materials
ω-pentadecalactone (PDL), diethyl sebacate (DES), N-methyldiethanolamine (MDEA), 3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DTSU), ethyl glycolate, ethyl 6-hydroxy hexanoate and methyl 10-hydroxydecanoate were purchased from Sigma Aldrich Chemical Co. and were used as received. Immobilized Candida antarctica lipase B (CALB) supported on acrylic resin (Novozym 435), potassium t-butoxide, chloroform, dichloromethane, hexane, pentane, diphenyl ether, tetrahydrofuran, ethylene diamine, triethylamine and chloroform-d were also obtained from Sigma Aldrich Chemical Co. The lipase catalyst was dried at 50 °C under 2.0 mmHg for 20 h prior to use. Plasmid DNA (pGL4.13) encoding the firefly luciferase (pLuc) and Luciferase Assay Buffer were obtained from Promega Co. (Madison, WI). GFP reporter gene pSicoR-GFP (pGFP) was obtained from Addgene.
2.2. Cell culture
Human embryonic kidney 293 (HEK293) cells and U87MG cells were obtained from American Type Culture Collection (Manassas, VA) and grown at 37°C under 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin. The primary mouse melanoma cells (2697T) 30 were provided by M. Bosenberg (Yale University), and were incubated at 37°C under 5% CO2 atmosphere in DMEM/F12 supplemented with 5% FBS, 1% NEAA, and 1% penicillin-streptomycin. Human umbilical vein endothelial cells (HUVECs) were purchased from Yale University Vascular Biology and Therapeutics core facility and cultured in M199 supplemented with 20% FBS, P/S, L-glutamine, and ECGS.
2.3. Instrument methods
1H and 13C NMR spectra were recorded on Bruker AVANCE spectrometer. The chemical shifts reported were referenced to internal tetramethylsilane (0.00 ppm) and chloroform-d was used for all NMR measurement. The number and weight average molecular weights (Mn and Mw, respectively) of polymers were measured by gel permeation chromatography (GPC) using a Waters HPLC system equipped with a model 1515 isocratic pump, a 717 plus autosampler, and a 2414 refractive index (RI) detector. Empower II GPC software was used for running the GPC instrument and for calculations. Columns and the RI detector were heated and maintained at 40 °C temperature during sample analysis. Chloroform was used as the eluent at a flow rate of 1.0 mL/min. Sample concentrations of 2 mg/mL and injection volumes of 100 μL were used. Polymer molecular weights were determined based on a conventional calibration curve generated by narrow polydispersity polystyrene standards from Aldrich Chemical Co. The morphology of polyplexes, which were stained with uranyl acetate, was visualized using a Zeiss EM 900 transmission electron microscope (TEM). ImageJ was used to analyze the average size of polyplexes on TEM images. Flow cytometry analysis were performed on an Attune NxT Flow Cytometer with a blue (488 nm) and a red (638 nm) laser.
2.4. Synthesis of ortho ester diester monomers
3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane (DETOSU) was prepared through the isomerization of DTSU according to previously described method 31 (Scheme 1A). Briefly, 18 g of DTSU was mixed with 20 g of potassium t-butoxide in 100 mL ethylene diamine. The mixture was heated to 100 °C under nitrogen for 16 hours. After that, the mixture was poured into 1000 mL of water, and the product was extracted with pentane and dried with K2CO3. The product was distilled under vacuum to remove the impurities. Following the synthesis of DETOSU, ethyl glycolate (n=1), ethyl 6-hydroxyhexanoate (n=5), or methyl 10-hydroxydecanoate (n=9) was mixed with DETOSU at a 2:1 molar ratio and the reaction was carried out at room temperature in THF catalyzed by supported p-toluenesulfonic acid. After 4 hours, the catalyst was removed by filtration and the solvent was evaporated under vacuum. Three distinct ortho ester diesters (OEDEs) were obtained with quantitative yield, designated as OEDE (1), OEDE (5) and OEDE (9), respectively. All the structures were confirmed by 1H and 13C NMR.
Scheme 1.
Synthesis of (A) ortho ester diester (OEDE) monomers and (B) oPACE polymers by a two-stage polymerization of OEDE (n) with PDL, DES, and MDEA.
2.5. Synthesis and purification of poly (amine-co-ester-co-ortho ester) (oPACE)
A reaction mixture containing PDL, DES, MDEA, OEDE (n=1, 5, 9), Novozym 435 catalyst (10wt% of total monomer), and diphenyl ether solvent (200 wt% versus total monomer) was prepared and purged with argon to remove air (Scheme 1B). The polymerization reactions were carried out at 90 °C in two stages: (1) in the first stage, the reaction mixtures were stirred under 1 atm of argon gas for 24 hours; (2) in the second stage, the reaction pressure was reduced to 1.6 mmHg and the reactions were continued for a further 72 h. The polymer products were isolated and purified by precipitation in hexane to extract and remove the residual diphenyl ether solvent from the polymers. Subsequently, the products were dissolved in dichloromethane and filtered to remove catalyst particles. Evaporation of the CH2Cl2 solvent from the filtrates at 40 °C under high vacuum (1.0 mmHg) yielded the purified polymers. The control terpolymer, poly (amine-co-ester) (PACE) was synthesized with a similar method using PDL, DES, and MDEA as monomers 11.
2.6. Polymer degradation study
A weight loss assay was used to characterize the degradation of the polymers. oPACE and PACE were coated on to the bottom of glass vials, covered with 150 mM sodium acetate buffer of pH 4, pH 5, pH 6 and PBS buffer, and incubated at 37 °C for different time. After incubation, the buffer was removed carefully, and the polymers were dried. The weight and molecular weight of the residual polymer were measured.
2.7. Polyplex preparation and characterization
To prepare DNA polyplexes at 100:1 polymer/DNA weight ratio, which was shown to be the optimized ratio in prior work 11, 4 μl of polymer solution (25 mg/ml in DMSO) was first diluted in 50 μl sodium acetate buffer (25 mM, pH = 5.6). After brief vortexing, the polymer solution was mixed with the same volume of a DNA solution in sodium acetate buffer containing 1 μg DNA and vortexed for additional 10 seconds. The polymer/DNA mixture was incubated at room temperature for 10 min before use. The size of the polyplexes was measured with dynamic laser scattering (DLS) on a Malvern Zetasizer. For the PicoGreen exclusion assay, polyplexes were prepared at different polymer/DNA weight ratio, while keeping the concentration of pDNA constant. All samples, as well as free pDNA solution, were analyzed with Quant-it PicoGreen dsDNA assay kit (Invitrogen). Since the PicoGreen can only bind to the free DNA, the reading reflects the concentration of the uncomplexed DNA.
2.8. Characterization of endosomal escape efficiency of polyplexes
To measure the pH of the local environment for the DNA delivered intracellularly by different polymers, plasmid DNA (pGL4.13, or pLuc) was double-labelled with pH-sensitive FITC and pH-insensitive Cy5 using Label IT® Tracker™ Intracellular Nucleic Acid Localization Kits (Mirus Bio) 32. The day before the transfection, U87MG cells were seeded in 24-well plate at a density of 150,000 cells/well. Polyplexes were prepared with the double-labelled pDNA and PACE or oPACE9-20. Cells were treated with the polyplexes for 1 hour at 37 °C before the polyplexes were removed with PBS washing. After another hour, the cells from 7 wells were collected. Cells from 3 wells were suspended in PBS containing 2% BSA, while cells in another 4 wells were suspended in 4 intracellular pH clamping buffer (pH=4.5, 6.0, 6.8, 7.5). The cells in each vial were further washed by pelleting and resuspending in the corresponding buffer. The signal from 50,000 treated cells was analyzed according to a method previously reported 33. The fluorescence of FITC and Cy5 was excited at 488 nm and 638 nm respectively, and was detected at 530 and 670 nm, respectively. The FITC/Cy5 ratio was calculated from the median fluorescence of all the live cells. The ratio from the cells incubated with the 4 intracellular clamping buffer generated a standard curve, which was used to convert the FITC/Cy5 ratio of the other three samples into pH values.
