Abstract
Alcoholism is a complex brain disease characterized by three distinct stages of the addiction cycle that manifest as neuroadaptive changes in the brain. One such stage of the addiction cycle is alcohol withdrawal and the negative affective states that promote drinking and maintain addiction. Repeated alcohol use, genetic predisposition to alcoholism and anxiety, and alcohol exposure during crucial developmental periods all contribute to the development of alcohol-induced withdrawal and negative affective symptoms. Epigenetic modifications within the amygdala have provided a molecular basis of these negative affective symptoms, also known as the dark side of addiction. Here, we propose that allostatic change within the epigenome in the amygdala is a prime mechanism of the biological basis of negative affective states resulting from, and contributing to, alcoholism. Acute alcohol exposure produces an anxiolytic response which is associated with the opening of chromatin due to increased histone acetylation, increased CREB binding protein (CBP) levels, and histone deacetylase (HDAC) inhibition. After chronic ethanol exposure, these changes return to baseline along with anxiety-like behaviors. However, during withdrawal, histone acetylation decreases due to increased HDAC activity and decreased CBP levels in the amygdala circuitry leading to the development of anxiety-like behaviors. Additionally, innately higher expression of the HDAC2 isoform leads to a deficit in global and gene-specific histone acetylation in the amygdala that is associated with a decrease in the expression of several synaptic plasticity-associated genes and maintaining heightened anxiety-like behavior and excessive alcohol intake. Adolescent alcohol exposure also leads to higher expression of HDAC2 and a deficit in histone acetylation leading to decreased expression of synaptic plasticity-associated genes and high anxiety and drinking behavior in adulthood. All these studies indicate that the epigenome can undergo allostatic reprogramming in the amygdaloid circuitry during various stages of alcohol exposure. Furthermore, opening the chromatin by inhibiting HDACs using pharmacological or genetic manipulations can lead to the attenuation of anxiety as well as alcohol intake. Chromatin remodeling provides a clear biological basis for the negative affective states seen during alcohol addiction and presents opportunities for novel drug development and treatment options.
Keywords: Alcoholism, Dark side, Addiction, Epigenetics, Gene Expression, Anxiety
1. Introduction
Alcohol use disorders (AUD) effect a significant portion of the population in the US, with a yearly prevalence of 13.9% and a 29.1% lifetime prevalence in recent epidemiological studies (Grant et al., 2015). AUDs are defined according to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-V) as patterns of ingestion and use involving tolerance to the effects of alcohol, craving, relapse from abstinence, and an inability to stop or reduce alcohol consumption patterns (American Psychiatric Association, 2013). Globally, 3.8% of all deaths and 4.6% of disability-adjusted life years, a measure of disease burden, are related to alcohol consumption (Rehm et al., 2009).
AUDs pose an even greater risk due to their well-documented comorbidity with anxiety disorders and other psychiatric diagnoses. Across multiple cultures and populations, patients with diagnosed alcohol abuse and/or dependence are 2–3 times more likely to have an anxiety disorder when compared to the general population (Birrell et al., 2015; Lai et al., 2015; Swendsen et al., 1998). Additionally, the presence of a diagnosed, unremitted anxiety and/or depressive disorder reliably predicts the first episode of alcohol dependence, and this relationship possibly interacts with sub-threshold alcohol use problems (Boschloo et al., 2013b). Personality traits encompassing the domain of negative emotionality are more highly correlated with alcohol dependence when compared to impulsive personality domains, indicating a potentially causative, or more likely cyclical, relationship between anxiety and AUDs (Birrell et al., 2015; Boschloo et al., 2013a). Additionally, age of first alcohol use in adolescents appears to be an important factor in promoting anxiety and AUD co-morbidity later in life. Early age of first alcohol use and later psychopathology are correlated (Grant et al., 2001), and early onset of anxiety disorders predicts first alcohol use in the general population (Birrell et al., 2015).
The social, economic, and individual burden exerted by AUDs and their comorbidity with anxiety highlights the need for increased understanding of the pathophysiology of the negative affective domains of alcohol addiction. Recent advances in the study of alcoholism have identified epigenetic pathways in the brain affected by various modes of alcohol exposure that modify neuronal morphology and are crucial for the development and maintenance of AUDs. Epigenetic processes involve chemical modifications of DNA and histones; particularly histone acetylation, histone methylation and DNA methylation mechanisms that are regulated by both acute and chronic ethanol exposure (Krishnan et al., 2014). In this review, we discuss the current understanding of the development of alcohol addiction and attempt to expand these theories to incorporate epigenomic allostasis into the framework of negative affective states to understand the neurobiology of AUDs.
2. Conceptual framework for the development of alcohol addiction
A heuristic framework encompassing the development of alcohol dependence from both a neurobiological and pathophysiological perspective has been developed in the alcohol addiction field. In particular, George Koob and colleagues have advanced the understanding of alcoholism as a disease involving specific neurocircuitry that are altered by the effects of alcohol exposure and chronic stress in the brain (Koob, 2003, 2008). He synthesized clinical and preclinical work to develop a heuristic framework composed of three distinct phases of the alcohol addiction cycle; 1) binge/intoxication, 2) withdrawal/negative affect, and 3) preoccupation/craving (Koob and Volkow, 2010). Each of these phases involves neuroadaptive changes in brain circuitry which we will briefly describe here (Koob and Volkow, 2016). For example, reward-related brain areas such as the ventral tegmental area (VTA), ventral pallidum, and nucleus accumbens (NAc) are involved in the binge/intoxication stage of acute alcohol use. Physiologically relevant ethanol concentrations directly increase the firing rate of dopaminergic VTA neurons (Brodie et al., 1990). Human imaging studies show striatal dopamine and endogenous opioids are released in the vicinity of the NAc following acute alcohol exposure (Boileau et al., 2003; Mitchell et al., 2012; Yoder et al., 2007).
The extended amygdala, composed of the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and a transitional zone of the NAc shell (Alheid, 2003), integrates brain stress systems to produce negative affective states including anxiety and dysphoria during the withdrawal stages of alcohol addiction (Koob and Volkow, 2016, 2010; Kyzar and Pandey, 2015). Brain stress pathways are activated during the withdrawal stage following repeated alcohol exposure, characterized by increased corticotrophin releasing factor (CRF) in the CeA and other brain regions (Merlo Pich et al., 1995), and injection of a CRF antagonist into the CeA can reduce ethanol self administration and the increased anxiety-like behaviors seen during withdrawal in ethanol-dependent rats (Funk et al., 2006; Koob, 2008). Additionally, brain-derived neurotrophic factor (BDNF) is increased by acute ethanol and subsequently decreased during withdrawal after chronic ethanol exposure in the CeA, and infusion of BDNF into CeA is able to attenuate anxiety-like behaviors in ethanol withdrawn rats (Pandey et al., 2008a). Dynorphin is a κ-opioid agonist that is increased in the CeA and NAc during withdrawal from alcohol and is possibly associated with the increased dysphoria seen in alcohol-withdrawn patients (Koob, 2008).
