Abstract
Rheumatoid arthritis (RA) is characterized by synovial joint inflammation and by development of pathogenic humoral and cellular autoimmunity to citrullinated proteins. Neutrophil extracellular traps (NETs) are a source of citrullinated autoantigens and activate RA synovial fibroblasts (FLS), cells crucial in joint damage. We investigated the molecular mechanisms by which NETs promote proinflammatory phenotypes in FLS, and whether these interactions generate pathogenic anti-citrulline adaptive immune responses. NETs containing citrullinated peptides are internalized by FLS through a RAGE-TLR9 pathway promoting FLS inflammatory phenotype and their upregulation of MHC class II. Once internalized, arthritogenic NET-peptides are loaded into FLS MHC class II and presented to Ag-specific T cells. HLADRB1*0401 transgenic mice immunized with mouse FLS loaded with NETs develop antibodies specific to citrullinated forms of relevant RA autoantigens implicated in RA pathogenesis as well as cartilage damage. These results implicate FLS as mediators in RA pathogenesis, through the internalization and presentation of NET citrullinated peptides to the adaptive immune system leading to pathogenic autoimmunity and cartilage damage.
Introduction
Rheumatoid arthritis (RA) is the second most prevalent autoimmune condition, affecting 1% of the world population. It is a chronic, systemic inflammatory disease that affects the peripheral synovial joints and is associated to high morbidity and enhanced mortality. A significant proportion of RA patients exhibit RA-related autoantibodies, which include rheumatoid factor and antibodies to citrullinated protein antigens (ACPAs)(1). RA is characterized by a prolonged (3–5 year) subclinical phase where ACPAs are detected before the onset of clinically apparent disease (2–5). ACPA reactivity is directed against various citrullinated intracellular and extracellular antigens including vimentin, histones, fibrinogen and enolase. T-cell responses to citrullinated peptides also develop in RA. Reactivity to citrullinated antigens correlates with the presence of the HLA-DRB1*04:01 shared epitope, which includes HLA-DRB1*04:01, *04:04 and *01:01, haplotypes associated with risk to develop RA (6, 7). Citrullination of specific anchor residues enhances the ability of peptides to bind and be presented by the MHC class II (MHCII) shared epitope alleles, allowing the activation and expansion of citrulline specific CD4+-T-cells, and the subsequent promotion of ACPA generation (8–12).
In early stages of RA, neutrophils are abundant in both synovial tissue and fluid, supporting an important role for these cells in the initial events contributing to the pathogenesis of this disease (13). Recent work from our group and others indicates that RA synovial and peripheral blood neutrophils display an enhanced capacity to form neutrophil extracellular traps (NETs) (14, 15). During NET formation, there is intracellular activation of peptidylarginine deiminase-4 (PAD4), a myeloid-specific PAD involved in citrullination, and neutrophils extrude a meshwork of nuclear material coupled to cytoplasmic and granular proteins. Due to PAD activation, proteins externalized in NETs become citrullinated and several of them have been characterized as important RA autoantigens (14). As such, NET formation may represent an important process leading to the citrullination of autoantigens that, in a genetically predisposed host, could promote activation of innate and adaptive immune responses and contribute to RA development.
One important cellular participant in RA is the fibroblast-like synoviocyte (FLS). These cells are major effectors in cartilage damage and participate in synovial inflammation in the rheumatoid joint. FLS express a variety of Toll-like receptors (TLRs) and have the capacity to act as antigen-presenting cells (APCs) in the synovium (16–19). Recent evidence demonstrates that FLS are activated by NETs, leading to upregulation of inflammatory cytokine and adhesion molecule synthesis (14). However, the mechanisms by which NETs activate FLS remain to be fully characterized. We hypothesized that specific citrullinated autoantigens contained in NETs can be taken up by FLS and presented to T cells in an MHCII-dependent manner, leading to Ag-specific enhanced T and B cell responses relevant to disease pathogenesis.
Results
ACPAs induce NETosis and recognize multiple citrullinated autoantigens exposed in NETs
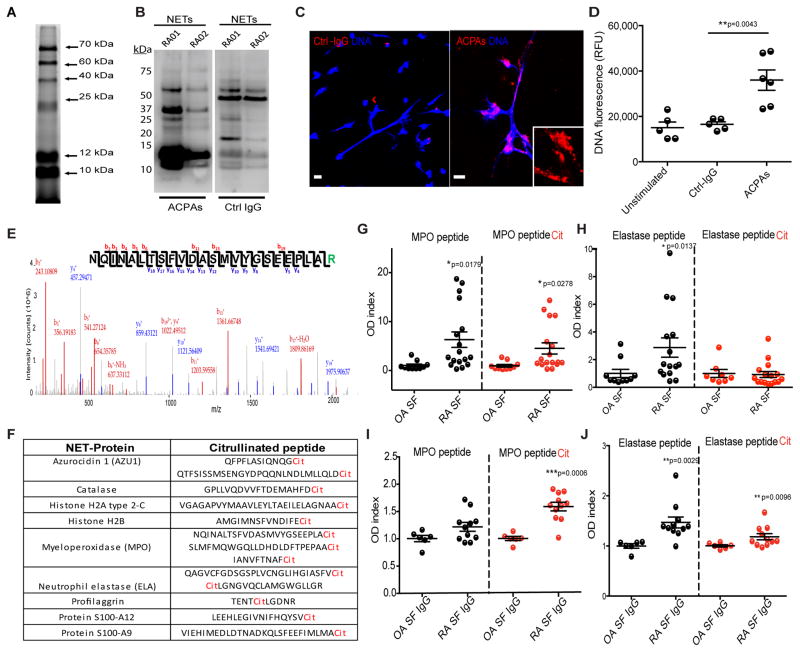
Putative arthritogenic peptides such as histone H3, H4 and vimentin have been reported to be citrullinated in NETs (14, 20, 21). As citrullination and response to citrullinated antigens are considered key in RA pathogenesis, we investigated whether other peptides are citrullinated in NETs and could function as autoantigens in this disease. Using a rhodamine phenylglyoxal-based probe to quantify citrullination, multiple citrullinated proteins were detected in NETs that were induced in control neutrophils by rheumatoid factor stimulation, a known inducer of NETosis12 (Fig. 1A). Western blot and immunofluorescence analyses demonstrated that ACPAs isolated from RA patients differentially recognized multiple citrullinated proteins in spontaneously generated RA NETs when compared to control IgGs, suggesting that RA-specific autoantigens are contained within these structures (Fig. 1B–C & Fig. S1). In addition, purified RA ACPAs enhanced NETosis in control neutrophils when compared to control IgG (Fig. 1D). These results confirm the hypothesis that RA NETs or NETs induced by RA-relevant stimuli externalize multiple citrullinated autoantigens; in turn, RA autoantibodies enhance NET formation.
Figure 1. ACPAs recognize multiple citrullinated peptides in NETs and induce NETosis.
A. Rhodamine-Phenylglyoxal probe against citrulline was utilized to detect specific citrullinated proteins in purified NETs generated by stimulating control neutrophils with IgM rheumatoid factor. B. ACPAs differentially recognize citrullinated autoantigens in NETs when compared to control IgG. Spontaneously generated NETs from peripheral blood neutrophils from two RA patients (RA01 and RA02) were isolated and resolved in SDS-PAGE. Western blot was performed using ACPAs or control IgG. C. ACPAs bind to NETs and D. enhance NETosis. Red represents control IgG (ctrl-IgG) or ACPAs, and blue is Hoechst. E. Representative PEAKS’ histogram of citrullinated peptides detected in NETs. F. Mass Spectrometry analysis demonstrates multiple citrullinated peptides in NETs. G–H ELISA analysis of synovial fluid from OA (n=10) and RA (n=17) patients to detect autoantibodies recognizing citrullinated or non-citrullinated forms of MPO and neutrophil elastase, respectively. I–J ELISA analysis of IgGs isolated from synovial fluid (SF) of OA (n=6) and RA (n=11) patients showing recognition of native or citrullinated MPO or elastase. A, B and C are representative of three independent experiments. Scale bars, 10μm. Results are the mean ± s.e.m. of n= 5–6. For statistical analyses Mann-Whitney U test was used.
