Summary
Novel influenza viruses continue to emerge posing zoonotic and potentially pandemic threats, avian influenza A/H7N9 being the most recent example. While closure of live poultry markets in mainland China was effective at aborting A/H7N9 outbreaks temporarily, they are difficult to sustain, given the current poultry production and marketing systems in China. We summarise interventions taken in mainland China to date. We provide evidence for other more sustainable but effective interventions in the live poultry market (LPM) systems that reduce risk of zoonotic influenza including “rest days” in LPM and banning live poultry in markets overnight. On the longer term, separation of live ducks and geese from terrestrial poultry in LPM systems can reduce the risk of emergence of zoonotic, epizootic (and potentially pandemic) viruses at source. Given evidence that A/H7N9 is now endemic in over half of the provinces in mainland China, and will continue to cause recurrent zoonotic disease in the winter months, such interventions should receive high priority in China as well as other Asian countries which are at risk of introduction of A/H7N9 through cross-border poultry movements. Such generic measures are likely to reduce current as well as future threats from zoonotic influenza.
Keywords: influenza, avian, zoonotic, pandemic, prevention, global public health, One Health, live poultry markets, poultry trade, H7N9, H5N1
Text
Epidemic and pandemic influenza pose unique threats to global health. Pandemic strains arise from animal viruses at unpredictable intervals, and in today’s globalised world, spread within weeks to affect many countries and continents. The novel A/H1N1 pandemic virus which was first recognised in April 2009 spread globally within a few months. The development and production of pandemic-specific vaccines was much slower than the timescale of global spread. For example, 37% of children in Hong Kong SAR, China had been infected by end of September 2009, long before any vaccines were available.1 It was indeed fortuitous that this pandemic caused lower mortality than the pandemics of 1918, 1957 or 1968.
Current zoonotic and pandemic threats
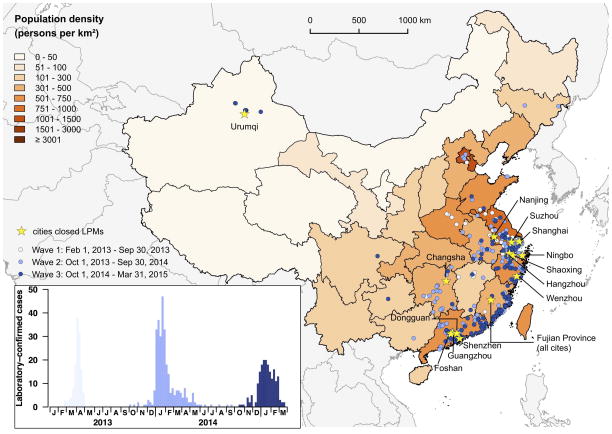
Highly pathogenic avian influenza (HPAI) A/H5N1 has caused zoonotic disease and led to pandemic concern during the past decade. Recent research has demonstrated that a relatively small number of mutations may permit this avian virus to develop capacity to spread from humans to other humans and thus potentially become pandemic. 2 A novel A/H7N9 virus emerged in Eastern China in 2013 and in many respects is even more threatening.3 Human infection appears to be mainly zoonotic in origin although limited non-sustained transmission between humans is reported.4 Genetic and biological characterization of A/H7N9 reveals that this virus is already better adapted to human nasal passages and tracheo-broncheal tree - a feature that is needed for a virus to be transmissible between humans - than any other avian virus known to date.3,5,6 Airborne transmission of influenza viruses between ferrets is the best experimental animal model for viruses that are transmissible in humans and A/H7N9 is partially transmissible between ferrets by the airborne route without prior adaptation, again unique for avian viruses (including H5N1).7 In less than two years, the A/H7N9 virus, so far only known to circulate in 17 of 31 provinces of mainland China, has led to more than 629 confirmed human cases and 251 deaths (as of Mar 26th 2015) (Yu H – personal communication). In contrast, since 2003 A/H5N1 has led to 784 confirmed human cases and 429 deaths (as of 3rd March 2015) acquired in 16 countries.8 The third-wave of zoonotic H7N9 disease has now occurred and future winter waves are inevitable (Figure 1). These epidemiological findings reinforce the experimental findings suggesting that A/H7N9 is unusually well adapted to the human respiratory tract.
Figure 1. Geographic distribution of A/H7N9 cases in mainland China and cities where closure of live poultry markets has been implemented.
Each of the three waves of infection is denoted in different shades of blue. Insert shows numbers of laboratory confirmed cases of human A/H7N9 disease in China from 01/02/2013 to 31/03/2015.
