Abstract
The ability to accurately remember distinct episodes is supported by high-level sensory discrimination. Performance on mnemonic similarity tasks, which test high-level discrimination, declines with advancing age in humans and these deficits have been linked to altered activity in hippocampal CA3 and dentate gyrus. Lesion studies in animal models, however, point to the perirhinal cortex as a brain region critical for sensory discriminations that serve memory. Reconciliation of the contributions of different regions within the cortical-hippocampal circuit requires the development of a discrimination paradigm comparable to the human mnemonic similarity task that can be used in rodents. In the present experiments, young and aged rats were cross-characterized on a spatial water maze task and two variants of an object discrimination task: one in which rats incrementally learned which object of a pair was rewarded and different pairs varied in their similarity (Experiment 1), and a second in which rats were tested on their ability to discriminate a learned target object from multiple lure objects with an increasing degree of feature overlap (Experiment 2). In Experiment 1, aged rats required more training than young to correctly discriminate between similar objects. Comparably, in Experiment 2, aged rats were impaired in discriminating a target object from lures when the pair shared more features. Discrimination deficits across experiments were correlated within individual aged rats, though, for the cohort tested, aged rats were not impaired overall in spatial learning and memory. This could suggest discrimination deficits emerging with age precede declines in spatial or episodic memory, an observation that has been made in humans. Findings of robust impairments in object discrimination abilities in the aged rats parallel results from human studies, supporting use of the developed tasks for mechanistic investigation of cortical-hippocampal circuit dysfunction in aging and disease.
Keywords: CA3, dentate gyrus, object recognition, pattern separation, perirhinal cortex
Introduction
Aging leads to deficits in hippocampal-dependent episodic memory, including a reduced ability to maintain rich contextual details of an experience (Cansino, 2009; McDonough et al., 2014), greater susceptibility to interference (Talamini and Gorree, 2012), and treatment of novel stimuli as familiar, deemed ‘false recognition’ (Yeung et al., 2013; Pidgeon and Morcom, 2014). Evidence from human studies suggests that these aspects of age-related memory decline arise at least in part from a decreased ability to discriminate between similar stimuli (Leal and Yassa, 2015). While age-related dysfunction within hippocampal circuits is well-documented (Rosenzweig and Barnes, 2003; Burke and Barnes, 2006; Wilson et al., 2006; Burke and Barnes, 2010), the emergence of disrupted function across cortical sub-regions of the medial temporal lobe has not been fully examined. Thus, despite the established importance of perirhinal cortex in forming high-order sensory representations and discriminating between them (Bartko et al., 2007a, 2007b; Byun and Lee, 2010; Graham et al., 2010; Ahn and Lee, 2015), the contribution of change in neural communication across these circuits to age-related discrimination deficits remains unknown.
Both animal and human studies have reported declines in stimulus discrimination abilities with age, yet distinct potential mechanisms have been highlighted between different species. The ability to discriminate between similar objects has been shown to require the perirhinal cortex in rats (Eacott et al., 2001; Bartko et al., 2007a, 2007b; Winters et al., 2010; Ahn and Lee, 2015; Hales et al., 2015) and monkeys (Buckley and Gaffan, 1997; Baxter and Murray, 2001; Bussey et al., 2002, 2003). On the other hand, the majority of neuroimaging studies that have directly investigated object discrimination abilities in humans have shown a consistent relationship between task performance and activation within the CA3/dentate gyrus circuit (Kirwan and Stark, 2007; Bakker et al., 2008; Lacy et al., 2011b; Yassa et al., 2011a, 2011b; Bakker et al., 2012; Motley and Kirwan, 2012; Reagh and Yassa, 2014; Bakker et al., 2015; Doxey and Kirwan, 2015). A reason for this apparent discrepancy is that human and animal studies of aged subjects have used different behavioral paradigms. Specifically, the rodent work has generally used spontaneous or novel object recognition tasks that rely on rats’ innate preference for novelty (Burke et al., 2011; Gámiz and Gallo, 2012), which itself is known to be compromised by aging both in humans (Daffner et al., 2006; Fandakova et al., 2014) and animal models (Burke et al., 2010). In contrast, research in humans have used variants of the ‘mnemonic similarity task’, which tests subjects’ abilities to discriminate between target and lure stimuli that vary in their distinctiveness (Toner et al., 2009; Yassa et al., 2011a, 2011b; Ryan et al., 2012; Stark et al., 2013, 2015; Reagh et al., 2016; Huffman and Stark, 2017). The goal of the current studies was to develop and validate a rodent target-lure discrimination task that would be comparable to the mnemonic similarity task using the Fischer 344 x Brown Norway (F344 x BN) hybrid rat model of cognitive aging. We show that aged relative to young rats were slower to learn to discriminate between similar object pairs than distinct pairs (Experiment 1). Further, when a learned target object was paired with multiple novel lure objects, aged rats were selectively impaired on trials with high target-lure similarity (Experiment 2). These findings in rats parallel data obtained from aged humans, and suggest the model is valid for further exploration of underlying hippocampal and cortical mechanisms responsible for stimulus discrimination abilities.
Materials and Methods
Animals
A total of 20 young adult (4 months old) and 10 aged (24 months old) male Fischer 344 x Brown Norway F1 hybrid rats from the NIA colony (Taconic) completed Experiment 1. A subset of the young group (N = 10) and the full aged group (N = 10) completed Experiment 2. Rats were housed individually in standard Plexiglas cages and maintained on a 12-h reversed light/dark cycle (lights off at 8:00 am). All manipulations were performed during the dark phase of the cycle. Rats were given 7 days to acclimate to the facility and were handled for a minimum of 4 days before behavioral testing began. To encourage appetitive behavior in discrimination experiments, rats were placed on a restricted feeding protocol in which 25±5 g moist chow (Teklad LM-485, Harlan Labs) was provided daily and drinking water was provided ad lib. Shaping began once rats reached approximately 85% of their baseline weights. Throughout the period of restricted feeding, rats were weighed daily and closely monitored to ensure they maintained an optimal body condition score of +2 to 3. The body condition score is assigned based on the presence of palpable fat deposits over the lumbar vertebrae and pelvic bones (Ullman-Culleré and Foltz, 1999; Hickman and Swan, 2010). All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Florida.
Spatial Learning and Memory: Morris water maze task
Prior to beginning Experiments 1 and 2, spatial learning and memory abilities were cross-characterized in the majority of young rats (N = 15) and all aged rats (N = 10) using established procedures (Gallagher et al., 1993; Bizon et al., 2009), as previously implemented in our laboratory (Hernandez et al., 2015; Johnson et al., 2016). The water maze consisted of a circular pool (1.83 m diameter, 58 cm height) surrounded by black curtains to which visible cues were fixed. A submersible platform 12 cm in diameter (HVS Image; Mountain View, CA) was positioned in one quadrant of the pool and remained in this location throughout training and testing. The pool was filled with water (~25°C) to a depth of 2 cm above the platform with white non-toxic tempera paint added to obscure the platform’s location. Behavior was monitored with an overhead camera and video tracking software (Water 2100, HVS Image).
Rats completed 3 swim trials of maximum 90 s duration each day for 8 consecutive days. Mean cumulative integrated path length (CIPL) values were calculated for each block of 5 training trials to provide an index of spatial learning (see Johnson et al. 2016 for detailed description of protocol and variables). Probe tests were conducted on the third trial of days 2, 4, 6, and 8. For these tests, the platform was lowered 20 cm below the water’s surface for the first 30 s of the trial and spatial memory was assessed based on proximity of the swim path to the target location. To quantify performance during the probe trials, a proximity measure and spatial learning index were calculated. For proximity, the cumulative search error was divided by the duration of the probe trial. Proximity scores for the 2nd, 3rd and 4th probe trials were weighted and summed to provide an overall measure of spatial learning ability (Gallagher et al., 1993; Bizon et al. 2009). Lower spatial learning indices indicate a more accurate search.
One day after completing the spatial memory task, rats were given a cued navigation test as a control for visual, sensorimotor, or motivational factors that could influence performance. The water level was lowered to 2 cm below the platform and a black ring was placed on top of the platform to increase its visibility. The platform was moved to a different quadrant of the maze for each of 6 trials. Rats were allotted a maximum of 90 s to reach the platform, with a 30 s interval provided between trials. Mean path length values across cued trials were calculated as an index of visual and swimming abilities.
Object Discrimination Apparatus
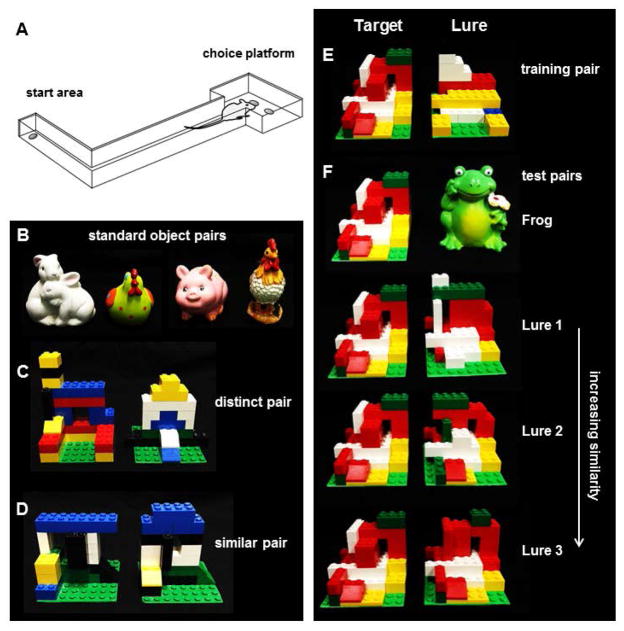
Discrimination tasks were carried out in an L-shaped track bounded by a start area and choice platform, separated by an arm 84 cm × 10.2 cm with walls 6.4 cm in height (Fig. 1A). The track was built out of plywood and sealed with waterproof black paint. In the start area, a single food well (2.5 cm diameter) was recessed 1 cm deep in the floor, 4 cm from the back wall. The choice platform measured 32 cm × 24 cm, also with walls 6.4 cm in height. Two food wells were centered relative to the platform entrance, 12 cm apart and positioned 7.6 cm from the back wall. The testing room was kept dimly lit with one white bulb angled to the wall near the start area and 2 red bulbs, one by the start area and one over the choice platform that provided extra illumination for purposes of video recording. This level of lighting was sufficient for the experimenters to comfortably see the objects. A webcam (Logitech; Newark, CA) was positioned 50 cm above the choice platform. Recording was carried out with a custom software interface (Collector; Burke/Maurer Laboratories, Gainesville, FL). A white noise machine was kept on to reduce the impact of extraneous noise on performance.