2.9. Acid-sensitivity of the oPACE polyplexes
Polyplexes of PACE/DNA or oPACE/DNA were prepared at 100:1 weight ratio, and then incubated in 150 mM pH 5 sodium acetate buffer containing 5 mM EDTA at 37 °C for 4 hours. Then, the acid-treated polyplexes and fresh polyplexes were incubated with different concentrations of heparin at 37 °C for 15 mins. The amount of gene released at different heparin concentration was quantified with PicoGreen assay and normalized to the reading the same concentration of free DNA in same buffer.
2.10. In vitro transfection of DNA and siRNA
For in vitro transfection, DNA polyplexes with polymer:DNA weight ratio of 100:1 were used unless otherwise noted. Cells were seeded in 24-well plates at density of 75,000 cells/well in 500 μl of medium one night before transfection. The growth medium was replaced with DMEM containing 10% FBS (without penicillin-streptomycin) and polyplexes containing 1ug of DNA was added to each well. Transfection using Lipofectamine 2000 (Invitrogen Corp.) was performed using the procedures provided by the manufacturer. The same amount of DNA was used for transfection with oPACE and Lipofectamine 2000.
For luciferase gene transfection assay, pLuc plasmid was used to prepare the polyplexes. Two days after transfection, the culture medium was removed and the cells were washed with cold PBS. Two hundred micro-liter Report Lysis Buffer (Promega) was added to each well. After a freeze-thaw cycle, cell lysate was collected. After a quick spin, 20 μl of cell lysate was mixed with Luciferase Assay Reagent. Luciferase expression in terms of relative light units (RLU) was measured with a GloMax® 20/20 Luminometer and normalized to the total amount of protein in the cell lysate. Total protein level was quantified using Pierce BCA protein assay kit (Pierce, Thermo Scientific). For GFP gene expression assay, pSicoR-GFP was used to prepare polyplexes. After 48 hours of incubation, the transfected cells in each well were harvested by trypsinization, washed with PBS and resuspended in PBS with 1% BSA. A total of 40,000 cells were analyzed with flow cytometry to detect GFP expressing cells.
For siRNA knockdown, HEK293 cells stably transfected with luciferase gene (HEK293-Luc) were seeded in 24-well plates at a density of 75,000 cells/well in 500 μl of medium one night before transfection. siRNA (5'-GCUAUGAAGCGCUAUGGGCUU-3') was used to knockdown the expression of luciferase. Polyplexes of siRNA/polymer were prepared at 100:1 polymer/siRNA weight ratio with a similar method for the preparation of pDNA/polymer polyplexes. Transfection using Lipofectamine RNAiMAX (Invitrogen Corp.) was performed using the procedures provided by the manufacturer. The same concentration of siRNA (10 nM) was used in transfection with polymer or Lipofectamine RNAiMAX. Two days after transfection, the luciferase expression level was measured with similar method for luciferase pDNA transfection, and was compared to no-treatment group.
2.11. In vitro toxicity test
The cytotoxicity of oPACE9-20, PACE, and PEI was studied against HEK293 and U87MG cells. The cells were seeded in 96-well plates one night before at an initial seeding density of 1.5×104 cells per well in 100 uL of DMEM. PACE/pLuc and oPACE9-20/pLuc polyplexes were prepared at 100:1 polymer:DNA weight ratio in sodium acetate buffer according to the previously described method. Polyplexes of PEI/pLuc were prepared at 3:1 polymer:DNA weight ratio with a similar method. Then, the growth medium was removed and replaced with 180 uL fresh DMEM, followed by addition of 20 uL of polyplexes solution at different concentrations so the final concentration of DNA was ranged from 0.15 ug/mL to 20 ug/mL. For control experiments, 20 uL sodium acetate buffer with the same amount of DNA and DMSO was added. After 48 hours incubation, the cells were assayed for metabolic activity using a standard MTT assay.
2.12. Lyophilization and storage of the polyplexes
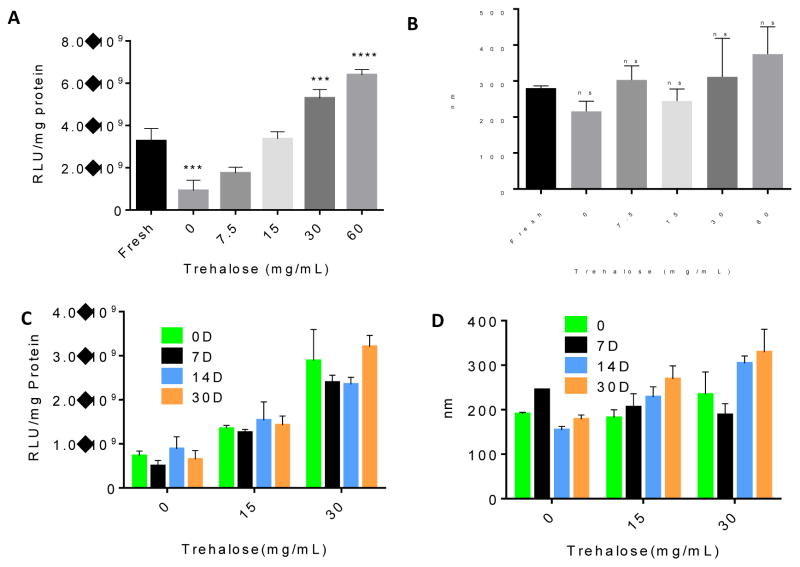
Polyplexes were prepared with oPACE9-20 and pLuc plasmid according to the method described above. Trehalose solutions of varying concentrations (15 mg/mL, 30 mg/mL, 60 mg/mL and 120 mg/mL in 25 mM sodium acetate buffer) was added to the polyplex suspension at a 1:1 volume ratio to get a final trehalose concentration of 7.5, 15, 30, and 60 mg/mL. The mixtures were then frozen in liquid nitrogen. After lyophilization for two days, the polyplexes were reconstructed in sodium acetate buffer and were used to treat U87MG cells. Some lyophilized polyplexes were stored in −20 °C for 7, 14 or 30 days. The gene transfection efficiency and diameter of the polyplexes were measured with the methods described above.
2.13. Statistical analysis
All experiments were performed with three replicates unless otherwise noted. Bar graphs represents mean with standard deviation. One-way ANOVA was used to analyze the statistical significance between samples. A p values smaller than 0.05 was considered significant in the analysis.
3. Results
3.1. Synthesis and characterization of ortho ester diester monomers
Here, we designed and synthesized a family of ortho ester diester (OEDE) monomers that are compatible with lipase catalysis. To prepare the OEDE monomers (Scheme 1A), we first synthesized the diketene acetal, 3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane (DETOSU), via the isomerization of 3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DTSU) 31. The 1H NMR resonances (Fig. S1) of the synthesized DETOSU were identical to those reported in the literature 34. Three different OEDEs were obtained with quantitative yield by the addition of either ethyl glycolate (n=1), ethyl 6-hydroxyhexanoate (n=5) or methyl 10-hydroxydecanoate (n=9) to DETOSU, which were named as OEDE (1), OEDE (5) and OEDE (9), respectively. The structure of the OEDE was confirmed by both 1H and 13C NMR spectroscopy (Fig. S2). For example, OEDE (5) exhibited 13C NMR resonances at 173.61 ppm due to the ester carbonyl groups and at 112.52 ppm due to the ortho ester groups. No absorption at ~150 ppm, which would result from unreacted ketene acetal, was observed. Elemental (C & H) analysis was also used to confirm the structure (theoretical/experimental weight ratio in percentage: 9.08/10.01 and 60.88/60.62, for H and C, respectively).