Epidemiological studies indicate that mild human A/H7N9 infections are much more common than that for A/H5N1, with an estimated ratio of 13 to 225 mild A/H7N9 infections for every diagnosed hospitalised A/H7N9 case.9–11 Evidence of mild undetected human H7N9 infections is supported by evidence of high (14.9%) sero-prevalence in poultry workers in Shenzhen.13 While this implies that the case fatality risk of A/H7N9 is much lower than suggested by crude case fatality risks documented in hospitalised patients, it also means that the number of human infections is many tens-of-thousands, much greater than that indicated by hospital-based surveillance.12 Every human infection affords this rapidly mutating virus with a fresh opportunity to adapt to transmit between humans.14 Indeed, one readily observes some viral mutations associated with mammalian adaption, for example, within the viral PB2 gene, taking place in viruses detected in humans as compared with viruses in poultry.15
Unlike HPAI A/H5N1, the A/H7N9 virus is asymptomatic in poultry, making its control and containment even more challenging. The lack of any avian illness means there is no incentive for the poultry industry to actively seek its presence. The virus remains undetected unless sought for by active surveillance within the live poultry market (LPM) system (supply farms, wholesale and retail market system) and thus, in practice, humans effectively serve as sentinels of poultry infection.
Within the last year we have seen emergence of other zoonotic avian influenza A viruses in Asia, including H10N8, H6N1, and H5N6.16–18 In the past few months, we have also seen a HPAI H5N8 virus derived from HPAI H5N1 clade 2.3.4.6 viruses make its way from Asia to Europe and North America affecting domestic poultry.19 Although this virus is not yet known to have infected humans, it likely has the potential to do so.
Options for response
How may one reduce the zoonotic and pandemic risks posed by these repeatedly emerging avian viruses? Systematically assessing the risk associated with such viruses20 and making vaccine seed strains (e.g. different antigenic variants of A/H5N1) that may be used for vaccine production for use in the event of pandemic emergence is a regular bi-annual WHO activity.21 Human vaccines for A/H7N9 virus are currently in clinical trials. However, it is neither feasible nor economical to take vaccine candidates for multiple virus subtypes and antigenic variants to phase 1 clinical trials to establish safety and immunogenicity or to develop vaccine stockpiles. Ideally, such pre-pandemic vaccine preparedness needs to be complemented with efforts to reduce emergence and transmission of such zoonotic and potentially pandemic viruses through evidence-based attempts to prevent their emergence at source.22 This may be attempted by identifying critical points along the pathway of their emergence and transmission at which we may intervene to minimise their zoonotic risks.23 Some of these measures such as farm biosecurity, surveillance and poultry vaccination (both advantages and disadvantages) have been previously discussed.23 Improved hygiene in people working in the poultry trade and consumers frequenting wholesale and retail LPM are important risk-reduction measures but perception of health hazards appears lacking. 24 This is partly because human zoonotic disease is rare although exposure to zoonotic viruses is common, resulting in a perceived disconnect between exposure and disease. There is a need for improved education and implementation of hygiene in such settings.
In this Personal Views article, we focus on evidence-based interventions within the LMP marketing systems in the Asian context. We use A/H7N9 and A/H5N1 as contemporary examples but the generic interventions we discuss are likely to apply to current and future zoonotic viruses.
Role of live poultry marketing systems
In China and some other parts of Asia (Vietnam, Indonesia), the poultry industry comprises two major sectors; an integrated meat and egg production system and a system that serves the long entrenched LPM trade. The former uses “Western-style” integrated chicken production systems and in China provides approximately 50% of the poultry demand to the consumer as chilled and frozen meat and fresh eggs or pasteurized egg products via supermarkets and chain-restaurants. The LPM system provides the remaining consumer demand as live poultry through a complex and non-uniform system of farm production, trucking to wholesale markets and distribution to retail markets and local restaurants. The wholesale and retail markets vary greatly in the type of birds they distribute; in some cities this trade is limited to land-based poultry while others also include waterfowl. The LPM system is less intensively regulated than the integrated production system.
