Abstract
Brain circuits that include the cortex and basal ganglia make up the bulk of the forebrain, and influence behaviors related to almost all aspects of affective, cognitive and sensorimotor functions. The learning of new actions as well as association of existing action repertoires with environmental events are key functions of this circuitry. Unfortunately, the cortico-basal ganglia circuitry is also the target for all drugs of abuse, including alcohol. This makes the circuitry susceptible to the actions of chronic alcohol exposure that impairs circuit function in ways that contribute to cognitive dysfunction and drug use disorders. In the present review, we describe the connectivity and functions of the associative, limbic and sensorimotor cortico-basal ganglia circuits. We then review the effects of acute and chronic alcohol exposure on circuit function. Finally, we review studies examining the roles of the different circuits and circuit elements in alcohol use and abuse. We attempt to synthesize information from a variety of studies in laboratory animals and humans to generate hypotheses about how the three circuits interact with each other and with the other brain circuits during exposure to alcohol and during the development of alcohol use disorders.
Brief description of cortico-basal ganglia circuitry
Circuits that connect the cerebral cortex to the basal ganglia, and the connections back through thalamus to the cortex are a major feature of the mammalian forebrain. Each area of allo-, meso-or neocortex projects to a striatal or striatal-like subregion, which then communicates to downstream basal ganglia regions that ultimately connect to thalamo-cortical neurons (Gerfen 1992, Haber and Calzavara 2009). Within the basal ganglia, regions including the globus pallidus internal and external segments (GPi and GPe respectively), and the substantia nigra reticulata (SNr) process striatal output to provide feedback that ultimately influences cortical output. Monoaminergic transmission from midbrain and brainstem regions modulates circuit function mainly through actions in the striatal regions, but also at other subregions within the circuitry (Molliver 1987, Berridge, Stratford et al. 1997, Delfs, Zhu et al. 1998, Beier, Steinberg et al. 2015, Lerner, Shilyansky et al. 2015) . These networks interface with the large thalamic network via thalamo-striatal projections. The overall loop of the cortico-basal ganglia-cortical (C-BG-C) system can be separated into numerous subcircuits based on anatomical and functional divisions (Alexander and Crutcher 1990). The best known among these are circuits in which neocortical structures, mesocortical structures (e.g. cingulate and piriform cortices) and allocortical regions (e.g. hippocampus and amygdala) project to well-known dorsal and ventral striatal regions, with processing/feedback loop networks influencing those cortical regions. However, the C-BG-C loop type networks are also maintained within the olfactory system where olfactory bulb and cortical inputs converge in the olfactory tubercle. The extended amygdala also has a C-BG-like network, with the lateral and basolateral amygdala serving as the cortical substrates, and the CeA and other downstream structures having features in common with the striatum (Swanson and Petrovich 1998). Cross-talk between the different C-BG-C networks also occurs at several levels throughout the cortex, BG and midbrain.
Other contributions to this volume will focus on the extended amygdala and prefrontal cortex, and thus, we will not cover these aspects of C-BG-C circuits except where needed to make key points about their role in the overall circuitry. Instead, our focus will be on the three predominant forebrain circuits known as the associative, limbic (aka cognitive) and sensorimotor (aka motor) C-BG-C circuits, and the interactions among these circuits (Haber 2003).
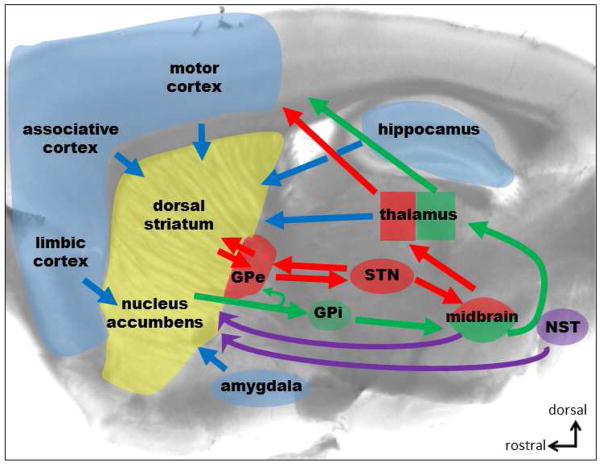
The three C-BG-C circuits on which we will focus share a basic cellular and molecular connectivity (Dudman and Gerfen 2015). Cortical regions send glutamatergic projections striatum that innervate both GABAergic medium spiny projection neurons (MSNs) and striatal interneurons. Specific thalamic subregions also send glutamatergic inputs to the MSNs and striatal interneurons. The striatum also receives dense dopaminergic projections from the midbrain, and much sparser monoaminergic inputs from other hindbrain regions that vary in different striatal subregions (Molliver 1987, Berridge, Stratford et al. 1997, Delfs, Zhu et al. 1998). The MSNs then send GABAergic projections to the GPe, GPi and SNr. Projections to GPi/SNr comprise the “direct” pathway that activates cortical output, while projections targeting the GPe via the “indirect” pathway inhibit cortical output. GABAergic projection neurons within the GPe project to the SNr and also form part of a reciprocal connection with the subthalamic nucleus (STN) in which the GPe contributes GABAergic innervation of STN, while STN sends glutamatergic afferents to GPe. The influence of these pathways on cortical function ultimately arises via GABAergic SNr projections to thalamus that gate thalamocortical glutamatergic inputs (Figure 1).
Figure 1.
Diagram of the cortex-basal ganglia-cortex loop showing the main projections that will be discussed in this review. Blue arrows represent all main glutamatergic inputs to the striatum and purple arrows the monoamine innervation from the midbrain dopamine neurons and the noradrenergic projection from the nucleus of the solitary tract (NST). Green and red arrows represent the two main output projections that form the direct- and indirect-pathway, respectively.
The cortical components of the associative circuit consist of areas that process sensory, motor and cognitive information at a stage with greater integration than primary sensory or motor cortices (Yin and Knowlton 2006). Examples include parts of the temporal and posterior parietal cortex, as well as prefrontal cortical regions. These cortical regions project predominantly to more medial areas of dorsal striatum (dorsomedial striatum, DMS) in the rodent brain (roughly equivalent to the primate caudate nucleus). Several anterior and medial and lateral thalamic regions project to DMS, and medial portions of the substantia nigra pars compacta (SNc) provide the bulk of dopaminergic innervation of this striatal subregion. The DMS MSNs project to the more medial parts of the GPe, GPi and SNr, and the GPe interacts with subregions of the STN. Ultimately, this circuit loop influences output from the same associative cortical areas that feed into the DMS (Balleine, Delgado et al. 2007) (Belin, Jonkman et al. 2009), although there is potential for cross-talk between circuits at several levels, which will be discussed later in this review.
