Abstract
Alcohol acts on numerous cellular and molecular targets to regulate neuronal communication within the brain. Chronic alcohol exposure and acute withdrawal generate prominent neuroadaptations at synapses, including compensatory effects on the expression, localization and function of synaptic proteins, channels and receptors.
The present article reviews the literature describing the synaptic effects of chronic alcohol exposure and their relevance for synaptic transmission in the central nervous system. This review is not meant to be comprehensive, but rather to highlight the effects that have been observed most consistently and that are thought to contribute to the development of alcohol dependence and the negative aspects of withdrawal. Specifically, we will focus on the major excitatory and inhibitory neurotransmitters in the brain, glutamate and GABA, respectively, and how their neuroadaptations after chronic alcohol exposure contributes to alcohol reinforcement, dependence and withdrawal.
Keywords: alcohol/ethanol, GABA, glutamate, synaptic transmission, presynaptic, postsynaptic, protein phosphorylation, intoxication, tolerance, dependence
1.0 Chronic Ethanol Effects on Synaptic Transmission
Alcohol use disorders (AUDs) cause large medical, economic and social burdens worldwide. It is widely accepted that alcohol/ethanol (EtOH) acts in the central nervous system (CNS) to alter neuronal function. The synapse is the central point of communication between neurons and it is among the most sensitive sites of ethanol’s actions. In the CNS, a number of cellular and molecular targets involved in synaptic transmission are profoundly altered by both acute and chronic EtOH exposure. Generally, low to intermediate concentrations of EtOH (5–50 mM, corresponding to a few drinks to complete intoxication) act on a variety of synaptic targets, including, but not limited to, ion channels, neurotransmitter receptors and intracellular signalling proteins.
Animal models of chronic EtOH exposure provide important information relevant to the human neurobiology and pathophysiology of long-term alcohol abuse. Chronic EtOH exposure produces both tolerance and dependence in humans, and critically, similar effects are observed in rodent and non-human primate models. Alcohol tolerance, which is acquired after repeated exposures, is characterized by decreased behavioral responses to EtOH, such that more EtOH is required to achieve the same intoxicating effects observed after an initial exposure. Alcohol dependence is a chronic relapsing disorder characterized by alcohol preoccupation, loss of control over intake and a negative emotional state (Koob and Volkow, 2010). Dependence is generally described by the symptomology elicited during and following EtOH withdrawal (Heilig et al., 2010; Koob, 2003; Koob and Volkow, 2010, 2016; Tsai and Coyle, 1998), including anxiety, dysphoria, increased seizure susceptibility, hyperalgesia and sleep disturbances (Enoch, 2008; Grobin et al., 1998; Kumar et al., 2009; Ron and Barak, 2016). Central to understanding and treating the pathophysiology of alcohol dependence is characterizing the brain region-specific neuroadaptions in glutamatergic and GABAergic synaptic transmission that occur with chronic alcohol exposure.
1.1 Glutamate and EtOH Effects
Acute EtOH generally inhibits glutamatergic neurotransmission, while chronic EtOH exposure and acute withdrawal tend to enhance it. It is important to note here that many of the studies that have investigated the effects of in vivo chronic ethanol exposure on synaptic function have been conducted during an acute in vitro withdrawal period (i.e. the absence of ethanol for ~2–8 hours) for technical reasons. Since the synapse is highly sensitive to the presence of EtOH, we will distinguish as best as possible experiments conducted under this acute in vitro withdrawal paradigm from work that assesses the effects of in vitro chronic EtOH exposure (usually in primary neuronal cultures), in vivo chronic EtOH exposure (where experiments are performed in the presence of EtOH) and in vivo withdrawal of chronic EtOH-exposed animals.
The effects of both acute EtOH and chronic EtOH/acute in vitro withdrawal on glutamatergic signaling are primarily centered on postsynaptic glutamate receptors (Bliss et al., 2014; Lovinger and Roberto, 2013; Roberto and Gilpin, 2014; Szumliski and Woodward, 2014). Glutamate receptors include three major classes of ionotropic receptors (iGluRs), 1) α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA receptors or AMPARs), 2) N-methyl-D-aspartate receptors (NMDA receptors or NMDARs) and 3) kainate receptors (KARs), as well as various sub-classes of metabotropic glutamate receptors (mGluRs) that are G protein-coupled receptors (GPCRs) (Lovinger and Roberto, 2013).
1.1.1 EtOH Effects on Ionotropic Glutamate Receptors
Ionotropic Glutamate Receptors (iGluRs) are ligand-gated ion channels (LGICs), which are well characterized targets for EtOH actions (Forstera et al., 2016; Lovinger, 1997; Szumliski and Woodward, 2014; Vengeliene et al., 2008). LGICs are heteromeric proteins that bind extracellular neurotransmitters or intracellular messengers and transduce that binding energy into the opening of an intrinsic ion pore (Collingridge et al., 2009). These receptors are present on all CNS neurons, where they mediate fast synaptic transmission and the activation of intracellular signalling. iGluRs are specifically activated by extracellular glutamate binding and transport cations into the cell, with each receptor class displaying different selectivity for sodium, potassium and calcium ions.
NMDA Receptors
Acute ethanol has generally inhibitory actions on iGluRs (although see (Lu and Yeh, 1999)). Most consistently, EtOH inhibits NMDAR function (Criswell et al., 2003; Dildy and Leslie, 1989; Hoffman et al., 1989; Lima-Landman and Albuquerque, 1989; Lovinger et al., 1989, 1990), as well as the synaptic responses mediated by NMDARs (Lovinger et al., 1990; Morrisett and Swartzwelder, 1993; Ren et al., 2013; Roberto et al., 2004b; Wang et al., 2007; Xu et al., 2015; Zhao et al., 2015). Functional NMDARs contain an obligatory NR1 subunit in combination with at least one NR2 or NR3 subunit. Although, EtOH inhibits all NMDAR subtypes, differences in their EtOH sensitivity are observed according to their subunit compositions. Generally, EtOH more potently inhibits receptors with NR1/2A or NR1/2B subunit compositions than NR1/2C-containing receptors (Chu et al., 1995; Jin et al., 2008; Jin and Woodward, 2006; Kuner et al., 1993; Lovinger, 1995; Lovinger and Roberto, 2013; Masood et al., 1994).
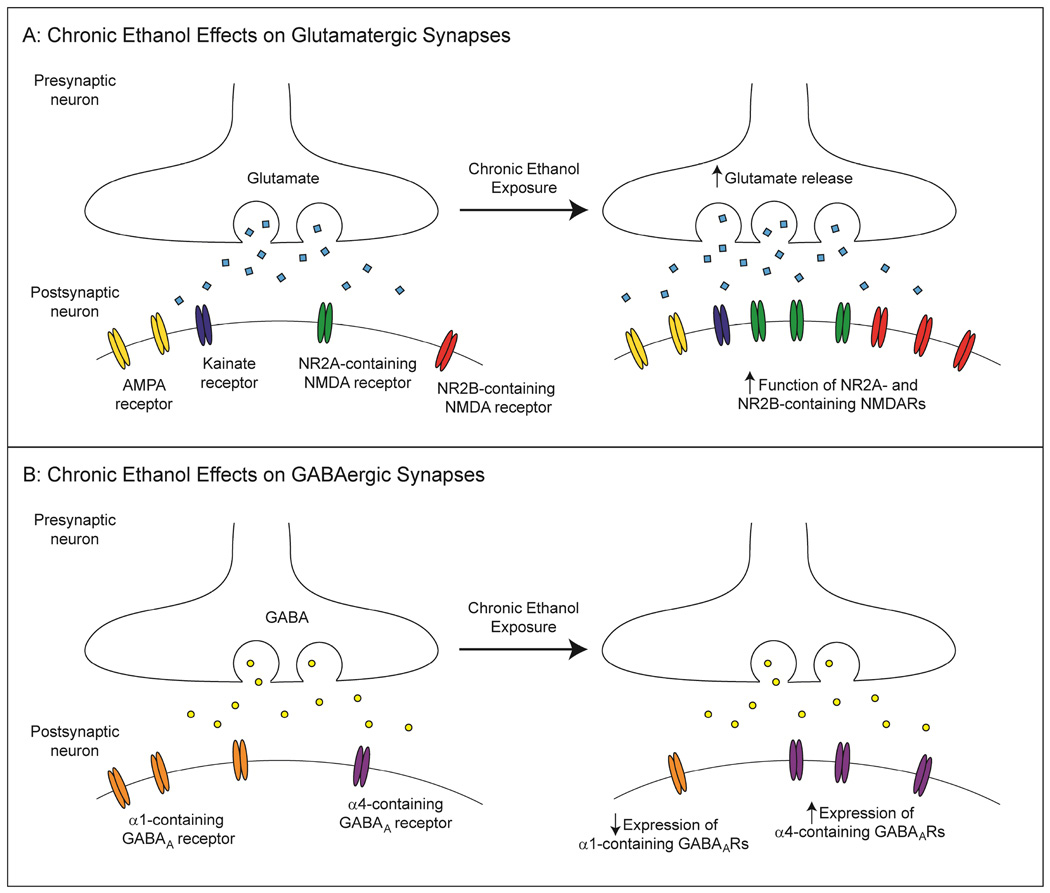
Chronic ethanol exposure tends to increase both the function of NMDARs and NMDAR-mediated glutamatergic synaptic transmission (see Figure 1A) (Cebere et al., 1999; Grover et al., 1998; Gulya et al., 1991; Lack et al., 2007; Smothers et al., 1997). These changes in NMDAR activation are consistently larger than the EtOH-induced activation of other ionotropic glutamate receptors (i.e. AMPA and kainite receptors) (Chandler et al., 1999; Chandler et al., 1997; Gulya et al., 1991; Smothers et al., 1997).
Figure 1.
Chronic ethanol effects on glutamatergic and GABAergic transmission. A: Schematic illustration of a glutamatergic synapse after chronic ethanol exposure. Presynaptically, glutamate release is enhanced. Postsynaptically, NMDAR function is increased, likely due to increased receptor expression. AMPA and kainite receptor function can also be enhanced by chronic ethanol, though these effects tend to be less consistent. B: A GABAergic synapse after chronic ethanol exposure. Presynaptically, GABA release is altered in a brain region-specific manner, potentially due to changes in GABABR function. Postsynaptically, GABAAR composition is altered by chronic ethanol, such that there is an increase in synaptic α4-containing receptor expression and a concomitant decrease in synaptic α1-containing receptor number, though the functional outcome of these changes is less clear.
