Abstract
Endocrine disrupting chemicals are ubiquitous chemicals that exhibit endocrine disrupting properties in both humans and animals. Female reproduction is an important process, which is regulated by hormones and is susceptible to the effects of exposure to endocrine disrupting chemicals. Disruptions in female reproductive functions by endocrine disrupting chemicals may result in subfertility, infertility, improper hormone production, estrous and menstrual cycle abnormalities, anovulation, and early reproductive senescence. This review summarizes the effects of a variety of synthetic endocrine disrupting chemicals during adult life. The chemicals covered in this review are pesticides (organochlorines, organophosphates, carbamates, pyrethroids, and triazines), heavy metals (arsenic, lead, and mercury), diethylstilbesterol, plasticizer alternatives (di-(2-ethylhexyl) phthalate and bisphenol A alternatives), 2,3,7,8-tetrachlorodibenzo-p-dioxin, nonylphenol, polychlorinated biphenyls, triclosan, and parabens. This review focuses on the hypothalamus, pituitary, ovary, and uterus because together they regulate normal female fertility and the onset of reproductive senescence. The literature shows that several endocrine disrupting chemicals have endocrine disrupting abilities in females during adult life, causing fertility abnormalities in both humans and animals.
Keywords: endocrine disrupting chemicals, adult, female, fertility
Introduction
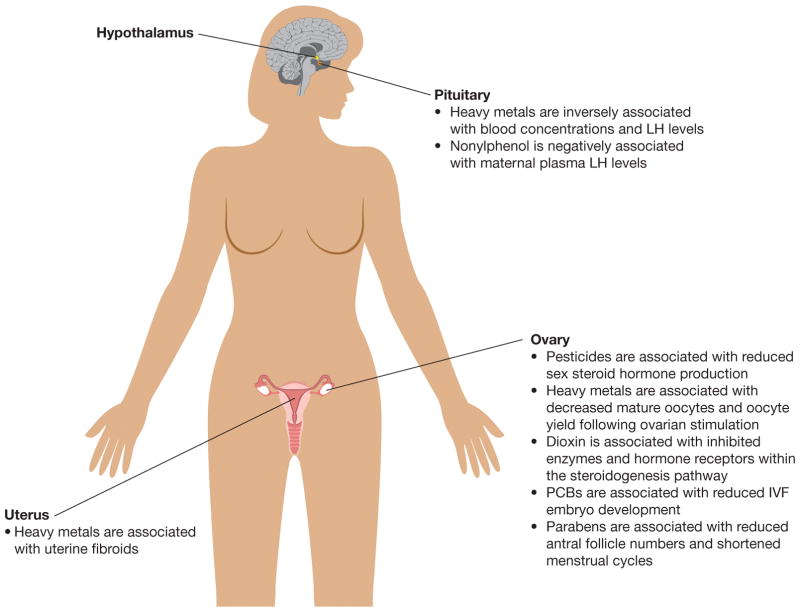
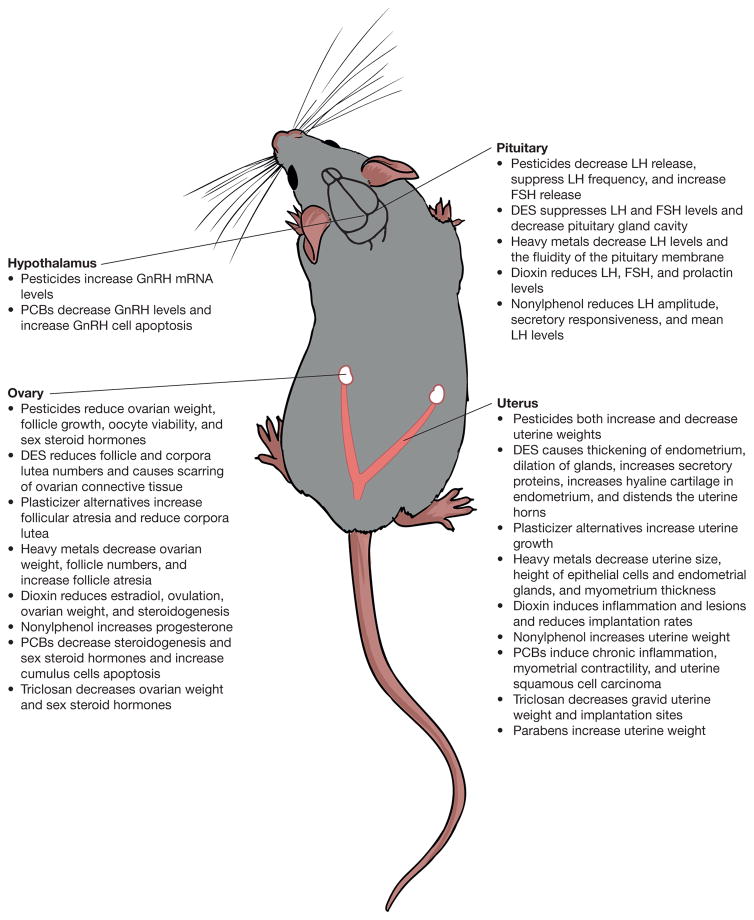
EDCs are chemicals that disrupt endocrine properties in animals by either mimicking or blocking endocrine actions. Specifically, EDCs can interfere with receptor binding, steroidogenesis, and metabolism of hormones (Sanderson 2006; Sweeney 2002). EDCs have been shown to disrupt female fertility in a wide range of species including humans (Figure 1 and Figure 2) (Patel et al. 2015; Woodruff and Walker 2008).
Figure 1.
Overview of the associations between exposure to pesticides, heavy metals, 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin), polychlorinated bisphenols, and parabens reproductive organs in adult women.
Figure 2.
Overview of the effects of pesticides, heavy metals, diethylstilbestrol, plasticizer alternatives, 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin), nonylphenol, polychlorinated bisphenols, triclosan, and parabens and their effects on the hypothalamus, pituitary, ovary, and uterus in adult female rodents.
Multiple recent reviews have previously summarized the effects of developmental exposure to EDCs (Katz et al. 2016; Walker and Gore 2016; Zama and Uzumcu 2010). Thus, this review focuses on adult exposure to synthetic EDCs such as pesticides (organochlorines, organophosphates, carbamates, pyrethroids, and triazines), heavy metals (arsenic, lead, and mercury), diethylstilbesterol, plasticizer alternatives (di-(2-ethylhexyl) phthalate and bisphenol A alternatives), 2,3,7,8-tetrachlorodibenzo-p-dioxin, nonylphenol, polychlorinated bisphenyls, triclosan, and parabens (Figures 1 and 2, Table 1). Further, this review focuses on the effects of the selected EDCs on the hypothalamus, pituitary, ovaries, and uterus in the adult female because normal function of these organs is required for normal fertility and onset of reproductive senescence.
Table 1.
Overview of the chemical names and associated abbreviations discussed in the review.
| Chemical Name | Chemical Abbreviation |
|---|---|
| dichlorodiphenyltrichloroethane | DDT |
| p,p’-dichlorodiphenyldichloroethylene | DDE |
| 2,2′,4,4′,5,5′-hexachlorobiphenyl | CB-153 |
| arsenic | As |
| lead | Pb |
| mercury | Hg |
| diethylstilbestrol | DES |
| bisphenol A | BPA |
| di(2-ethylhexyl) phthalate | DEHP |
| tri-2-ethylhexyl trimelliate | TETM |
| di-(2-ethylhexyl) terephthalate | DEHT |
| di-(isonyl)cyclohexanedicarboxylic acid | DINCH |
| di-isononyl phthalate | DINP |
| di-(2-ethylhexyl) adipate | DEHA |
| acetyl tri-n-butyl citrate | ATBC |
| bisphenol S | BPS |
| bisphenol B | BPB |
| bisphenol F | BPF |
| bisphenol AF | BPAF |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin | TCDD |
| nonylphenols | NP |
| polychlorinated biphenyls | PCB |
| 5-chloro-2-(2,4-dichlorophenoxy) phenol | Triclosan |
Pesticides
Pesticides are a group of agents used as insecticides, fungicides, herbicides, and rodenticides. Based on chemical composition, the major classes of pesticides include: 1) organochlorines (e.g., dichlorodiphenyltrichloroethane (DDT)), lindane, endosulfan, aldrin, dieldrin, chlordane, and methoxychlor), 2) organophosphates (e.g., parathion, malathion, diaznon, and glyphosate), 3) carbamates (e.g., carbaryl, carbofuran, and aminocarb), 4) pyrethroids (e.g., permethrin, cypermethrin, deltamethrin), and 5) triazines (e.g., atrazine). These pesticides have been shown to impair female reproduction by targeting a variety of reproductive tissues and functions. The sections below summarize some of the impacts of pesticide exposure on the hypothalamus, pituitary, ovary, uterus, fertility, and reproductive senescence (Figure 1 and Figure 2).
Hypothalamus and Pituitary
Currently, limited information is available on the effects of pesticide exposure during adulthood on the hypothalamus/pituitary (Figure 1 and Figure 2). The organophosphate pesticide chlorpyrifos (0.01 – 100 μM) and the organochlorine pesticide methoxychlor (0.01 – 100 μM) significantly increased gonadotropin releasing hormone (GnRH) mRNA levels in GT1-7 cells (Gore 2001). The carbamate molinate (25 and 50 mg/kg) suppressed LH pulse frequency, leading to delayed ovulation in rats (Stoker et al. 2005). Atrazine (75 mg/kg) both activated the release of pituitary hormones (Fraites et al. 2009) and it (100 – 200 mg/kg) inhibited LH release from the pituitary in rats (Foradori et al. 2011; Goldman et al. 2013).
Ovary
Although limited information is available on the effects of pesticides on the human ovary (Figure 1), animal studies have indicated that organochlorine pesticides adversely affect the ovary by reducing ovarian weight, follicle growth, and oocyte viability and/or or increasing atresia (Figure 2) (Borgeest et al. 2002; Tiemann 2008). For example, methoxychlor (32 – 200 mg/kg/day) decreased ovarian weight, increased the incidence of cystic ovaries, inhibited follicle growth, and induced atresia in rodents (Aoyama and Chapin 2014; Aoyama et al. 2012; Basavarajappa et al. 2012; Borgeest et al. 2004; Gupta et al. 2007; Gupta et al. 2009; Miller et al. 2005; Paulose et al. 2011; Paulose et al. 2012). Endosulfan (0.02 and 0.1 μg/mL) decreased oocyte viability and competence in buffalo oocytes in vitro (Nandi et al. 2011). It (11 mg/kg) also decreased the number of healthy follicles and increased the number of atretic follicles in rats (Koc et al. 2009). Malathion (1 and 10 nM) increased apoptosis in granulosa cells from goats (Bhardwaj and Saraf 2016). Similarly, pyrethroids (50 mg/kg) increased atresia in rats (Sangha et al. 2013) and a carbamate pesticide (2.5 and 1 mg/kg body weight) decreased the number of small follicles in mice (Shanthalatha et al. 2012).
Pesticide exposure also adversely affects the ability of the ovary to produce sex steroid hormones in women and animal models. For example, exposure to the organochlorine pesticide heptachlor was associated with a slower drop in the estradiol ratio and progesterone metabolites after ovulation in women (difference from estimated marginal mean of −2.00 was 0.263 with 95% CI: −0.001, −0.528) (Luderer et al. 2013). The organochlorine pesticides DDT and p,p’-dichlorodiphenyldichloroethylene (DDE) were associated with decreased progesterone levels and a shorter luteal phase in women (1.5 days at the highest quartile of DDT: 95% CI: −2.6, −3.0; or DDE: −2.6, −2.0) (Windham et al. 2005). The organochlorine pesticide methoxychlor (1 – 100 μg/mL) inhibited the production of estradiol, testosterone, androstenedione, and progesterone in isolated mouse antral follicles (Basavarajappa et al. 2011). The pyrethrin pesticide cypermethrin (50 mg/kg body weight) inhibited the activity of 3β-hydroxysteroid dehydrogenase (the enzyme that synthesizes progesterone production) in rats (Sangha et al. 2013) and it (10 – 100 ppm) inhibited progesterone secretion in the bovine corpus luteum (Gill et al. 2011). Atrazine (200 and 300 mg/kg) increased steroidogenic enzymes and sex steroid hormone levels in vivo (Quignot et al. 2012; Taketa et al. 2011). Further, atrazine (300 mg/kg) increased the estrogen-to-androgen ratio in rats (Quignot et al. 2012). It (10 μM) also increased progesterone and estradiol production and activity of aromatase (the enzyme that synthesizes estradiol from testosterone) in primary rat granulosa cells (Tinfo et al. 2011), and it (0.1 and 10 μM) disrupted steroidogenesis in swine granulosa cells (Basini et al. 2012).
