Summary
-7/del(7q) occurs in half of myeloid malignancies with adverse-risk cytogenetic features and is associated with poor survival. We identified the spectrum of mutations that co-occur with ‒7/del(7q) in forty patients with de novo or therapy-related myeloid neoplasms. -7/del(7q) leukemias have a distinct mutational profile characterized by low frequencies of alterations in genes encoding transcription factors, cohesin, and DNA-methylation-related proteins. In contrast, RAS pathway activating mutations occur in 50% of cases, a significantly higher frequency than other AMLs and higher than previously reported. Our data provide guidance for which pathways may be most relevant in the treatment of adverse-risk myeloid leukemia.
Keywords: Acute myeloid leukemia, therapy-related myeloid neoplasm, monosomy 7, mutations, CUX1, RAS pathway
Cytogenetic abnormalities remain the strongest independent predictor for response to therapy and survival in myeloid malignancies. Adverse-risk cytogenetic abnormalities occur in 20–30% of de novo acute myeloid malignancies (AML) and 70% of therapy-related myeloid neoplasms (t-MN) (Leith et al, 1997; Smith et al, 2003; Grimwade et al, 2010). The median overall survival for patients with high-risk abnormalities is less than one year, a rate that has only minimally improved over the last three decades (Smith et al. 2003; Grimwade et al. 2010). The most common high-risk cytogenetic abnormality is -7/del(7q), identified in half of all t-MN patients and half of adverse-risk de novo AML (Leith et al. 1997; Smith et al. 2003; Grimwade et al. 2010). While recent studies have focused on the genomics of low- and intermediate-risk AML, the genetic basis for adverse-risk AML/t-MN remains poorly understood. We previously mapped the commonly deleted segment of chromosome band 7q22 using RNA-sequencing and SNP-array analysis (McNerney et al, 2013). We identified the gene encoding the CUX1 transcription-factor to be a highly conserved, haploinsufficient myeloid tumor suppressor located within 7q22 (McNerney et al. 2013). Herein, we identify the genome-wide spectrum of somatic mutations that co-occur with -7/del(7q) and CUX1 loss. We found that the mutation profile of -7/del(7q) leukemias is significantly different from other AMLs and reveals therapeutic opportunities for improving the outcome for patients with high-risk disease.
Materials and methods
Methods are provided in Supplemental Materials.
Results/Discussion
We identified the somatic mutations in thirteen leukemia samples with -7/del(7q) (University of Chicago, [UC] cohort). Three patients had de novo AML and ten had t-MN (Table S1). We included t-MN and de novo AML samples as they are indistinguishable morphologically and clinically (Schoch et al, 2004), suggesting common biological features. It remains unknown, however, if t-MN and de novo AML with-7/del(7q) also have similar somatic mutations. Four samples had complex karyotypes, and three of these also had del(5q) (Tables S1). Two samples had a recurrent genetic variation as defined by the 2008 WHO category “AML with recurrent genetic abnormalities” (Swerdlow et al, 2008), which was inv(3). Complex karyotype, del(5q), and inv(3) frequently co-occur with loss of 7q (Swerdlow et al. 2008). Paired tumor and normal exome-sequencing was performed on six cases; seven others underwent RNA-sequencing of the leukemia sample with exome-sequencing of normal tissue. Thus, all samples received paired normal exome sequencing for somatic mutation detection. The median coverage of coding exons for tumor exomes was 130X, 72X for normal exomes, and 30X for RNA-sequenced tumors (Table S1). The median percentage of coding bases with sufficient depth for SNP identification (≥ 8X coverage) was 92.1% for tumor exomes, 83.6% for normal exomes, and 37.6% for RNA-sequenced tumors. Copy number analysis was available for eight leukemia samples (McNerney et al. 2013).