Figure 1.
Apparatus used in object discrimination training (Experiment 1) and mnemonic similarity tests (Experiment 2). (A) L-shaped maze bounded by start area and choice platform. (B, C, D) Object pairs used in Experiment 1. (B) shows standard object pairs used for initial training, (C) shows the distinct LEGO® object pair that shared 60% volume and 38% visible features, (D) shows the similar LEGO® pair that shared 87% volume and 63% visible features. (E, F) Target and lure objects used in Experiment 2. (E) Shows the target (S+) object used throughout the mnemonic similarity pre-training and tests, paired with a distinct object that shared 65% volume and 38% visible features. Rats were trained to associate the target with a food reward before moving on to discrimination tests. (F) Shows the familiar target object paired with each of 4 lure objects in order of increasing similarity: a distinct lure object (‘Frog’), Lure 1 that shared 89% volume and 50% visible features with the target, Lure 2 with 92% shared volume and 71% shared visible features, and Lure 3 with 95% shared volume and 90% shared visible features.
Experiment 1: Discrimination of target and single lure
Habituation and shaping
Discrimination sessions took place 5–6 days per week at approximately the same time each day. First, rats were habituated to the track for 1 to 3 days, depending on their natural tendency to explore. Each rat was placed individually in the start area and allowed 10 min to retrieve scattered pieces of Froot Loop cereal (Kellogg’s; Battle Creek, MI). Rats were then shaped to alternate between the start area and choice platform. For these sessions, one of the two food wells on the choice platform was baited with a piece of Froot Loop. Rats were required to leave the start area, traverse the length of the arm, retrieve the food reward, and return to the start area where a second food reward was provided. On the choice platform, the side of the baited well (left vs right) was randomized across trials. Shaping was considered complete once rats alternated 32 times within 20 min. Young rats achieved this criterion within 1–7 days (Mean = 3.6, SD = 1.54), while aged rats took between 4–15 days (Mean = 9.1, SD = 3.31). This extended period of shaping in the aged rats likely reflects the greater number of days required to reach 85% of baseline body weight on the restricted feeding protocol, and thus the slower associated increase in motivation to obtain food rewards.
Standard object discriminations
Rats were first trained on procedural aspects of the task by learning to discriminate between two ‘standard’ objects that differed in size, shape, and texture, measuring 5–10 cm in height by 5–8 cm in width (Fig. 1B). Two pairs of objects were used across all experiments: bunnies-hen and pig-rooster. Rats were required to learn that one of the two simultaneously presented objects in the pair was the target (S+), signaling a food reward, while the alternate object in the pair was never rewarded (S−). On each trial, the rat exited the start area and traversed the arm to the choice platform. The rat could then choose to displace one of the two objects covering the food wells. In the case of a correct response, the rat would receive a second food reward in the start area. In the case of an incorrect response, both objects and the un-retrieved piece of Froot Loop would be quickly removed from the choice platform by the experimenter, and the rat would return to the start area. A food reward was not provided in the start area on incorrect trials. The side of the baited well on the choice platform was pseudo-randomized across trials, such that an equal number of left- and right-rewarded trials were given in each session. Rats completed 32 trials in each session. Training proceeded by trial-and-error until rats reached criterion performance of ≥26 correct out of 32 trials (≥81.3% correct responses) and maintained performance at this level for 2 consecutive days. Each rat was required to achieve criterion on both standard object pairs before moving on to the next phase. The rewarded object within each pair (i.e., bunnies vs hen, pig vs rooster) and the order in which training for the two pairs took place (i.e., bunnies-hen first vs pig-rooster first) were counter-balanced across rats and age groups. To minimize the potential for scent-marking an object within sessions, two or three copies of each object were presented in rotation across trials. All copies of objects were thoroughly cleaned with 70% ethanol between test sessions.
LEGO® object discriminations
To test whether young and aged rats differed in their ability to learn to discriminate between distinct versus similar objects, two pairs of objects were created from LEGO® blocks (Enfield, CT; Fig. 1C and 1D). Object pairs were matched for overall volume, shape, and texture, while systematically varying shared visible features. Every object was built on a square piece of green plastic base sheet measuring 6.5 cm × 6.5 cm, and objects ranged from 6–9 cm in height. Feature overlap between the two objects in each pair was calculated in two ways. First, as a factor of total 3D volume of block ‘bits’, where a bit is the unit of LEGO® block corresponding to a single 3D pip. Overlap was also calculated as a factor of shared surface features on the front side of each object, visible to the rats as they entered the choice platform (see Supplemental Figure 1). Critically, the relative feature overlap between object pairs did not vary across the two methods of quantification. The first ‘distinct’ pair of objects, a relatively easy discrimination (Fig. 1C), shared 60% volume and 38% visible features (34 of 90 bits). The second ‘similar’ pair, a more difficult discrimination (Fig. 1D) shared 87% volume and 63% visible features (35 of 56 bits). Each rat was trained to criterion on both pairs of objects. As for the previous phase, the target object within each pair and order of training for the two pairs were counter-balanced across individual rats and age groups. In Experiment 1, discrimination abilities were assessed based on the number of days of training required and the number of incorrect responses made prior to reaching criterion performance.
Experiment 2: Discrimination of learned target from multiple novel lures
Pre-training on target object
Once rats had completed Experiment 1, they proceeded to Experiment 2. General procedures remained the same across both experiments. Rats were first pre-trained to identify the target object (S+) that would be rewarded throughout Experiment 2 (Fig. 1E). For these sessions, a relatively distinct object was used as the lure (S−). As in Experiment 1, these objects measured 6.5 cm × 6.5 cm in length and width, and 6–6.5 cm in height. This pair shared 65% volume and 38% visible features (21 of 56 bits), which was comparable to that of the ‘distinct’ pair from Experiment 1 (Fig. 1C). Rats were required to reach the same criterion of ≥81.3% correct responses in discriminating the target from the distinct lure for 1 day before moving on to tests.
Tests for discrimination of familiar target from novel lures
After achieving criterion in pre-training, rats were given 2 days off before their first test. Testing then proceeded every 3 days (day 1 test, days 2–3 off) until a total of 4 test sessions had been completed. This design was adopted to avoid over-familiarization with the lure objects. Each test session comprised 50 discrimination trials: 10 with a distinct control object (a frog figurine), 10 with each of 3 similar lure objects built from LEGO® blocks, and 10 with an identical copy of the target object. Lure objects are shown, with comparison to the target object, in Figure 1F. Objects were of the same overall dimensions as those used in Experiment 1 and in pre-training. The most distinct Lure 1 object shared 89% volume and 50% visible features (28 of 56 bits), next Lure 2 shared 92% volume and 71% visible features (34 of 48 bits), and the most similar Lure 3 object shared 95% volume and 90% visible features (43 of 48 bits). Discrimination trials with the distinct object were included as a positive control condition, to test whether rats would continue to discriminate between pairs with no feature overlap as in Experiment 1. Identical object discrimination trials were included to test the possibility that rats chose the target based on odor cues from the hidden food reward, or based on scent marks left on the objects on previous trials. Given that the target object was included in each of the 50 trials during each test session, 8 identical copies of this object were cycled in and out across trials. Similarly, 2 identical copies of each lure object were used in rotation across trials. All copies of objects were thoroughly cleaned with 70% ethanol between tests. For these tests, discrimination abilities were assessed based on percent correct responses made for each trial type. Performance was considered both for individual test sessions and collapsed as means across the 4 tests. Sessions were recorded and video files were reviewed with custom software (Collector/Minion; Burke/Maurer Laboratories, Gainesville, FL). Response selection behavior and reaction times were scored trial-by-trial for each test. Response selection was scored as a binary variable based on whether rats displayed ‘checking’. Checking was defined as hesitation or pausing in front of one of the two objects prior to withdrawing attention from that object, instead moving to investigate and/or displace the alternate object (see video in Supplemental Material). Reaction time was defined as the time elapsed between the video frame in which the tip of the rat’s nose crossed the threshold of the choice platform, and the video frame in which the rat initiated displacement of an object. During this time, the rat traversed the distance from the center point of the threshold to the center of a food well. Collector/Minion software allowed determination of reaction times for each frame of video recorded at 30 FPS, thus with precision to ±30 milliseconds.
Statistical Analyses
Data are presented as mean values ± SEMs. Analyses were performed with the Statistical Package for the Social Sciences (SPSS) v23 for Mac OS X. Behavioral variables were compared with repeated measures ANOVAs or t-tests, with experimental phase or similarity of object pairs as within subjects factors and age as a between subjects factor. Where relevant, performance (% correct responses) was compared to chance levels (i.e., 50% correct responses) with Bonferroni-corrected one sample t-tests. Choice of statistical test was dictated by assumptions of normality, assessed with Shapiro-Wilk tests, and homogeneity of variances, assessed with Levene’s tests. Results of these tests are stated where applicable. Post hoc analyses were performed with simple contrasts and a Bonferroni correction was applied. Relationships between spatial abilities and object discrimination performance in Experiments 1 and 2 were determined with two-tailed Spearman’s correlations on normalized variables. P-values less than 0.05 were considered statistically significant.