Uterus
Several studies have indicated that pesticides can disrupt uterine structure and/or function in animal models (Figure 2) (Gore et al. 2015). For example, the organochlorine pesticide methoxychlor (500 and 1,500 ppm) increased uterine weight in in rats (Aoyama et al. 2012; Yu et al. 2013), whereas carbendazim (500 mg and 1,000 mg/kg) decreased uterine weight in rats (Rama et al. 2014). Additionally, a mixture of organophosphate pesticides (dichlorovos, dimethoate, and malathion) (107.5 mg/kg) increased endometrial hyperplasia in rats (Aoyama et al. 2012; Yu et al. 2013). Expression profiling studies suggest that the organochlorine o,p-DDT (1 – 300 mg/kg) elicited estrogenic responses in uteri from immature ovariectomized mice and rats (Kwekel et al. 2013). However, pyrethroid metabolites (1 – 10 mg/kg) did not affect uterine weight in rats (Laffin et al. 2010).
Fertility
Several studies show that pesticide exposure is associated with reduced fertility in women and animal models. For example, 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB-153) and DDE exposure were associated with an increased risk of fetal loss in women (CB-153 odds ratio (OR): 2.4, 95% CI: 1.1, 5.5; DDE OR: 2.5, 95% CI: 0.9, 6.6) (Toft et al. 2010). Organochlorine pesticide exposure was associated with an increased time to pregnancy in women (fecundibility odds ratio (FOR): 0.46, 95% CI: 0.32, 0.66) (Chevrier et al. 2013). Furthermore, a mixture of organophosphate pesticides (43 and 107.5 mg/kg) (dichlorovos, dimethoate, and malathion) decreased pregnancy and live birth rates in Sprague-Dawley rats (Yu et al. 2013).
Reproductive Senescence
Limited data exist on the impact of pesticide exposure on reproductive senescence, but one cross-sectional showed that women with high levels of β-hexachlorocyclohexane and mirex had an earlier mean age at menopause compared to women with low levels of β-hexachlorocyclohexane and mirex (β-hexachlorocyclohexane average change in age of menopause β (SE) years: −0.07 (0.138); and mirex average change in age of menopause β (SE) year: −0.54 (0.084)) (Grindler et al. 2015). Another cross-sectional study showed that women with high serum levels of DDT, DDE, and β-hexachlorocyclohexane had an early age at menopause (5.7, 3.4, and 5.2 years earlier, respectively) (Akkina et al. 2004). Further, a case-control study showed that DDE exposure was borderline associated with an early age at natural menopause (OR: 1.4, 95% CI: 0.9, 2.1) (Cooper et al. 2002). Perinatal exposure to the organochlorine pesticide methoxychlor (20 μg/kg and 100 mg/kg) advanced the onset of reproductive senescence in rats (Gore et al. 2011). In contrast, the Agricultural Health Study showed that women who use pesticides have a later age at menopause (about 5 months) than women who do not use pesticides (hazard ratio: 0.77, 95% CI: 0.65, 0.92) (Farr et al. 2006).
Overall, several studies show that pesticide exposure impairs female reproduction by targeting a variety of reproductive tissues and function. Although the majority of studies report adverse effects of pesticide exposure, some studies report conflicting results. The divergent results may be due to inherent differences between animal models and humans, genetic variability between humans and animal strains/species, chemical exposures, and doses. Additionally, the mechanism of action for pesticide exposure should be further investigated to aid in the understanding the impact of chemical exposure on human and animal health.
Heavy Metals
Humans are exposed to a variety of heavy metals through several different routes such as cigarettes, alcoholic drinks, dietary supplements, and contaminated food, air, and water (Mathur and D’Cruz 2011). Specifically, arsenic (As) can be found naturally in the environment. Humans are generally exposed to As through ingestion of contaminated food and water. Some geographical areas have naturally higher levels of As in soil and ground water than others, resulting in an increased level of exposure for residents in that area (Ferguson et al. 2013). Lead (Pb) exposure can occur through inhalation of fossil fuel combustion products, drinking water contaminated by Pb used in pipes, and ingestion or inhalation of flakes of Pb-based paints (Ferguson et al. 2013). Human exposure to Pb via contaminated air and food is roughly equal (Rzymski et al. 2015). Mercury (Hg) is another heavy metal that can be found in air, soil, and fresh and salt waters. Human exposure to Hg can occur via inhalation as well as ingestion (Rzymski et al. 2015); however, the most common exposure route is ingestion of contaminated fish (Ferguson et al. 2013). Recent epidemiological and experimental studies focusing on the associations and the effects of heavy metal exposure on female reproduction are summarized in the sections below.
Hypothalamus and Pituitary
Information on heavy metal exposure and the effects on the hypothalamus and pituitary are scarce (Figure 1 and Figure 2). One study using data from 485 women who participated in the National Health and Nutrition Examination Survey (NHANES) reported an inverse association between inorganic Hg concentrations in the blood and LH levels (β coefficient: −0.0044, 95% CI: −0.0071, −0.0016; p=0.003) (Laks 2009). Similarly, exposure to Pb (0.05 mg/kg/day) decreased fluidity of the pituitary membrane in rats, a scenario that can impair secretion and receptor binding (Pillai et al. 2002). Further, sodium arsenite exposure (4 μg/mL and 0.4 ppm) via drinking water lowered levels of serum LH and follicle stimulating hormone (FSH) in rats (Chatterjee and Chatterji 2010; Chattopadhyay and Ghosh 2010). In contrast, Pb (0.05 mg/kg/day) exposure did not change circulating levels of LH, FSH, or dopamine in rats (Pillai et al. 2003).
Ovary
Epidemiological studies on the associations between heavy metal exposures and ovarian follicle numbers and health primarily focus on assisted reproductive technology outcomes (Figure 1 and Figure 2). One study reported that women undergoing IVF treatments in Taranto, Italy, an area known for environmental heavy metal contamination via industrial processes, have a significant elevation of follicular fluid concentrations of several heavy metals, including Pb (women in Taranto: 2.00 ± 2.01 vs. women outside Taranto: 0.68 ± 0.22; p=0.003). This study also found that women from Taranto have a significantly lower number of mature oocytes retrieved when compared to a control group living outside of Taranto (women in Taranto 6.7 ± 3.8 vs. women outside Taranto 9.5 ± 3.5; p=0.03) (Cavallini et al. 2016). Further, studies indicate that Hg concentration in hair is negatively correlated with oocyte yield (β: 0.38; p<0.05) and follicle number (β: 0.19; p=0.03) after ovarian stimulation (Dickerson et al. 2011) and that women with hair Hg concentrations above the EPA reference level of 1 ppm have significantly lower oocyte yields than those with hair Hg concentrations below 1 ppm (p=0.04) (Wright et al. 2015).
Few experimental studies examined the effects of heavy metal exposure on follicle numbers and follicle health (Figure 2). One study reported that exposure to sodium arsenite (0.4 ppm) via drinking water decreased ovarian weight and healthy follicle numbers and increased atresia in rats (Chattopadhyay and Ghosh 2010). Another study showed that dermal exposure to creams that contain high Hg levels caused significant accumulation of Hg in mouse ovaries (87.79 ± 26.20 ng/g and 3,515.61 ± 1,099.78 ng/g), which could alter reproductive outcomes (Al-Saleh et al. 2009).
Uterus
Limited data exist on the associations between heavy metal exposure and uterine outcomes (Figure 1 and Figure 2). Urinary levels of Pb and blood levels of Hg were significantly associated with fibroids in a sample of 99 women with fibroids and 374 control women (adjusted odds ratio (AOR): 1.31, 95% CI: 1.02, 1.69) (Johnstone et al. 2014). Sodium arsenite exposure via drinking water (4 μg/mL and 0.4 ppm) resulted in a decrease of uterine size, fewer invaginations of the uterine lumen, reduced height of luminal epithelial cells, fewer endometrial glands, and thinner myometrium in rats (Chatterjee and Chatterji 2010; Chattopadhyay and Ghosh 2010). Further, sodium arsenite exposure through drinking water (4 μg/mL) downregulated both mRNA expression and protein levels of ERα and reduced expression of vascular endothelial growth factor (VEGF), an estrogen responsive gene in the rat endometrium (Chatterjee and Chatterji 2010).
Fertility
Very few epidemiological studies have examined the associations between heavy metal exposure and fertility outcomes. Some studies reported associations between blood Pb levels and infertility (mean blood level of lead in women with unexplained infertility 130.0 ± 45.2 vs. mean blood level of lead in control women 78.3 ± 36.4 μ/L; p<0.001) (Rahman et al. 2013). Further, studies reported reduced fertility among dental health care workers who performed procedures that exposed them to Hg (fecundability ratio (FR): 0.63, 95% CI: 0.42, 0.96) (Colquitt 1995). Additionally, a significantly negative association was observed between blood Hg levels and fecundity in first time pregnant mothers (FR: 0.22, 95% CI: 0.07, 0.72) (Cole et al. 2006). Consistent with epidemiological studies, experimental studies showed that sodium arsenite in the drinking water (4 μg/mL and 0.4 ppm) caused constant diestrus in rats (Chatterjee and Chatterji 2010; Chattopadhyay and Ghosh 2010). In contrast, some studies reported no associations between blood levels of As, Pb, or Hg with fecundity (Bloom et al. 2011; Buck Louis 2014), infertility (Tanrikut et al. 2014), and fertilization rates in women undergoing IVF (Wright et al. 2015).
Although limited information exists on heavy metal exposure and fertility, several epidemiological studies showed associations between heavy metal exposure and adverse pregnancy outcomes. Blood and serum Pb levels were significantly higher in women with pre-eclampsia than in women without pre-eclampsia (blood Pb levels in women with pre-eclampsia: 37.68 ± 9.17 μg/dL vs. blood PB levels in women without pre-eclampsia: 14.5 ± 3.18 μg/dL; p<0.001; and serum Pb levels in women with pre-eclampsia: 27.18 ± 2.13 μg/dL vs. serum PB levels in women without pre-eclampsia: 18.23 ± 2.34 μg/dL; p<0.05) (Jameil 2014; Motawei et al. 2013). Further, high levels of As in drinking water and blood Pb levels were significantly associated with increased odds of spontaneous abortion (As in drinking water OR: 1.98, 95% CI: 1.27, 3.10 and blood Pb levels OR: 1.8, 95% CI: 1.1, 3.1 for every 5 μg/dL increase in blood Pb) (Borja-Aburto et al. 1999; Quansah et al. 2015). In contrast, some epidemiological studies reported no associations between maternal urinary As levels (Rahman et al. 2010) or maternal blood Pb levels (Sengupta et al. 2015) and adverse pregnancy outcomes.