To identify in more detail the proteins that are citrullinated in NETs, proteomic analysis was performed in NETs similarly induced in peripheral blood control neutrophils by rheumatoid factor stimulation (control IgM does not induce NETs12). This analysis detected several citrullinated proteins in these structures (Fig. 1E–F & Table S1) including azurocidin, catalase, histone H2B, myeloperoxidase (MPO), neutrophil elastase, profilaggrin, S100-A12, and S100-A9. Since MPO and neutrophil elastase play important roles in NET formation (22) and are abundant and citrullinated in NETs, we investigated whether RA patients develop autoAbs against citrullinated forms of these proteins. We selected two epitopes, IANVFTNAFR (citrullinated in MPO) and RLGNGVQCLAMGWGLLGR (citrullinated in neutrophil elastase), and generated synthetic peptides with or without citrullination sites. We identified the presence of autoantibodies against the citrullinated MPO peptide (cit-MPO) and against the native-form elastase peptide in RA synovial fluid but not in the synovial fluid from patients with osteoarthritis (OA) (Fig. 1G–H). Confirming these findings, IgGs purified from RA synovial fluid recognized cit-MPO and cit-ELA (Fig. 1I–J). These observations confirm and expand the repertoire of citrullinated molecules present in NETs to which RA patients develop autoantibody responses. These results also support previous observations that in RA, antibody responses to both native and citrullinated version of various proteins are detected (23).
Internalization of NET components by FLS promotes their inflammatory phenotype
We previously showed that both RA and OA FLS exposed to NETs become pro-inflammatory and synthesize significantly increased levels of IL-6 (14). We then assessed the putative mechanisms involved in NET-induced FLS activation. OA and RA FLS and control dermal fibroblasts were incubated in the presence or absence of spontaneously formed peripheral blood RA NETs for 2 hours. By confocal microscopy, we demonstrated that NETs are internalized by both OA and RA FLS, while skin fibroblasts showed minimal internalization (Fig. 2A–B). To determine whether the activation status of the cell has an influence in NETs’ internalization, skin fibroblasts harvested from psoriasis patients were also incubated with NETs from peripheral blood RA neutrophils. Psoriatic skin fibroblasts were also capable of internalizing NETs, suggesting that the activation status of the fibroblast has an impact in its ability to internalize NETs (Supplementary Fig. 2). Internalized NET components colocalized with the early endosome antigen-1 (EEA1) (24), implicating the endocytic pathway in NET internalization by FLS (Fig. 2A). To characterize the intracellular trafficking of NETs, FLS were pre-incubated with chloroquine (CQ), a lysosomotropic agent that prevents endosomal acidification, or with cytochalasin D (cyto D), an inhibitor of actin polymerization. FLS internalized NETs when exposed to cytoD, but not when cultured with CQ (Fig. 2C–D). By fluorescence microscopy visualization, we demonstrated significant decreases in the number of FLS positive for intracellular MPO, and Western blot analysis showed that MPO is not internalized by FLS treated with CQ (Fig. 2C & E). These observations indicate that NET internalization was impaired by antimalarials. Moreover, the levels of IL-6 synthesized by FLS significantly associate with the capacity to internalize NETs (Fig. 2F). These findings suggest that the internalization of NETs by FLS is independent of actin filaments and uses the endocytic pathway, most likely clathrin-coated vesicles. Furthermore, the release of proinflammatory cytokines by FLS triggered by NETs requires the internalization of these structures.
Figure 2. NETs are internalized by FLS into early endosomal antigen 1 (EEA1)-positive compartments.
RA-NETs were incubated with OA or RA FLS or skin fibroblasts for 2h. A. Internalized NETs colocalize with EEA1 compartments in FLS. Red represents MPO, green is EEA1 and blue is DNA. Results are representative of three independent experiments performed with confocal microscope, Scale bars, 10 μm. B. Western blot analysis of fibroblasts incubated in presence or absence of RA NETs shows that MPO bound to NETs is internalized by OA and RA FLS, but minimally by healthy control skin fibroblasts. Results are representative of three independent experiments C. Percentage of MPO positive cells (FLS that internalized NETs) decreased after chloroquine (CQ), but not cytochalasin D (cytoD) exposure. D. Representative confocal images after treatment with cytoD and CQ. Red represents MPO or F-actin, green is MPO and blue is DNA, Scale bars, 10 μm. E. Western blot analysis confirms that MPO internalization is impaired in FLS preincubated with CQ, but not in FLS pre-incubated with cytoD. F. IL-6 release by FLS is dependent on NET internalization. Results are the mean ± s.e.m. of n=4. For statistical analyses Mann-Whitney U test was used; ns= non-significant.
The RAGE/TLR9 signaling pathway mediates NET internalization by FLS
NETs contain DNA and nuclear and granule proteins (25). Given that unmethylated CpG sequences are recognized by TLR9, we tested whether this receptor was involved in the internalization of NETs by FLS. Western blot analysis demonstrated that OA and RA FLS pre-incubated with a TLR9 antagonist and then exposed to RA NETs are impaired in their ability to internalize MPO, a molecule present in NETs (Fig. 3A). In addition, IL-6 and IL-8 synthesis by FLS was significantly reduced in the presence of a TLR9 antagonist, with the effect being more predominant on IL-8 expression (Fig. 3B–C). Of note, the expression levels of TLR9 were significantly higher in OA and RA FLS than in control skin fibroblasts. Furthermore, TLR9 expression was significantly upregulated when FLS were incubated with NETs, suggesting a positive regulatory feedback loop (Fig. 3D). Given the role of TLR9 in the internalization of NETs and that this molecule is involved in type I interferon (IFN) synthesis in certain cell types, type I IFN genes were quantified in FLS after incubation with NETs for 24h. Indeed, IFN-α was significantly upregulated after NETs incubation (Fig. S3). Since TLR9 resides in endosomes, we hypothesized that a receptor present on the plasma membrane of FLS would mediate internalization of NETs. The receptor for advanced glycation end-products (RAGE) was previously reported to recognize HMGB1 (a molecule present in NETs)(26, 27), to promote DNA uptake into endosomes and to lower the immune recognition threshold for TLR9 activation (28). FLS were incubated with 2–4 μM RAGE peptide inhibitor for 30 min, followed by exposure to NETs for 1 hour. Co-immunoprecipitation analysis demonstrated that RAGE interacts with the active form of TLR9 (cleaved TLR9) to mediate NET internalization, and this interaction was abolished in the presence of a RAGE inhibitor (Fig. 3E & Fig. S4). We also detected a constitutive interaction of RAGE with the inactive form of TLR9, but this interaction was independent of NETs (Fig. 3E). We hypothesized that RAGE and the inactive form of TLR9 interact in the plasma membrane of FLS and that the functional interaction with the active form of TLR9 occurs intracellularly. Immunofluorescence of non-permeabilized cells demonstrated interaction of RAGE with TLR9 in the plasma membrane of FLS and this interaction was not perturbed by the presence of a RAGE inhibitor (Fig. 3F). Intracellularly, however, RAGE and TLR9 interactions were blocked with a RAGE inhibitor (Fig. 3F). These results suggest that NETs are internalized by FLS via a RAGE-TLR9 axis and that the proinflammatory profile induced in FLS is dependent on NET internalization.
Figure 3. RAGE-TLR9 axis mediates internalization of NETs by FLS.