Results
Experiment 1: Aged rats require more training on similar object discriminations
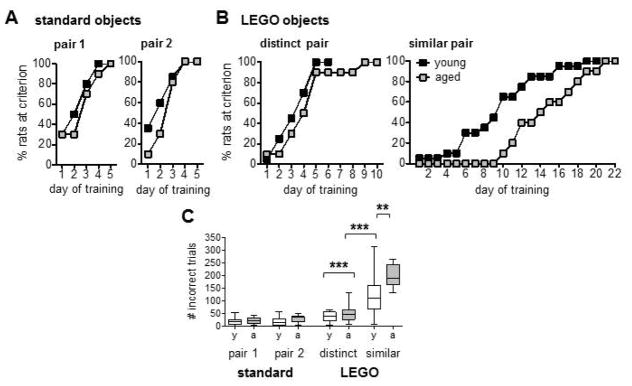
Young and aged rats achieved criterion within 5 days of training, for both the first and second standard pairs (Fig. 2A). Young rats learned the first pair within 2–5 days (Mean = 3.6, SD = 0.99) and aged within 4–5 days (Mean = 4.4, SD = 0.52). Similarly, young rats learned to discriminate the second pair within 2–5 days (Mean = 3.25, SD = 1.1) and aged within 2–5 days (Mean = 3.8, SD = 0.92). A repeated measures ANOVA comparing days to criterion on the two standard object pairs revealed main effects of pair (F(1,28) = 6.75, p < 0.02) and age (F(1,28) = 4.47, p < 0.04), but no pair x age interaction: p = 0.50). The results of this analysis reflect the fact that all rats took fewer days to reach criterion on the second of the two object pairs, and that aged rats took a greater number of days than young to reach criterion, across both object pairs. The amount of training required to achieve criterion was also analyzed as the number of incorrect trials completed, which does not artificially inflate duration of training based on days when rats nearly achieved, but just missed, the cut-off of ≥26 correct out of 32 trials. This analysis revealed that young and aged rats did not differ in number of incorrect trials for either standard object pair (Fig. 2C; effects of pair, age, pair x age interaction: p’s > 0.11). This lack of difference in training required to reach criterion for standard object discriminations strongly supports the conclusion that young and aged rats did not differ in their motivation or ability to physically perform object discrimination tasks.
Figure 2.
Results of object discrimination training in Experiment 1. (A) Cumulative frequency plots show the percentage of young (n = 20) and aged (n = 10) rats that achieved the required criterion of ≥81.3% correct responses across training sessions with standard object pairs. (B) Cumulative frequency plots show the percent of all young and aged rats that reached criterion across training sessions with the distinct and similar LEGO® object pairs. (C) Mean (±SEM) number of incorrect responses made by young and aged rats prior to achieving criterion performance on standard and LEGO® object discriminations, collapsed across all training days. Both age groups completed more incorrect trials in learning the similar pair than in learning the distinct pair (p’s < 0.001). Aged rats were no different than young in number of trials to learn the distinct discrimination (p = 0.23), but required a greater number of trials than young to reach criterion on the similar pair (p < 0.002). ** p < 0.01, *** p < 0.001.
The number of training days required for young and aged rats to achieve criterion performance on LEGO® object pairs is shown in Figure 2B and performance of rats across all training days for each of the two standard object pairs and the distinct and similar LEGO object pairs for are shown in Supplemental Figure 2A–D. Young rats learned to discriminate the distinct pair within 2–6 days (Mean = 4.6, SD = 1.3) and aged within 2–10 days (Mean = 5.2, SD = 2.1). In contrast, for the similar pair, young rats took 2–20 days (Mean = 10.8, SD = 4.4) and aged 10–22 days (Mean = 15.8, SD = 3.9). A repeated measures ANOVA comparing days to criterion on distinct versus similar pairs revealed statistically significant main effects of pair and age (F(1,28) values > 10.5, p’s < 0.003) and a pair x age interaction (F(1,28) = 6.32, p < 0.02). Between subjects contrasts showed no age difference in days to criterion for the distinct pair (F(1,28) = 1.12, p = 0.30), but a greater number of training days were required for aged relative to young in learning to discriminate the similar pair (F(1,28) = 9.46, p < 0.005).
The mean numbers of incorrect trials for LEGO® object pairs are shown in Figure 2C. A repeated measures ANOVA revealed a significant effect of similarity on the number of incorrect trials required to reach criterion (main effect of pair: F(1,28) = 79.9, p < 0.001). This analysis also revealed a statistically significant main effect of age (F(1,28) = 12.0, p < 0.002) and a pair x age interaction (F(1,28) = 7.38, p < 0.01). Within subjects contrasts confirmed that a greater number of incorrect trials were required for the similar objects relative to the distinct objects in both young (F(1,19) = 27.5, p < 0.001) and aged rats (F(1,9) = 57.5, p < 0.001). Between subjects contrasts showed no age difference in incorrect trials for the distinct pair (F(1,28) = 1.52, p = 0.23), but that aged rats required a greater number of incorrect trials than young to reach criterion on the similar pair (F(1,28) = 11.2, p < 0.002). For the distinct pair, the range in number of incorrect trials required was comparable between the young group (Range = 5–65 trials; Mean = 38.6, SD = 18.6) and aged group (Range = 9–67 trials, with one rat requiring 131 trials; Mean = 50.5, SD = 35). In contrast, for the similar pair, the full range of number of incorrect trials required to reach criterion for the aged group (Range = 132–265 trials; Mean = 196.5, SD = 44.6) exceeded the mean of the young (Range = 9–188 trials, with one rat requiring 313 trials; Mean = 116.5, SD = 68.4).
Experiment 2: Aging impairs discrimination performance based on target-lure similarity
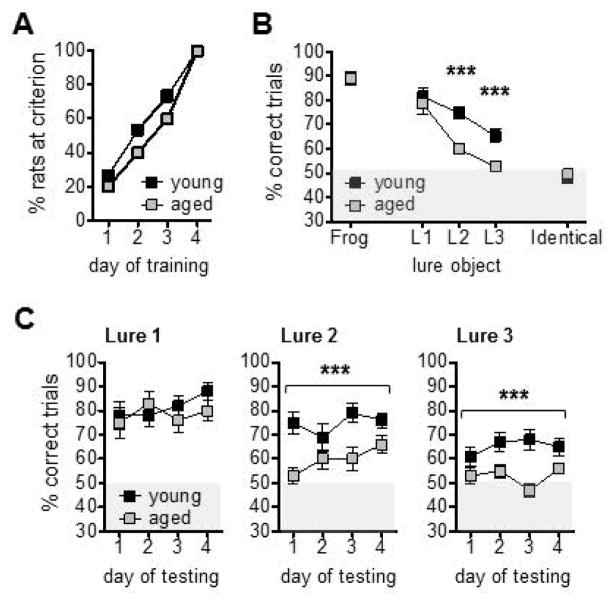
Rats quickly learned to discriminate between the target and the distinct lure object used for pre-training in Experiment 2 (Fig. 3A). Both young and aged groups reached criterion within 1–4 days (Supplemental Figure 2E). Young rats completed 2–35 incorrect trials within this period (Mean = 16.6, SD = 13) and aged 6–44 incorrect trials (Mean = 24.5, SD = 14.3). Young and aged rats did not differ in the number of days (t(18) = −0.94, p = 0.36) or incorrect trials (t(18) = −1.29, p = 0.21) required to reach criterion during this pre-training phase.
Figure 3.
Results of mnemonic similarity task pre-training and tests in Experiment 2. (A) Cumulative frequency plot showing the percentage of young (n = 10) and aged (n = 10) rats that achieved criterion of ≥81.3% correct responses across pre-training sessions with the target object and a distinct object. (B) Mean (±SEM) percent correct responses for each trial type presented on mnemonic similarity tests. Values are collapsed across four tests given over a period of 12 days, to a total of 40 trials with each lure object (distinct ‘Frog’ object, Lure objects 1–3, or an identical pair of copies of the target object). Both age groups showed poor performance on the high-similarity discriminations between the target and Lure 3 (L3), relative to lower-similarity discriminations with Lure 1 (L1) or Lure 2 (L2) (main effect of similarity: p < 0.001). Relative to young, aged rats showed impaired performance on Lure 2 and Lure 3 trials (p < 0.001). (C) Mean (±SEM) percent correct responses for each trial type plotted for each of the four test sessions. Performance did not improve significantly in young or aged rats across test days, for any trial type (p’s > 0.16). However, aged rats showed performance deficits on Lure 2 and Lure 3 trials across test days (p’s < 0.001). *** p < 0.001.
Mean performance across the four discrimination tests was calculated for each trial type. These values are shown in Figure 3B. Mean performance values on ‘Identical’ trials, for which 2 copies of the target object were presented, were excluded from analyses because they did not differ from chance in either the young (Mean = 48, SD = 7.2; t(9) = −0.89, p = 0.40) or aged rats (Mean = 49.5, SD = 4.2; t(9) = −0.38, p = 0.72), and did not change over the course of the 4 test days (repeated measures ANOVA, no main effect of day: F(3,54) = 0.13, p = 0.94). Mean values for all other trial types were entered into a repeated measures ANOVA with lure similarity as a within subjects factor and age as a between subjects factor. This analysis revealed significant main effects of lure similarity (F(3,54) = 53.0, p < 0.001) and age (F(1,18) = 14.1, p < 0.001), and a significant similarity x age interaction (F(3,54) = 3.93, p < 0.01). Within subjects contrasts showed a statistical trend for young rats to make fewer correct responses when the lure object was most similar to the target (Corrected α = 0.05/10 = 0.005; Lure 1 vs Lure 3 trials: F(1,9) = 2.06, p < 0.01). For aged rats, there was a statistically significant difference in the number of correct responses on both Lure 2 trials (F(1,9) = 29.7, p < 0.001) and Lure 3 trials (F(1,9) = 37.3, p < 0.001), relative to Lure 1. Between subjects contrasts confirmed aged rats were impaired on both Lure 2 (F(1,9) = 23.9, p < 0.001), and Lure 3 discriminations (F(1,9) = 17.6, p < 0.001), relative to young rats.
We next asked whether performance improved over the 4 days of discrimination tests (Fig. 3C). Percent correct responses for Lure 1, Lure 2, and Lure 3 trials were entered into separate repeated measures ANOVAs, with day as a within subjects factor and age as a between subjects factor. Performance on Lure 1 trials did not differ between young and aged rats (F(1,18) = 0.32, p = 0.58) and did not change over days (F(3,54) = 1.40, p = 0.25). The day x age interaction was also not statistically significant (F(3,54) = 1.17, p = 0.33). Performance on these trials was consistently above chance levels (i.e. 50% correct responses) across all test days (Means = 76.5–84 % correct, SDs = 13.5–18.7; t(19) values = 6.33–11.2, p’s < 0.001 for all comparisons, one sample t-tests). As for mean performance collapsed across test days (Fig. 3B), significant main effects of age were present for Lure 2 (F(1,18) = 23.9, p < 0.001) and Lure 3 (F(1,18) = 17.6, p < 0.001) trials across all test days. For both, main effects of day and day x age interactions were not statistically significant (Lure 2: p’s > 0.26, Lure 3: p’s > 0.18). For Lure 2 trials, young rats’ performance was significantly greater than chance across all test days, while aged rats’ performance only exceeded chance levels on day 4 (Corrected α = 0.05/8 = 0.006; p’s > 0.03 except for day 4: p < 0.002). Similarly, for Lure 3 trials, young rats maintained performance better than chance on days 2–4 (p’s < 0.003), while aged rats did not show levels that differed from chance on any of the 4 test days (p’s > 0.02). Thus, even with increasing experience across test sessions, aged rats show minimal improvement in their abilities to discriminate between similar objects.