Further, environmental As exposure though drinking water, maternal blood As, maternal hair As, and urinary As levels have been associated with infants being classified as low birth weight, small for gestational age, or having a comparatively lower birthweight levels (maternal hair As levels β: −193.5 ± 90.0; p=0.04 and urinary As levels (urinary monomethylarsonic acid (U-MMA) β: −24.4; 95% CI: −46.8, −2.0; p=0.03) (Bloom et al. 2014; Huyck et al. 2007; Laine et al. 2015). Further, environmental exposure to Hg and maternal blood Hg levels were significantly associated with reduced birth weight and low birth weight (0.29 – 0.62 ppm Hg in fish OR: 1.06, 95% CI: 1.02, 1.10; > 0.62 ppm Hg in fish OR: 1.04, 95% CI: 1.00, 1.09; total Hg blood levels 95% CI: −1.37, −0.13; inorganic mercury (iHg) 95% CI: −0.74, −0.08; and > 1.6 μg/L blood Hg level 95% CI: 1.04, 2.58) (Burch et al. 2014; Ou et al. 2015; Thomas et al. 2015). Similarly, high As levels in drinking water, urinary levels of As, (Laine et al. 2015) maternal blood Pb levels, placental Pb levels, maternal environmental Hg exposure, maternal hair Hg levels, and cord blood Hg levels were significantly associated with shortened gestation or preterm birth (As levels in drinking water p=0.018; urinary As levels −0.069 weeks gestation per unit increase in urinary inorganic arsenic (iAS), 95% CI: −0.13, −0.0043; p=0.03; maternal blood Pb levels OR: 5.51, 95% CI: 1.21, 25.15 for male infants only; > 0.17 – 0.29 ppm Hg in fish OR: 1.09, 95% CI: 1.06, 1.13; > 0.29–0.62 ppm Hg in fish OR: 1.09, 95% CI: 1.05, 1.13; maternal hair Hg levels AOR: 3.0, 95% CI: 1.3, 6.7; and cord blood Hg levels; p≤0.05) (Ahmad et al. 2001; Bloom et al. 2014; Burch et al. 2014; Dallaire et al. 2013; Ferguson et al. 2013; Perkins et al. 2014; Xue et al. 2007). In contrast, one study conducted in Inner Mongolia, China reported that infants born in areas with the highest levels of As (> 100 μg/L) were heavier on average when compared to those born in the lowest levels of As (< 20 μg/L) (Myers et al. 2010). However, several studies reported no associations between maternal As exposure via drinking water (Bloom et al. 2014; Ferguson et al. 2013), maternal blood As levels (Bermudez et al. 2015; Thomas et al. 2015), cord blood As levels (Bermudez et al. 2015), maternal urinary levels of As or Hg (Bashore et al. 2014; Laine et al. 2015; Ou et al. 2015), maternal blood Hg levels (Al-Saleh et al. 2014), cord blood Hg levels (Al-Saleh et al. 2014; Bashore et al. 2014), maternal urinary Pb levels (Sun et al. 2014), maternal blood Pb levels (Al-Saleh et al. 2014; Sun et al. 2014; Thomas et al. 2015), or cord blood Pb levels (Al-Saleh et al. 2014; Torres-Sanchez et al. 1999) and infant birthweight or incidence of low birth weight or small for gestational age infants.
Overall, heavy metal exposure has been shown to interfere with female reproduction in both experimental and epidemiological studies. Although some epidemiological studies agree with each other, some show conflicting results. The contrasting results seen between epidemiological studies may be due to differing sample sizes, use of different tissue types to measure heavy metals, and/or genetic variation between populations that were sampled in each study. The animal studies report similar findings, but different heavy metal exposures often elicit different results.
Diethylstilbestrol
Diethylstilbestrol (DES) is a non-steroidal estrogen that was first synthesized in 1938. DES use was approved by the United States Food and Drug Administration and was prescribed to pregnant women until the 1970s to prevent spontaneous abortions (Giusti et al. 1995). The use of DES as an anti-abortive drug was based on the assumption that DES, which had biological properties similar to estrogen, would restore hormonal balance in the pregnant mother. In turn, this would theoretically result in reduced complications of pregnancies and prevent miscarriages (Smith and Smith 1949). Unfortunately, prenatal exposure to DES has been shown to impair female reproductive tract development and increase breast cancer risk in offspring (Laitman 2002; Newbold et al. 2007; Tournaire et al. 2015). However, the effects of adult exposure to DES are less well studied, but are summarized below.
Hypothalamus and Pituitary
Currently, no information to our knowledge exists on the effects of adult exposure to DES on the hypothalamus and pituitary in women (Figure 1). However, daily exposure to DES (200 μg/kg body weight/day) suppressed serum LH and FSH levels in mice (Jaroenporn et al. 2007). Exposure to DES (5 mg/kg) also caused structural abnormalities in the pituitary gland such that the gland cavity size was decreased, but contained proliferated, tumor-like cells in rats (Zhao et al. 2010).
Ovary
Multiple studies demonstrated consistent effects of exposure to DES in adulthood on the ovary (Figure 1 and Figure 2). A common result seen from DES exposure (5 μg/g body weight and 50 – 2000 ppb) was a complete loss, degeneration, or a decrease in the number of corpora lutea in the adult ovary, suggesting that DES impaired ovulation (Hong et al. 2010; McAnulty and Skydsgaard 2005; Zhao et al. 2014). Additionally, DES exposure (200 μg/kg body weight/ day) decreased the numbers of primary, secondary, and pre-ovulatory follicles and increased atretic antral follicles in mice (Jaroenporn et al. 2007). DES (5 μg/g body weight and 250 – 2000 ppb) also caused atrophy of the ovary as well as the thickening and scarring of ovarian connective tissues in mice (Hong et al. 2010; McAnulty and Skydsgaard 2005). Further, DES (5 μg/g body weight) impaired reproductive activity in females by decreasing the maturation of ovarian follicles in mice (Hong et al. 2010).
Uterus
DES exposure during adulthood impairs the uterus (Figure 1 and Figure 2). Specifically, DES exposure (5 μg/g body weight) increased Sdd2 mRNA expression, which promoted programed cell death, and increased Psat1 mRNA expression, which inhibits uterine neoplasia (Hong et al. 2010). Further, adult exposure to DES (200 μg/kg body weight/ day) increased the thickening of the uterine endometrium, increased dilatation of uterine glands, and increased secretory proteins in mice (Jaroenporn et al. 2007). Additionally, DES exposure (250 – 2000 ppb) increased endometrial glandular hyperplasia and caused accumulation of hyaline cartilage in the endometrium of mice (McAnulty and Skydsgaard 2005). Finally, DES exposure (50 ppb) caused mouse uterine horns to become distended (Zhao et al. 2014).
Estrous Cyclicity and Fertility
DES exposure alters estrous cyclicity and fertility in animal models. Specifically, daily DES treatment (200 μg/kg body weight/ day) caused cornification of the vaginal epithelium (Jaroenporn et al. 2007) and it (250 – 2000 ppb) caused keratinization of the vagina (McAnulty and Skydsgaard 2005). Further, it (200 μg/kg body weight/day, 5μg/g body weight, and 50 ppb) decreased female fertility in mice by interfering with embryo transport, implantation, uterine receptivity, and preimplantation embryo development (Hong et al. 2010; Jaroenporn et al. 2007; Zhao et al. 2014).
Overall, DES exposure in adulthood has been shown to impair female reproductive outcomes in experimental studies. However, it has not been extensively studied in adult animals or women and the mechanism of action remains unclear. Thus, additional studies are needed to further elucidate the mechanism by which DES impairs female reproduction in adult animals and women.
Plasticizer Alternatives
Phthalates and bisphenol A (BPA) are plasticizers used to confer flexibility to plastic products. One of the most common phthalates, di-(2-ethylhexyl) phthalate (DEHP), leaches out of products and causes toxic effects. The use of DEHP has been challenged by European authorities because of its toxic properties (Bernard et al. 2014). Therefore, manufacturers began to replace DEHP with alternative plasticizers such as tri-2-ethylhexyl trimelliate (TETM), di-(2-ethylhexyl) terephthalate (DEHT), di-(isonyl)cyclohexanedicarboxylic acid (DINCH), di-isononyl phthalate (DINP), di-(2-ethylhexyl) adipate (DEHA), and acetyl tri-n-butyl citrate (ATBC). Another plasticizer, BPA, is a known reproductive toxicant and endocrine disrupting chemical (Peretz et al. 2014; Ziv-Gal and Flaws 2016). Within the last decade some restrictions have been placed on the use of BPA in children’s products and thermal receipt paper (Usman and Ahmad 2016), leading to the development of alternative bisphenol plasticizers such as bisphenol S (BPS), bisphenol B (BPB), bisphenol F (BPF), and bisphenol AF (BPAF) (Liao and Kannan 2014). These alternative bisphenols are structurally similar to BPA and are thought to have equivalent toxicological effects (North and Halden 2013), but this has not been studied in detail. The section below highlights the effects of DEHP and BPA alternatives on the hypothalamus, pituitary, ovary, uterus, fertility, and reproductive senescence in mammalian models.
Hypothalamus and Pituitary
Limited information is available on the effects of plasticizer alternatives on the adult mammalian hypothalamus and pituitary (Figure 1 and Figure 2). One study showed that the BPA alternative, BPF (20 – 500 mg/kg/day), did not alter histopathology in the rat pituitary (Higashihara et al. 2007).
Ovary
Few published studies report the effects of plasticizer alternatives on the adult mammalian ovary (Figure 1 and Figure 2). DEHA (1,000 and 2,000 mg/kg/day), a phthalate alternative, increased atresia in preantral and antral follicles (Miyata et al. 2006; Wato et al. 2009). ATBC (5 and 10 mg/kg/day), a phthalate alternative, decreased the numbers of primordial, primary, and secondary follicles, but did not alter the numbers of corpora lutea or atretic follicles in mouse ovaries (Rasmussen et al. 2016). In contrast the BPA analogue, BPF (20 – 500 mg/kg/day), did not cause histopathological changes in the rat ovary (Higashihara et al. 2007).
Uterus
Few studies have investigated the effects of BPA alternatives on the uterus (Figure 1 and Figure 2), but one study reported that BPF exposure (100 – 1,000 mg/kg/day) induced uterine growth in rats, indicating estrogenic activity (Rochester and Bolden 2015). Similarly, BPS (20 and 500 mg/kg/day) exhibited estrogenic activity by increasing the rate of uterine growth in rats, possibly through a mechanism involving estrogen receptors (Rochester and Bolden 2015). In contrast, a different study reported that BPF (20 – 500 mg/kg/day) did not cause histopathological changes in the rat uterus (Higashihara et al. 2007).
Fertility
Several studies show that plasticizer alternatives affect fertility in females. Exposure to ATBC (100 – 1,000 mg/kg/day), a phthalate alternative, decreased the number of implantation sites and litter size in rats (CPSC 2010), but it (5 – 10 mg/kg/day) did not affect implantation rates or litter size in mice (Rasmussen et al. 2016). Another phthalate alternative, DEHA (400 – 1080 mg/kg/day), caused prolonged pregnancies, smaller pups, and increased pup mortality rates in rats (CPSC 2010; Dalgaard et al. 2003). Additionally, DEHA (2,000 mg/kg/day) increased preimplantation loss rates in rats (Wato et al. 2009). In contrast, TETM (100 – 1,000 mg/kg/day), another phthalate alternative, did not affect the reproductive ability of maternal dams, viability or body weights of offspring (Van Vliet et al. 2011). Similarly, DINCH (1,000 mg/kg body weight/day), a phthalate alternative, did not cause maternal toxicity in rats (as reviewed in Van Vliet et al. 2011). DINP exposure (300 – 900 mg/kg BW/day) during pregnancy did not cause changes in maternal body weight gain during pregnancy, gestational length, postimplantation loss, litter size, sex ratio, or perinatal loss in rats (Boberg et al. 2011). BPF exposure (20 – 500 mg/kg/day) did not alter estrous cycles in rats (Higashihara et al. 2007). In contrast, DEHA (1,000 and 2,000 mg/kg/day) decreased average estrous cycle length in rats (Wato et al. 2009) and ATBC (5 mg/kg/day) decreased average estrous cycle length in mice (Rasmussen et al. 2016).
Overall, information regarding the effects of plasticizer alternatives for DEHP and BPA on female reproduction is scarce. From the limited information presented, there are conflicting results. The differing results are primarily due to the use of different chemicals, varying doses, and use of different animal models. There is a push to replace DEHP and BPA products with plasticizer alternatives, however, the lack of information on these plasticizer alternatives is concerning. Thus, there is a need for studies to examine the effects of plasticizer alternatives on female reproduction.
TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is perhaps the most potent polychlorinated dibenzodioxin and it has a long biological half-life of 2 – 5 years (Tavakoly Sany et al. 2015). TCDD is formed as a by-product during organic synthesis and burning, and it is a persistent environmental contaminant. Humans have been exposed to TCDD accidentally through contamination from Agent Orange during the Vietnam War and through an industrial explosion in Seveso, Italy. Both accidents resulted in high and long term TCDD exposure in humans. Recent studies indicate human and animal exposure to TCDD still occur (Tavakoly Sany et al. 2015). In this section, we briefly summarize recent research findings on the effects of adult exposure to TCDD on female reproduction.
Hypothalamus and Pituitary
Studies have shown that exposure to TCDD in adulthood can affect the hypothalamus and pituitary (Figure 2). In rats, exposure to TCDD (0.3 – 60 μg/kg/day) reduced preovulatory peak concentrations of FSH and LH, however, the mechanism is not clear (as reviewed in Petroff et al. 2001). In pig pituitary cell cultures, exposure to TCDD (100 nmol/L) altered the secretion of prolactin during both the follicular and luteal phases and induced LH secretion during the follicular phase (Jablonska et al. 2011).