We identified 40 mutations in the 6 exome-sequenced cases (Table 1). Twenty-one mutations were Sanger sequenced with a 100% validation rate (Table S2). Thirty-nine mutations were identified in the RNA-sequenced cases of which 30 were verified, and the validation rate was 93.8% (Table 1 and S2). One RNA-sequenced sample had fusion events identified by RNA-sequencing (McNerney et al. 2013) (Table S1). The average number of single nucleotide mutations and indels per sample was six (0.16 mutations/Mb), which is lower than previous reports (Link et al, 2011; TCGA 2013). This is possibly due to conservative mutation calling parameters and lower coverage in the current study, particularly for the RNA-sequenced samples. The median number of mutations for the RNA-sequenced samples was 3, compared to 5.5 in the exomes. There was no difference in the mutation load for t-MN patients as compared to de novo AML; however, there are only three de novo AMLs in this cohort. The fraction of mutations that were transversions was 32.5% and was similar when restricting the analysis to the t-MN samples (36.1%), consistent with prior reports (Link et al. 2011; TCGA 2013).
Table 1.
University of Chicago cohort mutations from exome and RNA-sequencing.
| Sample | Gene | Amino acid change | Deleteriousness (GERP score) | Cancer Gene Census gene | TCGA AML gene mutation frequency | cBioPortal gene mutation frequency in other tumors |
|---|---|---|---|---|---|---|
| A24 | CCDC33 | V341M | 2.67 | 0% | 7.1% bladder, 6.9% small cell lung, others | |
| A24 | CSMD2 | c.8047C>G, synonymous | −0.0615 | 0% | 34.7% melanoma, 24.1% lung small cell, others | |
| A24 | IMPG1 | c.2091G>A, synonymous | −8.35 | 0% | 12.4% melanoma, 6.2% lung squamous, others | |
| A24 | NRAS | G12D | 5.23 | Yes | 8.0% | 30.8% melanoma, 18.0% multiple myeloma, others |
| A24 | ROCK2 | S823* | nonsense | 0% | 7.1% bladder, 5.6% endometrial, others | |
| A24 | SMCHD1 | I183M | −2.61 | 0% | 6.0% endometrial, 5.1% cervical, others | |
| A24 | SPEF2 | E1521V | 4.38 | 0% | 17.2% melanoma, 13.8% lung small cell, others | |
| A24 | TET2 | Q1553* | nonsense | Yes | 8.5% | 6.9% colorectal, 6.9% lung small cell, others |
| A24 | VNN2 | A253T | 4.47 | 0% | 5.7% melanoma, 4.0% endometrial, others | |
| A24 | ZRSR2 | G268D | 5.09 | Yes | 0% | 2.4% endometrial, 2.3% bladder, others |
| A36 | COX7C | R57G | 3.7 | 0% | 1.8% pancreatic, 1.1% lung adeno., others | |
| A36 | FAM116B | Q479R | 4.72 | 0% | 5.6% colorectal, 1.8% pancreatic, others | |
| A36 | HEATR5B | A1534V | 5.43 | 0% | 7.7% cervical, 7.1% bladder, others | |
| A36 | KCTD17 | H94R | 3.58 | 0% | 2.2% melanoma, 1.4% colorectal, others | |
| A36 | TLN1 | R854H | 5.56 | 0% | 11.1% colorectal, 6.6% melanoma, others | |
| A74 | NRAS | G12S | 5.23 | Yes | 8.0% | 30.8% melanoma, 18.0% multiple myeloma, others |
