Abstract
BACKGROUND
Evidence suggests that angiotensin II AT1-receptor blockers (ARBs) may be protective against dementia, and studies in transgenic animals indicate that this may be due to improved amyloid-β (Aβ) clearance.
OBJECTIVE
We investigated whether taking ARBs was associated with an attenuation of age-related increases in cerebral Aβ retention, and reduced progression to dementia.
METHODS
Eight hundred seventy-one stroke-free and dementia-free older adults from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study underwent baseline lumbar puncture, and a subgroup (n=124) underwent 12 and 24 month follow-up lumbar puncture. Participants were followed at variable intervals for clinical progression to dementia. Linear mixed models and ANCOVA compared ARBs users with those taking other antihypertensives (O-antiHTN) or no antihypertensives (No-antiHTN) on cerebral spinal fluid (CSF) Aβ and phosphorylated tau (P-tau) levels. Cox regression and chi-square analyses compared groups on progression to dementia.
RESULTS
ARBs users exhibited greater vascular risk and lower educational attainment than the No-antiHTN group. Longitudinal analyses indicated higher CSF Aβ and lower P-tau in ARBs users versus other groups. Cross-sectional analyses revealed age-related decreases in CSF Aβ in other groups but not ARBs users. ARBs users were less likely to progress to dementia and showed reduced rate of progression relative to the No-antiHTN group.
DISCUSSION
Patients taking ARBs showed an attenuation of age-related decreases in CSF Aβ, a finding that is consistent with studies done in transgenic animals. These findings may partly explain why ARBs users show reduced progression to dementia despite their lower educational attainment and greater vascular risk burden.
Keywords: AT1-receptor blockers, antihypertensive medications, blood pressure, CSF biomarkers, amyloid-β, tau
Introduction
Blood pressure elevation is a risk factor for cognitive decline and Alzheimer’s dementia [1] and has been linked to increased amyloid beta (Aβ) retention with age [2, 3]. Numerous prior studies have examined whether antihypertensive treatment may aid preventative efforts, but results have been mixed [4]. There have been no published trials specifically for antihypertensive medications in the prevention of dementia, but there have been several secondary analyses of cognitive measures from trials involving primary cardiovascular outcomes. Although some of these studies have suggested potential benefits of antihypertensive medications in the prevention of dementia [5], others have found no effect [6]. One complexity involved in these studies is the diversity of available antihypertensive medications, which may work through a number of disparate physiological pathways, and may have pleiotropic effects on systemic and central nervous system pathways involved in neurodegeneration. Another difficulty lies in the fact that most prevention trials do not include biomarker outcomes, but rather rely on clinical outcomes such as progression to dementia or cognitive decline. Thus, the putative mechanism behind any potential preventative effect is typically speculative.
Angiotensin II AT1-receptor blockers (ARBs) may be of particular interest in the prevention of Alzheimer’s disease (AD), as findings from multiple observational studies [7–10] and experimental trials [11, 12] have suggested that these drugs may prevent or delay cognitive decline to a greater degree than other antihypertensive medicines [13]. Although these clinical associations are promising, the potential mechanism responsible for these observations remains unclear. Animal studies have suggested that AT1-receptor blockade may attenuate cognitive impairment by reducing amyloid-β (Aβ) levels [14–16], and that angiotensin II may exacerbate age-related changes in Aβ and phosphorylated tau (P-tau) [17, 18]. Furthermore, human autopsy studies have found that antemortem use of ARBs is associated with reduced Aβ and tau pathology in AD patient brains postmortem [19], and that angiotensin-converting enzyme (ACE) levels are increased in AD patient brains [20]. These studies suggest blockade of angiotensin II production with ACE inhibitors, or signaling with ARBs, may attenuate AD pathophysiology. However, ACE activity can aid in the enzymatic degradation of Aβ [21], and recent studies have found that lower cerebral spinal fluid (CSF) levels of ACE are associated with increased Aβ retention and brain atrophy [22, 23]. Thus, it has been hypothesized that ARBs may be more beneficial than ACE inhibitors in the prevention of AD by reducing AT1-receptor signaling without interfering with ACE-mediated Aβ degradation [24]. To date no studies have investigated CSF biomarkers of Aβ or P-tau in patients taking ARBs versus other antihypertensive medicines.
The present study sought to leverage data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study to investigate whether taking ARBs may be associated with attenuation of CSF biomarkers of AD during the prodromal phase of the disease. Our own work and that of others indicates that the vascular contribution to dementia may become increasingly important in advancing age, as very-old adults exhibit greater cerebrovascular pathology at autopsy [25], and the relationship between markers of vascular aging and markers of Alzheimer’s pathophysiology is particularly salient in those with the most advanced age [3, 26]. Finally, recent data indicate that substantial age-related increases in cerebral Aβ retention occur in a cumulative fashion in the general population [27]. Thus, we hypothesized that vascular protective factors, such as use of ARBs, may exert greater effects with aging, potentially stabilizing age-related changes in CSF biomarkers and leading to a cumulative attenuation of AD pathology over time.
