Abstract
Prohibitin (PHB) plays a role in regulation of ultraviolet B light (UVB)-induced apoptosis of human keratinocytes, HaCaT cells. The regulatory function of PHB appears to be associated with its lipid raft translocation. However, the detailed mechanism for PHB-mediated apoptosis of these keratinocytes upon UVB irradiation is not clear. In this report, we determined the role of lipid raft translocation of PHB in regulation of UVB-induced apoptosis. Our data show that upon UVB irradiation PHB is translocated from the non-raft membrane to the lipid rafts, which is correlated with a release of both Akt and Raf from membrane. Overexpression of Akt and/or Raf impedes UVB-induced lipid raft translocation of PHB. Immunoprecipitation analysis indicates that UVB alters the interactions among PHB, Akt, and Raf. Reduced expression of PHB leads to a decreased phosphorylation of Akt and ERK, as well as a decreased activity of Akt, and increased apoptosis of the cells upon UVB irradiation. These results suggest that PHB regulates UVB-induced apoptosis of keratinocytes via a mechanism that involves detachment from Akt and Raf on the plasma membrane, and sequential lipid raft translocation.
Keywords: Ultraviolet B light, apoptosis, prohibitin, lipid raft
Introduction
Lipid rafts play a role in regulation of UVB-induced apoptosis of skin cells [1–3]. By changing major components upon stimuli, lipid rafts regulate apoptotic signaling pathways by recruiting as well as inducing aggregation and activation of lipid raft-associated proteins, including but not limited to membrane receptors (such as Fas, CD5, and CD20), kinases (such as Akt, JNK, Src, and PKC), and calcium channels [4–11]. Our recent report indicated that PHB translocates onto lipid rafts upon UVB irradiation [3]. This report also indicated that reduced expression of PHB enhanced UVB-induced apoptosis [3]. However, the detailed mechanism of lipid raft translocation of PHB in regulation of UVB-induced apoptosis remains unknown.
Recent studies have found that PHB is essential in the activation of both PI3K/Akt and Raf/ERK signaling cascades [12–20]. PHB interacts with and is phosphorylated at threonine 258 by Akt [21], which was suggested to be critical for the activation of the Akt signaling cascade [15]. PHB regulates Raf activity via two mechanisms: (1) PHB interacts with Raf, which promotes Raf membrane localization and activation [12,16,22]; and (2) PHB may compete with Raf in binding and being phosphorylated by Akt [23]. Both PI3K/Akt and Raf/ERK signaling cascades are main cell survival signaling pathways mediating UVB-induced apoptosis in skin cells [24–29]. However, it is unknown if lipid raft translocation of PHB in regulation of UVB-induced apoptosis is mediated by PI3K/Akt and Raf/ERK signaling cascades. In this report, we reveal a novel mechanism demonstrating that lipid raft-translocation of PHB mediates UVB-induced apoptosis via alternating interactions and localization of PHB, Akt and Raf.
Materials and Methods
Cell culture
The HaCaT cells (human keratinocyte cell line, kindly provided by Dr. Nihal Ahamad, University of Wisconsin, Madison, WI) and H1299 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Mediatech, Manassas, VA) supplemented with 1% penicillin-streptomycin (Mediatech, Manassas, VA) and 10% fetal bovine serum (FBS, Denville Scientific, Metuchen, NJ). The cells were cultured at 37 °C with 5% CO2.
UVB irradiation
UVB light (290–320 nm) was generated from 15 W UVB tubes (UVP, Upland, CA). The intensity of UVB light was measured using a UVX digital radiometer (UVP, Upland, CA). The cells were irradiated with 50 mJ/cm2 or 5 mJ/cm2 of UVB light. The culture medium was replaced with PBS during exposure and was added back to the culture plates after UVB treatment.
Isolation of lipid raft
Lipid rafts fractions were isolated using Optiprep™ (Sigma, St. Louis, MO) density gradient (40% – 5%) as previously reported [3]. Briefly, the cells were collected, cross-linked with DTSSP, pelleted, and frozen at −80 °C overnight. Then the cells were lysed with cold 0.1% Triton X-100 membrane raft isolation buffer (50 mM Tris-HCl, PH 7.4, 150 mM NaCl, 5 mM EDTA and 0.1% Triton X-100, and the protease inhibitor cocktail). Equal volume of the lysates were applied to a discontinuous gradient (40% – 5%) and centrifuged using a SW60 rotor (Beckman Coulter, Brea, CA) at 31,300 rpm for 5 h at 4 °C. Five 0.8 mL fractions were collected from each gradient for future western blotting analysis.
Isolation of plasma membrane proteins
Plasma membrane extracts were prepared using Mem-PER™ plus membrane protein extraction kit (Pierce, Rockford, IL). Plasma membrane and cytosolic proteins were extracted according to the manufacturer’s protocol. Na+/K+ - ATPase and GAPDH were used as markers of plasma membrane and cytosolic proteins, respectively.
Lysis of cells
Cells were collected, pelleted, and lysed by Nonidet P-40 (NP-40) lysis buffer (2% NP-40, 80 mM NaCl, 100 mM Tris-HCl, 0.1% SDS) containing protease inhibitor cocktail (Calbiochem, La Jolla, CA). The cell lysate was incubated on ice for 15 min and centrifuged at 11,000 rpm for 10 min at 4 °C. The supernatant was collected and protein concentration was determined by Bio-Rad Dc Protein Assay kit (Bio-Rad, Hercules, CA).