| T03 | ANKRD32 | G875R | 5.19 | 0% | 4.2% colorectal, 2.8% endometrial, others | |
| T03 | DNAH1 | M2871T | 4.61 | 0% | 16.7% colorectal, 12.4% melanoma, others | |
| T03 | ELAC2 | M750T | 4.65 | 0% | 4.2% colorectal, 3.6% melanoma, others | |
| T03 | ETV6 | K403N | 3.53 | Yes | 1.0% | 5.6% colorectal, 3.6% bladder, others |
| T03 | EWSR1 | c.1291C>T, synonymous | 5.59 | Yes | 0.5% | 4.1% melanoma, 3.6% endometrial, others |
| T03 | EZH2 | G159R | 5.73 | Yes | 1.5% | 4.8% endometrial, 4.1% head neck, others |
| T03 | FLT3 | D835Y | 5.53 | Yes | 27.0% | 10.0% melanoma, 4.8% lung adeno., others |
| T03 | FRY | R1110* | nonsense | 0% | 11.1% colorectal, 9.1% melanoma, others | |
| T03 | HDAC5 | V311M | 3.98 | 0% | 4.2% colorectal, 3.6% endometrial, others | |
| T03 | LILRA6 | L115M | −1.17 | 0% | 6.9% small cell lung, 3.6% bladder, others | |
| T03 | MATR3 | R307G | 2.49 | 0% | 3.3% melanoma, 2.8% endometrial, others | |
| T03 | N4BP2L2 | Q441R | 4.22 | 0% | 4.4% endometrial, 3.6% bladder, others | |
| T03 | NUP153 | S902Y | 5.71 | 0% | 7.1% bladder, 5.2% endometrial, others | |
| T03 | PDE1B | I371T | 4.81 | 0% | 6.6% melanoma, 4.8% small cell lung, others | |
| T03 | PROS1 | M192V | −5.88 | 0% | 10.3% small cell lung, 7.0% lung adeno., others | |
| T03 | PTPN11 | F71L | 5.28 | Yes | 4.5% | 4.2% colorectal, 3.4% small cell lung, others |
| T03 | RIOK1 | M10T | 5.82 | 0% | 7.0% pancreatic, 5.8% melanoma, others | |
| T03 | TNPO2 | F873V | 4.42 | 0% | 4.5% gastric, 3.6% endometrial, others | |
| T03 | ZNF192 | L365V | 4.5 | 0% | 3.4% lung small cell, 3.4% lung squamous, others | |
| T03 | ZNF318 | Q219* | nonsense | 0% | 9.7% colorectal, 6.6% melanoma, others | |
| T12 | CDK2AP1 | H23R | 5.16 | 0% | 1.4% colorectal, 0.8% melanoma, others | |
| T12 | FBXO18 | A495T | 4.23 | 0% | 5.6% colorectal, 3.3% melanoma, others | |
| T16 | NUP210 | L1504I | −8.3 | 0% | 11.6% melanoma, 5.6% colorectal, others | |
| T16 | PPM1D | S446* | stop | 0% | 4.4% endometrial, 4.2% colorectal, others | |
| T16 | RUNX1 | R210K | 4.62 | Yes | 9.0% | 3.4% breast, 3.2% endometrial, others |
| T18 | CBL | Y368_E369insAD | indel | Yes | 1.0% | 5.5% melanoma, 4.4% endometrial, others |
| T18 | INPP1 | G178V | 4.89 | 0% | 3.6% bladder, 2.6% cervical, others | |
| T18 | SCN5A | R367C | 4.14 | 0% | 24.7% melanoma, 10.3% cervical, others | |
| T20 | GSTM5 | N85S | 3.43 | 0% | 2.2% melanoma, 1.7% lung squamous, others | |
| T20 | HERC2 | G1886R | 4.44 | 0.5% | 20.7% small cell lung, 19.4% colorectal, others | |
| T20 | MPEG1 | F444V | 5.38 | 0% | 4.2% colorectal, 3.4% lung small cell, others | |
| T20 | NAP1L4 | K26N | −0.991 | 0% | 6.9% small cell lung, 4.4% endometrial, others | |
| T20 | NRAS | G12D | 5.23 | Yes | 8.0% | 30.8% melanoma, 18.0% multiple myeloma, others |
| T45 | ADAMTS5 | N807S | 5.48 | 0% | 9.2% lung adeno., 7.7% gastric, others | |