OA and RA FLS were incubated in absence or presence of TLR9 inhibitors or control oligos and NETs for 72h. A. Western blot analysis shows intracellular MPO when FLS were incubated in presence or absence of TLR9 inhibitor. B. IL-6 and C. IL-8 quantification on FLS supernatants. D. qPCR analysis displays TLR9 mRNA expression in OA and RA FLS and skin fibroblasts in the presence or absence of NETs. Results for B–D are the mean ± s.e.m of four independent experiments. E. FLS were pre-treated with or without 2–4 μM RAGE inhibitor, and incubated with NETs for 1 hour. Co-immunoprecipitation was performed against RAGE and TLR9 was detected by Western blot. Anti-GFP was used as negative control. F. Plasma membrane (top panel) and intracellular (lower panel) detection of RAGE and TLR9 was performed on FLS pre-treated with or without RAGE inhibitor; red represents TLR9, green represents RAGE and blue is DNA of three independent experiments; white arrows highlight areas of colocalization of RAGE and TLR9. Scale bars, 5 μm. Mann-Whitney U test was used.
NET internalization induces MHCII upregulation in FLS in an IL-17B-dependent manner
FLS have the ability to acquire APC capabilities in the inflamed synovium (18), including MHCII upregulation. We tested the hypothesis that the uptake of NETs by FLS would lead to presentation of NET citrullinated peptides to the adaptive immune system in an MHCII-dependent manner. We observed that, upon NET internalization, MHCII was upregulated in FLS (Fig. 4A & Fig. S5) when compared to cells not exposed to NETs or isotype control. Flow cytometry analysis confirmed upregulation of MHCII intracellularly and in the plasma membrane of FLS incubated with NETs (Fig. 4B). This suggests that a molecule present in NETs may promote upregulation of FLS MHCII. IFN-γ is a known inducer of MHCII upregulation in APCs, including FLS(19). However, NETs showed no detectable levels of this cytokine by WB and ELISA (Fig. S6A–B). IFN-γ was also quantified by ELISA in supernatants of FLS incubated for up to 12 days with RA NETs and found to be undetectable (Fig. S6C). This ruled out the possibility that FLS were induced to produce IFN-γ in response to NETs. Members of the IL-17 family play important roles in inflammatory responses in RA(29). While most studies have focused on IL-17A, IL-17B synthesized by neutrophils was recently described as the most abundant IL-17 isoform in the RA synovium (30). Immunofluorescence and WB analyses demonstrated that RA NETs were decorated with significantly increased amounts of IL-17B but not IL-17A (Fig. 4C–D and Fig. S7). Neutralization of IL-17B in spontaneously generated RA-NETs induced significant decreases in expression of HLA-DRA and HLA-DRB MHCII molecules (Fig. 4E). Furthermore, incubation of RA-FLS with IL-17B or IL-17A led to upregulation of HLA-DRA and HLA-DRB mRNA (Fig. 4F). Mature MHCII compartments were detected in FLS incubated with recombinant IL-17B (Fig. 4F). Inhibition of IL17R utilizing a neutralizing antibody, did not impair NET internalization (Fig. S8), suggesting that RAGE-TLR9 is the main pathway of NET internalization and that internalization is independent of IL17R. Overall, these results indicate that IL-17B externalized in NETs can induce upregulation of MHCII in FLS.
Figure 4. IL-17B present in NETs upregulates MHCII in OA and RA FLS.
OA and RA FLS were incubated in presence or absence of spontaneously generated RA-NETs or 1000 U/mL IFN-γ A. Detection of MHCII in FLS by immunofluorescence; green represents MHCII and blue is DNA. Results are representative of three independent experiments, scale bars: 10μm. B. Plasma membrane and intracellular MHCI and MHCII were quantified by flow cytometry in RA FLS treated with NETs, IFN-γ or IFN-α for 5 days. C. IL-17B (red) is externalized in control (Ctrl) NETs generated with 1μg/mL of LPS and in spontaneously generated-RA NETs. D. IL-17B is detected in isolated NETs by Western Blot analysis. Each lane depicts independent NET isolation per group (Ctrl and RA). Mann-Whitney U test was used. Results are mean ± s.e.m. of 10 independent experiments,*p<0.05. E. OA FLS were incubated with RA-NETs in presence or absence of 1μg/mL IL-17B neutralizing Ab for 48h. Quantification of HLADR-A and DR-B mRNA was performed by real-time PCR. Bars mean ± s.e.m. of four independent experiments F. RA FLS were incubated with 100 ng of human recombinant IL-17A or IL-17B for 72h. qPCR and immunofluorescence analyses assessed MHCII mRNA expression and protein localization, respectively. ANOVA Bonferroni’s test was performed for B, E–F. Results are the mean ± s.e.m. of four independent experiments.
Arthritogenic NET peptides internalized by FLS colocalize with MHCII and are presented to antigen-specific CD4+T-cells
As FLS internalize molecules present in NETs and upregulate MHCII following this internalization, we tested whether these NET peptides were loaded onto the FLS MHCII compartment. After a 5-day incubation of FLS in the presence or absence of NETs, immunofluorescence confocal microscopy analysis demonstrated that MPO co-localizes with MHCII in both OA and RA FLS, suggesting that peptides derived from NETs are loaded onto MHCII compartments intracellularly (Fig. 5A). In addition, co-localization of NET-peptides and MHCII was detected in the plasma membrane of non-permeabilized FLS (Fig. 5B and Fig. S9), suggesting that the complex traffics to the plasma membrane to expose the peptide.
Figure 5. Arthritogenic peptides contained in NETs internalized by FLS are loaded into their MHCII compartment and presented to Ag-specific CD4+ T-cells.
A. Internalized NET-bound proteins colocalize with MHCII compartments in OA and RA FLS. B. Plasma membrane detection of MHCII and NET proteins in unpermeabilized FLS assessed by immunofluorescence. Red represents MPO or HC-gp39, green is MHCII and blue is DNA. C. Detection of mouse IL-2 after DRB1*04:01 RA FLS (with and without NETs) were incubated in the presence or absence of HC-gp39-specific CD4+ T-cell hybridomas for 5 days. Peptide is HC-gp39 263–275 (RSFTLASSETGVG). D Detection of various cytokines after DRB1*04:04 RA-FLS (with or without NETs) were incubated with haplotype matched cit-vimentin-specific CD4+ T-cells in the presence or absence of neutralizing antibodies against CD28 or MHCII. Mann-Whitney U test was used. Results are mean ± s.e.m. of four-six independent experiments. Scale bars, 10μm.
In addition to ACPAs, autoantibodies targeting native proteins have been described in RA. In particular, a subset of RA patients develops autoantibodies to human cartilage-glycoprotein 39 (HC-gp39) (31). To investigate whether non-citrullinated arthritogenic peptides are also contained within the NETs, WB analysis was performed and detected that HC-gp39 is synthesized by neutrophils and externalized in NETs (Fig. S6A). To determine the functionality of the peptide-MHCII complex with regards to the ability to activate antigen-specific T cells, we incubated FLS loaded with NETs with murine T cell hybridomas specific for immunodominant portions of HC gp-39 263–275 (RSFTLASSETGVG) and quantified IL-2 synthesis by the T cells. HC-gp39 T cell hybridomas synthesized significantly higher levels of IL-2 when incubated with FLS loaded with NETs than when exposed to FLS alone (Fig. 5C).