Materials and Methods
Data were obtained from the ADNI database (adni.loni.usc.edu). The primary goal of ADNI is to test whether neuroimaging, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. ADNI is the result of efforts of many co-investigators from a range of academic institutions and private corporations, and subjects have been recruited from more than 50 sites across the United States and Canada. Participants are recruited via newsletters, Web-based communication, direct mail, and press releases. Inclusion criteria include: age 55 to 91 years, permitted medications stable for 4 weeks, study partner who can accompany participant to visits, Geriatric Depression Scale less than 6, Hachinski Ischemic Score less than or equal to 4, adequate visual and auditory acuity, good general health, 6 grades of education or work history equivalent, and ability to speak English or Spanish fluently. Exclusion criteria for cognitively normal and MCI participants include any significant neurologic disease or history of significant head trauma. For more information, see www.adni-info.org.
Participants
Participants were 871 ADNI 1, ADNI-GO, and ADNI-2 participants who underwent lumbar puncture at their baseline evaluation and completed a clinical evaluation that included blood pressure assessment, medical history, and cognitive exam, and had at least one follow-up lumbar puncture. All participants were classified as either cognitively normal or having mild cognitive impairment (MCI) at baseline. Participants were followed with serial clinical assessments at varying intervals for different lengths of time, ranging from 6 to 96 months (mean=28.4). Criteria for MCI and dementia set forth by the ADNI study are described in detail elsewhere [28]. A subset of 124 participants underwent serial LP for evaluation of longitudinal change in CSF biomarkers.
Materials and Procedures
Cerebrospinal fluid (CSF) and genetic biomarkers
All participants underwent lumbar puncture and AD biomarkers were assayed from obtained CSF samples, including amyloid beta (Aβ1–42) and phosphorylated tau (P-tau) [29]. When available, data from multiple assays of a single sample were averaged to provide more robust estimates. Biomarker profiles were determined using previously reported cutoff values for CSF AD biomarkers in ADNI [30]: Aβ1–42, ≤ 192 pg/mL and P-tau, ≥ 23 pg/mL. All but two participants who were total tau (T-tau) positive were also P-tau positive, so all analyses were limited to P-tau. Samples were available for baseline and both 12 and 24 month follow-up on a participant subset (n=124).
Participants also underwent baseline venipuncture. Blood samples were used to determine apolipoprotein E (APOE)-ε4 carrier status, and participants were divided into those with versus without one or more copies of the APOE-ε4 allele. Those carrying the APOE ε2/ε4 genotype (n=12) were excluded given the ambiguity associated with the presence of both an allele imparting increased risk (ε4) and an allele with a possible protective impact (ε2).
Blood pressure assessment
Seated brachial artery systolic and diastolic blood pressures were obtained during the sample visit as the lumbar puncture and pulse pressure was calculated as systolic minus diastolic pressure.
Antihypertensive medications
Medications were reviewed at the time of baseline lumbar puncture and participants were divided into those taking antihypertensive medications versus those who were not. All major classes of antihypertensive medications were evaluated, including ARBs (Table 1), α-adrenergic blockers, β-adrenergic blockers, diuretics, calcium channel blockers, ACE inhibitors, direct vasodilators, and other mechanisms of action. In total over 140 antihypertensive drugs were screened. For all analyses, patients taking ARBs were compared with those taking other antihypertensive medicines (O-antiHTN) or no antihypertensive medicines (No-antiHTN).
Table 1.
List of ARBs
| ARBs | N |
|---|---|
| Candesartan | 9 |
| Irbesartan | 4 |
| Olmesartan | 10 |
| Valsartan | 30 |
| Losartan | 28 |
| Telmisartan | 8 |
| Eprosartan | 1 |
| Total | 90 |
Vascular Risk Factors
Participant vascular risk factor burden was determined during clinical interview and physical examination at study entry. For the purposes of the present study, participant medical history data was screened for vascular risk factors up to the date of baseline lumbar puncture using criteria derived from the Framingham profiles for risk of stroke and myocardial infarction [31]. Vascular risk factors included the following: a history of cardiovascular disease (i.e., myocardial infarction, intermittent claudication, angina, heart failure, or other evidence of coronary disease), dyslipidemia (i.e., hypercholesterolemia, low levels of high-density lipoprotein, or hypertriglyceridemia), hypertension, type 2 diabetes, atrial fibrillation, evidence of carotid artery disease, and transient ischemic attack or minor stroke. Body mass index (BMI) was calculated as the participant weight (kg) divided by height (meters) squared.
Statistical Analyses
Data were initially screened for influential outliers and departures from normality using indices of skewness and kurtosis. For longitudinal analyses, biomarker values were log-transformed due to kurtosis outside of acceptable range (+1 and -1). Raw values were used for cross-sectional analyses due to normalized distribution in this larger data sample. Both controlled and uncontrolled analyses were conducted. For all controlled analyses, covariates were limited to those demonstrated to influence CSF biomarker values in prior studies, including age [32], BMI [33], and APOE-ε4 carrier status [34], as well as gender. To investigate group differences and group x time interactions for CSF biomarker values among antihypertensive medication groups over all three time-points, a linear mixed models analysis was conducted with unstructured covariance structure and maximum likelihood estimation. Time was entered as a random effect, and group, group x time, age, BMI, and APOE-ε4 carrier status entered as fixed factors. Chi-square analyses examined whether the antihypertensive medication groups differed in the proportion of individuals exhibiting CSF biomarker values that were above or below established thresholds, and to compare across groups the proportion of participants who progressed to dementia over follow-up. Cox regression was used to compare the rate of progression to dementia, after controlling for age, gender, education, APOE4 carrier status, and BMI. All analyses were two-tailed with alpha set at p < .05.