Ovary
Studies have shown that TCDD can adversely affect adult ovaries (Figure 1 and Figure 2). Exposure to TCDD (5 – 200 ng/kg/week, 32 μg/kg/day) caused a reduction in ovarian weight, inhibition of estradiol production, and reduced follicular maturation and ovulation in rats (as reviewed in Bhattacharya and Keating 2012; Petroff et al. 2001). TCDD (20 – 125 ng/kg/day in vivo; 0.1 nM – 10 μM in vitro) also altered the expression of several enzymes and hormone receptors involved in the ovarian steroidogenesis pathway in both animal and human studies (Hutz et al. 2006; Karman et al. 2012a; Karman et al. 2012b; Patel et al. 2015; Petroff et al. 2001).
Uterus
Adult exposure to TCDD induces adverse effects on uterine tissues (Figure 2). Acute and chronic TCDD exposure (100 ng/kg/day) induced uterine tissue inflammation and increased the incidence of uterine lesions in rats (Yoshizawa et al. 2009). TCDD (30 μg/kg) also caused anti-uterotrophic effects in ovariectomized mice by inhibiting estrogen-mediated gene expression (Boverhof et al. 2008). Moreover, TCDD (30 μg/kg/day) inhibited estradiol-mediated uterine epithelial function via the aryl hydrocarbon receptor (Buchanan et al. 2000). During implantation, exposure to TCDD (1 and 10 μg/kg/day) decreased the number of successfully implanted embryos in mice (Kitajima et al. 2004).
Fertility
Adult TCDD exposure can reduce fertility. The comprehensive Seveso Women’s Health Study (SWHS) was conducted to examine the association between TCDD exposure and female fertility after an explosion in Italy exposed residents to high levels of TCDD (Eskenazi et al. 2000). This study found that TCDD exposure was associated with a longer time to pregnancy and infertility (time to pregnancy adjusted fecundability odds ratio (AFOR): 0.75, 95% CI: 0.60, 0.95; and infertility AOR: 1.9, 95% CI: 1.1, 3.2) (Eskenazi et al. 2010). In addition, another SWHS study showed that within the first 8 years after TCDD exposure in Italy, maternal serum TCDD levels were associated with lower birth weight and smaller gestational age (birth weight adjusted β: −92, 95% CI: −204, 19; and gestational age AOR: 1.4, 95% CI: 0.6, 2.9) (Eskenazi et al. 2003). In animal studies, TCDD (2 μg/kg body weight/day and 10 μg/kg) interfered with fertility parameters in mammalian species, including estrous cyclicity, time to pregnancy, maintenance of pregnancy, fetal development, and birth outcomes (as reviewed in Bhattacharya and Keating 2012; Hutz et al. 2006).
Reproductive Diseases and Senescence
TCDD exposure during different exposure windows has been associated with the occurrence of endometriosis in rodents, monkeys, and humans (Foster 2008; Rier 2002). In a recent epidemiology study, adult TCDD exposure was associated with an increased incidence of endometriosis (OR: 2.44, 95% CI: 1.04, 5.70; p=0.04) (Simsa et al. 2010). Moreover, in the SWHS study, serum TCDD levels were associated with an increased risk of early menopause in some women (20.4–34.2 ppt adjusted hazard ratio (AHR): 1.1, 95% CI: 0.7 – 1.8; p=0.77; 34.3–54.1 ppt AHR: 1.4, 95% CI: 0.9 – 2.3; p=0.14; 54.2–118 ppt AHR: 1.6, 95% CI: 0.9 – 2.6; p=0.10; and > 118 ppt AHR: 1.1, 95% CI: 0.6 – 1.9; p=0.82) (Eskenazi et al. 2005).
Several recent studies show that humans and animals are still exposed to dioxin in various ways (Tavakoly Sany et al. 2015). Available studies have shown that dioxins are reproductive toxicants and can interfere with several reproductive organs in females. These studies consistently show adverse effects of dioxins in women and animal models. Studies, however, are still needed to examine the potential and mechanisms of dioxin to human and animal health.
Nonylphenol
Nonylphenols (NP) are organic compounds with a nine-carbon alkyl chain bound to a phenol ring. NP is commonly used in pesticides, lubricating oils, and laundry or dish washing detergents. The demand for NP in 2010 was 380 million pounds in the United States (Careghini et al. 2015). Because of the widespread use of NP, it is present in soil and sediments, groundwater and surface water, food, and bottled water (Careghini et al. 2015). NP is a mixture of more than 100 isomers, but 4-NP makes up over 90% of the NP (Lu and Gan 2014).
Hypothalamus and Pituitary
Limited information is available on the effects of NP on the hypothalamus and pituitary (Figure 1 and Figure 2). In a Taiwanese study, 162 singleton pregnant women were recruited for a study designed to examine the association of urinary NP levels and sex steroid hormone levels in pregnant women (Chang et al. 2014). The results showed that urinary NP levels were negatively associated with maternal plasma LH levels, suggesting compromised LH negative feedback in the hypothalamus-pituitary-ovary axis during pregnancy (generalized estimating equation model, β: −0.23; p<0.01) (Chang et al. 2014). In adult ovariectomized rats, NP (10 mg injection) increased progesterone receptor mRNA in anterior pituitary, decreased the amplitude of LH pulses and mean LH levels without affecting LH pulse frequency, and attenuated LH secretory responsiveness to GnRH stimulation at the anterior pituitary level (Furuta et al. 2006).
Ovary and Uterus
Very few studies have been conducted on the effects of NP exposure on female reproductive organs (Figure 2). In a study that focused on the progesterone production by ovarian granulosa cells of rats, exposure to NPs (13 and 43 μM and 100 μg/kg/day) significantly increased progesterone production by increasing steroidogenic acute regulatory protein expression in both in vitro and in vivo experiments (Yu et al. 2011). In guinea pigs, NP administration (40 mg/kg/day) prevented uterine weight decline after castration and induced weak estrogenic effects on uterine tissue to maintain relatively normal histology in castrated animals (Danzo et al. 2002). Similarly, in adult ovariectomized rats, NP exposure (50 mg/kg) increased uterine weight during the 3-day uterotrophic assay (Laws et al. 2000). Further, one study showed that the uterotrophic action of NP is a direct result of interaction of NP (2.5 mg/kg) and uterine estrogen receptors in rats (Odum et al. 2001).
Fertility
Information on the effects of NP on female fertility is limited. Exposure to NPs has been associated with negative birth outcomes in humans, but the results are inconsistent. High maternal NP exposure during the second trimester has been associated with reduced gestational age, decreased birth body length and birth weight, and lower maternal weight gain (gestational age OR: 7.8; p<0.05; body length β: −0.47; p=0.04; birth weight OR: 1.18 for the 50th percentile, 2.12 for the 25th percentile, and 7.81 for the 10th percentile; and maternal weight gain: β: −1.55; p=0.02) (Chang et al. 2013; Tsai et al. 2013). However, a Chinese population-based study did not find any associations between NP exposure and negative birth outcomes (Tang et al. 2013). In animal studies, NP exposure (100 mg/kg) for 25 days significantly disrupted estrous cyclicity in rats (Laws et al. 2000).
Although NPs have been shown to be estrogenic and to have endocrine disrupting characteristics (Lu and Gan 2014; Ponzo and Silvia 2013), the effects of adult exposure to NPs on female fertility are not well studied. Given the wide use of NPs and their frequent detection in the environment (Lu and Gan 2014), more information is needed to determine the toxicity of NPs on female reproduction, especially their mechanisms of action.
Polychlorinated Biphenyls
Polychlorinated biphenyls (PCBs) are organochlorine compounds that were once widely manufactured worldwide for industrial use (Fernandez-Gonzalez et al. 2015). Although their production in the United States was banned in the 1970s and their use today is highly controlled, PCBs persist in the environment and accumulate in the food web (Bell 2014; Fernandez-Gonzalez et al. 2015). Different PCB congeners have been associated with reproductive dysfunction in females. The following sections summarize the effects of adult exposure to PCBs on the mammalian hypothalamus, pituitary, ovary, uterus, as well as fertility and reproductive senescence.
Hypothalamus and Pituitary
Current information is very limited on the effects of adult exposure to PCBs on the hypothalamus and pituitary (Figure 2). In vitro data suggest that PCB congeners (0.01 – 100 μM) caused an increase and then a decrease in mRNA and peptide levels of GnRH in hypothalamic mouse cells, and increased apoptosis of hypothalamic GnRH containing cells in a non-monotonic manner in mice (Bell 2014).
Ovary
Although PCB congeners have been shown to be present in the ovarian follicular fluid of women (0.37 /g wet weight), limited information is available on their effects on the human ovary (Figure 1) (Craig et al. 2011). One study found that contamination of human follicular fluid with endocrine disrupting chemicals including PCBs was associated with a decreased rate of in vitro fertilization and oocyte development into high-quality embryos (Petro et al. 2012). Studies in different experimental models and animals show that PCBs are able to alter ovarian steroidogenesis and oocyte health. For example, PCBs (1–100 ng/mL) decreased LH-stimulated luteal phase secretion of progesterone in bovine luteal cells, and altered the secretion of progesterone, testosterone, and estradiol in porcine ovarian follicular cells in vitro (Craig et al. 2011). Exposure to PCBs (12.5 – 50 mg/kg) also inhibited maturation and parthenogenetic activation of mouse oocytes in vivo, and increased apoptosis of cumulus cells (Liu et al. 2014). Additionally, PCB exposure in free-living female polar bears in Norway has been linked to decreased plasma levels of androstenedione and pregnenolone (Gustavson et al. 2015). Specifically, several hydroxylated PCBs were negatively associated with androstenedione levels: 3′-OH-CB180 (rs=−0.626, p=0.022), 3′-OH-CB138 (rs=−0.791, p=0.001), 4-OH-CB187 (rs=−0.626, p=0.022) and 4′-OH-CB172 (rs=−0.665, p=0.013), PCB-128 (rs=−0.543, p=0.037). Additionally, 4-OH-CB146 (rs=−0.643, p=0.018) and 4′-OH-CB172 (rs=−0.582, p=0.037) were negatively associated with pregnenolone levels (Gustavson et al. 2015).
Uterus
Very limited information is available on the effects of PCBs on uterine structure and function (Figure 1 and Figure 2). Chronic oral exposure to PCB congeners (1,000 and 4,600 μg/kg) is associated with an increased incidence of uterine squamous cell carcinoma in adult female rats (NTP 2010; Yoshizawa et al. 2009). Chronic oral exposure to PCBs (300 ng/kg – 3,000 μg/kg) also induced chronic active inflammation in rat uteri (Yoshizawa et al. 2009). A study of bovine reproductive tissues found that PCB exposure (1 – 100 ng/mL) increased myometrial contractility in vitro (Kotwica et al. 2006).
Fertility
Studies indicate that PCB exposure is associated with subfertility in women and animals. For example, a moderate to high PCB exposure index in women consuming contaminated fish is associated with shortened menstrual cycles (−1.03 days; 95% CI −1.88, −0.19) (Mendola et al. 1997). Exposure to different categories and congeners of PCBs in women has been consistently associated with a shorter duration of gestation (Dallaire et al. 2013; Kezios et al. 2012). For example, exposure to mono-ortho substituted PCBs is associated with a 2.1 day decrease in gestational length, whereas exposure to di-ortho substituted PCBs is associated with a 1.4 day reduction in gestational length (mono-ortho substituted PCB 95% CI: −4.13, −0.11; and di-ortho substituted PCB 95% CI: −2.9, 0.1) (Kezios et al. 2012). Several studies show that PCB exposure is also associated with diminished couple fecundity as measured by time to pregnancy, with a fecundability odds ratio < 1.0 denoting significantly reduced fecundability (reviewed in Buck Louis 2014). Certain PCB congeners may be associated with endometriosis (reviewed in Leon-Olea et al. 2014). Mono-ortho PCBs are associated with anovulation (OR: 2.36, 95% CI: 1.06, 5.28) (Gallo et al. 2016). Further, several PCB congeners are associated with uterine fibroids in women (Trabert et al. 2015). For instance, PCB 99, PCB 138, PCB 146, PCB 153, PCB 196, and PCB 206 have been significantly associated with uterine fibroids (PCB 99 OR: 1.64, 95% CI: 1.08, 2.49; PCB 138 OR: 1.64, 95% CI: 1.03, 2.59; PCB 146 OR: 1.54, 95% CI: 1.01, 2.37; PCB 153 OR: 1.88, 95% CI: 1.12, 3.13; PCB 196 OR: 1.60, 95% CI: 1.02, 2.51; and PCB 206 OR: 1.52, 95% CI: 1.01, 2.29) (Trabert et al. 2015). Similarly, PCBs may be associated with reproductive abnormalities in exposed marine mammal populations. A study from the United Kingdom shows that PCB exposure (6 – 18.5 mg/kg) may be associated with abortion, dystocia, still birth, and increased risk of reproductive disease in porpoises (Murphy et al. 2015).
