Abstract
Descriptions of the cognitive functions affected by alcohol use disorders (AUD) often highlight dysfunction of executive processes such attention, inhibitory control, working memory, and cognitive flexibility. Such complex cognitive functions have historically been ascribed to the prefrontal cortex. AUD, however, disrupts extensive areas of the brain. Structural and functional MRI studies suggest a central role for degradation of circuitry originating in the prefrontal cortex including nodes in widespread brain regions. This review features fronto-fugal circuits affected by AUD including frontocerebellar, frontolimbic, and frontostriatal networks and their relations to the salient, enduring, and debilitating cognitive and motor deficits reported in AUD.
Keywords: cerebellum, limbic system, hippocampus, striatum, circuitry, networks
INTRODUCTION
In the Diagnostic Statistical Manual of Mental Disorders 5th Edition (DSM-5) published in 2013, the biaxial distinction between alcohol abuse and alcohol dependence defined in the DSM-IV was replaced with an umbrella diagnosis of alcohol use disorder (AUD) with continua of symptoms. In the DSM-5 revision, the criterion of alcohol-related legal problems was dropped and a new craving criterion was added (Table 1). The criterion, “continued use despite untoward consequences,” a form of perseveration, present in both DSM-IV and DSM-5, also characterizes behavior in patients with frontal lobe lesions. Our work is based on the hypothesis that some people consume alcohol to excess, even though it interferes knowingly with their wellbeing, because alcohol changes the brain. Acute alcohol intoxication reversibly affects brain while continued consumption causes brain structural and functional brain changes, or neuroadaptations that can promote the need for further alcohol consumption. In this way, AUD is a self-perpetuating disorder.
Table 1.
DSM-IV versus DSM-5 criteria for Alcohol Abuse, Dependence, or Use Disorder
| DSM-IV Alcohol Abuse or Alcohol Dependence | DSM-5 Alcohol Use Disorder |
|---|---|
|
| |
| Two distinct but hierarchical constucts: alcohol abuse only diagnosed in absence of alcohol dependence | A single, unitary construct, with moderate and severe diagnoses based on number of criteria endorsed. |
| alcohol abuse: 1+ abuse criteria required | moderate AUD: 2–3 criteria required |
| alcohol dependence: 3+ dependence criteria required | severe AUD: 4+ criteria required |
|
| |
| Abuse Criteria: | Use Disorder Criteria: |
|
| |
| recurrent use resulting in failure to fulfill work, school, home obligations. | recurrent use resulting in failure to fulfill work, school, home obligations. |
| recurrent use when physically hazardous | recurrent use when physically hazardous |
| recurrent use despite social or interpersonal problems | recurrent use despite social or interpersonal problems |
| recurrent use despite legal problems | - |
|
|
|
| Dependence Criteria | |
|
|
|
| tolerance | tolerance |
| withdrawal or use to relieve/avoid withdrawal | withdrawal or use to relieve/avoid withdrawal |
| drinking in larger amounts or for longer than intended | drinking in larger amounts or for longer than intended |
| persistent desire or unsucessful attempts to reduce or stop drinking | persistent desire or unsucessful attempts to reduce or stop drinking |
| inordinate time spent obtaining alcohol | inordinate time spent obtaining alcohol |
| societal, occupational, recreational activites reduced | societal, occupational, recreational activites reduced |
| continued use despite physiological or psychological problems | continued use despite physiological or psychological problems |
Here we focus on neural nodes, networks, and functions damaged and potentially reparable in AUD based on findings from neuroimaging and neuropsychology and organize them according to three structural and functional brain systems: frontocerebellar, frontolimbic, and frontostriatal networks.
Prefrontal Cortex Anatomy and Function
The prefrontal cortex (PFC), delimited by the presence of a granular layer IV, has been subdivided based on Brodmann areas to include dorsolateral (Brodmann areas 8, 9, 46), ventrolateral (Brodmann areas 44, 45, lateral 47), orbitofrontal (also known as ventromedial, basal, or orbital and includes Brodmann areas orbital 47 and 11), and frontopolar (also known as anterior or rostral and includes Brodmann area 10) regions (Jacobsen, 1936). The PFC integrates information from other cortical and subcortical regions (Wilson et al., 2010): its principal role, as defined from a neuropsychological perspective, is executive functioning, which includes attentional and inhibitory control, working memory, and cognitive flexibility (Fuster, 1985; Goldman-Rakic, 1996; Mangels, 1997; Rezai et al., 1993; Zald and Andreotti, 2010).
Pattern of Neuropsychological Changes in AUD
Neuropsychological tests conducted in lesion (e.g., stroke, focal injury, surgery) studies identify disruptions in select functions associated with specific brain structures (e.g., Caramazza et al., 1990; Markowitsch, 1984; Vaina, 1989). The use of similar tests in AUD not only establishes a pattern of neuropsychological impairment and sparing, but also permits the investigation of brain structures potentially underlying the pattern of observed behaviors. Neuropsychological tests commonly administered to AUD subjects include tests of executive functions, working memory, declarative memory, visuospatial abilities, emotion, and motor ability. From these tests, AUD subjects relative to healthy controls have been found to have mild to moderate impairments of component processes involving executive function, working memory, declarative memory, visuospatial abilities, upper limb motor ability, and gait and balance (Fig. 1) (Bechara et al., 2001; Chanraud et al., 2007; Ellis and Oscar-Berman, 1989; Fama et al., 2009; Fernandez-Serrano et al., 2010; Maurage et al., 2014; Oscar-Berman and Marinkovic, 2007; Sullivan et al., 2002; Sullivan et al., 2000b; Sullivan et al., 2000c; Van Horn et al., 2006; Weafer and Fillmore, 2015). The pattern of neuropsychological deficits in AUD has been used to guide where to look in the brain for compromise.
Fig. 1.
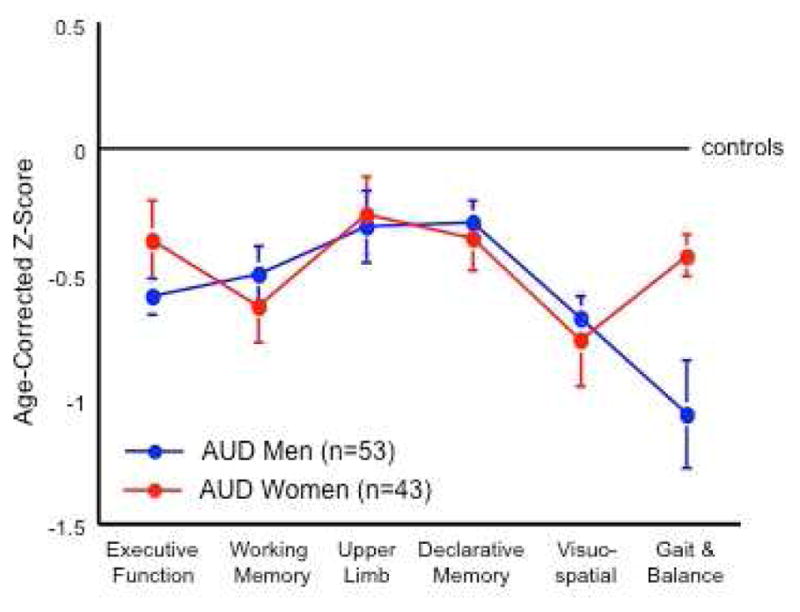
Profile of cognitive and motor deficits in men and women with Alcohol Use Disorders (AUD) relative to healthy control individuals. Apart from balance, AUD men and women show a similar pattern of impairment. Modified from Sullivan, E. V., Rosenbloom, M. J., Pfefferbaum, A., 2000c. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24, 611–621.