Discrimination performance depends on suppression of a perseverative side bias
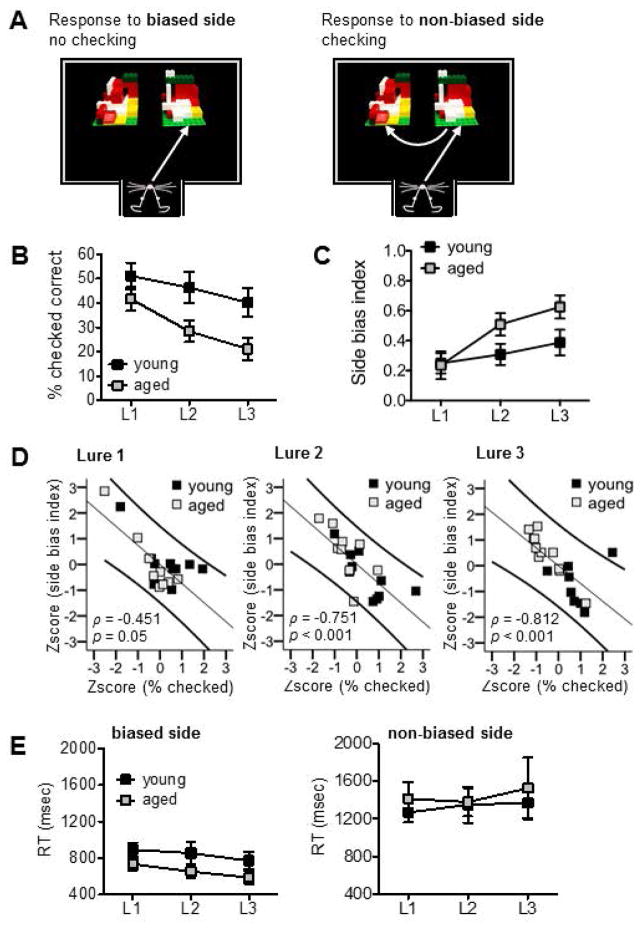
Potential variation in response strategies that rats employed on discrimination tests in Experiment 2 was assessed based on the presence of ‘checking’ behavior (see Figure 4A and video in Supplemental Materials) and duration of reaction times. The proportion of trials for which a rat displayed checking behavior was collapsed for each trial type across the 4 test sessions. The mean percent of correct discrimination trials for which rats displayed checking behavior, as a factor of lure similarity, is shown in Figure 4B. Incorrect trials were excluded from these analyses because a subset of rats made no errors, which restricted the number of data points available, and because rats very rarely showed checking behavior on incorrect trials. Mean values were entered into a repeated measures ANOVA with lure object as a within subjects factor and age as a between subjects factor. This analysis revealed significant main effects of lure object (F(2,36) = 11.5, p < 0.001) and age (F(1,18) = 5.72, p < 0.03), reflecting infrequent checking in the aged rats. The lure object x age interaction was not statistically significant (p = 0.29). Within subjects contrasts confirmed rats showed less checking on Lure 2 and Lure 3 trials, as compared to Lure 1 trials (F(1,18) values > 9.30, p’s < 0.007). There was also a statistical trend toward a difference in the proportion of Lure 2 trials with checking relative to Lure 3 trials (Corrected α = 0.05/3 = 0.017; F(1,18) = 4.99, p = 0.04).
Figure 4.
Results of behavioral analyses for mnemonic similarity tests in Experiment 2. (A) Schematic shows the distinction between a behavioral response to the rat’s inherently biased side, without checking, and to the non-biased side, with checking. (B) Mean (±SEM) percent of correct responses made across four test sessions in which young and aged rats showed checking behavior. Values are shown for each of the three Lure objects (L1, L2, L3). (C) Mean (±SEM) side bias index of young and aged rats across test sessions. A side bias index of 1 reflects complete bias to select the object presented on either the left or right side of the choice platform, while a side bias of 0 reflects no side bias and an equal number of responses made to the left and right sides. (D) Scatter plots show relationships between normalized mean side bias index values and normalized mean percent of correct trials with checking behavior, for Lure 1, Lure 2, and Lure 3 discriminations. Spearman’s rho correlation coefficients and p values are shown for each plot, inset. (E) Mean (±SEM) reaction time values for correct discrimination responses made by young and aged rats on mnemonic similarity tests. Values are collapsed for each trial type but considered separately based on whether rats selected the object presented on their inherently biased or non-biased side.
In tasks that require choice between two potential outcomes presented simultaneously, rats tend to initially default to an egocentric response-based strategy before learning to apply a more flexible task-specific strategy (Lee and Solivan, 2008; Jo and Lee, 2010; Lee and Byeon, 2014; Hernandez et al., 2015, 2017). For example, both young and aged rats develop a strong bias toward selecting an object on a specific side of a choice platform (i.e., left vs right) before learning to recognize and compare visual features and select a target object over a lure. Suppression of side bias as training progresses has been shown to correspond with an increase in correct responses guided by the task-specific rule (Lee and Byeon, 2014; Hernandez et al., 2015, 2017). Based on the fact that both young and aged rats showed more checking behavior and better performance on Lure 1 trials, but less checking and poorer performance on Lure 2 and Lure 3 trials (Fig. 3B and 4B), we asked whether rats showed evidence of suppressing a side bias when making correct responses across these trial types. A side bias index was calculated as the absolute value of (Total number left choices − Total number right choices) / Total number of trials. Values computed with this formula can reflect performance completely governed by a side bias (100% responses to one side, index = 1), or performance independent of a side bias (50% responses to each side, index = 0).
Mean side bias index as a factor of trial type is shown in Figure 4C. A repeated measures ANOVA with lure object as a within subjects factor and age as a between subjects factor revealed a significant main effect of lure object (F(2,36) = 9.93, p < 0.001). The main effect of age and lure x age interaction were not statistically significant (p’s > 0.11). Within subjects contrasts confirmed rats showed a greater side bias on Lure 3 trials than on Lure 1 trials (F(1,18) = 14.4, p < 0.001). Results of correlational analyses between checking behavior and side bias index are shown in Figure 4D. The proportion of trials with checking was inversely related to magnitude of the side bias index on Lure 2 (ρ = −0.751, p < 0.001) and Lure 3 trials (ρ = −0.812, p < 0.001). While there was not a significant relationship between these variables for Lure 1 trials after correcting for multiple tests (Corrected α = 0.05/3 = 0.02; ρ = −0.451, p = 0.05), this result shows a trend toward the same relationship as observed for Lure 2 and Lure 3. The reduced strength of this relationship for Lure 1 discriminations likely reflects ceiling effects, as performance on these trials was better across age groups. When young and aged rats were considered separately, a statistically significant inverse relationship was present for Lure 3 trials in the aged rats (ρ = −0.888, p < 0.001), with trends for Lure 1 and Lure 2 (Corrected α = 0.05/3 = 0.02; p’s = 0.03). No significant correlations were detected in the young rats for any trial type (Corrected α = 0.05/3 = 0.02; p’s > 0.04), also likely reflecting ceiling effects based on their better performance.
Mean reaction times are shown as a factor of trial type in Figure 4E, with trials on which rats responded to their biased side versus their non-biased side plotted separately. Within subjects comparisons collapsing across lure objects confirmed that rats’ reaction times were greater when responding to their non-biased side than when responding to their biased side (paired samples t-tests: p’s < 0.001). This directly reflects the increased frequency of checking behavior when presenting the target object on the non-biased side. A repeated measures ANOVA revealed a significant main effect of lure object on reaction times to the biased side (F(2,36) = 4.11, p < 0.03). The main effect of age and lure x age interaction were not statistically significant (p’s > 0.28), indicating no slowing of responding in the aged relative to young rats. Within subjects contrasts confirmed that, for side-biased responses, rats showed decreased reaction times on Lure 3 relative to Lure 1 trials (F(1,18) = 6.91, p < 0.02). A repeated measures ANOVA comparing reaction times to the non-biased side across lure objects and age revealed no significant effects of either of these factors (p’s > 0.50).
Object discrimination performance across experiments
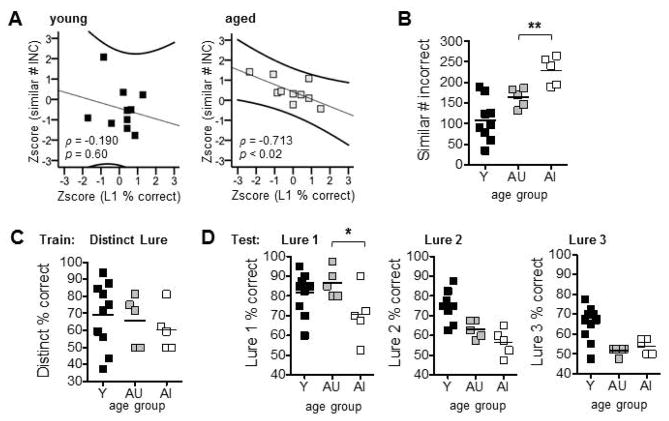
Correlational analyses were used to determine whether performance on the object discrimination tasks in Experiments 1 and 2 were related within individual rats. Given there was no difference between young and aged in the range of number of trials required to reach criterion for the distinct LEGO® object pair, numbers of incorrect responses required to reach criterion for the similar pair were normalized and Z-scores were entered as the only variable of interest from Experiment 1. Proportion of responses correct for Lure1, Lure 2, and Lure 3 trials were normalized and Z-scores were entered as variables of interest from Experiment 2. No correlations were detected between these variables when collapsing across age (p’s > 0.07), or in the young rats alone (p’s > 0.25; shown for Lure 1 trials in Fig. 5A). In contrast, for individual aged rats there was a significant inverse relationship between training required to reach criterion on similar discriminations and performance on Lure 1 trials (Fig. 5A; Corrected ® = 0.05/3 = 0.02; ρ = −0.713, p < 0.02). No such relationship was observed for Lure 2 or Lure 3 trials (p’s > 0.06). This was likely due to floor effects, with aged rats’ relatively poor performance on higher similarity pairs precluding the detection of a relationship.