| T45 | FGF18 | R34H | 4.24 | 0% | 2.2% melanoma, 1.6% lung adeno., others | |
| T45 | HIST1H2AL | L24I | 4.45 | 0% | 2.4% small cell lung, 2.0% bladder, others | |
| T45 | NRAS | G13C | 5.23 | Yes | 8.0% | 30.8% melanoma, 18.0% multiple myeloma, others |
| T45 | PAPPA2 | C1167F | 5.29 | 0.5% | 28.1% melanoma, 20.7% small cell lung, others | |
| T46 | BRCA2 | T2310P | 4.82 | Yes | 0% | 11.6% melanoma, 10.8% ovarian, others |
| T46 | CHM | c.1361G>A, synonymous | −1.01 | 0% | 4.2% colorectal, 4% endometrial, others | |
| T46 | GLB1L | I514T | 4.74 | 0% | 5.6% colorectal, 3.2% endometrial, others | |
| T46 | LRP5 | c.1876G>A | splice junction | 0.5% | 10.3% cervical, 9.1% melanoma, others | |
| T46 | MMP3 | K349fs | indel | 0% | 3.5% pancreatic, 3.2% melanoma, others | |
| T46 | NSD1 | Q1213* | stop | Yes | 0% | 10.8% head neck, 10.7% bladder, others |
| T47 | C10orf76 | Q267K | 5.71 | 0.5% | 2.8% colorectal, 2.4% small cell lung, others | |
| T47 | PLXNA2 | V475L | 3.2 | 0% | 11.1% colorectal, 7.7% endometrial, others | |
| T47 | TP53 | C275Y | 4.57 | Yes | 7.0% | 94.6% ovarian, 89.7% lung small cell, others |
| T47 | TXLNA | K427R | 5.32 | 0% | 3.4% lung small cell, 2.2% melanoma, others | |
| T50 | CCDC150 | T787I | 1.12 | 0.5% | 4.4% endometrial, 3.2% melanoma, others | |
| T50 | DLEC1 | c.2256C>T, synonymous | −9.17 | 0% | 10.0% melanoma, 5.6% endometrial, others | |
| T50 | DNAH5 | c.3206C>G, synonymous | −9.74 | 0.5% | 52.7% melanoma, 25.0% colorectal, others | |
| T50 | EWSR1 | Y170H | 5.14 | Yes | 0.5% | 4.1% melanoma, 3.6% endometrial, others |
| T50 | GOLGA3 | Q122P | 5.37 | 0% | 7.9% lung squamous, 5.9% gastric, others | |
| T50 | HECTD1 | L330Q | 5.72 | 0% | 7.7% endometrial, 7.1% bladder, others | |
| T50 | NLGN4X | R204H | 3.55 | 0% | 8.3% lung adeno., 7.4% melanoma, others | |
| T50 | PTPN11 | A72T | 5.28 | Yes | 4.5% | 4.2% colorectal, 3.4% small cell lung, others |
| T50 | SGOL1 | E212A | 3.12 | 0% | 7.1% bladder, 3.5% prostate, others | |
| T50 | SLC25A20 | S167N | 5.32 | 0% | 1.6% endometrial, 1.1% lung squamous, others | |
| T50 | SPTA1 | c.892G>A, synonymous | 2.59 | 0% | 30.6% lung adeno, 23.3% melanoma, others | |
| T50 | TRPV4 | N678S | 5.24 | 0% | 4.2% colorectal, 4.0% endometrial, others | |
| T52 | CPSF2 | V208M | 5.27 | 0% | 4.4% endometrial, 4.1% head neck, others | |
| T52 | TMCO1 | I154N | 5.82 | 0% | 1.8% pancreatic, 1.6% endometrial, others | |
| T52 | TP53 | Y220C | 4.93 | Yes | 7.0% | 94.6% ovarian, 89.7% lung small cell, others |
GERP, genomic evolutionary rate profiling score (Cooper, et al. 2005 Genome Research 15:901)
Cancer Gene Census data was downloaded March 2014 (Futreal, PA, et al. 2004 Nature Reviews Cancer 4:177).
cBioPortal data (Gao, J, et al. 2013 Science Signaling 6:pl1) represents the two tumor types with the highest frequency of mutations in that gene (accessed March 2014).
