Abstract
Cognitive changes that occur during mid-life and beyond are linked to both aging and the menopause transition. Studies in women suggest that the age at menopause onset can impact cognitive status later in life; yet, little is known about memory changes that occur during the transitional period to the post-menopausal state. The 4-vinylcyclohexene diepoxide (VCD) model simulates transitional menopause in rodents by depleting the immature ovarian follicle reserve and allowing animals to retain their follicle-deplete ovarian tissue, resulting in a profile similar to the majority of perimenopausal women. Here, Vehicle or VCD treatment was administered to ovary-intact adult and middle-aged Fischer-344 rats to assess the trajectory of cognitive change across time with normal aging and aging with transitional menopause via VCD-induced follicular depletion, as well as to evaluate whether age at the onset of follicular depletion plays a role in cognitive outcomes. Animals experiencing the onset of menopause at a younger age exhibited impaired spatial memory early in the transition to a follicle-deplete state. Additionally, at the mid- and post- follicular depletion time points, VCD-induced follicular depletion amplified an age effect on memory. Overall, these findings suggest that the age at the onset of menopause is a critical parameter to consider when evaluating learning and memory across the transition to reproductive senescence. From a translational perspective, this study illustrates how age at menopause onset might impact cognition in menopausal women, and provides insight into time points to explore for the window of opportunity for hormone therapy during the menopause transition period. Hormone therapy during this critical juncture might be especially efficacious at attenuating age- and menopause- related cognitive decline, producing healthy brain aging profiles in women who retain their ovaries throughout their lifespan.
Keywords: Menopause, Aging, VCD, Cognition, Learning, Memory, Hormone, Ovary
Introduction
Women typically begin to experience a natural transition to menopause, or the post-reproductive state, during the fifth decade of life (NAMS, 2015; Soules et al., 2001). This transitional stage, often referred to in the clinic as “perimenopause” or “climacteric,” is characterized by irregular menstrual cycles and erratic, fluctuating ovarian hormone levels that can last up to ten years before the final menstrual period (NAMS, 2015; Hoffman et al., 2012). Consequently, a range of physiological indicators often accompany the menopause transition, including vasomotor symptoms (e.g., hot flashes, night sweats), dyspareunia, genitourinary issues, sleep and mood alterations, and memory complaints (Al-Safi & Santoro, 2014; NAMS, 2015; Weber et al., 2014). Indeed, menopause and normal aging have each been associated with memory impairment (Tulving & Craik, 2000; Sullivan Mitchell & Fugate Woods, 2001). Both the type of menopause a woman experiences (transitional or surgical), and the age at which she experiences this climacteric change, may impact cognitive function later in life. Research in women suggests that oophorectomy, the surgical removal of the ovaries, detrimentally impacts cognition, and that this effect depends on the relationship to menopause status. Indeed, there is evidence that oophorectomy before the onset of the transition to menopause may result in a more negative impact on verbal memory and an increased risk of developing dementia than retaining the ovaries throughout the menopause transition, or compared to oophorectomy after the menopause transition is complete (Farrag et al., 2002; Nappi et al., 1999; Rocca et al., 2007, 2011, 2012). Thus, understanding the cognitive effects during and after transitional menopause could be fundamentally important to women who may need to undergo surgical menopause.
Menopause typically occurs around age 51; however, some women experience early-onset menopause, defined as the final menstrual period occurring before the age of 45. In addition, spontaneous premature ovarian insufficiency, wherein the final menstrual period occurring before age 40, affects a small percentage of women (Pal & Santoro, 2002; Shuster et al., 2010; Simpson and Rajkovic, 1999). Evaluating whether age at the onset of premature, early, and normal menopause impacts cognition may provide insight into the divergent effects observed for memory and other age-related health factors associated with menopause and the post-reproductive life stage.
In preclinical research, the gold standard for evaluating the impact of gonadal hormone loss and exogenous administration of hormone therapy on memory performance is ovariectomy (Ovx), or the surgical removal of the ovaries, which results in an abrupt loss of ovarian hormones. However, in the context of translational preclinical research, this classic technique only models a small percentage of women who undergo surgical menopause via bilateral oophorectomy (Centers for Disease Control and Prevention, 2010; Hall et al., 2010). Most women experience a gradual, natural menopause transition and typically maintain their reproductive organs into the post-menopausal life stage. While rodents are animal models often utilized in hormone research, rats and mice do not experience menopause; they undergo estropause (Finch, 2014; Meites & Lu, 1994). In contrast to human menopause, whereby immature follicles in the ovaries are depleted via natural atresia, rodents do not experience follicular loss to the same extent as women. Rather, the driving mechanism underlying reproductive senescence in rodents is a significant dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis in middle age (for review, see Downs and Wise, 2009; Finch, 2014; Wise, 2002; Wise et al., 1989, 1996, 1997, 1999). Thus, the introduction of 4-vinylcyclohexene diepoxide (VCD) as a rodent model of transitional menopause has given researchers another tool to model menopause in the preclinical laboratory (for review, see Koebele and Bimonte-Nelson, 2016). VCD targets primordial and primary ovarian follicles by initiating accelerated atresia, or programmed cell death, in the ovary (Hoyer et al., 2001; Springer et al., 1996c), resulting in follicular depletion and eventual ovarian failure in rodents (Borman et al., 1999; Flaws et al., 1994; Hirshfield, 1991; Hoyer et al., 2001; Hu et al., 2001a, 2001b; Kao et al., 1999; Mayer et al., 2002, 2004, 2005; Springer et al., 1996a, 1996b, 1996c). As such, VCD provides a translational tool for evaluating ovarian and hormone changes that occur across the transition to a follicle-deplete state. Indeed, compared to Ovx animals, VCD-treated animals exhibit hormone profiles more similar to transitionally menopausal women, and allow for the retention of follicle-deplete ovarian tissue like most women who transition to menopause without surgical intervention (Burger, 2006; Timiras et al., 1995; Frye et al., 2012). In rats, VCD treatment uniquely models perimenopause, in which follicular depletion is accelerated and gonadal hormone levels fluctuate over time (Frye et al., 2012; Mayer et al., 2002). As such, the VCD-induced menopause model is ideal for studying aging and the menopause transition, including the early stages of the transition when a multitude of physiological and affective symptoms begin to present in many women (Hale et al., 2014). Our laboratory has previously shown that middle-aged rats that experienced VCD-induced follicular depletion demonstrated impaired working and recent memory in the post-depletion time point compared to animals that did not have this VCD-induced accelerated follicular depletion, and compared to animals that underwent Ovx or VCD treatment followed by Ovx (Acosta et al., 2009). However, spatial memory performance has not yet been evaluated during the transition to a follicle-deplete state using a rat model.
Here, we aimed to elucidate the longitudinal cognitive effects of transitional menopause via VCD-induced follicular depletion in ovary-intact Fischer-344 rats, and whether there were differences in cognition depending on the age at which accelerated follicular depletion was experimentally initiated, with VCD treatment beginning at either six or twelve months of age. In addition, we also longitudinally assessed performance in vehicle-treated ovary-intact rats at two age cohorts, evaluating the younger group as they aged from adulthood (6 months) to middle age (12 months), and the older group as they aged from middle age (12 months) to aged (18 months). Thus, the goals of evaluation for each age cohort were manifold: (1) to longitudinally investigate learning and memory as experimentally-induced follicular depletion ensues, (2) to longitudinally evaluate learning and memory in the normally aging rat, (3) to assess the hormonal and ovarian changes that occur during VCD-induced follicular depletion that may relate to behavioral outcomes, and (4) to evaluate the hormonal and ovarian changes that occur with normal aging that may relate to behavioral outcomes. Given that some research in women suggests that the loss of ovarian hormones earlier in life (including non-surgical, naturally premature or early transitional menopause, as well as surgical menopause) may be detrimental to cognition (Ryan et al., 2014; Rocca et al., 2007), we hypothesize that if transitional menopause is induced at an earlier age, there will be a greater negative impact on cognition than if transitional menopause is induced at a later age. The VCD model affords us the opportunity to methodically test this hypothesis. Further, we predict that vehicle-treated, regularly aging animals will exhibit impaired memory performance as aging ensues; vehicle-treated controls were included to address this question. This inclusion of vehicle-treated, regularly aging animals also allows for a direct comparison to the VCD-treated animals with induced menopause across the transition to a follicle-deplete state.
Beginning at either six or twelve months of age, animals were trained on a water radial-arm maze, a complex spatial task requiring both working and reference memory. Of note, spatial memory necessitates the use of distal, extra-maze cues to solve a task, working memory is a type of short-term memory that needs to be updated, and reference memory is a form of long-term memory that stays constant (Bimonte-Nelson et al., 2015). Following training, subjects were administered VCD or vehicle treatment, and then repeatedly tested on the same water radial-arm maze task over a four-month period during the VCD-induced menopause transition. We examined the impact of altered hormone profiles on memory, how memory ability changes across this transition, and whether the age at follicular-depletion initiation influences cognitive performance. Animals were also tested on a task requiring cognitive flexibility, as well as a reference memory task following the water radial-arm maze evaluations to assess performance on unfamiliar tasks in the post-follicular depletion time point prior to sacrifice.
Methods
Animals
Animals used for this study were 56 female virgin Fischer-344 rats obtained from the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). At the beginning of the study, rats were either 6 months (n=28) or 12 months (n=28) of age. Upon arrival, rats were pair-housed, given food and water ad libitum, and maintained on a 12-hour light/dark cycle for the entirety of the study. Animals were given one week to acclimate in the vivarium prior to the commencement of the experiment. All procedures were approved by the Arizona State University Institutional Animal Care and Use Committee and adhered to National Institutes of Health standards.
Experimental timeline
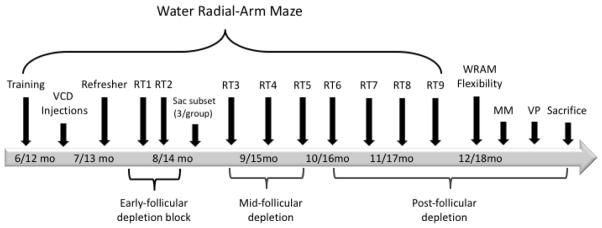
The following methods are described in chronological order of occurrence during the experiment. Prior to treatment, all animals were trained on the water radial-arm maze (WRAM), described in detail below. Pre-treatment training on the WRAM was conducted at six or twelve months of age and prior to VCD-induced menopause in order to obtain a baseline measure of learning and memory performance. Pre-treatment training also served to familiarize the animals with the task, allowing future evaluations of memory for a familiar task. Animals were randomly assigned to one of two treatments (balanced across age groups): Vehicle or VCD (to induce follicular depletion). After Vehicle or VCD treatment was administered, animals were subsequently given a two-day refresher session on the WRAM, followed by a two-day retest every other week for four months to obtain a behavioral profile across the early- , mid- , and post- follicular depletion time points. A subset of animals (n=3/group) were randomly selected for sacrifice during early follicular depletion. Following the final WRAM retest, the remaining animals were evaluated on several other tasks (described below) prior to sacrifice, including a flexibility measure where parameters had to be relearned for a revised WRAM task, the Morris water maze task, and the Visible Platform task. An overview of the study timeline can be found in Figure 1.
Figure 1.
Study Timeline. Age of the young and middle-aged animals in months (mo) at time of assessment is indicated in the arrow. Animals received training for 12 days on the water radial-arm maze (WRAM). A one-month interim occurred during which animals received Vehicle or VCD injections. After a two-day refresher, subjects were tested for two-day retests (RT) every other week for four months, capturing the VCD-treated animals’ transition from early follicular depletion to a post-follicle-deplete state. A subset of animals was sacrificed after RT2 to obtain a snapshot of serum hormone levels and ovarian follicular depletion early in the transition. Following WRAM RTs, remaining subjects were tested on a WRAM flexibility task, Morris water maze, and Visible Platform tasks prior to sacrifice.
Water radial-arm maze training
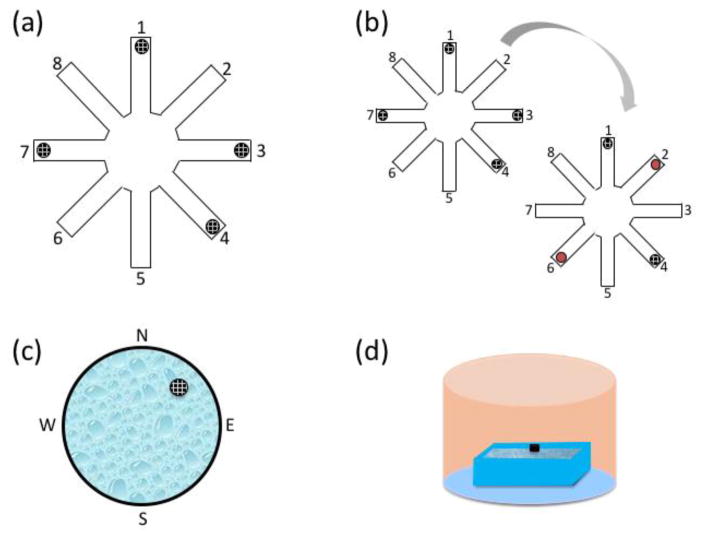
One week after arrival, and before any experimental hormonal manipulation, animals were trained for 12 days on the win-shift WRAM, evaluating spatial working and reference memory, as previously described (Figure 2a; Bimonte & Denenberg, 1999; Bimonte et al., 2000, 2002, 2003; Bimonte-Nelson et al., 2003, 2004). Briefly, the WRAM is an eight-arm apparatus (each arm 38.1 x 12.7 cm) filled with room temperature water (18–20°C) tinted with non-toxic, powdered black tempera paint. Four out of eight arms contained a hidden platform (11 cm diameter) just beneath the surface of the water. Each subject was assigned to a set of unique platform locations that remained fixed across all days of testing, including the training period and all subsequent retests. The room contained salient extra-maze spatial cues to aid in spatial navigation. All animals received a total of four trials per day of testing. Each rat was released from the start arm and had three minutes to find a hidden platform. If the platform was not located within the allotted three-minute trial, the experimenter led the animal to the nearest platform. Once the platform was located, the rat remained on the platform for 15 seconds before the experimenter returned the animal to its heated testing cage for an inter-trial interval (ITI) of 30 seconds. During the ITI, the just-found platform was removed from the maze, and the experimenter cleaned the water to remove any debris and to interrupt potential olfactory cues. The rat was placed back into the start arm and given three minutes to locate the next platform. Because one platform was removed after each trial, the working memory system was increasingly taxed as trials progressed. The daily testing session ended when the animal had located all four hidden platforms. Errors were quantified as an entry into a non-platformed arm; an arm entry was counted when the tip of the rat’s snout crossed a mark delineated on the outside of the arm (not visible from inside the maze; 11 cm into the arm).