Western blotting analysis
The proteins were boiled in 5-fold Laemmli buffer (0.25 M Tris–HCl, pH 6.8, 0.5 M DTT, 10% SDS, 50% glycerol and 0.03% bromophenol blue), separated on SDS-PAGE, and transferred to a nitrocellulose membrane. The membranes were blocked in 5% milk (w/v) in TBST buffer for 30 min at room temperature and then incubated with primary antibodies against PHB (sc-28259), Akt-1 (sc-55523), ERK (sc-135900), caspase-3 (sc-7148), Na+/K+ - ATPase (sc-21712), GAPDH (sc-25778) and Caveolin-1 (sc-894) (Santa Cruz Biotechnology, Santa Cruz, CA), p-Akt (9271s), and p-ERK (4377) (Cell Signaling, Danvers, MA), and β–actin (A5316) (Sigma, St. Louis, MO) at 4 °C overnight. After washing with TBST, the membrane was incubated with corresponding secondary horseradish peroxidase (HRP)-conjugated antibodies for 30 min at room temperature. The signals were detected using Supersignal West Pico chemilluminescent substrate (Pierce, Rockford, IL).
Immunoprecipitation
The cell lysate (150 μL) was incubated with 5 μL primary antibody against PHB (sc-28259), Raf-1 (sc-133), Akt-1 (sc-55523), p-Thr (sc-5267), p-Ser (sc-81514), or p-Tyr (sc-7020) (Santa Cruz Biotechnology, Santa Cruz, CA) with gentle rocking overnight at 4 °C. The protein A/G agarose beads (Pierce, Rockford, IL) were added followed by another 30 min gentle rocking at 4 °C. The cell lysate-beads mixture was centrifuged for 30 sec at 1,000 rpm in a benchtop centrifuge (Eppendorf, Hamburg, Germany) and washed three times with 700 μL 1X TBST at 4°C. The lysate-beads mixture was boiled in 5-fold Laemmli buffer, and then separated on SDS-PAGE. The samples were analyzed by western blotting.
Akt kinase activity
The cell lysates were prepared using Akt kinase assay kit (9840) (Cell Signaling, Danvers, MA). Akt kinase assay was performed according to the manufacturer’s protocol.
Flow cytometry
Cells were collected and pelleted by centrifugation, washed twice with PBS, fixed with 4% formaldehyde for 10 min and cooled on ice for 1 min. Cells were then blocked in PBS containing 1% BSA for 30 min. Cells were incubated with antibodies against PHB (ab75766) (Abcam, Cambridge, MA) or the isotype control anti-rabbit IgG, for 1 h at room temperature, washed twice with PBS, and incubated with FITC-conjugated secondary antibodies for 1 h. Cells were washed, and resuspended in PBS for cell surface PHB expression analysis on a FACSort analyzer (BD Biosciences, Franklin Lakes, NJ).
Immunofluorescence microscopy
Cells were incubated with 1 μg/mL FITC-conjugated cholera toxin β-subunit (Sigma, St. Louis, MO) for 15 min at 4 °C, and washed with PBS. Cells were fixed with 4% formaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 30 min, and then blocked in PBS containing 1% BSA for 30 min. Cells were incubated with antibody against PHB (ab75766) (Abcam, Cambridge, MA), Raf-1 (sc-133), and Akt-1 (sc-55523) (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature, washed twice with PBS, and then incubated with DyLight 549 secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h. Cells were washed with PBS, mounted on slides with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and analyzed by fluorescence microscopy (Nikon ECLIPS E600, Melville, NY).
PHB silencing using the RNA interference method
Cells were seeded in a 6-well tissue culture plate in antibiotic-free medium until 60–80% confluent. The medium was then replaced with 0.5 mL transfection mixture, containing either scrambled siRNA (60 nM, sc-37007) or PHB siRNA (60 nM, sc-37629), siRNA Transfection Medium and siRNA Transfection Reagent (Santa Cruz Biotechnology, Santa Cruz, CA). The cells were first incubated at 37 °C with 5% CO2 for 6 h and then incubated in 1.0 mL antibiotic-free DMEM with 20% FBS overnight. The cells were incubated for another 18–24 h with DMEM with 10% FBS before harvesting.
Generation of stable PHB shRNA cell line
HaCaT cells were transfected with pLKO.1-Neo-CMV-tGFP vectors containing PHB shRNA or scrambled control shRNA (Sigma, St. Louis, MO) using Lipofectamine LTX and PLUS following the manufacture’s protocol (Invitrogen, Carlsbad, CA). The transfected cells were maintained for 2 weeks in DMEM with G418. Stable cell lines were generated by isolating single cell-derived colonies. The PHB expression in each cell line was determined by western blot.
Construction of Akt and Raf expression vectors and transient transfection
Total RNAs from HaCaT cells were extracted using RNeasy Mini kit (Qiagen, Germantown, MD) and reverse-transcribed into first-strand DNA using oligo(dT)20 (Invitrogen, Carlsbad, CA). The full-length human Akt and Raf cDNA was prepared by PCR using the following primers:
Akt primer F: 5’-ATGCGGATCCCCACCATGAGCGACGTGGCTATTGTGAAG-3’;
Akt primer R: 5’-ATGCTCTAGATCAGGCCGTGCTGCTGGC-3’;
Raf primer F: 5’-ATGCGGATCCCCACCATGGAGCACATACAGGGAGCTT-3’;
Raf primer R: 5’-ATGCTCTAGACTAGAAGACAGGCAGCCTCG-3’).