In order to investigate whether use of ARBs was associated with an attenuation of age-related decreases in CSF Aβ in the larger cross-sectional sample, we employed multiple linear regression, ANCOVA with post-hoc least significant difference (LSD) tests, and chi-square analyses. All analyses investigated the relationship between age (continuous for regression analyses; age tertiles for ANCOVA and chi-square) and CSF biomarkers in all three medication groups, after controlling for gender, APOE-ε4 carrier status, and BMI. A small subgroup of participants (n=24) in the No-antiHTN group had no known history of hypertension or treatment with antihypertensive medicines but exhibited blood pressure levels consistent with stage II hypertension on baseline exam (systolic > 159 mmHg or diastolic > 99 mmHg). We repeated all cross-sectional analyses with and without this subgroup out of concern that they may represent a group with undiagnosed hypertension. The inclusion/exclusion of this group did not substantially influence the study findings so they remained in the No-antiHTN group for the results presented below.
Results
Clinical and demographic factors
When compared with the No-antiHTN group, the O-antiHTN group was significantly older, p < .001 and exhibited greater BMI, p < .001, systolic blood pressure, p < .001, pulse pressure, p < .001, and mean arterial pressure, p < .001, as well as higher proportions of individuals who were male, p = .03, and had a history of dyslipidemia, p < .001, cardiovascular disease, p < .001, type 2 diabetes, p = .001, carotid artery disease, p = .01, and TIA/minor stroke, p = .01. In a comparison between the No-antiHTN group and the ARBs group, ARBs users exhibited greater BMI, p < .001, systolic blood pressure, p = .01, pulse pressure, p < .02, a non-significant trend toward greater mean arterial pressure, p = .06, and lower educational attainment, p = .02, as well as higher proportions of individuals with a history of dyslipidemia, p < .001, cardiovascular disease, p < .001, type 2 diabetes, p < .001, and TIA/minor stroke, p = .02. Relative to the O-antiHTN group, those in the ARBs group were more likely to be female, p = .03. There were no other differences on any clinical or demographic measures among the groups, with all p’s > .10 (Table 2).
Table 2.
Group Comparisons on Clinical and Demographic Data
| Risk Factors | No-antiHTN | O-antiHTN | ARBs | F or χ2 | P-value |
|---|---|---|---|---|---|
| n = 438 | n = 343 | n = 90 | |||
| Age, yrs | 71.4 (7.2) | 74.0 (6.9) | 72.9 (6.8) | 10.29 | < .001 |
|
| |||||
| Education, yrs | 16.3 (2.7) | 16.0 (2.7) | 15.6 (3.0) | 2.91 | .06 |
|
| |||||
| Sex (% men) | 53.7% | 61.2% | 48.9% | 6.62 | .04 |
|
| |||||
| APOE4 (% ε4+) | 43.4% | 40.1% | 33.3% | 3.24 | .20 |
|
| |||||
| Diagnosis (% MCI) | 68.3% | 70.6% | 64.4% | 1.34 | .51 |
|
| |||||
| BMI (kg/m2) | 26.4 (4.6) | 27.8 (4.7) | 28.5 (4.9) | 13.42 | < .001 |
|
| |||||
| Systolic BP (mmHg) | 131.9 (16.4) | 138.5 (17.1) | 136.8 (15.5) | 15.52 | < .001 |
|
| |||||
| Diastolic BP (mmHg) | 74.7 (9.5) | 75.5 (10.0) | 75.5 (9.6) | 0.78 | .46 |
|
| |||||
| MABP (mmHg) | 93.8 (10.1) | 96.5 (10.5) | 95.9 (10.1) | 7.83 | .001 |
|
| |||||
| Pulse pressure (mmHg) | 57.2 (14.5) | 62.9 (15.6) | 61.3 (13.4) | 14.67 | < .001 |
| Vascular Risk Factors | |||||
| Cardiovascular disease | 3.2% | 16.6% | 12.2% | 41.56 | < .001 |
|
| |||||
| Dyslipidemia | 28.5% | 46.6% | 47.8% | 31.25 | < .001 |
|
| |||||
| Atrial fibrillation | 0.9% | 2.9% | 2.2% | 4.36 | .11 |
|
| |||||
| Type 2 diabetes | 3.2% | 8.5% | 13.3% | 17.35 | < .001 |
|
| |||||
| Carotid artery disease | 0.2% | 2.0% | 0% | 7.87 | .02 |
|
| |||||
| TIA / minor stroke | 1.6% | 5.5% | 5.6% | 8.29 | .02 |
Among those with serial CSF biomarker assessments (n=124), participants in the O-antiHTN group displayed greater vascular risk factor burden than those in the No-antiHTN including history of dyslipidemia, p = .04, and cardiovascular disease, p = .004, and a non-significant trend toward greater BMI, p = .07, and history of TIA/minor stroke, p = .06. Relative to the larger cross-sectional cohort, the longitudinal cohort who underwent serial CSF biomarker assessments were significantly older, p < .001 (72.2±7.2 vs. 75.3±5.6 years), and exhibited lower vascular risk factor burden, including lower BMI, p = .03, systolic blood pressure, p = .02, diastolic blood pressure, p < .001, and mean arterial pressure, p < .001. The longitudinal subgroup was also more likely to be male, p = .005, and less likely to have been diagnosed with MCI, p = .03 (data not shown).