3.2. Synthesis and characterization of oPACEs
OEDEs were successfully co-polymerized with ω-pentadecalactone (PDL), diethyl sebacate (DES) and N-methyldiethanolamine (MDEA) using Candida antarctica lipase B (CALB) as catalyst (Scheme 1B). The polymerization was carried out in two-stages: a first stage under 1 atm of inert gas for 24 hours, during which monomers were polymerized into oligomers; and a second stage under 1.6 mmHg for 72 hours, during which high molecular weight polymers were formed. The structures of oPACEs were confirmed and analyzed by NMR (Fig. S3, Table 1) and GPC (Table 1). The polymer products are named as oPACEn-x, where n indicates OEDE (n) units in the polymer chain, x is the molar percentage of OEDE units vs MDEA units. For example, oPACE9-20 represents the polymer containing 20 mol% OEDE (9) relative to MDEA unites in the polymer backbone.
Table 1.
Characterization data of oPACEs
| Name a | OEDE/DES/MDEA/PDL (feed molar ratio) | OEDE/DES/MDEA/PDL (molar ratio) b | Mwc | PDIc | Nitrogen content (wt%) |
|---|---|---|---|---|---|
| oPACE1-6 | 10/90/100/11 | 6/94/100/11 | 1777 | 1.20 | 4.3 |
| oPACE1-13 | 20/80/100/11 | 13/87/100/11 | 1827 | 1.21 | 4.2 |
| oPACE1-24 | 30/70/100/11 | 24/76/100/11 | 1874 | 1.21 | 4.0 |
| oPACE1-34 | 40/60/100/11 | 34/66/100/11 | 2169 | 1.28 | 3.8 |
| oPACE1-43 | 50/50/100/11 | 43/57/100/11 | 2064 | 1.26 | 3.7 |
| oPACE5-13 | 20/80/100/11 | 13/87/100/11 | 15963 | 2.13 | 4.0 |
| oPACE5-20 | 30/70/100/11 | 20/80/100/11 | 13175 | 2.04 | 3.8 |
| oPACE5-28 | 40/60/100/11 | 28/72/100/11 | 8767 | 2.00 | 3.6 |
| oPACE9-8 | 10/90/100/11 | 8/92/100/11 | 18533 | 2.6 | 4.1 |
| oPACE9-14 | 20/80/100/11 | 14/86/100/11 | 11187 | 2.09 | 3.9 |
| oPACE9-20 | 30/70/100/11 | 20/80/100/11 | 9162 | 1.91 | 3.7 |
| oPACE9-31 | 40/60/100/11 | 31/69/100/11 | 10092 | 1.99 | 3.3 |
| oPACE9-36 | 50/50/100/11 | 36/64/100/11 | 8126 | 1.89 | 3.2 |
| PACE | 0/100/100/11 | 0/100/100/11 | 9937 | 2.31 | 4.5 |
The polymers are named as oPACEn-x, where n indicates OEDE (n) units in the copolymer, and x is the molar percentage of OEDE units vs MDEA units.
Measured by 1H NMR spectroscopy.
Measured by GPC with narrow polydispersity polystyrene standards.
Polymers with a range of compositions were prepared and characterized (Table 1). All the quaterpolymers have nitrogen content ranging from 3.2% to 4.3%, which is much lower than PEI (~32.6 wt% of nitrogen). The molar ratio of OEDE was varied from 8% to 36% of MDEA. oPACEs containing OEDE (5) and OEDE (9) had MW ranging from 8 kDa to 19 kDa, indicating successful polymerization. However, polymerization with the most polar OEDE (1) resulted in formation of oligomers (MW~2 kDa) only. We also observed that the polymer products contain less OEDE than the feed monomer mixtures. These observations appear to be due to the lower activity of the lipase for the highly polar OEDEs, especially OEDE (1), as the acyl binding site in the lipase CALB is more accessible to non-polar substrates 35.
3.3. Acid-sensitivity of oPACE
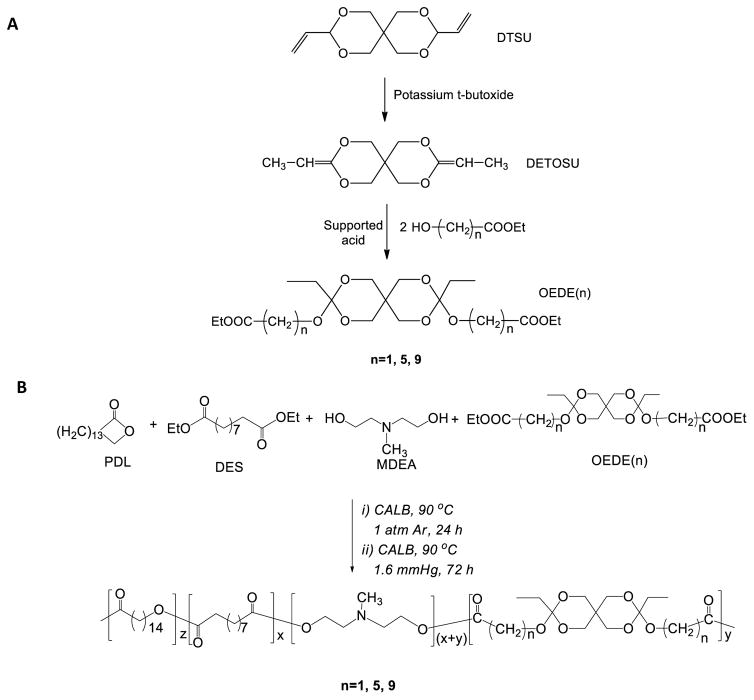
Degradation of oPACE in water was sensitive to pH (Fig. 1). For oPACE5-20, the rate of polymer weight loss was minimal at pH 7.4 and pH 6 during a 7-day incubation, moderate at pH 5.0, and rapid at pH 4.0. The half-life for weight loss was >168 hours at pH 7.4 and 6, ~24 hours at pH 5, and <1 hour at pH 4. In contrast, weight loss of PACE, the control terpolymer without ortho ester, was much slower: degradation was negligible at pH of 5, 6, or 7.4 during the 7-day incubation and was moderate at pH 4 (half-life ~120 hours). We also noted that the degradation of the oPACE5-20 copolymer occurred primarily via surface erosion mechanism: the Mw of the residual polymers was only decreased by 10–20% even after a substantial polymer weight loss of 70–90% (Fig. S4). This is similar to that reported for poly(ortho esters) previously 25–26.
Figure 1.
Acid sensitivity of oPACE. Weight loss vs time for oPACE5-20 (red) and PACE (black) upon incubation at 37 °C in (A) PBS at pH 7.4 or in sodium acetate buffer solutions at (B) pH 6.0, (C) 5.0, or (D) 4.0. The dry weight of the residual polymers is reported as a percentage of the initial polymer weight.