Generally, EtOH-treated cultured primary neurons and tissue/cultured neurons from EtOH-exposed animals were used to study EtOH’s effects on glutamate receptor activation and calcium influx. In these studies, chronic EtOH exposure (days to weeks) enhanced NMDAR agonist-induced increases in intracellular calcium (Chandler et al., 1997; Grover et al., 1998; Gulya et al., 1991; Iorio et al., 1992; Smothers et al., 1997) and upregulated the flow of ion current through the NMDAR pore (Floyd et al., 2003; Grover et al., 1998). Moreover, increased currents specific to NR2B-containing receptors have been observed in the basolateral amygdala (BLA) (Floyd et al., 2003)), bed nucleus of the stria terminalis (Kash et al., 2009) and central amygdala (CeA) (Roberto et al., 2006; Roberto et al., 2004b) of chronic EtOH-exposed rodents during acute in vitro withdrawal.
Notably, the effects of acute EtOH on NMDAR function remained intact, or were even enhanced, after chronic exposure in most brain regions (Floyd et al., 2003; Roberto et al., 2006; Roberto et al., 2004b). Tolerance to acute EtOH-induced inhibition has been reported in hippocampal slices (Grover et al., 1994; Miyakawa et al., 1997) and in medial septum neurons (Grover et al., 1998), but not, for example, in the BLA (Floyd et al., 2003) or CeA (Roberto et al., 2004b). Since increased NMDAR-mediated calcium influx is associated with increased susceptibility to the excitotoxic effects of NMDA (Chandler et al., 1993; Iorio et al., 1993), the combination of chronic EtOH/acute withdrawal-induced increases in NMDAR function and the conclusion of acute EtOH-induced NMDAR inhibition during the early stages of withdrawal are likely to generate a hyperexcitable state. Generally, this excitotoxicity during EtOH withdrawal contributes to alcohol-related neuronal loss in the brain (Bliss et al., 2014). Overall, these studies show that NMDAR function is still depressed by acute EtOH in animals exposed to chronic EtOH, and imply significantly enhanced NMDAR function during the acute stages of withdrawal to produce hyperexcitability/excitotoxicity.
The mechanisms underlying chronic EtOH’s enhancement of NMDAR function are still not fully understood. Similar to the acute effects of EtOH, studies on receptor subunit expression, function and location indicate that NR2B-containing receptors are most strongly affected by chronic EtOH exposure and acute in vitro withdrawal (Carpenter-Hyland et al., 2004; Floyd et al., 2003; Kash et al., 2009; Roberto et al., 2004b). Some studies, conducted both in vivo and in vitro, report increases in NR2B mRNA expression following chronic EtOH exposure (Follesa and Ticku, 1995; Hu et al., 1996; Kash et al., 2009; Roberto et al., 2006; Snell et al., 1996), but these results are not always consistent (Cebere et al., 1999; Floyd et al., 2003; Lack et al., 2005). Notably, postmortem human brain gene expression studies have observed a similar increase in NMDA Type Subunit 2B (GRIN2B) mRNA levels in both the prefrontal cortex and hippocampus of human alcoholics (Farris and Mayfield, 2014; Zhou et al., 2011) (for more details see article by Warden and Mayfield in this Special Issue). Increases in NR2B protein expression, and sometimes NR2A protein expression, have also been observed in rodents after both in vitro and in vivo chronic EtOH exposure (Kash et al., 2009; Kash et al., 2008; Obara et al., 2009; Snell et al., 1996). Furthermore, increased expression of mRNA and protein for other NR subunits and specific NR1 splice variants have been reported following chronic EtOH exposure (Roberto et al., 2006; Trevisan et al., 1994; Winkler et al., 1999) (but see (Morrow et al., 1994)), but a clear correlation with increased receptor function has not been observed.
For instance, a chronic EtOH exposure paradigm that produced alcohol-dependent rats significantly increased NR1, NR2A and NR2B mRNA transcription, protein expression and immunohistochemical staining in the CeA (Roberto et al., 2006). The protein levels of all subunits returned to control values at 1 week of withdrawal, but the NR1 and NR2B mRNA expression reversed, leading to a significant decrease at 1 week of withdrawal and recovery to control levels after 2 weeks (Roberto et al., 2006). These data suggest that prolonged EtOH exposure and withdrawal induced NMDAR neuroadaptations within CeA glutamatergic synapses by selectively altering the expression of its subunits and thus, their contribution to the final NMDAR subunit composition. Parallel functional studies in CeA neurons of EtOH-naïve rats showed that acute EtOH (5–66 mM) significantly decreases evoked NMDAR- and non-NMDAR-mediated excitatory postsynaptic currents (EPSCs), while chronic EtOH/acute in vitro withdrawal produced an enhancement of the acute EtOH-induced depression of NMDA-EPSCs (but not non-NMDAR-mediated EPSCs) (Roberto et al., 2004b). Similarly, local application of exogenous NMDA and acute EtOH elicited a greater inhibition of NMDAR currents in CeA slices taken from alcohol-dependent rats compared to those from naïve animals, suggesting that chronic EtOH sensitizes NMDARs to the acute effects of the drug. In addition, ifenprodil, a specific NR2A and NR2B subunit-specific antagonist, occludes EtOH’s effects and is more effective in blocking CeA NMDA-EPSCs in alcohol-dependent rats compared to naïve rats (Roberto et al., 2004b). Notably, acute EtOH significantly decreased the paired-pulse ratios of compound, non-NMDA and NMDA-EPSCs in CeA slices of alcohol-dependent rats, indicating that after chronic EtOH exposure there is an acute EtOH-induced increase in glutamate release (Roberto et al., 2004b). This finding was supported by in vivo experiments showing that infusion of EtOH via reverse microdialysis significantly increases glutamate release in CeA dialysate only in rats that underwent chronic EtOH exposure (Roberto et al., 2004b). Moreover, baseline CeA glutamate content was significantly higher in alcohol-dependent animals relative to naïve controls. These combined studies indicate that alcohol dependence and withdrawal lead to neuroadaptations of glutamatergic transmission at both pre- and postsynaptic sites in the CeA, and that CeA glutamatergic synapses may play an important role in the development of alcohol dependence.
Notably, chronic intermittent ethanol (CIE) induced a significant increase in NMDAR function in dopamine D1 receptor-containing medium spiny neurons (MSNs) during acute in vitro withdrawal, as measured by the NMDA/AMPA ratio and input-output curves of isolated NMDAR currents and a decrease in D1- MSNs in the mouse nucleus accumbens (NAcc) shell (Renteria et al., 2016). This reversal of NMDAR function may account for the CIE-induced alterations in neuronal plasticity. In fact, in ethanol naïve mice, low frequency stimulation induced synaptic depression (LTD) in D1+ MSNs, but induced synaptic potentiation (LTP) in these cells after chronic ethanol exposure (Renteria et al., 2016). These cell-type specific alterations in excitatory signaling in the NAcc shell may constitute an important neuroadaptation for the expression of increased ethanol consumption induced by intermittent ethanol vapor exposure.
In another set of elegant studies, the NMDA/AMPA current ratio of medial prefrontal cortex (mPFC) layer V pyramidal neurons was increased with both acute in vitro withdrawal from CIE and one week of in vivo withdrawal (Kroener et al., 2012). In the CIE mice, these changes were associated with increased NMDA-EPSCs and increased NMDA NR1 and NRB subunit expression. After one week of withdrawal, increased NR1 and NR2B expression was no longer observed, despite the persistent increase in synaptic NMDA currents. Notably, no change in expression of the AMPA GluR1 subunit was observed in either animal group. Finally, there were mPFC-related cognitive flexibility deficits in these CIE mice when tested up to one week into withdrawal using an attentional set-shifting task (Kroener et al., 2012). These results are consistent with those in human alcoholics, who show protracted deficits in executive function (cite), and overall, this work suggests that these behavioral deficits may be associated with the persistent alterations in mPFC glutamatergic signaling observed here.
Consistent with the Kroener study, CIE exposure (for 15 days) increased the baseline amplitude of evoked NMDA currents in mPFC layer V pyramidal neurons of rats withdrawn for 1 week or 4 weeks (Trantham-Davidson et al., 2014). However, this increase in evoked NMDA current after 1 week of withdrawal did not appear to be associated with increased NMDAR subunit expression (Trantham-Davidson et al., 2014). The reason for this discrepancy is not clear, but may relate to circuit specific changes that are not detected when analyzing tissue samples containing diverse neuronal types and synaptic sites of excitatory afferent projections from numerous brain regions (Trantham-Davidson et al., 2014). Notably, exposing rats to alcohol during early-mid adolescence (PD28–42) did not alter the expression of glutamatergic proteins in the adult mPFC. However, there was a pronounced reduction in dopamine D1 receptor modulation of both intrinsic firing and evoked NMDA currents in pyramidal cells, whereas D2 receptor function was unaltered (Trantham-Davidson et al., 2014; Trantham-Davidson et al., 2016).
Finally, glutamatergic transmission was enhanced in layer 2/3 pyramidal neurons from the infralimbic division of the mPFC in 48-hr withdrawn CIE mice compared to control mice (Pleil et al., 2015). While this study did not separate out NMDAR- and AMPAR-mediated currents, neurons from the IfL of CIE mice had larger sEPSC amplitudes, indicating altered postsynaptic receptor expression/function. Also, this enhancement of IfL glutamatergic transmission was accompanied by a reduction in sEPSC amplitudes in the medial subdivision of the central amygdala (CeA) of CIE mice.
One of the mechanisms driving increased NMDAR function following chronic ethanol exposure may involve its subcellular distribution and/or its phosphorylation state. For example, chronic EtOH exposure specifically increases the trafficking of NR2B-containing NMDARs to dendritic spines, enhancing glutamatergic transmission in cultured hippocampal neurons (Carpenter-Hyland et al., 2004). Additionally, several studies have investigated the effects of EtOH-induced kinase activity on NMDARs, with varying results. EtOH increased Fyn-mediated tyrosine phosphorylation of NR2B, resulting in an acute tolerance of NMDA currents to EtOH’s effects (Miyakawa et al., 1997) (for review see (Ron and Barak, 2016)), while brief EtOH exposure (seconds to min) rapidly increased NR2B-containing receptor function via tyrosine phosphorylation by a Fyn-like kinase (Wang et al., 2007; Yaka et al., 2003). Despite these results suggesting a role for Fyn-mediated NR2B tyrosine phosphorylation in EtOH effects on glutamatergic signaling, Woodward (2004) reported that in a co-expression system, Fyn kinase did not directly influence the acute EtOH sensitivity of NR1/NR2A receptor function (Woodward, 2004). Additionally, Wu et al. (2010) reported that a relatively short-term (2 weeks) chronic EtOH liquid-diet consumption altered hippocampal NMDAR activity, which was associated with decreased levels of phospho-Y-1472 NR2B and increased levels of STEP33 and phospho-p38 mitogen-activated protein kinase (pp38 MAPK), but not NMDAR subunit expression (Wu et al., 2010). Behavioral studies with these rats following an EtOH challenge also revealed a tolerance to EtOH’s sedative effects, suggesting that the NMDAR system’s functional and biochemical changes are associated with behavioral tolerance to its acute sedative effects after chronic exposure. Therefore, the question remains whether tyrosine phosphorylation or de-phosphorylation can directly modulate the acute and chronic effects of EtOH on NMDARs.