Figure 2.
Schematics of the behavioral battery used throughout the experiment. (a) The water radial-arm maze (WRAM) contains four hidden platforms beneath the surface of the water, represented by black circles. Subjects were each assigned a set of platform locations that remained constant throughout retests. Platform locations varied across animals and were counterbalanced for age and treatment group. After one platform was located on each trial, the just-found platform was removed from the maze for the remainder of the day. (b) The WRAM Flexibility task occurred after RT9. Each subject was given two familiar platform locations from their initial platform location assignment (e.g. arms 1,4), and two platform locations were flipped to be located in a novel arm (e.g, platforms in arms 3 and 7 were moved to arms 2 and 6, indicated by the red circles), resulting in two novel spatial locations that require updating. (c) The Morris water maze was a large round tub with a hidden platform submerged beneath the water’s surface in the northeast quadrant. The platform location remained constant across all baseline days and trials. Subjects were dropped off from each cardinal direction (north, south, east, and west) once per day. The order in which the drop off locations occurred was the same for all animals within a day, but varied across days. After the fourth trial on Day 5, the platform as completely removed from the maze to conduct the probe trial; animals were dropped off from the west for the probe trial. (d) The visible platform was a rectangular tub filled with clear water, with a black platform placed approximately 4 cm above the surface of the water. Opaque curtains were hung in a circular fashion around the room to block any spatial and geometric cues. Animals were dropped off from the south wall and were given 90s to reach the platform, which was located on the north wall. The platform location was the same for all animals within a trial, but varied across trials (left, center, and right of the drop off location).
VCD Injections
Animals in each age cohort were randomly assigned to either the Vehicle or VCD group. Animals were classified as “Young Vehicle” (n=13), “Young VCD” (n=15), “Middle-Aged Vehicle” (n=13), or “Middle-Aged VCD” (n=15) to signify whether animals were young (6 mo) or middle-aged (12 mo) at the beginning of the experiment, and whether they underwent VCD-induced follicular depletion. VCD was administered for a total of 15 days via intraperitoneal injection at 160 mg/kg/day (SenesTech Inc., Flagstaff, AZ) in 47% dimethyl sulfoxide (DMSO)/saline vehicle (Sigma-Aldrich, St. Louis, MO). The vehicle injection was 0.5 ml of 47% DMSO/saline solution. Daily VCD injection volume was determined by the animal’s weight. Animals were not injected if they decreased below 90% of their initial body weight; injections were resumed when weight was regained. VCD injections were administered on Monday, Tuesday, Thursday, and Friday; injections were not administered Wednesday, Saturday, or Sunday to allow animals to recover any weight lost, and were completed over the course of one month.
Water radial-arm maze refresher and retests
Following the initial training on the WRAM task and subsequent VCD or Vehicle administration, all animals were given a two-day refresher on the WRAM, with procedures and platform locations identical to the initial 12 days of training. Immediately following two days of refresher practice, the first WRAM retest took place. Each retest lasted two days. A total of nine retests occurred every other week for four months. Because ovarian follicular depletion via VCD takes approximately three months from the first injection (Mayer et al., 2002, 2004), these retests captured behavioral snapshots throughout the transition to the follicle-deplete state, as well as throughout aging independent of VCD-induced follicular depletion in Vehicle-treated groups.
Water radial-arm maze flexibility task
Following the final WRAM retest, the animals were evaluated for cognitive flexibility. That is, we tested cognitive flexibility by examining the animals’ ability to update previously learned information in the WRAM task. Specifically, we shifted the reward locations within the WRAM task so that the animals had to update formerly learned escape locations with new escape locations to successfully solve the task. Subjects were tested in the same room and apparatus as the previous WRAM retests, but two of their four previously-assigned platform locations were changed to new platform locations for each animal, as schematically represented in Figure 2b. Altering two of the platform locations required animals to update previously learned information about navigating to a particular arm to find a platform and escape the maze. All other maze procedures were identical to previous WRAM testing.
Morris water maze
After one day of rest, all of the animals were evaluated for spatial reference memory using the win-stay Morris water maze (MM; Figure 2c). The apparatus was a large round tub (188 cm diameter) filled with black-tinted water maintained at 18–20°C. One platform (11 cm diameter) was hidden just below the surface of the water in the northeast quadrant of the maze. This platform location remained constant across all days and trials. Salient spatial cues were present around the room to aid in spatial navigation to the platform (Morris et al., 1982). Each animal received four trials per day for five days. At the beginning of each trial, animals were dropped off from one of four starting points (north, south, east, or west). Drop-off points varied semi-randomly across days. Animals had 60 seconds to locate the platform before the experimenter led them to it. Once the platform was found, the rat remained on the platform for 15 seconds to allow for spatial localization before the experimenter returned it to the heated testing cage for an ITI of five to eight minutes. On the final day of MM, animals were given an additional probe trial in which the submerged platform was removed from the maze and animals swam freely in the maze for 60 seconds. The probe trial was implemented to evaluate whether the animals had spatially localized to the platform by quantifying the swim distance in the target quadrant versus the opposite quadrant. A video camera and tracking system (Ethovision; Noldus Instruments; Wageningen, The Netherlands) were utilized to measure each rat’s swim path(distance in cm) across all days and trials, as well as on the probe trial.
Visible platform
Animals were tested on the Visible Platform (VP) task as a measure of visual and motor competency to solve a water-escape task at the end of the behavioral assay battery. This is a non-spatial adaptation of the cue-navigation version of the spatial MM, which has been used to dissociate visual and motor acuity from place memory (Morris et al., 1982). The apparatus was a rectangular tub (100 x 60 cm) filled with clear water maintained at 18–20°C. A black platform (10 cm diameter) was placed 4 cm above the surface of the water. Opaque curtains were hung around the room to block out any potential spatial or geometric cues (Figure 2d). The rats were given six trials in one day. Animals were dropped off from a fixed location, while the platform location varied semi-randomly in three possible locations across trials. Each subject had 90 seconds to locate the platform, and was allowed to remain on the platform for 15 seconds before being returned to its heated home cage for an ITI of five to eight minutes.
Sacrifices
Subset Sacrifice
A subset of animals (n=3/group) was sacrificed on Day 52 of follicular depletion (i.e. approximately halfway to a follicle-deplete state). This subset is representative of the early menopause transition time point, wherein immature ovarian follicles are undergoing extensive atresia leading to a follicle-deplete state (Mayer et al., 2002, 2004). Procedures for the subset sacrifice and end sacrifice were identical and are described below.
End Sacrifice
Three additional animals died over the course of the study (one subject from the Middle-Aged-Vehicle group, one subject from Middle-Aged-VCD group, and one subject from the Young-VCD group). The remaining animals (N=41) were sacrificed one day after the final behavioral measure to obtain blood, ovaries, and uterine weights. Rats were deeply anesthetized with isoflurane anesthesia. Blood was collected via cardiocentesis prior to decapitation. Uterine horns and ovaries were dissected from the body cavity. Ovaries were removed from the tips of the uterine horn, trimmed of excess fat, and fixed in 4% paraformaldehyde until analysis. Uterine horns were trimmed of visible fat and wet weight was obtained as a marker of gonadal hormone stimulation.
Serum Hormone Measurements
At sacrifice, blood was collected via cardiocentesis and allowed to clot at 4°C (Vacutainer 367986, Becton Dickinson and Company, Franklin Lakes, NJ, USA). Serum was collected after centrifugation for 20 minutes at 2,000 rpm at 4°C and stored at −20°C until measurement by radioimmunoassay. Steroid hormone levels for 17β-estradiol, estrone, androstenedione, and progesterone were determined by radioimmunoassay using previously described methods (Acosta et al., 2010; Camp et al., 2012; Mennenga et al., 2015a, 2015b).
Briefly, 17β-estradiol was determined using a double antibody liquid-phase radioimmunoassay purchased from Beckman Coulter (Brea, CA), which employs estradiol-specific antibodies along with an 125I-labeled estradiol as the tracer. Interassay coefficients of variation for the assay average 8% at a mean value of 6 pg/ml. Functional sensitivity of the assay is 4 pg/ml. Estrone was determined using a double antibody liquid-phase radioimmunoassay purchased from Beckman Coulter (Brea, CA), that employs estrone-specific antibodies along with an 125I-labeled estrone as the tracer. Interassay coefficients of variation for the assay average 11% at a mean value of 90 pg/ml. Functional sensitivity of the assay is 16 pg/ml. Androstenedione was determined using a solid-phase radioimmunoassay purchased from Siemens (Los Angeles, CA), based on androstenedione-specific antibodies that are immobilized to the wall of polypropylene tubes and 125I-labeled androstenedione as the tracer. Interassay coefficients of variation for the assay average 3% at a mean value of 2.80 ng/ml. Functional sensitivity of the assay is 0.1 ng/ml. Progesterone was determined using a solid-phase radioimmunoassay, based on progesterone-specific antibodies that are immobilized to the wall of polypropylene tubes and 125I-labeled progesterone as the tracer. Interassay coefficients of variation for the assay average 4% at a mean value of 3.3 ng/ml. Functional sensitivity of the assay is 0.1 ng/ml.
Ovarian Follicle Counts
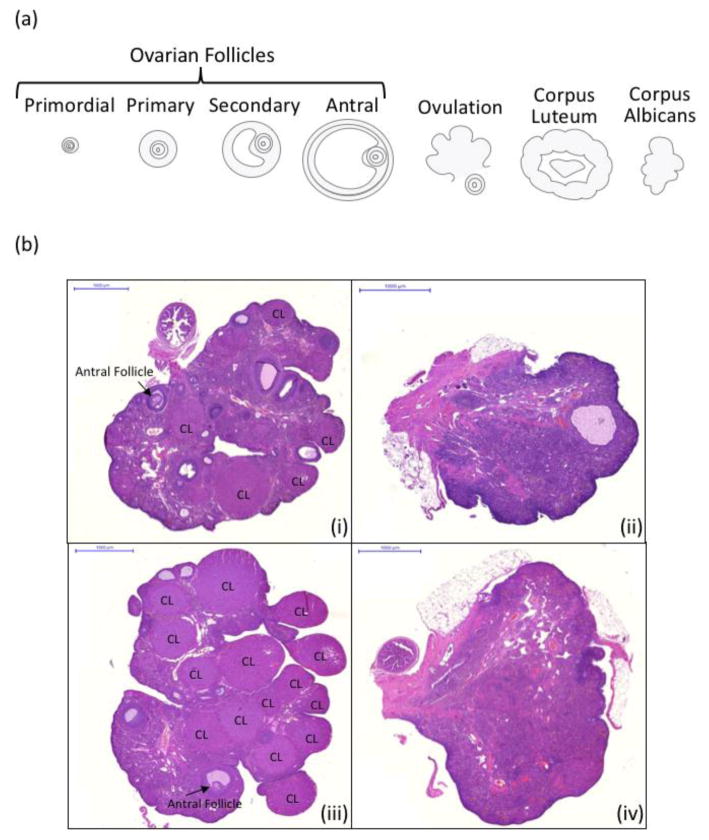
After post-fixing ovaries in 4% paraformaldehyde at sacrifice, one ovary from each subject was randomly selected for processing and quantification of primordial, primary, secondary, and antral follicles, as well as corpora lutea. Primordial follicles are considered to be non-growing, or resting-state, follicles within the ovary; these are the follicles targeted by VCD. Figure 3a provides a schematic of ovarian follicle types and corpora lutea, and 3b shows representative ovary micrographs from each group.
Figure 3.
(a) A schematic of the different phases of ovarian follicle growth, beginning with the primordial, resting follicle pool, and progressing from primary to secondary to antral (pre-ovulatory) stages of growth. Following ovulation of the mature egg, the remaining follicle becomes the corpus luteum, a temporary endocrine structure that secretes progesterone and low levels of estrogens. The corpus luteum eventually regresses into the corpus albicans, which no longer secretes ovarian hormones. Of these ovarian follicle stages, VCD accelerates atresia (programmed cell death) for primordial and primary ovarian follicles. (b) Representative ovary micrographs from each group. (i) Rat ovary from the Young-Vehicle group (ii) Rat ovary from the Young-VCD group (iii) Rat ovary from the Middle-Aged-Vehicle group (iv) Rat ovary from the Middle-Aged-VCD group. Note that long-term exposure to VCD is indicated by the loss of corpora lutea structures due to ovarian failure. All micrographs are depicted at 2x and the scale bar is 1000 μm.