The amplified Akt or Raf cDNA fragments were ligated into the BamH1-Xba1 sites of pcDNA6/V5-His (Invitrogen, Carlsbad, CA). Raf and Akt overexpression plasmids were purified using MidiPrep (Qiagen, Germantown, MD), followed by gene sequencing at Genomics Facility (Ohio University, Athens, OH). The expression vector(s) were transiently transfected into H1299 cells using Lipofectamine 2000 following the manufacture’s protocol (Invitrogen, Carlsbad, CA).
Clonogenic assay
Cells (6×103) were seeded in a 6-well tissue culture plate and incubated at 37 °C with 5% CO2 for 6 d. The cells were then washed with PBS twice and fixed in cold methanol for 10 min at −20 °C. The fixed cells were stained by 1% crystal violet in 25% methanol for another 10 min, and then washed with ddH2O. The colonies with a size more than 0.5 mm were counted.
Results
Lipid raft-association of PHB is correlated with translocation of Akt and Raf upon UVB irradiation
Since PHB serves as a scaffold in regulating Akt and Raf signaling [23], we examined whether lipid raft-translocation of PHB is correlated with a subcellular redistribution of Akt and Raf at 6 h post-UVB, the time that PHB took to translocate to lipid raft as we previously reported [3]. Caveolin-1, a lipid rafts-associated protein, was used as a marker. Our data indicated that the translocation of PHB into fraction 2 (lipid raft fraction) was accompanied by Akt and Raf translocation out of fraction 3 (non-rafts membrane fraction) in HaCaT cells at 6 h post-UVB (50 mJ/cm2) (Figures 1a). Further analysis using immunofluorescence staining showed that while PHB distributed homogenously in the unirradiated cells, it was accumulated and co-localized with cholera toxin B subunit (CTB) stained monosialoganglioside1 (GM1), a marker of lipid raft, in the HaCaT cells at 6 h post-UVB (50 mJ/cm2) (Figure 1b). Our data also showed that the increased association of PHB in lipid rafts was due to translocation but not induced expression of PHB since total PHB protein level remained almost the same within 24 h after UVB irradiation (Figure S1a), and the cell surface PHB protein level remained almost unchanged 6 h after UVB irradiation (Figure S1b).
Figure 1.
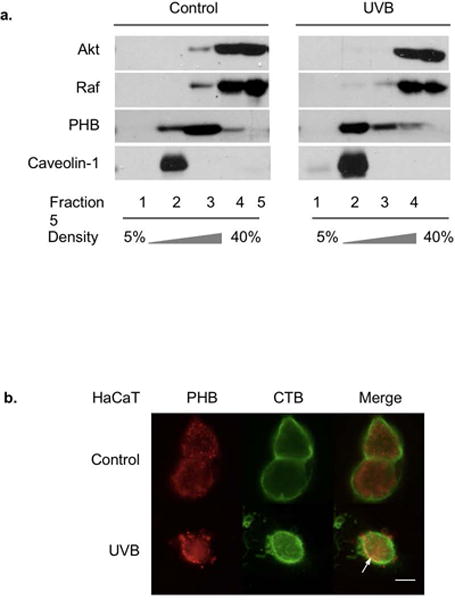
PHB translocation and expression after UVB irradiation. HaCaT cells were irradiated with UVB (50 mJ/cm2) and harvested at 6 h (a and b). (a) PHB, Akt, Raf, and Caveolin-1 in each fraction of the gradient were assessed by western blotting analysis. 30 mL of fractions (1, 3–5), and 15 mL of fractions (2) were subjected to the analysis. The error bars represent S.D. of three independent experiments. *: p<0.05 versus corresponding fraction 3 in control group. **: p<0.05 versus corresponding fraction 2 in control group. (b) The cells were incubated with FITC-conjugated CTB, followed by fixation, permeabilization, and development for PHB fluorescence detection. The arrow: PHB colocalization with lipid rafts. Scale bar, 10 μm. The data represents three independent experiments.
UVB-induced lipid raft translocation of PHB is dependent on expression levels of Akt and Raf
Since there appeared to be a relationship between lipid raft translocation of PHB and PHB binding to Akt and Raf, we determined whether overexpression of Akt, Raf or Akt plus Raf had an effect on the PHB membrane redistribution. The H1299 cell line was used in this study because of its high transfection efficiency. Our data showed that Akt and/or Raf were overexpressed in both unirradiated and UVB-irradiated H1299 cells (Figure 2a, Lanes 2–4 and 6–8 vs. 1 and 5). Further analysis using immunofluorescence staining showed that in H1299 cells, Akt and/or Raf were overexpressed highly in the cells, in both the plasma membrane and cytosol (Figure 2b). The fluorescence microscopic images revealed that PHB was homogeneously distributed without UVB irradiation and then co-localized with GM1 in H1299 cells transfected with empty vector at 6 h post-UVB (50 mJ/cm2) (Figure 2c, Vector), which indicated that PHB behaves similarly in H1299 cells and HaCaT cells (Figure 2c vs. 1b). In contrast, UVB has no effect on GM1 localization of PHB in the cells overexpressing Raf, Akt or both (Figure 2c). Interestingly, while overexpression of Akt or Akt+Raf inhibited the lipid raft translocation of PHB after UVB irradiation, overexpression of Raf appeared to promote the cell membrane translocation without UVB irradiation (Figure 2c). These results suggest that lipid raft translocation of PHB might due to an increased affinity between the lipid rafts and PHB; or a decreased affinity among PHB, Akt and Raf upon UVB irradiation.