3.4. Polyplex formation
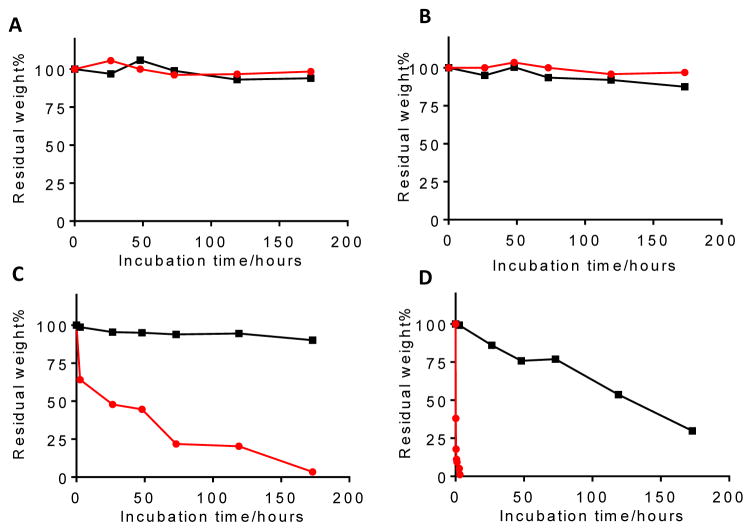
oPACE9-20 formed stable polyplexes with plasmid DNA. At a polymer:DNA weight ratio of 40:1 or higher, most of the pDNA (>80%) was complexed with polymer, as demonstrated by a PicoGreen exclusion assay (Fig. 2A). The size of the polyplexes decreased as the polymer:DNA weight ratio increased. At a ratio of 100:1 or higher, the diameter of the polyplexes was around 200 nm as measured by DLS (Fig. 2B). The polyplexes formed at 100:1 polymer:DNA weight ratio exhibited a well-defined spherical shape under TEM (Fig. 2C). A polymer to DNA weight ratio of 100:1 was used in all of the following studies unless otherwise noted.
Figure 2.
Characterization of oPACE/pDNA polyplexes. oPACE9-20/pLuc polyplexes were prepared at various polymer:DNA weight ratios, and (A) the amount of uncomplexed DNA was measured by PicoGreen assay and reported as a percentage of the total amount of DNA, and (B) their diameter was measured with DLS. (C) TEM image of the polyplexes prepared at 100:1 weight ratio. Scale bar indicates 500 nm.
3.5. Endosomal escape of pDNA/oPACE polyplexes
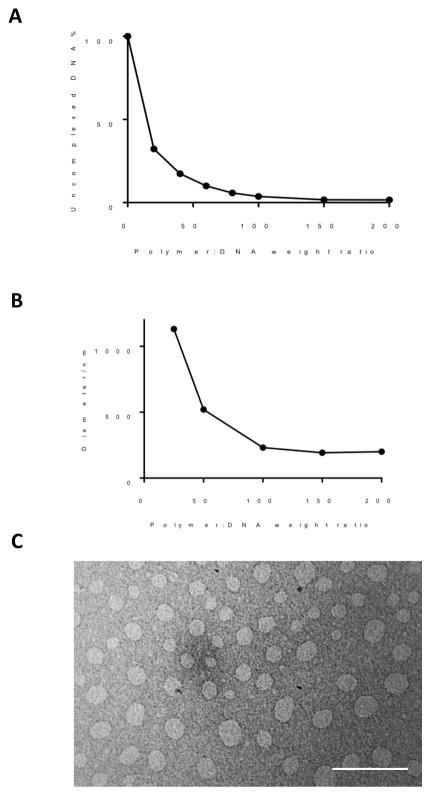
To test whether the acid-sensitive oPACE could facilitate endosomal escape, we labelled pLuc plasmids with both acid-sensitive FITC and acid-insensitive Cy5 and used a previously reported method to evaluate the pH environment of the intracellular DNA 32–33. At lower pH, FITC is quenched, leading to a low FITC/Cy5 fluorescence ratio; and vice versa. We prepared oPACE or PACE polyplexes with this plasmid, which were then used to transfect U87MG cells. After 1 hour of transfection, the polyplexes were removed, followed by another hour of incubation. Then, we used intracellular pH clamping buffers 36 to fix the pH of some of the transfected cells and generated separate pH calibration curves for PACE and oPACE polyplex (Fig. 3A). Using these standard curves, we measured the pH around the DNA delivered by oPACE or PACE polyplexes (Fig. 3B). The pH environment around the DNA delivered by oPACE polyplexes was 6.5 ± 0.05, compared to 5.8 ± 0.06 for the DNA delivered by PACE polyplexes. This result indicates that a greater fraction of the DNA delivered by oPACE polyplexes has escaped from the acidic endosome into cytosol, thus generating a higher average pH than DNA delivered by PACE polyplexes.
Figure 3.
oPACE facilitates endosomal escape of genes. U87MG cells were transfected with FITC-Cy5 double labelled DNA (pLuc) by PACE or oPACE9-20. (A) Standard curves between FITC/Cy5 fluorescence ratio (measured by FACS) and intracellular pH of cells. (B) The FITC/Cy5 ratio of the transfected U87MG cells was measured and converted into pH values using the standard curves. Error bars indicate standard deviation (n=3, ****: p<0.0001).
3.6. Acid-sensitivity of the pDNA/oPACE polyplexes
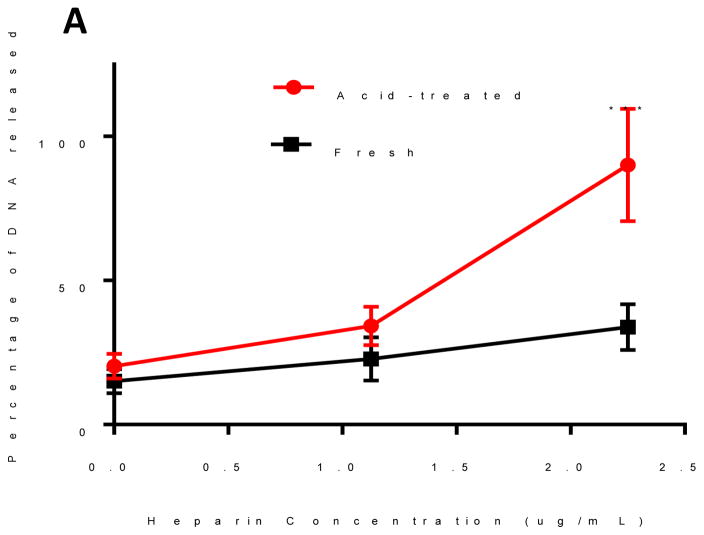
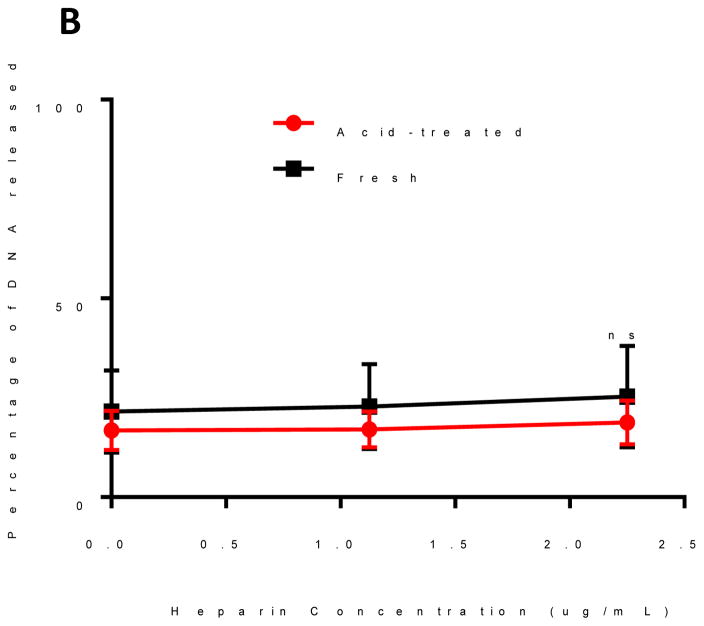
We hypothesized that the binding between oPACE and pDNA would be unstable after incubation in acidic environment because of the fast degradation of oPACE, which would lead to efficient unpackaging of pDNA from the polyplexes. This feature is potentially important for the unpackaging of pDNA after incubation in endosomes. To test this hypothesis, pLuc/oPACE9-20 and pLuc/PACE polyplexes were prepared and incubated at pH 5 and 37 °C for 4 hours. After acid-treatment, oPACE polyplexes exhibited a significantly increased instability in the presence of heparin (Fig. 4A): at a heparin concentration of 2.25ug/mL, 90% of the gene was released from the acid-treated oPACE polyplexes, while only 30% was released from the fresh polyplexes. In comparison, the pDNA/PACE polyplexes did not show significant differences in stability before and after acid-treatment (Fig. 4B).