Prefrontal Cortex in Normal Aging and AUD
Normal aging has profound effects on brain structure including expansion of the ventricles and decreases in tissue volume (e.g., Ge et al., 2002; Pfefferbaum et al., 1994; Pfefferbaum et al., 2013; Symonds et al., 1999; Tang et al., 2001). Relevant to the current discussion, the PFC naturally atrophies with age (Calso et al., 2016; Mander et al., 2013; Schretlen et al., 2000). Data from normal aging permits modeling of age-related brain changes (Cabeza and Dennis, 2013) and permits detection of age-disease interactions. Older individuals with AUD, for example, have disproportionate volume deficits in frontal gray and white matter relative to their younger counterparts, independent of age of AUD onset (Pfefferbaum et al., 1997).
Postmortem evaluation of AUD brains has noted biochemical disturbances in frontal regions including effects on gene, protein, and receptor expression (e.g., Alexander-Kaufman et al., 2006; Henriksson et al., 2008; Lewohl et al., 2000; Mayfield et al., 2002; Mitsuyama et al., 1998; Watanabe et al., 2009). Synaptic loss (i.e., Brodman’s area 10, Brun and Andersson, 2001), changes in neuronal morphology, and decreases in neuronal density have also been reported in the AUD frontal cortex (Harper and Kril, 1989; Harper et al., 1987; Kril et al., 1997). Indeed, neuronal loss in the frontal lobes in AUD might explain cognitive deficits that persist with abstinence.
Magnetic resonance imaging (MRI) tools have confirmed postmortem findings of a specific vulnerability of frontal regions in AUD relative to healthy control individuals including volume deficits (Cardenas et al., 2011; Chanraud et al., 2007; Fein et al., 2002; Jernigan et al., 1991; Kubota et al., 2001; Makris et al., 2008; Pfefferbaum et al., 1998; Sullivan et al., 1998), low rates of glucose metabolism (Moselhy et al., 2001; Volkow et al., 1990; Volkow et al., 2008), and depressed BOLD responses (e.g., during inhibition tasks Cservenka and Nagel, 2012; Li et al., 2009; Marinkovic et al., 2012; O’Daly et al., 2012), possibly related to altered resting state activity of executive control networks (Krmpotich et al., 2013; Weiland et al., 2014; Zhu et al., 2015). Additionally, MR spectroscopy (MRS) studies with voxels placed in frontal regions report lower levels of the brain metabolite, N-acetylaspartate (NAA) in recently sober AUD subjects relative to healthy controls (Bendszus et al., 2001; Durazzo et al., 2004; Durazzo et al., 2010; Fein et al., 1994; Jagannathan et al., 1996; Meyerhoff et al., 2004; Schweinsburg et al., 2003; Seitz et al., 1999). Although the frontal lobes are considered a primary site of pathology in AUD (Moselhy et al., 2001; Oscar-Berman and Hutner, 1993), expected correlations between relatively simple measures such as volumes of frontal lobes and performance on relevant neuropsychological tests in AUD have not always been forthcoming. Instead, circuitry originating and moving away from the frontal cortex (i.e., fronto-fugal) has been proposed to underlie some of the distinct cognitive and motor functions shown in behavioral studies to be impaired in AUD (Geschwind and Kaplan, 1962; Sullivan et al., 2003a; Sullivan and Pfefferbaum, 2005; Thiebaut de Schotten et al., 2015).
Frontocerebellar Systems
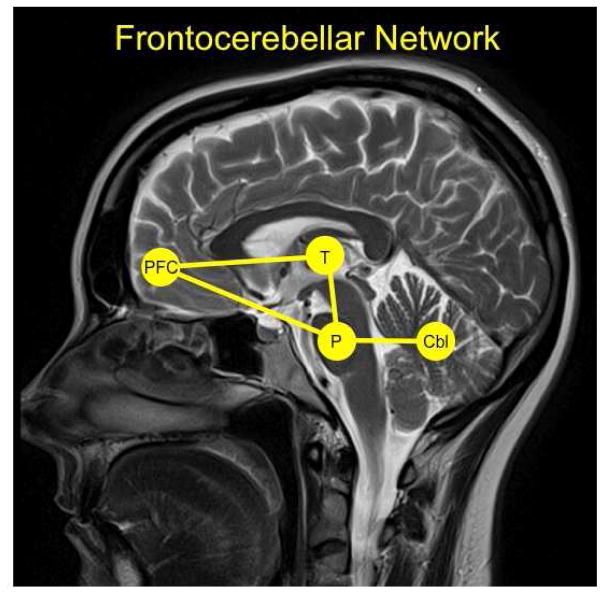
The frontocerebellar network includes efferent pathways from frontal lobes to cerebellum via pons and afferents to frontal lobes from cerebellum via thalamus (Fig. 2) (Ramnani and Miall, 2001). To our knowledge, the anatomy linking frontal brain regions with the cerebellum was first proposed by Dr. Henrietta C. Leiner (Leiner et al., 1986, 1989; Leiner et al., 1993a; Leiner et al., 1993b; Leiner et al., 1993c; Leiner et al., 1993d). Connections between the frontal cortex and cerebellum were confirmed by careful anatomic work in monkeys using viral tracing methods to identify parallel closed loops forming frontocerebellar circuits that support dissociable behaviors (Kelly and Strick, 2003; Middleton and Strick, 1994). Neuroimaging studies confirmed the presence of segregated corticocerebellar loops (Habas et al., 2009; Krienen and Buckner, 2009; Schmahmann and Pandya, 1997). Examples of these divergent but parallel loops include: (1) the motor network, involving motor lobules of the cerebellar vermis (e.g., IV, V, VI) and motor cerebral cortices (Biswal et al., 1995) (2) the executive network, involving the cerebellar neocortex (e.g., lobule VII, lobule VIII, Crus I, and Crus II) and PFC sites (e.g., Brodmann areas 9 and 46).
Fig. 2.
The frontocerebellar network with key nodes highlighted including the prefrontal cortex (PFC), thalamus (T), pons (P), and cerebellm (Cbl).
There is some initial evidence that a family history of AUD is associated with altered frontocerebellar connectivity in alcohol naïve youth (Herting et al., 2011). In adults with AUD, structural MRI has demonstrated that relative to healthy controls, the volumes of the pons, thalamus, cerebellar hemispheres, and cerebellar vermis are smaller (Fig. 3) (Chanraud et al., 2007; Pfefferbaum et al., 1992; Sullivan et al., 2000a; Sullivan et al., 1998; Sullivan et al., 2003b). These findings are apparently generalizable as AUD cohorts in both the US and France relative to healthy control individuals show similar volume deficits (i.e., smaller volumes of pons, thalamus, cerebellar hemispheres and vermis, and frontal lobes (Fig. 4) (Le Berre et al., 2014).
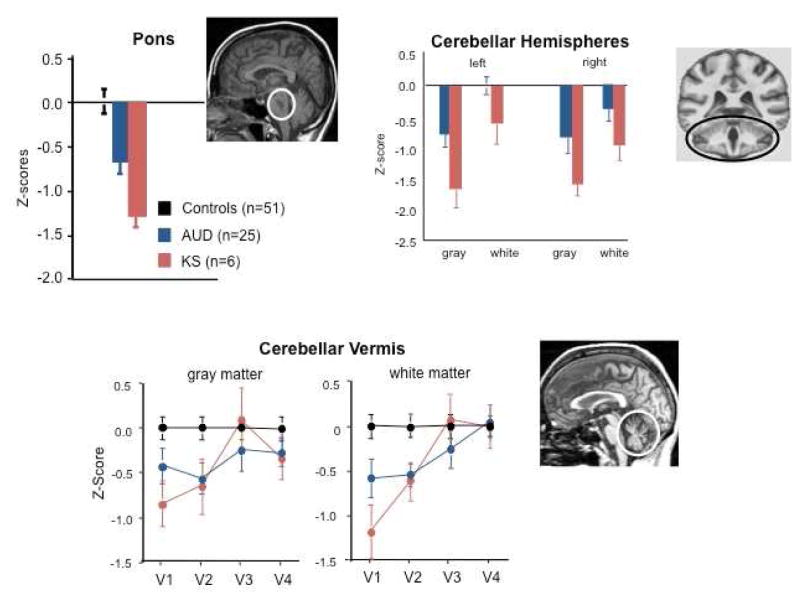
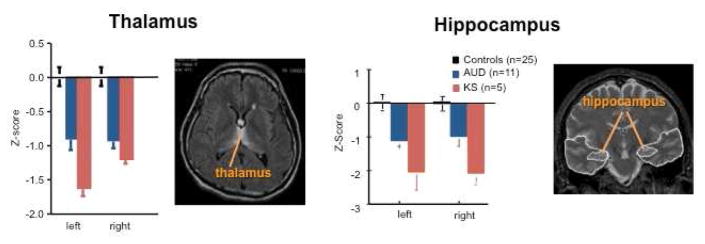
Fig. 3.