Figure 2.
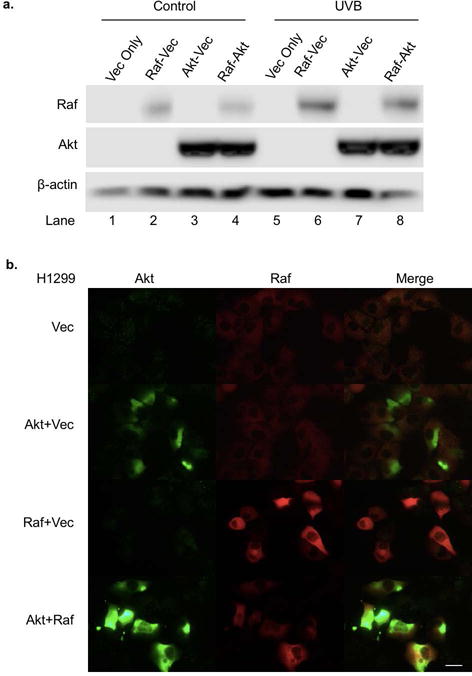
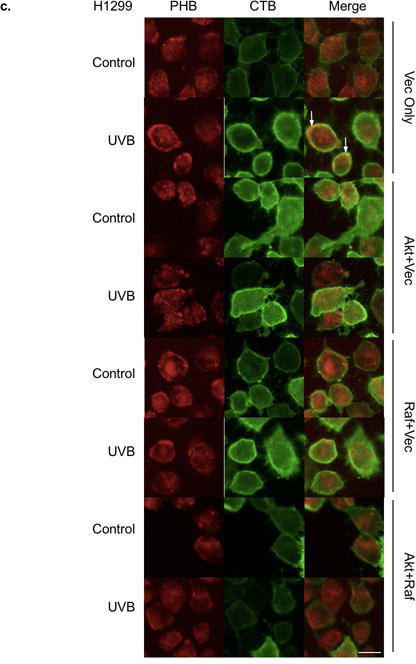
Effects of Akt and Raf overexpression on PHB translocation. H1299 cells were transfected with Akt and/or Raf expression plasmid. Empty plasmid was transfected into H1299 cells as a control. (a) The cells were lysed at 6 h post UVB (50 mJ/cm2) and the expression of Akt, Raf, and β-actin were assessed by western blotting analysis. The data represents three experiments. (b) The expressions of Akt and Raf in the transfected cells were detected by immunofluorescent staining. (c) The expressions of CTB and PHB in the transfected cells with or without UVB (50 mJ/cm2, 6 h) were detected by immunofluorescent staining. The arrow: PHB colocalization with lipid rafts. Scale bars, 20 μm. The data represents three independent experiments.
Figure 5.
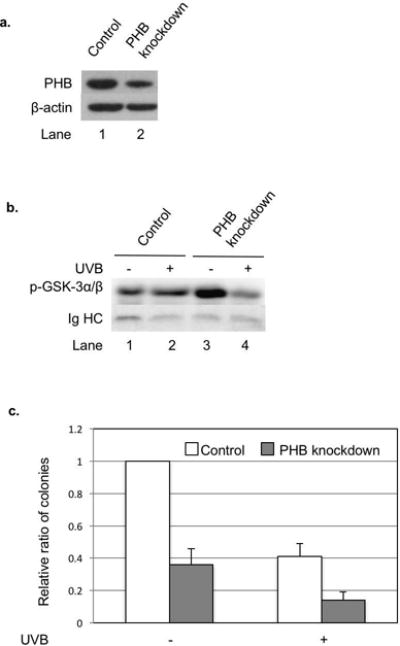
The effect of PHB knockdown on Akt kinase activity as well as cell survival and proliferation upon UVB irradiation. HaCaT cells were stably transfected with scrambled shRNA (control) or PHB shRNA (PHB knockdown). (a) PHB expression in the cells was examined by western blotting analysis. The data represents three experiments. (b) Phospho-Akt in the cells treated or not treated with UVB (50 mJ/cm2, 6 h) was immunoprecipitated by immoblilized p-Akt primary antibody and incubated with GSK 3 fusion protein. Then the phosphorylation of GSK-3α/β was detected by western blotting analysis. The data represents three experiments. (c) Survival and proliferation of the cells treated or not treated with UVB (5 mJ/cm2) were assessed by clonogenic assay. The data represents the means ± SD of three independent experiments.
PHB, Akt, and Raf interactions in HaCaT upon UVB irradiation
To further analyze the affinities among PHB, Akt and Raf, we analyzed the interaction between Akt-PHB, Raf-PHB and Akt-Raf after UVB irradiation using immunoprecipitation. Antibody heavy chain (HC) serves as the internal control of the co-immunoprecipitation experiment. Our data showed that the interaction between Akt-PHB decreased at 6 h post-UVB (50 mJ/cm2), while the interaction between Akt-Raf increased upon UVB irradiation (Figure 3a). The interaction between Raf-PHB was not significantly changed post-UVB (Figure 3a). Since UVB induces Akt phosphorylation and activation [30–34], we determined the role of Akt activation in regulating the binding of PHB with Akt by analyzing the Akt phosphorylation in different subcellular localization after UVB irradiation. GAPDH and Na+/K+-ATPase were used as markers of cytosolic protein and plasma membrane-associated protein, respectively. Western blotting analysis revealed that p-Akt increased in the cytosolic fraction, but decreased in the plasma membrane fraction at 6 h post-UVB (50 mJ/cm2) (Figure 3b). These results suggest that p-Akt might have a weaker affinity than Akt for binding to PHB, and, thus, became detached from PHB and subsequently freed from the plasma membrane to the cytosol.