The cortical components of the limbic circuit consist of mesocortical and prefrontal areas, as well as the allocortical regions, such as the hippocampal formation and basolateral amygdala (BLA) (Gerfen 1992, Yin and Knowlton 2006). These cortical regions project predominantly to the nucleus accumbens (NAc) (although the BLA does give rise to an appreciable projection to the DMS). The NAc can be further subdivided into the “core” and “shell” subregions, and these have been implicated in different aspects of learning and responses to substances of abuse as will be discussed. Thalamic projections to the NAc from antero-medial subregions are also part of the limbic circuitry. The dopaminergic input to the NAc comes predominantly from the ventral tegmental area (VTA). The NAc shell subregion also receives a more robust noradrenergic input in comparison to DS and the NAc core, and this arises from the nucleus of the solitary tract (Berridge, Stratford et al. 1997, Delfs, Zhu et al. 1998). Some MSNs within the NAc send their projections to the ventral mesencephalon (VM), while others innervate the ventral pallidum (VP). While the VM and VP projections have a superficial resemblance to the direct and indirect pathways, respectively, recent studies indicate that there is considerable mixing of MSN projections to the VP (Kupchik, Brown et al. 2015). Thus, the limbic striatal inputs and outputs are different from the dorsal striatal counterparts. The output from the limbic BG affects many parts of the limbic system, but also has more indirect influence on the associative and sensorimotor circuits. It should also be noted that parts of the limbic C-BG-C circuitry loop also include the extended amygdala network that will be described in a separate article in this volume. Our focus when discussing limbic circuitry will be on actions that differ from the negative affect/negative reinforcement functions described in that review.
The final large C-BG-C circuit that we will discuss is the sensorimotor circuit. As the name implies, this circuit includes the primary sensory and motor neocortices, along with some secondary regions such as supplementary motor areas (e.g. mouse M2 cortex). These cortical regions project into the dorsolateral striatum (DLS) in rodents (roughly equivalent to the putamen nucleus in primates). Indeed, there are several subcircuits within the corticostriatal sensorimotor projections that roughly correspond to different body parts, forming a very diffuse “humuncular” representation. No attempt will be made to differentiate these subcircuits in the present review. The intralaminar nuclei of the thalamus project to DLS, including several regions extending across the anterior-posterior extent of the thalamus. The dopaminergic DLS innervation comes mainly from the lateral portions of the SNc. The DLS MSNs innervate the lateral GPe, GPi and SNc, but there is also considerable overlap with regions receiving DMS input.
Within the dorsal striatum, subcompartments known as the striosome (or patch) and matrix participate in somewhat segregated C-BG-C circuits. These striatal subregions are characterized by different levels of a variety of neuronal proteins (e.g. higher expression of mu opiate receptors in striosome relative to matrix). Projections from cortex and other brain regions also selectively innervate striosomes vs. matrix (Kincaid and Wilson 1996, Crittenden and Graybiel 2011, Smith, Klug et al. 2016). This sub-compartmentalization runs parallel to the circuitry already discussed, at least at the corticostriatal level. However, the functions of patch and matrix subcompartments are still being sorted out, especially in relation to actions of drugs of abuse, and thus we will not have too much to say in this review about differential roles of these regions.
Circuitry roles in behavior, with emphasis on action control and abused substances
The C-BG-C circuitry influences a wide variety of behaviors. Most notable among these behaviors is movement, as evidenced by the severe movement impairments in neurological disorders involving the BG (e.g. Huntington’s and Parkinson’s diseases). The role the BG in movement has been strongly debated, with ideas including control of movement initiation and cessation, control of movement velocity and/or acceleration, movement sequencing, and generation of diverse movements (Kim, Barter et al. 2014, Jin and Costa 2015, Rueda-Orozco and Robbe 2015). All of these ideas have relatively strong experimental support, and thus it is possible that BG has diverse roles in movement control, or that there is a unifying role that is not easily fit into our heuristic categories. The hypotheses about diverse roles may also reflect variability in the BG subcircuit examined. Now that new approaches allow for more easily identification of different subcircuits, we can expect that, some of the issues will be clarified. Another important function of the BG is in the control of reward and reinforcement-based learning. This function overlaps to some extent with its role in movement control, as most learning and memory assays depend on performance of a particular set of movements. The NAc and downstream regions are implicated in responses to both rewarding and aversive stimuli, as well as in Pavlovian learning that involves conditioning stimuli to both appetitive and aversive unconditioned stimuli. These are brain areas high involved during the early stages of reward motivated learning and the response to substance of abuse. A NAc core and shell regions can be identified based on the inputs, projections patterns and immunochemistry markers, such as differential distribution of the calcium binding protein Calbindin D, as seen in rodents and humans (Groenewegen, Wright et al. 1999) (Voorn, Brady et al. 1994). The NAc core has multiple resembles with the dorsomedial striatum while the shell has more diverse features and can be further segregated into subregions (dorsomedial, intermediate, and ventrolateral) based on differences in gross projection patterns (Heimer, Alheid et al. 1997, Usuda, Tanaka et al. 1998) and immunoreactivity to peptides/neurotransmitters (Heimer, Alheid et al. 1997, Zahm 1998). These shell subregions appear to mediate different aspects of the response to drugs of abuse (Ikemoto 2007) and dopamine and reward seeking behavior (Fabbricatore, Ghitza et al. 2009, Reed, Hildebrand et al. 2015). Thus, through connections with limbic circuitry, including the extended amygdala, the ventral portions of the BG also integrate affective information with movement control.
Also, the C-BG-C circuitry appears to be involved in the cognitive aspect of this learning. Dopaminergic innervation of striatum has long been at the center of investigations of the BG role in reward motivated learning, reinforcement and memory. Indeed, there is ample evidence that activity of dopaminergic neurons is related to reward, and dopamine roles in reward and reinforcement-based learning have been studied extensively (Steinberg, Keiflin et al. 2013, Keiflin and Janak 2015). The different models will not be discussed extensively at present, but some aspects of the dopamine roles will be covered.
One overarching view of the C-BG-C circuits is that they form a sophisticated brain network for action control (Jin and Costa 2015, O'Doherty 2016). This idea encompasses all facets of action learning, including the association of unconditioned actions with new environmental events, the learning of new actions under the control of the internal and external context combined with outcomes produced by these actions, the cognitive components of action planning and selection, and many aspects of the performance of the actions themselves. By integrating information from a vast array of forebrain, midbrain and hindbrain sources, the mammalian C-BG-C circuits allow organisms to modify and extend their behavior repertoire to maximize the likelihood of survival and reproduction. Unfortunately, drugs of abuse and certain predispositions to excessive drug use can divert these circuits to behaviors that are maladaptive with respect to the normal goals of the organism. We will consider in-depth how ethanol interacts with C-BG-C circuits to produce these unfortunate consequences.
