Abstract
Purpose
Loneliness is a known risk factor for poor mental and physical health outcomes and quality of life in the general population, and preliminary research suggests that loneliness is linked to poorer health outcomes in cancer patients as well. Various aspects of the cancer experience contribute to patients feeling alone and misunderstood. Furthermore, loneliness theory suggests that negative social expectations, which may specifically relate to the cancer experience, precipitate and sustain loneliness. Cancer-specific tools are needed to assess key constructs of this theory. In the current study, we developed and tested measures of: (1) loneliness attributed to cancer (i.e., cancer-related loneliness), and (2) negative social expectations related to cancer.
Methods
First, we developed the items for the measures based on theory, prior research, and expert feedback. Next, we assessed the measures’ psychometric properties (i.e., internal consistency, construct validity) in a diverse sample of cancer patients.
Results
The final products included a 7-item unidimensional Cancer Loneliness Scale and a 5-item unidimensional Cancer-related Negative Social Expectations Scale. Evidence of excellent reliability and validity was found for both measures.
Conclusions
The resulting measures have both clinical and research utility.
Keywords: cancer, oncology, loneliness, social expectations, measurement, scale development
Feeling socially connected is a crucial aspect of quality of life; in the absence of social connection, individuals experience loneliness. Loneliness is a known risk factor for poor mental and physical health in the general population and is linked to poorer health in cancer patients as well [1–6]. Specifically, greater loneliness has predicted poorer immune functioning and greater depression, fatigue, pain, sleep disturbance, and incidence of cancer and all-cause mortality among cancer patients [5–8].
Loneliness theory suggests that loneliness is not merely a result of one’s actual isolation or number of social network members, but instead relates to perceived isolation and dissatisfaction with the quality of relationships [9,10]. Loneliness theory suggests that lonely individuals have more negative expectations of others and are viewed more negatively by others [2]. According to this theory, negative social cognitions (i.e., negative social expectations) lead to more negative interactions that, in turn, sustain both loneliness and the associated negative social expectations. Social conditions (e.g., social support quality) also impact the persistence of loneliness and negative social cognitions [2,11]. These cognitions are hypothesized to play a role in increasing health risk factors, such as poor sleep quality [2].
Various aspects of the cancer experience contribute to loneliness. For example, many cancer patients have heightened existential concerns but feel that family members do not share these concerns [12,13]. Additionally, some patients experience socially constraining behaviors (e.g., criticism, avoidance) when attempting to discuss cancer-related concerns. Social-cognitive processing theory and research suggest that these social constraints are associated with poor psychological outcomes, such as loneliness [14–16]. Furthermore, patients’ loneliness-related cognitions may specifically relate to the cancer experience (e.g., unrealistic expectations regarding others’ level of support during their illness) [12]. Thus, loneliness may be influenced by different social factors in cancer patients relative to the general population. Research supporting this hypothesis would justify tailoring loneliness interventions to address cancer-related experiences.
Although efficacious loneliness-reduction interventions have been developed [17], these interventions have rarely targeted cancer populations. Furthermore, loneliness interventions in cancer have not addressed maladaptive social cognitions (e.g., negative social expectations), despite the fact that targeting such cognitions was found to be most efficacious for reducing loneliness in a meta-analysis with general population samples [17].
Prior to the development of loneliness interventions for cancer populations, cancer-specific tools are needed to assess: (1) loneliness attributed to cancer (i.e., cancer-related loneliness), and (2) negative social expectations related to cancer that may precipitate and sustain loneliness. These measures could advance loneliness research in multiple respects. First, a tool assessing loneliness attributed to cancer may allow us to identify patients who warrant a cancer-specific loneliness intervention. Second, a measure of patients’ negative social expectations related to cancer could contribute to theory development and clinical care. To our knowledge, a measure of negative social expectations associated with loneliness has not been developed for any population. Empirical evidence that negative social expectations are correlated with cancer patients’ loneliness would provide further support for theory linking these variables [2]. Furthermore, researchers could empirically evaluate whether reduced negative social expectations mediate the effects of a loneliness intervention on cancer patients’ mental health and quality of life. To address the need for cancer-specific tools, we developed and tested measures of cancer-related loneliness and cancer-related negative social expectations in this study.
Methods
Generation of Initial Item Pools
Cancer Loneliness Scale
We developed the initial 15-item pool for the Cancer Loneliness Scale based on loneliness theory [2,9,18,19], previous general loneliness measures [10,20–22], and qualitative studies of loneliness in cancer populations [13,23]. A 5-point scale with item responses ranging from 1 (never) to 5 (always) was selected. To ensure that the scale comprehensively described cancer-related loneliness, we obtained feedback on the items from three experts in loneliness and social aspects of cancer and 15 cancer patients before administering the Cancer Loneliness Scale to assess its psychometric properties (see [12] for study methods). Overall, most participants stated that the potential items were easy to understand and included key content. The 7 items in the final pool are listed in Table 1.
Table 1.
Descriptive Statistics for Cancer Loneliness Scale Item Pool
| Item | M | SD | Item-total correlation (item pool) | Item-total correlation (final scale)a |
|---|---|---|---|---|
| 1. Since your cancer diagnosis, how often have you felt that people are around you but not with you? | 2.23 | 1.16 | 0.79 | |
| 2. How often do you feel left out because of your cancer? | 1.70 | 1.00 | 0.73 | |
| 3. Since your cancer diagnosis, how often have you felt that you were not important to others? | 1.71 | 1.01 | 0.82 | |
| 4. How often do you feel that there is no one you can share the ups and downs of cancer with? | 2.01 | 1.16 | 0.81 | |
| 5. How often does having cancer make you feel empty? | 2.10 | 1.22 | 0.78 | |
| 6. Since your cancer diagnosis, how often have you felt misunderstood even by your closest friends and family members? | 1.99 | 1.16 | 0.86 | 0.85 |
| 7. How often do you feel that others cannot provide the support you need to deal with your cancer? | 1.90 | 1.15 | 0.79 | 0.76 |
| 8. Since your cancer diagnosis, how often have you felt that you don’t have a lot in common with the people around you? | 1.89 | 1.04 | 0.73 | 0.72 |
| 9. How often do you feel that you cannot share personal thoughts about cancer with anyone? | 2.06 | 1.20 | 0.82 | 0.82 |
| 10. Since your cancer diagnosis, how often have you felt that you were not needed by others? | 1.75 | 1.09 | 0.82 | 0.80 |
| 11. How often does having cancer make you feel alone? | 2.03 | 1.08 | 0.83 | |
| 12. How often do you feel that no one really understands how cancer has affected you? | 2.47 | 1.37 | 0.84 | |
| 13. Since your cancer diagnosis, how often have you experienced a general sense of emptiness? | 2.13 | 1.11 | 0.81 | 0.78 |
| 14. How often does your cancer diagnosis make you feel isolated from others? | 2.96 | 1.07 | 0.86 | 0.82 |
| 15. Since your cancer diagnosis, how often have you felt you are no longer close to anyone? | 1.67 | 1.02 | 0.81 |
Note. All ns= 185. All ranges = 1.0–5.0.