Figure 3.
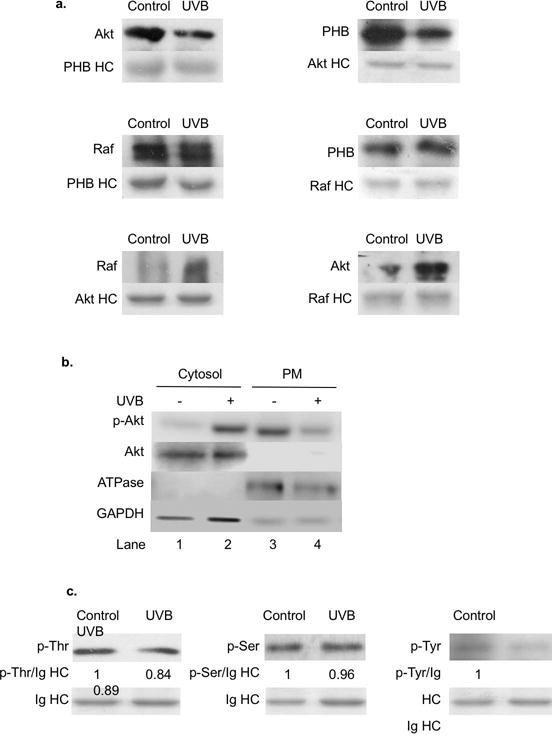
PHB interaction with Akt and Raf, PHB phosphorylation, and Akt phosphorylation in HaCaT cells upon UVB irradiation. HaCaT cells were irradiated with UVB (50 mJ/cm2) and harvested at 6 h post-UVB. (a) PHB, Akt, and Raf interactions were determined by immunoprecipitation followed by western blot analysis. The data represents three independent experiments. (b) The levels of p-Akt, Akt, ATPase, and GAPDH on plasma membrane or in cytosol were assessed by western blotting analysis. The data represents three experiments. (c) The phosphorylated threonine, serine, and tyrosine residues on immunoprecipitated PHB were determined by western blot analysis. The data represents three experiments.
Since activated Akt can phosphorylate PHB at Thr258 [17], we determined whether UVB-induced Akt phosphorylation is correlated to PHB Thr phosphorylation. Our data indicated that total Thr phosphorylation on PHB was slightly decreased at 6 h post-UVB, which correlated with the detachment and translocation of PHB from Akt and from the membrane into the lipid rafts (Figure 3c). In addition to Thr phosphorylation, we also analyzed Ser and Tyr phosphorylation on PHB as they are also associated with PHB activity [23]. Our data showed that while the Ser phosphorylation on PHB was not significantly changed, Tyr phosphorylation was also slightly decreased at 6 h post-UVB. These results suggest that the lipid raft translocation of PHB might be associated with PHB dephosphorylation at certain amino acid residues and, thus, alter the interaction among PHB, Akt and Raf.
Regulation of apoptosis by PHB knockdown in HaCaT upon UVB irradiation
We previously reported that reduced expression of PHB enhances UVB-induced apoptosis [3]. To further determine the regulatory mechanism of PHB-mediated apoptosis upon UVB irradiation, we examined the effect of PHB knockdown on phosphorylation of Akt and ERK (a downstream target activated by Raf). Our data showed that UVB induced phosphorylation of Akt and ERK in cells treated with scrambled or PHB siRNA (Figure 4a, lane 5 vs. lane 2, lane 6 vs. lane 3). Compared with scrambled siRNA, treating cells with PHB siRNA slightly increased Akt phosphorylation without UVB treatment (Figure 4a, lane 3 vs. lane 2), while it decreased the inducibility of Akt phosphorylation by UVB (Figure 4a, lane 6/3= 2.11 vs. lane 5/2= 7.63). Our data also showed that treating cells with PHB siRNA inhibited UVB-induced ERK phosphorylation (Figure 4a, lane 6 vs. lane 5). To confirm that Akt phosphorylation regulates UVB-induced apoptosis, we examined UVB-induced caspase-3 cleavage in cells treated with Akt kinase inhibitor (Akt I). Akt I inhibits Akt phosphorylation in vivo, and its IC50 is 58 nM for Akt1. Our data showed that treatment with Akt I resulted in a decrease in Akt phosphorylation, but an increase in UVB-induced caspase-3 cleavage (Figure 4b), confirming that Akt kinase activity protects cells from UVB-induced apoptosis [35,36].
Figure 4.
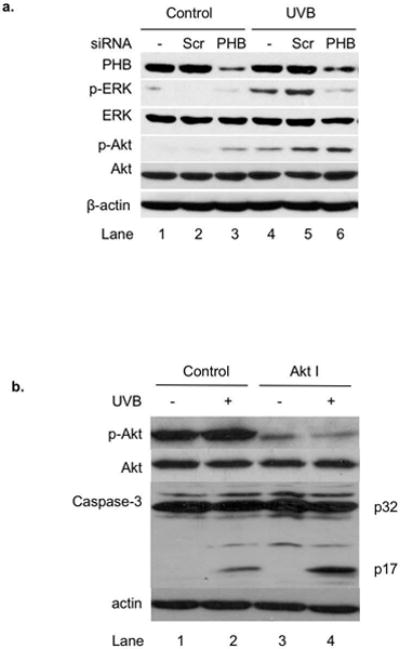
The role of PHB in UVB-induced apoptosis in HaCaT cells. (a) HaCaT cells were transiently transfected with scrambled siRNA or PHB siRNA. The cells without transfection were used as control. The levels of PHB, p-ERK, ERK, p-Akt, Akt, and β-actin in the transfected cells with or without UVB (50 mJ/cm2, 6 h) were assessed by western blotting analysis. The data represents three experiments. (b) The levels of p-Akt, Akt, caspase-3, and β-actin in Akt I (1 μM) and/ or UVB (50 mJ/cm2, 6 h) treated cells were assessed by western blotting analysis. The data represents three experiments.