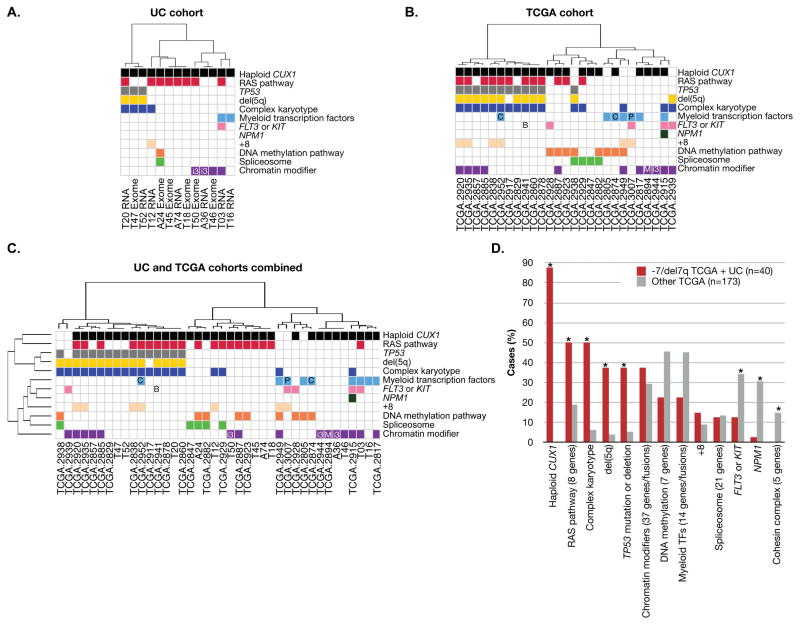
Driver mutations in AML genomes predominate in eight functional categories: tumor suppressors, signaling molecules, myeloid transcription factors, DNA-methylation regulators, chromatin modifiers, cohesin, spliceosome components, and NPM1 (Table S3) (TCGA 2013). Of these, the most frequently altered in the UC cohort was the RAS pathway, with activating mutations in 8/13 (61.5%) samples (Figure 1A). The mutations were comprised of those associated with juvenile myelomonocytic leukemia (JMML), including activating mutations of NRAS and PTPN11, and inactivating mutations of CBL (Table 1). The next most frequently altered pathway involved chromatin modifiers (4/13 cases, 31%). There was a paucity of mutations in the other major pathways.
Figure 1.
The pattern of somatic mutations in -7/del(7q) leukemias is distinct from other AML types. Categorization of genes within pathways is as defined (TCGA 2013) (Table S4). Mutations in genes not in these pathways are not shown. Samples are hierarchically clustered by Pearson correlation coefficients based on the presence or absence of mutations in these pathways using Ward’s method. Mutated pathways are shown for the UC cohort (A), the TCGA cohort (B), and the combined UC and TCGA cohorts (C). D. The frequency of the alteration in the combined UC (n=13) and TCGA (n=27) cohorts of ‒7/del(7q) leukemias (red bars, n=40) is shown in comparison to TCGA AML samples without -7/del(7q) (grey bars, n=173). The number of genes per category is indicated in parentheses. * indicates chi-squared test p < 0.05 comparing -7/del(7q) TCGA samples versus other TCGA samples. All recurrent genetic abnormalities according to the 2008 WHO classification “AML with recurrent genetic abnormalities” are indicated (Swerdlow et al. 2008), with an abbreviation within the relevant pathway. B: BCR-ABL fusion; C: CEBPA mutation; i3: inv(3)(q21q26.2) or t(3)(3;3)(q21;q26.2); M: MLLT3-MLL fusion; and P: PML-RAR fusion. Abbreviations: TF, transcription factor. Within the UC cohort, t-MN samples are named by TXX and de novo AML samples are named by AXX.