All these functions of the C-BG-C circuit are impacted by drugs of abuse and help to shape responses to these drugs. The majority of abused substances have “rewarding” properties, and some of these drugs, including ethanol, can be perceived as rewarding or aversive depending on factors such as context and dose. Drugs of abuse also produce reinforcement learning, fostering instrumentally-conditioned habitual actions including those related to the drugs themselves. Cognitive processes are also impaired by drugs of abuse, with ethanol having well known actions on judgement and memory (Sullivan, Harris et al. 2010) . Drugs of abuse have demonstrable effects on action production, timing and duration that involve the C-BG-C circuits. In subsequent sections of this review we will discuss the neural substrates of these ethanol effects, both acute and chronic, and how they set the stage for aberrant C-BG-C circuit function that drives ethanol seeking/taking and alcohol use disorders.
Ethanol effects on Associative and Sensory motor circuits
Ethanol produces its acute actions through low-affinity interactions with a variety of molecular targets (Howard, Slesinger et al. 2011, Lovinger and Roberto 2013). In the brain, concentrations ranging from 5–100 mM produce intoxication, increased sedation and ultimately coma and death. While there are many neuronal molecules and functions that are relatively unchanged by ethanol even at the high end of this concentration range, the drug still lacks the molecular specificity exhibited by other drugs of abuse (e.g. heroin), with no single molecule accounting for all acute actions of the drug. To understand the initial effects of ethanol on a given circuit it is important to determine what molecular and cellular targets are sensitive to ethanol in the neurons and synapses that make up that circuit. Information regarding alcohol effects on one circuit or brain region will not necessarily explain the alcohol effects on another circuit, given the diverse molecular composition of neuronal subtypes in different brain regions. Within the C-BG-C circuits this is a daunting task, given the large number of neuronal subtypes within the each subregion. Still, a great deal of progress has been made over the last few decades in understanding these ethanol actions and especially within the last few years, the pace of understanding has increased exponentially.
Ethanol increases the firing rate of dopaminergic neurons, especially the “tonic” firing exhibited by these continuously-active neurons (Mereu, Fadda et al. 1984, Gessa, Muntoni et al. 1985). This effect is best known to occur in the limbic VTA, but in fact ethanol also enhances firing of SNc dopaminergic neurons. The molecular targets of ethanol that produce this effect are still not fully characterized (Melis, Diana et al. 2009, Morikawa and Morrisett 2010). It does seem clear that this increase in firing underlies the increase in extracellular dopamine observed in the striatum (including dorsal striatum) following ethanol exposure in vivo (Yim, Schallert et al. 1998, Gonzales, Job et al. 2004). Ethanol-induced increases in DA and activation of dopamine receptors have been postulated to contribute to the rewarding effects of the drug, as well as locomotor activation and sensitization produced by ethanol at some doses in some mammalian species (Milton, Randall et al. 1995, Shen, Crabbe et al. 1995, Gonzales, Job et al. 2004, Abrahao, Quadros et al. 2012). However, increased DA could also contribute to the reinforcing effects of ethanol that drive habitual drug seeking and taking (Corbit, Nie et al. 2014). Within the SNc and dorsal striatum there is still much to be understood about the role of ethanol-stimulated DA levels, including the roles in subsequent abuse, and if there is any subregional heterogeneity in this ethanol actions.
Acute ethanol exposure inhibits DA release from terminals in the dorsal striatum, as observed in brain slice preparations (Budygin, Mathews et al. 2005), and in vivo (Wang, Palmer et al. 1997). This effect is generally observed only at relatively high ethanol concentrations, and thus it is not clear how relevant the effect is for acute intoxication. However, this mechanism may gain in significance during prolonged exposure to high ethanol concentrations, such those that occur during chronic alcohol abuse.
Repeated exposure to ethanol (often accompanied by several withdrawal/forced abstinence periods) alters several aspects of dopaminergic function in SNc and dorsal striatum (Rothblat, Rubin et al. 2001). It should be noted, however, that most of the studies examining these effects did not differentiate between different dorsal striatal subregions. Tissue levels of DA and DA metabolites are decreased in dorsal striatum, cortex and SN following chronic alcohol administration (Fadda, Argiolas et al. 1980, Kaneyuki, Morimasa et al. 1991), and a similar effect has also been observed following ethanol injections (Fadda, Mosca et al. 1989). Stimulated release of DA in DS is also reduced following chronic ethanol exposure in adolescent rat (Palm and Nylander 2014), and following drinking in adult monkeys (Siciliano, Calipari et al. 2015), and mixed effects have been seen in other assays of release (Hunt, Majchrowicz et al. 1979). Increased inhibition of DA release by kappa opiate receptors was also observed in monkey caudate following chronic ethanol drinking (Siciliano, Calipari et al. 2015). Increases in ligand binding to dopamine transporters and increased DA uptake in DS have also been reported following alcohol exposure or consumption (Jiao, Pare et al. 2006, Budygin, Oleson et al. 2007). Co-release of GABA from dopamine neuron projections to the dorsal striatum (Tritsch, Ding et al. 2012) was found to be depressed by acute ethanol concentrations seen during binge drinking (~30 mM) and deletion of the enzyme aldehyde dehydrogenase selectively in dopamine neurons where it is thought to mediate GABA synthesis caused an increase in ethanol drinking in mice (Kim, Ganesan et al. 2015).
D2 receptor binding was decreased following ethanol exposure or intake and withdrawal in rat, and this effect appeared to be larger in DS compared to NAc (Muller, Britton et al. 1980, Rommelspacher, Raeder et al. 1992). However, other studies found no change or increases in D2 receptor binding, and/or changes in D1 receptor binding following ethanol intake (Pellegrino and Druse 1992, De Montis, Gambarana et al. 1993, Lograno, Matteo et al. 1993, Kim, Lee et al. 1997, Souza-Formigoni, De Lucca et al. 1999). These differences could be due to numerous factors, including dose and duration of ethanol intake, timing of the assay relative to drinking/withdrawal, and which striatal subregion was examined. Altered D2 receptor occupancy has also been reported in humans with alcohol use disorders, with decreased receptor availability and negative correlation with craving being the most common findings (Ebert, Klein et al. 2002, Volkow, Wang et al. 2002, Heinz, Siessmeier et al. 2004, Heinz, Siessmeier et al. 2005, Watanabe, Kato et al. 2008, Rominger, Cumming et al. 2012). Stimulation of cAMP levels by D1 agonist is enhanced in DS slices, but not NAc, in ethanol-drinking rats (Nestby, Vanderschuren et al. 1999).