Many zoonotic avian influenza virus infections (A/H5N1, A/H7N9, A/H10N8) have been acquired through exposure to poultry at retail LPM. Retail LPM are a risk factor for human A/H5N1 and A/H7N9 infection25,26 and high avian influenza viral isolation rates in poultry and environmental viral contamination has been demonstrated in most instances where it has been systematically sought, in China, Indonesia and elsewhere with viruses such as H5N1 and H7N9.27–30 A recent extensive surveillance study in retail LPM in 5 provinces in mainland China found an overall A/H7N9 detection rate of 2.5% of faecal swabs collected.29
Reducing zoonotic risk
A number of interventions have been proven to be effective at reducing the risk of zoonotic infection. Closing wholesale and retail LPM have been shown to be associated with cessation of zoonotic outbreaks of A/H5N127 and A/H7N931,32 disease though proof of direct causality remains elusive. Such an intervention can however be costly to the existing poultry industry in the short term – for example the closure of wholesale and retail LPM in Shanghai, Hangzhou, Huzhou and Nanjing in Eastern China during the summer of 2013 is believed to have cost the poultry industry approximately US$ 8 billion.31
LMP closure
Given the repeated emergence of avian influenza viruses of zoonotic and pandemic threat, permanent closure of wholesale and retail LPM and its replacement with central slaughter of poultry and sale of frozen or chilled chicken carcasses has been advocated as a proactive countermeasure from the public health point of view. Such a change has been implemented since 1992 in Singapore and more recently in Taiwan, China.33 Singapore has not had zoonotic avian influenza in humans. However, popular support for implementing such a measure in mainland China has been wanting. Although more than 100 cases of A/H7N9 were reported from Guangdong province in 2014, only 42.3% of the general public and 9.6% of live poultry traders supported permanent termination of the live poultry trade.34 Interestingly, although perhaps unsurprisingly, among those who prefer to purchase live poultry, reasons for this practice differed between respondents in Hong Kong SAR and in Guangdong. The former gave better taste when cooking freshly killed chicken as important while the latter prioritised food security, i.e. lack of trust in the quality of chilled and frozen chicken (Cowling BJ-personal communication). In the face of resistance from the general public and the poultry trade, authorities in mainland China have implemented retail and wholesale LPM closure in key municipalities and provincial capitals for various durations as a reactive response to outbreaks of human A/H7N9 disease (Table, Figure 1). These decisions have been taken at the local, municipal and provincial level rather than by the central government. Thus there is wide variation in the measures adopted across different cities and the duration of their implementation.
Furthermore, centrally slaughtered chilled-chicken carcasses may not be a “zero-risk” option if the slaughtered poultry arise from poultry endemically infected with zoonotic avian influenza viruses. For example, HPAI H5N1 has been repeatedly isolated from duck meat imported into Japan and South Korea35 and thus slaughtered poultry carcasses could potentially be a source of human infection prior to cooking although such an event has not been directly documented. A change of consumption pattern from freshly killed to chilled chicken carcass may also increase risk of other food borne infections such as Campylobacter and Salmonella.
LPM rest days
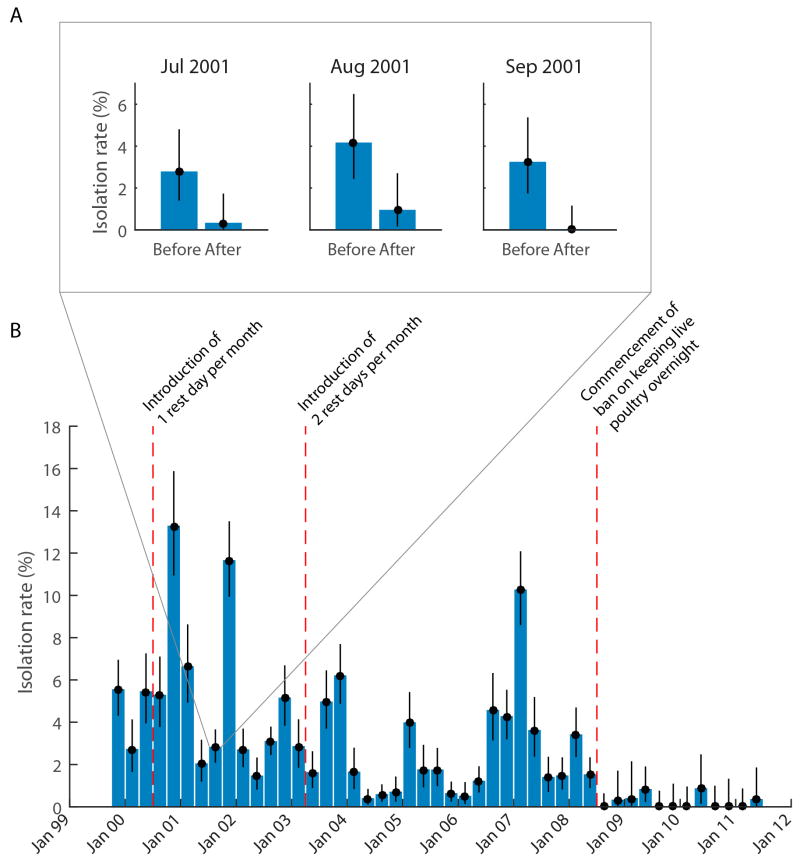
While the pros and cons of permanently shutting down retail and wholesale LPM in favour of centralised slaughter of poultry were being debated over the past decade in Hong Kong SAR, local studies have shown that a number of other interventions had significantly reduced the risk of virus amplification and maintenance within retail LPM, thus reducing zoonotic risks. “Rest days” in the whole-sale and retail LPM, where the markets are emptied of live poultry for a day, washed and cleaned and restocked with poultry the next day was introduced in 2001 (once a month during 2001–2003 and twice monthly from 2003–2008).23 Figure 2A shows the A/H9N2 virus isolation rates in LMP immediately prior to and after a “rest day”.36 The data demonstrate that these viruses are introduced into these markets infrequently, but once introduced, remain circulating within the retail LPM environment The setting where new susceptible poultry are added to the market every day provides an ideal milieu for such viruses to continue to amplify and be maintained. A/H9N2 virus circulation in retail LPM systematically sampled monthly from 1999 to 2011 showed the significant impact of these interventions (Figure 2B).37
Figure 2. Impact of interventions in retail live poultry markets on isolation of avian influenza viruses.