Ovarian tissues were processed for paraffin embedding, sectioned at 5 μm, mounted, and stained with hematoxylin and eosin Y-phloxine B. Primordial, primary, secondary, and antral follicles were counted for every 20th section. Corpora lutea were counted at a magnification of 10x (Spencer compound microscope; American Optical, Buffalo, NY). The total number of follicles was calculated using the following formula: Nt = (N0 x St x ts) / (S0 x d0), where Nt = total calculated number of follicles, N0 = number of follicles observed in the ovary, St = total number of sections in the ovary, ts = thickness of the section (μm) S0 = total number of sections observed, and d0 = mean diameter of the nucleus (Gougeon and Chainy, 1987). Ovarian follicle stage was determined using criteria from Haas et al., 2007. Briefly, primordial cells were denoted by the presence of a single layer of squamous granulosa cells surrounding an oocyte. Primary follicles presented with a single layer of cuboidal granulosa cells. Secondary follicle classification required several granulosa cell layers. Antral follicles were defined as having two or more layers of granulosa cells as well as a fluid-filled antral space within the follicle (Haas et al., 2007).
Statistical Analyses
All data analyses were completed using SPSS 23 software. For all analyses following Vehicle or VCD treatment assessments, two group comparisons were set a priori to compare treatment effects within each age group to determine if younger animals respond differently than older animals to the onset of VCD-induced follicular depletion. That is, an effect of Follicular Depletion was determined by comparing VCD and Vehicle treatments only in the young animals, as well as VCD and Vehicle treatments only in middle-aged animals.
Additionally, planned comparisons were set to compare age effects within each treatment group to understand, in both young and middle-aged animals, the impact of menopause induction versus normal aging. Specifically, an Age effect was evaluated within the VCD-treated group only, as well as within the Vehicle-treated group only. Unless otherwise noted, two-tailed tests were used with an alpha level set at 0.05. The Huynh-Feldt correction was applied to repeated measures analyses to account for potential violations of sphericity by altering degrees of freedom (but not the F-ratio) to reduce Type I error rate as a result of repeated measures analysis (Huynh & Feldt, 1976). For all planned comparison repeated measures ANOVAs, effect sizes are reported as generalized eta squared (ηG2; Olejnik and Algina, 2003; Bakeman, 2005). For all planned comparison ANOVAs with only one between-subjects independent variable (i.e., serum hormone levels and ovarian follicle counts), effect sizes are reported as eta squared (η2). These effect sizes are interpreted by standard guidelines for η2 outlined by Cohen, whereby 0.02 is a small effect, 0.13 is a medium effect, and 0.26 is a large effect (Cohen, 1988, 1992; Bakeman, 2005) Cohen’s d is reported for all pairwise comparisons as a measure of effect size, and is interpreted by the standard guidelines specified by Cohen, where 0.2 indicates a small effect, 0.5 indicates a medium effect, and 0.8 indicates a large effect (Cohen, 1988, 1992). Four animals were excluded for health-related concerns, and three animals were excluded from all analyses due to significant outlying scores that were greater than two standard deviations from the mean of the group’s performance when compared to other animals for WRAM performance.
Behavior data
Water radial-arm maze
Raw error scores from the WRAM were log transformed to account for extreme scores and the negative skew of error distribution as trials progress (Cohen et al., 2003). WRAM training data prior to the Vehicle or VCD treatments were analyzed using a nested repeated-measures ANOVA design with Age as the independent variable, and Days and Trials as repeated measures. The dependent measure was Total Errors committed. WRAM refresher, retest, and flexibility data following Vehicle or VCD treatments were also analyzed for Total Errors. Each retest (averaged across the two-day block) was assessed separately utilizing a nested repeated-measures ANOVA design. Days and Trials were repeated measures within each two-group a priori comparison, with four trials per day within each of the two-day retests.
Morris water maze
Data were analyzed using a nested repeated-measures ANOVA. Days and Trials were repeated measures in all comparisons, with four trials per day within each of the five days. The dependent measure assessed was Swim Distance (cm) to the platform. The probe analysis used percent of total Swim Distance (cm) in the Target versus Opposite Quadrant as the dependent measure.
Visible platform task
Visible platform data were analyzed using repeated-measures ANOVA with latency to platform (s) as the dependent measure and trials as the repeated measure.
Ovarian follicle and serum hormone level data
Ovarian follicle and serum hormone level data were analyzed using ANOVA. Quantification and analysis of ovarian follicles were divided into the following stages: primordial, primary, secondary, and antral follicles; corpora lutea were also assessed. Dependent variables for serum analyses included 17β-estradiol, androstenedione, progesterone, and estrone levels. Of note, for the estrone hormone assay, we did not collect a sufficient quantity of serum from seven animals at the end time point to complete the assay, and for the androstenedione hormone assay, we did not collect a sufficient quantity of serum from three animals at the end time point to complete the assay. Animals that were excluded for behavioral analyses were also excluded from serum and ovarian follicle analyses.
Results
Water Radial-Arm Maze Training
Evaluating Age effects: Do young and middle-aged ovary-intact rats differ in performance on the water radial-arm maze task?
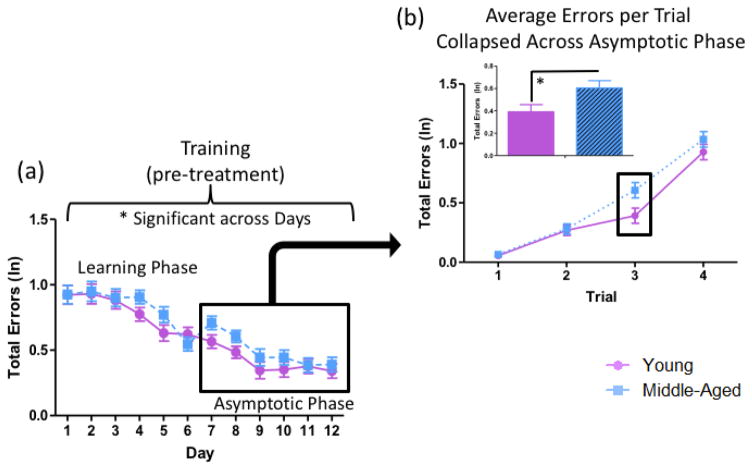
The ANOVA for Total Errors (ln) including all young and middle-aged animals prior to treatment administration across all days of training revealed a main effect of Age [F(1,44)=4.12, p<0.05, ηG2=0.003], wherein Middle-Aged animals made more errors than Young animals collapsed across all days of testing (Figure 4a). There was also a main effect of Day [F(10,445)=29.83, p<0.01, ηG2=0.14], such that animals decreased in errors across days of testing, and a main effect of Trial [F(3,129)=277.64, p<0.01, ηG2=0.35], wherein errors increased as trials progressed and working memory load increased.
Figure 4.
WRAM performance for Training (pre-treatment). (a) Across the 12 days of baseline testing, there was a main effect of Age, where Middle-Aged animals made more total errors than Young animals collapsed across all days of testing. (b) Average total errors (ln) per trial were evaluated for the asymptotic phase of testing (D7–12). Trial 3 alone, a higher working memory load trial, revealed a main effect of Age, where Middle-Aged animals made more errors than Young animals (* = p<0.05).
Previous studies examining the impact of gonadal hormones on spatial memory have observed marked effects on the WRAM when working memory load is taxed (Bimonte & Denenberg, 1999; Bimonte-Nelson et al., 2003, 2004; Braden et al., 2010). Working memory load effects often present in the latter part of testing, or the asymptotic phase, after animals have learned the rules of the task (Bimonte & Denenberg, 1999; Bimonte et al., 2000, 2003; Hyde et al., 2000; Mennenga et al., 2015b). Therefore, we assessed performance for the asymptotic phase, comprised of the last six days of testing (Days 7–12), based on previous WRAM findings from our laboratory (e.g. Bimonte & Denenberg, 1999; Braden et al., 2011; Mennenga et al., 2015b); of note, asymptotic performance is operationally defined as animals approaching their optimal performance. For this block, there was a marginal main effect of Age [F(1,44)=3.88, p=0.06, ηG2=0.01]. Each trial for the Day 7–12 block was assessed separately, and we found that there was a main effect of Age on Trial 3 [F(1,44)=5.58, p<0.05, ηG2=0.04], where the Middle-Aged group made more errors than the Young group (Figure 4b). However, by Trial 4, there was no longer an Age effect [F(1,44)=1.354, p=NS, ηG2=0.01], indicating that the working memory load on Trial 4 was sufficiently difficult to challenge both young and middle-aged animals.
Water Radial-Arm Maze Retests
Refresher practice. Does forgetting occur for the water radial-arm maze task across a one-month interval?
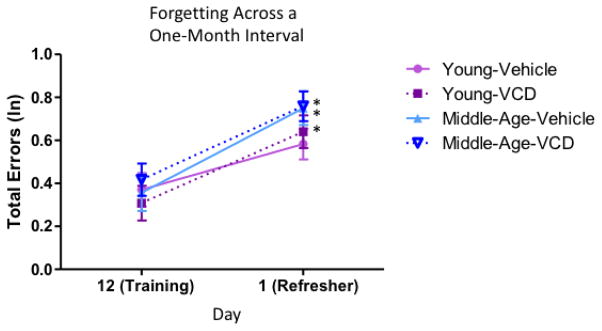
After initial WRAM training and then one month of Vehicle or VCD injections, animals in the Young group were eight months old, and animals in the Middle-Aged group were fourteen months old (Figure 1). Animals received two days of refresher practice on the WRAM, utilizing the same respective platform locations each animal previously learned during training, one month prior to starting the retest sessions. Performance was evaluated within each treatment group from the last day of training (prior to treatment administration) to the first day of the refresher practice block (after treatment) to assess forgetting over the one-month interim. For the Young Vehicle-treated group, there was no effect of Day [F(1,11)=2.72, p=NS, ηG2=0.05], suggesting that during the one-month interval where these animals received Vehicle injections and were not tested, they retained memory for their four assigned platform locations. However, the Young VCD-, Middle-Aged Vehicle-, and Middle-Aged VCD- treated groups had a main effect of Day (Young-VCD: [F(1,10)=8.76, p<0.05, ηG2=0.09; Middle-Aged Vehicle: [F(1,9)=15.72, p<0.01, ηG2=0.14]; Middle-Aged VCD: [F(1,12)=13.69, p<0.01, ηG2=0.11]) with errors increasing across the training to refresher interval, indicating forgetting across the month in which they received their VCD or Vehicle injections (Figure 5). The collected results from the refresher indicate that initiation of follicular depletion impacted the Young group’s ability to remember platform locations learned a month ago as compared to Vehicle-treated counterparts, and that Middle-Aged animals exhibited forgetting across the month delay regardless of treatment.
Figure 5.
Forgetting for WRAM performance across a one-month interval. Between the last day of training (prior to treatment) and the first day of the refresher one month later (after treatment), Young-Vehicle treated animals did not exhibit significant forgetting, but the Young-VCD, Middle-Age-Vehicle, and Middle-Aged-VCD showed forgetting (* = p<0.05).
Water radial-arm maze retests
Evaluating Follicular Depletion effects: Does follicular depletion across the menopause transition impact young and middle-aged animals differently?
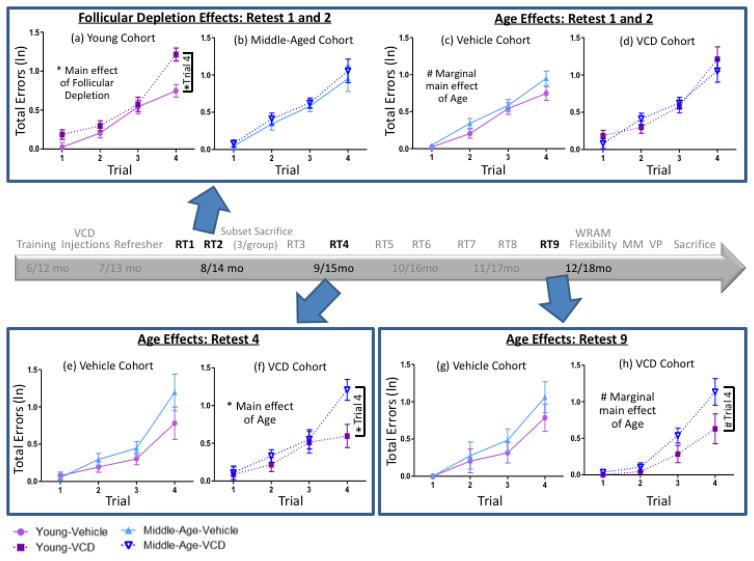
To evaluate how each age group responded to VCD-induced follicular depletion compared to age-matched Vehicle-treated animals, planned comparisons between VCD and Vehicle-treated animals were performed within the Young cohort and within the Middle-Aged cohort. Retests 1 and 2 were combined into a single block representing the early follicular depletion period. During this block, two group (Vehicle vs. VCD) planned comparisons for Young animals revealed a main effect of Follicular Depletion [F(1,21)=10.32, p<0.01, ηG2=0.07], with Young-VCD animals making more errors than Young-Vehicle animals. We also found a Trial x Follicular Depletion interaction [F(3,63)=3.56, p<0.05, ηG2=0.05], with Young-VCD animals making disproportionately more errors as trials and, respectively, working memory load increased, as compared to Young-Vehicle animals. On Trial 4, the highest working memory load trial, the Young VCD-treated group made more errors than the Young Vehicle-treated group [F(1,21)=15.93, p<0.01, d=1.66] (Figure 6a). Planned comparisons for the Middle-Aged cohort showed neither a main effect of Follicular Depletion [F(1,21)=0.61, p=NS, ηG2=0.004] nor a Trial x Follicular Depletion interaction [F(2,46)=0.07, p=NS, ηG2=0.002]. Moreover, Middle-Aged Vehicle- and Middle-Aged VCD-treated groups did not differ in performance on Trial 4 [F(1,21)=0.22, p=NS, d=0.196] (Figure 6b).
Figure 6.