The third stage of alcohol addiction involves preoccupation with drug taking/intoxication. This stage of the cycle involves neuroadaptation of cortical regions such as the prefrontal and orbitofrontal cortex as well as limbic structures with cortical connections (Koob and Volkow, 2016, 2010). The preoccupation stage of addiction also involves stress systems such as CRF in the extended amygdala circuitry (Shaham et al., 2003), and centrally-administered CRF antagonists can reduce ethanol self-administration after abstinence from chronic ethanol vapor exposure (Valdez et al., 2002).
2.1. Allostatic load and alcohol addiction
"Allostatic load" refers to the total burden of stress placed on an individual (McEwen, 1998; McEwen and Gianaros, 2011). Acute alcohol exposure leads to release of dopamine and opioid peptides in the NAc, as mentioned above, which is associated with the rewarding properties of alcohol as a stimulus. However, with repeated exposure to alcohol, the drug is consumed more often to avoid the negative reinforcing properties of alcohol withdrawal rather than to experience the positive reinforcement of acute alcohol-induced reward. This switch is a consequence of the allostatic load accrued during alcohol abuse, and is a crucial component in the progression from casual alcohol use to alcohol dependence and addiction (Koob, 2003; Koob and Le Moal, 2001). Notably, as an individual progresses from low levels of alcohol use to higher levels of use indicative of abuse and dependence, neuroadaptation occurs in discrete neurocircuitry (Koob and Volkow, 2010; Kyzar and Pandey, 2015). These structural and molecular changes that occur in the brain promote the continued consumption and abuse of alcohol, causing increasingly more severe neuroadaptive consequences. While these changes occur at all stages of the addiction cycle, the withdrawal/negative affective stage, also known as the "dark side" of addiction, has been singled out as a model from which to develop drugs targeting AUDs (Koob and Mason, 2016).
2.2. Utility of preclinical animal models in investigating the dark side of addiction
Preclinical animal models have proven especially useful in investigating the neural substrates of the negative affective states associated with alcohol withdrawal. Principal among these phenotypes is anxiety, which is rapidly induced upon alcohol withdrawal and is a primary motivating factor for relapse and further alcohol consumption (Koob et al., 2014; Pandey, 2003; Zimmermann et al., 2003). As both anxiety-related behavior and alcohol preference/consumption can be reliably measured in animal models, researchers are able to probe the neuroadaptations that occur after alcohol abuse and correlate with the dark side of addiction.
As mentioned above, the negative affective domain of alcohol addiction plays a role in maintaining the vicious cycle of addiction, as anxiety and depression can promote alcohol drinking behavior (Boschloo et al., 2013 a, b) until an individual becomes dependent and ceases alcohol intake. At this juncture, alcohol withdrawal can lead to the development of anxiety and depressive symptoms, thus promoting alcohol intake as means to avoid withdrawal symptoms and improve negative affect. The extended amygdaloid circuitry, particularly the the CeA, is crucial to the behavioral expression of these alcohol-induced affective states (Gilpin et al., 2015). Recent studies in the epigenetic field provide a novel molecular basis for the actions of alcohol, as acute alcohol and withdrawal from chronic ethanol exposure modify chromatin architecture bidirectionally in the amygdala. By either closing or opening the epigenome pharmacologically, alcohol-induced anxiolysis and alcohol withdrawal-induced anxiety-like behavior can be altered (Pandey et al., 2008a, 2015; Sakharkar et al., 2012; Teppen et al., 2015; You et al., 2014). The changes seen in the epigenome serve as a molecular correlate and potential drug development target to the negative affective domain of alcohol addiction (Figure 1).
Figure 1. The epigenetic allostasis model of the dark side of alcohol addiction.
The epigenome is dynamically altered by acute alcohol, chronic alcohol, and alcohol withdrawal in the amygdala and is crucial for the progression from casual use to addiction. Acute alcohol exposure opens the chromatin by inhibiting histone deacetylases (HDACs), thereby increasing histone acetylation around crucial synaptic plasticity-related genes such as brain-derived neurotrohic factor (Bdnf), activity regulated cytoskeleton-associated protein (Arc), and neuropeptide Y (Npy) in the amygdala, leading to anxiolysis. However, these biological parameters normalize (=) with continued alcohol exposure. During ethanol withdrawal, HDAC activity is increased leading to condensed chromatin structure and decreased expression of these genes as well as dendritic spine density. As the amygdala controls negative affective states, amygdalar chromatin conformation is critical for the development of anxiety seen in ethanol-withdrawn animals. Additionally, the condensed chromatin state is associated with greater ethanol preference and self-administration, ostensibly to relieve the negative affective states experienced. This switch from consuming alcohol for its pro-social, anxiolytic effects to drinking as a means to relieve negative affective states is critical to the addiction cycle and is reflected in the underlying epigenome of the amygdala ( Krishnan et al., 2014; Pandey et al., 2008a, b; Sakharkar et al., 2012; You et al., 2014).
Here, we describe the epigenetic, molecular, neuronal, and behavioral adaptations associated with the dark side of alcohol addiction in preclinical models and focus on developing novel treatments to target commonly affected pathways. The models discussed herein generally fall into four categories of alcohol exposure: 1) acute alcohol exposure; 2) repeated alcohol exposure; 3) genetic predisposition to alcohol addiction, and; 4) developmental exposure to alcohol. The primary goal of this review is to develop a model that integrates the observed epigenetic and neuronal adaptations seen in alcohol addiction with the heuristic framework of addiction and allostasis developed previously (Koob, 2003; Koob and Volkow, 2016).
3. Acute alcohol exposure, anxiolysis and the epigenome
3.1. Effects of acute alcohol on anxiety and behavioral responses
Alcohol consumption is widespread worldwide, and many people are able to regularly consume alcohol without developing dependence or abuse. However, acute alcohol causes alterations in cellular and molecular pathways in the brains of both addicted and non-addicted individuals, such as the well-studied ability of acute alcohol to increase dopamine neurotransmission in the brain reward circuitry (Boileau et al., 2003; Brodie et al., 1990), and these neuroadaptations go on to change behavior. For example, alcohol is often consumed for its positive effects with regard to social facilitation in humans (Beck and Treiman, 1996), and this effect is also seen in rodent models of acute alcohol exposure (Varlinskaya and Spear, 2002).