Items in this column were selected for the final scale
Cancer-related Negative Social Expectations Scale
To ensure representative content coverage, we drew upon theory [2,14,24] and existing research [12,13,16,23,25] to create five content domains for the Cancer-related Negative Social Expectations Scale item pool. We specifically drew upon our prior qualitative work which identified situations and thoughts that cancer patients associated with their loneliness [12]. Fourteen items were written to assess each of five content domains (see Table 2 for the content domains and Table 3 for the final pool of items). A 6-point response scale ranging from 1 (strongly disagree) to 6 (strongly agree) was used.
Table 2.
Description of Cancer-related Negative Social Expectations Scale Content Domains
| Domain | Rationale | Items from Table 3 | |
|---|---|---|---|
| 1 | Expecting others to listen or be available whenever the patient needs them. | Patients have reported feelings of loneliness when visits from family and friends and conversations about their illness did not occur as often as expected [23,12]. | 1, 5, 7, 11, 13 |
| 2 | Expecting a lack of understanding of cancer-related concerns. | Patients have reported feeling lonely when they believed others misunderstood their cancer-related experiences and changes [13,12]. | 2, 8 |
| 3 | Expecting that others will not understand existential thoughts. | Many cancer patients have reported new existential thoughts, including a newfound awareness of their mortality and unpredictable future, after their diagnosis. Patients have reported feeling lonely when they perceived that others did not share their heightened awareness about mortality [13]. | 4, 10 |
| 4 | Expecting sharing cancer-related concerns to burden others | For some patients, a lack of cancer-related disclosure is motivated by a desire to protect others from distress. Some patients with this thought pattern experience distress and disconnection from others when they fail to discuss cancer-related concerns [13,12]. Relatedly, protective buffering (e.g., hiding concerns and worries in an attempt to prevent others from experiencing distress) [25] has been associated with poorer psychological adjustment and lower levels of relationship satisfaction in cancer patients [25,48]. | 3, 9 |
| 5 | Expecting disclosure of their diagnosis or cancer-related concerns to worsen their relationships. | Some patients withhold cancer-related information from others due to concerns that disclosing their health status would negatively change the way others interact with them [12]. For example, patients expect others to avoid them or show discomfort during conversations. Thus, whereas domain 4 focuses on cancer-related disclosure burdening others, domain 5 focuses on patients’ anticipated distress as relationships change following disclosure. However, many patients have reported that withholding cancer-related information led to feelings of isolation [12], consistent with social-cognitive processing theory [24,14]. Specifically, processing cancer-related information with others has been found to facilitate psychological adjustment [49,50,16], an opportunity not available to non-disclosing patients. | 6, 12, 14 |
Table 3.
Descriptive Statistics for the Cancer-related Negative Social Expectations Scale Item Pool
| Item | Domain assessed | n | M | SD | Item-total correlation (item pool) | Item-total correlation (final scale)a |
|---|---|---|---|---|---|---|
| 1. If people stopped asking about my cancer, I would think that they don’t care. | 1 | 185 | 2.02 | 1.39 | 0.56 | |
| 2. People will not understand if I share my concerns about cancer. | 2 | 185 | 2.42 | 1.52 | 0.71 | |
| 3. If I shared my concerns about cancer with people then it would be too hard on them. | 4 | 185 | 2.49 | 1.51 | 0.69 | |
| 4. People would not understand my thoughts about death since my cancer diagnosis. | 3 | 185 | 2.88 | 1.70 | 0.75 | |
| 5. I expect people to always be available for me because of my cancer. | 1 | 185 | 1.97 | 1.45 | 0.16 | |
| 6. If I told people about my cancer experience, they would be nervous and uncomfortable around me. | 5 | 185 | 2.30 | 1.43 | 0.79 | 0.76 |
| 7. If people avoided discussing my cancer with me, I would think that they didn’t want to hear about it. | 1 | 185 | 2.68 | 1.60 | 0.67 | 0.67 |
| 8. People could not truly understand how I feel about my cancer diagnosis. | 2 | 185 | 3.12 | 1.79 | 0.77 | 0.76 |
| 9. I would burden people if I shared my thoughts and feelings about cancer with them. | 4 | 185 | 2.75 | 1.58 | 0.76 | 0.80 |
| 10. People would not understand my uncertainty about the future since my cancer diagnosis. | 3 | 185 | 3.05 | 1.78 | 0.78 | 0.77 |
| 11. I expect people to listen to me whenever I want to talk about my cancer. | 1 | 186 | 2.58 | 1.66 | 0.29 | |
| 12. If I told people about my cancer experience, our relationship would change for the worse. | 5 | 186 | 1.71 | 1.18 | 0.57 | |
| 13. If people avoided seeing or talking to me after my cancer diagnosis, I would think that they don’t care. | 1 | 186 | 2.42 | 1.61 | 0.65 | |
| 14. If I shared my concerns about cancer with people then they might hurt me with their reactions. | 5 | 186 | 1.83 | 1.19 | 0.64 |
Note. All ranges = 1.0-6.0.
Items in this column were selected for the final scale.