Within the dorsomedial (associative) and dorsolateral (sensorimotor) striatum effects of acute ethanol exposure on both glutamatergic and GABAergic synaptic transmission have been characterized within the last decade. It has long been known that ethanol acutely inhibits the function of NMDA-type ionotropic glutamate receptors (Hoffman, Rabe et al. 1989, Lovinger, White et al. 1989) (Woodward and Gonzales 1990), and this inhibition is also observed at synapses in the dorsal striatum (Yin, Park et al. 2007) (Wang, Lanfranco et al. 2010). This inhibitory action likely contributes to a decreased ability to induce long-term potentiation (LTP) of glutamatergic synapses in striatum (Yin, Park et al. 2007). As LTD is thought to contribute to learning and memory, suppression of NMDAR function likely underlies cognitive deficits associated with acute intoxication. Within the dorsomedial striatum, the inhibition observed in the presence of ethanol gives way to facilitation of NMDAR-mediated transmission upon cessation of a single exposure to acute ethanol (Wang, Carnicella et al. 2007). This rebound long-term facilitation (LTF) seems to set the stage for gradual induction of LTP at glutamatergic striatal synapses (Wang, Ben Hamida et al. 2012). Acute ethanol has opposite effects on GABAergic transmission in the DLS and DMS, where ethanol decreases and increases the frequency of unitary GABA synaptic responses, respectively. Repeated cycles of ethanol drinking diminished the acute effects of ethanol on GABA and decrease transmission in the CLS compared to naïve mice, suggesting occlusion of the acute ethanol effects (Wilcox, Cuzon Carlson et al. 2014). The net result of all these processes after multiple periods of ethanol exposure and withdrawal will be enhanced glutamatergic synaptic drive and decreased inhibition onto MSNs in the dorsomedial striatum that appears to contribute to the learning of goal-directed ethanol self-administration and escalated drinking (Wang, Cheng et al. 2015).
Neurons within the GPe are inhibited during acute ethanol exposure (Criswell, Simson et al. 1995, Abrahao, Chancey et al. 2016). A recent study has described ethanol inhibition of the activity of a subset of neurons in the GPe. This inhibitory effect is observed at ethanol concentrations associated with mild intoxication (e.g. 10 mM). The ethanol sensitive GPe neurons exhibit firing rates in the low frequency range, and are characterized by the expression of the transcription factors NPAS1 and Lhx6 (Abrahao, Chancey et al. 2016). The mechanism underlying this inhibitory ethanol action appears to be potentiation of the function of large-conductance (BK) potassium channels, a well-known target for acute ethanol actions (Dopico, Bukiya et al. 2014). In vivo, ethanol administration also inhibited firing in neurons with low baseline firing rates in the GPe of awake, freely-moving mice (Abrahao, Chancey et al. 2016). Interestingly, GPe neurons that project back to striatum (the “arkypallidal” neurons) appear to be among those inhibited by ethanol. Thus, ethanol can mediate disinhibition of striatum via suppression of the inhibitory arkypallidal input to the striatum, in addition to ethanol effects on intrastriatal synaptic connections.
Acute ethanol exposure also has effects on glial cells in the dorsal striatum (Adermark and Bowers 2016). Adermark and coworkers (2006) found that the input resistance and capacitance measured in striatal astrocytes was reduced during exposure to concentrations of ethanol associated with intoxication (Adermark and Lovinger 2006). This effect may involve inhibition of potassium channels and/or gap junctions, with the net effect of uncoupling the large astrocytic network normally seen in striatum. Ethanol also alters expression of neurotransmitter transporters in glia, with implications for glutamatergic transmission in striatum (Wu, Lee et al. 2010). The ethanol effects on astrocytes may also contribute to alterations in the extracellular levels of taurine and dopamine (Ericson, Chau et al. 2011). More work is needed to determine how ethanol effects on astroglia contribute to acute ethanol actions on striatal circuitry and behavior, as reviewed by Ruby et al. (Ruby, Adams et al. 2010).
Ethanol also alters another major form of synaptic plasticity in the dorsal striatum, namely long-term synaptic depression (LTD) (Xia, Li et al. 2006, Adermark, Talani et al. 2009, Adermark, Jonsson et al. 2011, DePoy, Daut et al. 2013). The mechanism of LTD induction and expression in DS involves a retrograde trans-synaptic mechanism driven by neuromodulators known as endocannabinoids (eCBs). The eCBs are lipid metabolites that are ubiquitous in the brain and body. Within the brain the eCBs primarily activate the cannabinoid 1 (CB1) subtype of G protein-coupled receptor (GPCR), the major molecular target of delta-9-tetrahydrocannabinol which is the predominant psychoactive ingredient in drugs derived from Cannabis sativa (e.g. marijuana, hashish, spice). This receptor is found predominantly on presynaptic axon terminals where it suppresses neurotransmitter release. Within the striatum, eCBs participate in LTD at both GABAergic and glutamatergic synapses (Calabresi, Maj et al. 1992, Gerdeman, Ronesi et al. 2002, Adermark, Talani et al. 2009, Mathur, Capik et al. 2011). The glutamatergic synapses that express LTD are made by afferents from the cortex, as these inputs, but not thalamic inputs, contain CB1 receptors (Wu, Kim et al. 2015). Acute ethanol exposure appears to enhance this form of LTD while inhibiting LTP in the DMS (Yin, Park et al. 2007). Chronic exposure to ethanol prevents the induction of this LTD in the DLS when examined in an ex vivo brain slice preparation (Xia, Li et al. 2006, Adermark, Talani et al. 2009, DePoy, Daut et al. 2013). This loss of LTD is observed with both passive ethanol exposure and after voluntary ethanol consumption in rat and mouse. It is not yet clear if the loss of LTD is due to a general impairment of CB1 signaling, occlusion of LTD by presynaptic depression induced by the in vivo ethanol exposure, or interference with some other signaling event involved in LTD induction. The inhibitory effect of a CB1 agonist is lost following chronic ethanol consumption (Adermark et al., 2011), indicating that CB1 activity or a downstream mechanism is impaired. A few days abstinence following chronic ethanol exposure appears to be sufficient to restore eCB-LTD at striatal glutamatergic synapses (Xia, Li et al. 2006).
The eCB-LTD observed at glutamatergic synapses in DLS is also impaired following chronic ethanol consumption (Adermark, Jonsson et al. 2011). This may involve a change in GABAergic tone at DLS synapses, but also appears to involve loss of CB1 agonist effects. It will be interesting to determine if the mechanisms of LTD loss at glutamatergic and GABAergic synapses are overlapping. It will also be important to learn if LTD at one or the other type of synapse is more sensitive to the effects of ethanol, as this will determine how the balance of striatal output is affected following chronic exposure.
Recent studies indicate that eCB-CB1 signaling is not the only system that can initiate presynaptic LTD at striatal synapses. Indeed, presynaptically-expressed LTD can be induced by activation of serotonin, opiate, and group II metabotropic glutamate receptors, among those that have been examined to date (Kahn, Alonso et al. 2001, Mathur, Capik et al. 2011, Atwood, Lovinger et al. 2014). Some of these LTD forms show mutual occlusion with eCB-LTD, indicating that they probably occur at the same synapses, and involve similar downstream signaling mechanisms (Atwood, Lovinger et al. 2014). It will be interesting to determine how chronic EtOH exposure affects these other presynaptic forms of LTD, and if similar mechanisms underlie these effects.