The effect of acute alcohol is not, however, limited to the mesolimbic dopamine pathway. Acute alcohol is a potent anxiolytic in both humans and rodents (Gilman et al., 2008; Moonat et al., 2011; Pandey et al., 2008a; Peterson et al., 1990), implicating limbic structures such as the extended amygdala in the response to acute alcohol intoxication. Given the anxiolytic profile of acute alcohol exposure with regards to both novelty-induced and socially-induced anxiety, it follows that specific cellular and molecular changes occur in the extended amygdala corresponding to these behavioral changes.
3.2. Effects of acute alcohol on amygdalar signaling pathways occur via epigenetic remodeling
Acute alcohol reduces anxiety-like behavior in various animal models (Moonat et al., 2011; Pandey et al., 2008a,b), and also effects numerous signaling cascades that may explain the behavioral responses (Moonat et al., 2010). The cyclic AMP response element binding protein (CREB) signaling pathway is rapidly induced during neuronal depolarization and other types of neuronal activation by the Gα subunit of G-protein-coupled receptors (GPCRs) and calcium signaling (Carlezon et al., 2005). In addition to its anxiolytic effects, acute alcohol activates CREB pathway signaling in the amygdala within one hour of exposure (Pandey et al., 2008a,b). In particular, phosphorylated CREB (pCREB), the active form of the transcription factor, and the histone acetyltransferase CREB-binding protein (CBP) are both increased in the CeA and medial nucleus of the amygdala (MeA) (Pandey et al., 2008a,b).
Notably, the CREB target genes brain-derived neurotrophic factor (Bdnf), activity-regulated cytoskeleton-associated protein (Arc), and neuropeptide Y (Npy) are all increased in the amygdala upon acute alcohol exposure corresponding with anxiolytic behavioral effects (Pandey et al., 2008a,b). The question emerged as to how the cellular signaling effects of alcohol on CREB and other kinases were related to downstream gene activation and the potential involvement of epigenetic mechanisms. CBP and its cofactor p300 are known histone acetyltransferases, covalently adding acetyl groups to histone tails in a manner that decreases interactions between adjacent chromatin complexes, opening the DNA to be read by transcription factors, and thereby facilitating increased transcription (Bannister and Kouzarides, 1996; Krishnan et al., 2014; Ogryzko et al., 1996). Predictably, global acetylation of histones H3 and H4 is increased in the CeA and MeA of rats exposed to acute alcohol. This corresponds with decreased activity of histone deacetylases (HDACs), a group of enzymes that remove these activating acetyl groups from chromatin, in the amygdala of rats (Pandey et al., 2008a; Sakharkar et al., 2012). These initial studies were the first to suggest that epigenetic remodeling is involved in acute alcohol exposure in the amygdala and identified HDACs as a potential hub for chromatin modifications related to anxiety and addiction (Figure 1). In addition, rapid tolerance to the anxiolytic effects of ethanol develops and is associated with molecular tolerance at the level of HDACs and histone acetylation in the amygdala of rats. Treatment with an HDAC inhibitor, trichostatin A (TSA), reverses the rapid tolerance to the anxiolytic effects of ethanol on histone acetylation in the amygdala (Sakharkar et al., 2012).
In addition to DNA and histone modifications, microRNAs (miRNAs) work to regulate both transcription and translation by binding to mRNAs, and in some cases to genomic DNA, and either stabilizing or destabilizing the transcript (He and Hannon, 2004). A recent microarray study identified a novel miRNA, miR-494, as operative in the effect of acute alcohol in the amygdala via its regulation of anxiolysis (Teppen et al., 2016). Acute alcohol reduced miR-494 in the amygdala, which is responsible for increased transcript levels of its target genes Cbp, p300, and Cbp/p300-interacting transactivator 2 (Cited2) and thus increased histone acetylation. Infusion of a specific antagomir to miR-494 directly into the CeA mimics the anxiolytic effect of acute alcohol via induction of Cbp, p300, and Cited2 and the resulting increase in histone acetylation (Teppen et al., 2016). This data points to the existence of miRNA-epigenome regulatory loops in mature neurons, such as those observed during neuronal differentiation (Liu et al., 2013; Sun et al., 2011), that control specific transcriptional states and present opportunities for new epigenetic-based drug development. Amygdaloid miRNA-494 may serve as a molecular target responsible for regulating chromatin remodeling and negative affective states. Future studies are needed to explore these possibilities in an alcohol dependence model.
4. Chronic alcohol exposure-induced withdrawal is anxiogenic and condenses the epigenome
Alcohol addiction involves chronic ingestion of the drug despite the negative consequences encountered. While acute ethanol opens the chromatin in the amygdala, sustained exposure to ethanol causes neuroadaptations that normalize the effects of ethanol on brain circuits and create behavioral tolerance (Kyzar and Pandey, 2015; Sakharkar et al., 2012; Figure 1). A crucial step in the transition from casual alcohol use to alcohol dependence and addiction is the switch from social consumption of alcohol for its rewarding effects to the consumption of alcohol in order to avoid the negative affective states, such as anxiety, that result from withdrawal (Koob, 2003; Koob et al., 2014; Koob and Volkow, 2010). The proposed epigenetic allostatic model fits nicely with the progression of the anxiolytic effects associated with casual alcohol drinking to the development of anxiety-like behaviors related to dependence (Figure 1).
Withdrawal from chronic ethanol treatment leads to a molecular phenotype that is opposite that of acute alcohol in the amygdala; namely, decreased pCREB, CBP, histone H3 and H4 acetylation, and synaptic plasticity-associated gene expression (Bdnf, Arc and Npy) as well as decreased dendritic spines. HDAC activity is also increased in the amygdala during the withdrawal period, while it is inhibited by acute ethanol exposure and normalized by chronic ethanol exposure in rats. The behavioral outcome of this molecular phenotype is markedly increased anxiety, which has been reported in numerous preclinical studies and is a hallmark of clinically significant withdrawal-related negative affect (Baldwin et al., 1991; Funk et al., 2007; Grant et al., 2015; Läck et al., 2007; Valdez et al., 2002). The HDAC inhibitor TSA reverses the anxiety-like behaviors seen in these animals (Pandey et al., 2008a). SAHA, another HDAC inhibitor, and 5-azacitidine (5-aza), a DNA methyltransferase (DNMT) inhibitor, are both effective in limiting excessive binge-like alcohol drinking in rodent models (Warnault et al., 2013). Additionally, NPY, BDNF and Arc protein and mRNA levels, along with tropomyosin receptor kinase B (TrkB; the receptor for BDNF), are decreased in the CeA and MeA during ethanol withdrawal (Pandey et al., 2008a,b; You et al., 2014). BDNF infusion into CeA during ethanol withdrawal attenuated anxiety-like behaviors and normalized BDNF signaling in the CeA of rats (Pandey et al., 2008b). TSA is also able to reverse BDNF, Arc and NPY expression deficits, along with the decreased dendritic spine density seen in the amygdala of alcohol-withdrawn rats (Pandey et al., 2008a; You et al., 2014). The deficits seen in dendritic spine morphology in the amygdala during ethanol withdrawal mimic those seen in neuronal culture models and in other brain regions such as the NAc (Carpenter-Hyland and Chandler, 2006; Spiga et al., 2014a, 2014b). These results suggest that acute and chronic ethanol exposure cause chromatin and synaptic remodeling in the amygdala, which is correlated with the anxiolytic and anxiogenic effects of acute ethanol exposure and withdrawal, respectively (Figure 1).