Mean ± SE of volumes (expressed as standardized Z-scores, adjusted for normal variation in intracranial volume and age) of pons, cerebellar hemispheres, and cerebellar vermis (quantified as areas V1–V4) in healthy control subjects (black), subjects with Alcohol Use Disorders (AUD, blue), and those with Korsakoff’s syndrome (KS, red). The expected value of the controls is 0 with a standard deviation = 1; low values of volume in AUD and KS groups reflect volume deficits. The pons, cerebellar hemispheres, and vermis each demonstrate a trend toward a graded effect from AUD to KS. Modified from Sullivan, E. V., Pfefferbaum, A., 2009. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 44, 155–165.
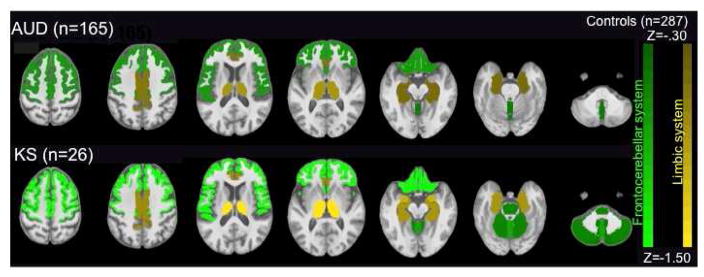
Fig. 4.
Results from a large-scale study pooling MRI data across 2 French sites (Orsay and Caen) and our US site, resulting in normative data from 287 healthy controls. Here, the age-corrected Z-scores derived from the common control group are now coded by color shading and the volumes of the regions of interest were identified with parcellation of selective brain structures—the green represents frontocerebellar regions and mustard represents nodes of the limbic system that showed volume deficits in AUD compared with controls. The brighter colors indicate even greater severity and extent of deficits in KS than AUD or controls, again indicative of the graded deficit concept. From Le Berre, A. P., Pitel, A. L., Chanraud, S., Beaunieux, H., Eustache, F., Martinot, J. L., Reynaud, M., Martelli, C., Rohlfing, T., Sullivan, E. V., Pfefferbaum, A., 2014. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp 35, 4635–4653.
In addition to identifying volume compromise in nodes of the frontocerebellar circuit, further support for the involvement of this network in AUD (Sullivan et al., 2003a; Sullivan and Pfefferbaum, 2005) emerged from work seeking a neural substrate for ataxia. Body sway, measured with posturographical recordings from a force platform, is far greater in AUD individuals, even after 1 month of sobriety, than in age-matched, low drinking individuals (e.g., Bauer, 1993; Diener et al., 1984a; Ledin and Odkvist, 1991; Scholz et al., 1986; Sullivan et al., 2000a). Correlational analyses revealed that a longer sway path during quiet standing in AUD is associated with a smaller volume of the anterior superior vermis of the cerebellum (Sullivan et al., 2000a; Sullivan et al., 2010a), recapitulating findings observed in patients with lesions of the cerebellar vermis (Diener et al., 1984b). Whereas those with lesions of the vermis do not benefit from visual cues (Diener et al., 1984b), those with AUD successfully used vision to normalize sway (Sullivan et al., 2006). These findings are in contrast to healthy aging men and women, wherein greater postural sway was associated with ventricular and sulcal enlargement and white matter hyperintensity burden (Sullivan et al., 2009). Truncal tremor, measured by analyzing the temporal frequency of the sway path, is also evident in individuals with AUD: tremor at 3–5 Hz (Koller et al., 1985) and 5–7 Hz has been reported (Sullivan et al., 2010b; Sullivan et al., 2015). As with sway path, tremor (both low- (i.e., 3–5 Hz) Sullivan et al., 2006 and high- (i.e., 5–7 Hz) ) in AUD is associated with vermian volume (Sullivan et al., 2015). Tremor elicited in these two frequency bands by cognitive challenge during quiet standing showed degradation of separate brain systems: the lower frequency tremor was associated with integrity of the cerebellar hemispheres and superior cingulate bundles, whereas higher frequency tremor correlated with motor cortex and internal capsule integrity (Sullivan et al., 2015).
Further evidence indicates that recruitment of functionally intact cerebellar loops in AUD can compensate for otherwise impaired performance on tasks requiring, for example, visuospatial or working-memory skills (Sullivan and Pfefferbaum, 2005). A biological interpretation is that the recruitment of an unaffected loop parallel to the affected one leads to rapid changes in connectivity, which depend on already present but “silent” connections rather than the creation of new ones (Nakayama et al., 2005). For example, in AUD, equivalent performance on a verbal working memory task is associated with increased, bilateral activation of Brodmann areas 44/45 (left frontal) and right superior cerebellum (Fig. 5) (Desmond et al., 2003; Desmond et al., 1997). In further investigation of verbal working memory, individuals with AUD activate frontal and cerebellar regions, a task accomplished by controls with only the frontal system (Desmond et al., 2003). Similarly, exploratory analysis of language skills revealed that, even though individuals with AUD show comparable performance in terms of error rates and response time relative to controls, they exhibit greater fMRI response in the left middle frontal gyrus, right superior frontal gyrus, and cerebellar vermis relative to controls (Chanraud-Guillermo et al., 2009). During an impulsivity task, AUD subjects with compromised frontocerebellar functional circuitry recruited an alternative network, including the premotor cortex, to perform well under low-risk, unambiguous conditions (Jung et al., 2014). In summary, the positive outcome of shift and expansion in functional anatomy can be apparently normal performance, but this comes at the price of usurping reserve that reduces processing capacity for conducting multiple tasks simultaneously and efficiently (e.g., De Rosa et al., 2004; Desmond et al., 2003; Jung et al., 2014; Pfefferbaum et al., 2001; Sullivan and Pfefferbaum, 2005; Tapert et al., 2001).
Fig. 5.
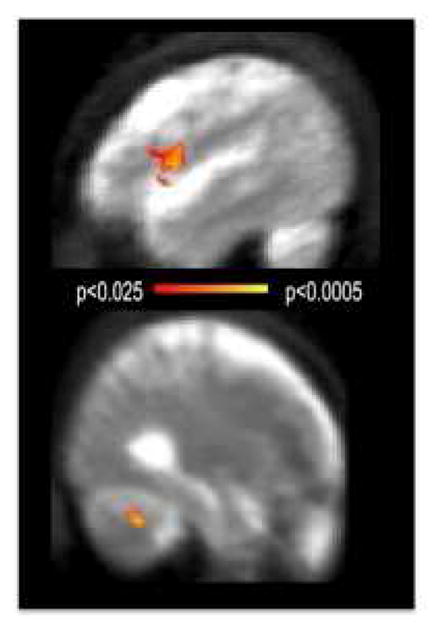
AUD activated frontal and cerebellar regions to maintain verbal material in working memory, a task accomplished by controls with the frontal system only.