Evaluation of Psychometric Properties
Participants
Following institutional review board approval, cancer patients were recruited from the Indiana Cancer Registry, a list of every cancer case in the state of Indiana. Eligibility status was determined by medical chart review and a telephone-based informed consent process. Eligibility criteria for the study included: (1) being diagnosed with cancer in 2013 or 2014; (2) receiving care for cancer at an Indiana University Health Hospital during 2013 or 2014; (3) being an English-speaking adult; and (4) having no evidence of serious cognitive impairment. Patients whose primary cancer diagnosis was brain cancer were excluded. Otherwise, all cancer types and stages were eligible because loneliness has not been found to differ by cancer type or stage [11].
Measures
In addition to the new measures described previously, validated measures of health and social well-being were administered to assess theory-based relationships with cancer-related loneliness and cancer-related negative social expectations:
PROMIS measures of social and health-related outcomes
NIH-funded PROMIS measures were used to assess emotional support, depressive and anxiety symptoms, and mental and physical quality of life. PROMIS measures have undergone rigorous reliability and validity testing [26–31]. Emotional support was assessed with the 4-item Emotional Support measure [32], which showed excellent internal consistency (α=0.95) in the current study. Depressive and anxiety symptoms were assessed with the 4-item Depression and Anxiety measures [33,34], both of which showed excellent internal consistency (αs=0.93 and 0.90, respectively) in the current study. The 4-item mental health and the 4-item physical health scales from the 10-item Global Health measure were used to assess quality of life [31]. In this study, internal consistency for the mental health scale was good (α=0.82), whereas it was poor for the physical health scale (α=0.27). To determine whether one item reduced the physical health scale’s alpha, we examined the alpha level with and without each item. Removing the pain item increased the alpha to 0.84; thus, analyses were conducted without this item.
Loneliness
General loneliness was measured with the 20-item UCLA Loneliness Scale-Version 3 [10]. The UCLA Loneliness Scale has shown excellent reliability and validity across studies, including studies with cancer patients [5,16,35]. In this study, internal consistency was excellent (α=0.94).
Social network characteristics
Three items adapted from the Social Network Index [36] were used to assess social network characteristics. The items included: (1) “How many relatives do you see or talk to on the phone at least once every 2 weeks?”; (2) “How many friends do you see or talk to on the phone at least once every 2 weeks?”; and (3) “How many cancer patients or survivors do you see or talk to on the phone at least once every 2 weeks?”
Demographic and medical characteristics
Age, gender, and cancer-related information were collected via medical records. Other demographics were self-reported.
Procedure
Potential participants were identified through the Indiana Cancer Registry. First, we reviewed patients’ medical records to confirm their eligibility. To ensure diversity, we used purposive sampling based on gender and race. Potentially eligible participants were mailed introductory letters and study information sheets. We called all prospective participants who did not opt out to describe the study. Informed consent was obtained by phone from all patients included in the study. Consenting patients were mailed a survey and a pre-paid, addressed envelope for returning the survey. Participants who returned their survey received a $25 gift card.
Analyses
Data screening and preliminary analyses
First, we examined the assumptions of normality and linearity. According to Kline’s [37] guidelines, the values for each variable were appropriate, except for the social network size items. After applying a winsorization transformation to outliers on social network size questions [38], all skew and kurtosis values were acceptable [37]. Second, we examined whether data were missing completely at random using Little’s Missing Completely at Random (MCAR) test [39]. Because our data were shown to be missing completely at random, χ2(2278)=2286.8, p=0.44, and we had little missingness, when an item was missing from a scale, we imputed the value of the strongest correlated item in that scale. Finally, descriptive statistics were calculated.
Assessment of item performance
Next we assessed item quality and eliminated items that performed poorly. Items could be removed at any stage of the project based on performance. For example, items could be removed for floor or ceiling effects (i.e., more than 80% endorsed the highest or lowest category), low factor loadings (i.e., <0.40), or low item-total correlations (i.e., <0.30). Items with similar content were compared, and items with the best performance were retained for the measures.
Assessment of dimensionality
The dimensionalities of both scales were assessed using confirmatory factor analyses in LISREL 8.8. Regarding the Cancer Loneliness Scale, unidimensionality was hypothesized. Regarding the dimensionality of the Cancer-related Negative Social Expectations Scale, three models specified a priori were compared to determine which model best fit the data: (1) a 5-dimensional model corresponded to the 5 content domains described previously; (2) a 4-dimensional model collapsed domains 2 (expecting a lack of understanding of cancer-related concerns) and 3 (expecting that others will not understand existential thoughts) due to conceptual similarity; and (3) a unidimensional model. All models were run using a robust maximum likelihood estimator.
To evaluate model fit, we examined the goodness-of-fit χ2 statistic, the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and the standardized root mean square residual (SRMR). Although model fit guidelines vary, we defined acceptable model fit as: (1) RMSEA<0.06; (2) CFI>0.95; (3) SRMR<0.08; and (4) a non-significant χ2 statistic [37,40]. Correlated residuals were allowed in cases where the correlation improved the model fit and where the item pairs shared minor secondary constructs that were not central to the constructs being measured.
Assessment of reliability and validity evidence
Reliability and validity analyses were also conducted. Alphas were obtained to assess the measures’ internal consistency. We also ran correlational analyses to assess construct validity using the final measures. First, we expected the Cancer-related Loneliness Scale to be positively related to general loneliness, anxiety, and depressive symptoms, and negatively related to emotional support and mental and physical quality of life, consistent with theory and prior research on general loneliness [1,2,5,9,11,19]. Second, we expected cancer-related loneliness to be negatively associated with the number of cancer patient contacts, but unrelated to the size of one’s total network of family and friends. Loneliness theory and research suggest that loneliness relates to dissatisfaction with relationships rather than one’s number of social network members [9,10]; however, in qualitative research, cancer patients have reported that having more contact with other cancer patients reduced their feelings of loneliness [41].
To assess the construct validity of the Cancer-related Negative Social Expectations Scale, we examined its relationships with theory-driven variables. First, we expected negative social expectations to be positively correlated with general loneliness, cancer-related loneliness, anxiety, and depressive symptoms and negatively correlated with mental and physical quality of life based on theory linking negative social expectations to loneliness and poor mental and physical health outcomes [2,42]. Second, we expected negative social expectations to be negatively correlated with emotional support, as these expectations are theorized to increase social behaviors that elicit negative social interactions [2]. Expected magnitudes for all hypothesized relationships appear in Table 4 and are based on loneliness theory and prior research.