Though much research discussed above on epigenetics has focused on histone acetylation, histone methylation plays an important role in gene regulation and is crucial in mediating chromatin conformation over multiple temporal scales (Krishnan et al., 2014). Histone methylation can either enhance or repress transcription of the underlying DNA sequence depending on the residue modified. For example, histone H3 lysine 9 dimethylation and trimethylation (H3K9me2/3) and H3K27me3 are considered repressive marks to transcription, while H3K4me2/3 is considered a mark of actively transcribed chromatin (Kouzarides, 2007; Krishnan et al., 2014). Interestingly, genome-wide association studies (GWAS) have identified the genomic region encompassing the histone demethylase KDM4C gene as a hotspot associated with alcohol withdrawal symptoms (Wang et al., 2012). As research into the negative affective states seen during alcohol withdrawal and addiction continues, additional epigenetic modifications such as histone methylation, phosphorylation, and others may emerge as novel biomarkers of alcohol-induced anxiety in brain circuits such as the amygdala (Krishnan et al., 2014; Berkel and Pandey, 2017).
5. Genetic predisposition to alcohol addiction and anxiety
5.1. Clinical overview of AUD genetics
The heritability of AUDs and associated phenotypes is estimated at 50–60% across populations (McGue, 1999; Slutske et al., 1999), indicating that genetic variation plays a significant role in the pathophysiology of these and related disorders. While GWAS have identified specific hotspots that encode risk for AUD such as N-methyl-D-aspartate (NMDA) receptor subunit 2B (GRIN2B) and aldehyde dehydrogenase 2 (ALDH2), most genetic risk for AUD is the result of common variation in hundreds of different genes (Forero et al., 2015; Heath et al., 2011; Kim et al., 2006).
As with other psychiatric diseases with genetic contributions, the behaviors that lead to AUDs occur more often in individuals with greater genetic risk for alcoholism. That is, a high genetic load resets an individual's baseline AUD risk and encourages greater alcohol consumption. This genetic risk involves neuronal pathways that are similar to those involved in the action of acute and chronic alcohol exposure.
5.2. Animal models of genetic risk for AUD display altered epigenomic conformation
In order to study the genetic risk for AUD in laboratory animals, researchers have selectively bred rodent stains to isolate alcohol-preferring animals from non-preferring animals. Interestingly, alcohol-preferring (P) rats display increased alcohol preference but also increased baseline anxiety-like behaviors compared to their non-preferring (NP) control rats (Stewart et al., 1993; Pandey et al., 2005; Moonat et al., 2011). A separate strain of rats also bred for alcohol preference, the Sardinian alcohol-preferring (sP) rats, also displays increased anxiety-like behaviors compared to Sardinian non-preferring control rats (sNP) (Colombo et al., 1995). An acute dose of alcohol is anxiolytic in P rats compared to baseline, but has no effect in NP rats (Moonat et al., 2011, 2013; Stewart et al., 1993). P rats treated with an acute dose of alcohol display levels of anxiety-like behavior that resemble untreated NP control rats, indicating that P rats may consume more alcohol to compensate for an increased susceptibility to anxiety and negative affective states.
Genetic models of alcohol addiction display altered neuronal signaling pathways in limbic circuitry that correspond with their increase in anxiety-like behaviors. For example, sP rats show increased levels of the pro-stress peptide corticotrophin-releasing factor (CRF) in the amygdala while P rats display decreased amygdalar CRF but increased CRF release in response to exogenous CRF administration (Ehlers et al., 1992; Richter et al., 2000). P rats also show decreased levels of the anxiolytic peptide NPY in the amygdala and hippocampus (Ehlers et al., 1998; Zhang et al., 2010). Infusion of NPY directly into the CeA of P rats causes anxiolysis and decreases alcohol intake as well as increased NPY expression likely due to increased pCREB levels (Zhang et al., 2010). In addition to NPY, other CREB target genes including BDNF and Arc show decreased mRNA and protein levels in the CeA and MeA of P rats compared to NP rats (Moonat et al., 2011; Prakash et al., 2008). As these proteins are crucial for the formation and maintenance of synapses, P rats show decreased dendritic spine density in both the CeA and MeA that returns to baseline (NP-like) levels with a single exposure to acute alcohol (Moonat et al., 2011). Levels of BDNF and Arc in the amygdala also return to control-like levels with a single dose of alcohol in P rats (Moonat et al., 2011).
Recent research suggests that global alterations to epigenetic machinery may underlie the transcriptional and circuit-level deficits seen in the amygdala of P rats and their ultimate contribution to increased alcohol consumption and anxiety-like behavior. P rats display higher HDAC activity in the amygdala that is the result of increased baseline expression of HDAC2, but not HDACs 1, 3, 4, 5 or 6, leading to a global decrease in activating H3K9, but not H3K14, acetylation in the CeA and MeA (Moonat et al., 2013; Sakharkar et al., 2014b). Acute alcohol exposure decreases HDAC2 expression in the amygdala of P rats, normalizing global histone acetylation levels, gene specific (Bdnf and Arc) histone acetylation, and produces anxiolytic effects. The HDAC inhibitor TSA is able to normalize the increased anxiety and alcohol drinking seen in P rats, ostensibly by reducing nuclear HDAC activity due to decreased HDAC2 expression in the amygdala and increased H3K9 acetylation in the CeA and MeA (Sakharkar et al., 2014b). TSA treatment also normalizes the deficits in H3K9/14 acetylation at the Npy gene promoter and Npy expression in the amygdala of P rats without producing any effects in NP rats. Interestingly, infusion of an Hdac2-specific siRNA directly into the CeA mimics the effect of acute alcohol by normalizing global and gene-specific histone acetylation, BDNF and Arc protein levels, dendritic spine density, and the increased alcohol preference and anxiety-like behavior seen at baseline in P rats (Moonat et al., 2013). This data suggests that innately higher expression of HDAC2 in the amygdala of alcohol-preferring rats condenses the chromatin around a specific genetic network to regulate synaptic remodeling that is crucial for the interplay between alcohol consumption and anxiety-like behavior. Additionally, the genetic risk for alcoholism may be at least partially encoded by genetic variation that effects the expression and functionality of epigenetic enzymes. The genetic risk for alcoholism increases the likelihood of harmful patterns of alcohol ingestion, and by extension the overall risk for AUD diagnosis (Figure 2). Interestingly, over-expression of HDAC2 in the hippocampus causes decreased LTP, dendritic spines and synapse number whereas HDAC2 knockout is associated with increased LTP, dendritic spines, and synapse number in the hippocampus (Guan et al., 2009). Together, these studies highlight the crucial role of HDAC2 gene in synaptic remodeling and the phenotypes of anxiety, alcoholism and cognition in animal models.