Longitudinal analyses
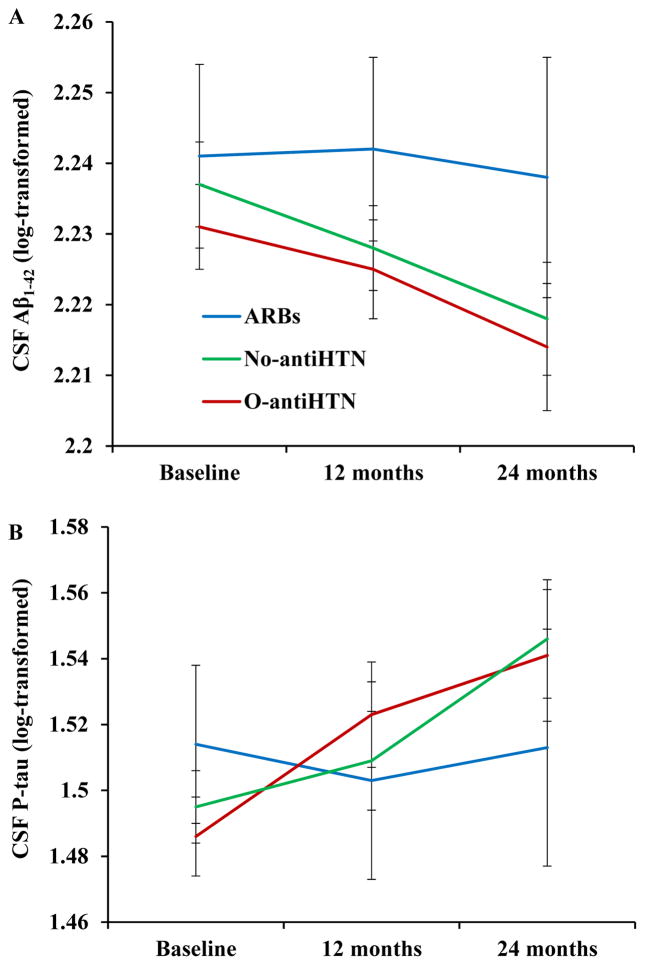
Longitudinal analysis of CSF AD biomarkers revealed a significant group x time interaction for CSF Aβ1–42 levels after controlling for all covariates, F(3, 148) = 7.01, p < .001, such that those in the ARBs group displayed an attenuation of CSF Aβ1–42 reduction over time (indicating less cerebral Aβ1–42 retention) relative to the No-antiHTN group, β = − 3.54, t(140) = −3.43, p = .001, and the O-antiHTN group, β = −3.80, t(145) = −3.32, p = .001 (Figure 1A). There was also a significant group × time interaction for CSF P-tau levels, F(3, 140) = 7.25, p < .001, such that those in the ARBs group showed less P-tau accumulation over time relative to the No-antiHTN group, β = 2.57, t(136) = 3.32, p = .001, and the O-antiHTN group, β = 2.73, t(136) = 3.14, p = .002 (Figure 1B).
Figure 1.
Participants taking ARBs showed attenuation of CSF Aβ1–42 reduction (A) and P-tau accumulation (B) over time, as well as fewer Aβ1–42 positive cases (C) and P-tau positive cases (D) over 24 month follow-up.
*p < .05
†non-significant trend, p < .07
Error bars represent standard error of the mean
Participants taking ARBs also exhibited significantly fewer Aβ1–42 positive cases relative to the O-antiHTN group at baseline, χ2 = 4.56, p = .03, and 12 month follow-up, χ2 = 5.24, p = .02, and displayed a nonsignificant trend towards fewer Aβ1–42 positive cases at 24 month follow-up, χ2 = 3.40, p = .07. (Figure 1C). Those taking ARBs also exhibited significantly fewer P-tau positive cases at 12 month follow-up, χ2 = 4.50, p = .03, and 24 month follow-up, χ2 = 6.44, p = .01, relative to the No-antiHTN group. When compared with those in the O-antiHTN group, participants taking ARBs showed a non-significant trend toward fewer P-tau positive cases at 12 month follow-up, χ2 = 3.40, p = .07, and significantly fewer cases at 24 month follow-up, χ2 = 4.69, p = .03 (Figure 1D).