Figure 5.
Analyses of relationships between individual rats’ abilities on object discrimination training in Experiment 1 and mnemonic discrimination tests in Experiment 2. (A) Scatter plots and results of correlation between number of incorrect (INC) responses made in reaching criterion on the similar LEGO® object pair in Experiment 1 and normalized % correct discrimination responses on Lure 1 trials (L1) in Experiment 2 for young (left panel) and aged (right panel) rats, considered separately. A significant inverse relationship was detected between the two variables for aged rats only (p < 0.02). (B) Mean (±SEM) number of incorrect responses made during similar object discrimination training in Experiment 1. Values are plotted for individual young (Y) rats, and individual aged rats segregated into unimpaired (AU) and impaired (AI) groups based on training required for similar object discriminations. Relative to AU, AI required a significantly greater number of incorrect trials to reach criterion on the similar pair (p < 0.008). (C,D) Mean (±SEM) percent correct discrimination responses on Experiment 2, for individual Y, AU, and AI rats. Separate plots are shown for the Distinct Lure object used in pre-training (C) and for each lure object used in tests (D). Relative to AU, AI showed impairments on Lure 1 trials (p < 0.05). * p < 0.05, ** p < 0.01.
To further probe the link between discrimination abilities assessed in Experiment 1 and Experiment 2, rats from the aged group were split based on their median number of incorrect trials required to reach criterion for similar object discriminations (Median = 188 trials). Rats that required the greatest number of trials to reach criterion (Range = 190–265; Mean = 229, SD = 34.8) were designated as Aged-Impaired (AI), while those requiring fewer trials (Range = 132–186; Mean = 164, SD = 23.4) were designated Aged-Unimpaired (AU) (Fig. 5B). The difference in number of incorrect trials required to reach criterion between these resulting subgroups was statistically significant (t(8) = 3.51, p < 0.008). Mean performance on the first 32 trials of pre-training for Experiment 2 with the distinct lure did not differ between AU and AI rats (Fig. 5C; t(8) = 0.57, p = 0.58), nor between young rats and aged rats of either group (independent samples t tests: p’s > 0.39), suggesting aged rats classified as impaired based on the duration of training required to reach criterion on the similar pair did not in fact suffer from degraded sensory abilities. Furthermore, relative to AU rats, AI rats showed poorer performance on Lure 1 discriminations in Experiment 2 (Fig. 5D; AU: Mean = 86.5%, SD = 7.42; AI: Mean = 70.5%, SD = 13.4; t(8) = 2.34, p < 0.05). Performance on Lure 2 and Lure 3 trials did not differ between AU and AI rats; all aged rats showed deficits on these discriminations relative to the young (Fig. 5D; p’s > 0.11).
Spatial learning and memory
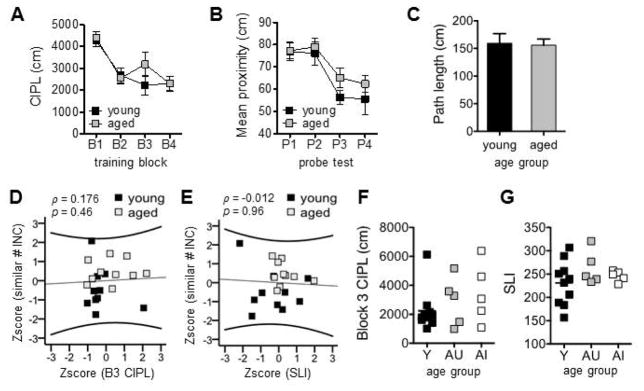
Given that age-related discrimination deficits can be predictive of memory loss, both in elderly humans (Stark et al., 2013) and animal models (LaSarge et al., 2007; Yoder et al., preliminary acceptance), rats were cross-characterized for spatial learning and memory abilities using a Morris water maze task (Gallagher et al., 1993; Bizon et al., 2009; Johnson et al., 2016). Cumulative integrated path length (CIPL) values across the 4 blocks of training trials are shown in Figure 6A. All rats learned to locate a hidden platform with training (main effect of training block: F(3,69) = 14.8, p < 0.001). Specifically, CIPL values for training blocks 2, 3, and 4 were significantly less than those on training block 1 (within subjects contrasts: F(1,23) values > 16.7, p’s < 0.001). In this experimental cohort, spatial learning abilities did not differ between young and aged groups (main effect of age: F(1,23) = 0.58, p = 0.46; block x age interaction: F(3,69) = 0.91, p= 0.44). Mean proximity values, reflecting distance from the rat’s location across the 30-second probe test to the learned platform location, across 4 probe tests for spatial memory are shown in Figure 6B (see Gallagher et al., 1993, Johnson et al., 2018 for detailed description of calculated values). Performance on probe tests of spatial memory improved over time (main effect of probe test: F(3,69) = 9.05, p < 0.001). Mean proximity to the target platform reflected a more targeted search strategy on probe tests 3 and 4, relative to the first probe test (within subjects contrasts: F(1,23) values > 13.5, p’s < 0.001). However, the main effects of age and probe test x age interaction were not statistically significant (F’s(1,23) < 1.31, p’s > 0.27). The spatial learning index (Gallagher et al., 1993; Bizon et al., 2009; Tomás Pereira and Burwell, 2015; Johnson et al., 2016), reflecting spatial memory based on change in proximity to the learned platform location with successive probe tests, also did not differ with age (young Mean = 236, SD = 40.8; aged Mean = 255, SD = 26.9; t(23) = −1.22, p = 0.23). Mean path lengths taken to locate a visible platform on cued navigation trials are shown in Figure 6C. Path lengths did not differ between young and aged rats (t(23) = −0.60, p = 0.95), indicating that sensorimotor abilities and motivation to perform the task were not influenced by age.
Figure 6.
Assessment of spatial learning and memory abilities on the water maze task, and relationship of spatial abilities to object discrimination training in Experiment 1. (A) Cumulative integrated path length (CIPL) values reflecting the total distance traveled (cm) prior to locating the hidden platform during spatial training, corrected for swim speed differences. All rats learned to locate the platform more effectively across training blocks (B1–B4; main effect of block: p < 0.001), but there was no difference in rate of acquisition between young and aged rats (p = 0.46). (B) Mean proximity (cm) to the platform’s target location during four probe tests of spatial reference memory (P1–P4). Proximity values decreased in both age groups across probe tests, reflecting an improvement in memory for the hidden platform’s location over time (main effect of test: p < 0.001); however, no difference was detected in spatial memory between young and aged (p = 0.27). (C) Swim path lengths (cm) of young and aged rats did not differ on cued navigation trials (p = 0.95). Graphs show means ± SEM. (D. E) Scatter plots show normalized number of incorrect responses required to learn to discriminate between the similar object pair in Experiment 1 and normalized measures of (D) spatial learning (block 3 CIPL values) and (E) spatial memory (spatial learning index; SLI). No relationship was detected between discrimination and spatial abilities in young or aged rats. (F, G) When aged rats were segregated into unimpaired (AU) and impaired (AI) groups based on similar object discrimination training required in Experiment 1, no differences between the two groups or with respect to young (Y) rats were observed in (F) block 3 CIPL values or (G) SLI values.
Relationship between object discrimination and spatial learning and memory abilities
Spatial memory assessed on the water maze task has previously been shown to not correlate with spatial discrimination abilities in young or aged rats (Johnson et al., 2016). Thus, we asked whether the same might be true of spatial memory and object discrimination abilities in rats from the present study. Given that number of training trials required to achieve criterion on the similar object pair from Experiment 1 was found to be predictive of performance on discrimination tests in Experiment 2 (Fig. 5), Z-scores for this variable were entered in correlational analyses. CIPL values for block 3 of training and SLI values reflecting performance on probe tests following blocks 2–4 of training were normalized and included as measures of spatial learning and memory, respectively. Data from block 3 of spatial training trials were analyzed because this was the block in which rats showed the greatest variability in behavioral performance (Fig. 6A).
When collapsing across age groups, there was no statistically significant correlation between object discrimination and spatial learning abilities (block 3 CIPL, Fig. 6D; ρ = 0.176, p = 0.46), or spatial memory performance (SLI, Fig. 6E: ρ = −0.012, p = 0.96). Separate consideration of young and aged groups also did not reveal correlations between these variables (p’s > 0.65). A comparison of CIPL values from rats classified as Aged-Unimpaired and Aged-Impaired based on their object discrimination abilities in Experiment 1 is shown in Figure 6F. CIPL values did not differ between the two aged sub-groups (t(8) = 0.48, p = 0.64). An equivalent comparison of SLI values is shown in Figure 6G. As for spatial learning, this index of spatial memory did not differ between aged sub-groups (t(8) = 1.0, p = 0.35). Together, these data suggest that object discrimination deficits in old animals may precede impairments in spatial learning and memory that are reported to occur in old age. An alternative explanation is that the water maze procedure used here is just not sensitive to detecting age-related performance declines in Fischer 344 x Brown Norway hybrid rats. Future experiments will need to characterize old rats on object discrimination tests and then age them for additional months, when water maze deficits have been reported to be more pronounced in this strain (McQuail and Nicolle, 2015). This will allow for the determination of whether deficits in the discrimination of similar objects can predict future spatial memory loss.