AMPA Receptors
Acute ethanol also inhibits the function of AMPARs (Akinshola et al., 2001; Akinshola et al., 2003; Dildy-Mayfield and Harris, 1992; Moykkynen et al., 2003; Nieber et al., 1998; Wirkner et al., 2000) although with lower potency than NMDARs (Frye and Fincher, 2000; Lovinger, 1995; Lovinger et al., 1989). EtOH slightly alters AMPAR-mediated synaptic responses [the amplitude or time course of EPSCs] at most synapses due to increased receptor desensitization (Ariwodola et al., 2003; Lovinger et al., 1990; Moykkynen et al., 2003; Moykkynen et al., 2009), but see (Mameli et al., 2005; Nie et al., 1993; Roberto et al., 2004b; Zhu et al., 2007).
The effects of chronic ethanol on AMPARs are more variable. Increased AMPAR subunit mRNA expression in the hippocampus (Bruckner et al., 1997) and protein expression in cortical cultures (Chandler et al., 1999) have been reported following chronic EtOH exposure. Notably, a postmortem human brain gene expression studies identified the AMPA Type Subunit 1 (GRIA1) as a hub gene in the human alcoholic prefrontal cortex (Farris and Mayfield, 2014) (for more details see article by Warden and Mayfield in this Special Issue). Alcohol also up-regulated neuronal activity dependent pentraxin (Narp) levels, concomitant with increased AMPAR subunit levels in the mouse NAcc (Ary et al., 2012). Additionally, Marty and Spigelman (2012) reported that the amplitude and conductance of AMPAR-mediated miniature EPSCs were enhanced in the NAcc of CIE-treated rats during acute in vitro withdrawal, due to an increase in a small fraction of functional postsynaptic GluA2-lacking AMPARs (Marty and Spigelman, 2012). Similarly, CIE/acute in vitro withdrawal induced a significant increase in baseline AMPAR-mediated signaling in D1+ MSNs, but not D1- MSNs, in the rat NAcc (Renteria et al., 2016). Other studies have demonstrated similar increases in AMPAR function in cerebellar Purkinje neurons (Netzeband et al., 1999), and AMPAR-mediated synaptic responses in the BLA during in vivo withdrawal (Lack et al., 2007). A more recent study from the McCool group (Christian et al., 2012) specifically dissected CIE- and withdrawal-induced changes in glutamatergic signaling using electrophysiological and biochemical approaches, with a particular focus on cortical glutamatergic inputs to BLA principal neurons. Both animal treatments induced postsynaptic alterations in the AMPAR that were associated with its surface expression, and AMPAR subunit phosphorylation that was associated with changes in total protein levels and/or the phosphorylation status of several key, plasticity-associated protein kinases, such as calcium/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC). Together, these data show that CIE- and withdrawal-induced changes in BLA glutamatergic signaling both functionally and biochemically mimic plasticity-related states that likely contribute to the long-term increases in anxiety-like behavior that result from chronic EtOH exposure (Christian et al., 2012). The same team of investigators (Christian et al., 2013) also reported that withdrawal from chronic EtOH exposure also induced presynaptic alterations at thalamic-BLA synapses. The functional changes observed include altered paired-pulse ratios, decreased failure rates of unitary events and increased concentrations of synaptic glutamate release. These alterations were also associated with increased expression of vesicle-associated proteins. Overall, these data demonstrate that chronic EtOH modulation of glutamate neurotransmission in the rat basolateral amygdala is afferent-specific (Christian et al., 2013).
Notably, high-dose EtOH (BAL 0.32 g/dl) exposure during the equivalent to the last trimester of human pregnancy (postnatal days P3–5) can persistently increase the frequency of spontaneous and miniature EPSCs in the BLA of P36–50 rats, correlating with an increase in anxiety-like behaviors (Baculis et al., 2015). A similar paradigm of early EtOH exposure did not significantly affect the frequency, amplitude, rise-time and half-width of sEPSCs in hippocampal CA3 pyramidal neurons 2 weeks later (Baculis and Valenzuela, 2015). Previous studies also reported that AMPAR expression and function in the hippocampus are not altered following chronic EtOH exposure (e.g. (Smothers et al., 1997)). Chronic EtOH appears to produce variable effects on glutamatergic transmission, though this may stem from differences in brain regions and type of tissue/cell preparation, the paradigm of EtOH exposure, and whether experiments were performed immediately prior to the end of drug exposure or during acute in vitro or in vivo withdrawal.
Kainate Receptors
Although, acute EtOH also inhibits KAR-mediated responses at quite low (10 mM) concentrations (Costa et al., 2000; Lack et al., 2008; Valenzuela et al., 1998; Weiner et al., 1999) it is unclear whether EtOH’s actions on KAR function are direct or indirect (Dildy-Mayfield and Harris, 1992). More consistently, chronic EtOH exposure/acute in vitro withdrawal increased KAR subunit protein expression and receptor function in cultured hippocampal neurons (Carta et al., 2002), as well as KAR-mediated synaptic transmission in the basolateral amygdala (Lack et al., 2009). However, KAR expression was unchanged in cultured cortical neurons treated with chronic ethanol (Chandler et al., 1999).
Overall, acute EtOH’s effects on iGluRs are generally thought to dampen neuronal excitability across the brain by reducing excitatory synaptic drive and inhibiting the synaptic plasticity that requires iGluR activation. Chronic EtOH exposure tends to have the opposite effect of enhancing iGluR function, though there generally remains a lack of tolerance to the acute effects of the drug. This combination of chronic EtOH-induced increases in iGluR function and the conclusion of acute EtOH-induced iGluR inhibition are likely to generate the hyperexcitable state that characterizes early withdrawal.
1.1.2 EtOH Effects on Metabotropic Glutamate Receptors
The majority of the brain’s glutamate receptors are G protein-coupled receptors (GPCRs) and are termed metabotropic glutamate receptors (mGluRs). mGluRs bind glutamate, which alters their protein conformation to activate intracellular signaling proteins, known as G proteins. GPCRs act on three main G protein subclasses: Gi/o, Gs and Gq (Wickman and Clapham, 1995). Gi/o proteins tend to inhibit neuronal function via the downstream effects of its α and β/γ protein subunits. Specifically, Gi/o α subunits inhibit adenylyl cylase (AC) activity to prevent the production of the second messenger 3’,5’-cyclic adenosine monophosphate (cAMP), while its β/γ subunits activate G protein-activated inward rectifier potassium channels (GIRKs) to inhibit neuronal activity and inhibit voltage-gated calcium channels to prevent neurotransmitter release (Dolphin, 2003a, b; Elmslie, 2003; Wickman and Clapham, 1995; Wu and Saggau, 1997). In contrast, Gs α subunits stimulate AC/cAMP formation to promote synaptic transmission and inhibit some potassium channels (Wickman and Clapham, 1995). Finally, the Gq α subunits induce protein and lipid signaling pathways to activate ion channels that induce neuronal excitability, inhibit potassium channels and increase neurotransmitter release (Wickman and Clapham, 1995). Overall, Gs and Gq activation generally have net excitatory effects on neuronal activity and synaptic transmission. Therefore, mGluRs can directly alter cellular physiology and can also induce long-lasting effects on neuronal function.
The direct effects of acute alcohol on GPCRs and G proteins tend to be small and the physiological consequences unclear. Early studies demonstrated that acute EtOH stimulated cAMP formation (Luthin and Tabakoff, 1984; Rabin and Molinoff, 1981), likely via actions on AC (Bjork et al., 2008). More recent work confirms that acute EtOH exposure increases neurotransmitter release via AC activity (Cruz et al., 2012; Kelm et al., 2008; Roberto et al., 2010; Ron and Barak, 2016; Talani and Lovinger, 2015). Of particular interest here, acute EtOH inhibits the activation of some Gq-type GPCRs, including mGluRs (Kelm et al., 2011; Ron and Barak, 2016). More consistently, chronic alcohol exposure enhances mGluR expression/function in several brain regions. Specifically, chronic ethanol activates mGluR-associated intracellular signaling pathways, such as mGluR5 in the NAcc (Cozzoli et al., 2012; Cozzoli et al., 2009). However, while chronic EtOH increases both mGluR1 and mGluR5 protein expression in the NAcc and amygdala (Cozzoli et al., 2014; Obara et al., 2009; Szumlinski et al., 2008; Szumliski and Woodward, 2014), the enhancement of NAcc mGluR5 downstream signaling is not always associated with its increased expression (Szumlinski et al., 2008). Chronic EtOH exposure also decreased mGluR-induced dendritic calcium signals in cultured cerebellar Purkinje neurons (Netzeband et al., 2002).
1.1.3 Ethanol Effects on Glutamate Release
Chronic EtOH treatment increased synaptic glutamate release in several amygdalar nuclei (Lack et al., 2007; Roberto et al., 2004b), while also decreasing glutamate reuptake (Melendez et al., 2005a; Melendez et al., 2005b) (see Figure 1A). A similar decrease in glutamate reuptake leads to increased basal extracellular glutamate in the NAcc core after alcohol consumption (Pati et al., 2016), though there are likely to be multiple other factors that contribute to these observed changes following chronic EtOH exposure and withdrawal.
Chronic EtOH exposure and withdrawal also increase extracellular glutamate levels in the central amygdala, hippocampus and striatum, as measured with in vivo microdialysis (Dahchour and De Witte, 1999, 2003; Roberto et al., 2004b; Rossetti and Carboni, 1995). However, in the lateral subdivision of the CeA, Pleil et al. (2015) observed a lower sEPSC frequency in 48 hr withdrawn CIE mice compared to control mice, indicating reduced glutamate release in this region (Pleil et al., 2015). Since these electrophysiology recordings assess local synaptic currents from a restricted population of neurons (lateral CeA), while in vivo microdialysis measures regional neurochemistry, it is not so surprising that these results differ. Furthermore, it is important to keep in mind that both synaptic and non-synaptic sources of glutamate contribute to the extracellular pool measured with microdialysis methods (Kalivas, 2009; Rao et al., 2015).
Finally, gene expression studies in the frontal cortex of human alcoholics revealed an up-regulation in several genes associated with glutamatergic synaptic transmission, including the NMDA Type Subunit 1 (GRIN1), dynamin (DNM1), syntaxin (STX1A), synapsin 1 (SYN1), synaptophysin (SYP), and the vesicular glutamate transporter 1 (VGLUT1, SLC17A7) (Ponomarev et al., 2012), suggesting that chronic ethanol exposure produces a coordinated increase in glutamatergic release (for more details see article by Warden and Mayfield in this Special Issue).