A) Bar chart showing A/H9N2 isolation rates and 95% confidence intervals before and after a “rest day” in 8 live poultry market stalls in Hong Kong SAR in three consecutive months in 2001. Faecal swabs were collected immediately prior to and after the market “rest day” during which the market was emptied of live poultry for one day. The reduction in virus isolation rates for each month was statistically significant.36 B) The effect of interventions on isolation rates of H9N2 viruses in retail live poultry markets in Hong Kong SAR during the period 1999–2011. Systematic surveillance in live poultry markets carried out monthly from 1999 to 2011 with fecal droppings from poultry cages being collected and tested for influenza virus isolation (n=53, 541 swabs tested). The data is presented as three monthly aggregate mean isolation rates with 95% confidence intervals. The impact of the introduction of one rest day per month, two rest days per month and banning keeping of live poultry overnight within the market is shown.37
Ban holding poultry overnight in LMP
Since 2008, Hong Kong SAR implemented a ban on keeping live poultry overnight in retail LPM; i.e. poultry that are unsold by evening are slaughtered for sale as chicken carcass. The retail market is therefore emptied overnight and re-stocked next morning. This intervention dramatically further reduced viral detection rates within retail LPM (Figure 2B).37 A major reason for the effectiveness of this intervention is that incoming chicken are not in the market for long enough to be newly infected, shed virus and infect others as simulated in mathematical models.38 The emptying of the market of live poultry overnight deprives the virus of a permissive host for replication. Disinfection and cleaning of markets while live poultry remain within them is unlikely have this impact. It is important that faecal matter, drinking water used by poultry and poultry feed is removed and replenished. Virus survival in water is longer than that on surfaces.39
Relevance and impact
The importance of both wholesale and retail LMP in virus amplification has been noted in the spread of H7N9.40 This is also demonstrated in a recent study in Guangdong where none of the 8 farms tested had evidence of A/H7N9 virus, but 16 of 36 LPM and 3 of 6 wholesale markets were infected.30 There is so far no direct evidence of the impact of LMP rest days on A/H7N9 virus activity and such studies are urgently needed. Thus, even if a complete change in the poultry trade from one where live birds are sold to customers to one where they are slaughtered centrally with chilled or frozen chicken retailed to customers is not possible in the short term, the aforementioned less disruptive interventions in the wholesale and retail LMP are likely to have a profound effect in reducing zoonotic risk, thus pandemic risk. These interventions appear to have protected Hong Kong from zoonotic avian influenza for the past decade; all cases of zoonotic in the period diagnosed in Hong Kong were acquired in mainland China.