Discussion
The current experiments developed a rodent task that requires distinction of a familiar target object from a range of novel lures, and validated this task against animals’ abilities to acquire discriminations of objects that share visual features. This task emulates visual discrimination tests used in human subjects, sometimes referred to as the ‘mnemonic similarity task’ (Toner et al., 2009; Lacy et al., 2011a; Reagh et al., 2014; Reagh and Yassa, 2014; Bennett et al., 2015; Stark et al., 2015; Bennett and Stark, 2016; Reagh et al., 2016). Validity of the rodent task is based on our findings that accurate discrimination of the familiar target from novel lures is contingent on the similarity of the lure, and that aged rats are selectively impaired in discriminating the familiar target from similar lures, compared to young animals. Moreover, aged rats’ performance on high-similarity discriminations in Experiment 2 correlated with the amount of training required to reach a criterion level of performance in discriminating similar objects in Experiment 1. This rodent task can now be applied to investigation of cortical-hippocampal circuit dynamics that support stimulus discrimination, in the context of age-related memory loss and cognitive manifestations of early-stage Alzheimer’s disease.
Cross-species consensus that stimulus discrimination abilities are sensitive to aging
A number of types of visual discrimination tasks have been implemented in animal models. Variants of spontaneous or novel object recognition tasks, which rely on rats’ inherent preference for novelty, have been used to gauge visual discrimination abilities across perceptual gradients (Bartko et al., 2007a, 2007b; Forwood et al., 2007). Age-related deficits specific to more complex or similar stimuli have been shown using this approach in rats (Burke et al., 2011; Gámiz and Gallo, 2012). Tasks relying on spontaneous novelty detection, however, are variable with regards to the extent that the hippocampus is engaged (Mumby, 2001; Warburton and Brown, 2015), and large numbers of animals are often necessary to detect group differences. Moreover, an animal’s motivation to explore even novel objects can quickly decrease as a function of trial number. Thus, the current experiments tested an animal’s ability to discriminate between two simultaneously presented objects, with one rewarded (S+) and the other not (S−). While this design has been widely used in rats with three-dimensional objects (Bartko et al., 2007b), and more recently with presentation of two-dimensional images on a touchscreen (Bussey et al., 2008; Talpos et al., 2012; Ahn and Lee, 2015), the results of Experiment 1 provide a first demonstration of age differences in acquiring similar object discriminations in a rodent model. This simultaneous object discrimination approach was also extended to create a rodent target-lure task that more closely approximates the human mnemonic similarity task (Experiment 2). Similar to humans, young rats’ performance across the different stimulus pairs was contingent on the similarity of the lure objects to the known target. Moreover, like elderly subjects, aged rats were selectively impaired on high-similarity object discriminations, but performed comparable to young when the target and lure did not share features.
An additional similarity between the results of the current experiments and studies in humans is the relative sensitivity of the object discrimination assessment tool to age-related dysfunction. In prior reports from elderly humans, performance on the discrimination test is impaired relative to young and middle-aged adults, while performance on a metric of recognition memory is not (Yassa et al., 2011b; Stark et al., 2013; Reagh et al., 2016). Furthermore, a stronger relationship has been detected between age and performance on the discrimination test than age and performance on a delayed recall test from standardized neuropsychological screening tools (e.g., the RAVLT; Stark et al., 2013). Finally, when elderly subjects were grouped based on delayed recall performance, significant discrimination deficits were observed even in aged individuals who were unimpaired on the delayed recall measure (Stark et al., 2013). These observations are consistent with our findings that aged F344 x BN rats in the present cohort did not show spatial learning or memory impairments relative to their young counterparts, even though these aged rats showed consistent, correlated deficits on similar object discriminations across Experiments 1 and 2. Interestingly, in F344 rats that show more robust water maze deficits with advancing age, as compared to hybrid strains (LaSarge and Nicolle, 2009), sensory discrimination abilities are highly predictive of spatial memory impairments (LaSarge et al., 2007; Yoder et al., preliminary acceptance). Together, these data suggest application of the rodent target-lure discrimination task could permit detection of the earliest signs of age-related neurobiological change across cortical-hippocampal circuits.
Several important distinctions between the rodent target-lure discrimination task and the human mnemonic similarity task are noteworthy. While testing in humans uses trial-unique stimuli, rats train over multiple trials to identify the target that signals presence of food reward. Thus, rats are provided with food motivation to incrementally learn a rule. This could potentially recruit brain circuits responsible for decision-making and response selection in addition to the medial temporal lobe cortices and hippocampus. Along these lines, in the rat target-lure task the stimuli are not trial-unique, but rather presented several times over the course of days. Reagh and Yassa (2014) have shown that the repeated presentation of a target leads to worse lure performance in young subjects, perhaps through a mechanism by which familiarity-based responses are enhanced as objects become semanticized through multiple exposures. Under this framework, the current data would suggest that the aged rats have an increased rate of semanticization compared to the young rats, which is consistent with previous spontaneous object recognition data from young and aged rats (Burke et al., 2010; 2011). Future experiments will be needed to confirm that rodent target-lure discrimination tasks rely on comparable circuits to those engaged by the mnemonic similarity task in humans.
Finally, in the rat object discrimination tasks used to date, the animals are presented with a forced choice. This is distinct from the original mnemonic similarity tasks in which subjects are asked to judge only a single item as new or old. Critically, a recent variant of the mnemonic similarity task in which the subjects were simultaneously presented with an object from the encoding phase and a similar lure in a forced-choice format, which is more comparable to the rat studies used here, has confirmed age-related impairments in discriminating between similar objects (Huffman and Stark, 2017). Thus, the old/new and forced-choice test formats appear to be equally sensitive to detecting age-associated deficits.
Response strategy, reaction times, and performance on target-lure discrimination tests
To gain further insight into strategies guiding response selection on object discrimination test, checking behavior (for example see video, Supplemental Material) and reaction times were scored on a trial-by-trial basis. This analysis was motivated by observations that rats often default to simple, rule-based response strategies on forced-choice tasks, and must learn to suppress their initial strategy in order to apply a more complex task-specific strategy. For example, in an object-place paired-associate (OPPA) task that requires learning object-reward associations contingent on spatial location within the test apparatus, rats initially adopt a side-biased strategy to obtain the concealed food reward 50% of the time (Lee and Solivan, 2008; Jo and Lee, 2010; Lee and Byeon, 2014; Hernandez et al., 2015, 2017). We observed the same effect during initial object discrimination training in Experiment 1 (data not shown), and a side-biased strategy remained evident on trials with greater target-lure similarity during tests in Experiment 2. During later stages of acquisition of the OPPA task, rats begin to hesitate before displacing the incorrect object on the biased side, instead moving to the non-biased side of the platform to make a correct response. In their analysis of OPPA acquisition, Lee and Byeon (2014) described this behavior as inhibition-and-push response (or IPR), while we refer to the same behavior as ‘checking’ in the present analyses. Checking behavior is reminiscent of vicarious trial and error (or VTE) behavior observed when rats pause at a choice point in a maze (Muenzinger and Gentry, 1931; Tolman, 1939), which is proposed to reflect flexible deliberation during decision-making (Redish, 2016).
Trial-by-trial analysis of checking behavior on mnemonic similarity tests confirmed that, overall, performance corresponded with the ability to suppress a side-biased response strategy. Specifically, better performance on Lure 1 trials was associated with reduced side bias and increased checking, while poorer performance on Lure 2 and Lure 3 trials was associated with greater side bias and decreased checking. Given that aged rats showed less checking behavior than young across all trial types, it is likely that age-related deficits on the task can be explained in part by perseveration of a side-biased strategy. Though all rats appeared to default to their side bias when faced with high-similarity discriminations, this effect was more pronounced in the aged group. These data are consistent with findings that aged rats show impaired flexibility relative to young on reversal learning or set-shifting tasks (Beas et al., 2013, 2016), and that aged rats more readily adopt a response-based strategy than spatial place-based strategy on a T-maze navigation task (Tomás Pereira et al., 2015).
In young adult rats, in vivo recordings have revealed that single units within the prefrontal cortex can be selectively responsive when suppressing side bias during acquisition of the OPPA task (Lee and Byeon, 2014). Similar experiments have also shown that once rats learned to suppress their side bias and adopt the object-in-place rule that OPPA requires, neuronal firing was increasingly synchronized and phase-locked to theta oscillations across the prefrontal cortex and hippocampus (Kim et al., 2011). These results suggest prefrontal cortical regions may contribute by gating hippocampal-dependent discrimination responses, depending on the relative difficulty of the task. An implication of the prefrontal cortex together with hippocampus in mnemonic discrimination is supported by one recent study in humans, in which activity across the two regions was associated with failure to correctly identify similar lure stimuli as novel (Pidgeon and Morcom, 2016). Overall, our results indicating that aged rats are selectively impaired on high-similarity discriminations, but are also slow to overcome a perseverative response bias, suggests that interaction of these two sets of abilities is required to perform the task. The mnemonic similarity task may therefore be particularly sensitive to coordinated function of the prefrontal cortex and medial temporal lobe, and could provide an opportunity to assess age-related imbalance across these brain circuits.
Studies of cognition across the human lifespan consistently show behavioral slowing in older age, and this can be an accurate predictor of broader cognitive and physical decline (Hong et al., 2015; Aichele et al., 2016; Finkel et al., 2016). To determine whether aged rats from the current experimental cohorts showed evidence of behavioral slowing or general physical impairments, we also carried out a trial-by-trial analysis of reaction times across mnemonic similarity tests. First, a pronounced effect of response side was observed on reaction times, irrespective of age. Reaction times for trials when rats correctly responded to their biased side were significantly shorter than those when rats correctly responded to their non-biased side. This can easily be explained by the fact that pausing and checking the biased side of the platform before self-correcting and responding on the non-biased side takes longer than moving directly from the threshold of the choice platform to the biased side. Separate analyses of reaction times to the biased versus non-biased side showed no difference in their duration between young and aged groups. In fact, examination of individual rats’ reaction times (not shown) revealed the majority of aged rats had shorter reaction times than the median value of young rats when correctly responding to the biased side of the platform, across trial types. Reaction times were comparable between young and aged when responding to the non-biased side of the platform, indicating that checking behavior was not slowed in the aged group by physical impediments. Together with behavioral analyses of correct responses, side bias, and checking, these data emphasize that physical or sensory impairments did not influence mnemonic similarity task performance in the aged group.