1.2 GABA and EtOH Effects
The γ-aminobutyric acid (GABA) system plays a well-established role in the behavioral and cellular effects of acute and chronic alcohol consumption (Koob, 2009). There are 3 main types of GABA receptors; GABAAR and GABACR are LGICs, while the GABABR is a GPCR (Aguayo et al., 2002; Forstera et al., 2016; Harris, 1999; Lovinger, 1997). Early rodent studies showed that systemic administration of GABAAR agonists increased voluntary EtOH consumption, whereas GABAAR antagonists and benzodiazepine inverse agonists decreased drinking (Boyle et al., 1993; Rassnick et al., 1993). Additionally, intra-amygdala infusion of GABAA antagonists decreased EtOH drinking by non-dependent rats (Hyytia and Koob, 1995), while a similar study using a GABAAR agonist suppressed drinking by alcohol-dependent rats without affecting intake by the non-dependent controls (Roberts et al., 1996). GABABR agonists also reduced the alcohol intake, anxiety-like behaviors and seizure susceptibility of alcohol-dependent rodents (Colombo et al., 2000; File et al., 1991; Knapp et al., 2007; Walker and Koob, 2007).
The GABA-mimetic effects of EtOH are well described and further support the relevance of the GABAergic system, and so in this section we will focus mostly on the effects of ethanol on GABAAR transmission. In particular, GABAARs can be potentiated by low to intermediate concentrations of ethanol (5–50mM, corresponding to a few drinks up to complete intoxication). In recent years there has been an increase in the understanding of ethanol effects on GABAA and GABAB receptors, however, several critical questions remain. What are EtOH’s mechanisms of action on GABAA and GABAB receptors? Are these mechanisms direct or indirect? Which GABAA subunits compositions are most influenced by EtOH, and in what way?
1.2.1 EtOH Effects on GABAA Receptors
GABAARs are pentameric “cys-loop” LGICs that are activated by extracellular GABA binding and transport chloride ions. This class of channels is characterized by an obligatory cysteine double bond in the N-terminal binding domain. Other “cys-loop” LGICs include the strychnine-sensitive glycine receptor (GlyR), nicotinic acetylcholine receptor (nAChR) and serotonin 3 receptor (5-HT3). Acute EtOH tends to enhance cys-loop LGIC function (Aguayo et al., 2002; Forstera et al., 2016; Harris, 1999; Lovinger, 1997) by increasing their probability of channel opening and/or increasing agonist affinity (Tonner and Miller, 1995; Welsh et al., 2009). This EtOH effect has been observed in both synaptic and extrasynaptic receptors (Eggers and Berger, 2004; Sebe et al., 2003; Ye et al., 2001; Ziskind-Conhaim et al., 2003). For example, acute EtOH increases the amplitude and/or duration of GABAAR and GlyR-mediated inhibitory postsynaptic currents (IPSCs) (Sebe et al., 2003; Ziskind-Conhaim et al., 2003). It is important to note, that acute EtOH can also inhibit nAChRs and GABAARs (Aguayo et al., 2002; Cardoso et al., 1999; Davis and de Fiebre, 2006; Marszalec et al., 1994; Roberto et al., 2003).
Generally acute EtOH facilitates GABAAR function. There are 19 subunit proteins (α1-6, β1–3, γ1–3, δ, ε, θ, π and ρ1–3) that can combine in specific patterns to form different types of GABAARs (Aguayo et al., 2002; Forstera et al., 2016; Harris, 1999; Lovinger, 1997). Most GABAARs comprise 2α and 2β subunits, together with a single γ2 or δ subunit. The differential subunit composition of GABAARs determines its ligand binding affinity and gating properties. For instance, recombinant α4β2γ2 GABAARs are less sensitive to GABA and benzodiazepines compared to α1β2γ2 receptors (Whittemore et al., 1996). GABAAR subunit composition also affects the receptor localization, such that receptors containing the γ subunit are expressed within the synapse where they mediate phasic inhibition, while receptors containing α4, α5, α6 and δ subunits are largely extra- or non-synaptic (Cherubini and Conti, 2001; McKernan and Whiting, 1996). In contrast, extrasynaptic high affinity GABAARs that contain the δ receptor subunit produce a tonic GABAAR-mediated current in many CNS neurons (Hanchar et al., 2005). Many studies have shown that EtOH potentiates the function of synaptic α/β/γ-containing receptors and extrasynaptic GABAAR containing either α4 or α6 paired with β and δ subunits (Harris et al., 2008; Herman et al., 2013; Lobo and Harris, 2008; McCool et al., 2003; Mihic, 1996; Olsen et al., 2007). These findings remain inconsistent, and have not been uniformly replicated across all studies on EtOH/GABAARs in heterologous systems (reviewed in (Aguayo et al., 2002; Forstera et al., 2016; Lovinger and Homanics, 2007; Lovinger and Roberto, 2013)). Acute EtOH potentiation of extrasynaptic tonic current has been observed in recordings from the cerebellum, hippocampus, accumbens, amygdala and thalamus (Botta et al., 2007; Chandra et al., 2006; Forstera et al., 2016; Glykys et al., 2007; Hanchar et al., 2005; Herman et al., 2013; Jia et al., 2008; Liang et al., 2014; Pirker et al., 2000; Valenzuela and Jotty, 2015; Wei et al., 2004).
Chronic ethanol exposure induces many neuroadaptations within GABAergic synapses in a brain region-specific manner. The effects of chronic EtOH are critical for the development of ethanol dependence (Eckardt et al., 1998; Grobin et al., 1998) and accumulating evidence suggests a role for the GABAAR system in chronic EtOH-induced tolerance for the anxiolytic, sedative, ataxic and positive reinforcing effects of acute EtOH (Kumar et al., 2004; Kumar et al., 2009; Kumar et al., 2002). Specifically, these behaviors involve marked adaptations in GABAergic signaling, including GABAA receptor subunit expression (Grobin et al., 1998) and pharmacology (see Figure 1B) (Kang et al., 1998b).
Chronic EtOH differentially altered the expression of specific GABAAR subunits at both the transcriptional and translational levels in several brain regions. While it can be difficult to compare the work of multiple groups even within a single region due to differences in the EtOH exposure paradigm (in vitro vs. in vivo), here we describe the major findings in three brain regions that are known to play central roles in the development of alcohol dependence: 1) the cerebral cortex, 2) the hippocampus and 3) the amygdala.
Cerebral Cortex
In the cerebral cortex, chronic EtOH decreased mRNA and protein expression of the α1, α2 and α3 subunits (Devaud et al., 1997; Devaud et al., 1995) and increased expression of the α4, β1, β2, β3, γ1 and γ2 subunits (Devaud et al., 1997; Devaud et al., 1995). Notably, human RNAseq studies have also identified several genes encoding for GABAAR subunits that were associated with lifetime alcohol consumption, including α3 (GABRA3) in the prefrontal cortex (Farris and Mayfield, 2014)and α2 (GABRA2), γ1 (GABRG1) and γ2 (GABRG2) in the hippocampus (Enoch et al., 2012; Zhou et al., 2011) (for more details see article by Warden and Mayfield in this Special Issue). These changes in the subunit expression could affect the assembly of GABAARs and thus their ligand binding affinities and function. For instance, cerebral cortical synaptoneurosomes exhibit decreased sensitivity to GABA (Morrow et al., 1988) and cortical membrane vesicles (microsacs) to benzodiazepines (Buck and Harris, 1990) after chronic EtOH treatment. Similarly, acute EtOH no longer facilitated GABA- or muscimol-stimulated Cl- uptake in the cortex (Morrow et al., 1988) and cerebellum (Allan and Harris, 1987) after chronic EtOH exposure. More recently, the Morrow laboratory has investigated two distinct populations of synaptic and extrasynaptic α4-containing GABAARs in cultured rat cortical neurons (Carlson et al., 2016a; Carlson et al., 2016b). They found that chronic EtOH treatment alters the abundance of both types of subunits, while six weeks of chronic EtOH diet, but not 2 weeks, increased mouse thalamic GABAAR α4 subunit levels (Werner et al., 2016). Chronic EtOH also decreased α1 mRNA and increased α6 mRNA in the cerebellum, and altered δ protein levels in the rat cerebellum and hippocampus (Mhatre and Ticku, 1994; Morrow et al., 1992). However, a down-regulation of native δ subunit-containing GABAAR assemblies in the cerebellum has also been observed after rats received chronic EtOH administration (Marutha Ravindran et al., 2007), while no changes in δ subunit protein levels were reported in the cerebral cortex (Marutha Ravindran et al., 2007). Finally, CIE exposure did not affect the amplitude of baseline evoked IPSCs in Layer V pyramidal neurons of mPFC at 1 and 4 weeks of withdrawal (Trantham-Davidson et al., 2014).
Hippocampus
Alterations in GABAAR expression induced by chronic EtOH exposure can also vary based on the time period of exposure (as alluded to above), and these temporal effects are demonstrated by work that assessed changes in hippocampus subunit expression at multiple time points during a single study. Accordingly, chronic EtOH consumption for 40 days, but not 14 days, produced a significant increase in the level of GABAAR α4 subunit protein expression in the hippocampus (Matthews et al., 1998). However, neither exposure altered α1, α2, α3, β(2/3), or γ2 protein levels (Charlton et al., 1997; Matthews et al., 1998). Hippocampal α1 subunit immunoreactivity and mRNA content were also significantly reduced and α5 mRNA content was increased after 12 weeks of EtOH exposure, but not after 4 weeks of exposure. In contrast, chronic EtOH consumption for both 14 days (Devaud et al., 1997) and 40 days (Devaud et al., 1997; Matthews et al., 1998) increased cerebral cortical GABAAR α4 subunit expression, but decreased α1 subunit expression (Devaud et al., 1997; Matthews et al., 1998). Overall, these findings indicate that chronic EtOH consumption differentially alters GABAAR expression in both a temporal- and brain region-specific manner (Grobin et al., 1998).
Other studies from the Spiegelman and Olsen laboratories used a longer CIE treatment (120 day paradigm that ended with 2 days of in vivo withdrawal) in rats to correlate observed alternations in GABAAR subunit expression with changes in the channel’s function. These animals had increased levels of α4, γ2S and γ1 in the hippocampus, a decrease in α1 and δ expression and no significant change in α5 (Cagetti et al., 2003; Mahmoudi et al., 1997). This increased hippocampal α4 subunit protein expression was paralleled by alterations in the pharmacological responses of GABAARs to benzodiazepine agonists and inverse agonists (Cagetti et al., 2003). CA1 pyramidal neurons from these CIE rats also exhibited decreases in the GABAAR miniature IPSCs (mIPSCs) amplitude and decay time, which can be interpreted as altered receptor expression/composition, and reflects the observed changes in the expression of α1 and α4 subunits (described above). The mIPSC frequency was also slightly decreased, suggesting that chronic EtOH exposure may also be associated with a presynaptic decrease in GABA release at these synapses. These adaptive changes in GABAAR expression are thought to lead to a pronounced hypofunction of GABAergic neurotransmission and possibly the development of tolerance to the acute effects of EtOH at these synapses. Additionally some of these GABA system neuroadaptations persist during abstinence, including the decrease in hippocampal GABAAR activity detailed above, which lasts for at least 40 days into withdrawal (Cagetti et al., 2003; Kang et al., 1996; Liang et al., 2004; Liang et al., 2009).