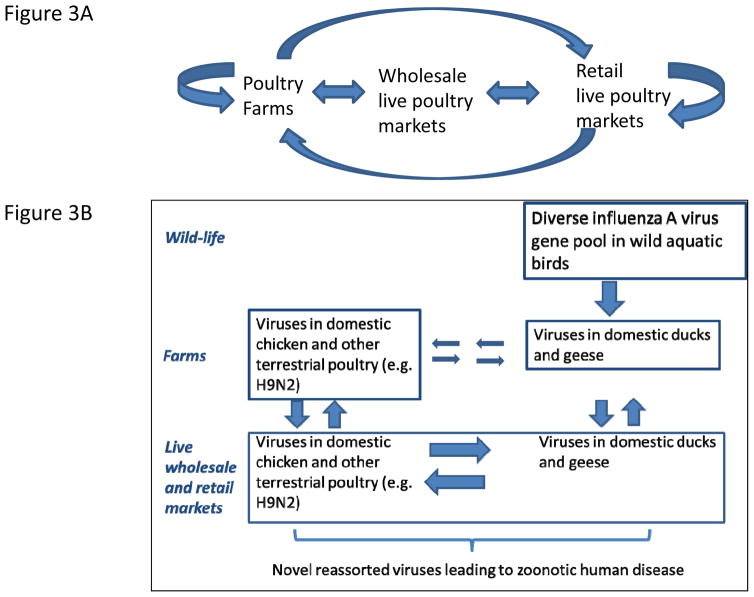
In fact, these LPM interventions are not only effective in minimising the risk of zoonoses, they would also reduce the spread of avian influenza viruses to, and between poultry farms. 41,42 A case-control study during an A/H5N1 outbreak in poultry farms in Hong Kong SAR in 2002 showed that virus amplification within retail LPM was the source of virus introduction to farms through the movement of fomites (e.g. poultry cages) and personnel (Figure 3A).41 Poultry cages should be of easily cleanable material (i.e. polypropylene rather than wood or bamboo) and cleaned before moving between LMPs and farms.23 Live poultry markets have also been shown to be the source of virus introduction back to farms in USA.42
Figure 3. Avian influenza virus transmission and amplification within the poultry marketing chain (A) and influenza virus gene-transfer between wild birds, aquatic and terrestrial poultry (B).
Mixing of ducks and geese with chicken and other terrestrial poultry (figure 3B) allows reassortment of viruses these viruses with viruses established in terrestrial poultry such as H9N2 leading to the emergence of novel influenza viruses with zoonotic potential.43–46
Reducing risk of emergence at source
The last two decades have seen the emergence of a number of viruses with zoonotic potential, including subtypes A/H5N1, A/H9N2, A/H7N9, A/H6N1, A/H10N8 and A/H5N6. Studies on the evolutionary pathways for the emergence of these viruses reveal important commonalities. Both A/H7N9 and A/H10N8 derived their HA and NA gene segments from viruses of wild aquatic birds giving rise to intermediate viruses within domestic ducks.43–45 However, such viruses are poorly adapted to terrestrial poultry (e.g. chicken) and they underwent further genetic reassortment with A/H9N2 viruses endemic in terrestrial poultry to give rise to viruses well adapted in chickens. 43–45 Thus, it is the mixing of the viruses of domestic ducks and terrestrial poultry (e.g. chicken, quail) in the marketing chain that has been crucial to the emergence of both A/H7N9 and A/H10N8 viruses (Figure 3B). Indeed, this was also the underlying mechanism in the emergence of HPAI A/H5N1 viruses in 1997 and in 2004, with domestic geese as well as ducks being involved.46 Thus, separation of terrestrial poultry (chicken, quail, pheasant, chukka) from domestic ducks and geese in the wholesale and retail marketing chain will contribute substantially to the long-term reduction of risk of emergence of new avian influenza viruses adapted to chicken and other terrestrial poultry, which in turn appear to be those that have the greatest zoonotic and pandemic potential. While it is clearly not possible to prevent the mingling of ducks from chickens in rural backyard settings, this is probably not as important as the large-scale intermingling and genetic reassortment of viruses that takes place within the wholesale and retail poultry marketing systems in China and other parts of Asia.38 Re-engineering these poultry marketing systems to separate aquatic from terrestrial poultry in the longer term is sustainable and not beyond the realms of feasibility in Asia. Such an intervention was introduced in Hong Kong SAR following the initial H5N1 outbreak in 1997 and continues to date, with aquatic poultry (ducks, geese) being centrally slaughtered though terrestrial poultry continued to be sold in retail LPM.23
Conclusions
Over the past two decades, many newly emerged avian influenza viruses have posed zoonotic threats. Avian influenza viruses also contribute to pandemic emergence, although it is difficult to accurately assess pandemic risk attributable to an individual virus. Thus, rather than target individual virus subtypes and strains, generic measures targeting critical points along the pathways of virus emergence are attractive interventions. There is increasing evidence that sustainable interventions in live poultry marketing systems can reduce opportunities for transmission of avian influenza to humans, thus reducing risk of zoonotic (and possibly, pandemic) influenza. Ideally, such interventions would involve a complete transition from sale of live poultry in wholesale and retail LPMs to centralised slaughter and sale of chilled or frozen poultry. The latter already comprises about 50% of the total poultry consumption in mainland China. However, there are major short term hurdles in implementation, both in terms of cultural preference and perceptions of food security. Therefore, in the interim, less disruptive measures including “rest days” and banning live poultry overnight can be considered, interventions that have been clearly shown to reduce risk, both for human health as well as for the poultry industry. On a longer term basis, there is accumulating evidence that separation of the wholesale and retail marketing of ducks and geese from terrestrial poultry will reduce viral genetic reassortments leading to emergence of zoonotic, epizootic (and potentially pandemic) viruses at source. This would indeed be an excellent illustration of the application of the “One Health” concept and one that would benefit both human and animal health (e.g. HPAI H5N1) in the longer term. As a contemporary example, such interventions should receive high priority in mainland China, as well as other Asian countries such as Vietnam which are at risk of introduction of A/H7N9 through legal and illegal movements of live poultry across borders. Importantly, because of their generic nature, such measures are likely to sustainably reduce zoonotic and pandemic threats posed by avian influenza viruses in general.