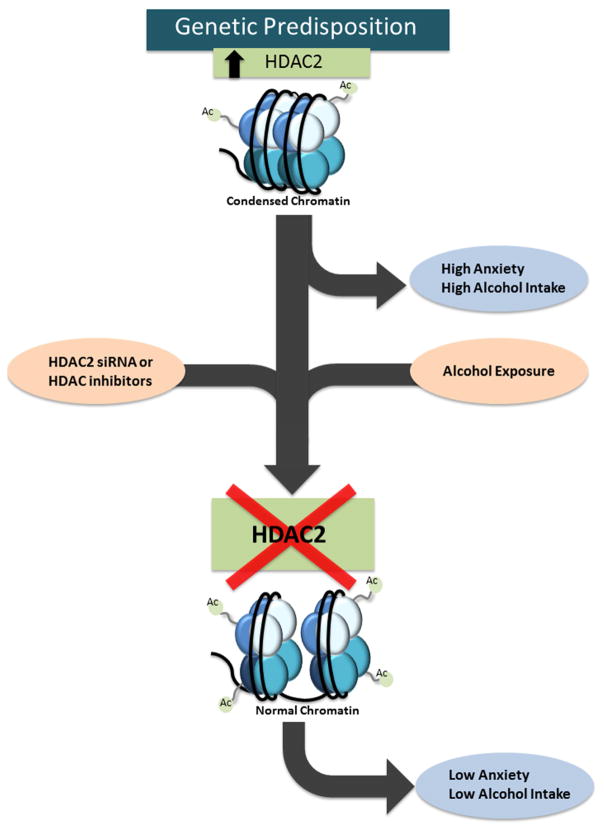
Figure 2. Innately condensed chromatin leads negative affective states and alcoholism.
Animals bred for alcohol preference, such as alcohol-preferring (P) rats, display increased histone deacetylase (HDAC) activity and HDAC2 levels in the amygdala compared to non-preferring (NP) rats, leading to decreased histone acetylation around synaptic plasticity-related genes such as brain-derived neurotrophic factor (Bdnf) and activity regulated cytoskeleton-associated protein (Arc). The resultant decrease in BDNF and Arc expression contributes to decreased amygdaloid synaptic plasticity, increased anxiety-like behaviors, and increased alcohol preference. However, HDAC inhibition by trichostatin A (TSA) or specific inhibition of HDAC2 via small interfering RNA (siRNA) normalizes the histone acetylation deficits in the amgydala of P rats and also attenuates anxiety-like and alcohol drinking behaviors (Moonat et al., 2013; Sakharkar et al., 2014b).
6. Developmental alcohol exposure causes long-lasting alterations to anxiety and drinking behavior
Exposure to alcohol during crucial developmental periods can cause lasting consequences to neurocircuits and behavior. It has long been appreciated that exposure to alcohol in utero leads to fetal alcohol spectrum disorders (FASD), but more recent research has concluded that exposure to alcohol during adolescence also causes long-lasting alterations to brain and behavior (Coriate et al., 2013; Kyzar et al., 2016a). Both FASD and adolescent alcohol exposure cause changes in the expression of epigenetic enzymes and chromatin conformation.
6.1. Epigenetic alterations in FASD
FASD is defined as a group of disorders resulting from in utero alcohol exposure characterized by altered facial appearance, microcephaly, intellectual disability, behavioral issues, and an increased propensity towards risky behaviors including alcohol abuse (Coriale et al., 2013; Sokol et al., 2003). Preclinical studies show that fetal alcohol exposure causes long-lasting effects, particularly in the hypothalamus, that affect an individual's ability to respond to stressful stimuli (Govorko et al., 2012). In particular, fetal alcohol exposure increases repressive histone marks such as H3K9me2 and DNA methylation and decreases activating histone marks such as acetylation and H3K4me2 specifically around the pro-opiomelanocortin (Pomc) gene, which is a crucial player in the stress-responsive hypothalamic-pituitary-adrenal (HPA) axis and alters stress-related behaviors in adulthood (Bekdash et al., 2014; Govorko et al., 2012). Interestingly, these epigenetic effects can be mitigated by choline supplementation during the fetal period (Bekdash et al., 2013). The brain-specific changes in Pomc expression and promoter modifications, as well as behavioral changes seen in animals exposed to fetal alcohol, are passed along through the male germline transgenerationally, indicating that epigenetic alterations may contribute to the inherited risk for stress-related alcohol addiction (Govorko et al., 2012; Sarkar, 2016).
As mentioned above, the hypothalamus is crucial for the response to stress, and many neurobehavioral studies of FASD have focused on this brain area. Importantly, the hypothalamus and the amygdala, as well as other crucial limbic regions, are highly interconnected and function in tandem to modulate the stress response (Buijs and Van Eden, 2000; Ulrich-Lai and Herman, 2009). Future studies into the epigenetic effects of FASD should also investigate the involvement of the extended amygdala in individuals exposed to fetal alcohol and also in the potential transgenerational epigenetic effects of fetal alcohol exposure.