Reproductive Senescence
Limited information is available on the effect of PCBs on reproductive senescence, but one study in rats found that prenatal exposure to a PCB mixture (1 mg/kg) increased estrous cycle lengths throughout the life-cycle, suggestive of an aging phenotype (Walker et al. 2013).
In conclusion, there is currently limited information available from experimental studies about the impact of PCBs on the female reproductive system. More studies are needed to fully understand the effects and mechanism of action of different PCB congeners on the hypothalamus, pituitary, ovary, and uterus. This is especially important because there are well-documented associations between PCBs and subfertility in women and other mammalian species (Buck Louis 2014; Gallo et al. 2016; Mendola et al. 1997; Murphy et al. 2015), although it is difficult to ascertain whether these associations are a direct cause-effect relationship. Further investigation is needed of these associations and should include efforts to relate PCB exposure in human and animal populations to effects observed in laboratory studies.
Triclosan
Triclosan (5-chloro-2-(2,4-dichlorophenoxy) phenol) is an anti-bacterial agent that has been used in the United States for over 40 years. It is found in many personal care products and consumer products including anti-bacterial soap, mouthwash, toothpaste, surgical scrubs, and sutures (Fang et al. 2010). As of 2002, over 1,500 tons of triclosan were produced annually around the world (Dann and Hontela 2011). Triclosan usually enters the body by ingestion or dermal contact, but it has also been shown to enter the body from inhalation of products including spray deodorants or air fresheners (Yang et al. 2015). The different sections below summarize the effects of triclosan on female reproduction.
Hypothalamus, Pituitary, Ovary and Uterus
No information to our knowledge is available on the effects of triclosan on the hypothalamus and pituitary (Figure 1 and Figure 2). Further, very little information is available on the effects of triclosan on the ovary and uterus (Figure 1 and Figure 2). Dermal triclosan exposure (125 mg/kg body weight/day) decreased ovary weights in adult mice (Fang et al. 2015), but aerosol inhalation of triclosan (0 – 0.40 mg/L) did not affect ovary weights in rats (Yang et al. 2015). Subcutaneous injections of triclosan (18 and 27 mg/day) decreased the number of implantation sites in the uteri of adult rats (Crawford and Decatanzaro 2012). Further, triclosan exposure (600 mg/kg/day) decreased gravid uterine weights in rats (Feng et al. 2016). Triclosan exposure decreased sex steroid hormone levels in rats. Specifically, pregnant rats exposed to triclosan (30 – 600 mg/kg/day) through oral gavage decreased progesterone, estradiol, testosterone, and prolactin levels (Feng et al. 2016).
Fertility
Few studies are currently available on the effects of triclosan on human and animal fertility. Triclosan exposure has been associated with a reduction in fecundity, suggested by an increased time to pregnancy (FOR: 0.84, 95% CI: 0.72, 0.97) (Velez et al. 2015). Triclosan in drinking water (1 – 50 mg/kg/day) decreased live birth index and 6-day survival index in rats (Rodriguez and Sanchez 2010). Further, triclosan (10 and 100 mg/kg) increased fetal loss rate percentage, increased abortion rate, and decreased the number of live fetuses in mice (Wang et al. 2015).
Overall, very few studies have examined the effects of triclosan on adult female reproduction in humans and other mammals. Although some studies report similar effects of triclosan exposure, others show conflicting results. The differing results between studies may be due to the use of different animal models or exposure routes. Because triclosan can be found in consumer products and personal care products, more studies are needed to determine whether and how triclosan exposure affects female reproduction.
Parabens
Parabens are a group of alkyl esters of p-hydroxybenzoic acid that are used as antimicrobial preservatives in personal care products and different foods. Besides being found in personal care products and food products, parabens are found in indoor dust. One study examined samples from the United States, China, Korea, and Japan discovered that the average paraben daily intake via ingestion of dust was 0.2 – 1.2 ng/kg/day (Wang et al. 2012). Estimated daily human exposure to parabens via personal care products was 5 – 50 μg/kg/day for adults and 15 – 230 μg/kg/day for infants and toddlers (Guo and Kannan 2013). The sections below discuss the known effects of parabens on female reproduction.
Hypothalamus, Pituitary, Ovary, and Uterus
To our knowledge, no information is available on exposure to parabens on the hypothalamus and pituitary and little information is available on the effects of paraben exposure during adulthood on the ovary and uterus (Figure 1 and Figure 2). In humans, increased levels of propylparaben (87.8 – 727 μg/L) were associated with a trend of lower antral follicle counts (estimated mean percent change in antral follicle count −16.3, 95% CI: −30.8, 1.3; p=0.07) (Smith et al. 2013). A study of Japanese university students showed that higher urinary estrogen-equivalent total paraben and butyl paraben concentrations were associated with shortened menstrual cycles (total paraben AOR: 0.73, 95% CI: 0.56, 0.96; p=0.027; and butyl paraben AOR: 0.83, 95% CI: 0.70, 0.99; p=0.037) (Nishihama et al. 2016). Further, subcutaneous exposure of multiple parabens (6 – 210 mg/kg) increased uterine weight in ovariectomized mice (Lemini et al. 2003) and implants containing isobutylparaben (approximately 4.36 mg/L/day) increased dam uterine weights in rats (Kawaguchi et al. 2009).
Fertility
To date, few studies are available on the effects of parabens on animal and human fertility. In humans, maternal paraben exposure was positively associated with birth weight (β: 193, (−4 – 389)) (Philippat et al. 2014). A study examining a variety of parabens in pregnant mothers associated paraben concentrations with increased odds of preterm, decreased gestational age at birth, decreased birth weight, and decreased body length (butylparaben preterm birth OR: 60.77, 95% CI: 2.60, 1417.93; butylparaben gestational age β: −3.04, 95% CI: −5.09, −0.99; butylparaben birth weight β: −480.40, 95% CI: −976.68, 15.89; propylparaben body length β: −1.06, 95% CI: −2.06, −0.05) (Geer et al. 2017). In another study, increased amounts of butylparaben in urine were associated with decreased estradiol, along with a decreased ratio of estradiol/progesterone in women (butylparaben estradiol 95% CI: 16.92, 0.00; butylparaben estradiol/progesterone 95% CI: 18.31, 0.38) (Aker et al. 2016). Further, female urinary concentrations of methylparaben and ethylparaben were associated with decreased fecundity in women (methyl paraben FOR: 0.72, 95% CI: 0.51,1.03 and AFOR 0.66, 95% CI: 0.45, 0.97; and ethylparaben FOR: 0.66, 95% CI: 0.47, 0.93 and AFOR: 0.66, 95% CI: 0.46, 0.95) (Smarr et al. 2016).
Oral exposure to methylparaben (0.1050 mg/kg/bw) increased litter size, however, it also increased pup mortality from postnatal day (PND) 7 and onwards in rats, possibly due to histological abnormalities in the mammary glands of the dams (Manservisi et al. 2015). Another study reported that butylparaben (100 – 200 mg/kg) exposure during pregnancy increased the proportion of live pups, and decreased the proportion of pups surviving until weaning in rats (Kang et al. 2002). Further, butylparaben exposure (160 – 1000 mg/kg/day) decreased the percent of males and caused a trend of reduced live birth rates in rats (Zhang et al. 2014).
To date, relatively little information is available on the impact of adult exposure of parabens on human and other mammal reproduction. Many studies have focused on birth outcomes, but given the limited information, additional studies are needed to determine whether paraben exposure affects reproductive outcomes.
Summary and Future Directions
Overall, the current literature shows that adult exposure to pesticides, heavy metals, diethylstilbestrol, DEHP and BPA alternatives, TCDD, nonylphenol, polychlorinated bisphenols, triclosan, and parabens may be associated with deleterious effects on adult female reproduction. Epidemiological data show that the selected EDCs are associated with adverse fertility outcomes such as reduced gestational age, weight, pregnancy gain, increased risk for miscarriage, and time to pregnancy in women, but some of these studies have limited sample sizes, exposure information, and/or outcome data. Experimental data show that EDC exposure during adulthood caused a range of effects such as estrous cyclicity abnormalities, decreased pregnancy rates, decreased pup survival, and increased onset of reproductive senescence. However, limited information is available on the mechanisms by which these selected chemicals impair reproduction. An understanding of the mechanisms of action of EDCs effects on adult female fertility is important to better understand environmentally-induced reproductive disease. Some studies report that EDCs exert toxicity through estrogen receptors, aryl hydrocarbon receptors, and peroxisome proliferator-activated receptor activation mechanisms, but they have not examined other potential pathways (Hannon and Flaws 2015). The doses reported in various studies vary greatly and the complete dose-response relationships of several chemicals are unclear. It is important to include a wide range of doses in studies to better understand the impact and mechanism of action of EDCs on female reproductive outcomes. Further work should be done to examine the direct effects of EDCs on the hypothalamus, pituitary, ovary, and uterus using environmentally relevant doses of chemicals because these organs regulate female fertility and the onset of reproductive senescence. EDC effects on these organs could be involved in the underlying mechanisms by which EDCs cause reproductive abnormalities and diseases.
Collectively, the data presented in this review can be categorized by the strength of evidence presented.
Strong evidence exists that:
Pesticide exposure during adulthood impaired ovarian follicular health in animal models, decreased ovarian sex steroid hormone production in animal models, reduced fertility in animal models, and was associated with reduced sex steroid hormone production, fertility, and a decreased mean age at menopause in women.
Heavy metal exposure was negatively associated with ovarian follicular health, fecundity, and pregnancy outcomes in women.
DES exposure during adulthood suppressed gonadotropin secretion and caused structural abnormalities in the pituitary, decreased ovarian follicular health, impaired uterus structure, and reduced fertility in animal models.
DEHP and BPA alternative exposure during adulthood decreased fertility in several animal models.
TCDD exposure during adulthood impaired ovarian sex steroid hormone production, reduced ovarian follicular maturation, and disrupted uterine functions in animal models. Adult human exposure to TCDD was associated with decreased fertility, time to pregnancy, and endometriosis in women.
Nonylphenol exposure during adulthood disrupted ovarian sex steroid hormone production and induced estrogenic effects in the uterus in several animal models.
PCB exposure during adulthood altered ovarian steroidogenesis and oocyte health, increased incidence of uterine squamous cell carcinoma, and increased uterine inflammation in animal models. Adult exposure to PCBs were associated with subfertility, endometriosis, and uterine fibroids in women.
Triclosan exposure during adulthood decreased sex steroid hormone levels and the number of live fetuses in animal models.
Paraben exposure was associated with decreased serum sex steroid hormone levels and decreased fecundity in women.
Limited evidence exists that:
Pesticide exposure during adulthood affects the hypothalamus and pituitary in animal models and women. Pesticide exposure increased GnRH mRNA levels, suppressed LH pulse frequency, and inhibited LH release in animal models, however, human data are lacking.
Heavy metal exposure during adulthood decreased serum LH levels in animal models, but no information is available on human exposure.
DES exposure during adulthood impairs gonadotropin secretion in animal models, however, there is a lack of information on the effects of DES exposure in women during adulthood.
TCDD exposure during adulthood reduced peak concentrations of FSH and LH in animal models, however, human data are lacking.
PCB exposure altered the mRNA and peptide levels of GnRH in hypothalamic cells, but research on the effects of adult exposure to PCBs on the hypothalamus and pituitary are not available.
Insufficient evidence exists to make conclusions about the effects of DEHP and BPA alternatives on the hypothalamus and pituitary. Further, insufficient evidence exists to make concluctions about the effects of nonylphenols, triclosan, and paraben exposure on the reproductive organs in animal models and women.
Future studies need to:
Use internal dose levels of EDCs that mimic human exposures.
Elucidate a mechanism of action for the EDCs presented in this review.
Include additional reproductive endpoints to further characterize the effects of adult exposure to EDCs on the hypothalamus, pituitary, ovary, uterus, fertility, and reproductive senescence.
Acknowledgments
This work was supported by NIH T32 ES007326 (SR, CC), NIH R56 ES025147 (JF, CC), and Environmental Toxicology Fellowship (CQ)
Footnotes
Declaration of Interest
The authors have no conflicts of interedt that could be perceived as prejudicing the impartiality of the research reported.