As NETs contain citrullinated vimentin, among other citrullinated peptides, and to corroborate that FLS can present citrullinated peptides to antigen-specific T cells, DRB1*04:04 RA FLS loaded with NETs were incubated with DRB1*04:04 cit-vimentin-specific CD4+T cells isolated and expanded from RA patients (Fig. S10). After 5 days of incubation, cit-vimentin-specific CD4+ T-cells displayed significant increases in secretion of IFN-γ, TNF-α, IL-10 and IL-1ra (Fig. 5D) when compared with T cells exposed to FLS alone. The release of these cytokines was reduced when cells were incubated with neutralizing antibodies against MHCII or against CD28 (Fig. 5D), indicating that costimulation is required. To test specificity of FLS toward citrullinated peptides, CD4+ T cells against citrullinated Aggrecan 225 were co-cultured with FLS. FLS were unable to activate Aggrecan 225 CD4+ T cells (Fig. S11), suggesting that specific citrullinated peptides are presented by FLS. Results indicate that FLS that internalize NET components can present arthritogenic peptides to Ag-specific T cells and activate adaptive immunity.
Humanized HLA-DRB1*04:01 transgenic mice develop ACPAs in response to immunization with FLS loaded with NETs
To confirm that NETs are an important source of citrullinated peptides and that FLS that internalize NETs can induce adaptive immune responses characteristic of RA in vivo, we used the humanized HLA-DRB1*04:01 transgenic mouse model(32). These mice are DRB1*0401.AEo; therefore they lack endogenous class II molecules (both I-A and I-E chains). In this model, transgenic expression of this DR1 allele confers susceptibility to inflammatory arthritis induced by immunization with various stimuli including citrullinated fibrinogen (33). Synovial tissue was harvested from healthy HLA-DRB1*04:01 mice to isolate and expand FLS in culture (Fig. 6A). Mouse FLS were incubated for 3 days in the presence or absence of NETs isolated from peripheral blood RA neutrophils. Internalization of NETs by mouse FLS was confirmed by intracellular immunofluorescence against human MPO (Fig. 6A). A total of 100,000 FLS with or without internalized NETs were injected in one knee of each HLA-DRB1*04:01 mouse (Fig. 6A). After seven rounds of injections performed every other week, serum ACPA levels were quantified using a commercial assay (anti-CCP, Wuhan Huamei Biotech Co). High titers of anti-CCP antibodies were detected in the sera of mice that received intra-articular injections of FLS loaded with NETs when compared to animals that received FLS alone (Fig. 6B). Dot-blot analysis demonstrated that sera from animals immunized with FLS loaded with NETs developed antibodies recognizing cit-histone H3, H4 and cit-MPO as well as anti-NET antibodies (Fig. 6C). In addition, serum samples from mice immunized with FLS loaded with NETs differentially recognized proteins contained in RA-NETs, when compared to serum from animals that were immunized with FLS alone (Fig. 6D). Splenocytes from mice injected with FLS loaded with NETs displayed a significant response to citrullinated peptides (a cocktail of cit-H3, cit-H4, cit-MPO and cit-vimentin) when compared to a cocktail of native peptides, as assessed by IL-2 synthesis (Fig. 6E). ACPA generation in these animals was dependent on CD4+ T cells, since CD4+-T cell-depleted animals (using an antibody approach) injected with FLS loaded with NETs displayed significantly decreased levels of ACPAs (Fig. 6F and Fig. S12).
Figure 6. DRB1*04:01 humanized mice that receive intraarticular injections of mouse FLS loaded with RA-NETs develop ACPAs and cartilage damage.
DRB1*04:01 FLS were isolated and incubated with human RA-NETs for 3 days prior to intra-articular injection. A. Internalization of NETs by FLS was assessed by immunofluorescence using an antibody against MPO (red). B. Serum ACPA levels at various time-points in animals immunized with FLS alone or FLS loaded with NETs (n=5/group). Results represent mean ± s.e.m. Mann-Whitney U test was used C. Sera from 3 mice immunized for 7 weeks with FLS or with FLS loaded with NETs was analyzed by dot-blot against citrullinated proteins. D. Western blot analysis to detect serum autoantibodies recognizing human NET proteins (arrows) in DRB1*04:01 animals that received FLS alone or FLS+NETs; each lane depicts two independent NET isolations. E. IL-2 synthesis by DRB1*04:01 mouse splenocytes incubated with a cocktail of native (pep; H3, H4, MPO and vimentin) or citrullinated peptides (cit pep; a cocktail of cit-H3, cit-H4, cit-MPO and cit-vimentin), when comparing animals immunized with FLS loaded with NETs (DR4 + NETs) with animals immunized with FLS alone (DR4). PHA is used as positive control. Results represent mean ± s.e.m of six independent experiments. Mann-Whitney U test was used. F. CD4+ T cell-depleted animals immunized with FLS loaded with NETs demonstrate significantly lower titers of ACPAs (n=4), as measured by ELISA, when compared with non-T cell depleted mice. Results represent mean ± s.e.m. Mann-Whitney U test was used. G. Percentage of FLS that migrate to the spleen and lymph nodes (LNs) after intraarticular injection of FLS loaded with NETs or untreated mice (n= 4–5). Results represent % mean ± s.e.m of FLS positive for CellTraceViolet (%CTV). Mann-Whitney U test was used. H. Epitope Chip analysis to quantify antibodies recognizing specific epitopes of RA-relevant autoantigens in animals immunized with FLS loaded with NETs when compared with animals immunized with FLS alone. Heat map represents the average of mean fluorescent units (MFI) of 5 animals/group. I. Sagittal sections of cartilage of the injected tibiofemoral compartment were stained with Safranin O (Saf-O) demonstrating impaired cartilage integrity (arrowhead) in animals immunized with FLS loaded with NETs when compared to animals immunized with FLS alone. J. Percentage of cartilage loss of the femurs and tibias of animals immunized with FLS or FLS loaded with NETs. Results represent mean ± s.e.m of 2–3 independent experiments. Mann-Whitney U test was used.
Given that we detected systemic ACPAs in animals immunized with FLS loaded with NETs, we hypothesized that FLS can migrate to the spleen and/or lymph nodes to interact with T-cells. Indeed, labeled FLS loaded with NETs were detected in spleen and lymph nodes of animals immunized with FLS when compared to naïve mice (Fig. 6G). These results indicate that induction of antigen-specific T cell responses and ACPA synthesis by FLS loaded with NETs likely occurs both intra-articularly and outside the joint.
An antigen array was used to identify the repertoire of autoantibodies generated after immunization. Animals immunized with FLS loaded with NETs displayed elevated levels of antibodies recognizing citrullinated histones cit-H2A, cit-H2B, and cit-H3 (Fig. 6H). Supporting our observation that NETs activate HCgp39 T cell hybridomas, mice immunized with FLS+NETs also displayed elevated levels of antibodies against HCgp39 (HCgp39-154-169, 258–279, 322–337 and 344–363 epitopes; Fig. 5c & Fig. 6H). Antibodies recognizing α-enolase (α-enolase 414–433), citrullinated fibrinogen and citrullinated vimentin (vim 58–77 cit3) were also present in animals immunized with FLS-NET, when compared to those immunized with FLS alone (Fig. 6H & Fig. S13). Other antibodies identified included those recognizing cartilage components, such as biglycan (epitope 238–257, 247–266, 247–266 cit3) suggesting that NETs may also modify FLS behavior potentially promoting cartilage damage. Indeed, while animals immunized with FLS alone or FLS loaded with NETs did not develop overt arthritis, safranin-O staining of murine synovial cartilage showed disruption in cartilage integrity, significantly increased cartilage loss and cartilage irregularity and higher prevalence of pannus in those animals immunized with FLS+NETs when compared to those immunized with FLS alone (Fig. 6I and 6J). Overall, these results suggest that, in the presence of the shared epitope, FLS that have internalized citrullinated peptides present in NETs can induce adaptive immunity in vivo, effectively activating cit-antigen-specific T cells which could in turn promote ACPA generation, as well as the promotion of synovial cartilage degradation.