6.2. Adolescent ethanol exposure increases risk for alcoholism and anxiety in adulthood and alters the amygdalar epigenome
Adolescence is a critical period for brain development (Keshavan et al., 2014; Kyzar et al., 2016a; Crews et al., 2016). While most organs do not undergo noteworthy changes during the lifespan with regards to structure and function, the brain goes through important changes during early life and adolescence. Specifically, adolescence sees a decrease in the amount of grey matter due to synaptic pruning and an increase in white matter due to ongoing myelination (Keshavan et al., 2014). Additionally, evolutionarily older structures such as the limbic system mature at a more rapid rate that the phylogenetically younger executive control structures in the prefrontal cortex, leading to behavioral manifestations including increased risk-taking and abuse of alcohol and other drugs (De Bellis et al., 2005; Crews et al., 2016; Keshavan et al., 2014). These individual scale changes manifest on a sociocultural scale in the high prevalence of binge drinking, where alcohol consumption raises blood ethanol content above 80 mg/dL during a single drinking session, during adolescence and young adulthood (Lopez-Caneda et al., 2014; Miller et al., 2007; Patrick et al., 2013).
Epidemiological studies show that binge drinking during adolescence increases the risk for alcohol problems and AUD diagnosis during adulthood. In particular, individuals first exposed to alcohol during ages 11–14 have a 10–15 fold increased risk of developing alcohol dependence in adulthood compared to individuals who had their first experience with alcohol at age 19 or later (DeWit et al., 2000; Grant et al., 2001). This increased environmental risk has led researchers to use animal models to investigate the neuroadaptations occurring in the brain of adolescents exposed to alcohol that may persist until adulthood and exert lasting effects on behavior (Kyzar et al., 2016a). Importantly, numerous independent studies show that exposure to alcohol during adolescence increases the preference for alcohol in adulthood compared to non-ethanol exposed animals, thus recapitulating the clinical phenotype observed in epidemiological studies (Alaux-Cantin et al., 2013; Broadwater and Spear, 2013; Gilpin et al., 2012; Pandey et al., 2015; Sakharkar et al., 2016). Additionally, adults exposed to repeated and/or intermittent binge-like concentrations of ethanol in adolescence respond less aversively and more positively to ethanol exposure in adulthood (Kyzar et al., 2016a; Spear and Swartzwelder, 2014). Finally, exposure to the same schedule of alcohol later in life does not appear to cause the persistent effects seen after adolescent exposure, indicating that a critical period of neurodevelopment exists with regard to the effects of alcohol on the brain (Spear and Swartzwelder, 2014; Vetreno et al., 2014).
The behavioral alterations seen in adult rodents exposed to adolescent alcohol are indicative of long-lasting adaptations in neural circuitry responsible for the regulation of negative affective states. Other groups have investigated the involvement of epigenetic mechanisms in executive regions such as the prefrontal cortex in adulthood after adolescent alcohol, finding for instance that DNA methylation and neuroimmune mechanisms are likely involved (Barbier et al., 2015; Crews et a., 2016; Montesinos et al., 2016). However, for the sake of this review we focus on the long-lasting changes seen in limbic circuitry such as the amygdala.
Acute alcohol exposure in adolescent rats produces anxiolytic effects and inhibits HDAC activity in the amygdala. However, adolescent rats require a higher dose of ethanol as compared to adult rats to produce anxiolytic effects and inhibition of HDAC activity in the amygdala (Sakharkar et al., 2012; 2014b). Adolescent intermittent ethanol (AIE) exposure (8 total intraperitoneal (i.p.) injections of 2 g/kg alcohol on a 2 days on/2 days off schedule during postnatal days (PND) 28–41) leads to increased anxiety-like behaviors and alcohol preference in adulthood along with increased HDAC2 in the CeA and MeA (Pandey et al., 2015). This is accompanied by decreased histone acetylation both globally and at the promoters of Bdnf and Arc, leading to decreased BDNF and Arc expression and a decrease in dendritic spine density. The increased anxiety-like behaviors and increased alcohol preference as well as deficits in histone acetylation (H3K9/14 acetylation) at the Bdnf and Arc promoters in the amygdala seen in AIE adults are abolished during treatment with the HDAC inhibitor TSA (Pandey et al., 2015). It is interesting to note that AIE-induced increases in HDAC2 and deficits in histone acetylation (H3K9) and anxiety-like behaviors during the 24 hours after initial withdrawal after last AIE persistent into adulthood, suggesting that AIE produces enduring effects on HDAC2-induced chromatin remodeling in the amygdala and anxiety-like behaviors in rats (Pandey et al., 2015).
TSA treatment also reverses the decreased histone acetylation seen in the CA1, CA2, and CA3 regions of the hippocampus after AIE that is likely a result of increased HDAC activity in the hippocampus (Sakharkar et al., 2016). In addition to histone acetylation, AIE causes a long-lasting decrease in lysine-specific demethylase 1 (LSD1), and in particular the neuron-specific splice variant termed LSD1+8a for its inclusion of mini-exon 8a into the full-length transcript (Kyzar et al., 2016b; Zibetti et al., 2010). Additionally, repressive H3K9me2 is increased in the CeA and MeA of adult AIE rats compared to control rats, with no change observed in the activating H3K4me2 mark. Interestingly, acute alcohol exposure in adulthood is anxiolytic in saline-exposed control (AIS) rats, but normalizes the increased anxiety-like behavior in AIE rats while also normalizing amygdala Lsd1+8a mRNA expression and H3K9me2 occupancy at the Bdnf exon IV promoter (Kyzar et al., 2016b). This resembles the normalization of anxiety-like behavior in P rats after a single dose of acute alcohol (Moonat et al., 2011, 2013), possibly indicating shared pathophysiology via common epigenetic mechanisms. These results further implicate condensed chromatin confirmation due to increased HDAC2 and decreased LSD1+8a in the amygdala in the expression of alcohol- and anxiety-related behaviors, and also demonstrate the potential efficacy of epigenetic drugs such as HDAC inhibitors in the treatment of alcoholism and comorbid anxiety (Figure 3).
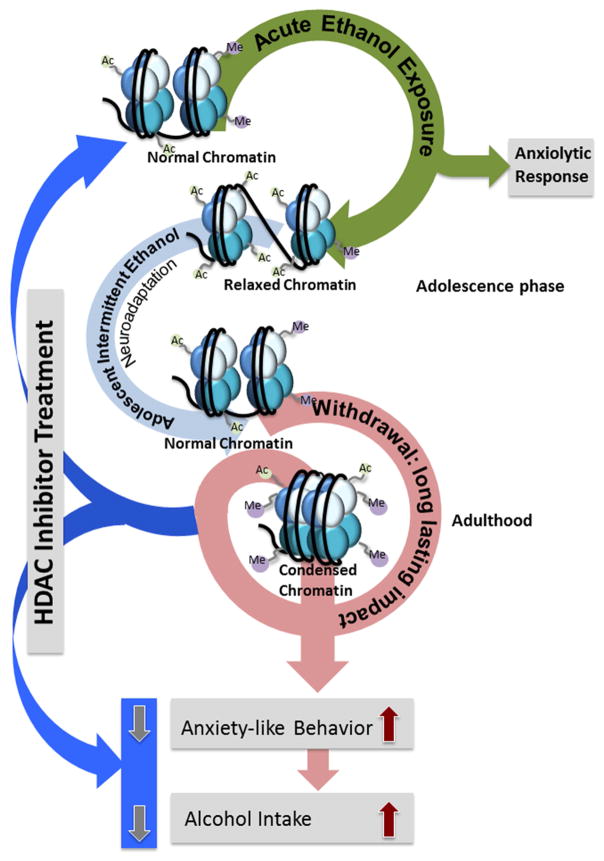
Figure 3. Alcohol exposure during development leads to persistent changes in epigenetic mechanisms in amygdala and produces long-lasting anxiety and excessive alcohol intake in adulthood.