Figure 4.
Acid-sensitivity of oPACE/pDNA polyplexes. Percentage of DNA released from fresh and acid-treated (A) oPACE/DNA polyplexes and (B) PACE/DNA polyplexes upon incubation with heparin at different concentrations. The amount of DNA released was measured with PicoGreen and the acid-treatment condition was pH 5 at 37 °C for 4 hours. Error bars indicate standard deviation (n=3, ***: p<0.001, ns=not significant).
3.7. In vitro characterization
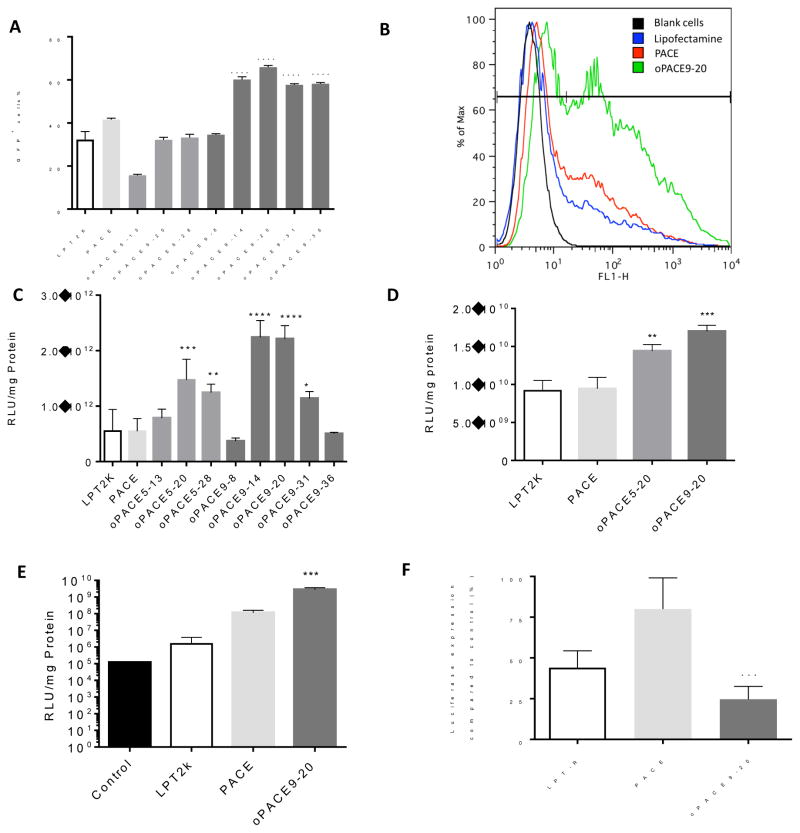
To test for transfection efficiency, polyplexes of oPACE and pDNA encoding GFP (pGFP) were added to U87MG cells in culture and the percentage of transfected cells was measured by flow cytometry (Fig. 5A and 5B). Several oPACE polymers containing OEDE (9) exhibited significantly higher transfection efficiency than PACE or lipofectamine (Fig. 5A). The most efficient polymer, oPACE9-20, transfected 67% of U87MG cells, while PACE only transfected 41% and lipofectamine transfected 28% (Fig. 5B). oPACE9-20 transfected more U87MG cells and resulted in a significantly higher average fluorescence intensity than PACE and lipofectamine 2000. The transfection efficiency was dependent on the ratio and type of OEDE.
Figure 5.
Gene transfection efficiency of oPACEs. (A) GFP expression of U87MG cells 2 days after transfection of pGFP by different vectors. (B) Flow cytometry histogram of U87MG cells transfected with pGFP. Luciferase expression of (C) HEK293, (D) primary mouse melanoma, and (E) HUVEC cells 2 days after transfection of pLuc by different vectors. (F) Knockdown of luciferase expression in HEK293-Luc cells by siRNA delivered by different vectors two days after transfection. All polyplexes were prepared at a 100:1 polymer:gene weight ratio. Lipofectamine 2000 (LPT2K) and Lipofectamine RNAiMAX (LPT-R) transfection was performed according to manufacturer’s protocol. The same amount of DNA was used in all samples. Error bars indicate standard deviation (n=3; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001, all indicating statistical difference with PACE).
oPACEs were also used to deliver a pDNA encoding luciferase (pLuc) into various types of cells. In HEK293 cells (Fig. 5C), many of the oPACEs showed significantly higher transfection efficiency than Lipofectamine 2000 or PACE. The best polymer, oPACE9-20, showed 4 times higher transfection efficiency than PACE or lipofectamine (LPT). The transfection efficiency was dependent on the mole percentage of OEDE, suggesting that the acid sensitivity is playing an important role. For example, the efficiency for oPACE9 peaks around 14 or 20 mol% of OEDE (9), which is 6, 2 and 4 times higher than polymer containing 8 mol%, 30 mol% or 35 mol% of OEDE (9), respectively. A similar trend was found for polymers containing OEDE (5). At similar molar ratio, polymers containing OEDE (9) were more efficient than polymers containing OEDE (5), possibly due to the higher hydrophobicity of OEDE (9).
To test the efficiency of the polymers in transfecting primary cells, some oPACE polymers were used to transfect a primary mouse melanoma cell line (Fig. 5D). Both oPACE5-20 and oPACE9-20 exhibited significantly higher transfection efficiency than PACE and Lipofectamine 2000. oPACE9-20 is 2 times more efficient than PACE or Lipofectamine. We also screened the polymers for transfection efficiency in human umbilical vein endothelial cells (HUVEC), which are primary cells that are difficult to transfect 37–38 (Fig. 5E). We found that oPACE was 25 times more efficient in transfecting HUVECs than PACE. Lipofectamine exhibited limited transfection efficiency, possibly due to the high toxicity to HUVECs.
To examine the potential of oPACE to transfect cells with other types of genetic materials, we also tested the efficiency of oPACE9-20 in siRNA delivery (Fig. 5F). We used a HEK293 cell line that is stably transfected with luciferase genes. Even by delivering a very low dose of siRNA, oPACE9-20 was still able to knockdown the luciferase expression by 75%. In comparison, PACE and Lipofectamine RNAiMAX (LPT-R) only demonstrated a 25% and 66% knockdown, respectively.