Table 4.
Correlations for Assessment of Construct Validity
| Cancer-related Loneliness | Cancer-related Negative Social Expectations | |||
|---|---|---|---|---|
|
| ||||
| Expectation | Result | Expectation | Result | |
| Cancer-related Negative Social Expectations | +Strong | 0.70** | — | — |
| Emotional Support | −Moderate | −0.66** | −Moderate | −0.48** |
| Depressive Symptoms | +Strong | 0.54** | +Moderate | 0.41** |
| Anxiety | +Strong | 0.55** | +Moderate | 0.41** |
| Mental Quality of Life | −Strong | −0.54** | −Moderate | −0.43** |
| Physical Quality of Life | −Moderate | −0.33** | −Moderate | −0.31** |
| General Loneliness | +Strong | 0.67** | +Strong | 0.47** |
| Number of Relatives | No relationship | 0.41** | — | — |
| Number of Friends | No relationship | 0.44** | — | — |
| Number of Cancer Patients | −Weak | −0.14 | — | — |
Note. N=186. The hypotheses regarding magnitude were supported in the bolded results.
p < 0.01.
Sample size considerations
A sample with a minimum of 5 participants per pathway is thought to be required for sufficient power to detect effects based on simulations [37]. The largest number of pathways among all of our hypothesized models was 24. Thus, a minimum final sample size of 120 is thought to provide sufficient statistical power for all proposed models.
Results
Sample Characteristics
A total of 380 patients randomly selected within race and gender categories were deemed eligible based on medical chart review and were sent introductory letters. Of the 380 patients sent introductory letters, 36 (9%) were ineligible. Of the remaining 344 patients, 215 (63%) consented to participate, 47 (14%) declined participation, and 82 (24%) could not be reached via phone. Of the 215 consenters, 186 (87%) returned their surveys, 27 (13%) were lost to followup, 1 withdrew from the study, and 1 died. Sample characteristics are shown in Table 5.
Table 5.
Sample Characteristics (N = 186)
| Characteristic | N (%) | M (SD) | Range |
|---|---|---|---|
| Average age | 59.3 (12.6) | 21.0–87.0 | |
| Female gender | 95 (51.1) | ||
| Race/ethnicity | |||
| White | 138 (74.2) | ||
| Black or African American | 41 (22.0) | ||
| Other race | 7 (3.8) | ||
| Marital status | |||
| Married/living with partner | 126 (67.7) | ||
| Divorced, separated, or widowed | 39 (21.0) | ||
| Never married | 21 (11.3) | ||
| Education level | |||
| Elementary or some high school | 14 (7.5) | ||
| High school graduate | 63 (33.9) | ||
| Some college or technical school | 56 (30.1) | ||
| College graduate | 53 (28.5) | ||
| Employment status | |||
| Employed full or part-time | 77 (41.4) | ||
| Retired | 64 (34.4) | ||
| Unemployed due to disability | 31 (16.7) | ||
| Other | 13 (7.0) | ||
| Missing | 1 (0.5) | ||
| Cancer types | |||
| Breast | 29 (15.6) | ||
| Prostate | 20 (10.8) | ||
| Skin | 14 (7.5) | ||
| Uterine | 13 (7.0) | ||
| Kidney | 12 (6.5) | ||
| Lung | 11 (5.9) | ||
| Colon | 11 (5.9) | ||
| Other types (31 total other types) | 75 (40.3) | ||
| Unknown primary | 1 (0.5) | ||
| Cancer stage | |||
| Early stage | 117 (62.9) | ||
| Late stage | 46 (24.70) | ||
| N/A staging system | 9 (4.80) | ||
| Missing | 14 (7.50) | ||
| Months since diagnosis | 16.8 (3.2) | 1.0–24.3 | |
| Cancer treatments received | |||
| Surgery | 154 (82.80) | ||
| Chemotherapy | 71 (38.20) | ||
| Radiation | 61 (32.80) | ||
| Hormone therapy | 36 (19.40) | ||
| Immunotherapy | 15 (8.10) | ||
| Stem cell transplant | 4 (2.20) | ||
| Other | 2 (1.10) | ||
| Emotional support | 17.0 (3.7) | 4.0–20.0 | |
| Depression | 7.1 (3.5) | 4.0–18.0 | |
| Anxiety | 7.3 (3.4) | 4.0–19.0 | |
| Loneliness | 37.0 (11.3) | 20.0–78.0 | |
| Mental quality of life | 14.2 (3.1) | 6.0–20.0 | |
| Physical quality of life | 12.3 (2.3) | 6.0–17.0 | |
| Number of relativesa | 5.9 (4.27) | 0.0–30.0 | |
| Number of friendsb | 7.9 (11.06) | 0.0–100.0 | |
| Number of cancer patientsc | 1.7 (2.52) | 0.0–25.0 |
Number of relatives participant reported seeing or talking to on the phone at least once every 2 weeks. Values are not winsorized.
Number of friends participant reported seeing or talking to on the phone at least once every 2 weeks. Values are not winsorized.
Number of cancer patients participant reported seeing or talking to on the phone at least once every 2 weeks. Values are not winsorized
Cancer Loneliness Scale Item Selection, Factor Structure, and Reliability
First, we selected the items for the Cancer Loneliness Scale. All of the items from the item pool performed well (e.g., all response categories were endorsed, all had adequate item-total correlations) (see Table 1 for item descriptive statistics and Online Resource 1 for inter-item correlations). Thus, no items were initially eliminated due to poor performance. Next, the first and second authors grouped the items with content overlap (e.g., items assessing general feelings of aloneness, items assessing the feeling that they have no one with whom to share cancer-related thoughts) and selected one representative item from each group with the highest item-total correlation. A total of 7 items from the 15-item pool were retained for the final measure (see Table 1 for the final list of items).