Additionally, Cagetti et al. identified several pharmacological alterations in the properties of GABAergic synapses that were consistent with their observed changes in subunit expression (Cagetti et al., 2003). Overall, CIE primarily decreased CA1 pyramidal neuron mIPSC sensitivity to positive modulation (by compounds such as diazepam and the steroid anesthetic alphaxalone); however, mIPSC potentiation by bretazenil, a α-preferring benzodiazepine ligand, was maintained, and mIPSC potentiation by Ro15-4513 was increased (Cagetti et al., 2003; Liang et al., 2009). Critically, the loss of the effect of diazepam and alphaxalone on mIPSCs in the CIE rats compared to the control rats (Cagetti et al., 2003) reflects the loss of α1 and γ-subunits, respectively. In contrast, drugs with some selectivity for α4-subunits [e.g.; RO 15-4513 and a benzodiazepine inverse agonist (DMCM)] showed an increased modulation of mIPSCs, potentially due to the observed increase in α4 subunit expression (Kang et al., 1998a; Kang et al., 1996; Kang et al., 1998b). Notably, electrically-evoked IPSCs were still sensitive to alphaxalone (Kang et al., 1998b), suggesting differences in the populations of GABAARs that underlie evoked and miniature IPSCs in terms of their sensitivity to chronic EtOH exposure. In addition, the acute effect of EtOH on evoked IPSCs was significantly increased in hippocampal slices from chronic ethanol exposed rats (Kang et al., 1998a; Kang et al., 1998b).
Liang et al., (Liang et al., 2004) have also compared the effects of chronic EtOH exposure on synaptic and extrasynaptic GABAAR function in hippocampal CA1 neurons and observed similar effects of chronic EtOH exposure on synaptic mIPSCs and the extrasynaptic tonic conductance. Specifically, both mIPSCs and the tonic current exhibit a high tolerance to doses of diazepam and zolpidem that are selective for α1-containing GABAARs (Cagetti et al., 2003). Previous reports identified that chronic EtOH exposure decreased the benzodiazepine-sensitive αl-subunits and increased the benzodiazepine-insensitive α4-subunits at synaptic receptors (Grobin et al., 2000). Accordingly, THIP (a high affinity and efficacy agonist of the α4-containing GABAAR) activated the tonic GABA current and depressed mIPSCs in slices from control rats; the THIP effect on tonic conductance was lost after chronic EtOH exposure and it now strongly increased mIPSCs (Liang et al., 2004). As observed for glutamatergic signaling, high-dose EtOH (BAL 0.32 g/dl) exposure during pregnancy did not significantly affect the frequency, amplitude, rise-time and half-width of sIPSCs in hippocampal CA3 pyramidal neurons (Baculis and Valenzuela, 2015).
In the last decade, non-human primates (Cynomolgus macaques) have emerged as a powerful model for studying the neurobiology of long-term EtOH consumption (Vivian et al., 2001). The first evidence of GABAergic synapse neuroadaptation in the monkey hippocampus was obtained using a paradigm of ethanol-self administration where cynomolgus macaques were trained using operant 4% EtOH solution self-administration and then given 22 hr daily access to the ethanol solution (Vivian et al., 2001). For the electrophysiology studies, tissue slices were prepared immediately following the last day of 18 months of daily EtOH drinking, and a significant increase in paired-pulse facilitation (PPF) of GABAA IPSCs in hippocampal dentate granule cells was observed (Ariwodola et al., 2003; Weiner, 2005). These findings are indicate a decrease in hippocampal GABA release after chronic ethanol exposure, similar to that observed in CIE rats (Cagetti et al., 2003).
Amygdala
In the amygdala, α1 and α4 subunit expression was significantly decreased after two weeks of chronic EtOH consumption (Papadeas et al., 2001), while (1–12) weeks of EtOH exposure led to decreased α4 subunit expression in the NAc, but no change in α1 subunit expression (Charlton et al., 1997; Ortiz et al., 1995). Additionally, muscimol-stimulated Cl-uptake was enhanced in the extended amygdala, but not the NAc of EtOH-dependent rats (after acute in vitro withdrawal) (Papadeas et al., 2001). Western blot analysis of surface subunit levels also revealed selective decreases in α1 and δ and increases in α4, a5, and γ2 GABAAR subunits in rat NAc after CIE treatment and withdrawal (Liang et al., 2014). Additionally, in this study there was decreased in the ethanol and Ro15-4513 potentiation of extrasynaptic GABAARs and decreased diazepam sensitivity of both tonic and phasic GABAAR signaling, suggesting a reduction in somatic GABAergic synapses onto NAcc MSNs in CIE rats (Liang et al., 2014). Collectively, these results suggest that chronic EtOH exposure alters GABAAR expression in the amygdala and NAcc and that decreased expression of α4 subunits is associated with increases in receptor function in the amygdala, but not the NAcc (Papadeas et al., 2001).
In the CeA of alcohol-dependent rats (with acute in vitro withdrawal), the baseline frequency of mIPSCs and the baseline evoked IPSCs was significantly higher, while the baseline PPF ratios of IPSCs was significantly lower, suggesting that basal amygdala GABA release is augmented by chronic EtOH exposure (Roberto et al., 2004a). In addition, acute EtOH superfusion significantly enhanced evoked IPSCs and mIPSC frequencies to an extent equivalent to that in slices from non-dependent rats, suggesting lack of tolerance for these acute EtOH effects (Roberto et al., 2004a). Further, in vivo EtOH administration directly into the CeA via a microdialysis probe dose-dependently increased CeA GABA release in both alcohol-dependent and non-dependent rats. Interestingly, in dependent rats there was about a 4-fold increase in baseline GABA microdialysate content compared to the non-dependent rats, further suggesting increased GABAergic ‘tone’ during alcohol dependence (Roberto et al., 2004a). These studies point to a dysregulation by acute and chronic EtOH of presynaptic GABAergic function in the CeA. Recent studies in mice also show that CIE produced profound and long-lasting changes in local inhibitory circuits in the CeA (Herman and Roberto, 2016). In particular, a loss of tonic inhibition in a population of neurons occurred in parallel with an increase in phasic and tonic signaling mediated by δ-containing GABAAR. These effects include a complete loss of tonic inhibition that persisted 5–7 days into withdrawal in a subtype of CeA neurons (Herman and Roberto, 2016).
Electrophysiology experiments conducted using the same cynomolgus macaque model of long-term EtOH consumption as described earlier, showed a decrease in the effect of flunitrazepam on the currents gated by exogenous GABA application in acutely dissociated amygdala neurons from ethanol-exposed animals compared to control animals (Anderson et al., 2007; Floyd et al., 2004). However, the modest inhibition of GABA-gated currents induced by acute EtOH was not affected by the chronic EtOH consumption. In addition, chronic EtOH significantly reduced amygdala mRNA expression levels for GABAAR β1 and γ2 subunits. Overall, these findings demonstrate that chronic EtOH self-administration reduces the benzodiazepine sensitivity of amygdala GABAARs and this reduced sensitivity may reflect decreased expression of the γ subunit. Therefore, correlations between chronic EtOH-induced GABAAR subunit expression and channel function have been observed in multiple brain regions, though, it remains unclear whether this link is causal and whether/how it contributes to the development of dependence.
Molecular Mechanisms
The mechanisms underlying alcohol-induced adaptation of GABAARs, both in terms of their expression and composition, can be varied and are not well understood. GABAAR membrane expression involves a highly regulated process of synthesis, assembly, trafficking, endocytosis, and recycling or degradation. Therefore, chronic EtOH could act on a variety of cellular processes to change the overall number and/or composition of GABAARs, including endocytosis and recycling/degradation to selectively remove membrane receptors and/or trafficking of newly synthesized receptors to the cell surface. Several groups have shown that the altered GABAAR function could stem from altered subunit assembly and does not necessarily require changes in the total number of receptors expressed on the membrane (Devaud et al., 1995; Kumar et al., 2009; Morrow et al., 1992). These chronic EtOH-induced changes in GABAAR composition (and consequent change in receptor pharmacological properties) are hypothesized to contribute to the development of alcohol dependence (Kumar et al., 2004).
Notably, chronic EtOH administration can affect GABAAR internalization via clathrin and the adaptor complex (AP). For example, chronic EtOH exposure selectively increases the internalization of α1-containing GABAARs into clathrin coated vesicles of the cerebral cortex, with no change in the internalization of α4 GABAARs (Kumar et al., 2003). Decreases in α1 GABAAR subunit peptides, as well as concomitant increases in α4, have been also observed in the synaptic fraction following chronic EtOH exposure, while the clathrin-α1-GABAAR complex is increased in the intracellular fraction (Kumar et al., 2004). Additionally, the presence of β2 and/or γ 2GABAAR subunits allowed for receptor recognition by AP-2, resulting in clathrin-dependent endocytosis (Herring et al., 2003; Kittler et al., 2008).
Although, many protein kinases, including PKC, PKA and fyn, regulate GABAAR trafficking, the role of these protein kinases is not completely understood following chronic EtOH exposure. In the cerebral cortex, chronic EtOH increases the expression of α4-, β2-, and β3- GABAAR subunits and decreases α1, α2, and α3, and the differential PKC-dependent phosphorylation of these GABAAR subunits is thought to be involved in the selective endocytosis of specific GABAARs (Brandon et al., 2002; Mohler et al., 1996). Moreover, in a separate study in the cerebral cortex, chronic EtOH consumption decreases the association of PKCγ with α1-containing GABAARs and increases the association of PKCγ with α4 GABAARs, which is accompanied by a decreased α1 subunit expression and increased α4 expression at the cell surface (Kumar et al., 2002). Considering that phosphorylation of GABAAR subunits reduces their binding to AP-2 (which is necessary for clathrin-dependent internalization, as described above) (Kittler et al., 2008), the increased association of PKCγ with α4 GABAARs may prevent their internalization (Kumar et al., 2004). Conversely, a reduction in PKC-dependent GABAAR phosphorylation disinhibits receptor binding to AP-2 to enable GABAAR endocytosis (Terunuma et al., 2008).