Connectivity analyses of fMRI data collected at rest has identified intrinsic functional connectivity networks that are more activated and synchronous at rest than while engaged in a task (Biswal et al., 1995; Raichle and Snyder, 2007; Van Dijk et al., 2010). The initially identified network, referred to as the default mode network (DMN), has as a seed – the start point of the correlation between that region and the activity in the rest of the brain – the posterior cingulate cortex: its activity is correlated with medial PFC and other sites, making a functional network. DMN integrity may provide an index of neural health and flexibility (Chanraud et al., 2011). Individuals with AUD showed the same pattern of activation synchrony as controls but had less robust synchrony between posterior cingulate and cerebellar regions (Chanraud et al., 2011). During a spatial working memory task, whereas controls recruited prefrontal-cerebellar regions VI/Crus I known to subserve working memory, individuals with AUD recruited two other parallel frontocerebellar loops: dorsolateral PFC-cerebellar VIII system during rest and dorsolateral PFC-cerebellar VI system while task-engaged. Greater synchronous activity between cerebellar lobule VIII and dorsolateral PFC at rest and greater activation within cerebellar lobule VI and dorsolateral PFC while preforming a task predicted better working memory performance. Thus, higher intrinsic cerebellar activity in AUD was an adequate condition for triggering task-relevant activity in the frontal cortex required for normal working memory performance (Chanraud et al., 2013). Together, these findings suggest that despite equivalence in behavioral performance, either a functional reorganization of brain systems or an invocation of inappropriate brain systems has occurred as a consequence of AUD.
Frontolimbic Systems
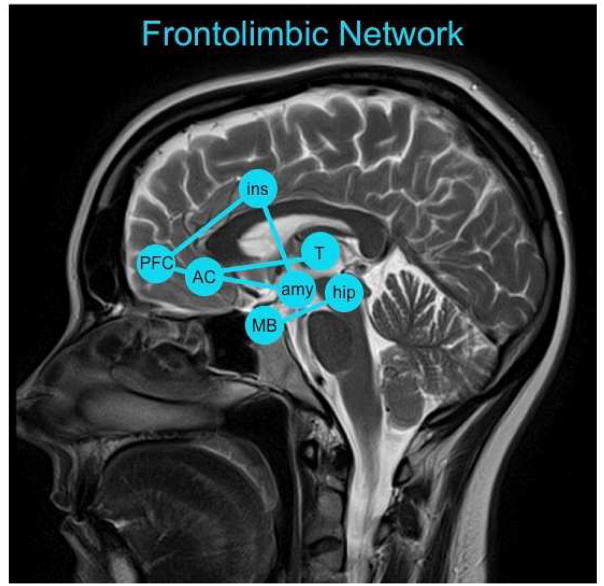
Herein, the frontolimbic network includes connections between the PFC and the insula, anterior cingulate, amygdala, hippocampus, mammillary bodies, and thalamus (Fig. 6) (Broca, 1878; Newman and Harris, 2009; Papez, 1937). Although the limbic system is highly interconnected with the nucleus accumbens (Morgane et al., 2005), this and other basal ganglia structures will be discussed in the section on Frontostriatal Systems. Frontolimbic circuitry was identified as relevant to AUD (e.g., Harris et al., 2008; Lingford-Hughes et al., 2012; Wrase et al., 2008) in the search of a neural substrate for mild memory impairment (Cermak, 1987; Fama et al., 2009; Goldman et al., 1991; Oscar-Berman and Ellis, 1987). This circuitry is also relevant to allostatic load (e.g., Herman, 2012) and negative emotions (e.g., irritability, anxiety, depression, the so-called, “dark side of addiction”) that emerge when alcohol is not available (Koob and Le Moal, 2005; Lu and Richardson, 2014).
Fig. 6.
The frontolimbic network with key nodes highlighted including the prefrontal cortex (PFC), insula (ins), anterior cingulate (AC), amygdala (amy), hippocampus (hip), mammillary bodies (MB), and thalamus (T).
The sequelae of ‘uncomplicated” AUD marked by mild to moderate memory impairment has been compared with individuals with AUD that have Korsakoff’s syndrome (KS), which is marked by dense anterograde amnesia, the result of thiamine deficiency and Wernicke’s encephalopathy (WE) (Dreyfus and Victor, 1961). Classically, WE is characterized by the detection of three clinical signs: ophthalmoplegia, ataxia, and confusion. In “uncomplicated” AUD, however, the incidence of neuropathological signs of WE seen postmortem remains higher than recognized in vivo (Harper, 1983; Harper et al., 1986). In an attempt to diagnose WE using objective in vivo criteria, a chart review of postmortem cases evaluated 4 general operational markers in place of the 3 traditional, clinical signs: signs of oculomotor abnormalities in place of ophthalmoplegia, any indication of cerebellar dysfunction in place of ataxia, altered mental state in place of confusion, and additionally noting evidence for dietary deficiency. Having only 2 of 4 of these more generalized indicators correlated with presence of neuropathology indicative of WE (Caine et al., 1997). Using operationalized definitions of the 4 signs, we evaluated 56 AUD subjects relative to 38 healthy controls and found that although none of the AUD subjects presented with the 3 classical signs, a large proportion of this group met 1 (>50%) or 2 (16%) criteria; only about one quarter of the group were free of signs. The pattern of functional impairments quantified using neuropsychological tests across the AUD group, without consideration of the criteria, indicated only mild to moderate deficits in a few functional domains. However, when the AUD group was divided by the number of criteria met, AUD individuals meeting no criteria performed at normal levels in all domains, those meeting 1 criterion showed modest selective compromise, and those meeting 2 criteria were the most widely and severely impaired (Pitel et al., 2011). This AUD cohort was also used to demonstrate that poor memory performance was related to lower blood levels of the biologically relevant form of thiamine, thiamine diphosphate (TDP) (Pitel et al., 2011).
Although amnesic disorders are often attributed to disturbances of the hippocampus (Sullivan et al., 2000a; Sullivan et al., 2002), the classical neuropathology of KS has been primarily linked to atrophy of the mammillary bodies (Bigler et al., 1989). Compared with controls, KS subjects show profound bilateral volume deficits of the mammillary bodies: uncomplicated, non-amnesic individuals with AUD also have mammillary bodies volume deficits, but to a milder degree. The thalamus and hippocampus likewise show graded volume deficits in these study populations (Fig. 7) (Le Berre et al., 2014; Sullivan et al., 1999; Sullivan and Pfefferbaum, 2009). Although the mammillary bodies appear to be the primary site of damage in KS, input from other brain regions, including the frontal cortex, hippocampus, cerebellum, and amygdala, may have a modulatory role on memory function in AUD (Kril and Harper, 2012; Poldrack and Gabrieli, 1997).
Fig. 7.
Mean ± SE of volumes (expressed as standardized Z-scores, adjusted for normal variation in intracranial volume and age) of thalamus and hippocampus in healthy control subjects (black), subjects with Alcohol Use Disorders (AUD, blue), and those with Korsakoff’s syndrome (KS, red). The thalamus and hippocampus both demonstrate a trend toward a graded effect from AUD to KS. Modified from Sullivan, E. V., Pfefferbaum, A., 2009. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 44, 155–165.
As was discussed for the frontocerebellar systems, there is evidence of sparing of particular structures of the frontolimbic system. For example, inferior cingulate cortical volume appears to be relatively spared in AUD and may compensate for compromised frontal regions and contribute to successful recall (Rosenbloom et al., 2009). In a face-name associative learning task, those with AUD had preserved limbic activation but lower cerebellar (Crus II) activation than controls (Pitel et al., 2013). This finding could be the result of functional reorganization: functional connectivity analysis at rest showed that in controls, the left hippocampus and left Crus II are positively synchronized, while in AUD these two regions are negatively synchronized (Pitel et al., 2013).