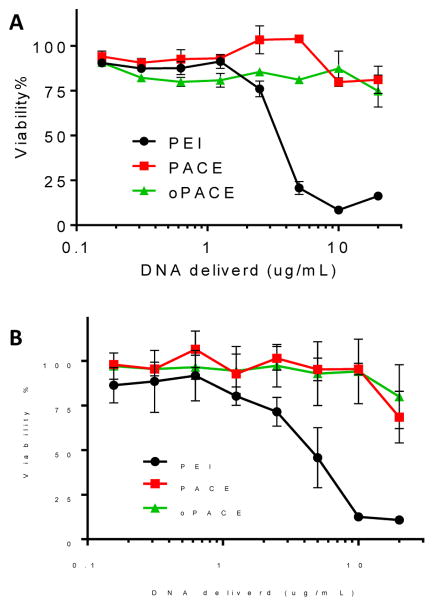
Polyplexes prepared with oPACE are non-toxic (Fig. 6). Polyplexes were prepared with PACE and oPACE9-20 at 100:1 polymer:DNA weight ratio. Similar to PACE, oPACE9-20 could deliver a high concentration of pDNA (20 ug/mL) with minimal toxicity on both HEK293 and U87MG cells. Although PEI polyplexes were prepared at a much lower polymer:DNA weight ratio (3:1) according to previously reported protocol 11, they exhibited much higher toxicity: more than 50% of the cells were killed at 5 ug/mL of DNA delivered.
Figure 6.
oPACE polyplexes were non-toxic. (A) U87MG and (B) HEK293 cells were treated with oPACE9-20/pLuc, PACE/pLuc or PEI/pLuc polyplexes. Cell viability was measured 2 days later with MTT assay and reported as percentage of the cells treated by control vehicle. Error bars indicate standard deviation (n=3).
3.8. Lyophilization of the polyplexes
We developed methods to lyophilize the polyplexes for concentration and storage stability of the polyplexes. The polyplexes were prepared with oPACE9-20 and pLuc at 100:1 polymer:DNA weight ratio and lyophilized with the presence of 0, 7.5, 15, 30, and 60mg/mL of trehalose. Without the addition of trehalose, the lyophilized polyplexes was 3 times less efficient in transfecting U87MG cells than the fresh ones (Fig. 7A). However, the addition of trehalose during lyophilization significantly increased the transfection efficiency: after lyophilization, the polyplexes lyophilized with 15 mg/mL trehalose exhibited a comparable transfection efficiency to the fresh polyplexes, while the polyplexes lyophilized with 30 or 60 mg/mL trehalose exhibited a higher transfection efficiency than the fresh polyplexes. There was no significant change in the size of the polyplexes after lyophilization (Fig. 7B). After storing the lyophilized polyplexes at -20°C for a month, no significant change was found in their transfection efficiency (Fig. 7C) or diameter (Fig. 7D), indicating the high stability of the lyophilized formulation.
Figure 7.
Lyophilization and storage of oPACE/DNA polyplexes. oPACE9-20/pLuc polyplexes were lyophilized with trehalose of different concentration. (A) The gene transfection efficiency of the lyophilized polyplexes was tested on U87MG cells with a luciferase assay, and (B) their diameter was measured with DLS. The freshly prepared polyplexes were included for comparison. In another study, oPACE9-20/pLuc polyplexes were lyophilized with 0, 15 or 30 mg/mL of trehalose and stored at -20°C for 0, 7, 14, or 30 days. Their (C) gene transfection efficiency on U87MG cells and (D) diameter before and after storage was measured. Error bars indicate standard deviation (n=3, ***: p<0.001, ****: p<0.0001, ns=no significant difference, all indicating statistical difference with fresh polyplexes).
4. Discussion
4.1. Multifunctional polymers synthesized by novel chemistry
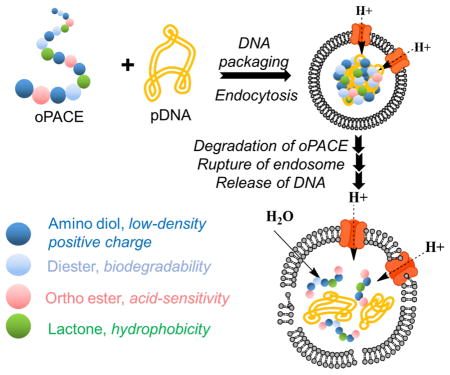
Here we rationally designed and synthesized a family of poly (amine-co-ester-co-ortho esters) that have high multi-functionality. The polymers have biodegradability, low cationic charge, high hydrophobicity, and acid-sensitivity, all of which are crucial for efficient and safe gene delivery. Biodegradability is important to avoid bioaccumulation. Low charge is important because polyplexes with excess positive charge can interact with cellular components and inhibit normal cellular processes, which leads to substantial toxicity 39–42. Hydrophobicity is another desired feature to help stabilize the polyplexes, especially for polymers with low cationic density 11. Acid-sensitivity is important for endosomal escape and unpackaging of genes. Nanoparticles are taken up by cells through endocytosis: nanoparticles are internalized within endosomes, where acidification occurs within 2–3 min due to an ATPase-mediated proton influx 43. The endosomes can mature to become late endosomes or lysosomes, in which the pH can be as low as 4 44. The escape of genes from the endolysosomes is a major barrier for gene transfection 3. An acid-sensitive polymer can help with endosomal escape through colloid osmotic mechanism: upon exposure to the acidic environment, the polymer will degrade promptly and create a large amount of small molecular weight degradation products inside the endosomes 14–18. This increases the intra-endosomal osmotic pressure and enhances the breakdown of endosomes. At the same time, the degradation of the polymer will make it easier for the genetic materials to be released from the polyplexes 14, 16, 19.
The synthesis of the multifunctional oPACE was enabled by novel chemistry. To the best of our knowledge, this is the first report showing the compatibility of ortho ester functional groups in lipase catalysis, owing to the novel ortho ester diester monomers we designed for this study (Scheme 1A). Also, it is the first report of polymers containing ortho ester groups, large lactone groups, esters and tertiary amines, resulting a new class of multi-functional polymers.
The chemistry we used is also tunable and provides the simplicity and versatility to allow easy access to the range of polymer structures that is needed to test hypotheses regarding polymer multifunctionality. For example, the OEDE content in the polymers can be varied over a wide range (8–36%), which allows fine tuning of the acid sensitivity of the polymers. In contrast, although poly (ortho ester amide) and poly (ortho ester amidine) copolymers were recently prepared in multiple steps 29, 45 for gene delivery, the synthetic methods do not allow the optimization of the polymer structure. As a result, none of these prior polymers showed high enough efficiency in delivering genes and induced high toxicity.
4.2. oPACE facilitates the endosomal escape and unpackaging of genes
As shown in the pH-dependent degradation study, the oPACE remained stable at physiological pH (7.4) and the pH found in tumor microenvironment (6–7) 46 (Fig. 1). This implies that the oPACE polyplexes will be stable in the pH of systematic circulation and tumor microenvironment so that the genetic materials are efficiently protected. However, under endolysosomal pH (4–5), the oPACE degraded rapidly. We demonstrated that the oPACE promotes the endosomal escape of DNA (Fig. 3). The environmental pH surrounding oPACE polyplexes (pH 6.5 ± 0.05) is higher than that surrounding PACE polyplexes (pH 5.8 ± 0.06), which indicates that more oPACE polyplexes have escaped from the endolysosome and entered cytosol after transfecting U87MG cells. These results are consistent with a recent report on confocal laser scanning microscopy experiments revealing the endosomal escape of polyplex particles formed from DNA and a similar polymer with tertiary amino groups, PEG-poly(lactone-co-β-amino ester).47 Using the same technique, Akinc et al. measured the pH environment of the DNA delivered by different vectors 2 hours after transfection in NIH 3T3 cells 33. The measured pH for DNA delivered by poly (L-lysine) (PLL), linear PEI (LPEI), PEI, and lipofectamine were 4.5, 5.0, 5.9, and 7.1, respectively. Among these vectors, PLL was least efficient in facilitating endosomal escape and the pH around the DNA delivered by PLL was similar to lysosomal pH. LPEI and PEI could facilitate endosomal escape through proton sponge mechanism. Lipofectamine was most efficient in facilitating endosomal escape, presumably because of the high efficiency of the cationic lipids in destabilizing the endosomal membranes.48
We also demonstrated that the oPACE/DNA polyplexes are sensitive to acidic environments (Fig. 4): after incubation in pH 5, the binding between oPACE and DNA became less stable and more prone to competitive disruption by heparin. We believe that this reflects the degradation of oPACE polymers in acidic environment resulting in small molecular weight polymers that were less efficient in condensing DNA. Thus, after exposure to the acidic endosomes, the release of DNA from the polyplexes is easier because of the looser binding between DNA and the polymer, which will make the DNA more accessible for expression.
