Abstract
This report describes an outbreak at a dog daycare facility where 13 of 52 dogs developed suspected canine papillomavirus (CPV) infection. Based on contact tracing, subclinical CPV shedding was speculated. Active surveillance, exclusion of animals with active or recent infection and cohort formation may have been effective in stopping the outbreak.
Résumé
Éclosion du papillomavirus oral canin dans une garderie canine. Ce rapport décrit l’éclosion dans une garderie canine où 13 des 52 chiens ont développé une infection suspectée par le virus du papillome canin (VPC). En se basant sur le retraçage des contacts, on a émis la supposition d’une excrétion subclinique du VPC. Une surveillance active, l’exclusion des animaux avec une infection active ou récente et la formation d’une cohorte ont pu être efficaces pour freiner l’éclosion.
(Traduit par Isabelle Vallières)
Hospital acquired infections (HAIs) in human healthcare settings are of great health and economic concern (1). Human HAIs have been associated with numerous factors, including weakened immune systems of patients, poor compliance of healthcare staff with procedures such as hand hygiene and high patient-patient interaction (2,3). While traditionally the focus on HAIs is transmission within hospital environments, it is increasingly clear that there can be a strong influence of the community, as patients who are admitted shedding a pathogen (clinically or asymptomatically) can be important sources of infection (4,5). Similar risk factors and transmission dynamics exist in veterinary hospitals and settings such as dog daycares and boarding facilities, in which there may be mixing of animals from different origins. Concentrated populations, admission of animals from diverse backgrounds, direct animal contact, and fomites, among other factors, can create an ideal environment for pathogen transmission (6). Little has been published on pathogen outbreaks and control measures in these animal group settings. However, anecdotally, outbreaks in facilities such as dog daycares are not uncommon and under-reporting is a considerable issue where investigation may be limited or there is reluctance to report problems.
Canine papillomavirus (CPV) is a double-stranded, non-enveloped, DNA virus. Papillomaviruses can be found in various mammalian species, but are highly host-specific with numerous types identified in dogs (7). Infection can be transmitted by direct contact with the papilloma(s) of an infected dog or contact with the virus in the environment. The virus requires microabrasions to access the basal layer of the skin to establish an infection (8). It is not known if dogs need to have visible lesions to be infectious. After an approximately 4-week incubation period (9,10), lesions of varying size and number may become apparent, although subclinical infections are believed to also occur. The most common presentation is the development of oral lesions (papillomatosis) in young dogs, but cutaneous papillomatosis is also possible (9,10).
In most cases, lesions are mild and result in little apparent discomfort or complications, and spontaneous regression typically occurs over 4 to 8 wk (10). However, severe clinical signs can be seen in some animals. In rare cases, lesions can be so severe that they create difficulty eating and drinking and can be a cause of respiratory obstruction (8).
A definitive diagnosis of CPV can only be obtained through histopathology, polymerase chain reaction (PCR), immunohistochemistry, in situ hybridization, or electron microscopy of biopsy samples. Additional diagnostics are needed to determine the CPV type. Given the generally limited severity or long-term health consequences of CPV infection, relatively short duration of clinical signs, and typically self-limiting nature of the disease, confirmatory testing is not often pursued. Several approaches have been suggested for treatment (e.g., surgical excision, vaccination, antimicrobials); however, data on efficacy are lacking due to limited study, the transient nature of lesions and concerns about the use of antimicrobials when not indicated (7,8,11–14).
Although CPV has been described since the 19th century, anecdotally frequently observed in dogs in group settings and is highly transmissible, little published information is available on disease occurrence (9). In 1 CPV outbreak in a dog breeding facility 10% (40/400) of dogs, all approximately 3.5 mo of age, were affected (15). Furthermore, limited information is available on best practices that can be implemented to prevent or control CPV transmission and resulting outbreaks.
At the University of Guelph, Ontario Veterinary College, a dog daycare facility was established as a branch of the community practice program. Daycare dogs were kept separated from community practice patients, although there was a shared entrance and lobby. Procedural separation from veterinary practice patients included separate dedicated items, runs, and common use areas. At the daycare, dogs were managed as a single group, allowed to directly interact with other attendees and animal care attendants were responsible for monitoring the dogs. Common use toys and water dishes were available. Routine environmental cleaning and disinfection practices, using accelerated hydrogen peroxide, occurred once daily.
On September 6, 2011 an animal care attendant noted oral lesions in a 9-month-old dog (D1) at the dog daycare facility. The dog was subsequently examined by a facility veterinarian and papillomavirus infection was presumptively diagnosed. The dog was immediately excluded from the daycare facility until free of oral lesions for 2 wk. On September 14, 2011 similar oral lesions were noted on another dog (D2) at the daycare facility and were also diagnosed by a facility veterinarian as suspected papillomavirus infection. During a conversation with the owner of D2, it was reported that these lesions had been present for approximately 3 to 4 wk. This dog was excluded from the facility as per the previous dog.