In addition to its role as a substrate for memory, the frontolimbic system is involved with emotional regulation. In social drinkers, acute alcohol attenuates the response to fearful stimuli in limbic regions (Gilman and Hommer, 2008). In another fMRI study, control subjects showed stronger activation in the amygdala and hippocampus when viewing faces with emotional relative to neutral expressions, whereas individuals with AUD responded in an undifferentiated manner to all facial expressions (Marinkovic et al., 2009). The observation that individuals with AUD respond to emotionally-valenced stimuli in an undifferentiated manner is consistent with clinical evidence of their interpersonal difficulties and may constitute a relapse factor (Kornreich et al., 2002). In the AUD participants, amygdala activity was inversely correlated with an increase in lateral PFC activity as a function of behavioral deficits (Marinkovic et al., 2009). PFC activity as a compensation for blunted limbic activity is in agreement with distributed network engagement and suggests that in AUD, the brain relies on frontal rather than temporal areas to maintain behavioral adequacy when faced with emotionally or socially challenging situations.
Frontostriatal Systems
The frontostriatal circuits connect frontal lobe regions with the basal ganglia (Fig. 8) (Alexander et al., 1986; Tekin and Cummings, 2002). Interest in the frontostriatal system in AUD emerged from the rich literature on dopaminergic activity in substance dependence (e.g., Noble, 1996). Single photon emission computed tomography (SPECT) and Positron Emission Tomography (PET) studies have quantified and reported disrupted dopamine transmission in AUD (Boileau et al., 2003; Ebert et al., 2002; Guardia et al., 2000; Heinz et al., 2004; Laine et al., 1999a; Laine et al., 1999b; Martinez et al., 2005; Repo et al., 1999; Tiihonen et al., 1995; Tiihonen et al., 1998; Volkow et al., 1996; Yoder et al., 2005).
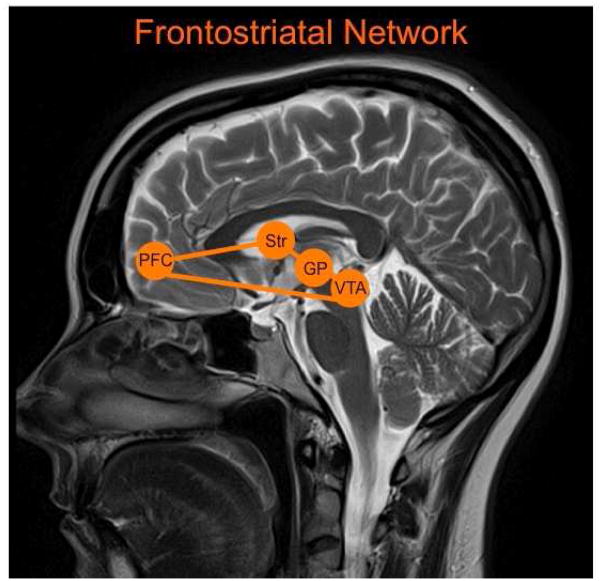
Fig. 8.
The frontostriatal network with key nodes highlighted including the prefrontal cortex (PFC), striatum (Str), globus pallidus (GP), and ventral tegmental area (VTA).
Nodes of the frontostriatal system are affected by AUD (e.g., Namura and Hishikawa, 1997). Volumes of the caudate, putamen, and nucleus accumbens are smaller in AUD men than age-matched control men, regardless of length of sobriety (Fig. 9) (Makris et al., 2008; Sullivan et al., 2005; Wrase et al., 2008). Whether smaller volumes (Benegal et al., 2007) or altered activation patterns (Acheson et al., 2009; Andrews et al., 2011; Casement et al., 2015; Heitzeg et al., 2008; Heitzeg et al., 2010; Nees et al., 2012; Yau et al., 2012) in reward-related regions can predict higher risk for AUD remains equivocal. While there are inherent difficulties in resolving brain volumes as a function of premorbid condition or outcome of alcohol exposure, another confounding factor may be sex: binge drinking men showed smaller prefrontal, striatal, and medial temporal lobe volumes relative to non-binge drinking men, but binge drinking women showed the inverse pattern of larger volumes of these areas relative to non-binge drinking women (Kvamme et al., 2016). A recent study of 872 Mexican-American individuals evaluating subcortical volumes suggested that amygdala volume, but not volumes of hippocampus, caudate, putamen, pallidum, or thalamus, is sensitive to genetic predisposition for AUD (Dager et al., 2015). In an fMRI study, adolescents that were family history positive for AUD showed a pattern of nucleus accumbens connectivity to supplementary sensorimotor area and precuneus that correlated positively with and mediated the relationship with sensation seeking and also with total volume of alcohol consumed (Weiland et al., 2013). This latter finding indicates that a number of logistical and scientific challenges, including the use of prospective, longitudinal neuroimaging data from alcohol-naïve pre-adolescents, remain to be addressed to distinguish clearly neurodevelopmental precursors from the consequences of alcohol exposure (Fishbein et al., 2016).
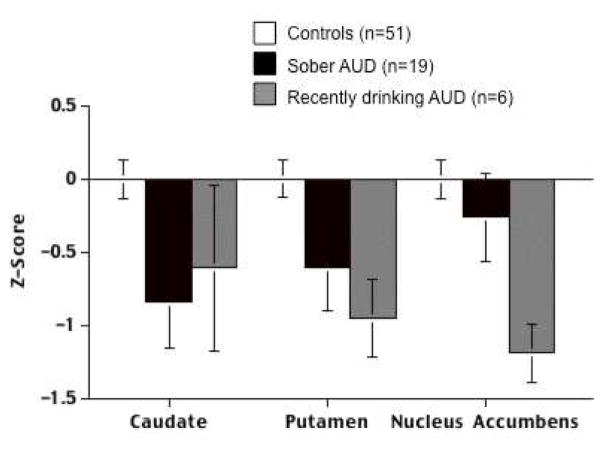
Fig. 9.
Volume measures (expressed as age- and intracranial volume -corrected Z scores) for mean bilateral volumes of caudate, putamen, and nucleus accumbens for 51 control subjects, 6 AUD subjects who had been drinking in the past 3 weeks, and 19 AUD subjects with a median sobriety of 1 year. Modified from Sullivan, E. V., Deshmukh, A., De Rosa, E., Rosenbloom, M. J., Pfefferbaum, A., 2005. Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biological Psychiatry 57, 768–776.
One of the first relevant fMRI studies showed that alcohol-associated visual stimuli activated the ventral putamen in AUD but not control subjects (Braus et al., 2001). This was followed by several visual cue-reactivity fMRI studies in AUD showing brain regions, particularly the basal ganglia (Wrase et al., 2002), including the putamen, nucleus accumbens, and medial PFC, responding more intensely to images of alcohol vs. other images (Grusser et al., 2004; Ihssen et al., 2011; Myrick et al., 2004; Myrick et al., 2008; Seo et al., 2011; Wrase et al., 2006). Olfactory (Bragulat et al., 2008; Kareken et al., 2010; Kareken et al., 2004; Schacht et al., 2013; Schacht et al., 2011) and taste cues (Claus et al., 2011; Feldstein Ewing et al., 2010; Filbey et al., 2008a; Filbey et al., 2008b; Myrick et al., 2010) resulted in similar activation patterns in mesocortical structures. It appears that brain activation in individuals with AUD is biased towards responding to alcohol-associated cues, as the ventral striatum shows greater activation in response to alcohol cues but lower activation during anticipation of monetary gain in AUD relative to control individuals (Beck et al., 2009; Wrase et al., 2007). Additional non-drug, monetary incentive, reward-task based fMRI studies also demonstrate altered basal ganglia functioning in AUD relative to healthy subjects (Bjork et al., 2012; de Greck et al., 2009; Forbes et al., 2014).