Ethanol effects on Limbic circuits
The mesopallidal, mesoaccumbens, and nigrostriatal dopamine systems are very responsive to acute ethanol administration (Budygin, Phillips et al. 2001, Robinson, Volz et al. 2005, Robinson, Howard et al. 2009), more sensitive than the nigropallidal dopamine system. Indeed, the brain regions with greatest increases in ethanol-induced dopamine release are the VP, NAc, and DS, but not the GP (Melendez, Rodd-Henricks et al. 2003). As described in the previous section of this review, ethanol acts acutely on multiple targets within the VTA/SN to increase the firing of dopamine neurons. The dopaminergic neurotransmission in ventral and dorsal striatum differentially modulates the behavioral response to ethanol and mediate different aspects of its reinforcing properties (see below) but there is also evidence that they act in concert to mediate the different responses to ethanol.
Pretreatment with ethanol was shown to further enhance the dopamine levels in the NAc shell in response to a challenge dose of ethanol in the posterior VTA, indicating that VTA neurons sensitize to this acute effect of ethanol causing larger ethanol-induced dopamine release in the shell (Ding, Rodd et al. 2009). At the same time, a recent report shows blunted dopamine levels after chronic ethanol exposure, an effect mediated by kappa opioid receptors (Karkhanis, Huggins et al. 2016). Stress also can change the acute ethanol response in the VTA from inhibition in naïve animals to excitation of GABA neurons in the VTA of animals pre-expose to stress. As consequences of this switch, dopamine neuron firing is decreased and the ethanol-induced dopamine signals in the NAc is blunted by stress pre-exposure (Ostroumov, Thomas et al. 2016). It is possible that this effects of stress can explain, at least partially, the discrepancy on the sensitized or blunted dopamine levels in response to acute ethanol after chronic exposure. (Valenta, Job et al. 2013)
Several lines of evidence indicate that ethanol interferes with endogenous opioid mechanisms which are closely linked with dopamine transmission in the mesolimbic pathway (Herz 1997). Acute ethanol administration induced a transient decrease in pro-enk mRNA expression in the VTA and a prolonged increase in the NAc core and shell regions that peaked 2 h after ethanol exposure (Mendez and Morales-Mulia 2006, Chang, Barson et al. 2010). Local administration of opioid receptor antagonist naloxone in the NAc, but not the VTA (Valenta, Job et al. 2013), reduced ethanol intake (Barson, Carr et al. 2009). Using displacement of radiolabeled μ opioid receptor agonist before and immediately after ethanol consumption in humans, ethanol drinking was shown to induce opioid release in the NAc and orbitofrontal cortex (Mitchell, O'Neil et al. 2012). Further, local administration of opioid receptor antagonist in the anterior cingulate cortex disrupted expression of an ethanol-induced conditioned place preference (Gremel, Young et al. 2011).
Ethanol, acutely and chronically, affects synaptic transmission and plasticity in the NAc. Chronic intermittent ethanol enhances glutamatergic transmission onto a subtype of MSNs, D1-receptor expressing MSNs selectively and it was not seen in D1-receptor negative MSNs in the NAc (Renteria, Maier et al. 2017). Chronic ethanol also impairs long-term depression (LTD) in D1-receptor expressing MSNs in the NAc (Renteria, Jeanes et al. 2014, Renteria, Maier et al. 2017). Activation of NMDA-type glutamate receptors in the NAc core, medial prefrontal (mPFC) and insula is necessary for aversion-resistant alcohol consumption in rats. Further, aversion-resistant intake was associated with expression in the NAc core of a new type of NMDA receptor containing the Grin2C (or NR2C) subunit that lacks the Mg2+ block and remains active at hyperpolarizing potentials (Seif, Chang et al. 2013).
Ethanol actions on GABA transmission in the NAc shell are thought to contribute to reinforcing effects of oral alcohol intake. More specifically, knockdown of γ-subunit of GABA-A receptors in the medial but not lateral shell, reduced alcohol intake (Nie, Rewal et al. 2011). Also, the alpha4 subunit of the ionotropic GABA receptors is also thought to play a role in ethanol reinforcement (Rewal, Donahue et al. 2012).
Abstinence after prolonged access to ethanol increased the firing of NAc core neurons by reducing small-conductance calcium-activated potassium channel (SK) currents and decreased SK3 but not SK2 subunit protein expression (Hopf, Bowers et al. 2010). Thus SK channels have been proposed as a novel target for treating alcohol use disorders (Hopf, Seif et al. 2011).
Ethanol actions on many components of the extended amygdala circuitry are now well documented (McCool 2011, Kash 2012, Wills, Klug et al. 2012, Gilpin, Herman et al. 2015). This system has clear roles in the anxiolytic effects of acute alcohol, and the anxiogenic effects of abstinence/withdrawal following chronic exposure (Koob 2004). Indeed, there is strong evidence that the buildup of allostatic changes in extended amygdala and the contribution of this circuitry to negative consequences of withdrawal contributes to relapse to drinking (Koob 2014). A recent study showed that varenicline, a partial agonist for nicotinic receptor widely used to treat nicotine addiction and being tested for the treatment of alcohol dependence, disrupt amygdala response to fearful faces in heavy drinkers (Gowin, Vatsalya et al. 2016).
What is less well understood is how this circuitry may interact with the C-BG-C circuits to alter goal-directed and habitual alcohol seeking and taking. There are several circuit interaction points at which this could occur. For example, the BLA projects to both the NAc and DMS, providing avenues for direct communication between extended amygdala and both the associative and limbic circuits. Indeed, there is evidence that BLA provides important information about positive and negative outcomes that helps to guide goal directed behavior (Hart, Leung et al. 2014) (Maren 2003). It is likely that ethanol effects on BLA neuronal function will impact communication to both striatal subregions, thereby influencing how outcomes control behavior. For example, ethanol-induced alterations in synaptic inhibition within BLA (Silberman, Bajo et al. 2009) (Talani and Lovinger 2015) could reduce transmission of goal-related information to DMS and NAc, thus weakening their control of behavior and by default strengthening the influence of the sensorimotor circuit. Indeed, Packard and coworkers have shown that manipulation of BLA function alters the extent to which DLS controls behavior (Packard and Gabriele 2009). Exploration of specific projections from BLA to different components associative and limbic circuit will be necessary to understand how the negative symptoms associated with ethanol withdrawal alter instrumental ethanol seeking and taking.
The central amygdala (CeA) and BNST are also key components of the extended amygdala circuitry that are affected by ethanol exposure and influence drinking, withdrawal and relapse. These forebrain regions can influence the function of the three C-BG-C circuits through projections to the midbrain. The BNST and CeA project to both the VTA and SNc, providing GABAergic and glutamatergic inputs that can directly or indirectly influence the dopaminergic connections with key roles in reward- and reinforcement-based learning (Watabe-Uchida, Zhu et al. 2012, Jennings, Sparta et al. 2013). Newly-discovered monosynaptic GABAergic projections from the BNST to the DS (Smith et al., 2016) may also provide another source of integration between the extended amygdala and associative basal ganglia that could mediate stress effects on operant and ethanol-related behaviors. As yet, there is little evidence as to whether and how these projections influence the functions of the C-BG-C circuits, let alone how ethanol alters these influences, and this is a research area that is primed for exploration.