WRAM performance across the transition to follicular depletion. (a) Within the Young cohort, a main effect of Follicular Depletion was observed, where VCD-treated animals made more errors than Vehicle-treated animals, and this was particularly evident on Trial 4 early in follicular depletion. (c) Within the Vehicle cohort, there was a marginal main effect of Age where Middle-Aged animals tended to make more errors than Young animals. (b,d) There were no significant differences within the Middle-Aged cohort or the VCD cohort early in follicular depletion. (e) There were no differences in performance in the Vehicle cohort in mid-follicular depletion. (f) Within the VCD cohort, there was a main effect of Age where Middle-Aged animals made more errors than Young animals collapsed across trial, and this was particularly evident on Trial 4 in mid-follicular depletion. (g) There were no differences in performance in the Vehicle-treated cohort in post-follicular depletion. (h) For the VCD cohort, there was a marginal main effect of Age collapsed across trials, and a marginal Age effect on Trial 4, where Middle-Aged animals made more errors than Young animals in post-follicular depletion (* = p<0.05, # = p<0.10).
Following this initial early menopause transition block, no Follicular Depletion effects were observed in the planned comparisons within the Young cohort and within the Middle-Aged cohort on retests three through nine for Total Errors, indicating that VCD-induced follicular depletion had a transient impairing effect on spatial memory for young animals, but not middle-aged animals, early in the menopause transition.
Evaluating Age effects: Does follicular depletion influence age effects on cognition?
Another goal of the study was to evaluate whether menopause exacerbates age-related changes in spatial memory performance. Therefore, we compared Young and Middle-Aged animals within the Vehicle-treated cohort and within the VCD-treated cohort. During the early follicular depletion block (Retest 1 and 2), a marginal main effect of Age was observed for Vehicle-treated animals [F(1,20)=3.56, p=0.07, η2=0.02], where Middle-Aged Vehicle-treated animals made marginally more errors than Young Vehicle-treated animals (Figure 6c). For VCD-treated animals, an age effect was not observed, indicating that Young VCD-treated animals performed similarly to Middle-Aged VCD-treated animals (Figure 6d).
No differences were observed on Retest 3. However, on Retest 4, as the VCD-treated cohort transitioned to a mid-follicle deplete state, we observed that for VCD-treated animals, there was a main effect of Age [F(1,16)=5.38, p<0.05, ηG2=0.04] and a marginal Age x Trial interaction [F(3,48)=2.77, p=0.05, ηG2=0.06]. On Trial 4, for animals that received VCD, Middle-Aged animals, which were 15 months old at this time point, made more errors than Young animals, which were 9 months old at this time point [F(1,16)=8.75, p<0.01, d=1.40], indicating that VCD-induced follicular depletion imparted an age effect (Figure 6f). On the other hand, for the Vehicle-treated cohort, there was neither a main effect of Age [F(1,14)=2.21, p=NS, ηG2=0.03] nor an Age x Trial interaction [F(2,23)=1.11, p=NS, ηG2=0.03]. On Trial 4, there was no Age effect for animals that received Vehicle treatment (Figure 6e). Thus, menopause induction revealed an age effect that was not seen in Vehicle-treated animals.
No age effects were observed on Retests 5, 6, 7, or 8 within each treatment cohort. On Retest 9 – a post-follicular depletion time point – for VCD-treated animals, there was a marginal main effect of Age [F(1,16)=4.34, p=0.05, ηG2==0.06]. On Trial 4, there was a marginal effect of Age for animals that received VCD-induced follicular depletion, whereby Middle-Aged animals, now 18 months old, trended toward making more errors than Young animals, now 12 months old [F(1,16)=3.42, p=0.08, d=0.88], indicating that VCD-induced follicular depletion marginally imparted an age effect in the post-follicular depletion time point (Figure 6h). For Vehicle-treated animals, there was no main effect of Age [F(1,14)=1.27, p=NS, ηG2=0.02] and no observed differences in performance on Trial 4 (Figure 6g).
Water Radial-Arm Maze Flexibility Task
After Retest 9, platform location assignments were altered for all animals; two of their assigned locations remained the same, and two locations were changed to different arms, such that there were two familiar and two novel platform locations for this task (Figure 2b). Animals that were Young animals at VCD or Vehicle treatment were now 12 months of age, and Middle-Aged animals were now 18 months of age at the flexibility task assessment.
Evaluating Follicular Depletion effects: Does follicular depletion impact cognitive flexibility in young and middle-aged animals differently?
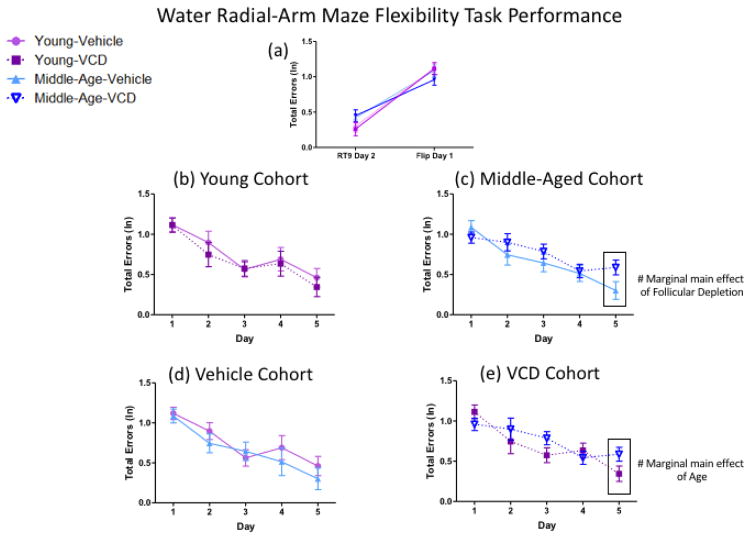
From the last day of Retest 9 to the first day of the WRAM Flexibility task, there was a main effect of Day for the Young cohort [F(1,15)=80.14, p<0.01, ηG2=0.39] as well as for the Middle-Aged cohort [F(1,15)=30.97, p<0.01, ηG2=0.27] wherein all animals made more errors on the test day of the platform location flip as compared to the last day of WRAM testing with all platforms in the familiar spatial location; since there was no interaction with Follicular Depletion for either age group, the platform location flip impaired performance in both ages regardless of Vehicle or VCD treatment (Figure 7a). Across the five days of flipped testing, there was a main effect of Day within each age (Young: [F(3,52)=12.96, p<0.01, ηG2=0.15]; Middle-Aged: [F(4,60)=13.28, p<0.01, ηG2=0.13]), where errors decreased across days, indicating that all animals learned across the testing period (Figure 7b–c); again, a lack of interaction with Follicular Depletion within each age indicated that learning occurred similarly regardless of vehicle treatment or VCD-induced follicular depletion. On the final day of the flexibility task, for the Middle-Aged cohort, there was a marginal main effect of Follicular Depletion [F(1,15)=3.97, p=0.07, ηG2=0.06], where VCD-treated animals trended toward making more errors than Vehicle-treated counterparts, suggesting that follicular depletion in middle-aged animals results in poorer performance compared to Vehicle-treated counterparts for a flexibility measure (Figure 7c); however, the Young cohort did not differ in performance, regardless of ovarian status.
Figure 7.
Water radial-arm maze flexibility task performance. (a) From the last day of RT9 to the first day of the flexibility task with two flipped platform locations, all subjects had impaired performance on the first day of exposure to the two flipped spatial locations, regardless of age or follicular depletion status. (b) No differences in performance were noted within the Young cohort across days, regardless of follicular depletion status. (c) Within the Middle-Aged cohort, on the final day of the flexibility task, there was a marginal main effect of Follicular Depletion, where VCD-treated animals made more errors than Vehicle-treated counterparts. (d) No differences in performance were noted within the Vehicle cohort across days, regardless of age. (e) Within the VCD cohort, on the final day of the flexibility task, there was a marginal main effect of Age, where older animals made more errors than younger animals. (# = p<0.10).
Evaluating Age effects: Does follicular depletion influence age effects for cognitive flexibility?
From the last day of Retest 9 to the first day of the WRAM Flexibility task, there was a main effect of Day for the Vehicle cohort [F(1,14)=50.28, p<0.01, ηG2=0.32] as well as for the VCD cohort [F(1,16)=51.64, p<0.01, ηG2=0.34], wherein all animals made more errors on the day of the flip, regardless of age (Figure 7a). Across the five days of testing, there was a main effect of Day (Vehicle: [F(3,56)=14.55, p<0.01, ηG2=0.15]; VCD: [F(4,64)=10.54, p<0.01, ηG2=0.13]) indicating that all animals learned across the testing period. On the final day of the flexibility task, in the VCD cohort, there was a marginal main effect of Age [F(1,16)=3.52, p=0.08, ηG2=0.05] where older animals tended to make more errors than younger animals; of note, this age effect did not occur in the Vehicle cohort. Again, we see that follicular depletion exacerbated memory deficits in older, but not younger, animals, while there were no age effects in animals that did not undergo follicular depletion (Figure 7d–e).
Morris Water Maze
Evaluating Follicular Depletion effects: Does follicular depletion impact young and middle-aged animals differently for a novel reference memory task?
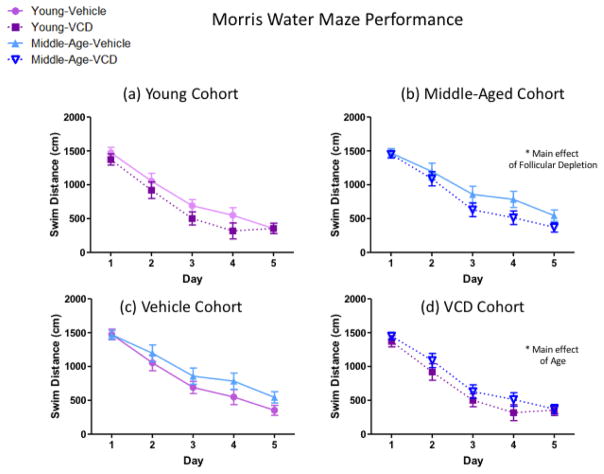
For the Young cohort, across the five days of MM testing, there were no Follicular Depletion effects. There was a main effect of Day [F(4,60)=74.85, p<0.01, ηG2=0.49] indicating learning across days for all Young animals (Figure 8a). Planned comparisons for Middle-Aged animals revealed a main effect of Follicular Depletion [F(1,15)=5.24, p<0.05, ηG2=0.04], where VCD-treated animals had a lower swim distance compared to Vehicle-treated counterparts, indicating that ovarian follicle depletion occurring in middle-age may aid reference memory for a novel task as compared to non-follicle deplete animals of the same age. There was also a main effect of Day [F(4,60)=37.62, p<0.01, ηG2=0.44] demonstrating learning across days for all Middle-Aged animals (Figure 8b).
Figure 8.
Morris water maze performance, average swim distance across four trials per day. (a) Swim distance did not vary in the Young cohort, regardless of follicular depletion status. (b) Within the Middle-Aged cohort, there was a main effect of Follicular Depletion, where VCD-treated animals swam less distance than Vehicle-treated animals. (c) Swim distance did not vary in the Vehicle cohort, regardless of age. (d) Within the VCD cohort, there was a main effect of Age, where Young animals swam less distance than Middle-Aged animals across days. (* = p<0.05).
Overnight forgetting was assessed as a measure of memory retention for a reference memory task across an overnight interval. We examined performance on the last trial of each day (Trial 4) and the first trial of each day (Trial 1). When collapsed across overnight intervals, for Middle-Aged animals, there was a marginal Trial x Follicular Depletion interaction [F(1,15)=3.52, p=0.08, ηG2=0.03], where on the first trial of the day, although it did not reach significance, Middle-Aged VCD-treated animals had a lower swim distance to the platform than Vehicle-treated counterparts [F(1,15)=4.32, p=0.06, d=1.02], suggesting better memory retention during the overnight interval for older follicle-deplete animals compared to their Vehicle-treated counterparts. There were no effects of follicular depletion between Young groups for overnight forgetting.
Evaluating Age effects: Does follicular depletion influence age effects for a novel reference memory task?
For the Vehicle-treated cohort, across the five days of MM testing, there were no Age effects. There was a main effect of Day [F(4,56)=41.98, p<0.01, ηG2=0.41] indicating learning across days for all Vehicle-treated animals (Figure 8c). Planned comparisons for the VCD-treated cohort revealed a main effect of Age [F(1,16)=5.42, p<0.05, ηG2=0.02], where Young animals had less swim distance compared to Middle-Aged animals, indicating that older follicle-deplete animals were impaired compared to their younger counterparts. There was also a main effect of Day [F(4,64)=63.75, p<0.01, ηG2=0.53] indicating learning across days for all VCD-treated animals (Figure 8d). Overnight forgetting planned comparisons did not reveal effects of Age in either Vehicle- or VCD- treated cohorts.