References
- Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK. Arsenic in drinking water and pregnancy outcomes. Environmental Health Perspectives. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–37. doi: 10.1016/j.envres.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina J, Reif J, Keefe T, Bachand A. Age at natural menopause and exposure to organochlorine pesticides in Hispanic women. J Toxicol Environ Health A. 2004;67:1407–1422. doi: 10.1080/15287390490483845. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Shinwari N, Al-Amodi M. Accumulation of mercury in ovaries of mice after the application of skin-lightening creams. Biol Trace Elem Res. 2009;131:43–54. doi: 10.1007/s12011-009-8341-x. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Shinwari N, Mashhour A, Rabah A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int J Hyg Environ Health. 2014;217:205–218. doi: 10.1016/j.ijheh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Chapin RE. Reproductive toxicities of methoxychlor based on estrogenic properties of the compound and its estrogenic metabolite, hydroxyphenyltrichloroethane. Vitam Horm. 2014;94:193–210. doi: 10.1016/B978-0-12-800095-3.00007-9. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Hojo H, Takahashi KL, Shimizu-Endo N, Araki M, Takeuchi-Kashimoto Y, Saka M, Teramoto S. Two-generation reproduction toxicity study in rats with methoxychlor. Congenit Anom (Kyoto) 2012;52:28–41. doi: 10.1111/j.1741-4520.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa MS, Craig ZR, Hernandez-Ochoa I, Paulose T, Leslie TC, Flaws JA. Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2011;253:161–169. doi: 10.1016/j.taap.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa MS, Hernandez-Ochoa I, Wang W, Flaws JA. Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles. Reprod Toxicol. 2012;34:16–21. doi: 10.1016/j.reprotox.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashore CJ, Geer LA, He X, Puett R, Parsons PJ, Palmer CD, Steuerwald AJ, Abulafia O, Dalloul M, Sapkota A. Maternal mercury exposure, season of conception and adverse birth outcomes in an urban immigrant community in Brooklyn, New York, U.S.A. Int J Environ Res Public Health. 2014;11:8414–8442. doi: 10.3390/ijerph110808414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basini G, Bianchi F, Bussolati S, Baioni L, Ramoni R, Grolli S, Conti V, Bianchi F, Grasselli F. Atrazine disrupts steroidogenesis, VEGF and NO production in swine granulosa cells. Ecotoxicol Environ Saf. 2012;85:59–63. doi: 10.1016/j.ecoenv.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Bell MR. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr Opin Pharmacol. 2014;19:134–144. doi: 10.1016/j.coph.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L, Garcia-Vicent C, Lopez J, Torro MI, Lurbe E. Assessment of ten trace elements in umbilical cord blood and maternal blood: association with birth weight. J Transl Med. 2015;13:291. doi: 10.1186/s12967-015-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard L, Decaudin B, Lecoeur M, Richard D, Bourdeaux D, Cueff R, Sautou V. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: a review. Talanta. 2014;129:39–54. doi: 10.1016/j.talanta.2014.04.069. [DOI] [PubMed] [Google Scholar]
- Bhardwaj JK, Saraf P. Transmission electron microscopic analysis of malathion-induced cytotoxicity in granulosa cells of caprine antral follicles. Ultrastruct Pathol. 2016;40:43–50. doi: 10.3109/01913123.2015.1088908. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol Appl Pharmacol. 2012;261:227–235. doi: 10.1016/j.taap.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Louis GM, Sundaram R, Kostyniak PJ, Jain J. Associations between blood metals and fecundity among women residing in New York State. Reprod Toxicol. 2011;31:158–163. doi: 10.1016/j.reprotox.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Surdu S, Neamtiu IA, Gurzau ES. Maternal arsenic exposure and birth outcomes: a comprehensive review of the epidemiologic literature focused on drinking water. Int J Hyg Environ Health. 2014;217:709–719. doi: 10.1016/j.ijheh.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C, Hass U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31:200–209. doi: 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Greenfeld C, Tomic D, Flaws JA. The effects of endocrine disrupting chemicals on the ovary. Front Biosci. 2002;7:d1941–1948. doi: 10.2741/borgees. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, Flaws JA. Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–1835. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J. Blood lead levels measured prospectively and risk of spontaneous abortion. American Journal of Epidemiology. 1999;150:590–597. doi: 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Williams KJ, Zacharewski TR. Inhibition of estrogen-mediated uterine gene expression responses by dioxin. Mol Pharmacol. 2008;73:82–93. doi: 10.1124/mol.107.040451. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Sato T, Peterson RE, Cooke PS. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol Sci. 2000;57:302–311. doi: 10.1093/toxsci/57.2.302. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM. Persistent environmental pollutants and couple fecundity: an overview. Reproduction. 2014;147:R97–r104. doi: 10.1530/REP-13-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB, Wagner Robb S, Puett R, Cai B, Wilkerson R, Karmaus W, Vena J, Svendsen E. Mercury in fish and adverse reproductive outcomes: results from South Carolina. Int J Health Geogr. 2014;13:30. doi: 10.1186/1476-072X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res Int. 2015;22:5711–5741. doi: 10.1007/s11356-014-3974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini A, Lippolis C, Vacca M, Nardelli C, Castegna A, Arnesano F, Carella N, Depalo R. The Effects of Chronic Lifelong Activation of the AHR Pathway by Industrial Chemical Pollutants on Female Human Reproduction. PLoS One. 2016;11:e0152181. doi: 10.1371/journal.pone.0152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Chen ML, Liao KW, Tsai YA, Mao IF, Wang TH, Hwang SM, Chang YJ, Tsai MS. The association between maternal nonylphenol exposure and parity on neonatal birth weight: a cohort study in Taiwan. Chemosphere. 2013;93:1145–1152. doi: 10.1016/j.chemosphere.2013.06.048. [DOI] [PubMed] [Google Scholar]
- Chang CH, Tsai MS, Lin CL, Hou JW, Wang TH, Tsai YA, Liao KW, Mao IF, Chen ML. The association between nonylphenols and sexual hormones levels among pregnant women: a cohort study in Taiwan. PLoS One. 2014;9:e104245. doi: 10.1371/journal.pone.0104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Chatterji U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod Biol Endocrinol. 2010;8:80. doi: 10.1186/1477-7827-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Ghosh D. The involvement of hypophyseal-gonadal and hypophyseal-adrenal axes in arsenic-mediated ovarian and uterine toxicity: modulation by hCG. J Biochem Mol Toxicol. 2010;24:29–41. doi: 10.1002/jbt.20309. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, Cordier S. Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology. 2013;24:251–260. doi: 10.1097/EDE.0b013e31827f53ec. [DOI] [PubMed] [Google Scholar]
- Cole DC, Wainman B, Sanin LH, Weber JP, Muggah H, Ibrahim S. Environmental contaminant levels and fecundability among non-smoking couples. Reproductive Toxicology. 2006;22:13–19. doi: 10.1016/j.reprotox.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Colquitt PJ. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occupational and Environmental Medicine. 1995;52:214. doi: 10.1136/oem.52.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Savitz DA, Millikan R, Chiu Kit T. Organochlorine exposure and age at natural menopause. Epidemiology. 2002;13:729–733. doi: 10.1097/00001648-200211000-00021. [DOI] [PubMed] [Google Scholar]
- CPSC. Review of Exposure and Toxicity Data for Phthalate Substitutes. 2010. pp. 1–106. [Google Scholar]
- Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- Crawford BR, Decatanzaro D. Disruption of blastocyst implantation by triclosan in mice: impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol. 2012;34:607–613. doi: 10.1016/j.reprotox.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Dalgaard M, Hass U, Vinggaard AM, Jarfelt K, Lam HR, Sorensen IK, Sommer HM, Ladefoged O. Di(2-ethylhexyl) adipate (DEHA) induced developmental toxicity but not antiandrogenic effects in pre- and postnatally exposed Wistar rats. Reprod Toxicol. 2003;17:163–170. doi: 10.1016/s0890-6238(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Dallaire R, Dewailly E, Ayotte P, Forget-Dubois N, Jacobson SW, Jacobson JL, Muckle G. Exposure to organochlorines and mercury through fish and marine mammal consumption: associations with growth and duration of gestation among Inuit newborns. Environ Int. 2013;54:85–91. doi: 10.1016/j.envint.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. Journal of Applied Toxicology. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Shappell HW, Banerjee A, Hachey DL. Effects of nonylphenol, 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p’-DDE), and pentachlorophenol on the adult female guinea pig reproductive tract. Reprod Toxicol. 2002;16:29–43. doi: 10.1016/s0890-6238(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Dickerson EH, Sathyapalan T, Knight R, Maguiness SM, Killick SR, Robinson J, Atkin SL. Endocrine disruptor & nutritional effects of heavy metals in ovarian hyperstimulation. J Assist Reprod Genet. 2011;28:1223–1228. doi: 10.1007/s10815-011-9652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG., Jr Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect. 2003;111:947–953. doi: 10.1289/ehp.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D, Jr, Mocarelli P. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005;113:858–862. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology. 2010;21:224–231. doi: 10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J-L, Vanlandingham MM, Juliar BE, Olson RG, Patton ER, Beland FA. Dose-response assessment of the dermal toxicity of triclosan in B6C3F1 mice. Toxicology Research. 2015;4:867–877. [Google Scholar]
- Fang J-L, Stingley RL, Beland FA, Harrouk W, Lumpkins DL, Howard P. Occurrence, Efficacy, Metabolism, and Toxicity of Triclosan. Journal of Environmental Science and Health, Part C. 2010;28:147–171. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- Farr SL, Cai J, Savitz DA, Sandler DP, Hoppin JA, Cooper GS. Pesticide exposure and timing of menopause: the Agricultural Health Study. Am J Epidemiol. 2006;163:731–742. doi: 10.1093/aje/kwj099. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z, Shao B. Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PLoS One. 2016;11:e0154758. doi: 10.1371/journal.pone.0154758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, O’Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2013;16:69–113. doi: 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Yebra-Pimentel I, Martinez-Carballo E, Simal-Gandara J. A Critical Review about Human Exposure to Polychlorinated Dibenzo-p-Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs) and Polychlorinated Biphenyls (PCBs) through Foods. Crit Rev Food Sci Nutr. 2015;55:1590–1617. doi: 10.1080/10408398.2012.710279. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Quihuis AM, Lacagnina AF, Breckenridge CB, Handa RJ. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol Reprod. 2011;85:684–689. doi: 10.1095/biolreprod.111.092452. [DOI] [PubMed] [Google Scholar]
- Foster WG. Endocrine toxicants including 2,3,7,8-terachlorodibenzo-p-dioxin (TCDD) and dioxin-like chemicals and endometriosis: is there a link? J Toxicol Environ Health B Crit Rev. 2008;11:177–187. doi: 10.1080/10937400701873456. [DOI] [PubMed] [Google Scholar]
- Fraites MJ, Cooper RL, Buckalew A, Jayaraman S, Mills L, Laws SC. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol Sci. 2009;112:88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- Furuta M, Funabashi T, Kawaguchi M, Nakamura TJ, Mitsushima D, Kimura F. Effects of p-nonylphenol and 4-tert-octylphenol on the anterior pituitary functions in adult ovariectomized rats. Neuroendocrinology. 2006;84:14–20. doi: 10.1159/000096093. [DOI] [PubMed] [Google Scholar]