One theory of the neural correlates of the transition from casual to compulsive, uncontrolled consumption of alcohol is a shift from goal-directed behaviors executed by regions of the PFC to more automatic, habitual behaviors driven by appetitive regions such as the caudate and putamen (Camchong et al., 2014; Everitt and Robbins, 2005; Sjoerds et al., 2013). Indeed, tests of this hypothesis using implicit tasks (Ames et al., 2014; Virag et al., 2015) support a dual processing framework: habits are mediated through neural circuitry dependent on the striatum, while more controlled behaviors are mediated through neural circuitry more dependent on the PFC (Fein and Cardenas, 2015). Another way disturbances to these systems have been reported is by demonstrating greater synchrony in executive control networks (including regions such as the anterior cingulate cortex and dorsolateral PFC) and lower synchrony in appetitive drive networks (including regions such as the nucleus accumbens, thalamus, and caudate) in abstinent AUD relative to healthy control individuals (Camchong et al., 2013a; Camchong et al., 2013b, c). Other studies evaluating the neural substrate of compulsive alcohol use have reported a shift in alcohol-cue reactivity from ventral to dorsal striatum (Vollstadt-Klein et al., 2010). A more refined framework suggests that the shift from goal-directed to habitual behaviors is mediated via subtly different cortical to subcortical circuitry: dorsal prelimbic PFC to nucleus accumbens core playing a role in the acquisition of goal-directed actions while the more ventral infralimbic PFC to nucleus accumbens shell circuitry plays a role in the expression of habitual behavior (Barker et al., 2015). Indeed, in nonhuman primates, the nucleus accumbens shell and core subregions are organized in a series of parallel circuits linked in an ascending spiral to the dorsal striatum in a manner that could account for the transition from goal-directed to habitual behaviors during the development of addiction (Renteria et al., 2016). Whether these bidirectional nucleus accumbens circuits are relevant to human addiction will require further evaluation and the development of imaging methods with higher resolution and greater detection capabilities for microstructure and neurochemistry (Lucas-Neto et al., 2015).
Highlighting the relevance of frontostriatal systems to behavioral regulation in AUD, abnormal connectivity (e.g., between the dorsolateral PFC and striatum) predicts impairments in learning (Park et al., 2010) and response inhibition (Courtney et al., 2013; Muller-Oehring et al., 2013; Schulte et al., 2012). Impaired functional connectivity may in fact be due to compromised anatomical integrity of corticostriatal fibers (Yeh et al., 2009).
Summary and Conclusions
For exposition, the three systems described herein were represented as independent and non-overlapping and as mediating specific neuropsychological deficits in AUD. Our work and much of the literature cited includes data from normal aging to permits detection of age-disease interactions. In other words, the deficits in brain and behavior described are a function of AUD and not merely aging. The literature reviewed herein suggests that frontocerebellar systems mediate motor and working memory deficits, frontolimbic systems mediate semantic memory impairments and emotional dysregulation, and frontostriatal systems mediate the transition from goal-directed to habitual behaviors in AUD. These frontally-based networks, however, are highly interconnected and whether a certain brain region (e.g., thalamus) is included in one system or another can depend on the scope and perspective of the work (e.g., psychology or imaging) (e.g., Fein and Cardenas, 2015; Yan and Li, 2009) or the behavior evaluated (e.g., affect may be subserved by frontolimbic or frontostriatal systems) (Alexander et al., 1986; Schmahmann and Pandya, 2008). Although we have described fronto-fugal pathways, the cerebellum, limbic system, and basal ganglia are also interconnected. For example, the output of the cerebellum, the dentate nucleus, projects to the input structure of the basal ganglia, the striatum (Hoshi et al., 2005). The ventral tegmental area can act as a mediator of dialogue between these systems because dopamine projections target, among other regions, the cerebellum, thalamus, hypothalamus, nucleus accumbens, amygdala, hippocampus, and prefrontal cortices (Oades and Halliday, 1987). Information from these subcortical regions may also be integrated in regions not discussed herein, such as the inferior parietal lobule, which has inputs from the hippocampus and cerebellum and subserves functions including attention and calibration of eye-hand coordination (Clower et al., 2001). Together, current data demonstrate that the cognitive and motor impairments noted in AUD are the result of disturbances in the coordinated activity of a number of regions interacting with frontal structures, rather than a single area in the PFC. We speculate that a more refined knowledge of the structures and functions that are compromised and which are spared in AUD could enhance therapeutic efforts to redirect neural recruitment from the usual but disrupted paths and networks to functional alternatives, possibly through behavioral therapy. This knowledge might also inform pharmacological efforts for initiating sobriety, maintaining abstinence and promoting recovery.
Highlights.
Brain structures and functions damaged in Alcohol Use Disorders (AUD) are presented based on findings from neuroimaging and neuropsychology studies.
Compromised networks are organized according to three structural and functional brain systems: frontocerebellar, frontolimbic, and frontostriatal.
Frontocerebellar systems underlie motor and working memory deficits in AUD.
Frontolimbic systems underlie declarative memory and emotional regulation deficits in AUD.
Frontostriatal systems are involved in the transition from casual to compulsive alcohol consumption.
Acknowledgments
This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) including K05 AA017168, R37 AA010723, R01 AA012388, R01 AA005965, U01 AA013521, U01AA17923
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I. Differential protein expression in the prefrontal white matter of human alcoholics: a proteomics study. Mol Psychiatry. 2006;11:56–65. doi: 10.1038/sj.mp.4001741. [DOI] [PubMed] [Google Scholar]
- Ames SL, Grenard JL, He Q, Stacy AW, Wong SW, Xiao L, Xue G, Bechara A. Functional imaging of an alcohol-Implicit Association Test (IAT) Addict Biol. 2014;19:467–481. doi: 10.1111/adb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ. Corticostriatal circuitry and habitual ethanol seeking. Alcohol. 2015;49:817–824. doi: 10.1016/j.alcohol.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO. Motoric signs of CNS dysfunction associated with alcohol and cocaine withdrawal. Psychiatry Research. 1993;47:69–77. doi: 10.1016/0165-1781(93)90056-m. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Nelson JE, Schmidt RD. Mammillary body atrophy identified by magnetic resonance imaging in alcohol amnestic (Korsakoff’s) syndrome. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1989;2:189–201. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum Brain Mapp. 2012;33:2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Broca P. Anatomie comparee des circonvolutions cerebrales: Le grand lobe limbique et la scissure limbique dans la serie des mammifères. Revue d’Anthropologie. 1878;1:385–498. [Google Scholar]
- Brun A, Andersson J. Frontal dysfunction and frontal cortical synapse loss in alcoholism--the main cause of alcohol dementia? Dement Geriatr Cogn Disord. 2001;12:289–294. doi: 10.1159/000051271. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. Frontal lobes and aging: deterioration and compensation. In: Stuss DT, Knight RT, editors. Principles of frontal lobe functioning. Oxford University Press; New York: 2013. [Google Scholar]
- Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: Identification of Wernicke’s encephalopathy. Journal of Neurology Neurosurgery and Psychiatry. 1997;62:51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calso C, Besnard J, Allain P. Normal aging of frontal lobe functions. Geriatr Psychol Neuropsychiatr Vieil. 2016;14:77–85. doi: 10.1684/pnv.2016.0586. [DOI] [PubMed] [Google Scholar]