After the items were selected, we examined the hypothesized unidimensional factor structure of the Cancer Loneliness Scale. The model showed mixed evidence of adequate fit: SRMR=0.03; RMSEA=0.09; CFI=0.99; χ2(14)=36.12, p=0.001. Modification indices suggested correlating certain residuals. We chose to correlate the residuals of items 13 and 14 because it was also theoretically relevant; specifically, items and 13 and 14 both focus on core emotions related to loneliness (i.e., emptiness, isolation) rather than perceptions of lack of support. Figure 1 shows the final model. Overall, the final model showed evidence of excellent fit: SRMR=0.02; RMSEA=0.03; CFI=1.00; χ2(13)=15.73, p=0.26. The internal consistency for the single-factor Cancer Loneliness Scale was also excellent (α=0.94).
Figure 1. Test of Cancer-related Loneliness Model.
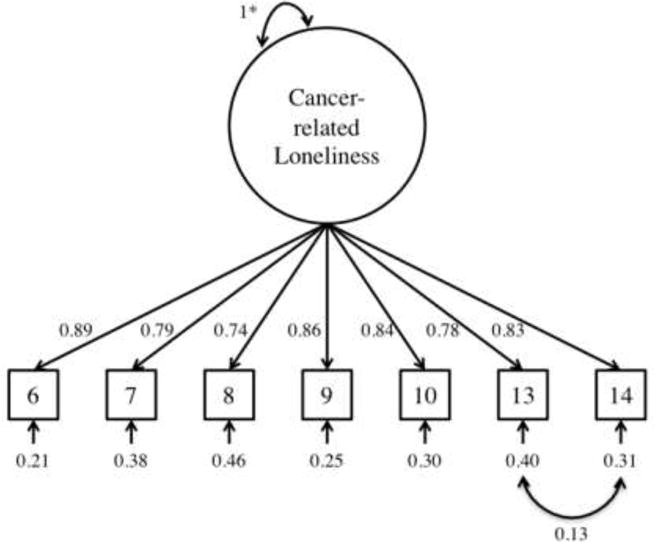
Note. All parameters statistically significant at p < 0.05.
*Factor variance fixed to 1.0.
Cancer Loneliness Scale Construct Validity
To assess the construct validity of the Cancer Loneliness Scale, we examined relationships between cancer-related loneliness and theory-based characteristics (see Table 4). A composite score was calculated by summing the final items, with higher scores indicating greater cancer-related loneliness. First, as hypothesized, cancer-related loneliness was positively correlated with general loneliness, anxiety symptoms, and depressive symptoms and negatively correlated with mental quality of life, physical quality of life, and emotional support. Contrary to our hypothesis, cancer-related loneliness was unrelated to the number of cancer patients with whom participants had regular contact. Also contrary to our hypothesis, cancer-related loneliness was positively correlated with the number of relatives and friends with whom participants had regular contact.
Cancer-Related Negative Social Expectations Scale Item Selection, Factor Structure, and Reliability
First, we examined the performance of potential items for the Cancer-related Negative Social Expectations Scale (see Table 3 for item descriptive statistics and Online Resource 2 for inter-item correlations). Two items (5 and 11) were removed from the 14-item pool because they had low inter-item correlations (i.e., many p-values>0.05) and low item-total correlations (i.e., 0.16 and 0.29, respectively). The other items performed well and were initially retained.
Next, we examined the three contending factor structure models. Regarding the 5-dimensional model, there was mixed evidence of adequate fit: SRMR=0.06; RMSEA=0.07; CFI=0.99; χ2(47)=92.68, p<0.001. In addition, there were invalid parameter estimates, with correlations among some of the factors being above 1.0. Similar to the 5-dimensional model, the 4-dimensional model showed mixed evidence of adequate fit: SRMR=0.04; RMSEA=0.07; CFI=0.99; χ2(49)=92.10, p<0.001. Furthermore, high correlations among the 4 factors (e.g., psi=0.99) were problematic and suggested that the factors were not separate. Finally, we examined the unidimensional model, which also showed mixed evidence of adequate fit: SRMR=0.05;CFI=0.98; RMSEA=0.09; χ2(54)=134.24, p<0.001. After reviewing the three models, we rejected the 5- and 4-dimensional models because the estimated correlations among the factors were too high and sometimes invalid. We retained the unidimensional model as the best representation of our data.
Next, we shortened the measure to increase its practicality for use with cancer populations. We retained one item from each conceptual domain for representative content coverage; the item with the highest item-total correlation was retained (see Table 3 for the final list of five items). Thus, nine of the 14 items were discarded. Subsequently, we re-ran the unidimensional model with the final five items. The resulting model showed mixed evidence of adequate fit: SRMR=0.03; RMSEA=0.11; CFI=0.99; χ2(5)=15.5, p=0.01. Modification indices suggested correlating certain residuals. We chose to correlate the residuals of items 8 and 10 because it was also theoretically appropriate; specifically, items 8 and 10 focus on others’ lack of understanding and have similar wording. Figure 2 shows the final model. The resulting model showed excellent fit overall: SRMR=0.02; RMSEA=0.03; CFI=1.00; χ2(4)=4.70, p=0.32. The internal consistency coefficient for the brief, single-factor scale was also excellent (α=0.90).
Figure 2. Test of the Final Cancer-related Negative Social Expectations Scale Model.
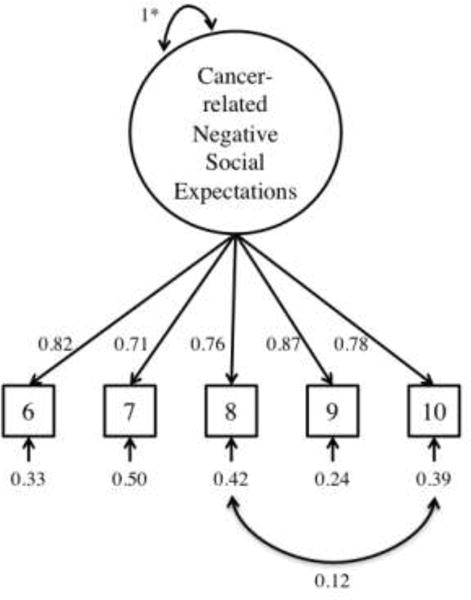
Note. All parameters statistically significant at p < 0.05.