Cross-sectional analyses
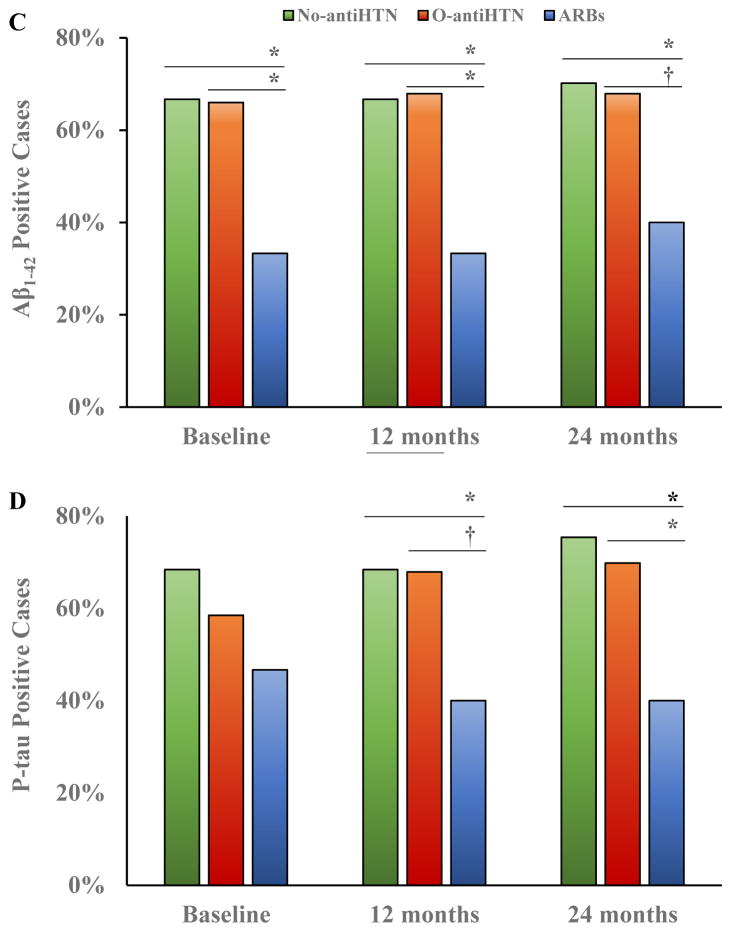
Results of the 3 × 3 ANCOVA analysis indicated a significant medication group × age-group (tertiles) interaction in relation to CSF Aβ1–42 levels, F(4, 842) = 3.90, p < .01, η2 = .02, after controlling for gender, APOE-ε4 carrier status, and BMI. Simple main effects analyses revealed significant group differences among those age 70–75 years, F(2, 284) = 3.43, p = .03, η2 = .02, with those taking ARBs exhibiting higher CSF Aβ1–42 than those in the No-antiHTN group, p = .01, and a non-significant trend toward higher levels than the O-antiHTN group, p = .07, after controlling for age, gender, APOE-ε4 carrier status, and BMI. In uncontrolled analyses there were significant group differences among those age 76–91 years, F(2, 301) = 3.43, p = .05, η2 = .02, with those in the O-antiHTN group showing lower CSF Aβ1–42 than those in the ARBs, p = .05, or No-antiHTN, p = .04, groups, but the omnibus test showed a non-significant trend after including all covariates, p = .06 (Figure 2A). Chi-square analyses demonstrated no significant differences in the proportion of Aβ1–42 positive cases across medication and age groups, but there were non-significant trends towards fewer Aβ1–42 positive cases in the ARBs group versus the No-antiHTN group among participants age 70–75 years, χ2 = 3.28, p = .07, and between the ARBs and O-antiHTN groups among participants age 76–91 years, χ2 = 3.52, p = .06 (Figure 2B).
Figure 2.
Among those ages 70–75, participants taking ARBs exhibited higher CSF Aβ1–42, with a non-significant trend toward higher levels at ages 76–91 years (A). There were also non-significant trends towards fewer Aβ1–42 positive cases in the ARBs group relative to the No-antiHTN group in those ages 70–75 and fewer cases relative to the O-antiHTN group in those ages 76–91 (B). There were age-related decreases in CSF Aβ1–42 in the No-antiHTN and O-antiHTN groups, but not the ARBs group (C) and age-related increases in the proportion of Aβ1–42 positive individuals in the No-antiHTN and O-antiHTN groups, but not the ARBs group (D).
ns = non-significant, p > .10
*p < .05
**p < .01
***p < .001
†non-significant trend, p < .07
Error bars represent standard error of the mean
Additional analyses indicated age-related decline across age tertiles in CSF Aβ1–42 among participants in the No-antiHTN group, F(2, 323) = 8.44, p < .001, η2 = .04, and the O-antiHTN group, F(11, 328) = 14.21, p < .001, η2 = .08, but there was no age-related decrease in CSF Aβ1–42 among those taking ARBs, F(2,81) = 1.71, p = .19, η2 = .04 (Figure 2C). Chi-square analyses demonstrated a substantial increase in the proportion of Aβ1–42 positive cases with increasing age among the No-antiHTN group, χ2 = 6.37, p = .04, and O-antiHTN group, χ2 = 11.423, p < .01, but there was no age-related change among those taking ARBs, χ2 = 1.44, p = .49 (Figure 2D).