- Camchong J, Endres M, Fein G. Decision making, risky behavior, and alcoholism. Handb Clin Neurol. 2014;125:227–236. doi: 10.1016/B978-0-444-62619-6.00014-8. [DOI] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2013a;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, Fein G. Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug Alcohol Depend. 2013b;131:56–65. doi: 10.1016/j.drugalcdep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, Fein G. Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcohol Clin Exp Res. 2013c;37:794–803. doi: 10.1111/acer.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza R, Cereti Mazza MT, De Cicco L. Preparation and activity of metal complexes with heteroatomic organic ligands. Preliminary note. Boll Soc Ital Biol Sper. 1990;66:717–724. [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement MD, Shaw DS, Sitnick SL, Musselman SC, Forbes EE. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Social cognitive and affective neuroscience. 2015;10:416–423. doi: 10.1093/scan/nsu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak LS. Models of memory loss in Korsakoff and alcoholic patients. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism. Guilford Press; New York: 1987. pp. 207–226. [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud-Guillermo S, Andoh J, Martelli C, Artiges E, Pallier C, Aubin HJ, Martinot JL, Reynaud M. Imaging of language-related brain regions in detoxified alcoholics. Alcohol Clin Exp Res. 2009;33:977–984. doi: 10.1111/j.1530-0277.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2013;18:593–604. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an FMRI study of youth at high risk for alcoholism. Alcohol Clin Exp Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW, Jr, Curran JE, Knowles E, Sprooten E, Goring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, Glahn DC. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics--a fMRI study. Hum Brain Mapp. 2009;30:1691–1704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, De Rosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased fronto-cerebellar activation in alcoholics during verbal working memory: An fMRI study. NeuroImage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bacher M, Guschlbauer B. Improvement in ataxia in alcoholic cerebellar atrophy through alcohol abstinence. Journal of Neurology. 1984a;231:258–262. doi: 10.1007/BF00313662. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984b;57:134–142. doi: 10.1016/0013-4694(84)90172-x. [DOI] [PubMed] [Google Scholar]
- Dreyfus PM, Victor M. Effects of thiamine deficiency on the central nervous system. Am J Clin Nutr. 1961;9:414–425. doi: 10.1093/ajcn/9.4.414. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Klein T, Lohrmann C, van Elst LT, Hesslinger B, Juengling FD. Different striatal dopamine D2 receptor occupancy in alcohol dependent patients with or without physical withdrawal symptoms --a study using IBZM-SPECT. J Neural Transm (Vienna) 2002;109:1215–1219. doi: 10.1007/s00702-002-0749-9. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries. Psychological Bulletin. 1989;106:128–147. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcoholism Clinical and Experimental Research. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Cardenas VA. Neuroplasticity in Human Alcoholism: Studies of Extended Abstinence with Potential Treatment Implications. Alcohol Res. 2015;37:125–141. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Meyerhoff D, Di Sclafani V, Ezekiel F, Poole N, MacKay S, Dillon WP, Constans J-M, Weiner MW. 1H magnetic resonance spectroscopic imaging separates neuronal from glial changes in alcohol-related brain atrophy. In: Lancaster F, editor. Alcohol and Glial Cells, NIAAA Research Monograph # 27. US Government Printing Office; Bethesda, MD: 1994. pp. 227–241. [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcohol Clin Exp Res. 2010;34:396–403. doi: 10.1111/j.1530-0277.2009.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008a;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008b;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Rose EJ, Darcey VL, Belcher AM, VanMeter JW. Neurodevelopmental Precursors and Consequences of Substance Use during Adolescence: Promises and Pitfalls of Longitudinal Neuroimaging Strategies. Front Hum Neurosci. 2016;10:296. doi: 10.3389/fnhum.2016.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One. 2014;9:e94640. doi: 10.1371/journal.pone.0094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex, mediator of cross-temporal contingencies. Human Neurobiol. 1985;4:169–179. [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Kaplan E. A human cerebral deconnection syndrome. A preliminary report. Neurology. 1962;12:675–685. doi: 10.1212/wnl.12.10.675. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Christiansen BA, Brown SA, Smith GT. Alcoholism and memory - Broadening the scope of alcohol-expectancy research. Psychological Bulletin. 1991;110:137–146. doi: 10.1037/0033-2909.110.1.137. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, Prat G, Carrio I, Casas M. Striatal dopaminergic D(2) receptor density measured by [(123)I]iodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry. 2000;157:127–129. doi: 10.1176/ajp.157.1.127. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. Journal of Neurological Science. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper CG. The incidence of Wernicke’s encephalopathy in Australia: A neuropathological study of 131 cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1983;46:593–598. doi: 10.1136/jnnp.46.7.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. Journal of Neurology Neurosurgery and Psychiatry. 1986;49:341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? British Medical Journal. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson R, Kuzmin A, Okvist A, Harper C, Sheedy D, Garrick T, Yakovleva T, Bakalkin G. Elevated synaptophysin I in the prefrontal cortex of human chronic alcoholics. Synapse. 2008;62:829–833. doi: 10.1002/syn.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol Res. 2012;34:441–447. [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates. I. The functions of the frontal associations areas in monkeys. Comparative Psychology Monographs. 1936;13:3–60. [Google Scholar]
- Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magnetic Resonance Imaging. 1996;14:553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak L. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jung YC, Schulte T, Muller-Oehring EM, Namkoong K, Pfefferbaum A, Sullivan EV. Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology (Berl) 2014;231:4443–4453. doi: 10.1007/s00213-014-3594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller W, O’Hara R, Dorus W, Bauer J. Tremor in chronic alcoholism. Neurology. 1985;35:1660–1662. doi: 10.1212/wnl.35.11.1660. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuroanatomy and neuropathology associated with Korsakoff’s syndrome. Neuropsychol Rev. 2012;22:72–80. doi: 10.1007/s11065-012-9195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL. Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug Alcohol Depend. 2013;129:1–7. doi: 10.1016/j.drugalcdep.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme TL, Schmidt C, Strelchuk D, Chang-Webb YC, Baek K, Voon V. Sexually dimorphic brain volume interaction in college-aged binge drinkers. Neuroimage Clin. 2016;10:310–317. doi: 10.1016/j.nicl.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Rasanen P, Tiihonen J. Dopamine transporter availability and depressive symptoms during alcohol withdrawal. Psychiatry Res. 1999a;90:153–157. doi: 10.1016/s0925-4927(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, Niemela O, Hillbom M. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999b;4:189–191. 104–185. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, Reynaud M, Martelli C, Rohlfing T, Sullivan EV, Pfefferbaum A. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp. 2014;35:4635–4653. doi: 10.1002/hbm.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledin T, Odkvist L. Abstinent chronic alcoholics investigated by dynamic posturography, ocular smooth pursuit and visual suppression. Acta Otolaryngology. 1991;111:646–655. doi: 10.3109/00016489109138395. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends in Neurosciences. 1993a;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993b;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The role of the cerebellum in the human brain. Trends Neurosci. 1993c;16:453–454. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The role of the cerebellum in the human brain - reply. Trends in Neurosciences. 1993d;16:453–454. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Reid AG, Myers J, Feeney A, Hammers A, Taylor LG, Rosso L, Turkheimer F, Brooks DJ, Grasby P, Nutt DJ. A [11C]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced alpha5 benzodiazepine receptors in limbic regions. J Psychopharmacol. 2012;26:273–281. doi: 10.1177/0269881110379509. [DOI] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Neto L, Reimao S, Oliveira E, Rainha-Campos A, Sousa J, Nunes RG, Goncalves-Ferreira A, Campos JG. Advanced MR Imaging of the Human Nucleus Accumbens--Additional Guiding Tool for Deep Brain Stimulation. Neuromodulation. 2015;18:341–348. doi: 10.1111/ner.12289. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased Volume of the Brain Reward System in Alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ. Can amnesia be caused by damage of a single brain structure? Cortex. 1984;20:27–45. doi: 10.1016/s0010-9452(84)80021-0. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Maurage P, de Timary P, Billieux J, Collignon M, Heeren A. Attentional alterations in alcohol dependence are underpinned by specific executive control deficits. Alcohol Clin Exp Res. 2014;38:2105–2112. doi: 10.1111/acer.12444. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Mitsuyama H, Little KY, Sieghart W, Devaud LL, Morrow AL. GABA(A) receptor alpha1, alpha4, and beta3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcohol Clin Exp Res. 1998;22:815–822. [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol and Alcoholism. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T. Midbrain-driven emotion and reward processing in alcoholism. Neuropsychopharmacology. 2013;38:1844–1853. doi: 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–372. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. Journal of Neuroscience. 2005;25:4040–4051. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura I, Hishikawa Y. Cerebral damages in alcoholic patients visualized by MRI; with special reference to frontal atrophy, basal ganglia and white matter lesion. Nihon Rinsho. 1997;55(Suppl):288–298. [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, Garavan H, Heinz A, Gallinat J, Lathrop M, Mann K, Artiges E, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka MN, Spanagel R, Struve M, Loth E, Schumann G, Flor H, Consortium I. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JD, Harris JC. The scientific contributions of Paul D. MacLean (1913–2007) J Nerv Ment Dis. 2009;197:3–5. doi: 10.1097/NMD.0b013e31818ec5d9. [DOI] [PubMed] [Google Scholar]