Following the 2 identified suspect cases of CPV and concern about likely transmission to daycare dogs from these cases, an active surveillance program was established. Animal care attendants performed daily oral and external evaluations on all dogs at time of admission, with hand hygiene carried out between oral examinations. If clinical signs of CPV infection were found (e.g., newly visible raised lesion in the oral cavity or elsewhere) the affected dog was excluded from the daycare and not permitted to return until 2 wk after cessation of clinical signs (as determined by a veterinarian). A suspect CPV case was defined as a dog with clinical signs compatible with CPV infection in the form of oral or cutaneous papillomatosis that was supported by examination by a veterinarian. All cases were considered suspect as additional diagnostics (e.g., biopsy) were not pursued by clients. Electronic daily dog attendance logs were kept for the daycare and subsequently reviewed to determine the population at risk and inform hypotheses for transmission within the facility. Contact tracing was conducted whereby dog attendance logs of all suspect CPV cases were reviewed to determine if they had contact with other affected dogs with an attempt to estimate an infectious period.
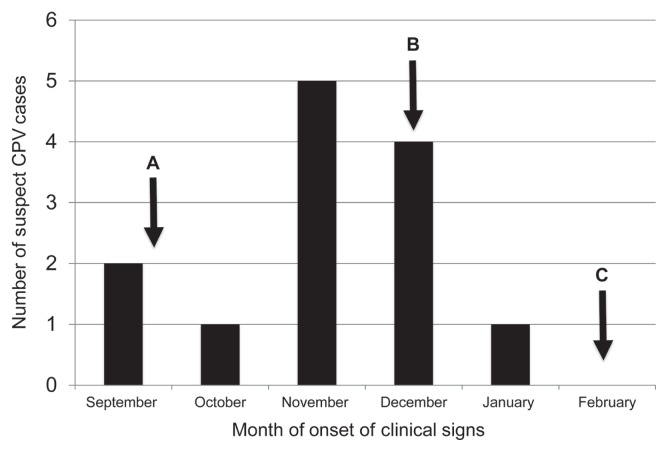
The outbreak period was defined from September 6, 2011 to March 30, 2012 (the date of identification of the first CPV case to an estimated 2 incubation periods after exclusion of the last case, respectively). In total, during the 7 mo, 52 dogs attended the daycare facility, ranging from 1 to 78 total visits (mean 15 visits). During the outbreak period, 13 (25%) of these dogs were diagnosed with suspect CPV infection: September (n = 2), October (n = 1), November (n = 5), December (n = 4), January (n = 1) (Figure 1). All suspect dogs had oral lesions; no cutaneous lesions were reported. The ages of 7 of the suspect dogs were known (range: 4 mo to 7 y; mean: 1.8 y; median: 11 mo). The incidence rate for the 7-month outbreak period was 1.5 suspect CPV cases per 100 dog-days at risk (calculated using the 11 incident cases identified after active surveillance was initiated and the dog-days at risk for this period determined from the attendance logs). Of the suspected dogs, 7 (54%) returned to the daycare facility after resolution of clinical signs (median: 40 d after exclusion; range: 29 to 85 d). Information was not available on additional details of cases, such as severity of illness, breed, comorbidity and specific duration of clinical signs.
Figure 1.
Epidemic curve of suspected CPV cases in dog daycare facility (2011–2012). A — Active surveillance initiated. B — First cohort system put in place on December 13, 2011. C — Existing cohorts consolidated February 21, 2012 and continued to April 1, 2012.
Due to the continued case identification despite active surveillance and immediate exclusion, effective December 13, 2011 a cohort system was implemented. This involved the creation of 10 cohorts (4 to 5 dogs per cohort) and each daycare attendee was assigned to 1 of these cohorts based on dog-dog compatibility and planned attendance. Dogs only interacted with other dogs in their cohort and measures were taken to reduce indirect contact between the different cohort members (e.g., water dishes and toys were changed between cohorts; common indoor and outdoor exercise areas were used by all cohorts). Due to logistics and decreasing daycare attendees, existing cohorts were consolidated into 4 cohorts (3 to 10 dogs per cohort) that remained from February 21, 2012 to March 30, 2012.
During the outbreak, clients were provided an information sheet about CPV, including how dogs become infected, clinical course and duration, general information about the outbreak at the facility, and steps that were being taken to protect participating dogs. Clients were asked to report observed signs in their dogs consistent with CPV infection. On March 30, 2012 the outbreak was considered over as no new cases had been identified within 2 incubation periods.
Despite the anecdotally frequent occurrence of oral CPV, there are minimal publications on CPV outbreaks, management, and prevention. This may be due to the transient nature and spontaneous regression of CPV clinical signs and minimum severity in most of the infected animals. However, despite the often minor severity of lesions, CPV is highly infectious, as documented in this outbreak and another study (15), can lead to severe disease in some dogs and therefore should be addressed by prevention and response actions in canine group settings and veterinary facilities.