The actions of acute and chronic ethanol exposure on hippocampal physiology are well characterized. In general, the focus has been on how these actions influence hippocampal-based learning and memory (e.g. spatial learning) (Matthews and Silvers 2004). The hippocampus also contributes to context-dependent reinstatement of cocaine use (Ramirez, Bell et al. 2009, Lasseter, Xie et al. 2010), but it is unclear if it has a similar role with respect to ethanol reinstatement. The ventral hippocampus is implicated in the processing of emotional information, and it is this region that projects most heavily to the NAc as part of the limbic circuit (Dudman and Gerfen 2015). The input from the hippocampus to the limbic circuit can help to integrate spatial and emotional information with reward processing and pavlovian learning processes (Robbins, Ersche et al. 2008). There is a greater need to integrate the studies of ethanol actions in hippocampus within the larger context of limbic circuit function, perhaps through examination of connections with other regions in the circuit.
Roles of Limbic circuit in alcohol seeking taking
Core and shell regions of the NAc play different roles in mediating the reinforcing effects of ethanol as well as ethanol seeking behavior. Once operant ethanol consumption is established, ethanol seeking triggered by environmental context cues requires dopamine D1 receptor activation in the core and shell (Chaudhri, Sahuque et al. 2009). However, core is important for cue-induced ethanol-seeking while the shell contributes to the contextual influence on ethanol-seeking (Chaudhri, Sahuque et al. 2010) (Corbit, Fischbach et al. 2016).
The posterior VTA is important in mediating the reinforcing effects of ethanol and the acquisition of ethanol taking. Rats self-administered ethanol directly into the posterior VTA (Rodd-Henricks, McKinzie et al. 2000) and selectively bred alcohol-preferring P-rats self-administered lower concentrations than Wistar rats, indicating a heightened sensitivity (Gatto, McBride et al. 1994, Rodd, Melendez et al. 2004). Operant responding can be established for ethanol self-administration in the shell, but not the core NAc region (Engleman, Ding et al. 2009). Thus, similarly to other drugs of abuse (Rodd-Henricks, McKinzie et al. 2002), the mesopontine dopamine system and the NAc shell region are critical in mediating the reinforcing effect of ethanol and likely to contribute to the initial stages of acquisition of ethanol drinking behavior.
Dopamine in the NAc shell is involved in the incentive motivation for alcohol, whereas dopamine in the DLS comes into play when obtaining alcohol requires high levels of effort (Robinson, Howard et al. 2009, Spoelder, Hesseling et al. 2016). These studies also show that dopamine neurotransmission is enhanced at several striatal subregions which might act in concert to mediate different aspects of ethanol-related behaviors.
Varenicline, a partial agonist for nicotinic receptor used to treat nicotine addiction and being tested for the treatment of alcohol dependence, attenuated context induced relapse to alcohol seeking when administered bilaterally in the NAc but not the VTA (Lacroix, Pettorelli et al. 2016).
In addition, there is ample evidence (much of it discussed in this special issue) that ethanol alters neural and glial function in forebrain nuclei including amygdala, hippocampus, hypothalamus, the bed nucleus of the stria terminalis (BNST), septal nucleus and other cholinergic forebrain regions, and thalamus, not to mention numerous brainstem and cerebellar areas. These are just a few examples of how ethanol effects in brain regions not always thought of as part of the canonical C-BG-C circuits can interact with the three C-BG-C circuits. While it is not yet possible to place all these actions into their proper role within the context of the neural actions of alcohol, a clearer picture has begun to emerge of how the circuits interact. These interactions facilitate crosstalk between brain circuits involved in different aspects of drinking and alcohol use disorders. To gain a fuller understanding of all the stages of use and abuse it will be necessary to gather more information about what ethanol does in each of these brain regions and circuits, and how the different regions and circuits contribute to drinking and use disorders.
Conclusions and a general hypothesis for the progression of alcohol use and abuse
It is now apparent that acute and chronic ethanol exposure alters synaptic function and neuronal connectivity in a variety of brain regions, affecting multiple brain circuits. It does so by affecting both excitatory and inhibitory neurotransmission as well as altering the release of neuromodulators such as dopamine, opioids and likely also acetylcholine. It is reasonable to speculate that each of these direct and indirect actions of ethanol on the different brain circuits contributes to specific aspects of intoxication, ethanol seeking and drinking, and alcohol use disorders. In order to parse out their contributions, detailed knowledge of the specific cognitive and behavioral functions subserved by each of these brain circuits is needed. While significant progress has been made in this regard over the past decades, more work is needed for addressing the specific role of neuronal circuits and pathways, rather than nuclei as a whole. Moving forward, it is also crucial to improve our understanding of how the different circuits interact with each other to produce more complex behaviors such as the constellation of behavioral changes that follow ethanol use and abuse.
A general hypothesis for the loss of control over ethanol drinking could be extended from the drug addiction field that considers impaired inhibitory control from cortical circuits as a root cause of the compulsive drug taking. Impaired inhibitory control over ethanol drinking could then simply arise from a reduced ability by the cortex to engage subcortical inhibitory circuits that restrain reward seeking. As such, cortical circuits mediating volitional control over behavior could be rendered less effective in suppressing a well-learned stimulus-response that gets then reliably triggered as an automated motor response. Alternatively, an enhancement in the ability of cues/stimuli to trigger the behavioral response (ethanol seeking/taking) could be equally effective in shifting the balance of “go”/”no-go” subcortical circuits and weakening the influence of the cortical circuits in mediating behavioral inhibition.
Highlights.
Brain circuitry that includes the cortex and basal ganglia has key roles in action control and action learning
This circuitry can be subdivided into 3 parallel cortico-basal ganglia-cortical loops, the associative, limbic and sensorimotor subcircuits
Ethanol alters several aspects of the function of all 3 circuits, both during acute exposure and after chronic exposure or consumption
Molecular targets of ethanol action in these circuits include GABAergic synapses, dopaminergic transmission, cholinergic transmission, endogenous opiates, NMDA-type glutamate receptors and potassium channels.