Regression analyses confirmed a highly significant relationship between age and CSF Aβ1–42 in the total sample, such that Aβ1–42 levels decreased with age, ΔR2 = .028, β = −.17, p < .001. This relationship was clearly observed in the No-antiHTN group, ΔR2 = .02, β = −.15, p = .001, and the O-antiHTN group, ΔR2 = .06, β = −.24, p < .001, but there was no relationship between age and Aβ1–42 among patients taking ARBs, ΔR2 = .001, β = −0.04, p = .72.
There was no medication group × age group interaction or medication group main effects in relation to CSF P-tau, and regression analyses indicated no relationship between age and P-tau in the total sample or any participant subgroup, all p’s > .23 (data not shown).
Progression to Dementia
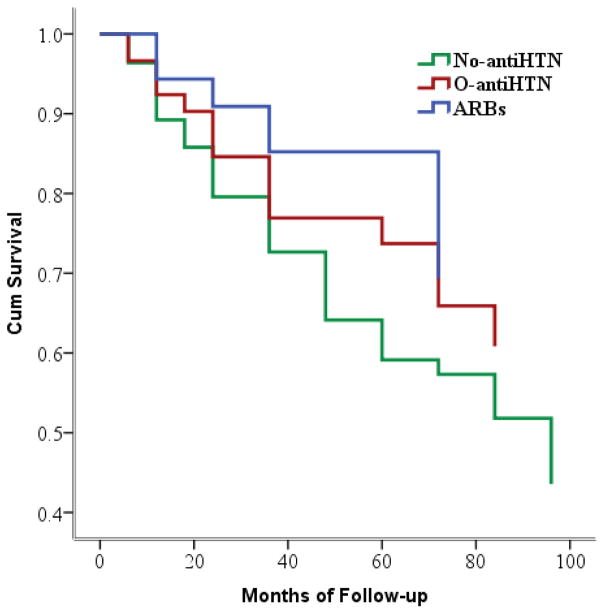
Chi-square analyses revealed a significant group difference in the likelihood of progressing to dementia across groups, such that participants taking ARBs were approximately half as likely as those in the No-antiHTN group to progress to dementia over follow-up (12.2% vs. 23.5%, respectively), χ2 = 8.50, p = .01. Those in the O-antiHTN group did not differ significantly from the ARBs or No-antiHTN groups in the frequency of dementia, both p’s > .10. Cox regression analyses indicated that those taking ARBs showed reduced progression to dementia relative the No-antiHTN group, p = .025, hazard ratio = 0.683, but not the O-antiHTN group, after controlling for age, gender, education, APOE-ε4 carrier status, and BMI (Figure 3).
Figure 3.
Participants taking ARBs displayed reduced progression to dementia relative to the No-antiHTN group over follow-up. The O-antiHTN group did not differ from either other group in rate of progression.
Discussion
To our knowledge, this is the first study to demonstrate an association between use of a specific class of antihypertensive medications and CSF biomarkers of Alzheimer’s pathophysiology. The longitudinal findings specifically indicated that older adults taking ARBs showed an attenuation of CSF Aβ1–42 reduction and P-tau accumulation over 24 months, relative to those taking other antihypertensive drugs or not taking antihypertensive drugs, potentially suggesting that ARBs may reduce cerebral amyloidosis and tau-mediated neurodegeneration. Cross-sectional findings indicated that although age was strongly associated with a reduction in CSF Aβ1–42 in individuals taking other antihypertensive medicines or no antihypertensive medicines, there was no relationship between age and CSF Aβ1–42 in those taking ARBs. Additionally, participants taking ARBs had higher CSF Aβ1–42 levels than the other groups in the center age tertile (70–75 years) and showed a non-significant trend toward higher levels than those taking other antihypertensive medications in the older age tertile (76–91 years). Finally, participants taking ARBs were less likely to progress to dementia relative to those not taking antihypertensive medications, despite the increased vascular risk factor burden and lower educational attainment in the ARBs group. Together these findings could suggest that ARBs may attenuate age-related reduction in CSF Aβ, potentially indicating decreased cerebral Aβ retention which may contribute to the reduced cognitive decline observed in patients taking these medications.
Collectively, these results are consistent with several prior studies suggesting a protective effect of ARBs in Alzheimer’s dementia [7, 8, 13], and animal studies indicating reduced cerebral Aβ deposition in transgenic mice treated with ARBs [14]. The findings are also consistent with neuropathological studies indicating fewer neuritic plaques and neurofibrillary tangles in medicated hypertensive patients [35], particularly those taking ARBs [36], compared to unmedicated hypertensives or normotensives.
Several mechanisms have been proposed to account for the apparent protective effect of ARBs, including their role in remodeling of cerebral microvasculature [36], inducing neural differentiation and DNA repair [37], reversing oxidative stress and inflammation, and preventing ischemic brain injury [38]. Animal studies have suggested that ARBs may also directly impact Aβ accumulation [14, 16], which is consistent with the present study findings. Wang and colleagues (2007) investigated 55 antihypertensive medications representing all drug classes in a transgenic mouse model (Tg2576) of AD, and reported that treatment with only one drug, valsartan (an ARB), improved Aβ clearance and disrupted the formation of high-molecular-weight oligomeric peptides [14]. Sample size limitations in the present study precluded examination of the differential influence of specific drugs within the ARBs class of antihypertensives. Future clinical trials assessing the influence of ARBs on change in CSF AD biomarkers with age may further elucidate which specific drugs have the most salient effects on AD pathophysiology.