Exposure to adolescent intermittent ethanol (AIE) during this critical developmental period causes increased HDAC2 in the amygdala leading to decreased global and Bdnf- and Arc-specific histone acetylation. Decreased BDNF and Arc expression in the adult amygdala of AIE animals is accompanied by increased alcohol preference and anxiety-like behaviors. Aside from histone acetylation, lysine demethylase 1 (LSD1) and particularly the neuron-specific splice variant Lsd1+8a, are decreased in the AIE adult amygdala. This decrease is accompanied by a resultant increase in histone H3K9 dimethylation (H3K9me2) both globally and at the Bdnf exon IV promoter, possibly explaining the decreased BDNF expression seen in these rats. Thus, increased HDAC2 and decreased LSD1 associated with AIE-induced chromatin remodeling may provide novel targets for intervention in alcohol addiction and comorbid anxiety (Kyzar et al., 2016a, b; Pandey et al., 2015; Sakharkar et al., 2014a).
7. Conclusion
In this review, we consolidate recent findings regarding epigenetics and chromatin conformation in brain structures responsible for the regulation of negative affective states, such as the amygdala, into the existing heuristic framework of the allostatic model of alcohol addiction. Acute alcohol exposure is anxiolytic and inhibits HDAC activity, increases histone acetylation, and increases expression of genes such as Bdnf, Arc, and Npy, leading to an increase in dendritic spine density in the amygdala. However, with repeated ethanol exposure these parameters adapt and return to baseline levels until ethanol is no longer administered. During withdrawal, the chromatin condenses partly as a result of increased HDAC activity and decreased histone acetylation, leading to decreased expression of same genes and dendritic spine density in the amygdala and development of anxiety-like behaviors (Pandey et al., 2008a; You et al., 2014). Animals bred for their alcohol preference (e.g., P and sP rats) show increased baseline anxiety-like behaviors correlating with a condensed chromatin structure due to higher HDAC2 expression and decreased dendritic spines and synaptic plasticity-associated gene expression in the amygdala (Figure 2). Notably, HDAC inhibitors and HDAC2 siRNA are able to reverse the molecular and circuit-level deficits in P rats leading to normalization of anxiety-like behavior and attenuation of excessive alcohol intake (Moonat et al., 2013; Sakharkar et al., 2014). Lastly, exposure to alcohol during fetal development and adolescence causes alterations to the epigenetic machinery in brain areas involved in controlling negative affective states. In particular, adolescent alcohol exposure increases HDAC2 levels and decreases LSD1+8a levels in the amygdala at adulthood leading to decreased histone acetylation and increased H3K9me2 correlated with decreased dendritic branching, increased alcohol preference and anxiety-like behaviors that, again, are corrected with HDAC inhibitor treatment (Figure 3).
These studies point to a novel molecular model that integrates anxiety-like behavior, alcohol consumption, and limbic function. During alcohol-induced anxiolysis, the chromatin in the amygdala is open, allowing for the expression of neurotrophins and other genes involved in synaptic function. However, during withdrawal (Figure 1), or alternatively with the introduction of genetic or environmental risk factors for alcohol abuse (Figures 2 & 3), the chromatin in the amygdala is condensed around genes including Bdnf and Arc leading to a decrease in the number of dendritic spines and increase anxiogenesis.
Future directions regarding the epigenetic modulation of alcohol consumption, anxiety, and other affective states will be greatly influenced by novel genomic technologies. For example, recent studies used epigenetic enzymes fused to zinc finger proteins to specifically modify the chromatin at distinct genetic loci involved in drug addiction (Heller et al., 2016, 2014; Kyzar and Banerjee, 2016). The ability to experimentally modify specific segments of chromatin will allow researchers to associate causality with epigenetic modifications with regard to behavioral effects, thereby opening novel pathways for drug delivery and development. Finally, further epigenetic research into the interplay between alcoholism and other psychiatric diagnoses will bring clarity to the neuroscience field and allow scientists to better understand the molecular and cellular functions of the amygdala and other complex brain regions.
Highlights.
This review article develops an allostatic model of the epigenome in the amygdala during alcoholism that may be associated with the dark side of addiction.
Neuroadaptive changes due to alcohol exposure develop via epigenetic modifications.
Innately higher expression of HDAC2 may be involved in condensed chomatin architecture that is operative in anxiety and alcohol intake.
Alcohol-induced epigenetic reprogramming during adolescence produces enduring effects on the epigenome in the amygdala and on anxiety and alcohol intake.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants RO1AA-010005, RO1AA-021662, P50AA-022538 (Center for Alcohol Research in Epigenetics), UO1AA-019971, U24AA-024605 (Neurobiology of Adolescent Drinking in Adulthood project), and by the Department of Veterans Affairs (I01BX000143, Merit Review Grant; Senior Research Career Scientist award) to SCP and a fellowship F30 AA024948 grant to EJK. Authors would like to thank Luiza Kulikowska for editing and helping in the preparation of figures.