4.3. High efficiency in delivering genes into various cell lines
oPACE is a highly efficient, versatile, and non-toxic delivery vector for genetic materials. oPACE is significantly more efficient than PACE or lipofectamine in delivering luciferase- and GFP-encoding plasmid DNA into two different cell lines (HEK293 and U87MG) (Fig. 5A–C). oPACE is also efficient in transfecting mouse melanoma primary cells (Fig. 5D). In addition, the oPACE polymers have high efficiency in transfecting pDNA into HUVEC cells and are more efficient than PACE and lipofectamine (Fig. 5E). Endothelial cells play an important role in both cardiovascular diseases and cancers, as they are involved in vascular function and angiogenesis. However, effective and safe gene delivery to primary endothelial cells in the presence of serum proteins is known to be extremely challenging 37. Specifically, HUVEC cells are known for its difficulty in gene transfection.38,49 Although polymeric gene delivery systems to HUVEC have been reported 37, they usually suffer from high toxicity or low efficiency. We believe that the high efficiency of oPACE polymers results from their multi-functionality, which entails the ability to facilitate endosomal escape and gene unpackaging in cytosol. Preliminary results also suggest that oPACE can facilitate delivery of siRNA into cells (Fig. 5F). It is likely that oPACE also facilitates the escape of siRNA from endolysosomes, thus preventing the lysosomal degradation of the labile siRNA.50–51 All the above transfections were done in medium with normal (10%) serum to better mimic the in vivo conditions and demonstrate the stability of the polyplexes in serum containing medium.
4.4. Lyophilization for long term storage
Lyophilization of polyplexes is a common strategy to allow long term storage of the polyplexes. In addition, a solid or concentrated dosage of polyplexes is important for many applications 51, such as in intracranial delivery, where the injection volume is strictly limited 52. Lyophilization of the polyplexes is an ideal way to solve this problem, because the dried powder can be easily stored and reconstituted at the desired concentration. However, lyophilization without lyoprotectant led to diminishment of transfection efficiency compared to fresh polyplexes. The addition of trehalose helped to maintain the transfection efficiency of the particles upon lyophilization (Fig. 7A and 7B). The transfection efficiency was increased after lyophilization with high concentration of trehalose, possibly due to the lyoprotectant’s ability to increase the stability of the polyplexes after suspending in serum-containing buffers 53. The transfection efficiency of the lyophilized polyplexes remained stable for a month, suggesting high storage stability of the polyplexes (Fig. 7C and 7D).
5. Conclusions
In this study, we designed and synthesized a novel family of poly(amine-co-ester-co-ortho esters). By designing a novel ortho ester diester monomer, we demonstrated the compatibility of ortho ester group with lipase catalysis and successfully incorporated ortho ester groups, large lactone groups, esters and tertiary amines into the polymer backbone. We verified the hypothesis that the acid-sensitive polymer facilitates efficient endosomal escape and gene unpackaging, which are key factors for efficient gene delivery. As a result, polyplexes of oPACE exhibited high efficiency in delivering DNA into various cell lines and primary cells without toxicity. The oPACE polyplexes can be lyophilized and stored for up to a month without loss of activity. Thus, oPACE is an efficient and safe delivery platform for various applications in gene delivery.
Acknowledgments
We thank Marcus Bosenberg for providing the mouse melanoma primary cells. This work was supported by US National Institutes of Health (grant numbers CA149128 and HL125892).
Footnotes
Supporting Information Available
The following files are available free of charge.
1H-NMR spectrum of DETOSU, 1H-NMR and 13C-NMR spectra of OEDE (5), 1H NMR spectrum of oPACE5-20, molecular weight change of PACE and oPACE5-20 after incubation at different pH.
References
- 1.Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 2.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012–an update. The journal of gene medicine. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 3.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 4.Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Advanced drug delivery reviews. 1998;34(1):3–19. doi: 10.1016/S0169-409X(00)00133-2. [DOI] [PubMed] [Google Scholar]
- 5.Davis ME. Non-viral gene delivery systems. Current opinion in biotechnology. 2002;13(2):128–131. doi: 10.1016/S0958-1669(02)00294-X. [DOI] [PubMed] [Google Scholar]
- 6.Li S-D, Huang L. Non-viral is superior to viral gene delivery. J Control Release. 2007;123(3):181–183. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chemical reviews. 2008;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 8.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nature biotechnology. 2000;18(1):33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 9.Lynn DM, Langer R. Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. Journal of the American Chemical Society. 2000;122(44):10761–10768. doi: 10.1021/ja0015388. [DOI] [Google Scholar]
- 10.Godbey W, Wu KK, Mikos AG. Poly (ethylenimine) and its role in gene delivery. J Control Release. 1999;60(2):149–160. doi: 10.1016/S0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nature materials. 2012;11(1):82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Jiang ZZ, Zhou JB, Zhang SM, Saltzman WM. Enzyme-synthesized poly(amine-co-esters) as nonviral vectors for gene delivery. J Biomed Mater Res A. 2011;96A(2):456–465. doi: 10.1002/Jbm.A.32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behr J-P. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA International Journal for Chemistry. 1997;51(1–2):34–36. [Google Scholar]
- 14.Ko IK, Ziady A, Lu S, Kwon YJ. Acid-degradable cationic methacrylamide polymerized in the presence of plasmid DNA as tunable non-viral gene carrier. Biomaterials. 2008;29(28):3872–3881. doi: 10.1016/j.biomaterials.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JL, Schubert S, Wich PR, Cui L, Cohen JA, Mynar JL, Fréchet JMJ. Acid-Degradable Cationic Dextran Particles for the Delivery of siRNA Therapeutics. Bioconjugate Chem. 2011;22(6):1056–1065. doi: 10.1021/bc100542r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim MS, Kwon YJ. Controlled delivery of plasmid DNA and siRNA to intracellular targets using ketalized polyethylenimine. Biomacromolecules. 2008;9(2):444–455. doi: 10.1021/bm7007313. [DOI] [PubMed] [Google Scholar]
- 17.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Fréchet JMJ. A macromolecular delivery vehicle for protein-based vaccines: Acid-degradable protein-loaded microgels. Proceedings of the National Academy of Sciences. 2003;100(9):4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Fréchet JMJ. Acid-Degradable Particles for Protein-Based Vaccines: Enhanced Survival Rate for Tumor-Challenged Mice Using Ovalbumin Model. Bioconjugate Chem. 2004;15(6):1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 19.Goh SL, Murthy N, Xu M, Fréchet JMJ. Cross-Linked Microparticles as Carriers for the Delivery of Plasmid DNA for Vaccine Development. Bioconjugate Chem. 2004;15(3):467–474. doi: 10.1021/bc034159n. [DOI] [PubMed] [Google Scholar]