Discussion
Increasing experimental evidence suggests that FLS in the RA synovium can act as immune sentinels. Their interaction with various leukocytes may promote intra-articular and peripheral inflammation and immune responses characteristic of this disease (34, 35). However, it has been unclear how presentation of citrullinated autoantigens, key targets of the immune system in RA, to the adaptive immune system occurs in the synovium and the role of FLS in this process. We now describe neutrophil-FLS interactions in the RA synovium may play crucial roles in the promotion of joint damage and in the development of systemic dysregulated innate and adaptive immunity against citrullinated intracellular autoantigens. We found that NETs containing citrullinated and arthritogenic peptides are internalized by FLS through a RAGE-TLR9 endocytic pathway, leading to a proinflammatory phenotype in these cells. NET internalization promotes upregulation of MHCII in the FLS, loading of NET peptides into the MHCII, trafficking to the FLS cell membrane and presentation to antigen-specific T cells. This promotes T cell activation and modulation of B cell responses leading to the generation of ACPAs, the propagation of inflammatory responses and cartilage damage.
A key histologic finding of the RA joint is a hyperplastic synovial lining and an invasive, inflammatory pannus across the surface of synovial joints. In addition to FLS, a distinct structural cell, the RA synovium contains macrophages, lymphocytes and neutrophils and appears to function as a tertiary lymphoid structure (36). FLS display many proinflammatory properties that contribute to RA pathogenesis, including their ability to function as APCs through upregulation of MHCII following exposure to inflammatory stimuli, and their expression of costimulatory molecules that can provide second signals leading to T cell activation (18). During active phases of disease and in early disease, large numbers of activated neutrophils are found in the synovial fluid of RA patients as well as at the cartilage pannus interface, where they may interact with FLS (37)(38). In addition, T cell-FLS interactions are readily observed in the inflamed synovium, promote T cell recruitment and Th1 and Th17 differentiation (39).
We previously showed that the RA synovium, characterized by ACPA and rheumatoid factor generation and increased proinflammatory cytokines, is highly conducive to NET formation. In turn, NETs may provide the immune system with access to enhanced sources of citrullinated proteins and thereby may represent an early event preceding epitope spreading. We now add additional evidence on the role of NETs as a source of immunogenic peptides by identifying several specific citrullinated antigens present in these structures that are recognized by ACPAs. In turn, ACPAs induce NET production potentially creating a vicious inflammatory cycle in the synovium and in the periphery. Indeed, this loop may promote disease by allowing an expansion of the citrulline specificities in RA. Our observations could help explain the broad array of citrullinated antigens seen by the antibody and T cell repertoire of RA patients. Whether citrullination of these proteins involved in NET formation alters not only immunogenicity but also their physiologic function remains to be determinedand was not explored in this manuscript.
NETs externalize substantial amounts of DNA bound to granular proteins and this could be a mechanism by which FLS internalize NET components. RAGE promotes uptake of alarmin:DNA complexes into endosomes and lowers immune recognition threshold for TLR9 activation in other cell types (28). Indeed, this pathway is operational in FLS and mediates NET internalization. This is also in accordance with recent observations that some NET proteins (e.g., LL37, HMGB1) promote APC activation by facilitating antigen uptake, interaction with endosomal TLRs and inflammatory cytokine release (40). Beyond nucleic acids, it is possible that other molecules present in NETs could bind to RAGE and this was not explored in this study.
In the case of the RA synovium, we propose that NET internalization augments cytokine synthesis by FLS and leads to their upregulation of MHCII, thereby enhancing APC capabilities. Specifically, we showed that IL-17B is externalized by NETs and promotes MHCII upregulation in the FLS and hat this phenomenon is IFN-γ-independent. Our results support and expand previous observations that IL-17B induces neutrophilia, is expressed by synovial neutrophils and is the predominant IL-17 cytokine in the RA synovium(41, 42), while FLS express the IL-17RB receptor(30). Overall, our observations suggest that proteins present in NETs can significantly alter the FLS phenotype and endow these cells with APC capabilities.
Citrullinated peptides are preferentially recognized by the HLA-DRB1*04:01/04 alleles, leading to their presentation to autoreactive T cells which then have the ability to promote ACPA generation, a feature of severe erosive RA. (10, 43, 44). In this context, we found that HLA-DRB1*04:01/04-positive RA-FLS that internalized NETs efficiently stimulated haplotype-matched Ag-specific T cells in vitro, while HLA-DRB1*04:01 transgenic mice developed ACPAs, enhanced T cell responses and cartilage damage when immunized with syngeneic FLS loaded with RA-NETs. These findings support a crucial interplay between genetic susceptibility factors and environmental stimuli (for example microbes known to promote NETosis) in promoting and amplifying local and systemic inflammatory responses. While the administration of NET-loaded FLS to these mice did not lead to overt arthritis, they displayed disruptions in cartilage integrity that suggest a pathogenic local effect in the joint. It is likely that additional inflammatory triggers may be needed to promote full blown disease, and this is indeed supported not only by other animal models of arthritis(44) but also by the observation that ACPA development and immune dysregulation precede overt RA by many years(4).
B cells isolated from RA synovial tissue produce antibodies to NET targets and selectively recognize NETs synthesized by RA neutrophils(45). It is then conceivable that the activation of antigen-specific T cells by FLS loaded with NETs promotes ACPA production by B cells present in the synovium and in the periphery and this is supported by our observation that in vivo T cell depletion abrogates ACPA production induced by NET-loaded FLS transfer. Our results support the concept that Th cells with TCRs specific for processed NET peptides (including citrullinated and non-citrullinated peptides derived from pro-arthritogenic proteins) are present in the RA synovium and respond to local APCs, such as FLS, loaded with citrullinated NET antigens. Our findings are also consistent with previous evidence that NET products can be taken up by professional APCs. Indeed, myeloid DCs that internalize NET components induce autoimmunity when injected into naive mice(46).
Neutrophils may play additional roles in the resolution of inflammation in RA(47). While FLS that internalized NETs activate d T cells and induced proinflammatory cytokine synthesis, they also induced higher synthesis of the anti-inflammatory IL-1 receptor antagonist and it will be important to assess if these interactions also mediate resolution of inflammatory responses in RA. Furthermore, it is important to emphasize that other mechanisms inducing citrullination likely play important roles in subsets of RA patients(48).
Our observations highlight a novel mechanism that promotes immune dysregulation and pathogenic autoimmunity in RA and further supports the rationale for testing NETosis inhibitors and strategies that disrupt specific cell-cell interactions in the synovial joint in future clinical trials in RA and, potentially, other chronic inflammatory conditions. Of note, antimalarials have been widely used in RA for many years, although their exact mechanism of action to explain efficacy remain to be determined. As the internalization of NETs is decreased in the presence of antimalarials, our observations may provide an additional mechanism of action for this group of drugs and suggest that further exploring strategies that limit the interactions of NETs with FLS and other target cells may have potential therapeutic benefit.
Materials and Methods
See supplementary material for additional materials and methods.
Study design
The study investigated the interactions between RA FLS and NETs and their role in driving adaptive immunity in this disease. Observations using human cells in vitro were also corroborated in animal models that recapitulate the genetic predisposition found in human. Mice sample size and number of in vitro experiments using murine and human samples was chosen based on previous publications and no randomization was performed to select groups (14, 26). Number of samples used per experiment is explained in each figure legend.
Patient recruitment, cell isolation and culture and generation of T cell hybridomas and antigen-specific T cells
Patient selection
Patients recruited fulfilled the 1987 ACR criteria for RA or were diagnosed with OA based on clinical and radiographic features, and confirmed by pathological findings at joint surgery (49). Healthy controls were recruited by advertisement. All individuals gave written informed consent and enrolled in a protocol approved by the NIAMS/NIDDK Institutional Review Board (IRB 01-AR-0227), the University of Michigan IRB (IRB HUM00043667 or HUM00045058), Benaroya Research Institute IRB (IRB07109-139) or Karolinska Institute IRB (2003-138, 2010/935-31/3 and 2011/583-32). Peripheral blood was obtained by venipuncture and collected in EDTA-containing tubes, and fractionated via Ficoll-Paque Plus (GE Healthcare) gradient. Neutrophils were isolated by dextran sedimentation and hypotonic salt solution as previously described (14).