To determine whether the inhibition of PHB-mediated Akt phosphorylation leads to the inhibition of Akt kinase activity upon UVB irradiation, we established a stable PHB shRNA knockdown HaCaT cell line (HaCaTPHB-KD). Our data showed that PHB expression levels were reduced significantly in HaCaTPHB-KD compared to HaCaT cells (Figure 5a). Akt kinase activity assay showed that the phosphorylation of GSK-3α/β increased in HaCaT cells, but deceased in HaCaTPHB-KD cells at 6 h post-UVB (50 mJ/cm2) (Figure 5b), indicating that Akt kinase activity was regulated by PHB upon UVB irradiation. In addition, HaCaTPHB-KD cells formed fewer colonies than HaCaT cells after UVB irradiation (5 mJ/cm2) (Figure 5c), demonstrating that reduced expression of PHB enhances UVB-induced HaCaT cell growth arrest and death.
Discussion
Our previous report indicated that PHB-mediated apoptosis is correlated with a lipid raft translocation of PHB upon UVB irradiation [3], suggesting a potential novel mechanism involving PHB in regulation of an apoptotic pathway. In this report, we explored the role of lipid raft translocation of PHB in regulation of Akt and Raf-mediated apoptosis upon UVB irradiation. Our results indicated that the lipid raft translocation of PHB was accompanied with the detachment of Akt and Raf from the non-raft membrane upon UVB irradiation (Figure 1a). The freed Akt and Raf are likely to be released into the cytosol from the membrane since we didn’t detect any Akt and Raf in the lipid rafts fraction (Figure 1a). However, we didn’t detect an increased level of Akt or Raf in the cytosol either, which could be due to the fact that Akt and Raf mainly reside in the cytosol and a slight increase due to translocation from the membrane might not be detected. The detachment of PHB from Akt was also indicated by the reduced total Thr phosphorylation on PHB (Figure 3c) as previous reports indicated that Akt interacts with and phosphorylates PHB at Thr 258 [21,37].
The lipid raft translocation of PHB may be due to a reduced affinity of PHB to Akt and Raf, since overexpression of Akt and/or Raf, which increased Akt and Raf in both cytosol and plasma membrane, allows PHB to be retained on plasma membrane post-UVB (Figure 2c). The affinity of PHB for Akt and Raf appears to be regulated by the activation of Akt and Raf. Our results indicated that the phosphorylation of Akt, an indication of Akt activation, was reduced in the plasma membrane (Figure 3b) when PHB dissociated from Akt (Figure 3a). This agrees with the previous report that demonstrates that the PHB-Akt interaction is critical for the activation of the Akt signaling cascade [15,21,23]. While we don’t have data to demonstrate Raf activation on the membrane post-UVB, we showed that Raf detached from membrane (Figure 1a), which can lead to Raf activation as suggested in a previous report [16,38]. The increased phosphorylation of ERK (Figure 4a) also supports the theory that Raf was activated after detached from PHB. Moreover, we show that the interaction between Akt and Raf was increased upon UVB irradiation (Figure 3a), which could be a result of the release of Akt and Raf from PHB on the membrane, allowing them to bind to each other in the cytosol as previously reported [23,39–41].
Our previous report showed that reduced expression of PHB enhances UVB-induced apoptosis [3]. Since PHB mediates the activation of Akt and Raf on the membrane upon UVB as showed above, we determined whether PHB protects cells from apoptosis via activating PHB-dependent Akt and Raf pathways after UVB irradiation. Our data showed that PHB knockdown inhibited the phosphorylation of Akt and ERK (Figure 4a), as well as inhibited the Akt kinase activity (Figure 5b) upon UVB irradiation. The PHB knockdown-inhibited Akt and Raf activation is correlated with cell growth arrest and death (Figure 5c) in addition to apoptosis after UVB irradiation, as we previously reported [3]. These results and previous report indicate that PHB is essential for Akt and Raf activation on the membrane, which plays a critical role in protecting cells from UVB-induced apoptosis. Based on all these evidence, we propose a model (Figure 6) that UVB irradiation induces activation of PHB-associated Akt and Raf on the plasma membrane, which leads to detachment from PHB and sequent release into the cytosol in order to regulate downstream anti-apoptotic signaling pathway. While the freed PHB on the plasma membrane translocates onto lipid rafts, the activated Akt and Raf in the cytosol will gradually form Akt-Raf dimers and be deactivated.
Figure 6.