RNA-sequencing to detect somatic mutations is limited to identification of expressed mutations. Mutations in genes that are not expressed, expressed at low levels, or mutations that cause nonsense-mediated decay will be missed. Therefore, to extend our findings to a larger, independent cohort, and to exclude the possibility that RNA-sequencing biased the discovery of mutations in pathways, mutations in -7/del(7q) AML samples from The Cancer Genome Atlas (TCGA) were assessed (TCGA 2013). Of the 200 TCGA samples with exome or whole genome sequencing, 21 had -7/del(7q) by cytogenetic analysis. Six additional samples with >30 Mb deletions involving 7q identified by SNP array were also included, for a total of 27 cases with ‒7/del(7q) in the TCGA cohort. -7/del(7q) deletions spanned CUX1 in 22/27 cases, the remaining 5 cases had deletions that spanned EZH2 on 7q36.
The patterns of mutations seen in the TCGA -7/del(7q) samples reflected the results of the UC cohort (Figure 1B). RAS pathway activating mutations were prevalent, occurring in 44% of cases (Table S4). These included mutations of NRAS, KRAS, RIT1, and deletions or mutations of NF1. In contrast, RAS pathway mutations occurred in 19% of the other 173 TCGA samples (chi-squared p=0.0033). We note that RAS pathway mutations were restricted to those cases with deletions of CUX1, occurring in 12/22 (55%, p=0.00014). RAS pathway mutational status did not influence median overall survival within the -7/del(7q) TCGA subset (10.0±22.8 months without RAS pathway mutations, n=15; 9.4±15.5 months for patients with RAS pathway mutations, n=12).
The TCGA cohort replicated the finding that genes encoding chromatin modifiers were mutated at similar rates in -7/del(7q) cases (41%) as compared to others (30%, p=0.24), whereas alterations in other major leukemogenic pathways were underrepresented. There were fewer mutations in the genes encoding the signaling molecules, FLT3 or KIT, (p=0.045), the cohesin complex (p=0.031), and NPM1 (p=0.0034). Thirty percent of -7/del(7q) AML had alterations in the DNA methylation pathway, as compared to 46% of others, but this did not reach statistical significance (p=0.12).
Myeloid transcription factor alterations (Table S3) were decreased in ‒7/del(7q) leukemias. Whereas 45% of AML samples without -7/del(7q) had disruption of at least one myeloid transcription factor gene, the frequency was 26% (7/27) in the TCGA -7/del(7q) cases (p=0.061). The frequency of myeloid transcription factor mutations was markedly lower within those TCGA samples with deletions of CUX1, occurring in only 18% (4/22) of cases (p=0.014), indicating that CUX1 deletions are mutually exclusive with mutations of other myeloid transcription factor genes.
The high rate of TP53 mutations or deletions (20% UC and 44% TCGA) in -7/del(7q) samples compared to others (5%, p=0.0001, TCGA cohort), is driven by the strong association between del(5q) and TP53 mutations. With one exception, all of the fifteen TP53 mutations or deletions in the combined cohorts occurred in samples that also had del(5q) (Cochran-Mantel-Haenszel test p=3.5e-07).