Isolation of FLS and dermal fibroblasts
Human OA and RA FLS were obtained as previously described (18). In brief, FLS were obtained by collagenase (Worthington Biochemical) digestion of human synovial tissue obtained at arthroplasty or synovectomy from RA or OA joints. Cells were maintained in CMRL medium (Invitrogen Life Technologies) and used after passage 4 from primary cultures. Healthy control h uman dermal fibroblasts (gift from Elena Romm, NIH) and psoriasis human dermal fibroblasts (gift from JT Elder, University of Michigan) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (Invitrogen), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown in a humidified incubator with 5% CO2at 37°C.
Generation of HC-gp39 T-cell hybridomas
The generation and characterization of murine MHCII-restricted T cell hybridomas specific for a 13-aminoacid peptide of the arthritogenic HC-gp39 was previously described (50). These cells were cultured in RPMI 1640 medium (Invitrogen) with 0.6 mM sodium pyruvate, 1mM HEPES, and 0.055 mM β-mercaptoethanol. All cell cultures were supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown in a humidified incubator with 5% CO2at 37°C.
Cloning of citrulline specific T cells
T cell clones specific for citrullinated-vimentin restricted by HLA DRB1*04:04 were generated by staining PBMC samples directly ex vivo as described (51). Briefly, tetramer-positive CD4+ cells were sorted on a FACS Aria II at single-cell purity. Clones were expanded in 96-well plates in the presence of 1.0 × 105 irradiated PBMC and 2 μg/ml PHA (Remel Inc.) and screened by re-staining with tetramers. Antigen-specific T cell proliferation was assessed by stimulating 1.0 × 104 T cells in wells with 1×105 irradiated allogenic PBMC in the presence of 10 μg/ml peptide. After 72 hours cells were pulsed with H3-thymidine for an additional 24 hours and thymidine uptake measured by liquid scintillation counting. Fig. S3 displays the specificity of the response to citrullinated versus native forms of vimentin, validation of immunogenicity and the proliferative response of the clone. Aggrecan-specific T cell clones were used as negative control as aggrecan is not expressed in NETs.
Quantification of NET formation by plate assay
NET quantification was performed as described (14). Sytox green is a non-permeable DNA dye that will bind to DNA present in NETs generated during neutrophil culture. Briefly, neutrophils were resuspended in RPMI without phenol red containing 0.2 μM Sytox green (Invitrogen) for 2–3 h. Neutrophils (2 × 105) were incubated in presence or absence of Ctrl-IgG or ACPAs in 96-well black plate for 1h at 37°C. Fluorescence was measured in a Biotek Synergy H1 Hybrid Reader (Biotek). Results were reported as DNA relative fluorescence units (RFU).
Isolation and purification of ACPAs
Plasma samples were obtained from RA patients (n = 38) with high anti-CCP2 antibody levels (more than 3 times cut-off levels). IgGs were purified from plasma samples on Protein G columns followed by anti-CCP2 IgG affinity purification on CCP2 columns as described(52). IgGs from OA and from ACPA-seropositive RA synovial fluids were purified using the Melon Gel IgG purification kit (ThermoFisher) according to manufacturer’s protocol.
NET isolation
NETs were isolated as described (14). Briefly, RA neutrophils were purified and seeded in 24-well tissue culture plates in RPMI without phenol or stimuli and incubated for 2 hours at 37°C. Supernatants were harvested and NETs were digested with 10U/mL of micrococcal nuclease (Thermofisher) for 15 min at 37°C. Supernatants were collected and centrifuged at 300-x g for 5 min at 4°C. NET supernatants were transferred to a fresh tube and stored at −80°C until used.
Detection of citrullinated proteins using Rhodamine-Phenylglyoxal probe
Isolated NETs from control neutrophils stimulated with rheumatoid factor (14, 53) were analyzed using the rhodamine-phenylglyoxal (Rh-PG) probe, as described(54). Briefly, a 30 μL aliquot of each sample was prepared in 50 mM HEPES and treated with 20% trichloroacetic acid and 0.1 mM Rh-PG for 30 min at 37°C. Samples were quenched with citrulline (Sigma), cooled on ice for 30 min, and centrifuged at 14,000 rpm for 15 min at 4°C. The supernatant was removed and samples were washed with cold acetone and dried at 100°C for 5 min. After resuspending in 50 mM HEPES, samples were separated by SDS-PAGE (12%; 170 V; 50 min) and imaged on a Typhoon Imager (Ex. 532 nm; Em. 580 nm).
Assessment of FLS-T cell interactions
FLS were cultured in the presence or absence of spontaneously generated RA NETs for 3 days, washed twice with PBS to remove non-internalized NETs, then plated at a density of 100,000 cells/well in T-cell hybridoma medium or human T-cell medium. HC-gp-39 specific T cell hybridomas or human cit-vimentin specific T cells were added to FLS cultures at a ratio of 1:2 (FLS: T-cell) and incubated in presence or absence of 20 μg/mL of neutralizing antibodies against CD28 or MHCII for 5 days. Supernatants were then collected and centrifuged at 300 xg for 5 min at RT. Mouse IL-2 was quantified using ELISA Ready-SET-Go (eBioscience) for the hybridoma experiments and Bio-Plex pro-human cytokines assay (Bio-Rad) was performed for the human cit-vimentin T cell experiments, according to manufacturers’ recommendations. Aggrecan-specific T cell clones were used as negative control.
Generation of mouse FLS and in vivo administration of FLS with and without NETs
Breeding pairs of DR4-Tg mice were a kind gift from Dr. Chella David (Mayo Clinic) and were housed and bred at the NIH animal facility. These mice are DRB1*0401.AEo and lack endogenous class II molecules (both I-A and I-E chains)(33). All animal studies were performed according to the guidelines established by NIAMS LACU and following approved protocol A013-08-05. Eight-week old DRB1*04:01 female mice were euthanized and synovium from the tibiofemoral compartment was isolated and dissected. A piece of synovial tissue was incubated in 12-well plate with CMRL 1066 medium supplemented with 10% FBS, 1% L-Glutamine and 1% Pen-Strep. DRB1*04:01 FLS were used at passage four to ensure purity of the cells. FLS were cultured in the presence or absence of 50 μg of human RA NETs, isolated as above, for three days prior to injection. FLS were washed with PBS, detached with Trypsin, centrifuged 300 x g for 5 min at RT, washed and resuspended in 1 x HBSS. Uptake of NETs by FLS was confirmed by immunofluorescence microscopy. Twelve-week old DRB1*04:01 female mice were anesthetized using isoflurane vaporization and the left hindleg was shaved to expose the knee joint. A total of 1 × 105 DRB1*04:01 FLS with or without uploaded NETs were injected into the synovial space using a 27-gauge needle. This procedure was performed every 14 days for a total of 14 weeks (7 injections) and mice euthanized at 24 weeks of age.