- Gallo MV, Ravenscroft J, Carpenter DO, Frye C, Cook B, Schell LM Akwesasne Task Force On The E. Endocrine disrupting chemicals and ovulation: Is there a relationship? Environ Res. 2016;151:410–418. doi: 10.1016/j.envres.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Geer LA, Pycke BF, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 2017;323:177–183. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SA, Rizvi F, Khan MZ, Khan A. Toxic effects of cypermethrin and methamidophos on bovine corpus luteal cells and progesterone production. Exp Toxicol Pathol. 2011;63:131–135. doi: 10.1016/j.etp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Davis LK, Murr AS, Cooper RL. Atrazine-induced elevation or attenuation of the LH surge in the ovariectomized, estrogen-primed female rat: role of adrenal progesterone. Reproduction. 2013;146:305–314. doi: 10.1530/REP-13-0011. [DOI] [PubMed] [Google Scholar]
- Gore AC. Environmental toxicant effects on neuroendocrine function. Endocrine. 2001;14:235–246. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36:E1–e150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in U.S. women. PLoS One. 2015;10:e0116057. doi: 10.1371/journal.pone.0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013;47:14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Aberdeen G, Babus JK, Albrecht ED, Flaws JA. Methoxychlor and its metabolites inhibit growth and induce atresia of baboon antral follicles. Toxicol Pathol. 2007;35:649–656. doi: 10.1080/01926230701459960. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Meachum S, Hernandez-Ochoa I, Peretz J, Yao HH, Flaws JA. Methoxychlor inhibits growth of antral follicles by altering cell cycle regulators. Toxicol Appl Pharmacol. 2009;240:1–7. doi: 10.1016/j.taap.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson L, Ciesielski TM, Bytingsvik J, Styrishave B, Hansen M, Lie E, Aars J, Jenssen BM. Hydroxylated polychlorinated biphenyls decrease circulating steroids in female polar bears (Ursus maritimus) Environ Res. 2015;138:191–201. doi: 10.1016/j.envres.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015;6:8. doi: 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara N, Shiraishi K, Miyata K, Oshima Y, Minobe Y, Yamasaki K. Subacute oral toxicity study of bisphenol F based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol. 2007;81:825–832. doi: 10.1007/s00204-007-0223-4. [DOI] [PubMed] [Google Scholar]
- Hong Y, Wang J, Zhang P, Yang S, Song K, Yu F, Liu W. Histopathological and gene expression analysis of mice exposed to diethylstilbestrol. Toxicol Mech Methods. 2010;20:105–111. doi: 10.3109/15376510903572631. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Carvan MJ, Baldridge MG, Conley LK, Heiden TK. Environmental toxicants and effects on female reproductive function. Tren Reprod Bio. 2006;2:1–11. doi: 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, Dobson CB, Frelich J, Hoffman E, Yousuf J, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- Jablonska O, Piasecka J, Petroff BK, Nynca A, Siawrys G, Wasowska B, Zmijewska A, Lewczuk B, Ciereszko RE. In vitro effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on ovarian, pituitary, and pineal function in pigs. Theriogenology. 2011;76:921–932. doi: 10.1016/j.theriogenology.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Jameil NA. Maternal serum lead levels and risk of preeclampsia in pregnant women: a cohort study in a maternity hospital, Riyadh, Saudi Arabia. Int J Clin Exp Pathol. 2014;7:3182–3189. [PMC free article] [PubMed] [Google Scholar]
- Jaroenporn S, Malaivijitnond S, Wattanasirmkit K, Watanabe G, Taya K, Cherdshewasart W. Assessment of fertility and reproductive toxicity in adult female mice after long-term exposure to Pueraria mirifica herb. J Reprod Dev. 2007;53:995–1005. doi: 10.1262/jrd.18151. [DOI] [PubMed] [Google Scholar]
- Johnstone EB, Louis GM, Parsons PJ, Steuerwald AJ, Palmer CD, Chen Z, Sun L, Hammoud AO, Dorais J, Peterson CM. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod Toxicol. 2014;49:27–32. doi: 10.1016/j.reprotox.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KS, Che JH, Ryu DY, Kim TW, Li GX, Lee YS. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben) J Vet Med Sci. 2002;64:227–235. doi: 10.1292/jvms.64.227. [DOI] [PubMed] [Google Scholar]
- Karman BN, Basavarajappa MS, Craig ZR, Flaws JA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates the aryl hydrocarbon receptor and alters sex steroid hormone secretion without affecting growth of mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2012a;261:88–96. doi: 10.1016/j.taap.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman BN, Basavarajappa MS, Hannon P, Flaws JA. Dioxin exposure reduces the steroidogenic capacity of mouse antral follicles mainly at the level of HSD17B1 without altering atresia. Toxicol Appl Pharmacol. 2012b;264:1–12. doi: 10.1016/j.taap.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TA, Yang Q, Trevino LS, Walker CL, Al-Hendy A. Endocrine-disrupting chemicals and uterine fibroids. Fertil Steril. 2016;106:967–977. doi: 10.1016/j.fertnstert.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Morohoshi K, Masuda J, Watanabe G, Morita M, Imai H, Taya K, Himi T. Maternal isobutyl-paraben exposure decreases the plasma corticosterone level in dams and sensitivity to estrogen in female offspring rats. J Vet Med Sci. 2009;71:1027–1033. doi: 10.1292/jvms.71.1027. [DOI] [PubMed] [Google Scholar]
- Kezios KL, Liu X, Cirillio PM, Kalantzi OI, Wang Y, Petreas MX, Park JS, Bradwin G, Cohn BA, Factor-Litvak P. Prenatal polychlorinated biphenyl exposure is associated with decreased gestational length but not birth weight: archived samples from the Child Health and Development Studies pregnancy cohort. Environ Health. 2012;11:49. doi: 10.1186/1476-069X-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Fujishita A, Masuzaki H, Koji T, Ishimaru T. Expression of the arylhydrocarbon receptor in the peri-implantation period of the mouse uterus and the impact of dioxin on mouse implantation. Arch Histol Cytol. 2004;67:465–474. doi: 10.1679/aohc.67.465. [DOI] [PubMed] [Google Scholar]
- Koc ND, Kayhan FE, Sesal C, Muslu MN. Dose-dependent effects of endosulfan and malathion on adult Wistar albino rat ovaries. Pak J Biol Sci. 2009;12:498–503. doi: 10.3923/pjbs.2009.498.503. [DOI] [PubMed] [Google Scholar]
- Kotwica J, Wrobel M, Mlynarczuk J. The influence of polychlorinated biphenyls (PCBs) and phytoestrogens in vitro on functioning of reproductive tract in cow. Reprod Biol. 2006;6(Suppl 1):189–194. [PubMed] [Google Scholar]
- Kwekel JC, Forgacs AL, Williams KJ, Zacharewski TR. o-p’-DDT-mediated uterotrophy and gene expression in immature C57BL/6 mice and Sprague-Dawley rats. Toxicol Appl Pharmacol. 2013;273:532–541. doi: 10.1016/j.taap.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Laffin B, Chavez M, Pine M. The pyrethroid metabolites 3-phenoxybenzoic acid and 3-phenoxybenzyl alcohol do not exhibit estrogenic activity in the MCF-7 human breast carcinoma cell line or Sprague-Dawley rats. Toxicology. 2010;267:39–44. doi: 10.1016/j.tox.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, Herring AH, Styblo M, Garcia-Vargas GG, Fry RC. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123:186–192. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitman CJ. DES exposure and the aging woman: mothers and daughters. Curr Womens Health Rep. 2002;2:390–393. [PubMed] [Google Scholar]
- Laks DR. Assessment of chronic mercury exposure within the U.S. population, National Health and Nutrition Examination Survey, 1999–2006. Biometals. 2009;22:1103–1114. doi: 10.1007/s10534-009-9261-0. [DOI] [PubMed] [Google Scholar]
- Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health. 2003;19:69–79. doi: 10.1191/0748233703th177oa. [DOI] [PubMed] [Google Scholar]
- Leon-Olea M, Martyniuk CJ, Orlando EF, Ottinger MA, Rosenfeld CS, Wolstenholme JT, Trudeau VL. Current concepts in neuroendocrine disruption. Gen Comp Endocrinol. 2014;203:158–173. doi: 10.1016/j.ygcen.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Additives & Contaminants: Part A. 2014;31:319–329. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Liu S, Jiang L, Meng X, Han X, Cheng D, Zhang T, Miao Y. Effects of Aroclor 1254 on in vivo oocyte maturation in the mouse. PLoS One. 2014;9:e102064. doi: 10.1371/journal.pone.0102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Gan J. Analysis, toxicity, occurrence and biodegradation of nonylphenol isomers: a review. Environ Int. 2014;73:334–345. doi: 10.1016/j.envint.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Luderer U, Kesner JS, Fuller JM, Krieg EF, Jr, Meadows JW, Tramma SL, Yang H, Baker D. Effects of gestational and lactational exposure to heptachlor epoxide on age at puberty and reproductive function in men and women. Environ Res. 2013;121:84–94. doi: 10.1016/j.envres.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Manservisi F, Gopalakrishnan K, Tibaldi E, Hysi A, Iezzi M, Lambertini L, Teitelbaum S, Chen J, Belpoggi F. Effect of maternal exposure to endocrine disrupting chemicals on reproduction and mammary gland development in female Sprague-Dawley rats. Reprod Toxicol. 2015;54:110–119. doi: 10.1016/j.reprotox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur PP, D’Cruz SC. The effect of environmental contaminants on testicular function. Asian J Androl. 2011;13:585–591. doi: 10.1038/aja.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty PA, Skydsgaard M. Diethylstilbestrol (DES): Carcinogenic Potential in Xpa−/−, Xpa−/−/p53+/−, and Wild-Type Mice During 9 Months’ Dietary Exposure. Toxicologic Pathology. 2005;33:609–620. doi: 10.1080/01926230500261377. [DOI] [PubMed] [Google Scholar]
- Mendola P, Buck GM, Sever LE, Zielezny M, Vena JE. Consumption of PCB-contaminated freshwater fish and shortened menstrual cycle length. Am J Epidemiol. 1997;146:955–960. doi: 10.1093/oxfordjournals.aje.a009222. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Miyata K, Shiraishi K, Houshuyama S, Imatanaka N, Umano T, Minobe Y, Yamasaki K. Subacute oral toxicity study of di(2-ethylhexyl)adipate based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol. 2006;80:181–186. doi: 10.1007/s00204-005-0030-8. [DOI] [PubMed] [Google Scholar]
- Motawei SM, Attalla SM, Gouda HE, El-Harouny MA, El-Mansoury AM. Lead level in pregnant women suffering from pre-eclampsia in Dakahlia, Egypt. Int J Occup Environ Med. 2013;4:36–44. [PubMed] [Google Scholar]
- Murphy S, Barber JL, Learmonth JA, Read FL, Deaville R, Perkins MW, Brownlow A, Davison N, Penrose R, Pierce GJ, et al. Reproductive Failure in UK Harbour Porpoises Phocoena phocoena: Legacy of Pollutant Exposure? PLoS One. 2015;10:e0131085. doi: 10.1371/journal.pone.0131085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, Mendola P. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. Journal of Epidemiology and Community Health. 2010;64:325–329. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- Nandi S, Gupta PS, Roy SC, Selvaraju S, Ravindra JP. Chlorpyrifos and endosulfan affect buffalo oocyte maturation, fertilization, and embryo development in vitro directly and through cumulus cells. Environ Toxicol. 2011;26:57–67. doi: 10.1002/tox.20529. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog. 2007;46:783–796. doi: 10.1002/mc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama Y, Yoshinaga J, Iida A, Konishi S, Imai H, Yoneyama M, Nakajima D, Shiraishi H. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol. 2016;63:107–113. doi: 10.1016/j.reprotox.2016.05.010. [DOI] [PubMed] [Google Scholar]
- North EJ, Halden RU. Plastics and environmental health: the road ahead. Rev Environ Health. 2013;28:1–8. doi: 10.1515/reveh-2012-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in female harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 2010:1–174. [PubMed] [Google Scholar]
- Odum J, Tinwell H, Van Miller J, Joiner R, Ashby J. The uterotrophic activity of nonylphenol in the rat is not mediated by aromatase enzyme induction. Toxicol Lett. 2001;118:165–169. doi: 10.1016/s0378-4274(00)00293-9. [DOI] [PubMed] [Google Scholar]
- Ou L, Chen C, Chen L, Wang H, Yang T, Xie H, Tong Y, Hu D, Zhang W, Wang X. Low-level prenatal mercury exposure in north China: an exploratory study of anthropometric effects. Environ Sci Technol. 2015;49:6899–6908. doi: 10.1021/es5055868. [DOI] [PubMed] [Google Scholar]
- Patel S, Zhou C, Rattan S, Flaws JA. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol Reprod. 2015;93:20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose T, Hernandez-Ochoa I, Basavarajappa MS, Peretz J, Flaws JA. Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol Sci. 2011;120:447–459. doi: 10.1093/toxsci/kfr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose T, Tannenbaum LV, Borgeest C, Flaws JA. Methoxychlor-induced ovarian follicle toxicity in mice: dose and exposure duration-dependent effects. Birth Defects Res B Dev Reprod Toxicol. 2012;95:219–224. doi: 10.1002/bdrb.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann Epidemiol. 2014;24:915–919. doi: 10.1016/j.annepidem.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro EM, Leroy JL, Covaci A, Fransen E, De Neubourg D, Dirtu AC, De Pauw I, Bols PE. Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod. 2012;27:1025–1033. doi: 10.1093/humrep/der448. [DOI] [PubMed] [Google Scholar]