The origin of CPV in this outbreak cannot be established. The index case (D1) could have become infected through contact with animals outside of the daycare (community acquired) or through contact with an infectious dog or contaminated environment in the daycare facility. Due to the relatively long incubation period of CPV, any of these sources is possible.
Contact tracing based on electronic records and cohort assignments allowed evaluation of CPV transmission properties although there was high dog commingling during the first several months of the outbreak, making this evaluation difficult. With 1 exception, all dogs that became infected had 1 or more CPV transmission opportunities within the daycare population [present on the same day and time and with likely contact as a dog incubating CPV infection, with this contact occurring approximately 1 incubation period (4 wk) before the onset of clinical signs]. The exception was D13 whose only contact with a previously infected dog in the facility occurred approximately 2 mo before developing clinical signs. This suggests that an incubation period of greater than 4 wk may be possible, although other sources of infection cannot be excluded. For all other cases, transmission opportunities fell within the 4-week incubation period.
Three cases could each be traced back to a single previously infected dog. If it is assumed these dog-dog interactions were responsible for CPV transmission, dogs would have been infectious from 3 to 14 d prior to case identification (presumably when clinical signs began). Subclinical shedding has not been described for CPV. Since indirect transmission, including environmental contamination and additional external sources of CPV cannot be excluded for these cases, this area deserves further investigation.
There is limited information on the epidemiology of CPV in group settings and utility of control measures to stop an existing CPV outbreak. In the outbreak reported here cohort formation, active surveillance, and exclusion of animals with lesions may have been beneficial in decreasing case numbers by decreasing direct and environmental exposure to the virus. Early case identification (through active surveillance or client reporting of clinical signs) can also be helpful in preventing secondary transmission and occurrence of an outbreak. Due to the nature of this outbreak and data available, the true effect of these measures on halting the outbreak cannot be determined. It is possible an agent other than CPV was responsible for the lesions as confirmatory diagnostics were not pursued by the clients; however, this seems unlikely as in the authors’ opinion no other agents are consistent with the observed lesions and outbreak.
As many veterinary clinics have dog daycare facilities as an added form of revenue, outbreaks within the hospital setting could occur. Although most cases of CPV are mild and self-resolving, it is highly infectious, potentially with subclinical shedding, and an outbreak in hospital patients could be severe. The incorporation of infection control practices aimed at CPV and similar pathogens is important for all clinics, especially those with multiple (potentially mixing) animal groups. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Reed D, Kemmerly SA. Infection control and prevention: A review of hospital-acquired infections and the economic implications. Ochsner J. 2009;9:27–31. [PMC free article] [PubMed] [Google Scholar]
- 2.Sydnor ERM, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24:141–173. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson JK. Preventing healthcare-associated infection: Risks, health-care systems and behaviour. Intern Med J. 2009;39:574–581. doi: 10.1111/j.1445-5994.2009.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 6.Stull JW, Weese JS. Hospital-associated infections in small animal practice. Vet Clin Small Anim. 2015;45:217–233. doi: 10.1016/j.cvsm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange CE, Favrot C. Canine papillomatosis. Vet Clin North Am Small Anim Pract. 2011;41:1183–1195. doi: 10.1016/j.cvsm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Sykes JE, Luff JA. Viral papillomatosis. In: Sykes J, editor. Canine and Feline Infectious Diseases. St. Louis, Missouri: Elsevier; 2013. pp. 261–268. [Google Scholar]
- 9.M’Fadeyan J, Hobday F. Note on the experimental transmission of warts in the dog. J Comp Pathol Ther. 1898;11:341–344. [Google Scholar]
- 10.Chambers VC, Evans CA. Canine oral papillomatosis. I. Virus assay and observations on the various stages of the experimental infection. Cancer Res. 1959;19:1188–1195. [PubMed] [Google Scholar]
- 11.Lange CE, Tobler K, Schraner EM, et al. Complete canine papillomavirus life cycle in pigmented lesion. Vet Microbiol. 2013;162:388–395. doi: 10.1016/j.vetmic.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Bredal WP, Thoresen SI, Rimstad E, Aleksandersen M, Nafstad PH. Diagnosis and clinical course of canine oral papillomavirus infection. J Small Anim Pract. 1996;37:138–142. doi: 10.1111/j.1748-5827.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 13.Yağci BB, Ural K, Ocal N, Hayardedeoğlu AE. Azithromycin therapy of papillomatosis in dogs: A prospective, randomized, double-blinded, placebo-controlled clinical trial. Vet Dermatology. 2008;19:194–198. doi: 10.1111/j.1365-3164.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 14.Weese JS, Giguère S, Guardabassi L, et al. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med. 2015;29:487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yhee JY, Kwon BJ, Kim JH, et al. Characterization of canine oral papillomavirus by histopathology and genetic analysis in Korea. J Vet Sci. 2010;11:21–25. doi: 10.4142/jvs.2010.11.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]