Epitope mapping by Antigen Array
Antigens were diluted to 0.2 mg/ml in PBS or water and robotically spotted onto SuperEpoxy 2 Microarray Substrate Slides (ArrayIt) as described (56). A total of 330 RA-associated autoantigens were used, of which 52 are citrullinated, 263 are native, and 9 are control. Arrays were circumscribed using a hydrophobic Aqua-Hold Pap Pen 2 (Fisher Scientific). Chip was blocked overnight with PBS containing 3% FCS and 0.05% Tween 20. Arrays were probed with 1:300 diluted mouse sera, washed and incubated 1:2,000 dilution of Cy3-conjugated goat anti-mouse IgG+IgM secondary antibody (Jackson ImmunoResearch). Arrays were scanned using the GenePix 4000 scanner at wavelength 532 nm, and the median pixel intensities of the features and background values were determined using GenePix Pro version 3.0 software (Molecular Devices). Results were expressed as median fluorescence units, representing the median values from 4–8 identical replicates of an antigen on each array following subtraction of the median values of both intra-slide negative control (BSA) and inter-slide negative control (blank well features). Investigator that performed the array experiments was blinded to the experimental conditions used foreach sample.
Statistical analysis
Sample size for experiments using human samples was determined using similar patient numbers per experimental condition as in our previous publications assessing inhibition of NET responses12. No samples, mice or data points were excluded from the reported analysis once obtained. Data was analyzed using GraphPad Prism software. For samples with non-Gaussian distribution we employed Mann-Whitney U test. For multiple comparisons, we used ANOVA Bonferroni’s test. Results are presented as the mean ±s.e.m.
Supplementary Material
Fig S1. ACPAs recognize NET peptides.
Fig S2. Skin fibroblasts from subjects with psoriasis (Pso) internalize NETs.
Fig S3. NETs upregulate type I IFN inducible genes in FLS.
Fig S4. Endogenous expression of RAGE and TLR9 in FLS.
Fig S5. Upregulation of MHCII in OA-FLS incubated with NETs.
Fig S6. IFN-γ is not detected in NETs or FLS supernatants.
Fig S7. Detection of IL-17A and 17B in neutrophil lysate and NETs.
Fig S8. NETs are internalized by a TLR9-dependent and IL17R-independent mechanism.
Fig S9. FLS do not express HC-gp-39 or MPO.
Fig S10. Characterization of Vimentin (Vim) epitope binding to HLA DRB1*04:04 and T cell clone specificity.
Fig S11. Cytokine profile of co-cultures of aggrecan 225-CD4+ T cells and haplotype-matched FLS.
Fig S12. GK1.5 antibody deplete specifically CD4 T cells but not CD8 T cells.
Fig S13. DRB1*04:01 transgenic mice immunized with FLS loaded with NETs develop in vivo antibody responses to citrullinated peptides
Table S1. Mass spectrometry analysis demonstrates multiple citrullinated peptides in NETs induced with IgM rheumatoid factor.
Acknowledgments
We thank the Office of Science and Technology (NIAMS/NIH), Dr. Ming Zhou (Frederick National Laboratory for Cancer Research), Dr. Wanxia Tsai (NIAMS), Mr.Cliff Rims (Benaroya Research Institute) and Mr. Phillip Campbell (University of Michigan) for excellent technical assistance. We also thank Dr. JT Elder (University of Michigan) and Dr. Elena Romm (NIH) for providing skin fibroblasts.
Funding: Supported by the Intramural Research Program at NIAMS/NIH (ZIAAR041199). Also supported by NIH through PHS grants GM079357 (PRT) and GM110394 (PRT), 5 U01 AI101981-04 (JHB is sub investigator), W81XWH-15-1-003 (JHB and EJ), R01AR06367603 (to WHR), 5UM1AI 10055703 (DAF), and by the Rheumatology Research Foundation (MJK).
Footnotes
Author contributions: CCR, PMC, EM, NL, HV, EJ, YL, KLB, HW, AIC performed the experiments; CCR, NL, HU, WHR, VH, PRT, JHB, and MJK analyzed the data and performed statistical analyses; CCR, HU, EJ, WHR, JHB, DAF and MJK, provided scientific input, were involved in overall design and/or manuscript preparation.
Competing interests: PRT has a patent on Rh-PG probe and is a consultant to Padlock Therapeutics, a wholly owned subsidiary of Bristol Myers Squibb
Data and materials availability: deposited as additional material
References
- 1.Holers VM. Autoimmunity to citrullinated proteins and the initiation of rheumatoid arthritis. Current opinion in immunology. 2013 Dec;25:728. doi: 10.1016/j.coi.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, Stenlund H, Ronnelid J, Klareskog L, Rantapaa-Dahlqvist S. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis and rheumatism. 2013 Apr;65:899. doi: 10.1002/art.37835. [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature reviews. Rheumatology. 2012 Oct;8:573. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 4.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis and rheumatism. 2004 Feb;50:380. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 5.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, Engstrom M, Grunewald J, Nyren S, Eklund A, Klareskog L, Skold CM, Catrina AI. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis & rheumatology. 2014 Jan;66:31. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 6.Blass S, Engel JM, Burmester GR. The immunologic homunculus in rheumatoid arthritis. Arthritis and rheumatism. 1999 Dec;42:2499. doi: 10.1002/1529-0131(199912)42:12<2499::AID-ANR1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis and rheumatism. 1987 Nov;30:1205. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. Journal of immunology. 2003 Jul 15;171:538. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 9.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, Wijeyewickrema LC, Eckle SB, van Heemst J, Pike RN, McCluskey J, Toes RE, La Gruta NL, Purcell AW, Reid HH, Thomas R, Rossjohn J. A molecular basis for the association of the HLA-DRB1 locus, citrullination and rheumatoid arthritis. The Journal of experimental medicine. 2013 Nov 18;210:2569. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidd BA, Ho PP, Sharpe O, Zhao X, Tomooka BH, Kanter JL, Steinman L, Robinson WH. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis research & therapy. 2008;10:R119. doi: 10.1186/ar2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent C, de Keyser F, Masson-Bessiere C, Sebbag M, Veys EM, Serre G. Anti-perinuclear factor compared with the so called “antikeratin” antibodies, antibodies to human epidermis filaggrin in the diagnosis of arthritides. Annals of the rheumatic diseases. 1999 Jan;58:42. doi: 10.1136/ard.58.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, Lebeer K, Wyns B, Vincent C, Mielants H, Boullart L, Serre G, Veys EM, De Keyser F. Rheumatoid factor, anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate and extra-articular manifestations. Annals of the rheumatic diseases. 2004 Dec;63:1587. doi: 10.1136/ard.2003.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheumatic diseases clinics of North America. 1995 Aug;21:691. [PubMed] [Google Scholar]
- 14.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Science translational medicine. 2013 Mar 27;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis research & therapy. 2014;16:R122. doi: 10.1186/ar4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radstake TR, Franke B, Hanssen S, Netea MG, Welsing P, Barrera P, Joosten LA, van Riel PL, van den Berg WB. The Toll-like receptor 4 Asp299Gly functional variant is associated with decreased rheumatoid arthritis disease susceptibility but does not influence disease severity and/or outcome. Arthritis and rheumatism. 2004 Mar;50:999. doi: 10.1002/art.20114. [DOI] [PubMed] [Google Scholar]
- 17.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis and rheumatism. 2005 Sep;52:2656. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 18.Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C, Somers EC, Chung KC, Urquhart AG, Lundy SK, Kovats S, Fox DA. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis and rheumatism. 2007 May;56:1497. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- 19.Boots AM, Wimmers-Bertens AJ, Rijnders AW. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology. 1994 Jun;82:268. [PMC free article] [PubMed] [Google Scholar]
- 20.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, Kirou KA, Hellmich B, Knuckley B, Thompson PR, Crow MK, Mikuls TR, Csernok E, Radic M. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis and rheumatism. 2012 Apr;64:982. doi: 10.1002/art.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F, Boscaro F, Panza F, Puxeddu I, Rovero P, Migliorini P. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Annals of the rheumatic diseases. 2014 Jul;73:1414. doi: 10.1136/annrheumdis-2012-202765. [DOI] [PubMed] [Google Scholar]