One further consideration in interpreting age-related deficits on the discrimination tasks is whether aged rats simply find higher-similarity discriminations more difficult. Differentiation of the factor of ‘feature similarity’ from a more general factor of ‘task difficulty’ can be problematic. One approach adopted in human studies is to include simple discrimination conditions in which stimuli differ only on a single feature, such as size or color. Of note, control subjects show approximately the same accuracy when discriminating between basic shapes that differ slightly in size or color (Barense et al., 2007; Ryan et al., 2012; Behrmann et al., 2016) as when discriminating between complex ‘fribble’ or ‘greeble’ stimuli that share multiple overlapping features (Gauthier and Tarr, 1997; Williams and Simons, 2000; Barense et al., 2007). Patients with medial temporal lobe damage encompassing both hippocampus and perirhinal cortex were not impaired relative to controls on difficult size and color discriminations, yet showed significant deficits on high-similarity complex stimulus discriminations (Barense et al., 2007; Behrmann et al., 2016). The results of one study in elderly human subjects further suggest that mnemonic discrimination deficits emerge without concurrent impairments in difficult simple visual discriminations (Ryan et al., 2012). Together with functional neuroimaging data and the interpretation that perirhinal cortical-hippocampal function is critical to mnemonic discrimination abilities, these findings imply age-related deficits in the present experiments cannot be fully explained by task difficulty.
Domain-general discrimination deficits with aging as they relate to cortical-hippocampal circuit-level dysfunction
Availability of a rodent mnemonic similarity task provides an opportunity to address complementary gaps in the human and animal literature on the neurobiological underpinnings of stimulus discrimination. In humans, functional neuroimaging studies have most consistently linked visual discrimination of similar stimuli to activity in hippocampal CA3 and dentate gyrus (Kirwan and Stark, 2007; Bakker et al., 2008; Yassa et al., 2011a, 2011b; Yassa and Stark, 2011; Bakker et al., 2012; Motley and Kirwan, 2012; Reagh and Yassa, 2014; Doxey and Kirwan, 2015). Whereas, in animals, the majority of studies have tested a role for the perirhinal cortex in high-similarity object discriminations (Eacott et al., 2001; Bussey et al., 2002, 2003, Bartko et al., 2007a, 2007b; Winters et al., 2010; Ahn and Lee, 2015). To date, a single functional imaging study has found that altered perirhinal activity in the elderly is correlated with discrimination deficits (Ryan et al., 2012). Animal models point to a potential role for both hippocampal and perirhinal cortical dysfunction in similarity-dependent discrimination impairments; results from aged rats in the present study recapitulate similar data from young rats with hippocampal or dentate gyrus lesions (McTighe et al., 2009; Talpos et al., 2010; Oomen et al., 2015), as well as those data mentioned from young rats and monkeys with perirhinal cortical lesions (e.g., Bussey et al., 2002, 2003, Bartko et al., 2007a, 2007b). Thus, a likely possibility is that it may not be isolated dysfunction within either of these brain regions in old age, but rather integrity of cortical inputs from the rhinal cortices to the hippocampus, in particular via the perforant path, that underlie age-related visual discrimination impairments (Bennett and Stark, 2015; Gray and Barnes, 2015; Leal and Yassa, 2015; Bennett and Stark, 2016).
This interpretation is supported by a study in young adult human subjects, which examined mnemonic discrimination task-related activity across the medial temporal lobe and hippocampal sub-regions and found both cortical and CA3/dentate gyrus activity to correlate with performance (Reagh and Yassa, 2014). While this has not yet been extended to elderly subjects, it is interesting to consider their findings with respect to measurement of stimulus discrimination abilities across sensory domains and a potentially indispensable role of the hippocampus in performing this function to support memory fidelity. Reagh and Yassa (2014) reported that activity in lateral entorhinal and perirhinal cortices was linked to accurate discrimination of object stimuli, while activity in medial entorhinal and parahippocampal cortices was linked to discriminating between spatial locations on the screen. This is consistent with prior knowledge that lateral entorhinal and perirhinal cortical inputs provide visual and object information to the hippocampus, while medial entorhinal cortical inputs provide spatial information (Burwell et al., 1998; Hargreaves et al., 2005; Witter and Moser, 2006; Henriksen et al., 2010; Deshmukh and Knierim, 2011; Hartzell et al., 2013). However, a relationship between CA3/dentate gyrus activity and performance was observed across both object and spatial task variants (Reagh and Yassa, 2014). This reinforces the conclusion that, via its cortical inputs, the dentate gyrus plays a critical and domain-general role in distinguishing between similar stimuli (O’Reilly and McClelland, 1994; Treves et al., 2008; Yassa and Stark, 2011; Kesner and Rolls, 2015; Knierim and Neunuebel, 2016). In further support, studies in animal models have identified a role for the hippocampus and particularly the dentate gyrus in discrimination of olfactory stimuli (Weeden et al., 2014), spatial locations (Gilbert et al., 1998, 2001), and spatial positions presented on a touchscreen (McTighe et al., 2009; Talpos et al., 2010; Oomen et al., 2015).
Considered together, this growing body of data supports hippocampal involvement in stimulus discrimination across modalities. However, it is critical to note that the streams of information that support hippocampal computations for stimulus discrimination (i.e., the lateral entorhinal/perirhinal cortical circuit versus the medial entorhinal/parahippocampal cortical circuit) are not equally vulnerable to old age. Studies comparing multiple domains of discrimination and memory indicate that the discrimination of similar object features is more sensitive to aging than discrimination of similar spatial locations (Reagh et al., 2016). Furthermore, a recent study reported that performance on neuropsychological tests for early cognitive decline was predictive of memory for objects, but not memory of scenes (Fidalgo et al., 2016). Cross-domain discrimination behavior has not yet been assessed within individual animal subjects, to our knowledge. Nonetheless, it is interesting to note that age-related object discrimination impairments observed in the current experiments are more profound than age-related spatial discrimination impairments using the same F344 x BN hybrid rat model of cognitive aging (Johnson et al., 2016). Specifically, effect sizes for age differences in incorrect trials required to learn similar object discriminations in Experiment 1 (Cohen’s d = 1.34, 95% confidence interval (CI) = −22–20) and performance on Lure 2 (d = 2.30, CI = −0.56–5.16) and Lure 3 trials (d = 1.98, CI = −0.79–4.75) in Experiment 2 are greater than that observed for the age difference in performance on high-similarity spatial discriminations in our previously published study (d = 0.99, CI = 0.95–1.03) (Johnson et al., 2016). Critically, these findings are consistent with knowledge that lateral entorhinal and perirhinal cortices, which are more likely to contribute to visual and object discriminations, show earlier evidence of neuropathology with advanced age and progression to Alzheimer’s disease (Braak and Braak, 1991; Braak et al., 1993; Khan et al., 2014; Hirni et al., 2016; Krumm et al., 2016). With this highly sensitive behavioral index of aging and cognitive decline now identified in humans and animal models, future experiments focused on perirhinal and lateral entorhinal cortical inputs to the hippocampus will likely begin to clarify sensitivity of this circuit to aging.
Supplementary Material
Acknowledgments
We thank Sai Balusu, Sarah Barthle, Deandra Chetram, Gabriela Colon, Nardin Derias, and Kaeli Fertal for assistance with behavioral experiments. This work was carried out with funds from the National Institute on Aging (R01 AG049722, R03 AG049411), a McKnight Brain Institute Fellowship (SAJ), the McKnight Brain Research Foundation, UF Research Seed Opportunity Fund, and a UF University Scholars Program Awards (SMT).
Grant sponsor: NIH / NIA; Grant number: R01 AG 049722
Grant sponsor: NIH / NIA; Grant number: R03 AG 049411
Grant sponsor: McKnight Brain Institute Fellowship to SAJ
Grant sponsor: McKnight Brain Research Foundation
Grant sponsor: UF Research Seed Opportunity Fund
Grant sponsor: UF University Scholars Program Award to SMT
References
- Ahn J-R, Lee I. Neural correlates of object-associated choice behavior in the perirhinal cortex of rats. J Neurosci Off J Soc Neurosci. 2015;35:1692–1705. doi: 10.1523/JNEUROSCI.3160-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichele S, Rabbitt P, Ghisletta P. Think Fast, Feel Fine, Live Long: A 29-Year Study of Cognition, Health, and Survival in Middle-Aged and Older Adults. Psychol Sci. 2016;27:518–529. doi: 10.1177/0956797615626906. [DOI] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem Cold Spring Harb N. 2007a;14:821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci Off J Soc Neurosci. 2007b;27:2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Impairments in visual discrimination learning and recognition memory produced by neurotoxic lesions of rhinal cortex in rhesus monkeys. Eur J Neurosci. 2001;13:1228–1238. doi: 10.1046/j.0953-816x.2001.01491.x. [DOI] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Banuelos C, Setlow B, Bizon JL. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Lee ACH, Geskin JZ, Graham KS, Barense MD. Temporal lobe contribution to perceptual function: A tale of three patient groups. Neuropsychologia. 2016;90:33–45. doi: 10.1016/j.neuropsychologia.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Huffman DJ, Stark CEL. Limbic Tract Integrity Contributes to Pattern Separation Performance Across the Lifespan. Cereb Cortex. 2015;25:2988–2999. doi: 10.1093/cercor/bhu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Stark CEL. Mnemonic discrimination relates to perforant path integrity: An ultra-high resolution diffusion tensor imaging study. Neurobiol Learn Mem. 2015 doi: 10.1016/j.nlm.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Stark CEL. Mnemonic discrimination relates to perforant path integrity: An ultra-high resolution diffusion tensor imaging study. Neurobiol Learn Mem. 2016;129:107–112. doi: 10.1016/j.nlm.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111:467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behav Neurosci. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Shapiro ML, O’Malley MT, Eichenbaum H. Positional firing properties of perirhinal cortex neurons. Neuroreport. 1998;9:3013–3018. doi: 10.1097/00001756-199809140-00017. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem Cold Spring Harb N. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing “declarative” vs. “perceptual-mnemonic” views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Byun J, Lee I. Disambiguation of similar object-place paired associations and the roles of the brain structures in the medial temporal lobe. Exp Neurobiol. 2010;19:15–22. doi: 10.5607/en.2010.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S. Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. Int J Psychophysiol Off J Int Organ Psychophysiol. 2009;71:64–69. doi: 10.1016/j.ijpsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased responsiveness to novelty is associated with successful cognitive aging. J Cogn Neurosci. 2006;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey CR, Kirwan CB. Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe: AGE AND PATTERN SEPARATION. Hippocampus. 2015;25:524–533. doi: 10.1002/hipo.22389. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Machin PE, Gaffan EA. Elemental and configural visual discrimination learning following lesions to perirhinal cortex in the rat. Behav Brain Res. 2001;124:55–70. doi: 10.1016/s0166-4328(01)00234-0. [DOI] [PubMed] [Google Scholar]