This is the first description of the genome-wide mutation burden in high-risk myeloid leukemia with -7/del(7q). The analysis of additional patients in larger studies will be necessary to confirm the current findings. We did not observe differences in the mutational spectrum in t-MN or de novo AML. Across all -7/del(7q) cases, we observed a higher frequency of RAS pathway mutations (50% of UC and TCGA combined) than previously reported (14%) (Side et al, 2004), suggesting that haploinsufficiency of a gene(s) on chromosome 7 cooperates with RAS in AML pathogenesis. The finding of a low number of transcription factor alterations, particularly in those samples with a deletion of CUX1, is consistent with a transcription factor role for the gene(s) on chromosome 7, such as CUX1 (McNerney et al. 2013). Of note, CUX1 is mutated in 7–10% of endometrial carcinoma, gastric adenocarcinoma, and melanoma (Cerami et al, 2012). Our analysis of TCGA data revealed that RAS pathway mutations are over twice as frequent in CUX1-mutated solid tumors within these three diseases (p<0.01). Indeed, a striking 80% of endometrial and melanoma cancers with mutated CUX1 also have activating RAS pathway mutations, suggesting that cooperation between CUX1 and RAS may be a tumorigenic mechanism that extends beyond hematologic malignancies. As drugs targeting the RAS pathway advance, therapeutic inhibition of RAS, in addition to targeting pathways triggered by CUX1 haploinsufficiency, may cooperate to improve the outcome for patients with high-risk myeloid neoplasms.
Supplementary Material
Acknowledgments
Next-generation sequencing was performed at the University of Chicago High-throughput Genome Analysis Core. Sanger sequencing was performed at the University of Chicago Comprehensive Cancer Center Genomics Core. This work was supported by a Leukemia and Lymphoma Society Fellow award (M.E.M), the Cancer Research Foundation, National Institutes of Health (CA40046; M.M.L. and R.A.L.), and the Chicago Cancer Genomes Project. Computational infrastructure and bioinformatics support were kindly provided by Robert Grossman.
Footnotes
Authorship contributions: M.E.M. designed research, performed experiments, analyzed and interpreted data, and wrote the manuscript; C.D.B. assisted in sequencing data analysis and edited the manuscript; A.L.P. generated exome libraries and performed Sanger sequencing; M.B. collected biospecimens and generated lymphoblastoid cell lines; R.A.L. collected biospecimens and edited the manuscript; J.A. performed morphologic analysis, collected biospecimens, and edited the manuscript; M.M.L. designed research, performed cytogenetic analysis of leukemia samples, collected biospecimens, and edited the manuscript; and K.P.W. designed research, interpreted data, and edited the manuscript.
Conflict of interest: The authors do not have any competing financial interests in relation to the work described.
References
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discovery. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- Link DC, Schuettpelz LG, Shen D, Wang J, Walter MJ, Kulkarni S, Payton JE, Ivanovich J, Goodfellow PJ, Le Beau M, Koboldt DC, Dooling DJ, Fulton RS, Bender RH, Fulton LL, Delehaunty KD, Fronick CC, Appelbaum EL, Schmidt H, Abbott R, O’Laughlin M, Chen K, McLellan MD, Varghese N, Nagarajan R, Heath S, Graubert TA, Ding L, Ley TJ, Zambetti GP, Wilson RK, Mardis ER. Identification of a novel TP53 cancer susceptibility mutation through whole-genome sequencing of a patient with therapy-related AML. Journal of the American Medical Association. 2011;305:1568–1576. doi: 10.1001/jama.2011.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerney ME, Brown CD, Wang X, Bartom ET, Karmakar S, Bandlamudi C, Yu S, Ko J, Sandall BP, Stricker T, Anastasi J, Grossman RL, Cunningham JM, Le Beau MM, White KP. CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood. 2013;121:975–983. doi: 10.1182/blood-2012-04-426965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- Side LE, Curtiss NP, Teel K, Kratz C, Wang PW, Larson RA, Le Beau MM, Shannon KM. RAS, FLT3, and TP53 mutations in therapy-related myeloid malignancies with abnormalities of chromosomes 5 and 7. Genes Chromosomes Cancer. 2004;39:217–223. doi: 10.1002/gcc.10320. [DOI] [PubMed] [Google Scholar]
- Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of Tumours of Haematopoieitc and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- TCGA. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New England Journal of Medicine. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.