Interestingly, EtOH alters PKA expression and translocation (Diamond and Gordon, 1994; Newton and Messing, 2006) and chronic PKA activation in cerebellar granule cells increases α1 subunit membrane expression (Ives et al., 2002), suggesting that PKA might also regulate GABAAR membrane localization after chronic EtOH exposure. Recently, the Morrow laboratory reported that either PKA activation or PKC inhibition prevented ethanol-induced increases in α4 subunit expression and decreases in the decay of GABA mIPSCs, whereas PKA inhibition had no effect (Bohnsack et al., 2016; Carlson et al., 2016a). This work suggests that PKA and PKC have opposing effects on synaptic α4-containing GABAARs, with PKA activation negatively modulating, and PKC activation positively modulating, their abundance and function (Bohnsack et al., 2016; Carlson et al., 2016a). Furthermore, they reported that PKA activation decreases synaptic α4 expression via increased β3 S408/409 phosphorylation, while PKC activation acts via γ2 S327 phosphorylation to increase α4. Additionally, PKA activation increases extrasynaptic α4 and δ subunit expression (Bohnsack et al., 2016). Similar mechanisms and regulatory phosphorylation patterns (PKCγ and PKCδ) have been also observed in thalamic GABAAR α4 subunit expression following acute and chronic EtOH administration where they are likely dependent on neuroactive steroids (Werner et al., 2016).
Additionally, post-translational modifications, such as the phosphorylation of GABAARs, have been demonstrated to modulate receptor function and play a role in ethanol dependence. CAM kinase II (CAMKII) or tyrosine kinase activity phosphorylates GABAARs to enhance their function (Churn et al., 2002; Valenzuela et al., 1995), and in the rat hippocampus, chronic EtOH exposure decreases tyrosine kinase phosphorylation of α1 subunits, increases its phosphorylation of β2 subunits and has no effect on γ2 subunits (Marutha Ravindran et al., 2007). Overall, it is hypothesized that chronic EtOH exposure may produce persistent changes in second messenger systems, such as kinases, to alter GABAARs composition and expression, and ultimately function. This possibility is complicated by the in vivo heterogeneity of GABAARs, and it is important to note that there has been no direct demonstration of EtOH-induced phosphorylation of GABAARs altering receptor function. Thus, the exact mechanisms employed by chronic EtOH to regulate GABAA receptor function remain unknown at this point.
1.2.2 EtOH Effects on GABAB Receptors
GABAB receptors tend to localize to the presynaptic terminal, where they can act as auto-receptors to limit GABA release, as well as postsynaptic sites, and are thought to play a critical role in the effects of ethanol (Ariwodola and Weiner, 2004). Accordingly, GABABR antagonists enhance the effects of acute EtOH on GABAAR-mediated transmission in the hippocampus (Ariwodola and Weiner, 2004; Wan et al., 1996; Wu and Saggau, 1994) and NAc (Nie et al., 2000). These effects appear to be brain region-specific, as the presence of GABABRs accounted for the difference in sensitivity to EtOH’s influences on GABA transmission in specific subfields of the hippocampus (Weiner et al., 1997), and GABABRs did not influence GABA release from neurons in the CeA (Roberto et al., 2003). Thus, the presence or absence of presynaptic GABABRs may be an important determinant for the regional specificity of ethanol to affect GABA transmission (Ariwodola and Weiner, 2004).
Several studies have investigated the effects of chronic EtOH exposure on the role of the GABABRs in regulating GABA release, but the results appear to be mixed and perhaps brain region-specific. For example, Peris et al., (Peris et al., 1997) showed that chronic EtOH treatment (along with acute in vitro withdrawal) increased hippocampal 3H-GABA release, while decreasing long-term potentiation (LTP). Baclofen (a GABABR agonist) decreased this stimulated presynaptic release, while 2-OH-saclofen (a GABABR antagonist) enhanced it, suggesting presynaptic GABAB autoreceptor activity. This chronic EtOH-induced change in presynaptic regulation of GABA release may potentially represent an underlying mechanism for the concomitant reduction in hippocampal LTP observed after chronic EtOH exposure (Peris et al., 1997).
In a second hippocampal study, the role of postsynaptic GABABRs in EtOH’s effects was assessed. Baclofen produced a concentration-dependent hyperpolarization in CA1 pyramidal neurons, and this sensitivity was unchanged by chronic ethanol exposure/acute in vitro withdrawal, suggesting that these GABABRs were essentially insensitive to EtOH (Frye and Fincher, 1996).
In contrast, in the ventral tegmental area (VTA) systemic ETOH (and acute in vitro withdrawal) employed presynaptic GABABRs to produce a long-lasting potentiation of GABAergic synapses (Melis et al., 2002). These ethanol-exposed VTA neurons also had higher mIPSC frequencies (but not amplitudes) compared to controls, supporting an increased probability of action potential-independent GABA release after EtOH exposure (Melis et al., 2002).
Finally, in the CeA, we have previously shown that chronic ethanol exposure/acute in vitro withdrawal produces neuroadaptations in GABABR function (Roberto et al., 2008). Overall, chronic EtOH reduced the sensitivity of GABA IPSCs to CGP 55845A (a GABABR antagonist) and baclofen, suggesting an overall downregulation of GABABR function. Specifically, CGP 55845A significantly increased the amplitude of evoked IPSCs and decreased PPF ratios in the CeA of naïve rats, indicating that presynaptic GABABRs basally constrain GABA release. In alcohol-dependent rats, however, CGP 55845A has no effect on evoked IPSCs and PPF ratios. Conversely, baclofen markedly depressed evoked IPSCs in the CeA of naïve rats and chronic ethanol exposure attenuated this effect. Collectively, these data suggest that the downregulation of the GABABR system associated with EtOH-dependence may partially explain the increased GABAergic tone reported in dependent rats (Roberto et al., 2008).
1.2.3 EtOH Effects on GABA Release
While most of the results described above relate to ethanol’s effects on postsynaptic GABAARs to alter GABAergic transmission, EtOH can also regulate GABA release through its actions on GABAARs (Criswell and Breese, 2005; Siggins et al., 2005; Weiner and Valenzuela, 2006). Specifically, acute EtOH increases GABA release in several brain regions, including the cerebellum, hippocampus, VTA, hypoglossal nucleus, BLA and CeA (Ariwodola and Weiner, 2004; Kelm et al., 2007, 2011; Ming et al., 2006; Roberto et al., 2003; Sebe et al., 2003; Theile et al., 2008; Zhu and Lovinger, 2006; Ziskind-Conhaim et al., 2003). It is important to note that in these studies, EtOH-induced increases in spontaneous and/or miniature GABAergic IPSCs frequencies reflect enhanced GABA release, while changes in m/sIPSC amplitudes and/or kinetics are considered an index of postsynaptic GABAAR sensitivity (i.e. localization or function) (De Koninck and Mody, 1994; Otis et al., 1994). Accordingly, the studies described above consistently reported that acute EtOH increased s/mIPSC frequencies when applied at concentrations that are associated with intoxication (Ariwodola and Weiner, 2004; Kelm et al., 2007; Roberto et al., 2003; Theile et al., 2008; Zhu and Lovinger, 2006), but this is not true of all regions (e.g. the thalamus) (Jia et al., 2008). Furthermore, these studies observed that EtOH’s actions to promote GABA release are rapid in onset and rapidly reversible following EtOH washout from the tissue.
Only a few studies reported that chronic EtOH exposure alters GABAergic transmission via changes in presynaptic GABA release, and the results appear to be highly dependent on the paradigm of exposure (in vitro vs. in vivo). For instance, in vivo chronic EtOH exposure paired with acute in vitro withdrawal decreased LTP via increased electrically-stimulated (but not basal) GABA release in the CA1 region of hippocampal slices (Tremwel et al., 1994). This effect was likely produced by alterations in the muscarinic receptor regulation of GABA release, and not changed GABA uptake or GABAAR function (Hu et al., 1999), as well as by altered GABAB autoreceptor function (as described above) (Peris et al., 1997). Chronic EtOH consumption also induces tolerance to the impairing effects of acute EtOH treatment on induction of LTP in rat hippocampal CA1 slices (Fujii et al., 2008). In addition, voluntary EtOH drinking is associated with a significant increase in GABAergic synapse paired-pulse plasticity (consistent with a reduction in GABA release probability) in cynomolgus macaque dentate gyrus neurons (Weiner, 2004).
In the CeA, we have shown (Roberto et al., 2010; Roberto et al., 2004a) that chronic EtOH exposure can affect GABA release, perhaps via an action on GABAergic terminals. Using in vitro electrophysiology and in vivo microdialysis, baseline GABA release was higher in CeA from EtOH-dependent rats compared to non-dependent rats (Roberto et al., 2010; Roberto et al., 2004a). However, Pleil et al. (2015) observed no change in GABA release in the CeA of 48 hr withdrawn CIE mice compared to control mice; though there was decreased GABA release in the infralimbic mPFC cortex of these EtOH-exposed mice (Pleil et al., 2015). Collectively, these studies strengthen the possibility that chronic EtOH, as well as acute EtOH, may alter the function of the GABAergic synapses by acting at presynaptic terminals.
Overall, we conclude that chronic ethanol exposure can produce critical neuroadaptations at GABAergic synapses to alter local inhibition. These adaptations appear to be both brain region- and temporally-specific, and more studies are required to precisely determine the necessary EtOH exposure paradigms that elicit these synaptic changes, as well as their underlying molecular mechanisms and behavioral consequences with respect to withdrawal and dependence.
1.3 Conclusions
Therefore, chronic alcohol exposure produces significant neuroadaptation at GABAergic and glutamatergic synapses, particularly with regards to the expression, localization and function of synaptic proteins, channels and receptors, and these effects can be both cell type- and brain region-specific. The development of alcohol tolerance and dependence are of particular clinical relevance, and it is likely that these adaptive changes in GABA and glutamate receptor function play a critical role in this processes. Indeed, it is well known that chronic EtOH treatment can produce tolerance and physical dependence (Chandler et al., 1998) and that withdrawal following long-term EtOH consumption is associated with increased neuronal excitability (Kliethermes, 2005; Weiner and Valenzuela, 2006). These alterations have been hypothesized to represent, in part, a compensatory adaptation to the in vitro acute facilitatory effects of EtOH on GABAergic synapses (Siggins et al., 2005; Weiner and Valenzuela, 2006), as well as its inhibitory effects on glutamatergic synapses (Szumliski and Woodward, 2014).