- Petroff BK, Roby KF, Gao X, Son D, Williams S, Johnson D, Rozman KK, Terranova PF. A review of mechanisms controlling ovulation with implications for the anovulatory effects of polychlorinated dibenzo-p-dioxins in rodents. Toxicology. 2001;158:91–107. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25:625–635. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Laxmi Priya PN, Gupta S. Effects of combined exposure to lead and cadmium on pituitary membrane of female rats. Arch Toxicol. 2002;76:671–675. doi: 10.1007/s00204-002-0399-6. [DOI] [PubMed] [Google Scholar]
- Pillai A, Priya L, Gupta S. Effects of combined exposure to lead and cadmium on the hypothalamic-pituitary axis function in proestrous rats. Food Chem Toxicol. 2003;41:379–384. doi: 10.1016/s0278-6915(02)00247-8. [DOI] [PubMed] [Google Scholar]
- Ponzo OJ, Silvia C. Evidence of reproductive disruption associated with neuroendocrine changes induced by UV-B filters, phthalates and nonylphenol during sexual maturation in rats of both gender. Toxicology. 2013;311:41–51. doi: 10.1016/j.tox.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, Cobbina SJ, Nketiah-Amponsah E, Namujju PB, Obiri S, et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect. 2015;123:412–421. doi: 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignot N, Arnaud M, Robidel F, Lecomte A, Tournier M, Cren-Olive C, Barouki R, Lemazurier E. Characterization of endocrine-disrupting chemicals based on hormonal balance disruption in male and female adult rats. Reprod Toxicol. 2012;33:339–352. doi: 10.1016/j.reprotox.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, Vahter M. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21:797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- Rahman SN, Fatima P, Chowdhury AQ, Rahman MW. Blood level of lead in women with unexplained infertility. Mymensingh Med J. 2013;22:508–512. [PubMed] [Google Scholar]
- Rama EM, Bortolan S, Vieira ML, Gerardin DC, Moreira EG. Reproductive and possible hormonal effects of carbendazim. Regul Toxicol Pharmacol. 2014;69:476–486. doi: 10.1016/j.yrtph.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Rasmussen LM, Sen N, Liu X, Craig ZR. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J Appl Toxicol. 2016 doi: 10.1002/jat.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rier SE. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201–212. doi: 10.1111/j.1749-6632.2002.tb02781.x. discussion 230–202, 396–406. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PE, Sanchez MS. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A. 2010;73:1678–1688. doi: 10.1080/15287394.2010.516241. [DOI] [PubMed] [Google Scholar]
- Rzymski P, Tomczyk K, Rzymski P, Poniedzialek B, Opala T, Wilczak M. Impact of heavy metals on the female reproductive system. Ann Agric Environ Med. 2015;22:259–264. doi: 10.5604/12321966.1152077. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94:3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Sangha GK, Kaur K, Khera KS. Cypermethrin induced pathological and biochemical changes in reproductive organs of female rats. J Environ Biol. 2013;34:99–105. [PubMed] [Google Scholar]
- Sengupta P, Banerjee R, Nath S, Das S, Banerjee S. Metals and female reproductive toxicity. Human and Experimental Toxicology. 2015;34:679–697. doi: 10.1177/0960327114559611. [DOI] [PubMed] [Google Scholar]
- Shanthalatha A, Madhuranath BN, Yajurvedi HN. Effect of methomyl formulation, a carbamate pesticide on ovarian follicular development and fertility in albino mice. J Environ Biol. 2012;33:33–37. [PubMed] [Google Scholar]
- Simsa P, Mihalyi A, Schoeters G, Koppen G, Kyama CM, Den Hond EM, Fulop V, D’Hooghe TM. Increased exposure to dioxin-like compounds is associated with endometriosis in a case-control study in women. Reprod Biomed Online. 2010;20:681–688. doi: 10.1016/j.rbmo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Smarr MM, Sundaram R, Honda M, Kannan K, Buck Louis GM. Urinary Concentrations of Parabens and Other Antimicrobial Chemicals and Their Association with Couples’ Fecundity. Environ Health Perspect. 2016 doi: 10.1289/EHP189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KW, Souter I, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ Health Perspect. 2013;121:1299–1305. doi: 10.1289/ehp.1205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith OW, Smith GV. The influence of diethylstilbestrol on the progress and outcome of pregnancy as based on a comparison of treated with untreated primigravidas. Am J Obstet Gynecol. 1949;58:994–1009. doi: 10.1016/0002-9378(49)90204-5. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Perreault SD, Bremser K, Marshall RS, Murr A, Cooper RL. Acute exposure to molinate alters neuroendocrine control of ovulation in the rat. Toxicol Sci. 2005;84:38–48. doi: 10.1093/toxsci/kfi064. [DOI] [PubMed] [Google Scholar]
- Sun H, Chen W, Wang D, Jin Y, Chen X, Xu Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere. 2014;108:33–39. doi: 10.1016/j.chemosphere.2014.02.080. [DOI] [PubMed] [Google Scholar]
- Sweeney T. Is exposure to endocrine disrupting compounds during fetal/post-natal development affecting the reproductive potential of farm animals? Domest Anim Endocrinol. 2002;23:203–209. doi: 10.1016/s0739-7240(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Taketa Y, Yoshida M, Inoue K, Takahashi M, Sakamoto Y, Watanabe G, Taya K, Yamate J, Nishikawa A. Differential stimulation pathways of progesterone secretion from newly formed corpora lutea in rats treated with ethylene glycol monomethyl ether, sulpiride, or atrazine. Toxicol Sci. 2011;121:267–278. doi: 10.1093/toxsci/kfr062. [DOI] [PubMed] [Google Scholar]
- Tang R, Chen MJ, Ding GD, Chen XJ, Han XM, Zhou K, Chen LM, Xia YK, Tian Y, Wang XR. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115–120. doi: 10.1016/j.envpol.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Tanrikut E, Karaer A, Celik O, Celik E, Otlu B, Yilmaz E, Ozgul O. Role of endometrial concentrations of heavy metals (cadmium, lead, mercury and arsenic) in the aetiology of unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2014;179:187–190. doi: 10.1016/j.ejogrb.2014.05.039. [DOI] [PubMed] [Google Scholar]
- Tavakoly Sany SB, Hashim R, Salleh A, Rezayi M, Karlen DJ, Razavizadeh BB, Abouzari-Lotf E. Dioxin risk assessment: mechanisms of action and possible toxicity in human health. Environ Sci Pollut Res Int. 2015;22:19434–19450. doi: 10.1007/s11356-015-5597-x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ Res. 2015;140:430–439. doi: 10.1016/j.envres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25:316–326. doi: 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tinfo NS, Hotchkiss MG, Buckalew AR, Zorrilla LM, Cooper RL, Laws SC. Understanding the effects of atrazine on steroidogenesis in rat granulosa and H295R adrenal cortical carcinoma cells. Reprod Toxicol. 2011;31:184–193. doi: 10.1016/j.reprotox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Toft G, Thulstrup AM, Jonsson BA, Pedersen HS, Ludwicki JK, Zvezday V, Bonde JP. Fetal loss and maternal serum levels of 2,2′,4,4′,5,5′-hexachlorbiphenyl (CB-153) and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p’-DDE) exposure: a cohort study in Greenland and two European populations. Environ Health. 2010;9:22. doi: 10.1186/1476-069X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez LE, Berkowitz G, Lopez-Carrillo L, Torres-Arreola L, Rios C, Lopez-Cervantes M. Intrauterine lead exposure and preterm birth. Environmental Research. 1999;81:297–301. doi: 10.1006/enrs.1999.3984. [DOI] [PubMed] [Google Scholar]
- Tournaire M, Devouche E, Espie M, Asselain B, Levadou A, Cabau A, Dunbavand A, Grosclaude P, Epelboin S. Cancer Risk in Women Exposed to Diethylstilbestrol in Utero. Therapie. 2015;70:433–441. doi: 10.2515/therapie/2015030. [DOI] [PubMed] [Google Scholar]
- Trabert B, Chen Z, Kannan K, Peterson CM, Pollack AZ, Sun L, Buck Louis GM. Persistent organic pollutants (POPs) and fibroids: results from the ENDO study. J Expo Sci Environ Epidemiol. 2015;25:278–285. doi: 10.1038/jes.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Chang CH, Tsai YA, Liao KW, Mao IF, Wang TH, Hwang SM, Chang YJ, Chen ML. Neonatal outcomes of intrauterine nonylphenol exposure--a longitudinal cohort study in Taiwan. Sci Total Environ. 2013;458–460:367–373. doi: 10.1016/j.scitotenv.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Usman A, Ahmad M. From BPA to its analogues: Is it a safe journey? Chemosphere. 2016;158:131–142. doi: 10.1016/j.chemosphere.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM. A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol. 2011;31:551–560. doi: 10.1038/jp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril. 2015;103:1011–1020. e1012. doi: 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Walker DM, Gore AC. Epigenetic impacts of endocrine disruptors in the brain. Front Neuroendocrinol. 2016 doi: 10.1016/j.yfrne.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liao C, Liu F, Wu Q, Guo Y, Moon HB, Nakata H, Kannan K. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ Sci Technol. 2012;46:11584–11593. doi: 10.1021/es303516u. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Chen L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci Rep. 2015;5:18252. doi: 10.1038/srep18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wato E, Asahiyama M, Suzuki A, Funyu S, Amano Y. Collaborative work on evaluation of ovarian toxicity. 9) Effects of 2- or 4-week repeated dose studies and fertility study of di(2-ethylhexyl)adipate (DEHA) in female rats. J Toxicol Sci. 2009;34(Suppl 1):SP101–109. doi: 10.2131/jts.34.s101. [DOI] [PubMed] [Google Scholar]
- Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16:182–190. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Walker CL. Fetal and early postnatal environmental exposures and reproductive health effects in the female. Fertil Steril. 2008;89:e47–e51. doi: 10.1016/j.fertnstert.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DL, Afeiche MC, Ehrlich S, Smith K, Williams PL, Chavarro JE, Batsis M, Toth TL, Hauser R. Hair mercury concentrations and in vitro fertilization (IVF) outcomes among women from a fertility clinic. Reprod Toxicol. 2015;51:125–132. doi: 10.1016/j.reprotox.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environmental Health Perspectives. 2007;115:42–47. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, Kwon JT, Shim I, Kim HM, Kim P, Kim JC, Lee K. Evaluation of toxicity to triclosan in rats following 28 days of exposure to aerosol inhalation. Regul Toxicol Pharmacol. 2015;71:259–268. doi: 10.1016/j.yrtph.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Brix AE, Sells DM, Jokinen MP, Wyde M, Orzech DP, Kissling GE, Walker NJ, Nyska A. Reproductive lesions in female Harlan Sprague-Dawley rats following two-year oral treatment with dioxin and dioxin-like compounds. Toxicol Pathol. 2009;37:921–937. doi: 10.1177/0192623309351721. [DOI] [PubMed] [Google Scholar]
- Yu PL, Lin HW, Wang SW, Wang PS. Effects of nonylphenol on the production of progesterone on the rats granulosa cells. J Cell Biochem. 2011;112:2627–2636. doi: 10.1002/jcb.23189. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yang A, Zhang J, Hu S. Maternal exposure to the mixture of organophosphorus pesticides induces reproductive dysfunction in the offspring. Environ Toxicol. 2013;28:507–515. doi: 10.1002/tox.20741. [DOI] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: an ovarian perspective. Front Neuroendocrinol. 2010;31:420–439. doi: 10.1016/j.yfrne.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong L, Ding S, Qiao P, Wang C, Zhang M, Zhang L, Du Q, Li Y, Tang N, et al. Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E(2) levels in male rat offspring. Environ Toxicol Pharmacol. 2014;37:705–717. doi: 10.1016/j.etap.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhou J, El Zowalaty AE, Li R, Dudley EA, Ye X. Timing and recovery of postweaning exposure to diethylstilbestrol on early pregnancy in CD-1 mice. Reprod Toxicol. 2014;49:48–54. doi: 10.1016/j.reprotox.2014.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Shi Z, Yuan F, Li G, Sun Y, Zhang Y, Wang Z. Melatonin modulates the effects of diethylstilbestrol (DES) on the anterior pituitary of the female Wistar rat. Folia Histochemica et Cytobiologica. 2010;48:278–283. doi: 10.2478/v10042-010-0023-1. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]