Table 1.
Implementation of interventions in live poultry markets in response to influenza A(H7N9) epidemics in China.
| Province | City | Temporary LPM closure
|
Permanent LPM closure
|
Interventions on non-closed LPMs | ||
|---|---|---|---|---|---|---|
| Starting date | Duration | Starting date | Areas | |||
| Zhejiang^ | Hangzhou | 21–30 Jan 2014 | 1–6 months | 15 Feb 2014 | downtown area | Daily disinfection, LPMs should not open more than 10 consecutive days, at least 3 rest-days every month, ban on overnight stay of live poultry in LPMs, temporary LPM closure is initiated by H7N9 virus detection in poultry |
| Ningbo | 26–30 Jan 2014 | 4–6 months | 1 Jul 2014 | 4 districts of the city | ||
| Shaoxing | 23 Jan 2014 | 4–6 months | 1 Jul 2014 | 3 districts of the the city | ||
| Wenzhou | 8 Feb 2014 | 5 months | 1 Jul 2014 in | 5 districts of the city | ||
| Guangdong | Guangzhou | 23 Jan–15 Feb 2014 | 1 day–2 weeks | _ | Daily disinfection, weekly cleaning, monthly rest-day | |
| Shenzhen | 31 Jan 2014 | 2 weeks | _ | Temporary LPM closure due to detection of H7N9 virus in poultry | ||
| Foshan | 7–25 Jan 2014 | 2 days–2 weeks | Jun 2014 | downtown areas of different districts in the city | Ban on overnight stay of live poultry in retail markets, daily disinfection, weekly cleaning, and monthly rest-day | |
| Dongguan | - | - | - | Temporary LPM closure due to detection of H7N9 virus in poultry | ||
| Jiangsu | Nanjing# | 1 Apr 2014 | 5 months | 1 Jan 2015 | 6 districts of the main urban area of the city | Temporary LPM closure due to detection of H7N9 virus in poultry, market disinfection and cleaning |
| 5 Nov 2014 | 2 months | |||||
| Suzhou | 12 Jan 2015 (4 districts) | - | 12 Dec 2014 | Gusu district | 1 rest-day per week, ban on live poultry stock during rest-days | |
| Shanghai | Shanghai | 31 Jan 2014 | 3 months | 1 May 2014 | Jing’an district | 1 rest-day weekly for wholesale markets, 1 rest-day bi-weekly for retail markets |
| Hunan | Changsha | 19 Feb 2014 | 1 week | _ | Regular disinfection, cleaning, rest-day of LPMs | |
| Fujian | All cities | * | * | _ | Regular disinfection, cleaning, rest-day of LPMs | |
| Xinjiang | Urumqi | 13 Jan 2015 | - | - | - | |
Starting on 1 Jul 2014 in the main urban area of all municipalities that have administrative districts in Zhejiang province.
LPM closure was only implemented in 6 districts of the main urban area in Nanjing.
Rest-days were implemented in districts/counties/cities with identification of H7N9 human case(s).
Abbreviation: LPM - live poultry market
Acknowledgments
The authors thank Peng Wu and Bingyi Yang for technical support. This was supported in part from the Area of Excellence Scheme of the University Grants Committee [AoE/M-12/06] of the Hong Kong Special Administrative Region Government, China; the National Institute of Allergy and Infectious Diseases (Contract HHSN272201400006C); the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558); a commissioned grant from the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region; the US National Institutes of Health (Comprehensive International Program for Research on AIDS grant U19 AI51915) and the China-US Collaborative Program on Emerging and Re-emerging Infectious Diseases, grants from the Ministry of Science and Technology, China (2012 ZX10004-201). The funding sources played no role in drafting the manuscript.
Footnotes
Contributors: MP, BJC, JTW,LF,YG, HY, GML were involved in the conception of the ideas laid out in this manuscript; LF and HY collated the data in Table 1; BJC and JTW prepared figures; MP wrote the first draft and all co-authors critically commented on the text.