The associative and sensorimotor circuits control goal-directed and habitual ethanol seeking, respectively
Chronic ethanol exposure fosters habitual learning and habitual ethanol seeking
Ethanol effects on the limbic circuit mediate the rewarding effects of the drug, and facilitate the ability of ethanol-related environmental stimuli to enhance drinking
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao KP, Chancey JH, Chan CS, Lovinger DM. Ethanol-Sensitive Pacemaker Neurons in the Mouse External Globus Pallidus. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, I, Quadros M, Andrade AL, Souza-Formigoni ML. Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology. 2012;62(2):882–889. doi: 10.1016/j.neuropharm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Adermark L, Bowers MS. Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcohol Clin Exp Res. 2016;40(9):1802–1816. doi: 10.1111/acer.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Ericson M, Soderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61(7):1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Ethanol effects on electrophysiological properties of astrocytes in striatal brain slices. Neuropharmacology. 2006;51(7–8):1099–1108. doi: 10.1016/j.neuropharm.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29(1):32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, Mathur BN. Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci. 2014;37(11):663–673. doi: 10.1016/j.tins.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009;98(4):453–459. doi: 10.1016/j.physbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162(3):622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27(3):230–241. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Mathews TA, Lapa GB, Jones SR. Local effects of acute ethanol on dopamine neurotransmission in the ventral striatum in C57BL/6 mice. Eur J Pharmacol. 2005;523(1–3):40–45. doi: 10.1016/j.ejphar.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007;193(4):495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297(1):27–34. [PubMed] [Google Scholar]
- Calabresi P, Maj R, Mercuri NB, Bernardi G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett. 1992;142(1):95–99. doi: 10.1016/0304-3940(92)90628-k. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34(5):761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207(2):303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur J Neurosci. 2016;43(9):1229–1236. doi: 10.1111/ejn.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci. 2014;8:301. doi: 10.3389/fnbeh.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Simson PE, Knapp DJ, Devaud LL, McCown TJ, Duncan GE, Morrow AL, Breese GR. Effect of zolpidem on gamma-aminobutyric acid (GABA)-induced inhibition predicts the interaction of ethanol with GABA on individual neurons in several rat brain regions. J Pharmacol Exp Ther. 1995;273(1):526–536. [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Montis MG, Gambarana C, Gessa GL, Meloni D, Tagliamonte A, Stefanini E. Reduced [3H]SCH 23390 binding and DA-sensitive adenylyl cyclase in the limbic system of ethanol-preferring rats. Alcohol Alcohol. 1993;28(4):397–400. [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806(2):127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110(36):14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33(9):1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Bukiya AN, Martin GE. Ethanol modulation of mammalian BK channels in excitable tissues: molecular targets and their possible contribution to alcohol-induced altered behavior. Front Physiol. 2014;5:466. doi: 10.3389/fphys.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Gerfen CR. The Rat Nervous System. 4. San Diego: Academic Press; 2015. Chapter 17 - The Basal Ganglia A2 - Paxinos, George; pp. 391–440. [Google Scholar]
- Ebert D, Klein T, Lohrmann C, van Elst LT, Hesslinger B, Juengling FD. Different striatal dopamine D2 receptor occupancy in alcohol dependent patients with or without physical withdrawal symptoms --a study using IBZM-SPECT. J Neural Transm (Vienna) 2002;109(9):1215–1219. doi: 10.1007/s00702-002-0749-9. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33(12):2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Chau P, Clarke RB, Adermark L, Soderpalm B. Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict Biol. 2011;16(3):377–385. doi: 10.1111/j.1369-1600.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Fadda F, Argiolas A, Melis MR, Serra G. Effect of acute and chronic ethanol on dopamine synthesis in the caudate nucleus, substantia nigra and frontal cortex. Boll Soc Ital Biol Sper. 1980;56(24):2553–2558. [PubMed] [Google Scholar]
- Fadda F, Mosca E, Colombo G, Gessa GL. Effect of spontaneous ingestion of ethanol on brain dopamine metabolism. Life Sci. 1989;44(4):281–287. doi: 10.1016/0024-3205(89)90186-0. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11(6):557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5(5):446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348(1):201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77(10):859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Vatsalya V, Westman JG, Schwandt ML, Bartlett S, Heilig M, Momenan R, Ramchandani VA. The Effect of Varenicline on the Neural Processing of Fearful Faces and the Subjective Effects of Alcohol in Heavy Drinkers. Alcohol Clin Exp Res. 2016;40(5):979–987. doi: 10.1111/acer.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Young EA, Cunningham CL. Blockade of opioid receptors in anterior cingulate cortex disrupts ethanol-seeking behavior in mice. Behav Brain Res. 2011;219(2):358–362. doi: 10.1016/j.bbr.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78(2–3):69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol Learn Mem. 2014;108:104–118. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129(2):99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52(6):1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65(5):682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Bonci A. The SK channel as a novel target for treating alcohol use disorders. Channels (Austin) 2011;5(4):289–292. doi: 10.4161/chan.5.4.16577. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Slesinger PA, Davies DL, Das J, Trudell JR, Harris RA. Alcohol-binding sites in distinct brain proteins: the quest for atomic level resolution. Alcohol Clin Exp Res. 2011;35(9):1561–1573. doi: 10.1111/j.1530-0277.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Majchrowicz E, Dalton TK, Swartzwelder HS, Wixon H. Alterations in neurotransmitter activity after acute and chronic ethanol treatment: studies of transmitter interactions. Alcohol Clin Exp Res. 1979;3(4):359–363. doi: 10.1111/j.1530-0277.1979.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006;1073–1074:175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol. 2015;33:188–196. doi: 10.1016/j.conb.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Robbe D, Bockaert J, Manzoni OJ. Group 2 metabotropic glutamate receptors induced long term depression in mouse striatal slices. Neurosci Lett. 2001;316(3):178–182. doi: 10.1016/s0304-3940(01)02397-7. [DOI] [PubMed] [Google Scholar]
- Kaneyuki T, Morimasa T, Okada H, Shohmori T. The effect of acute and repeated ethanol administration on monoamines and their metabolites in brain regions of rats. Acta Med Okayama. 1991;45(4):201–208. doi: 10.18926/AMO/32171. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology. 2016;110(Pt A):190–197. doi: 10.1016/j.neuropharm.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46(4):303–308. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, Janak PH. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron. 2015;88(2):247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Chen L, Ding JB. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350(6256):102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MO, Lee YK, Choi WS, Kim JH, Hwang SK, Lee BJ, Kang SG, Kim K, Baik SH. Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol Cells. 1997;7(5):682–687. [PubMed] [Google Scholar]