- 22.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. The Journal of cell biology. 2010 Nov 1;191:677. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, Sonderstrup G, Monach P, Drijfhout JW, van Venrooij WJ, Utz PJ, Genovese MC, Robinson WH. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis and rheumatism. 2005 Sep;52:2645. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 24.Mishra A, Eathiraj S, Corvera S, Lambright DG. Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of Early Endosomal Autoantigen 1 (EEA1) Proceedings of the National Academy of Sciences of the United States of America. 2010 Jun 15;107:10866. doi: 10.1073/pnas.1000843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004 Mar 5;303:1532. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 26.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PloS one. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature immunology. 2007 May;8:487. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 28.Sirois CM, Jin T, Miller AL, Bertheloot D, Nakamura H, Horvath GL, Mian A, Jiang J, Schrum J, Bossaller L, Pelka K, Garbi N, Brewah Y, Tian J, Chang C, Chowdhury PS, Sims GP, Kolbeck R, Coyle AJ, Humbles AA, Xiao TS, Latz E. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. The Journal of experimental medicine. 2013 Oct 21;210:2447. doi: 10.1084/jem.20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011 Sep;134:8. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouri VP, Olkkonen J, Ainola M, Li TF, Bjorkman L, Konttinen YT, Mandelin J. Neutrophils produce interleukin-17B in rheumatoid synovial tissue. Rheumatology. 2014 Jan;53:39. doi: 10.1093/rheumatology/ket309. [DOI] [PubMed] [Google Scholar]
- 31.Sekine T, Masuko-Hongo K, Matsui T, Asahara H, Takigawa M, Nishioka K, Kato T. Recognition of YKL-39, a human cartilage related protein as a target antigen in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2001 Jan;60:49. doi: 10.1136/ard.60.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis and rheumatism. 2007 Jan;56:69. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 33.Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra H, David CS. Delineating the role of the HLA-DR4 “shared epitope” in susceptibility versus resistance to develop arthritis. Journal of immunology. 2008 Aug 15;181:2869. doi: 10.4049/jimmunol.181.4.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner JD, Filer A. The role of the synovial fibroblast in rheumatoid arthritis pathogenesis. Current opinion in rheumatology. 2015 Mar;27:175. doi: 10.1097/BOR.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 35.Fox DA, Gizinski A, Morgan R, Lundy SK. Cell-cell interactions in rheumatoid arthritis synovium. Rheumatic diseases clinics of North America. 2010 May;36:311. doi: 10.1016/j.rdc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noort AR, van Zoest KP, van Baarsen LG, Maracle CX, Helder B, Papazian N, Romera-Hernandez M, Tak PP, Cupedo T, Tas SW. Tertiary Lymphoid Structures in Rheumatoid Arthritis: NF-kappaB-Inducing Kinase-Positive Endothelial Cells as Central Players. The American journal of pathology. 2015 Jul;185:1935. doi: 10.1016/j.ajpath.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Raza K, Scheel-Toellner D, Lee CY, Pilling D, Curnow SJ, Falciani F, Trevino V, Kumar K, Assi LK, Lord JM, Gordon C, Buckley CD, Salmon M. Synovial fluid leukocyte apoptosis is inhibited in patients with very early rheumatoid arthritis. Arthritis research & therapy. 2006;8:R120. doi: 10.1186/ar2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bromley M, Woolley DE. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis and rheumatism. 1984 Aug;27:857. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- 39.Hu F, Li Y, Zheng L, Shi L, Liu H, Zhang X, Zhu H, Tang S, Zhu L, Xu L, Yang Y, Li Z. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and th17 cell responses in rheumatoid arthritis. PloS one. 2014;9:e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Science translational medicine. 2011 Mar 9;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. Journal of immunology. 2007 Nov 15;179:7128. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 42.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. Journal of immunology. 1998 Dec 1;161:6383. [PubMed] [Google Scholar]
- 43.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, Zendman AJ, Harris HE. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis research & therapy. 2005;7:R458. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, Shiao R, Fert-Bober J, Cheng D, Lahey LJ, Wong HH, Van Eyk J, Robinson WH, Sokolove J. Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis & rheumatology. 2015 Nov;67:2877. doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, Pitzalis C. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Annals of the rheumatic diseases. 2015 Dec 9; doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M, Guiducci C, Vitali C, Colombo MP. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012 Oct 11;120:3007. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 47.Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell’Accio F, Pitzalis C, Oliani SM, Jan LY, Perretti M. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Science translational medicine. 2015 Nov 25;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, Moutsopoulos NM, Andrade F. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Science translational medicine. 2016 Dec 14;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis and rheumatism. 2011 Jan;63:37. doi: 10.1002/art.30100. [DOI] [PubMed] [Google Scholar]
- 50.Tsark EC, Wang W, Teng YC, Arkfeld D, Dodge GR, Kovats S. Differential MHC class II-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. Journal of immunology. 2002 Dec 1;169:6625. doi: 10.4049/jimmunol.169.11.6625. [DOI] [PubMed] [Google Scholar]
- 51.James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, Peda M, Sandin C, Klareskog L, Malmstrom V, Buckner JH. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis & rheumatology. 2014 Jul;66:1712. doi: 10.1002/art.38637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ossipova E, Cerqueira CF, Reed E, Kharlamova N, Israelsson L, Holmdahl R, Nandakumar KS, Engstrom M, Harre U, Schett G, Catrina AI, Malmstrom V, Sommarin Y, Klareskog L, Jakobsson PJ, Lundberg K. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis research & therapy. 2014;16:R167. doi: 10.1186/ar4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen PP, Goni F, Houghten RA, Fong S, Goldfien R, Vaughan JH, Frangione B, Carson DA. Characterization of human rheumatoid factors with seven antiidiotypes induced by synthetic hypervariable region peptides. The Journal of experimental medicine. 1985 Aug 1;162:487. doi: 10.1084/jem.162.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bicker KL, Subramanian V, Chumanevich AA, Hofseth LJ, Thompson PR. Seeing citrulline: development of a phenylglyoxal-based probe to visualize protein citrullination. Journal of the American Chemical Society. 2012 Oct 17;134:17015. doi: 10.1021/ja308871v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmona-Rivera C, Simeonov DR, Cardillo ND, Gahl WA, Cadilla CL. A divalent interaction between HPS1 and HPS4 is required for the formation of the biogenesis of lysosome-related organelle complex-3 (BLOC-3) Biochimica et biophysica acta. 2013 Mar;1833:468. doi: 10.1016/j.bbamcr.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995 Oct 20;270:467. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. ACPAs recognize NET peptides.
Fig S2. Skin fibroblasts from subjects with psoriasis (Pso) internalize NETs.
Fig S3. NETs upregulate type I IFN inducible genes in FLS.
Fig S4. Endogenous expression of RAGE and TLR9 in FLS.
Fig S5. Upregulation of MHCII in OA-FLS incubated with NETs.
Fig S6. IFN-γ is not detected in NETs or FLS supernatants.
Fig S7. Detection of IL-17A and 17B in neutrophil lysate and NETs.
Fig S8. NETs are internalized by a TLR9-dependent and IL17R-independent mechanism.
Fig S9. FLS do not express HC-gp-39 or MPO.
Fig S10. Characterization of Vimentin (Vim) epitope binding to HLA DRB1*04:04 and T cell clone specificity.
Fig S11. Cytokine profile of co-cultures of aggrecan 225-CD4+ T cells and haplotype-matched FLS.
Fig S12. GK1.5 antibody deplete specifically CD4 T cells but not CD8 T cells.
Fig S13. DRB1*04:01 transgenic mice immunized with FLS loaded with NETs develop in vivo antibody responses to citrullinated peptides
Table S1. Mass spectrometry analysis demonstrates multiple citrullinated peptides in NETs induced with IgM rheumatoid factor.