Footnotes
Conflict of interest:
SCP reports that a US patent application entitled “Histone acetyltransferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th , 2006) is currently pending. EJ and HZ reported no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), Diagnostic and Statistical Manual of Mental Disorders 4th edition TR. 2013. [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bannister A, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, Sun H, Schuebel K, Zhou Z, Yuan Q, Vendruscolo LF, Goldman D, Heilig M. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. J Neurosci. 2015;35:6153–6164. doi: 10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KH, Treiman KA. The relationship of social context of drinking, perceived social norms, and parental influence to various drinking patterns of adolescents. Addict Behav. 1996;21:633–644. doi: 10.1016/0306-4603(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcohol Clin Exp Res. 2013;37:1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash R, Zhang C, Sarkar D. Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress. Alcohol Clin Exp Res. 2014;38:2323–2330. doi: 10.1111/acer.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel TDM, Pandey SC. Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13338. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Beekman aTF, Penninx BWJH. The role of negative emotionality and impulsivity in depressive/anxiety disorders and alcohol dependence. Psychol Med. 2013a;43:1241–1253. doi: 10.1017/S0033291712002152. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Van Den Brink W, Smit JH, Veltman DJ, Beekman ATF, Penninx BWJH. Depressive and anxiety disorders predicting first incidence of alcohol use disorders: Results of the netherlands study of depression and anxiety (nesda) J Clin Psychiatry. 2013b;74:1233–1240. doi: 10.4088/JCP.12m08159. [DOI] [PubMed] [Google Scholar]
- Birrell L, Newton NC, Teesson M, Tonks Z, Slade T. Anxiety disorders and first alcohol use in the general population. findings from a nationally representative sample. J Anxiety Dis. 2015;31:108–113. doi: 10.1016/j.janxdis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner Sa, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Pro Brain Res. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Coriale G, Fiorentino D, Di Lauro F, Marchitelli R, Scalese B, Fiore M, Maviglia M, Ceccanti M. Fetal Alcohol Spectrum Disorder (FASD): Neurobehavioral profile, indications for diagnosis and treatment. Riv Psichiatr. 2013;48:359–369. doi: 10.1708/1356.15062. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev. 2016;68:1074–1109. doi: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal Cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, Nemeroff CB. Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathé aa. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- Forero DA, López-León S, Shin HD, Park BL, Kim DJ. Meta-analysis of six genes (BDNF, DRD1, DRD3, DRD4, GRIN2B and MAOA) involved in neuroplasticity and the risk for alcohol dependence. Drug Alcohol Depend. 2015;149:259–263. doi: 10.1016/j.drugalcdep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani Va, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012:7. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harfold TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: 1 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou YL, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PAF, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: Findings and implications. Biol Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Peña CJ, Neve RL, Nestler EJ. Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J Neurosci. 2016;36:4690–4697. doi: 10.1523/JNEUROSCI.0013-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller Ea, Cates HM, Peña CJ, Sun H, Shao N, Feng J, Golden Sa, Herman JP, Walsh JJ, Mazei-Robison M, Ferguson D, Knight S, Gerber Ma, Nievera C, Han MH, Russo SJ, Tamminga CS, Neve RL, Shen L, Zhang HS, Zhang F, Nestler EJ. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat Neurosci. 2014;17:1720–1727. doi: 10.1038/nn.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JYF, Lewis Da, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park M, Yang SY, Jeong BS, Yoo HJ, Kim JW, Chung JH, Kim SA. Association study of polymorphisms in N-methyl-d-aspartate receptor 2B subunits (GRIN2B) gene with Korean alcoholism. Neurosci Res. 2006;56:220–223. doi: 10.1016/j.neures.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TDM, Pandey SC. The epigenetic landscape of alcoholism. Int Rev Neurobiol. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Banerjee R. Targeted epigenetic modulation of gene expression in the brain. J Neurosci. 2016;36:9283–9285. doi: 10.1523/JNEUROSCI.1990-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Floreani C, Teppen TL, Pandey SC. Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front Neurosci. 2016a;10:222. doi: 10.3389/fnins.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Pandey SC. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett. 2015;601:11–19. doi: 10.1016/j.neulet.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC. Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol. 2016b May 15; doi: 10.1111/adb.12404. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HMX, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013;27:1473–1483. doi: 10.1101/gad.209619.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Corral M, Doallo S, Cadaveira F. Evolution of the binge drinking pattern in college students: Neurophysiological correlates. Alcohol. 2014;48:407–418. doi: 10.1016/j.alcohol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Curr Dir Psychol Sci. 1999;8:109–115. [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodríguez-Arias M, Miñarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2016;53:159–171. doi: 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: A common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008a;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008b;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP responsive-element binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2015;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 2013;167:1019–1025. doi: 10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. J Stud Alcohol. 1990;51:114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Richter RM, Zorrilla EP, Basso aM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–1772. [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014a;17:2057–2067. doi: 10.1017/S1461145714001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC. A role for histone acetylation mechanisms in adolescent alcohol exposureinduced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 2016;221:4691–4703. doi: 10.1007/s00429-016-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014b;17:1207–1220. doi: 10.1017/S1461145714000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone Deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK. Male germline transmits fetal alcohol epigenetic marks for multiple generations: A review. Addict Biol. 2016;21:23–34. doi: 10.1111/adb.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Heath aC, Bucholz KK, Eisen Sa, Goldberg J, Lyons MJ, Tsuang MT. The heritability of alcoholism symptoms: “indicators of genetic and environmental influence in alcohol-dependent individuals” revisited. Alcohol Clin Exp Res. 1999;23:759–769. doi: 10.1111/j.1530-0277.1999.tb04181.x. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: A mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Mulas G, Piras F, Diana M. The “addicted” spine. Front Neuroanat. 2014a;8:110. doi: 10.3389/fnana.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Masala N, Cannizzaro C, Biggio G, Sanna E, Diana M. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc Natl Acad Sci U S A. 2014b;111:E3745–3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Sun G, Ye P, Murai K, Lang MF, Li S, Zhang H, Li W, Fu C, Yin J, Wang A, Ma X, Shi Y. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry. 1998;39:176–184. doi: 10.1016/s0010-440x(98)90058-x. [DOI] [PubMed] [Google Scholar]
- Teppen TL, Krishnan HR, Zhang H, Sakharkar AJ, Pandey SC. The potential role of amygdaloid microRNA-494 in alcohol-induced anxiolysis. Biol Psychiatry. 2016;80:711–719. doi: 10.1016/j.biopsych.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Liu X, Zhang Q, Wu LY, Zeng M. Genome-wide association study identifies 5q21 and 9p24.1 (KDM4C) loci associated with alcohol withdrawal symptoms. J Neural Transm. 2012;119:425–433. doi: 10.1007/s00702-011-0729-z. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine a, Barak S, Ron D. Chromatin remodeling--a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC. Neuropeptide y Signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res. 2010;34:451–461. doi: 10.1111/j.1530-0277.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibetti C, Adamo A, Binda C, Forneris F, Toffolo E, Verpelli C, Ginelli E, Mattevi A, Sala C, Battaglioli E. Alternative splicing of the histone demethylase LSD1/KDM1 contributes to the modulation of neurite morphogenesis in the mammalian nervous system. J Neurosci. 2010;30:2521–2532. doi: 10.1523/JNEUROSCI.5500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Wittchen HU, Höfler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychol Med. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]