*Factor variance fixed to 1.0.
Cancer-related Negative Social Expectations Scale Construct Validity
To assess the construct validity of the Cancer-related Negative Social Expectations Scale, we examined relationships between cancer-related negative social expectations and theory-based characteristics (see Table 4). A composite score was calculated by summing the final items, with higher scores indicating more negative social expectations. As expected, negative social expectations were positively correlated with general loneliness, cancer-related loneliness, anxiety symptoms, and depressive symptoms. Second, as expected, negative social expectations were negatively correlated with mental quality of life, physical quality of life, and emotional support.
Discussion
In the current study we developed a 7-item unidimensional Cancer Loneliness Scale and a 5-item unidimensional Cancer-related Negative Social Expectations Scale (see Tables 6 and 7 for final questionnaires). Evidence of good internal consistency and validity was found for both measures in a large, diverse sample of cancer patients. Results suggest the measures have both clinical and research utility.
Table 6.
Cancer Loneliness Scale
| The following statements describe how people sometimes feel after being diagnosed with cancer. For each statement, please indicate how often you have felt that way by writing a number in the space provided. | ||||
| NEVER | RARELY | SOMETIMES | OFTEN | ALWAYS |
| 1 | 2 | 3 | 4 | 5 |
| 1. Since your cancer diagnosis, how often have you felt misunderstood even by your closest friends and family members? | ______ | |||
| 2. How often do you feel that others cannot provide the support you need to deal with your cancer? | ______ | |||
| 3. Since your cancer diagnosis, how often have you felt that you don’t have a lot in common with the people around you? | ______ | |||
| 4. How often do you feel that you cannot share personal thoughts about cancer with anyone? | ______ | |||
| 5. Since your cancer diagnosis, how often have you felt that you were not needed by others? | ______ | |||
| 6. Since your cancer diagnosis, how often have you experienced a general sense of emptiness? | ______ | |||
| 7. How often does your cancer diagnosis make you feel isolated from others? | ______ | |||
Note. Items were re-numbered for the final scale. The items above are items 6, 7, 8, 9, 10, 13, and 14 from the original item pool.
Table 7.
Cancer-related Negative Social Expectations Scale
| Please respond to each item by marking one box per row. | ||||||
|---|---|---|---|---|---|---|
| Strongly disagree | Moderately disagree | Slightly disagree | Slightly agree | Moderately agree | Strongly agree | |
| 1. If I told people about my cancer experience, they would be nervous and uncomfortable around me……………………… | □ | □ | □ | □ | □ | □ |
|
| ||||||
| 2. If people avoided discussing my cancer with me, I would think that they didn’t want to hear about it……………………. | □ | □ | □ | □ | □ | □ |
|
| ||||||
| 3. People could not truly understand how I feel about my cancer diagnosis………………. | □ | □ | □ | □ | □ | □ |
|
| ||||||
| 4. I would burden people if I shared my thoughts and feelings about cancer with them………… | □ | □ | □ | □ | □ | □ |
|
| ||||||
| 5. People would not understand my uncertainty about the future since my cancer diagnosis………………………. | □ | □ | □ | □ | □ | □ |
Note. Items were re-numbered for the final scale. The items above are items 6, 7, 8, 9, and 10 from the original item pool.
Cancer Loneliness Scale
The Cancer Loneliness Scale was associated in expected directions with measures of mental and physical health, which provided evidence of construct validity. However, relationships between this scale and objective social network characteristics were not consistent with our predictions. For example, cancer-related loneliness was not significantly associated with the number of cancer patients with whom participants reported regular contact. This result suggests that, consistent with theory regarding non-patients [9,10], the quality of the interaction with fellow cancer patients may be more important than the quantity. Particularly, contact with other cancer patients may be a positive or negative experience depending on a number of factors, such as how well they feel understood.
Also contrary to our hypotheses, the number of friends and relatives with whom participants had regular contact was positively correlated with cancer-related loneliness. In the general loneliness literature, findings are mixed regarding relationships between loneliness and more objective social network characteristics (e.g., amount of time spent with others, size of social network), with some studies reporting significant negative associations [10,43] and others reporting null findings [9,10]. Thus, our findings contrast with the general literature. One potential explanation for our findings is that having more contact with others provided more opportunities to experience socially constraining behaviors or feel misunderstood with respect to the cancer experience, which led to greater loneliness.
Other explanations for the inconsistent findings regarding social network variables should also be considered. For instance, the results might reflect measurement error or the ambiguous nature of the relationship between the quantity of social contact and loneliness. Overall, evidence for the construct validity of the Cancer Loneliness Scale was deemed to be good, as the majority of theoretical relationships were found.
Cancer-related Negative Social Expectations Scale
The unidimensional model for the Cancer-related Negative Social Expectations Scale was retained, as it exhibited the best fit of the three factor structures that were examined. Results are consistent with the notion that patients typically endorse a single underlying pattern of thinking about relationships as opposed to having different types of expectations for different social situations.
The Cancer-related Negative Social Expectations Scale was associated in expected directions with measures of health and social well-being, which provided evidence of construct validity. First, as hypothesized, cancer-related negative social expectations were positively correlated with both general and cancer-related loneliness. To our knowledge, this is the first empirical examination of the relationship between negative social expectations and loneliness in any population, providing preliminary support for theory linking these constructs [2]. The association between negative social expectations and cancer-related loneliness (r=0.70) was stronger than the association between negative social expectations and general loneliness (r=0.47), which is expected because both the negative social expectations and cancer-related loneliness measures focus on cancer-specific experiences. The strong correlation between negative social expectations and cancer-related loneliness is consistent with theory [2] and provides evidence for the measure’s construct validity. Second, as hypothesized, negative social expectations were positively correlated with anxiety and depressive symptoms and negatively correlated with mental and physical quality of life and emotional support. Thus, overall, results provided evidence of excellent construct validity.