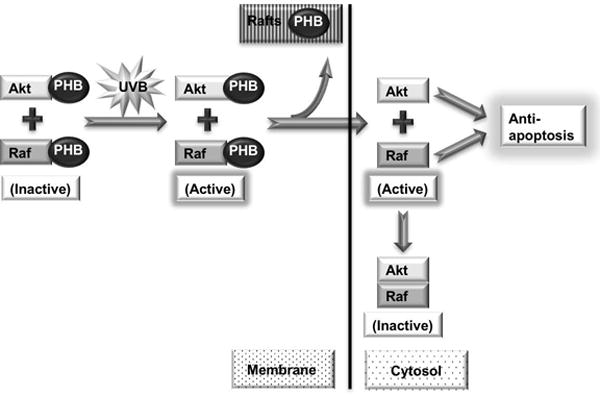
Proposed model for UVB-induced PHB-mediated apoptosis.
Supplementary Material
Figure S1. PHB expression after UVB irradiation. HaCaT cells were irradiated with UVB (50 mJ/cm2) and harvested at 6 h (b) or different time-points as indicated (a). (a) Total amount of PHB in different time-points post-UVB was assessed by western blotting analysis. The error bars represent S.D. of three independent experiments. (b) The cell surface expression of PHB was immunofluorescently labeled and detected by flow cytometry. The data represents three experiments.
Acknowledgments
Grant Sponsor: NIH RO1CA086928 (to S. Wu) and a graduate assistantship (to Q. Wu) from the Department of Chemistry and Biochemistry, Ohio University.
Q. Wu performed the research and analyzed the data. Q. Wu and S. Wu designed the study and wrote the paper. The authors thank Dr. K. S. George Parsons for her critical reading of this manuscript.
ABBREVIATIONS
- UVB
ultraviolet B light (290–320 nm)
- PHB
Prohibitin
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.George KS, Elyassaki W, Wu Q, Wu S. The role of cholesterol in UV light B-induced apoptosis. Photochem Photobiol. 2012;88(5):1191–1197. doi: 10.1111/j.1751-1097.2011.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elyassaki W, Wu S. Lipid rafts mediate ultraviolet light-induced Fas aggregation in M624 melanoma cells. Photochem Photobiol. 2006;82(3):787–792. doi: 10.1562/2005-12-09-RA-748. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q, Wu S. Lipid rafts association and anti-apoptotic function of prohibitin in ultraviolet B light-irradiated HaCaT keratinocytes. Experimental dermatology. 2012;21(8):640–642. doi: 10.1111/j.1600-0625.2012.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicol Appl Pharm. 2012;259(3):311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem. 2005;280(28):26425–26434. doi: 10.1074/jbc.M414569200. [DOI] [PubMed] [Google Scholar]
- 6.Charruyer A, Jean C, Colomba A, et al. PKCzeta protects against UV-C-induced apoptosis by inhibiting acid sphingomyelinase-dependent ceramide production. Biochem J. 2007;405(1):77–83. doi: 10.1042/BJ20061528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajate C, Mollinedo F. Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Pat Anticancer Drug Discov. 2011;6(3):274–283. doi: 10.2174/157489211796957766. [DOI] [PubMed] [Google Scholar]
- 8.Reis-Sobreiro M, Roue G, Moros A, et al. Lipid raft-mediated Akt signaling as a therapeutic target in mantle cell lymphoma. Blood Cancer J. 2013;3:e118. doi: 10.1038/bcj.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert S, Loranger A, Omary MB, Marceau N. Keratin impact on PKCdelta/ASMase regulation of hepatocyte lipid raft size: Implication in FasR-associated apoptosis. J Cell Sci. 2016 doi: 10.1242/jcs.171124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YN, Lin CI, Liao H, et al. Cholesterol overload induces apoptosis in SH-SY5Y human neuroblastoma cells through the up regulation of flotillin-2 in the lipid raft and the activation of BDNF/Trkb signaling. Neuroscience. 2016;328:201–209. doi: 10.1016/j.neuroscience.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Lin ML, Chen SS, Wu TS. Synthetic Bichalcone TSWU-BR23 Induces Apoptosis of Human Colon Cancer HT-29 Cells by p53-Mediated Mitochondrial Oligomerization of BAX/BAK and Lipid Raft Localization of CD95/FADD. Anticancer Res. 2015;35(10):5407–5416. [PubMed] [Google Scholar]
- 12.Doudican NA, Orlow SJ. Inhibition of the CRAF/prohibitin interaction reverses CRAF-dependent resistance to vemurafenib. Oncogene. 2016 doi: 10.1038/onc.2016.214. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Liang H, Zhang F, et al. Prohibitin overexpression predicts poor prognosis and promotes cell proliferation and invasion through ERK pathway activation in gallbladder cancer. J Exp Clin Cancer Res. 2016;35:68. doi: 10.1186/s13046-016-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ande SR, Gu Y, Nyomba BL, Mishra S. Insulin induced phosphorylation of prohibitin at tyrosine 114 recruits Shp1. Biochim Biophys Acta. 2009;1793(8):1372–1378. doi: 10.1016/j.bbamcr.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Ande SR, Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Biophys Res Commun. 2009;390(3):1023–1028. doi: 10.1016/j.bbrc.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 16.Rajalingam K, Wunder C, Brinkmann V, et al. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7(8):837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CF, Ho MY, Peng JM, et al. Raf activation by Ras and promotion of cellular metastasis require phosphorylation of prohibitin in the raft domain of the plasma membrane. Oncogene. 2013;32(6):777–787. doi: 10.1038/onc.2012.86. [DOI] [PubMed] [Google Scholar]