For a general review of brain-region specific EtOH actions on the GABA system see (Criswell and Breese, 2005; Siggins et al., 2005; Weiner and Valenzuela, 2006). From our review, it is clear that the majority of early studies characterizing chronic effects of EtOH on GABAergic transmission focused mainly on postsynaptic properties and the subunit composition of the GABAARs themselves (see Figure 1B). Some of the differences in the results reported across laboratories may reflect the differences in the chronic EtOH exposure duration and protocol, brain region examined, and methods of assessing receptor function. Most of these studies were in general agreement that chronic EtOH exposure and withdrawal did not result in dramatic decreases in the number of GABAARs in most brain regions. However, many of these studies reported marked alterations in the expression of specific GABAAR subunits and hypothesized that those changes in the subunit composition of the GABAARs may account for the physiological and pharmacological alterations in GABAergic signaling associated with chronic EtOH administration (Grobin et al., 1998).
Another area in which action of EtOH on GABA function has been implicated is in vivo withdrawal from chronic EtOH treatment. Withdrawal results in an increased sensitivity to the induction of seizures (Allan and Harris, 1987; Frye et al., 1983), and several studies have found that benzodiazepines and other drugs with GABAmimetic actions can reduce withdrawal-related hyperexcitability (Breese et al., 2006; McCown and Breese, 1990; McCown et al., 1985; Roberto et al., 2008; Ticku and Burch, 1980).
Likewise, in terms of chronic EtOH’s effects on glutamatergic signaling, the main effects appear to involve changes in the postsynaptic expression (see Figure 1A), composition and function of iGluRs (see (Bliss et al., 2014; Lovinger and Roberto, 2013; Rao et al., 2015; Szumliski and Woodward, 2014)) for more general reviews of EtOH’s effects on the glutamate system). Most significant were chronic EtOH-induced increases in the function of NR2B-containing NMDARs and NMDAR-mediated glutamatergic synaptic transmission (Carpenter-Hyland et al., 2004; Floyd et al., 2003; Kash et al., 2009; Roberto et al., 2004b). These findings were correlated with altered NMDAR subunit expression (and in particular NR2B levels) after both in vitro and in vivo chronic EtOH exposure (Follesa and Ticku, 1995; Hu et al., 1996; Kash et al., 2009; Roberto et al., 2006; Snell et al., 1996), but the results were not always consistent (Cebere et al., 1999; Floyd et al., 2003; Lack et al., 2005). More generally, EtOH’s actions on iGluRs dampen neuronal excitability by reducing excitatory synaptic drive and inhibiting the synaptic plasticity that requires iGluR activation. Chronic EtOH has the opposite effect and enhances iGluR function, though there generally remains a lack of tolerance to the acute effects of the drug. This combination of chronic EtOH-induced increases in iGluR function and the conclusion of acute EtOH-induced iGluR inhibition are likely to generate the hyperexcitable state that characterizes early withdrawal.
Collectively, these results offer strong support for the hypothesis that at least some of the actions of chronic EtOH are mediated by its effects on neural functions associated with GABA and glutamate synaptic transmission and these effects play an important role in the maintenance of addictive drinking behavior.
Chronic alcohol exposure produces neuroadaptation at GABA and glutamate synapses.
The main effects are altered GABAAR/NMDAR expression, composition and/or function.
It is hypothesized that these adaptations play a role in addictive drinking behavior.
Acknowledgments
This work was supported by National Institute of Alcohol Abuse and Alcoholism grants AA015566, AA006420, AA016985, AA017447, AA021491, AA013498 and the Pearson Center for Alcoholism and Addiction Research. This is manuscript number 29430 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marisa Roberto, The Scripps Research Institute.
Florence Varodayan, The Scripps Research Institute.
References
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABA(A) receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Akinshola BE, Stewart RR, Karvonen LL, Taylor RE, Liesi P. Involvement of non-NMDA receptors in the rescue of weaver cerebellar granule neurons and sensitivity to ethanol of cerebellar AMPA receptors in oocytes. Brain Res Mol Brain Res. 2001;93:8–17. doi: 10.1016/s0169-328x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Akinshola BE, Yasuda RP, Peoples RW, Taylor RE. Ethanol sensitivity of recombinant homomeric and heteromeric AMPA receptor subunits expressed in Xenopus oocytes. Alcohol Clin Exp Res. 2003;27:1876–1883. doi: 10.1097/01.ALC.0000098874.65490.52. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–1639. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK. Ethanol up-regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol. 2012;46:377–387. doi: 10.1016/j.alcohol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis BC, Diaz MR, Valenzuela CF. Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacol Biochem Behav. 2015;137:78–85. doi: 10.1016/j.pbb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis BC, Valenzuela CF. Ethanol exposure during the third trimester equivalent does not affect GABAA or AMPA receptor-mediated spontaneous synaptic transmission in rat CA3 pyramidal neurons. J Negat Results Biomed. 2015;14:19. doi: 10.1186/s12952-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork K, Rimondini R, Hansson AC, Terasmaa A, Hyytia P, Heilig M, Sommer WH. Modulation of voluntary ethanol consumption by beta-arrestin 2. Faseb J. 2008;22:2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Synaptic plasticity in health and disease: introduction and overview. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130129. doi: 10.1098/rstb.2013.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack JP, Carlson SL, Morrow AL. Differential regulation of synaptic and extrasynaptic alpha4 GABA(A) receptor populations by protein kinase A and protein kinase C in cultured cortical neurons. Neuropharmacology. 2016;105:124–132. doi: 10.1016/j.neuropharm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol. 2007;41:187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner MK, Rossner S, Arendt T. Differential changes in the expression of AMPA receptors genes in rat brain after chronic exposure to ethanol: an in situ hybridization study. J Hirnforsch. 1997;38:369–376. [PubMed] [Google Scholar]
- Buck KJ, Harris RA. Benzodiazepine agonist and inverse agonist actions on GABAA receptor-operated chloride channels. II. Chronic effects of ethanol. J Pharmacol Exp Ther. 1990;253:713–719. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Morrow AL. Ethanol Regulation of Synaptic GABAA alpha4 Receptors Is Prevented by Protein Kinase A Activation. J Pharmacol Exp Ther. 2016a;357:10–16. doi: 10.1124/jpet.115.230417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Patel V, Morrow AL. Regulation of Extrasynaptic GABAA alpha4 Receptors by Ethanol-Induced Protein Kinase A, but Not Protein Kinase C Activation in Cultured Rat Cerebral Cortical Neurons. J Pharmacol Exp Ther. 2016b;356:148–156. doi: 10.1124/jpet.115.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Olivera DS, Dettmer TS, Valenzuela CF. Ethanol withdrawal upregulates kainate receptors in cultured rat hippocampal neurons. Neurosci Lett. 2002;327:128–132. doi: 10.1016/s0304-3940(02)00399-3. [DOI] [PubMed] [Google Scholar]
- Cebere A, Cebers G, Liljequist S. Enhancement of NMDA-induced functional responses without concomitant NMDA receptor changes following chronic ethanol exposure in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:623–632. doi: 10.1007/s002109900133. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Newsom H, Sumners C, Crews F. Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J Neurochem. 1993;60:1578–1581. doi: 10.1111/j.1471-4159.1993.tb03326.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Norwood D, Sumners C, Crews FT. Chronic ethanol increases N-methyl-D-aspartate-stimulated nitric oxide formation but not receptor density in cultured cortical neurons. Mol Pharmacol. 1997;51:733–740. doi: 10.1124/mol.51.5.733. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton ME, Sweetnam PM, Fitzgerald LW, Terwilliger RZ, Nestler EJ, Duman RS. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- Churn SB, Rana A, Lee K, Parsons JT, De Blas A, Delorenzo RJ. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor alpha1 subunit modulates benzodiazepine binding. J Neurochem. 2002;82:1065–1076. doi: 10.1046/j.1471-4159.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I--Preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Costa ET, Soto EE, Cardoso RA, Olivera DS, Valenzuela CF. Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res. 2000;24:220–225. [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39:435–444. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther. 2003;304:192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Diamond I, Gordon AS. The role of adenosine in mediating cellular and molecular responses to ethanol. Exs. 1994;71:175–183. doi: 10.1007/978-3-0348-7330-7_18. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Acute and chronic ethanol exposure alters the function of hippocampal kainate receptors expressed in Xenopus oocytes. J Neurochem. 1992;58:1569–1572. doi: 10.1111/j.1471-4159.1992.tb11380.x. [DOI] [PubMed] [Google Scholar]
- Dildy JE, Leslie SW. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003a;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003b;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Berger AJ. Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol. 2004;91:2685–2695. doi: 10.1152/jn.00907.2003. [DOI] [PubMed] [Google Scholar]
- Elmslie KS. Neurotransmitter modulation of neuronal calcium channels. J Bioenerg Biomembr. 2003;35:477–489. doi: 10.1023/b:jobb.0000008021.55853.18. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naive P and NP rats. PLoS One. 2012;7:e29369. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Mayfield RD. RNA-Seq reveals novel transcriptional reorganization in human alcoholic brain. Int Rev Neurobiol. 2014;116:275–300. doi: 10.1016/B978-0-12-801105-8.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Gulati K. Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology. 1991;30:183–190. doi: 10.1016/0028-3908(91)90202-m. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Forstera B, Castro PA, Moraga-Cid G, Aguayo LG. Potentiation of Gamma Aminobutyric Acid Receptors (GABAAR) by Ethanol: How Are Inhibitory Receptors Affected? Front Cell Neurosci. 2016;10:114. doi: 10.3389/fncel.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, Fincher A. Sensitivity of postsynaptic GABAB receptors on hippocampal CA1 and CA3 pyramidal neurons to ethanol. Brain Res. 1996;735:239–248. doi: 10.1016/0006-8993(96)00579-3. [DOI] [PubMed] [Google Scholar]
- Frye GD, Fincher A. Sustained ethanol inhibition of native AMPA receptors on medial septum/diagonal band (MS/DB) neurons. Br J Pharmacol. 2000;129:87–94. doi: 10.1038/sj.bjp.0703039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA)mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res. 2008;1211:13–21. doi: 10.1016/j.brainres.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABA(A) receptor subunits in rat cortex in a region-specific manner. Alcohol Clin Exp Res. 2000;24:1137–1144. [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Grover CA, Frye GD, Griffith WH. Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res. 1994;642:70–76. doi: 10.1016/0006-8993(94)90906-7. [DOI] [PubMed] [Google Scholar]
- Grover CA, Wallace KA, Lindberg SA, Frye GD. Ethanol inhibition of NMDA currents in acutely dissociated medial septum/diagonal band neurons from ethanol dependent rats. Brain Res. 1998;782:43–52. doi: 10.1016/s0006-8993(97)01001-9. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci Signal. 2008;1:7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2016;21:72–86. doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Hu M, Walker DW, Vickroy TW, Peris J. Chronic ethanol exposure increases 3H–GABA release in rat hippocampus by presynaptic muscarinic receptor modulation. Alcohol Clin Exp Res. 1999;23:1587–1595. [PubMed] [Google Scholar]
- Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Iorio KR, Reinlib L, Tabakoff B, Hoffman PL. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41:1142–1148. [PubMed] [Google Scholar]
- Iorio KR, Tabakoff B, Hoffman PL. Glutamate-induced neurotoxicity is increased in cerebellar granule cells exposed chronically to ethanol. Eur J Pharmacol. 1993;248:209–212. doi: 10.1016/0926-6917(93)90045-r. [DOI] [PubMed] [Google Scholar]
- Ives JH, Drewery DL, Thompson CL. Differential cell surface expression of GABAA receptor alpha1, alpha6, beta2 and beta3 subunits in cultured mouse cerebellar granule cells influence of cAMP-activated signalling. J Neurochem. 2002;80:317–327. doi: 10.1046/j.0022-3042.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Smothers CT, Woodward JJ. Enhanced ethanol inhibition of recombinant N-methyl-D-aspartate receptors by magnesium: role of NR3A subunits. Alcohol Clin Exp Res. 2008;32:1059–1066. doi: 10.1111/j.1530-0277.2008.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30:673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998a;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABA(A) receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998b;22:2165–2173. [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008;33:1379–1390. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 Suppl. 2009;1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport. 1993;5:297–300. doi: 10.1097/00001756-199312000-00029. [DOI] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36:83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hoppocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;4:4. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Liang J, Lindemeyer AK, Suryanarayanan A, Meyer EM, Marty VN, Ahmad SO, Shao XM, Olsen RW, Spigelman I. Plasticity of GABA(A) receptor-mediated neurotransmission in the nucleus accumbens of alcohol-dependent rats. J Neurophysiol. 2014;112:39–50. doi: 10.1152/jn.00565.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- Lovinger DM. Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:267–282. doi: 10.1007/pl00005051. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic Effects Induced by Alcohol. In: Sommer WH, Spanagel R, editors. Current Topics in Behavioral Neurosciences. Vol. 13. Springer-Verlag Berlin-Heidelberg: 2013. pp. 31–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Yeh HH. Ethanol modulates AMPA-induced current responses of primary somatosensory cortical neurons. Neurochem Int. 1999;35:175–183. doi: 10.1016/s0197-0186(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Luthin GR, Tabakoff B. Activation of adenylate cyclase by alcohols requires the nucleotide-binding protein. J Pharmacol Exp Ther. 1984;228:579–587. [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalec W, Kurata Y, Hamilton BJ, Carter DB, Narahashi T. Selective effects of alcohols on gamma-aminobutyric acid A receptor subunits expressed in human embryonic kidney cells. J Pharmacol Exp Ther. 1994;269:157–163. [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci. 2012;6:86. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of the delta-subunit of GABA(A) receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” infrior collicular seizure activity: Evidence for kindling seizures associated with alcoholism. Alcohol. Clin. Exp. Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Frye GD, Breese GR. Evidence for site specific ethanol actions in the CNS. Alcohol Drug Res. 1985;6:423–429. [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005a;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005b;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M, Ticku MK. Chronic ethanol treatment upregulates the GABA receptor beta subunit expression. Brain Res Mol Brain Res. 1994;23:246–252. doi: 10.1016/0169-328x(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Mihic SJaHRA. Alcohol actions at the GABAA receptor/chloride channel complex. In: Deitrich R, Erwin VG, editors. Pharmacological effect of ethanol on the neurvous system. Boca Raton: CRC press; 1996. pp. 51–72. [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Luscher B, Rudolph U, Benson J, Benke D. The GABAA receptors. From subunits to diverse functions. Ion Channels. 1996;4:89–113. [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci. 1993;13:2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Bucci D, Smith FD. GABAA and NMDA receptor subunit mRNA expression in ethanol dependent rats. Alcohol Alcohol Suppl. 1994;2:89–95. [PubMed] [Google Scholar]
- Morrow AL, Herbert JS, Montpied P. Differential effects of chronic ethanol administration on GABA(A) receptor alpha1 and alpha6 subunit mRNA levels in rat cerebellum. Mol Cell Neurosci. 1992;3:251–258. doi: 10.1016/1044-7431(92)90045-4. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Karanian JW, Paul SM. Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl- uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- Moykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- Moykkynen TP, Coleman SK, Keinanen K, Lovinger DM, Korpi ER. Ethanol increases desensitization of recombinant GluR-D AMPA receptor and TARP combinations. Alcohol. 2009;43:277–284. doi: 10.1016/j.alcohol.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL. Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcohol Clin Exp Res. 2002;26:386–393. [PubMed] [Google Scholar]
- Netzeband JG, Trotter C, Caguioa JN, Gruol DL. Chronic ethanol exposure enhances AMPA-elicited Ca2+ signals in the somatic and dendritic regions of cerebellar Purkinje neurons. Neurochem Int. 1999;35:163–174. doi: 10.1016/s0197-0186(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293:654–661. [PubMed] [Google Scholar]
- Nie Z, Yuan X, Madamba SG, Siggins GR. Ethanol decreases glutamatergic synaptic transmission in rat nucleus accumbens in vitro: naloxone reversal. J Pharmacol Exp Ther. 1993;266:1705–1712. [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Sieler D, Illes P. Inhibition by ethanol of excitatory amino acid receptors in rat locus coeruleus neurons in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Pati D, Kelly K, Stennett B, Frazier CJ, Knackstedt LA. Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur J Neurosci. 2016;44:1896–1905. doi: 10.1111/ejn.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Eppler B, Hu M, Walker DW, Hunter BE, Mason K, Anderson KJ. Effects of chronic ethanol exposure on GABA receptors and GABAB receptor modulation of 3H–GABA release in the hippocampus. Alcohol Clin Exp Res. 1997;21:1047–1052. [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Molinoff PB. Activation of adenylate cyclase by ethanol in mouse striatal tissue. J Pharmacol Exp Ther. 1981;216:129–134. [PubMed] [Google Scholar]
- Rao PS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. doi: 10.3389/fnins.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Wu M, Peoples RW. A novel alcohol-sensitive position in the N-methyl-D-aspartate receptor GluN2A subunit M3 domain regulates agonist affinity and ion channel gating. Mol Pharmacol. 2013;84:501–510. doi: 10.1124/mol.113.085993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol Psychiatry. 2010:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW. Central amygdala neuroplasticity in alcohol dependence. In “Neurobiology of Alcohol Dependence”. In: Noronha A, Cui C, Harris A, Crabbe J, editors. Section II, Neurocircuitry and Neurosignaling of Alcohol Dependence. Elsevier Press; 2014. pp. 207–220. [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABA(A) and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumliski K, Woodward JJ. In: Glutamate Signaling in Alcohol Abuse and Dependence Section II, Neurocircuitry and Neurosignaling of Alcohol Dependence. Noronha A, Cui C, Harris A, Crabbe J, editors. Elsevier Press; 2014. pp. 173–206. [Google Scholar]
- Talani G, Lovinger DM. Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. 2015;49:781–794. doi: 10.1016/j.alcohol.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK, Burch T. Alterations in gamma-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J Neurochem. 1980;34:417–423. doi: 10.1111/j.1471-4159.1980.tb06612.x. [DOI] [PubMed] [Google Scholar]
- Tonner PH, Miller KW. Molecular sites of general anaesthetic action on acetylcholine receptors. Eur J Anaesthesiol. 1995;12:21–30. [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ. Binge-Like Alcohol Exposure during Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremwel MF, Hunter BE, Peris J. Chronic ethanol exposure enhances [3H]GABA release and does not affect GABAA receptor mediated 36Cl uptake. Synapse. 1994;17:149–154. doi: 10.1002/syn.890170302. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Bhave S, Hoffman P, Harris RA. Acute effects of ethanol on pharmacologically isolated kainate receptors in cerebellar granule neurons: comparison with NMDA and AMPA receptors. J Neurochem. 1998;71:1777–1780. doi: 10.1046/j.1471-4159.1998.71041777.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K. Mini-Review: Effects of Ethanol on GABAA Receptor-Mediated Neurotransmission in the Cerebellar Cortex--Recent Advances. Cerebellum. 2015;14:438–446. doi: 10.1007/s12311-014-0639-3. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res. 1995;31:165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci U S A. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Ariwodola OJ, Bates WH, Davenport AT, Daunais JB, Grant KA, Friedman DP. The effect of long-term voluntary ethanol consumption on GABAergic and glutamatergic synaptic transmission in the monkey CNS. Alcohol Clinical Experimental Research. 2004;28 [Google Scholar]
- Weiner JL, Ariwodola OJ, Bates WH, Bryant V, Silberman Y, Daunais JB. Presynaptic mechanisms underlying ethanol actions at GABAergic synapses in rat and monkey hippocampus. Alcohol Clinical Experimental Research. 2005;29 [Google Scholar]
- Weiner JL, Dunwiddie TV, Valenzuela CF. Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol Pharmacol. 1999;56:85–90. doi: 10.1124/mol.56.1.85. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ. Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther. 2009;330:198–205. doi: 10.1124/jpet.109.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Porcu P, Boyd KN, O’Buckley TK, Carter JM, Kumar S, Morrow AL. Ethanol-induced GABAA receptor alpha4 subunit plasticity involves phosphorylation and neuroactive steroids. Mol Cell Neurosci. 2016;72:1–8. doi: 10.1016/j.mcn.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Wickman KD, Clapham DE. G-protein regulation of ion channels. Curr Opin Neurobiol. 1995;5:278–285. doi: 10.1016/0959-4388(95)80039-5. [DOI] [PubMed] [Google Scholar]
- Winkler A, Mahal B, Kiianmaa K, Zieglgansberger W, Spanagel R. Effects of chronic alcohol consumption on the expression of different NR1 splice variants in the brain of AA and ANA lines of rats. Brain Res Mol Brain Res. 1999;72:166–175. doi: 10.1016/s0169-328x(99)00218-1. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Fyn kinase does not reduce ethanol inhibition of zinc-insensitive NR2A–containing N-methyl-D-aspartate receptors. Alcohol. 2004;34:101–105. doi: 10.1016/j.alcohol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Wu PH, Coultrap S, Browning MD, Proctor WR. Correlated changes in NMDA receptor phosphorylation, functional activity, and sedation by chronic ethanol consumption. J Neurochem. 2010;115:1112–1122. doi: 10.1111/j.1471-4159.2010.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Smothers CT, Woodward JJ. Cysteine substitution of transmembrane domain amino acids alters the ethanol inhibition of GluN1/GluN2A N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2015;353:91–101. doi: 10.1124/jpet.114.222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2001;296:77–83. [PubMed] [Google Scholar]
- Zhao Y, Ren H, Dwyer DS, Peoples RW. Different sites of alcohol action in the NMDA receptor GluN2A and GluN2B subunits. Neuropharmacology. 2015;97:240–250. doi: 10.1016/j.neuropharm.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yuan Q, Mash DC, Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc Natl Acad Sci U S A. 2011;108:6626–6631. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]