Mechanistic studies in animals have suggested that ARBs may reduce Aβ levels through changes in enzymatic degradation and modification [16, 39] or increased cellular turnover [19, 39, 40]. The hemodynamic effects of ARBs may also play a role in their relationship with AD biomarkers. We have recently reported that brachial artery pulse pressure is associated with both reduced CSF Aβ1–42 and increased P-tau [3], an effect that may be related to hemodynamic influences on the perivascular and/or transvascular clearance of Aβ [41, 42]. In the current study, patients taking ARBs exhibited intermediate pulse pressure values that fell between those taking other antihypertensive medications and those in the no treatment group, suggesting that reduced pulse pressure may only partially account for the study findings. Another possibility is that ARBs improved cerebral blood flow. Reduced cerebral blood flow is found in AD patients [30], where it is associated with regional Aβ deposition [43]. Some studies suggest that taking ARBs may lead to cognitive benefits through improved cerebral blood flow [44, 45]. Future experimental studies may provide greater insight into the mechanisms behind the ARBs-induced attenuation of cerebral Aβ retention.
The strengths of the current study include the large sample of participants with CSF biomarkers and longitudinal subgroup analysis. Limitations include the retrospective design and limited information regarding the duration of use of antihypertensive drugs and history of untreated hypertension. Another limitation is that participants were not randomly assigned to treatment groups, as they would be in a randomized clinical trial, but rather were assigned based on medication indications and other uncontrolled factors (e.g., access to healthcare). This creates a potential confound by indication whereby the participants in each medication group differ in meaningful ways beyond their medication regimen, which may account for any observed group differences. Importantly, we may infer that participants taking ARBs or other antihypertensive medicines were put on these drugs to treat hypertension, which is consistent with the observed group differences in blood pressure. We were unable to identify any other clinical or demographic differences between ARBs users and those taking other antihypertensive medications, except for gender and education, which were included as a covariates in all analyses.
A review of treatment guidelines suggests that older hypertensive patients may be put on ARBs after failure to adequately control blood pressure with first and second line agents (thiazide diuretics and calcium channel blockers, respectively) [46], but patients are more frequently started on ACEIs due to greater affordability. Thus, ARBs users may represent a subgroup with more severe hypertension prior to treatment control. As mentioned above, ARBs users exhibited lower educational attainment, which may represent a proxy measure of lower socioeconomic status and greater disease risk. These confounds would seem to increase rather than decrease the risk of cerebral amyloidosis and dementia in this patient subgroup, which is the opposite direction of the observed effects. However, a decline in blood pressure has also been observed prior to the diagnosis of dementia, despite the fact that baseline hypertension is a risk factor for dementia and is associated with cerebral amyloidosis [1, 3]. We conclude that although we cannot rule out the possibility that our findings represent the spurious result of a confound by indication, the expected direction of the confound is remains unclear. Consequently, the present study findings must be interpreted with caution until results are available from randomized controlled trials investigating ARBs in the prevention of cerebral amyloidosis and dementia. There are currently at least two ongoing trials involving the treatment of AD patients with ARBs, and cerebral amyloid retention will be included as outcome measures in these trials. Our findings suggest a small but cumulative effect of ARBs on Aβ retention and progression to dementia, potentially indicating that future trials focusing on early intervention and prevention may be of greatest benefit.
Another important limitation of all studies involving the ADNI sample is that this group of participants is comprised of individuals recruited from over 50 sites across the US and Canada with variable sampling bias and methodology, and inclusion/exclusion criteria that limited cerebrovascular disease. Variability in recruitment methods may result in a heterogeneous participant sample that may not be representative of the general population of older adults treated or untreated for hypertension regardless of antihypertensive medication class. For these reasons, replication of the study findings may be warranted, as the generalizability of these findings could be limited. Finally, variable length of follow-up may limit interpretation of our findings regarding the impact of ARBs treatment on progression to dementia. Despite these limitations, the study findings may have major treatment implications since hypertension is very common in older adults at risk for AD, yet most of the participants in this study were taking other antihypertensive drugs instead of ARBs. The current study findings suggest that greater use of ARBs to treat hypertension in the elderly might reduce the incidence of dementia through attenuation of age-related cerebral amyloid retention.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; ; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern Rev December 5, 2013, California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- 1.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 2.Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81:2024–2027. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW Alzheimer’s Disease Neuroimaging Initiative I. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 2015;72:546–553. doi: 10.1001/jamaneurol.2014.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C. Preventing Alzheimer’s disease by targeting vascular risk factors: hope and gap. J Alzheimers Dis. 2012;32:721–731. doi: 10.3233/JAD-2012-120922. [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C investigators H. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 6.Staessen JA, Thijs L, Richart T, Odili AN, Birkenhager WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- 7.Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu WC, Ho WC, Lin MH, Lee HH, Yeh YC, Wang JD, Chen PC Health Data Analysis in Taiwan Research G. Angiotension receptor blockers reduce the risk of dementia. J Hypertens. 2014;32:938–947. doi: 10.1097/HJH.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 9.Hanon O, Berrou JP, Negre-Pages L, Goch JH, Nadhazi Z, Petrella R, Sedefdjian A, Sevenier F, Shlyakhto EV, Pathak A. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive function And Systolic Blood Pressure Reduction open-label study. J Hypertens. 2008;26:1642–1650. doi: 10.1097/HJH.0b013e328301a280. [DOI] [PubMed] [Google Scholar]