- Noble EP. Alcoholism and the dopaminergic system: a review. Addiction Biology. 1996;1:333–348. doi: 10.1080/1355621961000124956. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Ellis RJ. Cognitive deficits related to memory impairments in alcoholism. Recent Developments in Alcoholism. 1987;5:59–80. doi: 10.1007/978-1-4899-1684-6_3. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage, NIAAA Research Monographs. 22. National Institutes of Health; Rockville, MD: 1993. pp. 121–156. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology. 1937;38:725–743. [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Signs of preclinical Wernicke’s Encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology. 2011;36:580–588. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Struct Funct. 2013;218:683–695. doi: 10.1007/s00429-012-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Functional anatomy of long-term memory. J Clin Neurophysiol. 1997;14:294–310. doi: 10.1097/00004691-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Miall C. Expanding cerebellar horizons. Trends in Cognitive Science. 2001;5:135–136. doi: 10.1016/s1364-6613(00)01635-1. [DOI] [PubMed] [Google Scholar]
- Renteria R, Jeanes ZM, Mangieri RA, Maier EY, Kircher DM, Buske TR, Morrisett RA. Using In Vitro Electrophysiology to Screen Medications: Accumbal Plasticity as an Engram of Alcohol Dependence. Int Rev Neurobiol. 2016;126:441–465. doi: 10.1016/bs.irn.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repo E, Kuikka JT, Bergstrom KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl) 1999;147:314–318. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- Rezai K, Andreasen NC, Alliger R, Cohen G, Swayze V, O’Leary DS. The neuropsychology of the prefrontal cortex. Archives of Neurology. 1993;50:636–642. doi: 10.1001/archneur.1993.00540060066020. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Contribution of regional white matter integrity to visuospatial construction accuracy, organizational strategy, and memory for a complex figure in abstinent alcoholics. Brain Imaging and Behavior. 2009;3:379–390. doi: 10.1007/s11682-009-9080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage. 2011;56:61–68. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The Cerebellum and Cognition. Academic Press; San Diego: 1997. pp. 31–60. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44:1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz E, Diener H, Dichgans J, Langohr H, Schied W, Schupmann A. Incidence of peripheral neuropathy and cerebellar ataxia in chronic alcoholics. Journal of Neurology. 1986;233:212–217. doi: 10.1007/BF00314021. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J Int Neuropsychol Soc. 2000;6:52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, Pfefferbaum A. Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biological Psychiatry. 2012;17:269–278. doi: 10.1016/j.biopsych.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. American Journal of Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcoholism: Clinical and Experimental Research. 1999;23:158–163. [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, Veltman DJ. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry. 2013;3:e337. doi: 10.1038/tp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biological Psychiatry. 2005;57:768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000a;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003a;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lane B, Rosenbloom MJ, Deshmukh A, Desmond J, Lim KO, Pfefferbaum A. In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcoholism: Clinical and Experimental Research. 1999;23:1629–1636. [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Lim KO, Marsh L, Pfefferbaum A. Patterns of regional cortical dysmorphology distinguishing schizophrenia and chronic alcoholism. Biol Psychiatry. 1998;43:118–131. doi: 10.1016/S0006-3223(97)00264-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005 doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology (Berl) 2010a;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiatry. 2010b;67:44–51. doi: 10.1016/j.biopsych.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A. Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging. 2009;30:793–807. doi: 10.1016/j.neurobiolaging.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000b;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000c;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A. Effects of alcohol dependence comorbidity and anti-psychotic medication on volumes of the thalamus and pons in schizophrenia. American Journal of Psychiatry. 2003b;160:1110–1116. doi: 10.1176/appi.ajp.160.6.1110. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Cognitive demands during quiet standing elicit truncal tremor in two frequency bands: differential relations to tissue integrity of corticospinal tracts and cortical targets. Front Hum Neurosci. 2015;9:175. doi: 10.3389/fnhum.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds LL, Archibald SL, Grant I, Zisook S, Jernigan TL. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? J Neuroimaging. 1999;9:201–209. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- Tang Y, Whitman GT, Lopez I, Baloh RW. Brain volume changes on longitudinal magnetic resonance imaging in normal older people. Journal of Neuroimaging. 2001;11:393–400. doi: 10.1111/j.1552-6569.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung E, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Ratiu P, Leslie A, Howells H, Cabanis E, Iba-Zizen MT, Plaisant O, Simmons A, Dronkers NF, Corkin S, Catani M. From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting Disconnection Syndromes. Cereb Cortex. 2015;25:4812–4827. doi: 10.1093/cercor/bhv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, Fohr J. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nat Med. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Vilkman H, Rasanen P, Ryynanen OP, Hakko H, Bergman J, Hamalainen T, Laakso A, Haaparanta-Solin M, Solin O, Kuoppamaki M, Syvalahti E, Hietala J. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry. 1998;3:156–161. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- Vaina LM. Selective impairment of visual motion interpretation following lesions of the right occipito-parietal area in humans. Biol Cybern. 1989;61:347–359. doi: 10.1007/BF00200800. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Yanos M, Schmitt PJ, Grafton ST. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31:1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Virag M, Janacsek K, Horvath A, Bujdoso Z, Fabo D, Nemeth D. Competition between frontal lobe functions and implicit sequence learning: evidence from the long-term effects of alcohol. Exp Brain Res. 2015;233:2081–2089. doi: 10.1007/s00221-015-4279-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism: Clinical and Experimental Research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Henriksson R, Ohnishi YN, Ohnishi YH, Harper C, Sheedy D, Garrick T, Nyberg F, Nestler EJ, Bakalkin G, Yakovleva T. FOSB proteins in the orbitofrontal and dorsolateral prefrontal cortices of human alcoholics. Addict Biol. 2009;14:294–297. doi: 10.1111/j.1369-1600.2009.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Alcohol-related cues potentiate alcohol impairment of behavioral control in drinkers. Psychol Addict Behav. 2015;29:290–299. doi: 10.1037/adb0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, Mayer AR, Hutchison KE. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res. 2014;38:2445–2453. doi: 10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 2010;33:533–540. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Heinz A. Cue-induced alcohol craving. Neurobiological correlates and clinical relevance. Nervenarzt. 2006;77:1051–1052. 1055–1058, 1060–1053. doi: 10.1007/s00115-006-2067-1. [DOI] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yan P, Li CS. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: A preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, SImson K, Durazzo TC, Gazdzinski S, Meyerhoff D. Tract-based spatial statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: Abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009 May 11; doi: 10.1016/j.pscychresns.2008.07.012. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48:3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol. 2015 doi: 10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]