Limitations and Future Directions
Limitations and future directions should be noted. First, although our consent and survey return rates were comparable to similar research [44], patients who participated may have differed from those who chose not to participate or could not be contacted. Second, replication of the current analyses in a larger, more diverse sample is needed. Third, future studies are required to assess additional aspects of validity (e.g., responsiveness to change, minimally important difference ranges). Fourth, examining whether cancer-related negative social expectations predict cancer-related loneliness over time would provide a more rigorous test of the theory [2], as well as a better indication of whether cognitive interventions should be tested to reduce loneliness in cancer patients. In addition, future studies should examine whether cancer-related loneliness or general loneliness is a stronger predictor of health outcomes over time. Lastly, there are no established clinical cut-offs for loneliness in the broader literature; a clinical cut-off would guide screening efforts.
Implications
The current project has implications for theory. First, to our knowledge, this is the first study to develop and evaluate measures of negative social expectations for any population and cancer-related loneliness. Thus, this study provided the first empirical tests of the degree to which negative social expectations are correlated with loneliness and health outcomes, and results were consistent with loneliness theory [2]. Additionally, this study expands on existing theory by examining these concepts in cancer patients, a population that may be at high risk of developing loneliness [11,45].
The project also has a number of implications for clinical practice and research. First, following further measure development, cancer patients who seek mental health care could be screened for cancer-related loneliness to see if they might benefit from an intervention to address loneliness. One key advantage of the Cancer Loneliness Scale is its brevity compared to existing measures of loneliness, increasing its practicality for use with cancer populations. Furthermore, if the current findings are replicated longitudinally, this would suggest that targeting negative social expectations in loneliness interventions might be beneficial. Therapists could identify potential negative social cognitions upon which to intervene based on patients’ item responses to the Cancer-related Negative Social Expectations Scale. Following the intervention, researchers could use the measure to evaluate whether reduced negative social expectations mediated the beneficial effects of a cognitive, loneliness-reduction intervention on cancer patients’ quality-of-life outcomes. Finally, the measures could be adapted to assess loneliness and negative social expectations in other medical populations.
Conclusions
Social connectedness is a critical component of quality of life, and loneliness is associated with poor mental and physical health outcomes in cancer patients [6,7,46,47]. Cancer patients may experience loneliness related to the cancer experience; thus, loneliness interventions in cancer should be tailored to address illness-related social conditions and negative social expectations. In the current study, we developed brief cancer-specific tools for use in future loneliness intervention trials with cancer patients. Development of theory-based loneliness reduction interventions may significantly improve cancer patients’ mental and physical quality of life.
Supplementary Material
Acknowledgments
This project was supported by the American Psychological Association. Rebecca Adams’s work was supported by R25CA117865 (V. Champion, PI) from the National Cancer Institute. Catherine Mosher’s work was supported by the National Cancer Institute under Grants K05CA175048 and K07CA168883. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors would like to thank the study participants and Madison Stout for her assistance.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Rebecca N. Adams, Department of Pediatrics, Stanford University School of Medicine, 780 Welch Road, MC 5776, Palo Alto, CA 94304, Phone: 1-650-736-1567, Fax: 1-650-736-6690
Catherine E. Mosher, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 124, Indianapolis, IN 46202
Kevin L. Rand, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 124, Indianapolis, IN 46202
Adam T. Hirsh, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 124, Indianapolis, IN 46202
Patrick O. Monahan, Department of Biostatistics, Indiana University School of Medicine and School of Public Health, 410 W. Tenth St., Suite 3000, Indianapolis, IN 46202
Rafat Abonour, Department of Medicine, Indiana University School of Medicine, 535 Barnhill Drive, Indiana Cancer Pavilion, Suite 446, Indianapolis IN 46202
Kurt Kroenke, Indiana University School of Medicine, Regenstrief Institute, Inc., VA HSR&D Center for Health Information and Communication, Regenstrief Institute, Inc. (RF), 1101 West Tenth Street, RF 221, Indianapolis, IN 46202
References
- 1.Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain, Behavior, and Immunity. 2003;17(1):98–105. doi: 10.1016/S0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 2.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychology and Aging. 2010;25(2):453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkley LC, Thisted RA, Masi CM, Cacioppo JT. Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychology and Aging. 2010;25(1):132–141. doi: 10.1037/a0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaremka LM, Andridge RR, Fagundes CP, Alfano CM, Povoski SP, Lipari AM, et al. Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychology. 2014;33(9):948–957. doi: 10.1037/a0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, et al. Loneliness promotes inflammation during acute stress. Psychological Science. 2013;24(7):1089–1097. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drageset J, Eide GE, Kirkevold M, Ranhoff AH. Emotional loneliness is associated with mortality among mentally intact nursing home residents with and without cancer: A five-year follow-up study. Journal of Clinical Nursing. 2013;22(1–2):106–114. doi: 10.1111/j.1365-2702.2012.04209.x. [DOI] [PubMed] [Google Scholar]
- 8.Nausheen B, Carr NJ, Peveler RC, Moss-Morris R, Verrill C, Robbins E, et al. Relationship between loneliness and proangiogenic cytokines in newly diagnosed tumors of colon and rectum. Psychosomatic Medicine. 2010;72(9):912–916. doi: 10.1097/PSY.0b013e3181f0bc1c. [DOI] [PubMed] [Google Scholar]
- 9.Peplau LA, Perlman D. Perspectives on loneliness. In: Peplau LA, Perlman D, editors. Loneliness: A sourcebook of current theory, research and therapy. New York: Wiley-Interscience; 1982. pp. 1–18. [Google Scholar]
- 10.Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601. [DOI] [PubMed] [Google Scholar]