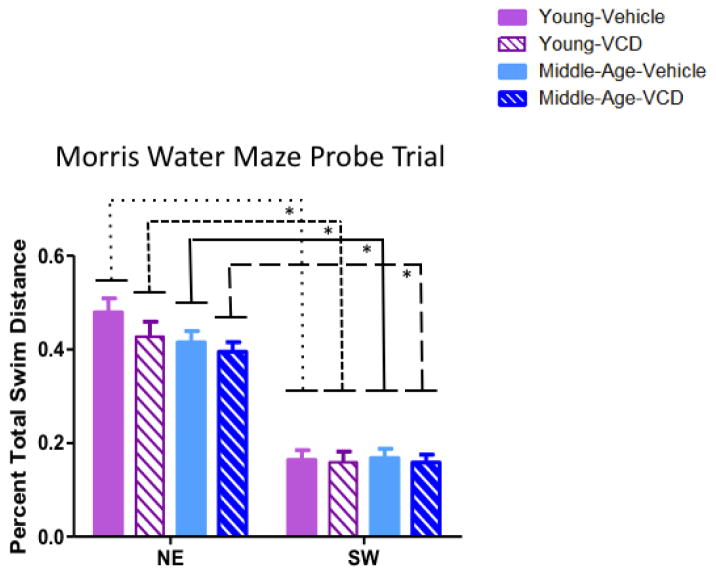
Probe trial performance
Each group was assessed separately for performance on the probe trial. Results indicated that there was a main effect of Quadrant for each group (Young-Vehicle: [F(1,8)=35.09, p<0.01, ηG2=0.77], Young-VCD: [F(1,7)=49.60, p<0.01, ηG2=0.85], Middle-Aged-Vehicle: [F(1,6)=29.43, p<0.01, ηG2=0.81], Middle-Aged-VCD: [F(1,9)=79.64, p<0.01, ηG2=0.83]), indicating that all animals, regardless of follicular depletion status or age, had a greater percent of total swim distance in the target quadrant; that is, they all localized to the northeast quadrant where the platform was previously located versus the opposite, southwest quadrant (Figure 9).
Figure 9.
Morris water maze probe trial performance. Each treatment group, assessed separately, had a greater percent of total swim distance in the northeast (target) quadrant compared to the southwest (opposite) quadrant during the probe trial, indicating all animals spatially localized to the platform location, regardless of age or follicular depletion status. (* = p<0.05).
Visible Platform
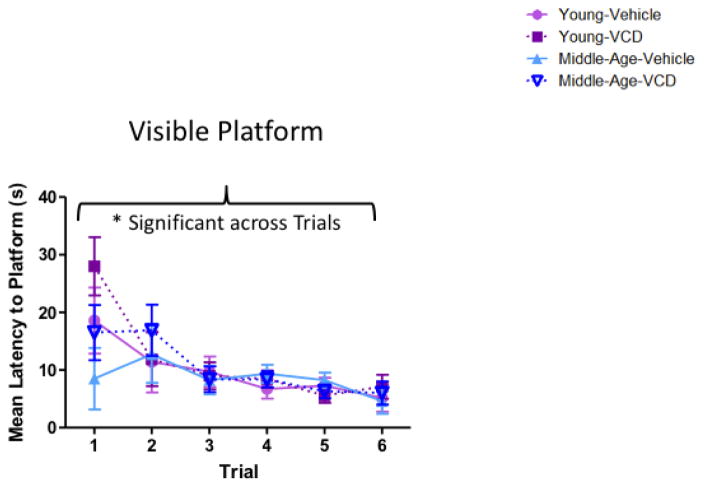
Evaluating Follicular Depletion effects: Does follicular depletion impact young and middle-aged animals differently for visual and motor competency?
Among Young animals as well as Middle-Aged animals, there were no main effects of Follicular Depletion. The mean escape latency for the last trial was 6.13±1.90 seconds for Young animals, and 5.31±1.15 seconds for Middle-Aged animals, indicating the ability to successfully perform the procedural components of a water escape task for both younger and aged cohorts (Figure 10).
Figure 10.
Visible platform performance. All animals, regardless of age or follicular depletion status, decreased latency (s) to the visible platform across six trials, confirming that all subjects could perform the motor and visual components of water maze tasks. (* = p<0.05).
Evaluating Age effects: Does follicular depletion influence age effects for visual and motor competency?
There was no effect of Age within Vehicle-treated animals, or within VCD-treated animals. The mean escape latency for the last trial was 4.88±0.92 seconds for Vehicle-treated animals, and 6.56±1.77 seconds for VCD-treated animals, indicating the ability to successfully perform the procedural components of a water escape task for both Vehicle- and VCD- treated cohorts (Figure 10).
Serum Hormone Levels
Serum hormone levels for 17β-estradiol, estrone, androstenedione, and progesterone were obtained for animals at the subset sacrifice time point and at the end sacrifice time point. Tables 1–4 include the mean ± SE, range, and median values for each hormone at each time point.
Table 1.
Mean± SE, range, and median of 17β-Estradiol levels (pg/ml) at subset and end time points
| Group | Age | Mean 17β-Estradiol level ± SE | Range of 17β-Estradiol levels | Median 17β-Estradiol levels |
|---|---|---|---|---|
| Young-Vehicle | 8 months | 10.07±1.32 | 8.6–12.7 | 8.90 |
| 12 months | 12.59±1.66 | 7.4–24.4 | 11.90 | |
| Young-VCD | 8 months | 10.47±2.57 | 6.1–15.0 | 10.30 |
| 12 months | 13.41±1.68 | 5.5–20.7 | 14.00 | |
| Middle-Aged- Vehicle | 14 months | 15.70±2.90 | 12.4–21.5 | 13.20 |
| 18 months | 13.61±4.23 | 6.1–38.5 | 10.10 | |
| Middle-Aged- VCD | 14 months | 10.57±1.20 | 8.7–12.8 | 10.20 |
| 18 months | 12.55±1.23 | 9.0–20.3 | 11.45 |
Table 4.
Mean± SE, range, and median of Progesterone (ng/ml) levels at subset and end time points
| Group | Age | Mean Progesterone level ± SE | Range of Progesterone levels | Median Progesterone levels |
|---|---|---|---|---|
| Young-Vehicle | 8 months | 27.77±6.72 | 18.9–39.2 | 19.20 |
| 12 months | 30.30±7.38 | 4.8–68.7 | 34.4 | |
| Young-VCD | 8 months | 11.23±3.41 | 5.2–17.0 | 11.50 |
| 12 months | 8.41±2.81 | 2.4–26.7 | 5.05 | |
| Middle-Aged- Vehicle | 14 months | 38.46±10.73 | 19.9–54.1 | 43.40 |
| 18 months | 40.04±10.74 | 9.7–76.8 | 45.5 | |
| Middle-Aged- VCD | 14 months | 50.67±11.26 | 29.4–67.7 | 54.9 |
| 18 months | 8.1±1.68 | 1.0–19.0 | 7.45 |
Subset sacrifice
Young animals (VCD: n=3; Vehicle: n=3) were 8 months old and Middle-Aged animals (VCD: n=3; Vehicle: n=3) were 14 months old at the time of serum analysis for the subset sacrifice.
Evaluating Follicular Depletion effects for the subset sacrifice: Does follicular depletion impact circulating ovarian hormone levels differently in young and middle-aged animals at the early follicular depletion time point?
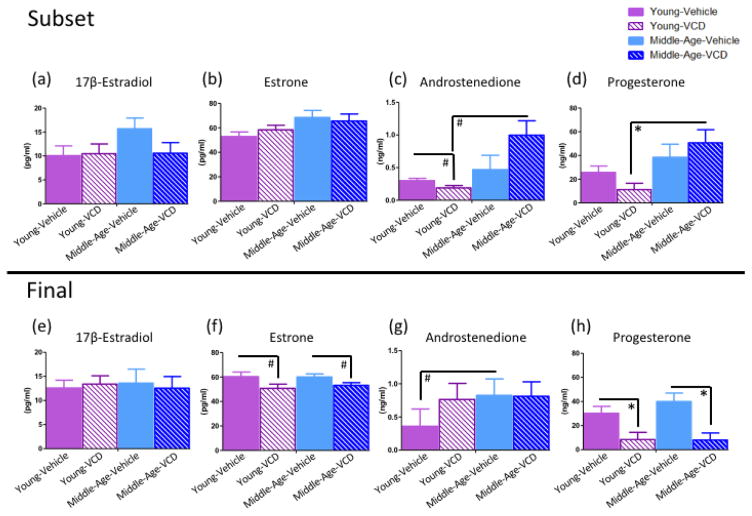
For the subset sacrifice, there were no Follicular Depletion effects within either age group for 17β-estradiol, estrone, or progesterone levels (Figures 11a, b, d, respectively), indicating that follicular depletion had no impact on these hormones within the Young cohort or within the Middle-Aged cohort early in the menopause transition (52 days after initiating VCD-induced follicular depletion). For androstenedione levels in the Young cohort, there was a marginal main effect of Follicular Depletion [F(1,4)=4.71, p=0.096, η2=0.53], where Vehicle-treated animals had marginally more androstenedione than VCD-treated animals. No differences in androstenedione levels were found in the Middle-Aged cohort at this time point (Figure 11c).
Figure 11.
Circulating serum hormone levels for the subset (a-d) and end sacrifice (e-h) time points. (a) Mean ± SEM for 17β-estradiol serum levels (pg/ml) for the subset sacrifice. No differences were observed among treatment groups at the early follicular depletion time point. (b) Mean ± SEM for estrone serum levels (pg/ml) for the subset sacrifice. No differences were observed among treatment groups at the early follicular depletion time point. (c) Mean ± SEM for androstenedione serum levels (ng/ml) for the subset sacrifice. Young-VCD animals had marginally less androstenedione compared to age-matched controls, and to Middle-Aged-VCD animals. (d) Mean ± SEM for progesterone serum levels (ng/ml) for the subset sacrifice. Young-VCD animals had less progesterone than Middle-Aged-VCD animals at the early follicular depletion time point. (e) Mean ± SEM for 17β-estradiol serum levels (pg/ml) for the end sacrifice. No differences were observed among treatment groups at the post-follicular depletion time point. (f) Mean ± SEM for estrone serum levels (pg/ml) for the end sacrifice. Within the Young cohort and within the Middle-Aged cohort, there were marginal main effects of Follicular Depletion where VCD-treated animals had marginally less estrone compared to Vehicle-treated animals within both age groups. (g) Mean ± SEM for androstenedione serum levels (ng/ml) for the end sacrifice. Middle-Aged Vehicle animals had marginally more androstenedione than Young Vehicle animals. (h) Mean ± SEM for progesterone serum levels (ng/ml) for the end sacrifice. Within the Young cohort and within the Middle-Aged cohort, there was a main effect of Follicular Depletion wherein VCD-treated animals had less progesterone than Vehicle-treated animals within both age groups. (* = p<0.05; # = p<0.10).
Evaluating Age effects for the subset sacrifice: Does follicular depletion influence age effects on circulating ovarian hormone levels at the early follicular depletion time point?
There were no Age effects within either treatment group for 17β-estradiol or estrone (Figures 11a, b, respectively), indicating that the age did not impact estrogen levels within the Vehicle cohort or within the VCD cohort early in the menopause transition. For androstenedione levels in the VCD-treated cohort, there was a marginal main effect of Age [F(1,4)=7.50, p=0.05, η2=0.65], wherein Middle-Aged animals had marginally higher androstenedione levels than Young animals (Figure 11c). For the Vehicle-treated cohort, there were no Age effects for androstenedione levels. For progesterone in the VCD-treated cohort, there was a main effect of Age [F(1,4)=11.24, p<0.05, η2=0.74], where Middle-Aged animals had more progesterone than Young animals (Figure 11d); however, there were no differences in progesterone levels for the Vehicle cohort at the subset sacrifice time point.
End sacrifice
Animals that were excluded for behavioral analyses were also excluded from serum analyses. Young animals (VCD: n=8; Vehicle: n=9) were 12 months old, and Middle-Aged animals (VCD: n=10; Vehicle: n=7) were 18 months old, at the end sacrifice time of serum analysis.
Evaluating Follicular Depletion effects for the end sacrifice: Does follicular depletion impact circulating ovarian hormone levels differently in young and middle-aged animals at the post-follicular depletion time point?
There were no Follicular Depletion effects within either age group for 17β-estradiol or androstenedione levels at the end sacrifice (Figures 11e, g, respectively), indicating that follicular depletion did not impact these hormones within the Young cohort or within the Middle-Aged cohort in the post-follicular depletion time point. For estrone levels, within the Young cohort as well as within the Middle-Aged cohort, there was a marginal main effect of Follicular Depletion (Young: [F(1,11)=3.62, p=0.08, η2=0.25]; Middle-Aged: [F(1,12)=4.31, p=0.06, η2=0.26]; Figure 11f), whereby VCD-treated animals had marginally less estrone compared to Vehicle-treated animals within both age groups. For progesterone levels within the Young cohort as well as within the Middle-Aged cohort, there was a main effect of Follicular Depletion (Young: [F(1,15)=6.98, p<0.05, η2=0.32]; Middle-Aged: [F(1,15)=12.35, p<0.01, η2=0.45]; Figure 11h) wherein VCD-treated animals had less progesterone than Vehicle-treated animals within both age groups. Because the majority of progesterone is produced by the corpus luteum after ovulation, these latter results suggest that the VCD-induced follicular depletion was effective in initiating ovarian failure.
Evaluating Age effects for the end sacrifice: Does follicular depletion influence age effects on circulating ovarian hormone levels at the post-follicular depletion time point?
There were no Age effects within either treatment group for 17β-estradiol, estrone, or progesterone levels (Figures 11e, f, h, respectively) indicating that age did not impact these serum hormone levels within the Vehicle cohort or within the VCD cohort in the post-follicular depletion time point. For androstenedione levels within the Vehicle cohort, there was a marginal main effect of Age [F(1,12)=3.97, p=0.07, η2=0.25] wherein Middle-Aged animals had marginally more androstenedione than Young animals (Figure 11g). However, within the VCD cohort, there were no age differences in androstenedione levels (Figure 11g), such that younger VCD-treated animals had similar androstenedione levels to older VCD-treated animals.