- 10.Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26:699–708. doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- 11.Kume K, Hanyu H, Sakurai H, Takada Y, Onuma T, Iwamoto T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr Gerontol Int. 2012;12:207–214. doi: 10.1111/j.1447-0594.2011.00746.x. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar I, Hart M, Chen YL, Mack W, Milberg W, Chui H, Lipsitz L. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Arch Intern Med. 2012;172:442–444. doi: 10.1001/archinternmed.2011.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi Marpillat N, Macquin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens. 2013;31:1073–1082. doi: 10.1097/HJH.0b013e3283603f53. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Liu S, Tanabe C, Maeda T, Zou K, Komano H. Differential effects of angiotensin II receptor blockers on Abeta generation. Neurosci Lett. 2014;567:51–56. doi: 10.1016/j.neulet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Mogi M, Li JM, Tsukuda K, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem Biophys Res Commun. 2008;375:446–449. doi: 10.1016/j.bbrc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Zhu D, Shi J, Zhang Y, Wang B, Liu W, Chen Z, Tong Q. Central angiotensin II stimulation promotes beta amyloid production in Sprague Dawley rats. PLoS One. 2011;6:e16037. doi: 10.1371/journal.pone.0016037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian M, Zhu D, Xie W, Shi J. Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 2012;586:3737–3745. doi: 10.1016/j.febslet.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol. 2012;69:1632–1638. doi: 10.1001/archneurol.2012.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miners JS, Ashby E, Van Helmond Z, Chalmers KA, Palmer LE, Love S, Kehoe PG. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer’s disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34:181–193. doi: 10.1111/j.1365-2990.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 21.Miners JS, Palmer JC, Tayler H, Palmer LE, Ashby E, Kehoe PG, Love S. Abeta degradation or cerebral perfusion? Divergent effects of multifunctional enzymes. Front Aging Neurosci. 2014;6:238. doi: 10.3389/fnagi.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jochemsen HM, van der Flier WM, Ashby EL, Teunissen CE, Jones RE, Wattjes MP, Scheltens P, Geerlings MI, Kehoe PG, Muller M. Angiotensin-converting enzyme in cerebrospinal fluid and risk of brain atrophy. J Alzheimers Dis. 2015;44:153–162. doi: 10.3233/JAD-131496. [DOI] [PubMed] [Google Scholar]
- 23.Jochemsen HM, Teunissen CE, Ashby EL, van der Flier WM, Jones RE, Geerlings MI, Scheltens P, Kehoe PG, Muller M. The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease. Alzheimers Res Ther. 2014;6:27. doi: 10.1186/alzrt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashby EL, Kehoe PG. Current status of renin-aldosterone angiotensin system-targeting anti-hypertensive drugs as therapeutic options for Alzheimer’s disease. Expert Opin Investig Drugs. 2013;22:1229–1242. doi: 10.1517/13543784.2013.812631. [DOI] [PubMed] [Google Scholar]
- 25.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Forster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gomez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Kohler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleo A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonca A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Mollergard HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodriguez-Rodriguez E, Roe CM, Rot U, Rowe CC, Ruther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schroder J, Schutte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin AK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H Amyloid Biomarker Study G. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jr, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ Alzheimer’s Disease Neuroimaging I. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidoni ED, Townley RA, Honea RA, Burns JM Alzheimer’s Disease Neuroimaging I. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuels SC, Silverman JM, Marin DB, Peskind ER, Younki SG, Greenberg DA, Schnur E, Santoro J, Davis KL. CSF beta-amyloid, cognition, and APOE genotype in Alzheimer’s disease. Neurology. 1999;52:547–551. doi: 10.1212/wnl.52.3.547. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, Haroutunian V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JW, Harding JW. The brain RAS and Alzheimer’s disease. Exp Neurol. 2010;223:326–333. doi: 10.1016/j.expneurol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens. 2015;28:289–299. doi: 10.1093/ajh/hpu197. [DOI] [PubMed] [Google Scholar]
- 38.Mogi M, Horiuchi M. Effects of angiotensin II receptor blockers on dementia. Hypertens Res. 2009;32:738–740. doi: 10.1038/hr.2009.110. [DOI] [PubMed] [Google Scholar]
- 39.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 40.Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattsson N, Tosun D, Insel PS, Simonson A, Jack CR, Jr, Beckett LA, Donohue M, Jagust W, Schuff N, Weiner MW Alzheimer’s Disease Neuroimaging I. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014;137:1550–1561. doi: 10.1093/brain/awu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajjar I, Hart M, Chen YL, Mack W, Novak V, HCC, Lipsitz L. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. J Am Geriatr Soc. 2013;61:194–201. doi: 10.1111/jgs.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 46.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]