- 20.Green JJ, Zugates GT, Tedford NC, Huang YH, Griffith LG, Lauffenburger DA, Sawicki JA, Langer R, Anderson DG. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv Mater. 2007;19(19):2836-+. doi: 10.1002/adma.200700371. [DOI] [Google Scholar]
- 21.Lu JS, Li NJ, Xu QF, Ge JF, Lu JM, Xia XW. Acetals moiety contained pH-sensitive amphiphilic copolymer self-assembly used for drug carrier. Polymer. 2010;51(8):1709–1715. doi: 10.1016/j.polymer.2009.12.034. [DOI] [Google Scholar]
- 22.Shim MS, Kwon YJ. Controlled cytoplasmic and nuclear localization of plasmid DNA and siRNA by differentially tailored polyethylenimine. Journal of Controlled Release. 2009;133(3):206–213. doi: 10.1016/j.jconrel.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Gu W, Chen L, Gao Y, Zhang Z, Li Y. A smart nanoassembly consisting of acid-labile vinyl ether PEG-DOPE and protamine for gene delivery: preparation and in vitro transfection. Biomacromolecules. 2008;9(11):3119–26. doi: 10.1021/bm800706f. [DOI] [PubMed] [Google Scholar]
- 24.Cordes EH, Bull HG. Mechanism and catalysis for hydrolysis of acetals, ketals, and ortho esters. Chemical Reviews. 1974;74(5):581–603. doi: 10.1021/cr60291a004. [DOI] [Google Scholar]
- 25.Heller J, Barr J. Poly(ortho esters)--from concept to reality. Biomacromolecules. 2004;5(5):1625–32. doi: 10.1021/bm040049n. [DOI] [PubMed] [Google Scholar]
- 26.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Advanced drug delivery reviews. 2002;54(7):1015–39. doi: 10.1016/S0169-409X(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 27.Heller J, Barr J, Ng SY, Shen HR, Schwach-Abdellaoui K, Einmahl S, Rothen-Weinhold A, Gurny R. Poly(ortho esters) - their development and some recent applications. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2000;50(1):121–8. doi: 10.1016/S0939-6411(00)00085-0. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Ge Q, Ting D, Nguyen D, Shen HR, Chen J, Eisen HN, Heller J, Langer R, Putnam D. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nature materials. 2004;3(3):190–6. doi: 10.1038/nmat1075. [DOI] [PubMed] [Google Scholar]
- 29.Tang R, Ji W, Wang C. Synthesis and characterization of new poly (ortho ester amidine) copolymers for non-viral gene delivery. Polymer. 2011;52(4):921–932. doi: 10.1016/j.polymer.2010.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41(5):544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helwing RF. Preparation of ketene acetals by rearrangement of allyl and substituted allyl acetals. Google Patents. 1985 [Google Scholar]
- 32.Yue Y, Jin F, Deng R, Cai J, Dai Z, Lin MCM, Kung H-F, Mattebjerg MA, Andresen TL, Wu C. Revisit complexation between DNA and polyethylenimine — Effect of length of free polycationic chains on gene transfection. J Control Release. 2011;152(1):143–151. doi: 10.1016/j.jconrel.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Akinc A, Langer R. Measuring the pH environment of DNA delivered using nonviral vectors: Implications for lysosomal trafficking. Biotechnol Bioeng. 2002;78(5):503–508. doi: 10.1002/bit.20215.abs. [DOI] [PubMed] [Google Scholar]
- 34.Crivello J, Malik R, Lai YL. Ketene acetal monomers: synthesis and characterization. Journal of Polymer Science Part A: Polymer Chemistry. 1996;34(15):3091–3102. doi: 10.1002/(SICI)1099-0518(19961115)34:15<3091::AID-POLA1>3.0.CO;2-0. [DOI] [Google Scholar]
- 35.Otto RT, Scheib H, Bornscheuer UT, Pleiss J, Syldatk C, Schmid RD. Substrate specificity of lipase B from Candida antarctica in the synthesis of arylaliphatic glycolipids. Journal of Molecular Catalysis B: Enzymatic. 2000;8(4–6):201–211. doi: 10.1016/S1381-1177(99)00058-2. [DOI] [Google Scholar]
- 36.Bayer N, Schober D, Prchla E, Murphy RF, Blaas D, Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. Journal of virology. 1998;72(12):9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconjugate Chem. 2006;17(5):1162–1169. doi: 10.1021/Bc0600968. [DOI] [PubMed] [Google Scholar]
- 38.Gresch O, Altrogge L. Transfection of difficult-to-transfect primary mammalian cells. Protein Expression in Mammalian Cells: Methods and Protocols. 2012:65–74. doi: 10.1007/978-1-61779-352-3_5. [DOI] [PubMed] [Google Scholar]
- 39.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 40.de Ilarduya CT, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. European journal of pharmaceutical sciences. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Gao X, Yao L, Song Q, Zhu L, Xia Z, Xia H, Jiang X, Chen J, Chen H. The association of autophagy with polyethylenimine-induced cytotoxity in nephritic and hepatic cell lines. Biomaterials. 2011;32(33):8613–8625. doi: 10.1016/j.biomaterials.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 42.Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BioImpacts: BI. 2011;1(1):23. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy RF, Powers S, Cantor CR. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. The Journal of cell biology. 1984;98(5):1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simões S, Moreira JN, Fonseca C, Düzgüneş N, de Lima MCP. On the formulation of pH-sensitive liposomes with long circulation times. Advanced drug delivery reviews. 2004;56(7):947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 45.Tang R, Palumbo RN, Ji W, Wang C. Poly (ortho ester amides): acid-labile temperature-responsive copolymers for potential biomedical applications. Biomacromolecules. 2009;10(4):722–727. doi: 10.1021/bm9000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer research. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 47.Chen Y, Li Y, Gao J, Cao Z, Jiang Q, Liu J, Jiang Z. Enzymatic PEGylated poly(lactone-co-β-amino ester) nanoparticles as biodegradable, biocompatible and stable vectors for gene delivery. ACS Appl Mater Interfaces. 2016;8:490–501. doi: 10.1021/acsami.5b09437. [DOI] [PubMed] [Google Scholar]
- 48.El Ouahabi A, Thiry M, Pector V, Fuks R, Ruysschaert JM, Vandenbranden M. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. FEBS letters. 1997;414(2):187–192. doi: 10.1016/S0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- 49.Zumbansen M, Altrogge LM, Spottke N, Spicker S, Offizier SM, Domzalski S, St Amand AL, Toell A, Leake D, Mueller-Hartmann HA. First siRNA library screening in hard-to-transfect HUVEC cells. J RNAi Gene Silencing. 2010;6(1):354–60. [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffelers RM, Woodle MC, Scaria P. Pharmaceutical prospects for RNA interference. Pharm Res. 2004;21(1):1–7. doi: 10.1023/B:PHAM.0000012145.49054.6c. [DOI] [PubMed] [Google Scholar]
- 51.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Advanced drug delivery reviews. 2006;58(15):1688–713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ, Jr, Piepmeier JM, Saltzman WM. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(29):11751–6. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzeng SY, Guerrero-Cázares H, Martinez EE, Sunshine JC, Quiñones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on Poly(β-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. 016/j.biomaterials.2011.04.016. For Table of Contents Only. [DOI] [PMC free article] [PubMed] [Google Scholar]