Ovarian Follicle Counts
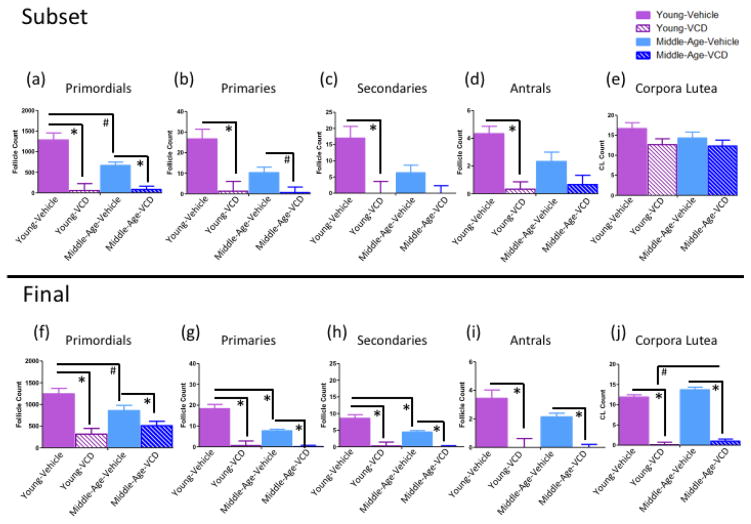
Ovarian follicle counts were obtained for both subset and end sacrifices (Figure 12).
Figure 12.
Ovarian follicle and corpora lutea counts for the subset (a-e) and end (f-j) sacrifice. (a-e) Young-VCD animals had fewer primordial, primary, secondary, and antral follicles compared to their Young-Vehicle controls at the subset time point. Middle-Aged-VCD animals had fewer primordial cells and marginally fewer primary cells compared to Middle-Aged-Vehicles, but did not differ from their Vehicle-treated counterparts for secondary or antral follicle counts at the subset time point. Animals did not differ in corpora lutea count, regardless of age or follicular depletion status, at the early follicular depletion time point. (f–j) Young and Middle-Aged VCD-treated animals had fewer primordial, primary, secondary, and antral follicle counts and fewer corpora lutea compared to their respective age-matched, Vehicle-treated controls, indicating that VCD treatment effectively depleted ovarian follicles. Middle-Aged Vehicle animals had marginally fewer primordial, and significantly fewer primary and secondary ovarian follicles than younger Vehicle-treated animals, but did not differ for antral follicle count or corpora lutea counts, at the end sacrifice time point, suggesting that rodents experience some follicular depletion with normal aging. (* = p<0.05; # = p<0.10).
Subset sacrifice
Evaluating Follicular Depletion effects at the subset sacrifice: Does follicular depletion impact ovarian follicle counts differently in young and middle-aged animals at the early follicular depletion time point?
Within the Young cohort there was a main effect of Follicular Depletion for primordial follicles [F(1,4)=26.40, p<0.01, η2=0.87, Figure 12a], primary follicles [F(1,4)=14.30, p<0.05, η2=0.78, Figure 12b], secondary follicles [F(1,4)=10.98, p<0.05, η2=0.73, Figure 12c], and antral follicles [F(1,4)=28.80, p<0.01, η2=0.88, Figure 12d], where VCD-treated animals had fewer ovarian follicles of each subtype than Vehicle-treated counterparts. Within the Middle-Aged cohort there was a main effect of Follicular Depletion for primordial follicles [F(1,4)=27.01, p<0.01, η2=0.87, Figure 12a] and a marginal main effect of Follicular Depletion [F(1,4)=6.42, p=0.06, η2=0.62, Figure 12b] for primary follicles, with VCD-treated animals tending to have fewer cells than Vehicle-treated counterparts. The Middle-Aged cohort did not have an observed difference in secondary or antral follicles at the subset time point (Figures c, d respectively). There were no observed differences in the number of corpora lutea in either the Young cohort or the Middle-Aged cohort at this time point (Figure 12e).
Evaluating Age effects at the subset sacrifice: Does follicular depletion influence age effects on ovarian follicle loss at the early follicular depletion time point?
Within the Vehicle cohort, there was a marginal main effect of Age [F(1,4)=5.50, p=0.08, η2=0.58], where Young animals had more primordial follicles than Middle-Aged animals (Figure 12a). There were no differences in primary, secondary, or antral follicles for the Vehicle cohort (Figures 12b, c, d, respectively). Within the VCD cohort there were no differences in any ovarian follicle subtype (Figures 12a–d). Neither the Vehicle cohort nor the VCD cohort exhibited differences in corpora lutea at this time point (Figure 12e).
End sacrifice
Animals that were excluded for behavioral analyses were also excluded from ovarian follicle count analyses.
Evaluating Follicular Depletion effects at the end sacrifice: Does follicular depletion impact ovarian follicle counts differently in young and middle-aged animals at the post-depletion time point?
Within the Young cohort, there was a main effect of Follicular Depletion for primordial follicles [F(1,15)=25.12, p<0.01, η2=0.63, Figure 12f], primary follicles [F(1,15)=34.72, p<0.01, η2=0.70, Figure 12g], secondary follicles [F(1,15)=27.44, p<0.01, η2=0.65, Figure 12h], antral follicles [F(1,15)=16.31, p<0.01, η2=0.52, Figure 12i], and corpora lutea [F(1,15)=200.89, p<0.01, η2=0.93, Figure 12j], where VCD-treated animals had fewer ovarian follicles and corpora lutea than Vehicle-treated counterparts. Within the Middle-Aged cohort, there was also a Follicular Depletion effect observed for primordial follicles [F(1,15)=4.79, p<0.05, η2=0.24, Figure 12f], primary follicles [F(1,15)=63.57, p<0.01, η2=0.81, Figure 12g], secondary follicles [F(1,15)=61.45, p<0.01, η2=0.80, Figure 15h], antral follicles [F(1,15)=41.36, p<0.01, η2=0.73, Figure 12i], and corpora lutea [F(1,15)=241.00, p<0.01, η2=0.94, Figure 12j], where VCD-treated animals had fewer ovarian follicles and corpora lutea than Vehicle-treated counterparts. In sum, VCD treatment effectively reduced ovarian follicle number in both age groups compared to Vehicle treatment.
Evaluating Age effects at the end sacrifice: Does follicular depletion influence age effects on ovarian follicle loss at the post-depletion time point?
Within the Vehicle cohort, there was a marginal effect of Age [F(1,14)=3.80, p=0.07, η2=0.21, Figure 12f] for primordial follicles, where Young animals had marginally more primordial follicles than Middle-Aged animals. In addition, for Vehicle-treated animals a main effect of Age was revealed for primary follicles [F(1,14)=10.00, p<0.01, η2=0.42, Figure 12g] and secondary follicles [F(1,14)=5.34, p<0.05, η2=0.28, Figure 12h], where Young animals had more of these follicle subtypes than Middle-Aged animals. However, there were no effects of Age for antral follicles or corpora lutea in the Vehicle cohort (Figures 12i, j, respectively). Within the VCD cohort, there were no effects of Age on primordial follicles, primary follicles, secondary follicles, or antral follicles (Figures 12f, g, h, i, respectively), suggesting that VCD reduced the number of follicles in the ovary without interactions with age. It is of note that none of the VCD-treated animals had quantifiable antral follicles at this time point, suggesting that these animals were no longer experiencing a normal ovarian cycle. However, within the VCD-treated animals, there was a marginal main effect of Age for corpora lutea counts [F(1,16)=3.66, p=0.07, η2=0.19, Figure 12j] where Middle-Aged VCD-treated animals had marginally more corpora lutea than Young VCD-treated animals. These collective results provide evidence for age-related follicle decline in Vehicle-treated rats, as well as substantial atresia of ovarian follicles in VCD-treated animals.
Uterine Horn Weights
There were no main effects of Age or Follicular Depletion on uterine horn wet weight (g) at either sacrifice time point. All uterine horns were similar in weight regardless of treatment. Given that estrogens have a well-known stimulatory effect on uterine tissue growth (Brody & Wiqvist, 1961; Kang et al., 1975) and there were no differences in circulating 17β-estradiol levels, this result was expected.
Discussion
Here, we performed a longitudinal study systematically evaluating the effects of age and ovarian status on spatial memory performance across the transition to menopause in a rodent model. The collective results indicate that: (1) age at the onset of transitional menopause impacts cognitive performance, with the onset of follicular depletion at a younger age negatively impacting spatial memory, and (2) follicular depletion exacerbates age-related memory changes later in the menopause transition. Furthermore, the impact of age and follicular depletion on spatial memory become evident when the working memory system was highly taxed, as demonstrated by the differences on trial four of the WRAM, when working memory load was the highest.
Inducing Transitional Menopause at a Younger Age Impairs Spatial Memory Early in Follicular Depletion
Early in the transition to menopause (Retests 1 and 2), VCD treatment impaired WRAM performance in the young animals, but not the middle-aged animals, compared to their respective age-matched Vehicle-treated controls (Figure 6a, b). In fact, at this time point, VCD-induced transitional menopause rendered younger animals' performance on the WRAM similar to that of middle-aged animals (Figure 6d). Because only the Young cohort was impaired with VCD treatment, this age-specific impairment suggests that undergoing the transition to menopause prior to middle-age is detrimental to memory performance, at least in the early stages of follicular depletion. This early stage of the VCD menopause model involves substantial ovarian follicle decline, particularly for the young animals, which is analogous to the beginning of the human menopause transition when women tend to report some memory complaints (Fugate Woods et al., 2000).
It is particularly interesting that these changes occur in adult animals undergoing follicular depletion, because advanced age is not a confounding factor for these animals due to the nature of our experimental design. Indeed, their age-matched Vehicle-treated counterparts performed better on the WRAM at the highest working memory load, indicating that follicular depletion and the associated hormonal and ovarian changes have a unique impact on adult animals that are not yet at the age that they would be naturally transitioning to a reproductively senescent state. Dissociating the effects of aging and follicular depletion is a tremendous benefit gained from using animal models. These variables are difficult to evaluate independently in women, because the onset of transitional menopause is typically concomitant with aging. Our results, if translated to women, suggest that an earlier onset of transitional menopause may result in disrupted cognition. It is notable that aging affects the functional connectivity and structure of brain areas important for learning and memory (Barnes et al., 1979; Poe et al, 2000) and these changes may impact strategy selection for solving behavioral tasks (Samson et al., 2015). As such, it is a possibility that younger animals relied more heavily on an allocentric spatial strategy to solve the WRAM compared to the strategies used by older animals. If induced transitional menopause impacted the capacity to use a hippocampal-dependent strategy or attend to the extramaze cues, this could, in part, explain their poor performance compared to age-matched counterparts. In addition, strategy selection for some spatial tasks has been shown to vary in female rats depending on estrous cycle phase (Korol et al., 2004), which may also have influenced subjects’ performance and maze-solving strategy.
Currently, there are no definitive clinical markers that identify the onset of the menopause transition. Menopause is confirmed retrospectively following one year without a menstrual period, and its onset is difficult to determine prospectively. Research suggests that changes in hypothalamic-pituitary-gonadal (HPG) axis function are detectable prior to the onset of menstrual or estrous cycle irregularities (Downs and Wise, 2009; Wise et al., 1989, 1996, 1997, 1999). It is possible that perturbations in HPG axis activity prior to the average onset of reproductive senescence — as is the case for early and premature onset of menopause — may result in unique cognitive and brain changes that ultimately impact the trajectory of cognitive and brain aging. Developing reliable measures to establish the impetus of the transition to reproductive senescence in women could provide the opportunity to intervene during a critical window early in the transition, which may be necessary to ascertain the beneficial effects of hormone therapy on menopause-related memory changes.
For Transitionally Menopausal Animals, Detrimental Age Effects Present in Mid- and Post- Follicular Depletion
The series of retests administered here throughout the transition into a follicle-deplete state allowed us to capture memory performance at several key time points in the menopause transition as well as across normal aging. Following the early menopause time point, as the transition to menopause progressed, an age-related effect of follicular depletion became apparent. During Retest 4 on the WRAM, during mid-follicular depletion, this age-related deficit was only evident in animals undergoing VCD-induced follicular depletion; Middle-Aged VCD-treated animals made more errors than Young VCD-treated animals, particularly at the highest working memory load trial. In contrast to the age-related change in the VCD cohort, there was no age-related change in the Vehicle-treated animals during Retest 4. Therefore, the detrimental cognitive effect of aging was only apparent in the transitional menopause model, but not in the naturally aging group.
Post-follicular depletion, at Retest 9, we saw a similar age-related impairment only in the VCD-treated animals, although the effect reached only marginal significance at this time point. Thus, during (at Retest 4) and after (at Retest 9) the transition to follicular depletion, older animals tended to perform worse than younger animals undergoing accelerated follicular depletion. Perhaps repeated testing on the WRAM (i.e., cognitive practice) resulted in a beneficial effect for memory maintenance, albeit only for animals aging independently of accelerated follicular depletion — a finding which has previously been shown in our and other laboratories (Talboom et al., 2008; Markowska and Savonenko, 2002b). Given that age-related impairment remained in the VCD cohort, this result could indicate that undergoing follicular depletion in middle age may obviate the beneficial effects of cognitive practice for a task that taxes the working memory system. Translationally, because the average lifespan in women is increasing (The World Factbook 2015 estimate; Xu et al., 2012; Singh et al., 1996; Murray et al., 2015), it is potentially of interest to slow the rate of ovarian follicle reserve loss in order to prolong the benefits of follicle-replete ovaries and normal circulating sex steroid hormone levels on memory as well as on a myriad of other body systems, such that age effects seen with follicular depletion would be delayed until later in life.
All Animals Showed Cognitive Flexibility to Learn a Revised Task, With Some Marginal Impairments of Transitional Menopause
After initial training on the WRAM task, half of the reinforced locations were switched so that we could assess cognitive flexibility. All animals were able to update previously learned information by shifting to new escape locations for the altered WRAM task, as indicated by improved performance across the five days of this WRAM flexibility task. On the final day, older VCD-treated animals performed marginally worse than their age-matched Vehicle controls, indicating that in a later stage of follicular depletion, the loss of ovarian follicles had a minor impact on performance on a novel flexibility task. Within the VCD-treated cohort, a marginal age effect was observed, with older animals tending to make more errors than younger animals; this effect did not occur within the Vehicle-treated cohort, indicating that follicular depletion may initiate age-related impairments for cognitive flexibility, similar to the impact of follicular depletion on the WRAM task in the mid- and post- menopausal time points.