- 18.Polier G, Neumann J, Thuaud F, et al. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem Biol. 2012;19(9):1093–1104. doi: 10.1016/j.chembiol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhu B, Zhai J, Zhu H, Kyprianou N. Prohibitin regulates TGF-beta induced apoptosis as a downstream effector of Smad-dependent and -independent signaling. Prostate. 2010;70(1):17–26. doi: 10.1002/pros.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan TL, Wang PW. Explore the Molecular Mechanism of Apoptosis Induced by Tanshinone IIA on Activated Rat Hepatic Stellate Cells. Evid Based Complement Alternat Med. 2012;2012:734987. doi: 10.1155/2012/734987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han EK, McGonigal T, Butler C, Giranda VL, Luo Y. Characterization of Akt overexpression in MiaPaCa-2 cells: prohibitin is an Akt substrate both in vitro and in cells. Anticancer Res. 2008;28(2A):957–963. [PubMed] [Google Scholar]
- 22.Rajalingam K, Rudel T. Ras-Raf signaling needs prohibitin. Cell Cycle. 2005;4(11):1503–1505. doi: 10.4161/cc.4.11.2142. [DOI] [PubMed] [Google Scholar]
- 23.Mishra S, Ande SR, Nyomba BL. The role of prohibitin in cell signaling. Febs J. 2010;277(19):3937–3946. doi: 10.1111/j.1742-4658.2010.07809.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith KA, Tong X, Abu-Yousif AO, et al. UVB radiation-induced beta-catenin signaling is enhanced by COX-2 expression in keratinocytes. Mol Carcinog. 2012;51(9):734–745. doi: 10.1002/mc.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu W, Wu S. Mechanism for dynamic regulation of iNOS expression after UVB-irradiation. Mol Carcinog. 2013;52(8):627–633. doi: 10.1002/mc.21898. [DOI] [PubMed] [Google Scholar]
- 26.Narayanapillai S, Agarwal C, Deep G, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced DNA-damage and apoptosis by enhancing interleukin-12 expression in JB6 cells and SKH-1 hairless mouse skin. Mol Carcinog. 2014;53(6):471–479. doi: 10.1002/mc.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han W, He YY. Requirement for metalloproteinase-dependent ERK and AKT activation in UVB-induced G1-S cell cycle progression of human keratinocytes. Photochem Photobiol. 2009;85(4):997–1003. doi: 10.1111/j.1751-1097.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chilampalli C, Guillermo R, Zhang X, et al. Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action. BMC Cancer. 2011;11:456. doi: 10.1186/1471-2407-11-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin F, Xu W, Guan C, et al. Niacin protects against UVB radiation-induced apoptosis in cultured human skin keratinocytes. Int J Mol Med. 2012;29(4):593–600. doi: 10.3892/ijmm.2012.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butts BD, Kwei KA, Bowden GT, Briehl MM. Elevated basal reactive oxygen species and phospho-Akt in murine keratinocytes resistant to ultraviolet B-induced apoptosis. Mol Carcinog. 2003;37(3):149–157. doi: 10.1002/mc.10131. [DOI] [PubMed] [Google Scholar]
- 31.Mildner M, Eckhart L, Lengauer B, Tschachler E. Hepatocyte growth factor/scatter factor inhibits UVB-induced apoptosis of human keratinocytes but not of keratinocyte-derived cell lines via the phosphatidylinositol 3-kinase/AKT pathway. J Biol Chem. 2002;277(16):14146–14152. doi: 10.1074/jbc.M110687200. [DOI] [PubMed] [Google Scholar]
- 32.Decraene D, Van Laethem A, Agostinis P, et al. AKT status controls susceptibility of malignant keratinocytes to the early-activated and UVB-induced apoptotic pathway. J Invest Dermatol. 2004;123(1):207–212. doi: 10.1111/j.0022-202X.2004.22702.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim W, Yang HJ, Youn H, Yun YJ, Seong KM, Youn B. Myricetin inhibits Akt survival signaling and induces Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated HaCaT human immortalized keratinocytes. J Radiat Res. 2010;51(3):285–296. doi: 10.1269/jrr.09141. [DOI] [PubMed] [Google Scholar]
- 34.Chou WW, Chen KC, Wang YS, Wang JY, Liang CL, Juo SH. The role of SIRT1/AKT/ERK pathway in ultraviolet B induced damage on human retinal pigment epithelial cells. Toxicol In Vitro. 2013;27(6):1728–1736. doi: 10.1016/j.tiv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Ibuki Y, Goto R. Suppression of apoptosis by UVB irradiation: survival signaling via PI3-kinase/Akt pathway. Biochem Biophys Res Commun. 2000;279(3):872–878. doi: 10.1006/bbrc.2000.4018. [DOI] [PubMed] [Google Scholar]
- 36.Saegusa J, Hsu DK, Liu W, et al. Galectin-3 protects keratinocytes from UVB-induced apoptosis by enhancing AKT activation and suppressing ERK activation. J Invest Dermatol. 2008;128(10):2403–2411. doi: 10.1038/jid.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurugi H, Rajalingam K. A role for prohibitin in mast cell activation: location matters. Sci Signal. 2013;6(292):pe29. doi: 10.1126/scisignal.2004646. [DOI] [PubMed] [Google Scholar]
- 38.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286(5445):1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 40.Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286(5445):1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 41.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277(34):31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PHB expression after UVB irradiation. HaCaT cells were irradiated with UVB (50 mJ/cm2) and harvested at 6 h (b) or different time-points as indicated (a). (a) Total amount of PHB in different time-points post-UVB was assessed by western blotting analysis. The error bars represent S.D. of three independent experiments. (b) The cell surface expression of PHB was immunofluorescently labeled and detected by flow cytometry. The data represents three experiments.