Conflicts of interests: Authors have nothing to disclose.
References
- 1.Wu JT, Leung K, Perera RA, et al. Inferring influenza infection attack rate from seroprevalence data. PLoS Pathog. 2014;10:e1004054. doi: 10.1371/journal.ppat.1004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai M, Herfst S, Sorrell EM, et al. Transmission of influenza A/H5N1 viruses in mammals. Virus Res. 2013;178:15–20. doi: 10.1016/j.virusres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 4.Xiao XC, Li KB, Chen ZQ, et al. Transmission of avian influenza A(H7N9) virus from father to child: a report of limited person-to-person transmission, Guangzhou, China, January 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.25.20837. pii: 20837. [DOI] [PubMed] [Google Scholar]
- 5.van Riel D, Leijten LM, de Graaf M, et al. Novel avian-origin influenza A(H7N9) virus attaches to epithelium in both upper and lower respiratory tract of humans. Am J Pathol. 2013;183:1137–43. doi: 10.1016/j.ajpath.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan MC, Chan RW, Chan LL, et al. Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med. 2013;1:534–42. doi: 10.1016/S2213-2600(13)70138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Wang D, Kelvin DJ, et al. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science. 2013;341:183–6. doi: 10.1126/science.1239844. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. [accessed 27 March 2015];Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2015. 2015 http://www.who.int/influenza/human_animal_interface/EN_GIP_20150303cumulativeNumberH5N1cases.pdf?ua=1.
- 9.Ip DK, Liao Q, Wu P, et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: case series. BMJ. 2013;346:f3693. doi: 10.1136/bmj.f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Cowling BJ, Feng L, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382:138–45. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viboud C, Simonsen L. Timely estimates of influenza A H7N9 infection severity. Lancet. 2013;382:106–8. doi: 10.1016/S0140-6736(13)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L, Wu JT, Liu X, et al. Clinical severity of human infections with avian influenza A(H7N9) virus, China, 2013/14. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.49.20984. pii=20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Fang S, Lu X, et al. Seroprevalence to avian influenza A(H7N9) virus among poultry workers and the general population in southern China:a longitudinal study. Clin Infect Dis. 2014;59:e76–83. doi: 10.1093/cid/ciu399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–61. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Yang L, Gao R, et al. Genetic tuning of the novel avian influenza A(H7N9) virus during interspecies transmission, China, 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.25.20836. pii: 20836. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Yuan H, Gao R, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–21. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Cui L, Yu H, Ge Y, Tang F, et al. Whole-Genome Sequence of a Reassortant H5N6 Avian Influenza Virus Isolated from a Live Poultry Market in China, 2013. Genome Announc. 2014;2 doi: 10.1128/genomeA.00706-14. pii: e00706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J, Zhang L, Kan X, et al. Origin and molecular characteristics of a novel 2013 avian influenza A(H6N1) virus causing human infection in Taiwan. Clin Infect Dis. 2013;57:1367–8. doi: 10.1093/cid/cit479. [DOI] [PubMed] [Google Scholar]
- 19.Jhung MA, Nelson DI Centers for Disease Control and Prevention (CDC) Outbreaks of avian influenza A (H5N2), (H5N8), and (H5N1) among birds—United States, December 2014–January 2015. MMWR Morb Mortal Wkly Rep. 2015;64:111. [PMC free article] [PubMed] [Google Scholar]
- 20.Cox NJ, Trock SC, Burke SA. Pandemic Preparedness and the Influenza Risk Assessment Tool (IRAT) Curr Top Microbiol Immunol. 2014;385:119–36. doi: 10.1007/82_2014_419. [DOI] [PubMed] [Google Scholar]
- 21.WHO. [accessed 201 January 2015];Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. 2015 http://www.who.int/influenza/vaccines/virus/201409_zoonotic_vaccinevirusupdate.pdf?ua=1.