Follicular Depletion Improves Reference Memory Performance for Older Animals, but Impairs Performance Compared to Younger Transitionally Menopausal Animals
Morris water maze results indicated that the younger animals learned the task similarly regardless of ovarian status. On the other hand, VCD treatment improved MM performance in middle-aged animals, as measured by decreased swim distance to the platform on the last day of testing. Ovarian follicular depletion in older animals bestowed a beneficial effect on learning and memory retention for a novel spatial reference memory task, providing evidence that the negative memory effects often noted in the post-menopausal state may not extend to all learning and memory domains. It is noteworthy that the VCD-treated animals had been in a post-follicle deplete state for several months at this time point and likely had a more stable circulating hormone profile, including significantly lower progesterone levels that could benefit spatial reference memory performance (discussed below). Of note, within the VCD-treated cohort, middle-aged animals had a greater swim distance to the platform, interpreted as poorer performance, compared to younger animals. Since a concordant age-related difference was not seen in Vehicle-treated animals, this suggests again that VCD induced follicular depletion revealed age-related effects. It is noteworthy that all groups were effectively utilizing a spatial strategy to solve the MM, regardless of ovarian status or age, as indicated by the probe trial. Additionally, the Visible Platform task verified that, after six months of testing, the animals included in these analyses were still able to see as well as perform the motor-related components of the task, even with advanced age.
Endocrine and Ovarian Markers
VCD-Induced Follicular Depletion Alters Ovarian Hormone Profiles
A hallmark of the human transition into a post-menopausal state is a marked decrease in circulating ovarian hormone levels. During the transitional phase, serum hormone levels may be erratic due to irregular ovulation patterns and disrupted hypothalamic-pituitary-ovarian communication. VCD treatment models this perimenopausal time point during which gonadal hormone levels are in a state of flux resulting from accelerated follicular depletion. Measurement of the four primary ovarian hormones, 17β-estradiol, estrone, androstenedione, and progesterone at the subset sacrifice time point (that is, at the early follicular depletion time point), revealed that older VCD-treated animals had marginally increased androstenedione as well as significantly increased progesterone levels compared to younger VCD-treated animals. These results possibly indicate that the younger animals experienced a more rapid decline in ovarian follicle reserve, even though estrogen levels were not different at this time point. At the post-menopausal time point, both Young and Middle-Aged VCD-treated cohorts had less progesterone and marginally less estrone than Vehicle-treated counterparts. A key characteristic of menopause is the decline in circulating progesterone levels resulting from anovulation and, consequently, no formation of the corpora lutea to secrete progesterone. Furthermore, exogenous progesterone alone (Bimonte-Nelson et al., 2004, 2006), as well as exogenous estrone alone (Engler-Chiurazzi et al., 2012), have been implicated in impaired spatial memory performance in middle-aged Ovx rats. Perhaps the elevated progesterone levels, as well as the marginally elevated estrone levels, in the Vehicle-treated animals observed at the end time point can partially explain the older VCD-treated animals’ advantage over their Vehicle-treated counterparts in the MM task discussed above.
The initial, foundational research using VCD-induced follicular depletion as a model of transitional menopause was completed in mice; VCD treatment was initiated early in life and resulted in a decline in progesterone and androstenedione, as well as undetectable 17β-estradiol levels, compared to age-matched controls (Mayer et al., 2004). Other VCD studies in both rats and mice have found no differences in 17β-estradiol levels at multiple time points compared to age-matched controls (Lohff et al., 2005; Mayer et al., 2002; Frye et al., 2012). These experiments consistently report increased FSH levels, reduced ovarian follicle counts, and extended estrous cycles following VCD treatment. Mice tended to transition into persistent diestrus (acyclicity) following VCD treatment (Lohff et al., 2005 Mayer et al., 2004), while rats had extended periods of persistent estrous (and detectable 17β-estradiol levels) during VCD-induced disrupted cyclicity (Frye et al., 2012; Mayer et al., 2002). Thus, this suggests that in rats, VCD-induced follicular depletion is a unique model of the perimenopause time point, during which ovarian cyclicity is disrupted and ovarian hormone levels may be fluctuating and at some time points elevated prior to the eventual decline of these circulating hormones. Indeed, our serum hormone levels with VCD-induced follicular depletion reported here are consistent with the observations reported in the Frye et al. (2012) and Mayer et al. (2002) publications using the same VCD dosing regimen. A limitation to this study is that we did not collect blood at multiple points across time within the same animal. It is possible that we may have observed fluctuating patterns of ovarian hormones that are similar to women’s circulating ovarian hormone patterns during the perimenopause. Human research suggests that it is not necessarily the final decline in circulating ovarian hormones, but the erratic fluctuations during the transition, in conjunction with changes in affect, stress, and physical health during midlife, that are associated with perceived cognitive changes as identified by self-reports (Sullivan Mitchell & Fugate Woods, 2001; Weber et al., 2012; 2013; 2014). Indeed, within the time constraint of the experimental design, all subjects, regardless of ovarian status, showed stable 17β-estradiol levels. Had we continued our assessments until a later time point or completed blood draws to evaluate hormone fluctuations across time, we may have observed altered 17β-estradiol profiles. Thus, this is an important future direction to pursue in order to fully understand the VCD model of transitional menopause in rats and its relationship to what is known about menopause in women.
VCD Successfully Induced Follicular Depletion Across Time and Normally Aging Rats Experience Some Follicular Atresia in Midlife
Recent clinical literature suggests that ovarian follicle counts are a contender as a reliable biomarker to determine reproductive status, over and above other measurements such as serum hormone levels alone. Indeed, serum hormone levels may not be a reliable measurement across the transition to menopause because they are in constant flux. More stable markers, such as antimüllerian hormone levels to estimate the non-growing follicle population (i.e., the ovarian follicle reserve), as well as antral follicle count, may be important in determining individual trajectories into the post-menopausal state (Anderson et al., 2012; Broer et al., 2011; Fleming et al., 2012). Ovarian follicle counts quantified here were an excellent guide in determining the rats’ progression into reproductive senescence and verifying the effectiveness of VCD-induced follicular depletion. At the subset sacrifice during the early menopause transition, a snapshot of the progression into follicle depletion was obtained. For primordial (non-growing) follicles, the VCD-treated animals had fewer follicles than Vehicle-treated animals in both age cohorts; for primary follicle counts, Young VCD-treated animals had fewer cells than age-matched counterparts, and Middle-Aged VCD-treated animals had marginally fewer. These results verify that VCD treatment effectively targets the resting ovarian follicle pool by initiating accelerated depletion of the pool. Interestingly, Young Vehicle-treated animals had marginally more primordial follicles than Middle-Aged Vehicle-treated counterparts, indicating that rats naturally experience some follicle depletion during aging; this loss of ovarian follicles is clearly not as extreme as is the case with VCD-induced ovarian changes, as evidenced by the fact that both VCD-treated age groups had significant follicular depletion of non-growing follicles at the time of the subset analysis. The antral follicle count, which is most often used in the clinical setting as a reliable marker of reproductive capacity, differed within the Young cohort, such that VCD-treated animals had fewer antral (pre-ovulatory) follicles compared to age-matched Vehicle-treated counterparts. The Middle-Aged cohort did not exhibit the same effect – VCD- and Vehicle- treated animals did not differ in antral follicle count at this subset snapshot. At the subset time point, within the Young and within the Middle-Aged cohorts, corpora lutea did not differ depending on treatment; this null effect was also seen within the VCD cohort and within the Vehicle cohort. Evidence of corpora lutea in the ovary at this early follicular depletion time point indicates that even animals undergoing accelerated follicular depletion still experience intermittent ovulatory cycles – akin to women’s irregular ovulatory cycles in the early menopause transition (O’Connor et al., 2009).
At the end of the study, within the Young cohort and within the Middle-Aged cohort, there were main effects of VCD-induced follicular depletion for all ovarian follicle types, as well as for corpora lutea, wherein animals that had undergone VCD-induced follicular depletion had fewer ovarian follicles and corpora lutea than Vehicle-treated animals. Indeed, VCD-treated animals had no quantifiable antral follicles at analysis, indicating the termination of normal ovulatory cycles and follicle maturation in these animals after the transition to a post-menopausal state. Although older VCD-treated animals had marginally more corpora lutea than younger VCD-treated counterparts, there was no difference in circulating progesterone levels between these groups at this time point. These results indicate that, despite VCD-treated animals experiencing intermittent ovulation, the few corpora lutea produced were not sufficient to generate a significant impact on resulting spatial memory performance. Similar to the results seen in the subset sacrifice, older Vehicle-treated animals had fewer primordial, primary, and secondary follicles than their younger counterparts, corroborating the idea that as aging ensues, rodents experience a natural decline in the ovarian follicle pool over time (for review, see Finch 2014), though not to the same extent as the profile of a VCD-induced follicle-deplete rat, or that of a menopausal woman. Within the VCD cohort, ovarian follicle counts did not differ between the ages, suggesting that overall, follicular depletion was successfully induced in both age groups with VCD treatment.
Broad Interpretations, Translational Implications, and Conclusions
To our knowledge, this is the first study to perform a longitudinal, repeated measures evaluation of ovary intact female rats comparing animals with or without VCD-induced follicular depletion at different ages to model human menopause. Several key findings are presented here: (1) undergoing the transition to menopause at a younger age is detrimental to memory performance compared to age-matched, reproductively competent subjects, at least during the early stages of the transition to reproductive senescence; (2) follicular depletion amplifies age-related differences in cognitive performance in the mid- and late- transitional menopause phases; and (3) in a post-follicle deplete state, ovarian follicular depletion in older animals may confer an enhanced capacity to solve a novel spatial memory task.
Of note, other laboratories have conducted cross-sectional studies looking at age-related memory decline in males (Bizon et al., 2009; Frick et al., 1995; Markowska, 1999b) and females (Markowska, 1999a, Markowska and Breckler, 1999). Ovx and sham female Fischer-344 rats have also been longitudinally evaluated for cognitive performance over a nine-month period, beginning in middle-age (Markowska & Savonenko, 2002a). Our laboratory recently demonstrated that cognitive practice can attenuate age-related decline in males and females by enhancing performance on the practiced task when tested at a later age compared to naïve, age-matched animals; additionally, cognitive practice bestows a sex-specific benefit for females to transfer enhanced performance capacity to a novel cognitive task (Talboom et al., 2014). It is possible that the animals’ previous, repeated experience on the complex WRAM task (i.e. cognitive practice) improved performance on the MM later in life. Similar findings for cognitive practice in females have also been seen in non-human primates (Lacreuse et al., 2005).
Many factors, including the appropriate timing, duration, hormone formulation, dose, and route of administration, are involved in developing hormone therapy regimens that will result in the attenuation of physiological menopause symptoms as well as provide potential beneficial effects for cognition. To add complexity to this puzzle, what is optimal for one woman may not have the same effect in another woman (for review, see Koebele & Bimonte-Nelson, 2015). Our findings provide further insight into the window of opportunity for hormone therapy by revealing that the age at the onset of the menopause transition matters for memory outcomes. The current study’s finding that early follicular depletion is detrimental to cognition corroborates clinical findings that younger women who experienced oophorectomy prior to the onset of natural menopause have poorer short-term verbal scores than those who experienced oopherectomy later in life (Nappi et al., 1999). Taken together, these findings indicate that age at menopause onset is an important parameter that impacts cognitive outcomes. Whether the age at onset of menopause, and further, the age at the onset of perimenopause, influences efficacy of hormone therapy is an important consideration as a next research step. Indeed, in the future, it will be important to experimentally evaluate whether the sensitive window(s) of follicular depletion and hormone loss map onto the sensitive window(s) for hormone therapy exposures to result in beneficial cognitive effects. Elucidating the impact of age, ovarian status, and hormone changes on spatial memory across the transition to a reproductively senescent state is key in developing personalized hormone therapy regimens that can be translated to the clinic for women experiencing the menopause transition. Understanding the dynamic nature of the menopausal hormone milieu and potential interactions with subsequent hormone therapy is key to deciphering parameters for optimal healthy brain aging profiles in women throughout their lifespans.
Table 2.
Mean± SE, range, and median of Estrone (pg/ml) levels at subset and end time points
| Group | Age | Mean Estrone level ± SE | Range of Estrone levels | Median Estrone levels |
|---|---|---|---|---|
| Young-Vehicle | 8 months | 52.9±4.74 | 45.8–61.9 | 51.00 |
| 12 months | 60.33±4.65 | 48.4–76.8 | 56.70 | |
| Young-VCD | 8 months | 58.37±2.36 | 53.9–61.9 | 59.30 |
| 12 months | 50.70±2.51 | 41.8–63.0 | 49.5 | |
| Middle-Aged- Vehicle | 14 months | 68.47±7.33 | 55.0–80.2 | 70.20 |
| 18 months | 60.02±1.80 | 55.5–66.1 | 59.35 | |
| Middle-Aged- VCD | 14 months | 65.57±3.50 | 61.3–72.5 | 62.90 |
| 18 months | 53.19±2.49 | 43.2–64.6 | 52.55 |
Table 3.