- Fandakova Y, Lindenberger U, Shing YL. Deficits in process-specific prefrontal and hippocampal activations contribute to adult age differences in episodic memory interference. Cereb Cortex N Y N 1991. 2014;24:1832–1844. doi: 10.1093/cercor/bht034. [DOI] [PubMed] [Google Scholar]
- Fidalgo CO, Changoor AT, Page-Gould E, Lee ACH, Barense MD. Early Cognitive Decline in Older Adults Better Predicts Object than Scene Recognition Performance. Hippocampus. 2016 doi: 10.1002/hipo.22658. [DOI] [PubMed] [Google Scholar]
- Finkel D, Ernsth-Bravell M, Pedersen NL. Temporal Dynamics of Motor Functioning and Cognitive Aging. J Gerontol A Biol Sci Med Sci. 2016;71:109–116. doi: 10.1093/gerona/glv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Bartko SJ, Saksida LM, Bussey TJ. Rats spontaneously discriminate purely visual, two-dimensional stimuli in tests of recognition memory and perceptual oddity. Behav Neurosci. 2007;121:1032–1042. doi: 10.1037/0735-7044.121.5.1032. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gámiz F, Gallo M. Spontaneous object recognition memory in aged rats: Complexity versus similarity. Learn Mem Cold Spring Harb N. 2012;19:444–448. doi: 10.1101/lm.027003.112. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Becoming a “Greeble” expert: exploring mechanisms for face recognition. Vision Res. 1997;37:1673–1682. doi: 10.1016/s0042-6989(96)00286-6. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Gray DT, Barnes CA. Distinguishing adaptive plasticity from vulnerability in the aging hippocampus. Neuroscience. 2015;309:17–28. doi: 10.1016/j.neuroscience.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JB, Broadbent NJ, Velu PD, Squire LR, Clark RE. Hippocampus, perirhinal cortex, and complex visual discriminations in rats and humans. Learn Mem Cold Spring Harb N. 2015;22:83–91. doi: 10.1101/lm.035840.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hartzell AL, Burke SN, Hoang LT, Lister JP, Rodriguez CN, Barnes CA. Transcription of the Immediate-Early Gene Arc in CA1 of the Hippocampus Reveals Activity Differences along the Proximodistal Axis That Are Attenuated by Advanced Age. J Neurosci. 2013;33:3424–3433. doi: 10.1523/JNEUROSCI.4727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser M-B, Moser EI. Spatial representation along the proximodistal axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Maurer AP, Reasor JE, Turner SM, Barthle SE, Johnson SA, Burke SN. Age-related impairments in object-place associations are not due to hippocampal dysfunction. Behav Neurosci. 2015;129:599–610. doi: 10.1037/bne0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Reasor JE, Truckenbrod LM, Lubke KN, Johnson SA, Bizon JL, Maurer AP, Burke SN. Medial prefrontal-perirhinal cortical communication is necessary for flexible response selection. Neurobiol Learn Mem. 2017;137:36–47. doi: 10.1016/j.nlm.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman DL, Swan M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci JAALAS. 2010;49:155–159. [PMC free article] [PubMed] [Google Scholar]
- Hirni DI, Kivisaari SL, Krumm S, Monsch AU, Berres M, Oeksuez F, Reinhardt J, Ulmer S, Kressig RW, Stippich C, Taylor KI. Neuropsychological Markers of Medial Perirhinal and Entorhinal Cortex Functioning are Impaired Twelve Years Preceding Diagnosis of Alzheimer’s Dementia. J Alzheimers Dis. 2016;52:573–580. doi: 10.3233/JAD-150158. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ng KK, Sim SKY, Ngeow MY, Zheng H, Lo JC, Chee MWL, Zhou J. Differential age-dependent associations of gray matter volume and white matter integrity with processing speed in healthy older adults. NeuroImage. 2015;123:42–50. doi: 10.1016/j.neuroimage.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Huffman DJ, Stark CEL. Age-related impairment on a forced-choice version of the Mnemonic Similarity Task. Behav Neurosci. 2017;131:55–67. doi: 10.1037/bne0000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee I. Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. J Neurosci Off J Soc Neurosci. 2010;30:9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Sacks PK, Turner SM, Gaynor LS, Ormerod BK, Maurer AP, Bizon JL, Burke SN. Discrimination performance in aging is vulnerable to interference and dissociable from spatial memory. Learn Mem Cold Spring Harb N. 2016;23:339–348. doi: 10.1101/lm.042069.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J Neurosci Off J Soc Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP. Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol Learn Mem. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm S, Kivisaari SL, Probst A, Monsch AU, Reinhardt J, Ulmer S, Stippich C, Kressig RW, Taylor KI. Cortical thinning of parahippocampal subregions in very early Alzheimer’s disease. Neurobiol Aging. 2016;38:188–196. doi: 10.1016/j.neurobiolaging.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011a;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem Cold Spring Harb N. 2011b;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Nicolle MM. Comparison of different cognitive rats models of human aging. In: Bizon JL, Woods AG, editors. Animal Models of Human Cognitive Aging. New York: Humana Press; 2009. pp. 73–102. [Google Scholar]
- Leal SL, Yassa MA. Neurocognitive Aging and the Hippocampus across Species. Trends Neurosci. 2015;38:800–812. doi: 10.1016/j.tins.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Byeon JS. Learning-dependent Changes in the Neuronal Correlates of Response Inhibition in the Prefrontal Cortex and Hippocampus. Exp Neurobiol. 2014;23:178–189. doi: 10.5607/en.2014.23.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn Mem Cold Spring Harb N. 2008;15:357–367. doi: 10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Cervantes SN, Gray SJ, Gallo DA. Memory’s aging echo: age-related decline in neural reactivation of perceptual details during recollection. NeuroImage. 2014;98:346–358. doi: 10.1016/j.neuroimage.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Nicolle MM. Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiol Aging. 2015;36:323–333. doi: 10.1016/j.neurobiolaging.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Motley SE, Kirwan CB. A Parametric Investigation of Pattern Separation Processes in the Medial Temporal Lobe. J Neurosci. 2012;32:13076–13084. doi: 10.1523/JNEUROSCI.5920-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzinger KF, Gentry E. Tone discrimination in white rats. J Comp Psychol. 1931;12:195–206. [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Kofink D, Preusser F, Mar AC, Saksida LM, Bussey TJ. A novel 2- and 3-choice touchscreen-based continuous trial-unique nonmatching-to-location task (cTUNL) sensitive to functional differences between dentate gyrus and CA3 subregions of the hippocampus. Psychopharmacology (Berl) 2015;232:3921–3933. doi: 10.1007/s00213-015-4019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Pidgeon LM, Morcom AM. Age-related increases in false recognition: the role of perceptual and conceptual similarity. [Accessed May 20, 2015];Front Aging Neurosci. 2014 :6. doi: 10.3389/fnagi.2014.00283. Available at: http://journal.frontiersin.org/article/10.3389/fnagi.2014.00283/abstract. [DOI] [PMC free article] [PubMed]
- Pidgeon LM, Morcom AM. Cortical pattern separation and item-specific memory encoding. Neuropsychologia. 2016;85:256–271. doi: 10.1016/j.neuropsychologia.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, Yassa MA. Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus. 2016;26:417–422. doi: 10.1002/hipo.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Roberts JM, Ly M, DiProspero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24:303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci U S A. 2014;111:E4264–4273. doi: 10.1073/pnas.1411250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–159. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cardoza JA, Barense MD, Kawa KH, Wallentin-Flores J, Arnold WT, Alexander GE. Age-related impairment in a complex object discrimination task that engages perirhinal cortex. Hippocampus. 2012;22:1978–1989. doi: 10.1002/hipo.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Stevenson R, Wu C, Rutledge S, Stark CEL. Stability of age-related deficits in the mnemonic similarity task across task variations. Behav Neurosci. 2015;129:257–268. doi: 10.1037/bne0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini LM, Gorree E. Aging memories: differential decay of episodic memory components. Learn Mem Cold Spring Harb N. 2012;19:239–246. doi: 10.1101/lm.024281.111. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Fletcher AC, Circelli C, Tricklebank MD, Dix SL. The pharmacological sensitivity of a touchscreen-based visual discrimination task in the rat using simple and perceptually challenging stimuli. Psychopharmacology (Berl) 2012;221:437–449. doi: 10.1007/s00213-011-2590-z. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Prediction of vicarious trial and error by means of the schematic sowbug. Psychol Rev. 1939;46:318–336. [Google Scholar]
- Tomás Pereira I, Burwell RD. Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci. 2015;129:533–539. doi: 10.1037/bne0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás Pereira I, Gallagher M, Rapp PR. Head west or left, east or right: interactions between memory systems in neurocognitive aging. Neurobiol Aging. 2015;36:3067–3078. doi: 10.1016/j.neurobiolaging.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem Cold Spring Harb N. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter MP, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Neural circuitry for rat recognition memory. Behav Brain Res. 2015;285:131–139. doi: 10.1016/j.bbr.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden CSS, Hu NJ, Ho LUN, Kesner RP. The role of the ventral dentate gyrus in olfactory pattern separation: ROLE OF THE VENTRAL DENTATE GYRUS. Hippocampus. 2014;24:553–559. doi: 10.1002/hipo.22248. [DOI] [PubMed] [Google Scholar]
- Williams P, Simons DJ. Detecting Changes in Novel, Complex Three-dimensional Objects. Vis Cogn. 2000;7:297–322. [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem. 2010;93:221–228. doi: 10.1016/j.nlm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung L-K, Ryan JD, Cowell RA, Barense MD. Recognition memory impairments caused by false recognition of novel objects. J Exp Psychol Gen. 2013;142:1384–1397. doi: 10.1037/a0034021. [DOI] [PubMed] [Google Scholar]
- Yoder WM, Gaynor L, Burke SN, Setlow B, Smith DW, Bizon JL. Interaction between age and perceptual similarity in olfactory discrimination learning in F344 rats: relationships with spatial learning. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2017.01.023. preliminary acceptance. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.