- 22.Heymann DL, Dixon M. Infections at the animal/human interface: shifting the paradigm from emergency response to prevention at source. Curr Top Microbiol Immunol. 2013;366:207–15. doi: 10.1007/82_2012_285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sims LD, Peiris M. One health: the Hong Kong experience with avian influenza. Curr Top Microbiol Immunol. 2013;365:281–98. doi: 10.1007/82_2012_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao QY, Lam WW, Dang VT, et al. What causes H5N1 avian influenza? Lay perceptions of H5N1 aetiology in South East and East Asia. J Public Health (Oxf) 2009 Dec;31(4):573–81. doi: 10.1093/pubmed/fdp043. [DOI] [PubMed] [Google Scholar]
- 25.Mounts AW, Kwong H, Izurieta HS, et al. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180:505–8. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Havers F, Chen E, et al. Risk factors for influenza A(H7N9) disease--China, 2013. Clin Infect Dis. 2014;59:787–94. doi: 10.1093/cid/ciu423. [DOI] [PubMed] [Google Scholar]
- 27.Shortridge KF, Peiris JS, Guan Y. The next influenza pandemic: lessons from Hong Kong. J Appl Microbiol. 2003;94(Suppl):70S–79S. doi: 10.1046/j.1365-2672.94.s1.8.x. [DOI] [PubMed] [Google Scholar]
- 28.Indriani R, Samaan G, Gultom A, et al. Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg infect Dis. 2010;16:1889–1895. doi: 10.3201/eid1612.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam TT, Zhou B, Wang J, et al. Dissemination, divergence and establishment of H7N9 influenza viruses in China. [Accessed 27 March 2015];Nature. 2015 doi: 10.1038/nature14348. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Li K, Luo L, et al. Detection of avian influenza A(H7N9) virus from live poultry markets in Guangzhou, China: a surveillance report. PLoS One. 2014;9:e107266. doi: 10.1371/journal.pone.0107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Wu JT, Cowling BJ, et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2014;383:541–8. doi: 10.1016/S0140-6736(13)61904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu P, Jiang H, Wu JT, et al. Poultry market closures and human infection with influenza A(H7N9) virus, China, 2013–14. Emerg Infect Dis. 2014;20:1891–4. doi: 10.3201/eid2011.140556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong HK, Goh CS, Chew ST, Lim CW, Lin YN, Chang SF, Yap HH, Chua SB. Prevention and control of avian influenza in Singapore. Ann Acad Med Singapore. 2008;37:504–9. [PubMed] [Google Scholar]
- 34.Yuan J, Liao Q, Xie CJ, et al. Attitudinal changes toward control measures in live poultry markets among the general public and live poultry traders, Guangzhou, China, January–February, 2014. Am J Infect Control. 2014;42:1322–4. doi: 10.1016/j.ajic.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Tumpey TM, Suarez DL, Perkins LE, Senne DA, Lee JG, Lee YJ, Mo IP, Sung HW, Swayne DE. Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat. J Virol. 2002;76:6344–55. doi: 10.1128/JVI.76.12.6344-6355.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kung NY, Guan Y, Perkins NR, Bissett L, Ellis T, Sims L, Morris RS, Shortridge KF, Peiris JS. The impact of a monthly rest day on avian influenza virus isolation rates in retail live poultry markets in Hong Kong. Avian Dis. 2003;47(3 Suppl):1037–41. doi: 10.1637/0005-2086-47.s3.1037. [DOI] [PubMed] [Google Scholar]
- 37.Leung YH, Lau EH, Zhang LJ, Guan Y, Cowling BJ, Peiris JS. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerg Infect Dis. 2012;18:1339–41. doi: 10.3201/eid1808.111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepin KM, Lloyd-Smith JO, Webb CT, et al. Minimizing the threat of pandemic emergence from avian influenza in poultry systems. BMC infect Dis. 2013;13:592. doi: 10.1186/1471-2334-13-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung YH, Zhang LJ, Chow CK, et al. Poultry drinking water used for avian influenza surveillance. Emerg Infect Dis. 2007;13:1380–2. doi: 10.3201/eid1309.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Organisation for Animal Health. (OIE) visit to the People’s Republic of China to investigate influenza A (H7N9) infections in poultry 25 April – 1 May 2013. http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/RD_China_H7N9_June2013.pdf.
- 41.Kung NY, Morris RS, Perkins NR, et al. Risk for infection with highly pathogenic influenza A virus (H5N1) in chickens, Hong Kong, 2002. Emerg Infect Dis. 2007;13:412–8. doi: 10.3201/eid1303.060365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trock SC, Huntley JP. Surveillance and control of avian influenza in the New York live bird markets. Avian Dis. 2010;54(1 Suppl):340–344. doi: 10.1637/8728-032409-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 43.Lam TT, Wang J, Shen Y, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013;502:241–4. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi W, Zhou X, Shi W, et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.25.20841. pii: 20841. [DOI] [PubMed] [Google Scholar]
- 45.Ma C, Lam TTY, Chai Y, et al. Emergence and evolution of H10 subtype influenza viruses in poultry in China: implications for the generation of a potential pandemic strain. J Virol. 2015 doi: 10.1128/JVI.03167-14. – e pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan Y, Smith GJ. The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res. 2013;178:35–43. doi: 10.1016/j.virusres.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]