- Kim N, Barter JW, Sukharnikova T, Yin HH. Striatal firing rate reflects head movement velocity. Eur J Neurosci. 2014;40(10):3481–3490. doi: 10.1111/ejn.12722. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374(4):578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68(8):1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18(9):1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix F, Pettorelli A, Maddux JM, Heidari-Jam A, Chaudhri N. Varenicline Reduces Context-Induced Relapse to Alcohol-Seeking through Actions in the Nucleus Accumbens. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171(3):830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, Deisseroth K. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162(3):635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lograno DE, Matteo F, Trabucchi M, Govoni S, Cagiano R, Lacomba C, Cuomo V. Effects of chronic ethanol intake at a low dose on the rat brain dopaminergic system. Alcohol. 1993;10(1):45–49. doi: 10.1016/0741-8329(93)90052-p. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243(4899):1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann N Y Acad Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Mathur BN, Capik NA, Alvarez VA, Lovinger DM. Serotonin induces long-term depression at corticostriatal synapses. J Neurosci. 2011;31(20):7402–7411. doi: 10.1523/JNEUROSCI.6250-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem. 2004;82(3):299–308. doi: 10.1016/j.nlm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61(7):1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: a dual-probe microdialysis study. Neuropsychopharmacology. 2003;28(5):939–946. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- Melis M, Diana M, Enrico P, Marinelli M, Brodie MS. Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol. 2009;43(7):531–539. doi: 10.1016/j.alcohol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Morales-Mulia M. Ethanol exposure differentially alters pro-enkephalin mRNA expression in regions of the mesocorticolimbic system. Psychopharmacology (Berl) 2006;189(1):117–124. doi: 10.1007/s00213-006-0503-3. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292(1):63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- Milton GV, Randall PK, Erickson CK. Low-dose effect of ethanol on locomotor activity induced by activation of the mesolimbic system. Alcohol Clin Exp Res. 1995;19(3):768–776. doi: 10.1111/j.1530-0277.1995.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O'Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4(116):116ra116. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7(6 Suppl):3S–23S. [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Britton RS, Seeman P. The effects of long-term ethanol on brain receptors for dopamine, acetylcholine, serotonin and noradrenaline. Eur J Pharmacol. 1980;65(1):31–37. doi: 10.1016/0014-2999(80)90205-8. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Mulder AH, Wardeh G, Hogenboom F, Schoffelmeer AN. Unrestricted free-choice ethanol self-administration in rats causes long-term neuroadaptations in the nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 1999;141(3):307–314. doi: 10.1007/s002130050838. [DOI] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A. 2011;108(11):4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Multiple Systems for the Motivational Control of Behavior and Associated Neural Substrates in Humans. Curr Top Behav Neurosci. 2016;27:291–312. doi: 10.1007/7854_2015_386. [DOI] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron. 2016;92(2):493–504. doi: 10.1016/j.neuron.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Gabriele A. Peripheral anxiogenic drug injections differentially affect cognitive and habit memory: role of basolateral amygdala. Neuroscience. 2009;164(2):457–462. doi: 10.1016/j.neuroscience.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Palm S, Nylander I. Dopamine release dynamics change during adolescence and after voluntary alcohol intake. PLoS One. 2014;9(5):e96337. doi: 10.1371/journal.pone.0096337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino SM, Druse MJ. The effects of chronic ethanol consumption on the mesolimbic and nigrostriatal dopamine systems. Alcohol Clin Exp Res. 1992;16(2):275–280. doi: 10.1111/j.1530-0277.1992.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30(5):901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Jeanes ZM, Morrisett RA. Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPV1 receptors. Alcohol Clin Exp Res. 2014;38(11):2763–2769. doi: 10.1111/acer.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2017;112(Pt A):164–171. doi: 10.1016/j.neuropharm.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Donahue R, Gill TM, Nie H, Ron D, Janak PH. Alpha4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of alcohol. Addict Biol. 2012;17(2):309–321. doi: 10.1111/j.1369-1600.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33(7):1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: in vivo and in vitro electrochemical measurements. Alcohol Clin Exp Res. 2005;29(5):746–755. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149(3):217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303(3):1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24(5):1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rominger A, Cumming P, Xiong G, Koller G, Boning G, Wulff M, Zwergal A, Forster S, Reilhac A, Munk O, Soyka M, Wangler B, Bartenstein P, la Fougere C, Pogarell O. [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol. 2012;17(2):490–503. doi: 10.1111/j.1369-1600.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Raeder C, Kaulen P, Bruning G. Adaptive changes of dopamine-D2 receptors in rat brain following ethanol withdrawal: a quantitative autoradiographic investigation. Alcohol. 1992;9(5):355–362. doi: 10.1016/0741-8329(92)90032-6. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider JS. Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci Lett. 2001;300(2):63–66. doi: 10.1016/s0304-3940(01)01548-8. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev. 2010;3(3):163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Robbe D. The striatum multiplexes contextual and kinematic information to constrain motor habits execution. Nat Neurosci. 2015;18(3):453–460. doi: 10.1038/nn.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Crabbe JC, Phillips TJ. Dopamine antagonist effects on locomotor activity in naive and ethanol-treated FAST and SLOW selected lines of mice. Psychopharmacology (Berl) 1995;118(1):28–36. doi: 10.1007/BF02245246. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. Voluntary ethanol intake predicts kappa-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci. 2015;35(15):5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43(7):509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, Hoffman H, Callaway EM, Gerfen CR, Jin X. Genetic-Based Dissection Unveils the Inputs and Outputs of Striatal Patch and Matrix Compartments. Neuron. 2016;91(5):1069–1084. doi: 10.1016/j.neuron.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Formigoni ML, De Lucca EM, Hipolide DC, Enns SC, Oliveira MG, Nobrega JN. Sensitization to ethanol's stimulant effect is associated with region-specific increases in brain D2 receptor binding. Psychopharmacology (Berl) 1999;146(3):262–267. doi: 10.1007/s002130051115. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Styles M, Baars AM, Lozeman-van 't Klooster JG, Lesscher HM, Vanderschuren LJ. Dopaminergic neurotransmission in ventral and dorsal striatum differentially modulates alcohol reinforcement. Eur J Neurosci. 2016 doi: 10.1111/ejn.13358. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16(7):966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A. Alcohol's effects on brain and behavior. Alcohol Res Health. 2010;33(1–2):127–143. [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Talani G, Lovinger DM. Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. 2015;49(8):781–794. doi: 10.1016/j.alcohol.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta JP, Job MO, Mangieri RA, Schier CJ, Howard EC, Gonzales RA. mu-opioid receptors in the stimulation of mesolimbic dopamine activity by ethanol and morphine in Long-Evans rats: a delayed effect of ethanol. Psychopharmacology (Berl) 2013;228(3):389–400. doi: 10.1007/s00213-013-3041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116(3):163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32(43):15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27(13):3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J Neurosci. 2015;35(33):11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30(30):10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol on striatal dopamine overflow and clearance: an in vivo electrochemical study. Alcohol. 1997;14(6):593–601. doi: 10.1016/s0741-8329(97)00054-2. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kato A, Sawara K, Butterworth RF, Sasaki T, Terasaki K, Sera K, Suzuki K. Selective alterations of brain dopamine D(2) receptor binding in cirrhotic patients: results of a (11)C-N-methylspiperone PET study. Metab Brain Dis. 2008;23(3):265–274. doi: 10.1007/s11011-008-9092-7. [DOI] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39(3):579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci U S A. 2012;109(5):E278–287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Gonzales RA. Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: reversal by glycine. J Neurochem. 1990;54(2):712–715. doi: 10.1111/j.1471-4159.1990.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee MR, Choi S, Kim T, Choi DS. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res. 2010;34(6):1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Kim JI, Tawfik VL, Lalchandani RR, Scherrer G, Ding JB. Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum. Cell Rep. 2015;10(1):75–87. doi: 10.1016/j.celrep.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2006;30(5):819–824. doi: 10.1111/j.1530-0277.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22(2):367–374. [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci. 2007;25(11):3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]