- 11.Deckx L, van den Akker M, Buntinx F. Risk factors for loneliness in patients with cancer: A systematic literature review and meta-analysis. European Journal of Oncology Nursing. 2014;18(5):466–477. doi: 10.1016/j.ejon.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Adams RN, Mosher CE, Abonour R, Robertson MJ, Champion VL, Kroenke K. Cognitive and situational precipitants of loneliness among patients with cancer: A qualitative analysis. Oncology Nursing Forum. 2016;43(2):156–163. doi: 10.1188/16.ONF.156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosedale M. Survivor loneliness of women following breast cancer. Oncology Nursing Forum. 2009;36:175–183. doi: 10.1188/09.ONF.175-183. [DOI] [PubMed] [Google Scholar]
- 14.Lepore S, Revenson T. Social constraints on disclosure and adjustment to cancer. Social and Personality Psychology Compass. 2007;1(1):313–333. doi: 10.1111/j.1751-9004.2007.00013.x. [DOI] [Google Scholar]
- 15.Adams RN, Winger JG, Mosher CE. A meta-analysis of the relationship between social constraints and distress in cancer patients. Journal of Behavioral Medicine. 2015;38(2):294–305. doi: 10.1007/s10865-014-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosher CE, Lepore S, Wu L, Austin J, Valdimarsdottir H, Rowley S, et al. Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. Journal of Health Psychology. 2012;17(7):1022–1032. doi: 10.1177/1359105311432490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Personality and Social Psychology Review. 2010;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutrona CE. Transition to college: Loneliness and the process of social adjustment. In: Peplau LA, Perlman D, editors. Loneliness: A sourcebook of current theory, research, and therapy. New York: Wiley-Interscience; 1982. pp. 291–309. [Google Scholar]
- 19.Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, et al. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40(6):1054–1085. doi: 10.1016/j.jrp.2005.11.007. [DOI] [Google Scholar]
- 20.de Jong-Gierveld J. Developing and testing a model of loneliness. Journal of Personality and Social Psychology. 1987;53(1):119–128. doi: 10.1037//0022-3514.53.1.119. [DOI] [PubMed] [Google Scholar]
- 21.de Jong-Gierveld J, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness confirmatory tests on survey data. Research on Aging. 2006;28(5):582–598. doi: 10.1177/0164027506289723. [DOI] [Google Scholar]
- 22.Vincenzi H, Grabosky F. Measuring the emotional/social aspects of loneliness and isolation. Journal of Social Behavior & Personality. 1987;2(2):257–270. [Google Scholar]
- 23.Sand L, Strang P, Milberg A. Dying cancer patients’ experiences of powerlessness and helplessness. Supportive Care in Cancer. 2008;16(7):853–862. doi: 10.1007/s00520. [DOI] [PubMed] [Google Scholar]
- 24.Lepore S. A social–cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial interventions for cancer. Washington, DC: American Psychological Association; 2001. pp. 99–118. [Google Scholar]
- 25.Manne S, Norton TR, Ostroff JS, Winkel G, Fox K, Grana G. Protective buffering and psychological distress among couples coping with breast cancer: The moderating role of relationship satisfaction. Journal of Family Psychology. 2007;21(3):380–388. doi: 10.1037/0893-3200.21.3.380. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, et al. Content validity of patient-reported outcome measures: Perspectives from a PROMIS meeting. Quality of Life Research. 2012;21(5):739–746. doi: 10.1007/s11136-009-9496-9. [DOI] [PubMed] [Google Scholar]
- 29.Badr H, Smith CB, Goldstein NE, Gomez JE, Redd WH. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: Results of a randomized pilot trial. Cancer. 2015;121(1):150–158. doi: 10.1002/cncr.29009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum G, Basen-Engquist K, Swartz MC, Parker PA, Carmack CL. Comparing PROMIS computer-adaptive tests to the Brief Symptom Inventory in patients with prostate cancer. Quality of Life Research. 2014;23(7):2031–2035. doi: 10.1007/s11136-014-0647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, Correia H, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychology. 2014;33(5):490–499. doi: 10.1037/hea0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Quality of Life Research. 2010;19(1):125–136. doi: 10.1007/s11136-009-9560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassar M, Crosby JW. A reliability generalization study of coefficient alpha for the UCLA Loneliness Scale. Journal of Personality Assessment. 2008;90(6):601–607. doi: 10.1080/00223890802388624. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. doi: 10.1001/jama. [DOI] [PubMed] [Google Scholar]
- 37.Kline RB. Principles and practice of structural equation modeling. Third. New York: The Guilford Press; 2011. [Google Scholar]
- 38.Tukey JW. The future of data analysis. The Annals of Mathematical Statistics. 1962;33(1):1–67. [Google Scholar]
- 39.Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. doi: 10.1080/01621459.1988.10478722. [DOI] [Google Scholar]
- 40.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 41.Egestad H. The significance of fellow patients for head and neck cancer patients in the radiation treatment period. European Journal of Oncology Nursing. 2013;17(5):618–624. doi: 10.1016/j.ejon.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Bredemeier K. A unified model of depression integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science. 2016:1–24. doi: 10.1177/2167702616628523. [DOI] [Google Scholar]
- 43.Pinquart M, Sorensen S. Influences on loneliness in older adults: A meta-analysis. Basic and Applied Social Psychology. 2001;23(4):245–266. doi: 10.1207/153248301753225702. [DOI] [Google Scholar]
- 44.Eakin EG, Strycker LA. Awareness and barriers to use of cancer support and information resources by HMO patients with breast, prostate, or colon cancer: Patient and provider perspectives. Psycho-Oncology. 2001;10(2):103–113. doi: 10.1002/pon.500. [DOI] [PubMed] [Google Scholar]
- 45.Wells M, Kelly D. The loneliness of cancer. European Journal of Oncology Nursing. 2008;12(5):410–411. doi: 10.1016/j.ejon.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaremka LM, Peng J, Bornstein R, Alfano CM, Andridge RR, Povoski SP, et al. Cognitive problems among breast cancer survivors: Loneliness enhances risk. Psycho-Oncology. 2014;23(12):1356–1364. doi: 10.1002/pon.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langer SL, Brown JD, Syrjala KL. Intrapersonal and interpersonal consequences of protective buffering among cancer patients and caregivers. Cancer. 2009;115(S18):4311–4325. doi: 10.1002/cncr.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepore S, Helgeson V. Social constraints, intrusive thoughts, and mental health after prostate cancer. Journal of Social and Clinical Psychology. 1998;17(1):89–106. doi: 10.1521/jscp.1998.17.1.89. [DOI] [Google Scholar]
- 50.Manne S, Sherman M, Ross S, Ostroff J, Heyman RE, Fox K. Couples’ support-related communication, psychological distress, and relationship satisfaction among women with early stage breast cancer. Journal of Consulting and Clinical Psychology. 2004;72(4):660–670. doi: 10.1037/0022-006X.72.4.660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