Mean± SE, range, and median of Androstenedione (ng/ml) levels at subset and end time points
| Group | Age | Mean Androstenedione level ± SE | Range of Androstenedione levels | Median Androstenedione levels |
|---|---|---|---|---|
| Young-Vehicle | 8 months | 0.30±0.03 | 0.24–0.34 | 0.32 |
| 12 months | 0.36±0.17 | Undetectable– 1.27 | 0.23 | |
| Young-VCD | 8 months | 0.19±0.04 | 0.11–0.24 | 0.22 |
| 12 months | 0.76±0.30 | 0.18–2.76 | 0.49 | |
| Middle-Aged-Vehicle | 14 months | 0.47±0.11 | 0.29–0.66 | 0.46 |
| 18 months | 0.83±0.16 | 0.20–1.47 | 0.70 | |
| Middle-Aged-VCD | 14 months | 1.00±0.29 | 0.48–1.49 | 1.02 |
| 18 months | 0.81±0.26 | Undetectable- 2.28 | 0.55 |
Highlights.
Longitudinal assessment of spatial memory in ovary-intact rats during aging
VCD transitional menopause model during aging
Greater memory impairment early in the transition was seen in young vs old animals
Follicular depletion amplified age-related memory changes later in the transition
Age at menopause transition onset is critical to consider for cognitive aging
Acknowledgments
Steroid hormone levels were determined in the Core Endocrine Laboratory of the Pennsylvania State University.
Funding: This research was funded by grants awarded to Heather A. Bimonte-Nelson from the National Institute on Aging (AG028084), state of Arizona, ADHS, and Alzheimer’s Disease Core Center Pilot grant program. Sarah E. Mennenga received funding from the NIH Initiative for Maximizing Student Development (IMSD) program (R25GM099650), the More Graduate Education at Mountain States Alliance (NSF), and the Western Alliance to Expand Student Opportunities Louis Stokes Alliance for Minority Participation Bridge to the Doctorate (WAESO-LSAMP-BD), National Science Foundation Cooperative Agreement HDR-1025879. Ryoko Hiroi received funding from the National Institute of Mental Health (F32 MH093145).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150(9):4248–59. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795–804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Safi Z, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertility and Sterility. 2014;101(4):905–915. doi: 10.1016/j.fertnstert.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Anderson R, Nelson S, Wallace W. Measuring anti-Müllerian hormone for the assessment of ovarian reserve: when and for whom is it indicated? Maturitas. 2012;71:28–33. doi: 10.1016/j.maturitas.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behavior Research Methods. 2005;37(3):379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. Journal of Comparative and Physiological Psychology. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–73. doi: 10.1016/s0306-4530(98)00068-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10101725. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(3–4):311–7. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not in aged, female rats. Neurosci Lett. 2002;328(1):50–4. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm ACEC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiology of aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12493549. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm ACEC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behavioral neuroscience. 2003;117(6):1395–406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm ACEC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behavioral neuroscience. 2004;118(4):707–14. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm ACC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. The European journal of neuroscience. 2006;24(1):229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Daniel JM, Koebele SV. The mazes. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Springer Publishing, Humana Press; NY: 2015. pp. 37–72. [Google Scholar]
- Bizon J, LaSarge C, Montgomery K, McDermott A, Setlow B, Griffith W. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of Aging. 2009;30(4):646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. A Single Dose of the Ovotoxicant 4-Vinylcyclohexene Diepoxide is Protective in Rat Primary Ovarian Follicles. 1999;158:244–252. doi: 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, … Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiology of learning and memory. 2010;93(3):444–53. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S, Wiqvist N. Ovarian hormones and uterine growth: effects of estradiol, progesterone and relaxin on cell growth and cell division in the rat uterus. Endocrinology. 1961;68:971–77. [Google Scholar]
- Broer S, Eijkemans M, Scheffer G, Rooij I, Vet A, Themmen A, Laven J, Jong F, Velde E, Fauser B, Broekmans F. Anti-Mullerian Hormone Predicts Menopause: A Long-Term Follow-Up Study in Normoovulatory Women. Journal of Clinical Endocrinology & Metabolism. 2011;96:25322539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- Burger HG. Physiology and endocrinology of the menopause. Medicine (Baltimore) 2006;34(1):27–30. [Google Scholar]
- Camp BW, Gerson JE, Tsang CW, Villa SR, Acosta JI, Blair Braden B, … Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings. The European journal of neuroscience. 2012;36(8):3086–95. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. CDC/NCHS National Hospital Discharge Survey. 2010;2010 [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–158. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis in the behavioral sciences. 3. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Downs J, Wise P. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Talboom JS, Braden BB, Tsang CW, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson HA. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Hormones and behavior. 2012;62(1):1–9. doi: 10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag AF, Khedr EM, Abdel-Aleem H, Rageh TA. Effects of Surgical Menopause on Cognitive Functions. Dement Geritar Cogn Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Finch C. The menopause and aging, a comparative perspective. The Journal of steroid biochemistry and molecular biology. 2014;142:132–41. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8(6):509–14. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Fleming R, Kelsey T, Anderson R, Wallace W, Nelson S. Interpreting human follicular recruitment and antimüllerian hormone concentrations throughout life. Fertility and Sterility. 2012;98:10971102. doi: 10.1016/j.fertnstert.2012.07.1114. [DOI] [PubMed] [Google Scholar]
- Frick K, Baxter M, Markowska A, Olton D, Price D. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16(2):149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frye JB, Lukefahr AL, Wright LE, Marion SL, Hoyer PB, Funk JL. Modeling Perimenopause in Sprague Dawley Rats by Chemical Manipulation of the Transition to Ovarian Failure. Comparative Medicine. 2012;62(3):193–202. doi: 10.1148/106.2.470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugate Woods N, Sullivan Mitchell E, Adams C. Memory functioning among midlife women: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7(4):257–265. [PubMed] [Google Scholar]
- Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. Journal of reproduction and fertility. 1987;81(2):433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- Haas J, Christian P, Hoyer P. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comparative medicine. 2007;57(5):443–9. [PubMed] [Google Scholar]
- Hale G, Robertson D, Burger H. The perimenopausal woman: Endocrinology and management. The Journal of Steroid Biochemistry and Molecular Biology. 2014;142:121–131. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;29:1–20. 24. [PubMed] [Google Scholar]
- Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG, Calver LE. In: Williams Gynecology. 2. Fried A, Boyle PJ, editors. McGraw-Hill; New York: 2012. [Google Scholar]
- Hoyer P, Devine P, Hu X, Thompson K, Sipes I. Ovarian Toxicity of 4-Vinylcyclohexene Diepoxide: A Mechanistic Model. Toxicologic Pathology. 2001;29(1):91–99. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes I, Hoyer P. Expression and Redistribution of Cellular Bad, Bax, and Bcl-xL Protein Is Associated with VCD-Induced Ovotoxicity in Rats. Biology of Reproduction. 2001a;65(5):1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Thompson K, Sipes I, Hoyer P. Apoptosis Induced in Rats by 4-Vinylcyclohexene Diepoxide Is Associated with Activation of the Caspase Cascades. Biology of Reproduction. 2001b;65(1):87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box Correlation for Degrees of Freedom from Sample Data in Randomized Bloc and Split-Plot Designs. J Educational Statistics. 1976;1(1):69–82. [Google Scholar]
- Hyde LA, Sherman GF, Denenberg VH. Non-spatial water radial-arm maze learning in mice. Brain Res. 2000;863(1–2):151–9. doi: 10.1016/S0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Kang Y, Anderson WA, DeSombre ER. Modulation of Uterine Morphology and Growth by Estradiol-17β and an Estrogen Antagonist. J Cell Biology. 1975;64:682–691. doi: 10.1083/jcb.64.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Sipes I, Hoyer P. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reproductive toxicology (Elmsford, NY) 1999;13(1):67–75. doi: 10.1016/S0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson H. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, Chennareddi L, Herndon JG. Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2005;119:118, e126. doi: 10.1037/0735-7044.119.1.118. [DOI] [PubMed] [Google Scholar]
- Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comparative Medicine. 2005;55(6):523–27. [PubMed] [Google Scholar]
- Markowska A. Life-long diet restriction failed to retard cognitive aging in Fischer-344 rat. Neurobiology of Aging. 1999a;20(2):177–189. doi: 10.1016/S0197-4580(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Markowska A. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999b;19(18):8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska A, Breckler S. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. The journals of gerontology. Series A, Biological sciences and medical sciences. 1999;54(12):B549–66. doi: 10.1093/gerona/54.12.B549. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savovnenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neuroscience. 2002a;22(24):10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002b;78:294e320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- Mayer L, Pearsall N, Christian P, Devine P, Payne C, McCuskey M, … Hoyer P. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reproductive Toxicology. 2002;16(6):775–781. doi: 10.1016/S0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Mayer L, Devine P, Dyer C, Hoyer P. The Follicle-Deplete Mouse Ovary Produces Androgen. Biology of Reproduction. 2004;71(1):130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- Mayer L, Dyer C, Eastgard R, Hoyer P, Banka C. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(9):1910–6. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- Meites J, Lu JKH. Reproductive aging and neuroendocrine function. In: Charlton HM, editor. Oxford Review of Reproductive Biology. Oxford Press; New York, NY, USA: 1994. [Google Scholar]
- Mennenga SE, Bimonte-Nelson HA. Translational cognitive endocrinology: designing rodent experiments with the goal to ultimately enhance cognitive health in women. Brain research. 2013;1514:50–62. doi: 10.1016/j.brainres.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga S, Koebele S, Mousa A, Alderete T, Tsang C, Acosta J, … Bimonte-Nelson H. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids. 2015a;99:16–25. doi: 10.1016/j.steroids.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga S, Gerson J, Koebele S, Kingston M, Tsang C, Engler-Chiurazzi E, … Bimonte-Nelson H. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology. 2015b;54:1–13. doi: 10.1016/j.psyneuen.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Garrud P, Rawlins J, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Murray C, Barber R, Foreman K, Ozgoren A, Abd-Allah F, … Vos T. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. The Lancet. 2015 doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- North American Menopause Society (NAMS) A Clinician’s Guide. 5 2015. [Google Scholar]
- O'Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178–1187. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychological methods. 2003;8(4):434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- Pal L, Santoro N. Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing research reviews. 2002;1(3):413–23. doi: 10.1016/s1568-1637(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Poe GR, Teed RGW, Insel N, White R, McNaughton BL, Barnes CA. Partial hippocampal inactivation: Effects on spatial memory performance in aged and young rats. Behavioral Neuroscience. 2000;114(5):940–949. doi: 10.1037//0735-7044.114.5.940. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahiskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca W, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Research. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca W, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, Oophorectomy, Estrogen, and the Risk of Dementia. Neurodegenerative Diseases. 2012;10(1–4):175–178. doi: 10.1159/000334764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Venkatesh A, Lester AW, Weinstein AT, Lipa P, Barnes CA. Age differences in strategy selection and risk preference during risk-based decision making. Behav Neurosci. 2015;129(2):138–148. doi: 10.1037/bne0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin B. Steroid hormones and cognitive functioning aging men: a mini-review. J Mol Neurosci. 2003;20(3):385–93. doi: 10.1385/JMN:20:3:385. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–6. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Rajkovic A. Ovarian differentiation and gonadal failure. American Journal of Medical Genetics. 1999;89(4):186–200. doi: 10.1002/(sici)1096-8628(19991229)89:4<186::aid-ajmg3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kochanek KD, MacDorman MF. Advance Report of Final Mortality Statistics, 1994. Monthly Vital Statistics Report CDC/NCHS. 1996;45(3S):1–80. [Google Scholar]
- Soules M, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: stages of reproductive aging workshop (STRAW) Fertility and Sterility. 2001;76(5):874–878. doi: 10.1016/S0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Springer L, Flaws J, Sipes I, Hoyer P. Follicular mechanisms associated with 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Reproductive toxicology (Elmsford, NY) 1996a;10(2):137–43. doi: 10.1016/0890-6238(95)02056-X. [DOI] [PubMed] [Google Scholar]
- Springer, McAsey, Flaws, Tilly, Sipes, Hoyer Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicology and applied pharmacology. 1996b;139(2):394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer L, Tilly J, Sipes I, Hoyer P. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicology and applied pharmacology. 1996c;139(2):402–10. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10(4):351–62. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- Talboom J, West S, Engler-Chiurazzi E, Enders C, Crain I, Bimonte-Nelson H. Learning to remember: cognitive training-induced attenuation of age-related memory decline depends on sex and cognitive demand, and can transfer to untrained cognitive domains. Neurobiology of Aging. 2014;35(12):27912802. doi: 10.1016/j.neurobiolaging.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timiras P, Quay W, Vernadakis A. Horm Aging. CRC Press; Boca Raton.NY: 1995. [Google Scholar]
- Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Weber M, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19(7):735–741. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of the transition stage. Menopause. 2013;20(5):511–7. doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: A systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2014;142:90–8. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine modulation and repercussions of female reproductive aging. J Phys Colloq. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Wise PM, Weiland NG, Scarbrough K, Sortino MA, Cohen IR, Larson GH. Changing hypothalamopituitary function: its role in aging of the female reproductive system. Horm Res. 1989;31:39–44. doi: 10.1159/000181084. [DOI] [PubMed] [Google Scholar]
- Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemarkers. Science. 1996;273:67–70. doi: 10.1126/science.273.5271.67. [DOI] [PubMed] [Google Scholar]
- Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough K, Harney JP, McShane T, Lloyd JM, Weiland NG. Aging of the female reproductive system: a window into brain aging. Recent Prog Horm Res. 1997;52:279–305. [PubMed] [Google Scholar]
- Wise P, Smith M, Dubal D, Wilson M, Krajnak K, Rosewell K. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20:243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- The World Factbook 2014–15. Washington, DC: Central Intelligence Agency; 2015. https://www.cia.gov/library/publications/the-world-factbook/index.html. [Google Scholar]
- Xu JQ, Kochanek KD, Murphy SL, Arias E. NCHS data brief. 168. Hyattsville, MD: National Center for Health Statistics; 2014. Mortality in the United States, 2012. [PubMed] [Google Scholar]